Abstract
This study evaluates the functional procoagulant features of plasma MP in order to explore the MP contribution to the hypercoagulable state of patients with Essential Thrombocythemia (ET). Platelet-free plasma samples were obtained from 73 ET patients (37 positive for the JAK2V617F mutation) and 72 control subjects. The calibrated automated thrombogram (CAT) was performed in plasma samples to determine thrombin generation of MP-associated tissue factor (TF) and procoagulant phospholipid (PPL) activity, and the STA Procoag PPL assay to measure MP-PPL activity only. Both thrombin generation and PPL procoagulant activities were found significantly elevated in ET patients compared to controls, and were associated to significantly higher levels of TF antigen and FVIIa/AT complex. Thrombin generation was significantly greater in JAK2-V617F positive compared to JAK2-V617F negative patients and normal subjects. Significant correlations were found between the PPL-assay and the different parameters of the CAT assay. No difference was seen between the thrombosis and no thrombosis group. Prospective studies are needed to test whether MP-associated thrombin generation and procoagulant activity may predict for thrombosis in these patients.
INTRODUCTION
Essential Thrombocythemia (ET) is a classic Philadelphia chromosome negative myeloproliferative neoplasm characterized by an increased rate of major arterial and venous thrombosis, and high frequency of microvascular occlusive symptoms, which are usually sensitive to low-dose aspirin [1]. Even though vascular complications represent the major cause of morbidity and mortality, the pathogenesis of the thrombotic diathesis typical of this disease is not yet fully understood. Among the mechanisms proposed, a prominent role has been attributed to both quantitative and qualitative abnormalities of platelets and leukocytes, arising from the clonal proliferation of hematopoietic progenitor cells [2-3]. These abnormalities are more commonly observed in ET patients carrying the acquired JAK2V717F mutation, which, found in about 60% of patients, is also considered another important mechanism for thrombosis [3-5].
In ET the hyper-viscosity of the blood, due to the grossly elevated platelet and leukocyte levels, together with the occurrence of platelet and leukocyte activation [6-7], can all favour an increased microparticle (MP) generation [8]. MP produced by both normal and cancer cells, depending on their origin, may express TF and/or procoagulant phospholipid (PPL) on their surface [9-10]. The TF content of MP contributes far more to the procoagulant activity than the procoagulant PS surface [11]. The increased number and thrombogenic activity of MP in thrombotic diseases indicate an important role of these cell-derived elements in the pathogenesis of thrombosis. Indeed, MP have been found altered in many diseases, including cancer, associated with an increased risk for both arterial and venous thrombosis [12-13].
Recently, in a small study, we could demonstrate significantly higher levels of circulating MP in ET patients [8]. In this larger study we explored the functional contribution of MP to the hypercoagulable state of ET by two different assays – the thrombin generation assay, measuring the combined procoagulant effect of TF and PPL, and a FXa based procoagulant activity assay, measuring the contribution of PPL alone. The circulating antigen levels of TF and FVIIa/AT complex (reflecting TF expression and exposure on cell surface), were determined as markers of blood clotting activation. All parameters were correlated with the incidence of the JAK2V617F mutation, type of therapy, and history of thrombosis.
MATERIALS AND METHODS
Study subjects
Seventy-two consecutive healthy controls (33 males and 39 females) and 73 ET patients (27 males and 46 females) diagnosed according to the WHO criteria [14] were consecutively enrolled. The patient and control characteristics are shown in Table 1. None of the healthy controls were taking any antiplatelet or anti-inflammatory drugs in the last 10 days prior blood drawing and none of them had history of thrombosis or other diseases with high risk for thrombosis (i.e. immunological or neoplastic diseases), or ongoing inflammatory status. In addition none of the female controls were taking oral contraceptives or hormone replacement therapy. None of the patients were taking anticoagulant or hormone replacement therapy. All investigations were approved by the local ethical committee (Comitato di Bioetica, Ospedale Papa Giovanni XXIII, Bergamo, Italy) and all of the study subjects gave informed written consent to the study. The procedures followed were in accordance with the Helsinki declaration of 1975 as revised in 2000.
Table 1.
Characteristics of patients and controls. Data are expressed as number or mean (range).
| Controls | ET | ET | |||
|---|---|---|---|---|---|
| JAK2V617F negative |
JAK2V617F positive |
||||
| Number | 71 | 73 | 34 | 39 | |
| Sex (M/F) | 33/38 | 27/46 | 16/18 | 13/26 | |
| Age (years) | 47 (26-70) | 57 (23-81)* | 54 (23-77) | 60 (35-81)§ | |
| Platelets (x10^9/L) | 249 (158-450) | 604 (223-1451)* | 666 (223-1451) | 556 (239-1427) | |
| WBC (x10^9/L) | 6.4 (3.7-10.3) | 7.4 (3.2-13.3) | 7.2 (3.2-11.7) | 7.54 (5.1-13.3) | |
| RBC (x10^6/L) | 4.8 (3.6-5.5) | 4.5 (3.0-6.6) | 4.4 (3.2-5.6) | 4.5 (3.0-6.6) | |
| HCT (%) | 42 (35-48) | 41 (33-48) | 40 (33-46) | 41 (34-48) | |
| THERAPY | NT | - | 11 | 4 | 7 |
| ASA | - | 29 | 18 | 11 | |
| HU | - | 5 | 3 | 2 | |
| HU+ASA | - | 28 | 9 | 19 | |
| THROMBOSIS (arterial/venous) | - | 26 (18/8) | 6 (6/3) | 20 (15/5)§ | |
indicates p value < 0.05. ET essential thrombocythemia. WBC= White Blood Cell count; NT= not treated; ASA= Aspirin; HU= Hydroxyurea.
Routine hematologic assays
White blood cell differential count, hematocrit, hemoglobin, red blood cell count, and platelet count were determined by a Sysmex- XE 2100 hematology analyzer (Sysmex, Kobe, Japan).
Blood collection and plasma preparation
Venous blood was collected by a team of experienced nurses from fasting patients and healthy subjects in the morning (09:00 to 11:00 am), and for patients also before any therapy, using a 21-gauge needle. Following the application of a light tourniquet, into 6 mL tubes containing 3.2% citrate (0.109 mol/L, 1:9 vol./vol.) (BD, Vacutainer™, Plymouth, UK), discarding the first 2-3 mL of blood. Anticoagulant was mixed during blood collection with gentle inversion of the tube. The transportation of blood tubes was carried out carefully to avoid unnecessary agitation. Samples were kept at room temperature (20-24 °C) and processed within 2 hours of collection.
Platelet poor plasma (PPP) was obtained by centrifugation at 1,500 g for 15 min at 24°C, and platelet free plasma (PFP) by further centrifugation of PPP at 18,000 g for 10 min at 20°C avoiding application of the centrifuge brake. Aliquots of PFP were snap-frozen in liquid nitrogen and stored at −80°C until use. The duration of storage of plasma samples before testing ranged from a min of 4 to a max of 6 months. Aliquots of 2 ml of frozen samples of PFP were thawed for 10 minutes at 37°C before use or until completely thawed. To remove MP, 250μL of PFP was centrifuged for 30 min at 18,000 g at 20°C, then 225 μL of supernatant (i.e. MP free plasma, MP-FP) was collected and used for the assays.
Thrombin generation assay
Thrombin generation was measured by the calibrated automated thrombogram method (CAT – from Thrombinoscope BV, Maastricht, Netherlands) [15]. 80 μl of plasma sample (PPP, PFP and MP-FP) were incubated for 10 minutes at 37°C with 20 μl of HEPES buffer. Coagulation was started by the addition of CaCl2 and a fluorogenic thrombin substrate (Z-Gly-Gly-Arg-AMC, Diagnostica Stago, Asnieres, France). Thrombin generation was measured using a Fluoroskan Ascent reader (Thermo Labsystems OY, Helsinki, Finland). Lag-time, endogenous thrombin potential (ETP), peak height and time to peak (ttP) were calculated using the Thrombinoscope software (Thrombinoscope BV, Netherlands). The lag-time, the initiation phase of coagulation, and the ttP, the time necessary to form the maximum concentration of thrombin, are expressed in minutes. Peak height, the maximum concentration of thrombin generated, is expressed in nM, while the ETP, defined as area under the curve, is expressed in nM*min. Thrombin generation test was performed in batch with inclusion of internal controls (2 levels) for validation of the runs. In addition, a normal pool platelet free plasma (n= 20 healthy donors) prepared in our laboratory was also included in each run to validate the results.
Plasma phospholipid procoagulant activity assay
The STA-Procoag-PPL (Diagnostica Stago, Asnieres, France) assay was used to evaluate the influence of plasma PPL on the activation of the coagulation cascade. The PPL assay measures clotting time, in the presence of FXa and CaCl2, of a system in which all the factors are present in physiological levels (supplied by P-PPL Depleted Plasma) except the PPL which are supplied by the plasma sample being tested. The assay was performed using a manual clot-timer (Heller Labs, San Diego, USA) on PFP and MP-FP. Results are expressed as coagulation time (sec), the shorter coagulation time the higher PPL activity. PPL assay was performed in batch with inclusion of positive and normal control samples, provided by the kit, for validation of the runs. The runs were then accepted as ‘valid’ if the results of these control samples were within the range of values as declared by the manufacturers. Furthermore, a normal pool platelet free plasma (n= 20 healthy donors) prepared in our laboratory was also included in each run to validate the results.
Tissue Factor and FVIIa-AT complex
Plasma levels of TF antigen (American Diagnostica, USA) and of FVIIa/AT complex (Diagnostica Stago, Asnieres, France) were measured by ELISA. The FVIIa/AT complex is considered an indirect marker of in vivo TF exposure and inhibition.
Statistical analysis
Results are expressed by mean ± standard error of the mean (SEM). Student’s t-test was employed to compare the difference between the mean values of different groups. Differences were considered significant at a p-value <0.05. Linear regression analysis was used to test the association between continuous variables. Multiple regression analysis was used to correct for differences in age and sex distribution, JAK2V617F mutational status and pharmacological treatment among groups. Regression coefficients are expressed as B, which represents the absolute change of the dependent variable increases by 1 unit. Statistical analyses were performed using the SPSS 15 statistical package (SPSS, Chicago, IL, USA).
RESULTS
Study subjects
ET patients had significantly higher total platelet counts compared to controls (Table 1). Among ET patients, 39 (53%) were JAK2V617F positive with < 50% allele burden, while the remaining were JAK2 negative. At enrolment into the study, 29 patients were receiving treatment with aspirin (ASA), 5 with hydroxyurea (HU), and 28 with HU in combination with ASA. Eleven patients were not receiving any cytoreductive or antiplatelet therapy. Twenty-six ET patients (35%) had a positive history for at least one major thrombotic event, and 68% of these were on arterial basis.
Thrombin generation and PPL activity are increased in ET patients
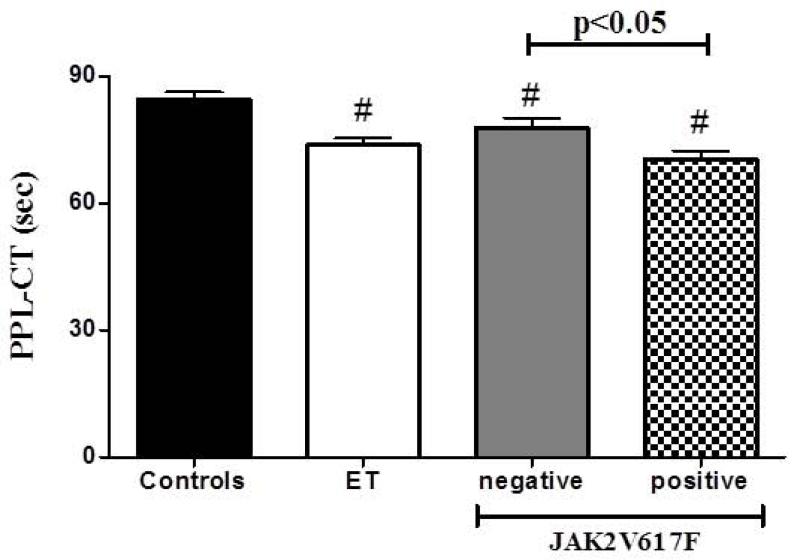
The evaluation of thrombin generation showed significantly higher procoagulant potential in plasma samples from ET patients (Figure 1) compared to those from controls, as indicated by the shorter lag-time (22.9±1.3 vs 29.4±2.0 min; p<0.01) and ttP (27.6±1.2 vs 33.4±1.8 min; p<0.05), and by the higher peak (50.5±3.3 vs 36.3±2.8 nM; p<0.01) and ETP (752±33 vs 546±42 nM*min) of the thrombin generation curves. Accordingly, results from the PPL assay (Figure 2) demonstrated significantly shorter clotting times (CT) in plasma samples from ET patients compared to controls (74 ±1.6 vs 84.7 ±1.6 sec; p<0.001). Statistically significant correlations (p<0.05) were found between the PPL-CT and the different parameters of the thrombin generation assay - positive with the kinetic parameters (lag-time and ttp) and negative with ETP and peak height (Figure 3).
Figure 1. MP-associated thrombin generation by the CAT assay.
Thrombin generation results (mean ± SD) are described in terms of lag-time, ttp, peak of thrombin and endogenous thrombin potential (ETP). Data obtained in ET patients are shown as overall group and according to JAK2V617F mutational status. # =p<0.05 vs controls.
Figure 2. MP-associated procoagulant activity.
The STA-Procoag-PPL assay was used to evaluate the influence of plasma PPL on the activation of the coagulation cascade. ET patients, and particularly those positive for JAK2V617F mutation, display shorter clotting times compared to controls, suggesting higher PPL-dependent procoagulant activity.
Figure 3. Correlations analysis between TG and PPL assays.
Clotting times from PPL assay (seconds) are plotted against the different parameters of thrombin generation curve, i.e. lag-time, time to peak (ttp), peak of thrombin and ETP.
Influence of JAK2-V617F mutation and type therapy on plasma procoagulant features of ET patients
In ET patients, data obtained by both the thrombin generation and the PPL assays were analyzed according to patients’ JAK2-V617F mutational status and type of therapy at the time of enrolment (Figure 1 and 2). The different parameters of the thrombin generation curves displayed a significantly higher procoagulant potential in JAK2V617F-positive compared to negative patients and controls. Particularly, plasma samples from JAK2V617F positive patients showed shorter lag-time (19.5±1.25 vs 24.5±8.1 min; p<0.05) and ttP (24.9±1.1 vs 30.2±1.8 min), and higher peak (56.5±5.1 vs 40.6±4.5 nM; p<0.05) and ETP (807±41.2 vs 616±61.5 nM*min) than negative patients. No significant differences were observed in the different parameters of TG between controls and negative patients.
Multiple regression analysis was performed to correct for age, gender and concomitant therapy. Correction for these variable revealed that the observed differences in all the thrombin generation parameters between ET patients and controls were largely explained by the JAK2V617F mutation.
Similarly, significantly shorter PPL-CT were generated by plasma samples from JAK2V617F positive compared to negative patients (70.42±2.1 vs 82.4±2.33 sec; p<0.05) and controls (70.4±2.1 vs 84.7±2.3 sec; p<0.05). A significant shorter PPL-CT generated by plasma samples from negative patients compared to controls (78±2.3 vs 84.7±2.3 sec; p<0.05) was also observed. After correction for gender, age, and therapy, JAK2V617F still remained a significant predictor of shorter PPL-CT (p=0.012, β= −0.311, B=−8.46).
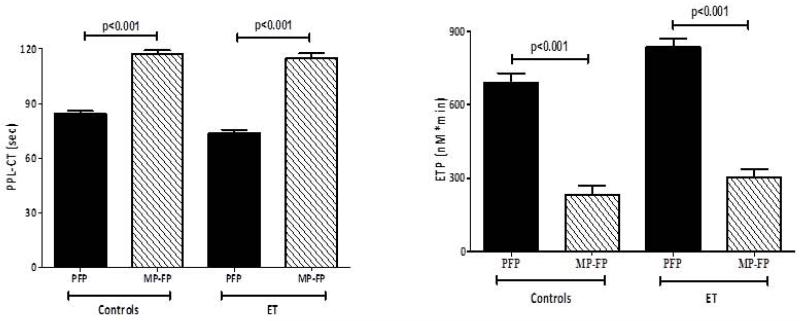
Removal of the MP fraction by high-speed centrifugation of plasma significantly reduced the procoagulant activity of samples from both ET and control subjects (Figure 4). The reduction was much greater in the thrombin generation assay (85% mean reduction) than in the PPL assay (20% mean reduction).
Figure 4. Effect of MP reduction on TG and PPL-1 assays.
Platelet free plasma (PFP) was centrifuged at high speed to pellet MP and supernatant (MP-FP) was tested for its capacity to induce thrombin generation (left panel, ETP) and procoagulant activity (right panel). Results, expressed as mean ± SD, compare PFP with MP-FP in controls and ET patients.
In patients on HU or HU-ASA treatments, the lag-time and ttp of thrombin generation and the CT of PPL assay were slightly, though not significantly, prolonged compared to non HU-treated patients (data not shown). No significant difference was seen between the thrombosis and no thrombosis history group with either the thrombin generation or PPL assays. No difference was found between arterial or venous thrombosis cases.
Circulating TF and FVIIa/AT complex
Plasma levels of TF antigen (246±23 vs 136±7.2 pg/ml, p<0.001) and FVIIa/AT (340±19 vs 247±15 pmol/L; p<0.01) were significantly elevated in patients compared to controls (Figure 5). Among patients the distribution of the two markers was not related to therapy or JAK2V617F mutation. No significant correlation was found between the two parameters.
Figure 5. Plasma levels of TF and FVIIa/AT complex.
Plasma levels of TF antigen and FVIIa/AT complex, an indirect marker of in vivo TF exposure and inhibition, were measured by ELISA in ET patients and controls. For ET patients data are shown as overall group and according to JAK2V617F mutational status. *=p<0.05 vs controls.
DISCUSSION
This study demonstrates that ET patients are characterized by increased plasma MP-related procoagulant activity as determined by both the thrombin generation and PPL assays. Similarly, raised levels of both TF and FVIIa/AT were observed in the same patients.
These data confirm the findings of our previous work, in which the thrombin generation analyses revealed that MP-rich plasma from 21 ET patients had an increased thrombin generation potential (i.e. shorter lag time and higher peak height) compared to plasma from controls. However, in that study we could not highlight differences between patients positive or negative for the JAK2V617F mutation, probably due to the small sample size. Differently, in the present paper, enrolling 73 ET patients, statistical analysis revealed that the group of subjects positive for the JAK2V617F mutation were significantly more procoagulant than the negative patients, as demonstrated by the shorter lag-time and time to peak, and the higher peak and ETP of thrombin generation and the shorter PPL-CT. Furthermore, multiple linear regression analysis indicated JAK2V617F as the major determinant of increased thrombin generation and shorter PPL-CT in our population.
Our findings are similar to those published by Duchemin et al [16] who demonstrated in a group of patients with myeloproliferative disorders that the JAK2V617F mutational status was responsible for a decreased sensitivity of thrombin generation to thrombomodulin inhibition, homozygous patients presenting with the lowest inhibition rate. As the removal of MP increased the sensitivity to thrombomodulin in plasma, the authors suggested also an influence of MP on the “ thrombomodulin resistance” observed in their study subjects [16].
It is now clear that patients with the JAK2V617F mutation are biologically distinct from those without the mutation [17], and typically present with higher leukocyte counts and increased thrombotic risk [5, 18]. In addition, we and other groups could demonstrate that ET patients positive for the JAK2V617F mutation are characterized by a more pronounced thrombophilic state compared to JAK2V617F negative patients, relating to blood clotting, neutrophil and endothelium activation, increased number of neutrophil/platelet mixed aggregates, and expression of platelet TF [19-20]. Furthermore, we also found a significant association between the presence of the JAK2V617F mutation and the occurrence of an acquired activated protein C resistant phenotype [15].
The risk of major thrombosis is higher in patients with ET who are older than 60 years and who have had a previous occlusive event. In this high-risk group, the non alkylating agent hydroxyurea significantly reduces the rate of vascular complications and is the treatment of choice. In the present study, treatment with hydroxyurea affected, through not significantly, the patients’ thrombin generation or PPL assay values, this supporting other clinical and biological data [16, 21-22]. The influence was seen only on the kinetic parameters of thrombin generation (lag-time and ttp), which are considered to reflect TF activity, but not on ETP and peak height. We cannot exclude that some of the antithrombotic effect of hydroxyurea described in patients may due to activation via a non-TF or non-PPL MP route so that it would not been seen by this study.
We could provide some evidence that the increased procoagulant activity of plasma was MP-related as removal of part of the MP population significantly reduced plasma procoagulant activity in both thrombin generation and PPL assays. The phenomenon was observed in plasma of both patients and controls and abolished the differences between the two groups.
Interestingly, MP-removal reduced the thrombin generation capacity by at least 85%, while only a 25% reduction was reached by the PPL assay. The present data are in agreement with previous observation showing that high speed centrifugation abolished thrombin generation capacity of plasma supernatants [23]. On this basis, it seems that the thrombin generation assay represents a more sensitive measure of procoagulant activity of MP carrying active TF than the PPL assay. In the thrombin generation assay used in this study, activation of plasma coagulation initiated by the addition of alone Ca2+, therefore, it is sensitive to all determinants (i.e. TF or PPL) present in the samples. Differently, the PPL assay is insensitive to TF and only measures PPL. Our data support findings demonstrating that TF and PPL has a much greater prothrombotic effect than PPL alone [11]. However, the fact that MP-removal reduced the PPL activity by 25% support the concept that also PPL can play an important role in the procoagulant activity of MP of our samples. That the two assays are related, but are not measuring the same coagulant determinants, is also supported by the fact that correlations between PPL assay and thrombin generation parameters are not very strict (R<0.5), although statistically significant.
After high speed centrifugation, it is possible that exosomes, and very small microparticles (carrying or not TF) are still present in plasma samples, thus contributing to their residual thrombin generation capacity and PPL activity. At today, the cellular origin of MP carrying TF still needs to be established and is important to consider that exchange of proteins along with MP of different types can take place. In this regard, we have to consider that ET resembles a chronic inflammatory disease which determines activation of the endothelium resulting in the production of TF-bearing MP. In our previous study significantly higher number of MP carrying TF were found in ET patients compared to controls, and, furthermore, TF was detected also on MP expressing both platelet and endothelial markers, suggesting these hybrid MP as one of the source of plasma TF [8]. Circulating MP released from various cell types are present in healthy individuals and the number and composition of their membranes vary in different disorders. Studies in normal subjects show that the predominant sources of the MP are platelet, endothelial, or leukocyte [24]. In ET patients a similar pattern of MP is seen as in healthy subjects, again the majority being of platelet origin [8]. Both these studies, using low sensitive techniques [8, 24], only found a very low number of TF positive MP (i.e. <1%), once more indicating the important positive role of TF activity on the procoagulant potential of the samples. It is uncertain whether, under normal conditions, MP are largely produced by platelets or may be generated to any significant degree by megakaryocytes, as recently reported [25].
Finally, our investigation shows raised plasma levels of both TF antigen and FVIIa/AT in ET patients, which were independent from JAK2V617F mutational status. There was no correlation between TF and FVIIa/AT as well as between TF and FVIIa/AT and thrombin generation or PPL assay. The TF assay utilized in the present study is immunologic and thus would measure all forms of TF including inactive and degraded TF. Therefore, lack of correlations with FVIIa-AT and thrombin generation may be expected as it reflects the total TF expression (both the active encrypted and inactive decrypted forms).
In summary, data from this study show that plasma from ET patients displayed increased thrombin generation potential and procoagulant activity compared to controls due to the presence of circulating MP, the highest values being observed in patients positive for JAK2V617F mutation. Therefore, this study adds new information to the mechanisms underlying the hypercoagulable state in patients with ET. Given the nature of the study, we could not evaluate the contribution of these assays to the thrombotic risk and prospective studies are needed to test whether MP-associated thrombin generation and procoagulant activity may predict for thrombosis in these patients. Furthermore, given the role of MP in tumor progression [12], future research is needed to understand whether MP may also have a role in the leukemic and/or fibrotic evolution of this disease.
Acknowledgments
This work is supported in part by the National Institutes of Health (Myeloproliferative Disorders-Research Consortium grant), and by grants of the Italian Association for Cancer Research (AIRC) (AIRC grant n. IG10558 and AIRC “5×1000” n. 12237).
Footnotes
Disclosure of Interest
Barry Woodhams is the Scientific Director of Stago, provided the kits for the experiments and revision of the paper.
REFERENCES
- 1.Tefferi A. Polycythemia vera and essential thrombocythemia: 2012 update on diagnosis, risk stratification, and management. Am J Hematol. 2012;87(3):285–93. doi: 10.1002/ajh.23135. [DOI] [PubMed] [Google Scholar]
- 2.Falanga A, et al. Pathogenesis of thrombosis in essential thrombocythemia and polycythemia vera: the role of neutrophils. Semin Hematol. 2005;42(4):239–47. doi: 10.1053/j.seminhematol.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 3.Marchetti M, Falanga A. Leukocytosis, JAK2V617F mutation, and hemostasis in myeloproliferative disorders. Pathophysiol Haemost Thromb. 2008;36(3-4):148–59. doi: 10.1159/000175153. [DOI] [PubMed] [Google Scholar]
- 4.Cheung B, et al. The presence of the JAK2 V617F mutation is associated with a higher haemoglobin and increased risk of thrombosis in essential thrombocythaemia. Br J Haematol. 2006;132(2):244–5. doi: 10.1111/j.1365-2141.2005.05858.x. [DOI] [PubMed] [Google Scholar]
- 5.Lussana F, et al. Association of V617F Jak2 mutation with the risk of thrombosis among patients with essential thrombocythaemia or idiopathic myelofibrosis: A systematic review. Thromb Res. 2009 doi: 10.1016/j.thromres.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Falanga A, et al. Polymorphonuclear leukocyte activation and hemostasis in patients with essential thrombocythemia and polycythemia vera. Blood. 2000;96(13):4261–6. [PubMed] [Google Scholar]
- 7.Arellano-Rodrigo E, et al. Increased platelet and leukocyte activation as contributing mechanisms for thrombosis in essential thrombocythemia and correlation with the JAK2 mutational status. Haematologica. 2006;91(2):169–75. [PubMed] [Google Scholar]
- 8.Trappenburg MC, et al. Elevated procoagulant microparticles expressing endothelial and platelet markers in essential thrombocythemia. Haematologica. 2009;94(7):911–8. doi: 10.3324/haematol.13774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enjeti AK, Lincz LF, Seldon M. Microparticles in health and disease. Semin Thromb Hemost. 2008;34(7):683–91. doi: 10.1055/s-0028-1104547. [DOI] [PubMed] [Google Scholar]
- 10.Morel O, et al. Cellular mechanisms underlying the formation of circulating microparticles. Arterioscler Thromb Vasc Biol. 2011;31(1):15–26. doi: 10.1161/ATVBAHA.109.200956. [DOI] [PubMed] [Google Scholar]
- 11.Owens AP, 3rd, Mackman N. Microparticles in hemostasis and thrombosis. Circ Res. 2011;108(10):1284–97. doi: 10.1161/CIRCRESAHA.110.233056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falanga A, Tartari CJ, Marchetti M. Microparticles in tumor progression. Thromb Res. 2012;129(Suppl 1):S132–6. doi: 10.1016/S0049-3848(12)70033-6. [DOI] [PubMed] [Google Scholar]
- 13.Davizon P, Lopez JA. Microparticles and thrombotic disease. Curr Opin Hematol. 2009;16(5):334–41. doi: 10.1097/MOH.0b013e32832ea49c. [DOI] [PubMed] [Google Scholar]
- 14.Thiele J, Kvasnicka HM. Chronic myeloproliferative disorders. The new WHO classification. Pathologe. 2001;22(6):429–43. doi: 10.1007/s002920100492. [DOI] [PubMed] [Google Scholar]
- 15.Marchetti M, et al. Thrombin generation and activated protein C resistance in patients with essential thrombocythemia and polycythemia vera. Blood. 2008;112(10):4061–8. doi: 10.1182/blood-2008-06-164087. [DOI] [PubMed] [Google Scholar]
- 16.Duchemin J, et al. Increased circulating procoagulant activity and thrombin generation in patients with myeloproliferative neoplasms. Thromb Res. 2010;126(3):238–42. doi: 10.1016/j.thromres.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 17.Barbui T, et al. Survival and disease progression in essential thrombocythemia are significantly influenced by accurate morphologic diagnosis: an international study. J Clin Oncol. 2011;29(23):3179–84. doi: 10.1200/JCO.2010.34.5298. [DOI] [PubMed] [Google Scholar]
- 18.Carobbio A, et al. Leukocytosis is a risk factor for thrombosis in essential thrombocythemia: interaction with treatment, standard risk factors, and Jak2 mutation status. Blood. 2007;109(6):2310–3. doi: 10.1182/blood-2006-09-046342. [DOI] [PubMed] [Google Scholar]
- 19.Falanga A, et al. V617F JAK-2 mutation in patients with essential thrombocythemia: relation to platelet, granulocyte, and plasma hemostatic and inflammatory molecules. Exp Hematol. 2007;35(5):702–11. doi: 10.1016/j.exphem.2007.01.053. [DOI] [PubMed] [Google Scholar]
- 20.Arellano-Rodrigo E, et al. Platelet turnover, coagulation factors, and soluble markers of platelet and endothelial activation in essential thrombocythemia: relationship with thrombosis occurrence and JAK2 V617F allele burden. Am J Hematol. 2009;84(2):102–8. doi: 10.1002/ajh.21338. [DOI] [PubMed] [Google Scholar]
- 21.Panova-Noeva M, et al. Platelet-induced thrombin generation by the calibrated automated thrombogram assay is increased in patients with essential thrombocythemia and polycythemia vera. Am J Hematol. 2011;86(4):337–42. doi: 10.1002/ajh.21974. [DOI] [PubMed] [Google Scholar]
- 22.Cortelazzo S, et al. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N Engl J Med. 1995;332(17):1132–6. doi: 10.1056/NEJM199504273321704. [DOI] [PubMed] [Google Scholar]
- 23.Haubold K, et al. Tissue factor procoagulant activity of plasma microparticles is increased in patients with early-stage prostate cancer. Thromb Haemost. 2009;101(6):1147–55. [PubMed] [Google Scholar]
- 24.Campello E, et al. Circulating microparticles in carriers of factor V Leiden with and without a history of venous thrombosis. Thromb Haemost. 2012;108(4):633–9. doi: 10.1160/TH12-05-0280. [DOI] [PubMed] [Google Scholar]
- 25.Italiano JE, Jr., Mairuhu AT, Flaumenhaft R. Clinical relevance of microparticles from platelets and megakaryocytes. Curr Opin Hematol. 2010;17(6):578–84. doi: 10.1097/MOH.0b013e32833e77ee. [DOI] [PMC free article] [PubMed] [Google Scholar]