Abstract
Background/Objective
To conduct a systematic review of all studies to determine whether there is an association between the Mediterranean diet (MeDi) and cognitive impairment.
Methods
We conducted a comprehensive search of the major databases and hand-searched proceedings of major neurology, psychiatry, and dementia conferences through November 2012. Prospective cohort studies examining the MeDi with longitudinal follow-up of at least 1 year and reporting cognitive outcomes (mild cognitive impairment [MCI] or Alzheimer’s disease [AD]) were included. The effect size was estimated as hazard-ratio (HR) with 95% confidence intervals (CIs) using the random-effects model. Heterogeneity was assessed using Cochran’s Q-test and I2-statistic.
Results
Out of the 664 studies screened, five studies met eligibility criteria. Higher adherence to the MeDi was associated with reduced risk of MCI and AD. The subjects in the highest MeDi tertile had 33% less risk (adjusted HR=0.67; 95% CI, 0.55–0.81; P<0.0001) of cognitive impairment (MCI or AD) as compared to the lowest MeDi score tertile. Among cognitively normal individuals, higher adherence to the MeDi was associated with a reduced risk of MCI (HR=0.73; 95% CI, 0.56–0.96; P=0.02) and AD (HR=0.64; 95% CI, 0.46–0.89; P=0.007). There was no significant heterogeneity in the analyses.
Conclusions
While the overall number of studies is small, pooled results suggest that a higher adherence to the MeDi is associated with a reduced risk of developing MCI and AD, and a reduced risk of progressing from MCI to AD. Further prospective-cohort studies with longer follow-up and randomized controlled trials are warranted to consolidate the evidence.
Keywords: Mediterranean diet, MCI, Mild Cognitive Impairment, Alzheimer’s disease, systematic review, meta-analysis
INTRODUCTION
Alzheimer’s disease (AD) is the sixth leading cause of mortality in the United States and the fifth leading cause for people aged ≥ 65 years [1]. Mild cognitive impairment (MCI) is an intermediate stage in the continuum from normal aging to dementia [2]. An individual with MCI has a 10-fold increased risk of developing dementia as compared to cognitively normal individuals [3]. Therefore, it is critical to identify potential protective factors for the development of MCI and progression to AD.
The Mediterranean diet (MeDi) is one factor that was initially shown to reduce of the risk of MCI and dementia [4–7]. MeDi is characterized by a high intake of vegetables, legumes, fruits, cereals, and unsaturated fatty acids [mostly in the form of olive oil], moderate to high intake of fish, low to moderate intake of dairy products, low intake of meat, and saturated fatty acids, and a regular but moderate intake of alcohol [7, 8]. A 9-point scale known as MeDi score was constructed to quantify the adherence to the Mediterranean diet [7, 9]. However, while some studies have reported that adherence to the MeDi is associated with reduced risk of MCI and AD [6, 10–12] other studies have reported no protective effects [13–15].
Therefore, we conducted a systematic review and meta-analysis of all the available studies and report the pooled results of the association between the MeDi and risk of MCI and AD from eligible prospective studies.
MATERIALS AND METHODS
This systematic review and meta-analysis followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement guidelines [16]. A protocol was designed a priori, which was registered with PROSPERO, registration number= PROSPERO 2013: CRD42013003868.
Eligibility criteria
To be eligible for inclusion in this meta-analysis, the study design had to be either a randomized controlled trial (RCT) or a cohort study with longitudinal follow up of at least 1 year. Case series, case-control, and cross-sectional studies were not considered. All studies also had to report adequate information to quantify MeDi and a risk estimate (hazard ratio [HR], relative risk [RR] or odds ratio [OR]) for a cognitive outcome, or data from which it could be calculated. Only dichotomous outcomes of MCI, dementia (all-cause or AD) or combined MCI/dementia were included. We did not limit our search strategy by the publication type or language status.
Multiple studies from a single cohort
We did identify multiple papers on MeDi from the same cohort (e.g. WHICAP 1992 and 1999 cohort). As a result, we used the following guidelines to determine which data to use in this meta-analysis. If the papers from same cohort reported different outcomes (i.e. MCI in one and AD in another), both of them were included. If the same outcome was presented in multiple papers, we only included the paper that reported a) maximum MeDi measures (both continuous and in tertile), and b) had the longest follow up. In case of disagreement, we conducted a sensitivity analysis. If there were multiple papers from the same cohort that used different study designs (cross sectional vs. cohort), only the cohort study that reported incident MCI or AD was included.
Literature search
We conducted a comprehensive search of six major databases (Ovid Medline In-Process & Other Non-Indexed Citations, Ovid MEDLINE, Ovid EMBASE, Ovid PsycInfo, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus) through November 2012, irrespective of language barriers. The comprehensive search was designed by an expert librarian (PJE), with input from the principal investigator. The detailed search strategy is available as an online appendix. We hand searched conference proceedings of the major neurology, psychiatry and dementia organizations from last 5 years for relevant abstracts and potential manuscripts, to minimize the publication bias. In addition, we queried experts (in MCI, and dementia) and reviewed the references for potentially eligible articles in order to identify all possible studies. In references of missing data, the corresponding authors of the articles were contacted for additional information.
Study selection
Two reviewers (BS and AJP) first independently screened the titles and abstracts of potentially eligible articles, followed by a comprehensive review of the full texts of the selected potentially eligible articles. Inter-reviewer agreement for study selection was assessed with Cohen’s weighted κ [17].
Data extraction
Two reviewers (BS and AJP) independently extracted data from the included studies using the standardized predesigned form and any disagreement was resolved by mutual consensus in the presence of a third investigator (MM). The following data were extracted from each study: author, country, publication year, number of participants, description of study participants, inclusion and exclusion criteria, outcome definition, the measure and strength of the association (HR, OR, or RR) with corresponding 95% confidence interval (CI), variables adjusted for in the multivariable analysis, the duration of follow-up, and missing data.
For exposure data, we included the definition and method used to calculate the MeDi score. For outcome data extraction, we included the criteria used to diagnose MCI, AD, and dementia and the risk estimates of participants with adherence to MeDi (using MeDi score).
Definitions for exposure and outcome
MeDi Adherence
We defined the adherence to the MeDi diet according to the MeDi score [7, 9]. MeDi score is a 9-point scale, in which a value of zero or one is assigned to the 9 components of the MeDi. A sex-specific median is assigned a value of 0 for consumption below the median and 1 for consumption at or above the median for beneficial components (vegetables, legumes, fruits, cereal, and fish). For components presumed to have adverse effects (meat, dairy products), a value of 1 for consumption below the median and 0 for consumption at or above the median is assigned. For fat intake, different studies have used the different versions; however in most of the studies they have used the ratio of monounsaturated fatty acids (MUFA) to saturated lipids/fatty acids, with a value of 1 assigned for high and 0 for low intake. In most of the studies, for alcohol intake, 0 is assigned for high/no intake (intake of 0 or ≥ 30g/day) and 1 for mild-to-moderate intake (> 0 to < 30 g per day). The total MeDi score ranges from 0 (minimal adherence) to 9 (maximal adherence). We divided the total score in to three tertiles according to adherence, MeDi scores 0–3 (low), 4–5 (middle), and 6–9 (higher) [6, 7, 11, 12, 14].
Outcomes of interest
Incident outcomes were divided into three groups; a) from cognitively normal at baseline to MCI, b) from cognitively normal at baseline to AD, and 3) conversion from MCI to AD.
Overall composite estimate of incident cognitive impairment, which includes any incident outcomes, either MCI or AD in cognitively normal subjects or AD in MCI subjects
Alzheimer’s disease
The diagnosis of AD was made according to the criteria of the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association [18].
Mild Cognitive Impairment
The MCI diagnosis are based on a full neuropsychological battery and reviewed by expert clinicians or neurologist or consensus/standard criteria [19].
QUALITY ASSESSMENT
Two reviewers (BS and AP) independently performed the quality assessment of the study methodology using the Newcastle-Ottawa Scale (NOS) [20] for observational studies. We used a “star system” in which a study was judged on three broad perspectives: selection of the study groups (four questions), comparability of the groups (two questions) and ascertainment of either the exposure/outcome of interest (three questions). All the questions received one star, except for comparability of the groups, where separate points were awarded for adjustment for age and sex (maximum two stars). Any disagreement was solved by mutual consensus in the presence of a third investigator (MM). No clinical trial was identified during the search and study selection.
STATISTICAL ANALYSIS
Categorical variables were estimated as mean number and frequency, while the continuous variables were estimated as means with standard deviation. We summarized the evidence for all the outcomes as HR with 95% CI. We used both the unadjusted and the adjusted multivariable models for the analysis. The DerSimonian-Laird random-effects model was used for the analysis [21]. The data points for the meta-analysis were entered as the logarithms of the HR, and their standard errors. Pooled results were reported separately for MCI, AD, and the composite end point of cognitive impairment. The results were reported separately for the MeDi adherence as a continuous score, as well as in tertiles.
We used the Cochran’s Q test to assess between-study heterogeneity. A P-value of <0.10 and I2 values of > 50% were considered as significant heterogeneity across the studies [22]. Statistical analyses were performed using Review Manager Version 5.1 [23]. A two-tailed P-value <0.05 was considered significant for all the analyses (except for heterogeneity).
We planned a priori hypotheses to explain the potential heterogeneity across studies by doing subgroup analysis of these factors: 1) methodological quality (NOS Score > 7 vs. ≤ 7) and 2) country of origin (US vs. Non-US).
Heterogeneity across the sub-groups was calculated with Cochran’s Q test [24], and the comparison of risk estimates were made with an interaction test [25]. Publication bias could not be assessed using the funnel plot as the number of included studies was < 10 [26, 27].
RESULTS
An initial comprehensive search identified a total of 738 records, of which 76 duplicate articles were excluded. Two additional articles were added through additional query of experts [28, 29]. Out of the 664 records initially screened from the titles and abstracts, 37 articles were selected for complete text review. The detailed study flow diagram is shown in figure 1. The inter-reviewer agreement for initial reference selection by reviewing abstract and titles, and reviewing complete articles were excellent, κ = 0.94 (95% CI= 0.89 – 1.00) and κ = 0.89 (95% CI= 0.69–1.00), respectively.
Figure 1.
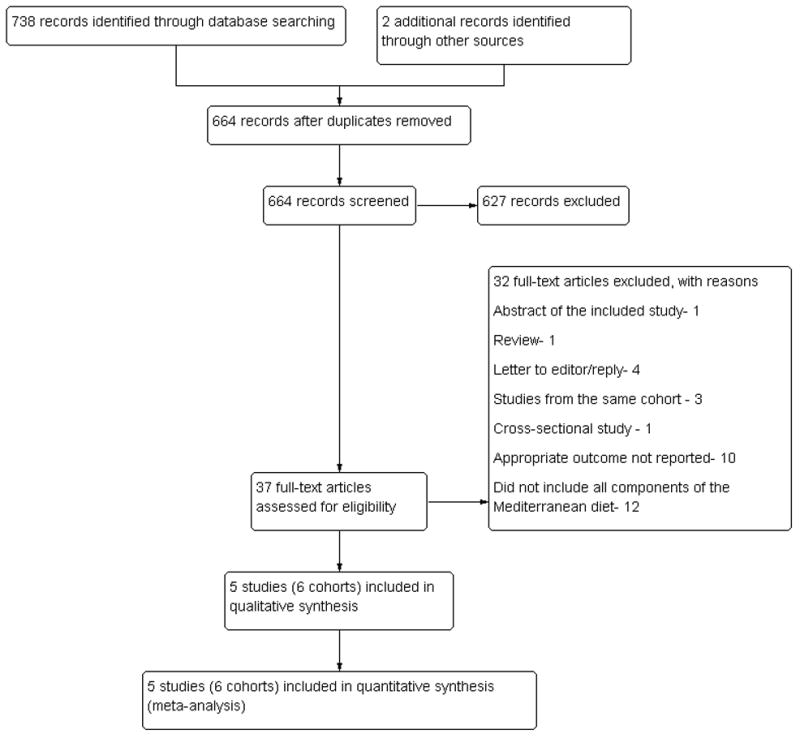
The study flowchart showing the study identification and selection
A total of 5 papers (6 cohorts) [4, 6, 11–14] met the eligibility criteria, of which three are from the US [6, 11, 12], one each from Australia [13] and France [4]. The Personality & Total Health (PATH) through life study investigators published two studies from the same cohort, with different follow-up duration of 4 [13] and 8 years [14]. For our primary analysis, we included the study with the longer follow-up duration [14] (which was published only as an abstract) and conducted a sensitivity analysis with the data from the full article [13]. The mean age of study participants varied from 62 years [13] to 80 years [11]. The study participants were ≥ 65 years of age in all except one study [13], in which the study participants were between 60 to 64 years of age. Length of follow-up ranged from 2.2 [11] to 8 years [14]. The study characteristics of the included studies are included in Table 1.
Table 1.
Characteristics of the longitudinal cohorts studies of Mediterranean diet and Incident cognitive impairment
| Author year country | Cohort inception year | N (%male) | Age, mean (SD) or median (IQR) | Follow-up, yrs, mean (SD) or median (IQR) | Subject selection | Mediterranean diet measures - MediScore (continuous); Tertiles | Outcome definition (N) | Adjustment | Sample size for adjusted outcome (outcome/total subjects) | Adjusted Hazard ratio (95% confidence interval) |
|---|---|---|---|---|---|---|---|---|---|---|
| Scarmeas, 2006, US [12] | WHICAP 1992 and WHICAP 1999 cohorts | 2226 (32) | 77.2 (6.6) | 4.0 (3.0) | Cognitively normal subjects (≥ 65 years) |
|
Alzheimer’s disease (262) | Cohort, age, sex, ethnicity, education, APOE, caloric intake, smoking, comorbidity index and BMI | 219/1759 | C = 0.91 (0.83–0.98) M vs. L = 0.85 (0.63–1.16) H vs. L = 0.6 (0.42–0.87) |
| Feart, 2009, France [4] | 1999–2000 | 1410 (37) | 75.9 | Median = 4.1 | Cognitively normal subjects (≥ 65 years) |
|
Alzheimer’s disease (66) | Age, sex, marital status, education, APOE, total energy intake, physical exercise, taking ≥5 medications/day, CES depression scale, BMI, hypertension, hypercholesterolemia, diabetes and tobacco use | 51/1410 | C = 0.99 (0.83–1.17) M vs. L = 0.93 (0.48–1.79) H vs. L =0.81 (0.37–1.75) |
| Scarmeas, 2009, US [6] | WHICAP 1992 and WHICAP 1999 cohorts | 482 (32) | 77.5 (6.6) | 4.3 (2.7) | Mild cognitive impairment (≥ 65 years) |
|
Alzheimer’s disease (106) | Age, sex, ethnicity, education, APOE, caloric intake, BMI and time between first dietary assessment and the first cognitive assessment | 96/409 | C = 0.89 (0.78–1.02) M vs. L = 0.55 (0.34–0.90) H vs. L = 0.52 (0.30–0.91) |
| Scarmeas, 2009, US [6] | WHICAP 1992 and WHICAP 1999 cohorts | 1393 (32) | 76.7 (6.5) | 4.5 (2.7) | Cognitively normal subjects (≥ 65 years) |
|
Mild cognitive impairment (275) | Age, sex, ethnicity, education, APOE, caloric intake, BMI and time between first dietary assessment and the first cognitive assessment | 241/1199 | C = 0.92 (0.85–0.99) M vs. L = 0.83(0.62–1.12) H vs. L = 0.72(0.52–1.00) |
| Roberts, 2010, US [11] | 2004–2007 | 1141 (53) | Event = 83.3 (78.7–86.2); No event = 79.7 (75.6– 84.2) | Median (IQR): 2.2 (1.7–2.6) | Cognitively normal or had MCI (70–89 years) |
|
Mild cognitive impairment (93) and dementia (23) | Age, Sex, education, total energy intake, APOE, stroke, coronary heart disease and depressive symptoms | 116/1141 | M vs. L = 0.79 (0.51–1.21) H vs. L = 0.75 (0.46–1.21) |
| Cherbuin, 2011, Australia [14] | 2001–2002 | 1367 | NA | 8 | Cognitively normal subjects (60–64 years) |
|
Mild cognitive impairment (NA) | Age, sex, education, APOE, BMI, total caloric intake, physical activity, stroke, hypertension, and diabetes | NA/1367 | C = 1.07 (0.85–1.35) |
WHICAP = Washington Heights-Inwood Columbia Aging Project; Continuous (C); Middle vs. low (M vs. L); High vs. Low (H vs. L); NA = not available; CIS = Center for Epidemiologic Studies; APOE = Apolipoprotein E
A total of 3636 participants from two studies [4, 12] and 3901 participants from three studies [6, 11, 14] were included in the analysis of incident MCI and AD among cognitively normal individuals at baseline. A total of 482 participants were included in the analysis examining MeDi and progression from MCI to AD.
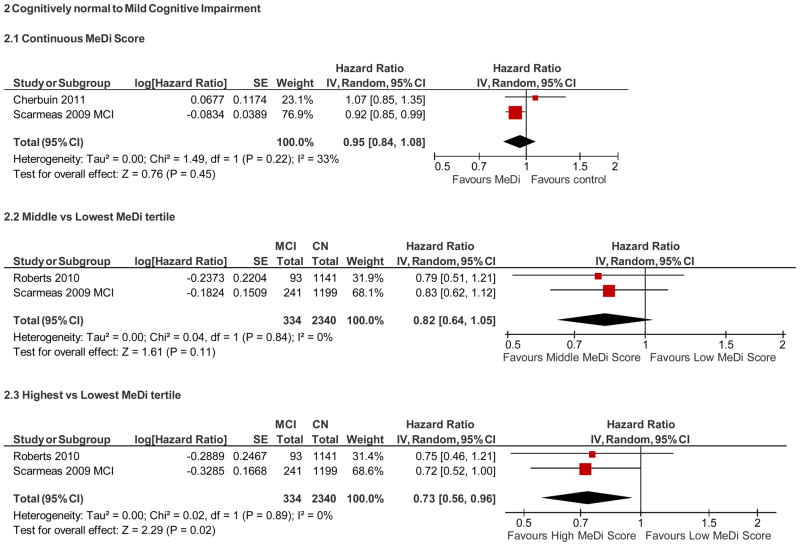
Cognitively normal at baseline to MCI (Figure 2)
Figure 2.
Summary of adherence to the Mediterranean diet and risk of mild cognitive impairment among cognitively normal individuals at baseline
When restricting the analyses to incident MCI, the MeDi score, as a continuous variable, was not associated with incident MCI (adjusted HR=0.95; 95% CI, 0.84 – 1.08, p = 0.45). When examining tertiles, the highest MeDi tertile (adjusted HR=0.73; 95% CI, 0.56– 0.96, p = 0.02) was associated with a reduced risk of MCI and there was also a trend for the middle tertile (HR=0.82; 95% CI, 0.64– 1.05, p = 0.11); compared to the lowest.
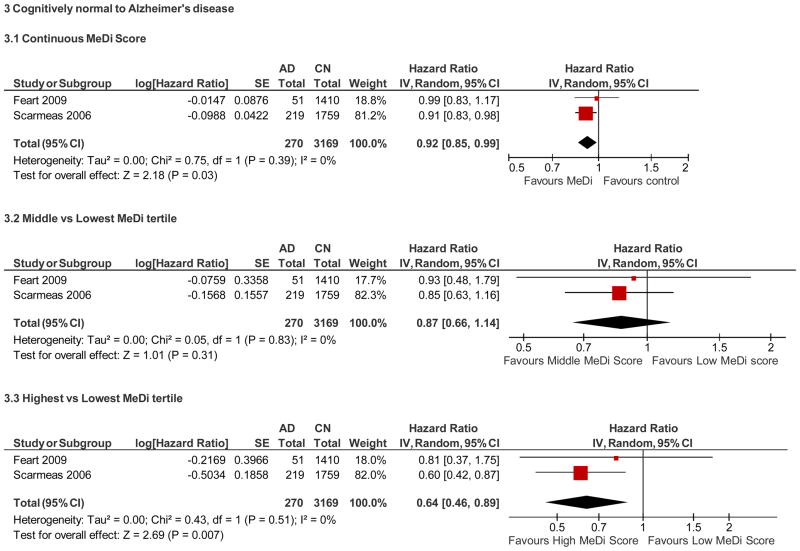
Cognitively normal at baseline to AD (Figure 3)
Figure 3.
Summary of adherence to the Mediterranean diet and risk of Alzheimer’s disease among cognitively normal individuals at baseline.
Among studies examining AD, each one-point increase in the MeDi score in cognitively normal individuals was associated with an 8% reduced risk of developing AD (adjusted HR, 0.92; 95% CI, 0.85 – 0.99), p= 0.03. Examining the MeDi in tertiles, subjects in the middle MeDi tertile had 13% (not significant) reduced risk (adjusted HR=0. 87; 95% CI, 0.66 – 1.14, P= 0.31), while the subjects in the higher tertile had 36% risk reduction (adjusted HR=0.64; 95% CI, 0.46– 0.89, P= 0.007) as compared to the lowest.
MCI to AD (Online Figure)
Continuous MeDi score showed a trend towards a reduced risk of progressing from MCI to AD (adjusted HR=0.89; 95% CI, 0.78 – 1.02, p=0.09). However, there was a significant risk reduction in MCI subjects among the middle MeDi tertile (adjusted HR=0.55; 95% CI, 0.34 – 0.90; P= 0.02) and highest MeDi tertile (adjusted HR=0.52; 95% CI, 0.30 – 0.91; P= 0.02) compared to the lowest tertile.
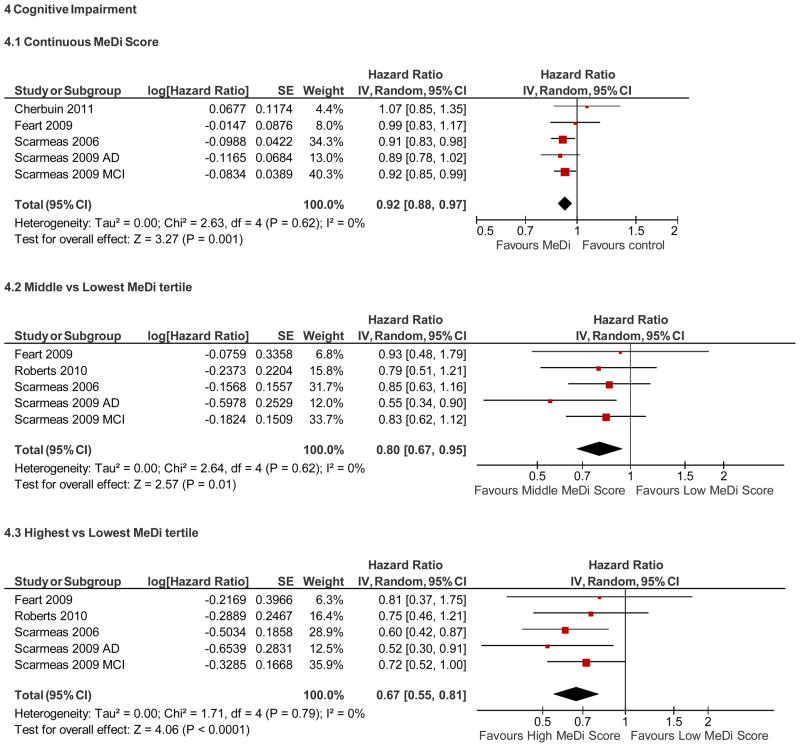
Risk of Cognitive impairment (Figure 4)
Figure 4.
Summary adherence to the Mediterranean diet and risk of cognitive impairment
A meta-analysis of all the studies showed that a one point increase in the MeDi score is associated with an 8% reduced risk of developing cognitive impairment in both unadjusted (HR, 0.92; 95% CI, 0.88 – 0.96) and adjusted (HR, 0.92; 95% CI, 0.88 – 0.97, p = 0.001) models, with no heterogeneity (I2 = 0%). Examining the MeDi in tertiles, there was a dose-response association such that subjects in the middle MeDi tertile had a 20% reduced risk (adjusted HR = 0.80; 95% CI, 0.67– 0.95; P = 0.01) while the highest MeDi tertile had 33% reduced risk (adjusted HR=0.67; 95% CI, 0.55– 0.81; P <0.0001). These findings are consistent with “level 2a evidence,” i.e., systematic review (with homogeneity) of cohort studies [30].
Quality of the included studies
All included studies were high quality, longitudinal, population-based studies with the total NOS score ≥ 8 (Table-2). The included studies varied according to the adjusting covariates; however, all the studies were adjusted for age, sex, education and Apolipoprotein E (APOE).
Table 2.
Quality assessment of the included studies using Newcastle-Ottawa Scale
| Scarmeas, 2009 [6] | Scarmeas, 2006 [12] | Roberts, 2010 [11] | Feart, 2009 [4] | Cherbuin, 2012 [13] | |
|---|---|---|---|---|---|
| Representativeness of the exposed cohort | |||||
| 1. Truly/somewhat representative of underlying population * | |||||
| 2. Selected group of users/no description of the derivation of the cohort | * | * | * | * | * |
| Selection of the non-exposed cohort | |||||
| 1. Drawn from the same community as the exposed cohort * | |||||
| 2. Drawn from a different source/no description of the derivation of the non-exposed cohort | * | * | * | * | * |
| Ascertainment of exposure | |||||
| 1. Secure record/structured interview * | |||||
| 2. Written self-report./no description | * | * | * | * | * |
| Demonstration that outcome of interest was not present at start of study | |||||
| 1. Yes * | |||||
| 2. No/unclear | * | * | * | * | * |
| Comparability of cohorts on the basis of the design or analysis | |||||
| 1. Study controls for main confounders (age/sex/education/APOE)* | ** | ||||
| 2. Study controls for any additional confounding factor (s) * | ** | ** | ** | ** | |
| Assessment of outcome | |||||
| 1. Independent blind assessment/record linkage * | |||||
| 2. Self-report/no description | * | * | * | * | * |
| Was follow-up long enough for outcomes to occur (follow-up >2 years) | |||||
| 1. Yes * | |||||
| 2. No | * | * | * | * | * |
| Adequacy of follow up of cohorts | |||||
| 1. Complete follow up/subjects lost to follow up unlikely to introduce bias * | |||||
| 2. Incomplete follow-up/no description of those lost/no statement | * | * | |||
| Total Score (0–9) | 8 | 8 | 9 | 8 | 9 |
Sensitivity and sub-group analyses
The PATH through life study investigators published two studies from same cohort, with different follow-up durations of 4 [13] and 8 years [14]. In one study [14], the outcome was reported as a HR and in the other [13] as an OR. A sensitivity analysis was conducted using the abstract data and full publication data separately. There was a mild reduction of 1% in risk estimate when we used the data from the published paper, this could be due to overestimation by using OR as an outcome measure [31]. There was disagreement between the two reviewers regarding the inclusion of a paper by Scarmeas et al [5] with longer duration of follow-up (5.4years), but lesser sample size (n=1476), and including only MeDi tertile measure; whereas the paper [12] included in the table-1 had larger sample size (n=1759) and in addition, had estimated for continuous MeDi scores. The number of outcomes in the adjusted models did not differ by much between the two studies (224–219=5). Therefore, we did a sensitivity analysis, including the study with longer duration of follow-up. We did not observe any difference in the results for the high vs. low MeDi score group. However, the results for the middle vs. low MeDi score became only borderline significant for a) overall cognitive impairment (HR=0.84, 95% CI= 0.71 – 1.00; p=0.05) and b) remained non-significant for MeDi diet and risk of AD in cognitively normal individuals at baseline (HR=0.98, 95% CI= 0.74, 1.30; p=0.90, I2=0%). Thus, inclusion of this paper in the main analysis could not have provided any additional advantage.
Out of the 5 included studies, two [6, 12] of the paper were from the same cohort, but with different outcomes, which could result in overrepresentation of the data from the same cohort. Therefore, we conducted a sensitivity analysis by excluding the paper with the smaller sample size [6]. After excluding this paper, the overall neuroprotective effect of MeDi was significant only for the highest MeDi tertile group (adjusted HR = 0.67; 95% CI 0.51 – 0.88, p = 0.004), but was non-significant for middle MeDi tertile group (adjusted HR = 0.85; 95% CI, 0.67– 1.07, p= 0.17) and continuous MeDi score (adjusted HR = 0.94; 95% CI, 0.87 – 1.02, p= 0.12).
We did not observe significant heterogeneity for any of the analyses. However, we conducted a pre-planned sub-group analysis based on the location of the study (online Table-1). For continuous MeDi scores, there was no difference between the studies conducted in the US (n = 3 studies; adjusted HR, 0.91; 95% CI, 0.87–0.96) and non-US (n=2 studies; adjusted HR, 1.02; 95% CI, 0.89 –1.17) (P value for difference between the sub-groups = 0.13). There was no significant difference in the cognitive impairment among the US and non-US populations for middle and highest MeDi tertiles in comparison to lowest MeDi tertile (P= 0.52 and 0.61, respectively). Sub-group analysis based on the pre-planned quality of studies could not be conducted, as all the studies were of high quality.
DISCUSSION
This meta-analysis found that a higher adherence to the MeDi is associated with a reduced risk of cognitive impairment, MCI and AD, as well as the transition from MCI to AD. The associations were significant even for the sensitivity analysis. This study suggests that there is evidence that MeDi may be neuroprotective for MCI and AD with higher adherence. However, given the limited number of studies, the results should be interpreted with caution. The findings of our study are similar to a recently published meta-analysis, which showed that adherence to MeDi is associated with a reduced risk of stroke, depression and cognitive impairment [32]. In the study by Psaltopoulou and colleagues [32], authors included both the case-control and longitudinal studies, whereas in this study, we have included only longitudinal studies. Another difference is in the inclusion criteria, we have included longitudinal studies measuring MeDi scores as continuous or tertile measures and reported the results separately; whereas, Psaltopoulou et al [32] included studies measuring MeDi scores as tertile only. Thus, we have included an additional study [13] in our systematic review and meta-analysis. Furthermore, we included studies using standard criteria to diagnose MCI and AD, and did not include studies using MMSE as a tool to diagnose dementia (as this could lead to heterogeneity), whereas, Psaltopoulou et al included studies using MMSE as a tool to diagnose dementia.
It is hypothesized that a composite score such as MeDi score would capture the possible additive and interactive effects among the diet components [6, 12]. Although, we did not find any heterogeneity in the analysis, the components of the MeDi in western countries would be different from the traditional MeDi diet, which has high components of the olive oil [39]. This difference in the beneficial components of MeDi could explain the difference in risk estimation among the studies. Diet is variable across regions. Given the methodology for computing the MeDi score, tertiles may not mean the same thing across locations, cultures, and populations. A low tertile in one group – for example, the French (with less fats, probably more fruit and vegetables, more wine), may be a high tertile in MN (where there is more meat and potatoes, less vegetables, more dairy). Thus, this could be a reason for the different results from the studies from different regions.
Variations in length of follow-up could be an important factor. Cognitive changes predate the development of AD pathology by decades [41]. The follow up duration of the studies in our meta-analysis ranged from 2.2 years [11] to 8 years [14], which may result in different risk estimates. Therefore, studies with longer follow-up duration are needed to estimate the neuroprotective effect of MeDi. Another important way to answer this question, whether MeDi reduces the cognitive impairment, would be a RCT. In a recent multicenter RCT, MeDi supplemented with extra-virgin olive oil or nuts have shown to reduce the incidence of major cardiovascular events in high risk cardiovascular disease patients [42]. Thus, RCT could be better choice to answer this important question in elderly patients at increased risk of cognitive impairment.
Higher adherence to MeDi has been shown to be associated with low level of C-reactive protein and lower interleukin levels [33–35]. Therefore a possible underlying mechanism for the neuroprotective effects of the MeDi could be due to its vascular properties and its ability to reduce inflammation, and oxidative stress, which are also associated with the pathophysiology of the degenerative disease [36–38]. Another reason for the beneficial effect of MeDi could be due to the beneficial effect of the individual components, vegetables [11], high ratio of monounsaturated fatty acids to saturated fatty acids [39], alcohol [11] and fish [40]. Another important pathway for the protective effect of MeDi could be due to the proposed cardio-protective role of MeDI, by lowering the risk of cardiovascular comorbidities, such as hypertension, dyslipidemia and coronary artery disease [38, 43, 44]. MeDi has been associated with a significant reduction in plasma glucose level, serum insulin levels and insulin resistance, and in ameliorating the features of metabolic syndrome and obesity [38, 45, 46].
Limitations
A major limitation of our meta-analysis is the small number of studies (<10), therefore we could not assess publication bias [27]. However, to overcome this limitation we hand-searched the abstracts of the major neurology, psychiatry, and dementia conferences and queried the content experts in dementia. We identified two additional papers by this method; however, they were excluded after full text review as they did not meet the eligibility criteria for our study. Another potential limitation could be the difference in the diet among the various regions; in particular, the amount of olive oil intake (which is associated with neuroprotection for cognitive decline) in Mediterranean regions is much higher than in other countries [47]. This could contribute to the differences in incident MCI and AD among low and middle MeDi tertile groups. Furthermore, there have been some concerns regarding the reliability of MeDi score as a tool to measure ‘adherence to the Mediterranean diet’, as the error variance of the observed-scores may vary after selection of food groups based on their median values by sex. Therefore, the pooled results should be interpreted with caution. However, MeDi score has shown acceptable performance in measuring adherence to MeDi [48].
Conclusion
Our systematic review and meta-analysis suggests that higher adherence to the Mediterranean diet is associated with a reduced risk of developing MCI and AD, and reduced risk of MCI conversion to AD. Findings from this study, suggest the need for further prospective longitudinal studies with longer follow-up and randomized controlled trials to determine whether adherence to the Mediterranean diet could reduce the risk of MCI and AD.
Supplementary Material
Acknowledgments
Study support: Supported by NIH grants P50 AG016574, U01 AG006786, K01 MH068351, and K01 AG028573, by the Robert Wood Johnson Foundation, and by the Robert H. and Clarice Smith and Abigail van Buren Alzheimer’s Disease Research Program, and was made possible by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Footnotes
Systematic review registration number: PROSPERO 2013: CRD42013003868
Disclosure: Drs. Singh, Parsaik and Mielke, and Ms. Erwin report no disclosures. Dr. Roberts receives research support from the NIH and Abbott Labs. Dr. Knopman serves as Deputy Editor for Neurology®; has served on a data safety monitoring board for Eli Lilly and Company; has served as a consultant for Elan/Janssen AI; is an investigator in clinical trials sponsored by Elan/Janssen AI, Baxter International Inc., and Forest Laboratories, Inc.; and receives research support from the NIH. Dr. Petersen serves on scientific advisory boards for the Alzheimer’s Association, the National Advisory Council on Aging (NIA), Elan/Janssen AI, Pfizer Inc (Wyeth), and GE Healthcare; receives publishing royalties from Mild Cognitive Impairment (Oxford University Press, 2003); serves as a consultant for Elan/Janssen AI and GE Healthcare; and receives research support from the NIH/NIA.
References
- 1.National Center for Health Statistics. [Accessed on March 23, 2013.];National Vital Statistics System. Multiple cause-of-death files. Available from: http://www.cdc.gov/nchs/data_access/Vitalstatsonline.htm.
- 2.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 3.Petersen RC. Clinical practice. Mild cognitive impairment. N Engl J Med. 2011;364:2227–2234. doi: 10.1056/NEJMcp0910237. [DOI] [PubMed] [Google Scholar]
- 4.Feart C, Samieri C, Rondeau V, Amieva H, Portet F, Dartigues JF, Scarmeas N, Barberger-Gateau P. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA. 2009;302:638–648. doi: 10.1001/jama.2009.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scarmeas N, Luchsinger JA, Schupf N, Brickman AM, Cosentino S, Tang MX, Stern Y. Physical activity, diet, and risk of Alzheimer disease. JAMA. 2009;302:627–637. doi: 10.1001/jama.2009.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Arch Neurol. 2009;66:216–225. doi: 10.1001/archneurol.2008.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348:2599–2608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 8.Willett WC, Sacks F, Trichopoulou A, Drescher G, Ferro-Luzzi A, Helsing E, Trichopoulos D. Mediterranean diet pyramid: A cultural model for healthy eating. Am J Clin Nutr. 1995;61:1402S–1406S. doi: 10.1093/ajcn/61.6.1402S. [DOI] [PubMed] [Google Scholar]
- 9.Trichopoulou A, Kouris-Blazos A, Wahlqvist ML, Gnardellis C, Lagiou P, Polychronopoulos E, Vassilakou T, Lipworth L, Trichopoulos D. Diet and overall survival in elderly people. BMJ. 1995;311:1457–1460. doi: 10.1136/bmj.311.7018.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sofi F, Abbate R, Gensini GF, Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: An updated systematic review and meta-analysis. Am J Clin Nutr. 2010;92:1189–1196. doi: 10.3945/ajcn.2010.29673. [DOI] [PubMed] [Google Scholar]
- 11.Roberts RO, Geda YE, Cerhan JR, Knopman DS, Cha RH, Christianson TJ, Pankratz VS, Ivnik RJ, Boeve BF, O’Connor HM, Petersen RC. Vegetables, unsaturated fats, moderate alcohol intake, and mild cognitive impairment. Dement Geriatr Cogn Disord. 2010;29:413–423. doi: 10.1159/000305099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol. 2006;59:912–921. doi: 10.1002/ana.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cherbuin N, Anstey KJ. The mediterranean diet is not related to cognitive change in a large prospective investigation: The PATH through life study. Am J Geriatr Psychiatry. 2012;20:635–639. doi: 10.1097/JGP.0b013e31823032a9. [DOI] [PubMed] [Google Scholar]
- 14.Cherbuin N, Kumar R, Anstey K. Caloric intake, but not the mediterranean diet, is associated with cognition and mild cognitive impairment. Alzheimers Dement. 2011;(1):S691. [Google Scholar]
- 15.Vercambre MN, Grodstein F, Berr C, Kang JH. Mediterranean Diet and Cognitive Decline in Women with Cardiovascular Disease or Risk Factors. J Acad Nutr Diet. 2012;112:816–823. doi: 10.1016/j.jand.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen J. A Coefficient of Agreement for Nominal Scales Educational and Psychological Measurement. Educ Psychol Meas. 1960;20:37–46. [Google Scholar]
- 18.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 19.Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 20.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. [Accessed January 23, 2012];The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [ http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm] website.
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 23.The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2011. [Google Scholar]
- 24.Cochran W. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- 25.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326:219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration; 2011. Available from www.cochrane-handbook.org. [Google Scholar]
- 27.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53:1119–1129. doi: 10.1016/s0895-4356(00)00242-0. [DOI] [PubMed] [Google Scholar]
- 28.Ye X, Scott T, Gao X, Maras JE, Bakun PJ, Tucker KL. Mediterranean diet, healthy eating index 2005, and cognitive function in middle-aged and older puerto rican adults. J Acad Nutr Diet. 2013;113:276–281. e273. doi: 10.1016/j.jand.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kesse-Guyot E, Andreeva VA, Lassale C, Ferry M, Jeandel C, Hercberg S, Galan P. Mediterranean diet and cognitive function: a French study. Am J Clin Nutr. 2013;97:369–376. doi: 10.3945/ajcn.112.047993. [DOI] [PubMed] [Google Scholar]
- 30. [accessed March 24, 2013];Oxford Centre for Evidence-based Medicine - Levels of Evidence. 2009 Mar; http://www.cebm.net/index.aspx?o=1025.
- 31.Sinclair JC, Bracken MB. Clinically useful measures of effect in binary analyses of randomized trials. J Clin Epidemiol. 1994;47:881–889. doi: 10.1016/0895-4356(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 32.Psaltopoulou T, Sergentanis TN, Panagiotakos DB, Sergentanis IN, Kosti R, Scarmeas N. Mediterranean diet and stroke, cognitive impairment, depression: A meta-analysis. Annals of Neurology. 2013 doi: 10.1002/ana.23944. [DOI] [PubMed] [Google Scholar]
- 33.Fung TT, McCullough ML, Newby PK, Manson JE, Meigs JB, Rifai N, Willett WC, Hu FB. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2005;82:163–173. doi: 10.1093/ajcn.82.1.163. [DOI] [PubMed] [Google Scholar]
- 34.Esposito K, Giugliano F, Di Palo C, Giugliano G, Marfella R, D’Andrea F, D’Armiento M, Giugliano D. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA. 2004;291:2978–2984. doi: 10.1001/jama.291.24.2978. [DOI] [PubMed] [Google Scholar]
- 35.Chrysohoou C, Panagiotakos DB, Pitsavos C, Das UN, Stefanadis C. Adherence to the Mediterranean diet attenuates inflammation and coagulation process in healthy adults: The ATTICA Study. J Am Coll Cardiol. 2004;44:152–158. doi: 10.1016/j.jacc.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 36.Steele M, Stuchbury G, Munch G. The molecular basis of the prevention of Alzheimer’s disease through healthy nutrition. Exp Gerontol. 2007;42:28–36. doi: 10.1016/j.exger.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Cummings JL. Alzheimer’s disease. N Engl J Med. 2004;351:56–67. doi: 10.1056/NEJMra040223. [DOI] [PubMed] [Google Scholar]
- 38.Frisardi V, Panza F, Seripa D, Imbimbo BP, Vendemiale G, Pilotto A, Solfrizzi V. Nutraceutical properties of Mediterranean diet and cognitive decline: possible underlying mechanisms. J Alzheimers Dis. 2010;22:715–740. doi: 10.3233/JAD-2010-100942. [DOI] [PubMed] [Google Scholar]
- 39.Panza F, Capurso C, D’Introno A, Colacicco AM, Del Parigi A, Gagliardi G, Breglia G, Capurso A, Solfrizzi V. Mediterranean diet, mild cognitive impairment, and Alzheimer’s disease. Exp Gerontol. 2007;42:6–7. doi: 10.1016/j.exger.2006.09.011. author reply 8–9. [DOI] [PubMed] [Google Scholar]
- 40.Eskelinen MH, Ngandu T, Helkala EL, Tuomilehto J, Nissinen A, Soininen H, Kivipelto M. Fat intake at midlife and cognitive impairment later in life: a population-based CAIDE study. Int J Geriatr Psychiatry. 2008;23:741–747. doi: 10.1002/gps.1969. [DOI] [PubMed] [Google Scholar]
- 41.Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, Holtzman DM, Santacruz A, Buckles V, Oliver A, Moulder K, Aisen PS, Ghetti B, Klunk WE, McDade E, Martins RN, Masters CL, Mayeux R, Ringman JM, Rossor MN, Schofield PR, Sperling RA, Salloway S, Morris JC. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Estruch R, Ros E, Salas-Salvado J, Covas MI, Pharm D, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pinto X, Basora J, Munoz MA, Sorli JV, Martinez JA, Martinez-Gonzalez MA. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet. N Engl J Med. 2013;368:1279–1290. doi: 10.1056/NEJMc1806491. [DOI] [PubMed] [Google Scholar]
- 43.Singh RB, Dubnov G, Niaz MA, Ghosh S, Singh R, Rastogi SS, Manor O, Pella D, Berry EM. Effect of an Indo-Mediterranean diet on progression of coronary artery disease in high risk patients (Indo-Mediterranean Diet Heart Study): a randomised single-blind trial. Lancet. 2002;360:1455–1461. doi: 10.1016/S0140-6736(02)11472-3. [DOI] [PubMed] [Google Scholar]
- 44.Psaltopoulou T, Kyrozis A, Stathopoulos P, Trichopoulos D, Vassilopoulos D, Trichopoulou A. Diet, physical activity and cognitive impairment among elders: the EPIC-Greece cohort (European Prospective Investigation into Cancer and Nutrition) Public Health Nutrition. 2008;11:1054–1062. doi: 10.1017/S1368980007001607. [DOI] [PubMed] [Google Scholar]
- 45.Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G, D’Armiento M, D’Andrea F, Giugliano D. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440–1446. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 46.Jula A, Marniemi J, Huupponen R, Virtanen A, Rastas M, Ronnemaa T. Effects of diet and simvastatin on serum lipids, insulin, and antioxidants in hypercholesterolemic men: a randomized controlled trial. JAMA. 2002;287:598–605. doi: 10.1001/jama.287.5.598. [DOI] [PubMed] [Google Scholar]
- 47.Martinez-Lapiscina EH, Clavero P, Toledo E, San Julian B, Sanchez-Tainta A, Corella D, Lamuela-Raventos RM, Martinez JA, Martinez-Gonzalez MA. Virgin olive oil supplementation and long-term cognition: the PREDIMED-NAVARRA randomized, trial. J Nutr Health Aging. 2013;17:544–552. doi: 10.1007/s12603-013-0027-6. [DOI] [PubMed] [Google Scholar]
- 48.Mila-Villarroel R, Bach-Faig A, Puig J, Puchal A, Farran A, Serra-Majem L, Carrasco JL. Comparison and evaluation of the reliability of indexes of adherence to the Mediterranean diet. Public Health Nutr. 2011;14:2338–2345. doi: 10.1017/S1368980011002606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.