Abstract
Background and Purpose
Aging is an important determinant of ischemic stroke outcomes. Both clinical and experimental stroke studies have shown that aging negatively correlates with infarct volumes but is associated with worsened functional recovery after stroke. This may correspond to a differing cellular and molecular response to stroke in the aged vs. young brain. It was hypothesized in this study that the smaller injury seen in the aged ischemic brain is due to structural differences in microvasculature with aging or differences in intra-ischemic tissue perfusion.
Methods
Both young and aged C57BL6 mice were subject to middle cerebral artery occlusion (MCAO) modeling. Laser Speckle Flowmetry (LSF) was utilized to study the functional dynamics of cerebral perfusion, and FITC-dextran staining was performed to examine the structural change in microvasculature. In separate cohorts, Cresyl violet (CV) staining and immunohistochemistry with CD31 and IgG antibodies were applied to further assess the microvascular density and blood brain barrier breakdown after stroke.
Results
No difference in cerebral blood flow was seen at the baseline, intra-ischemically and post-reperfusion in young vs. aged mice. FITC-dextran and CD31 staining did not show significant differences in the microvascular density between young and aged ischemic brains. More extravasation of IgG through the BBB was found in the young vs. aged cohort at both 24 and 72 hours after stroke.
Conclusions
Cerebrovascular dynamics and perfusion are not responsible for the different stroke phenotypes seen in the young vs. aged animals, which may be more related to different levels of BBB breakdown.
Keywords: Aging, Ischemic Stroke, Perfusion, Laser Speckle Flowmetry
Introduction
Aging is the most important non-modifiable risk factor for ischemic stroke 1 and an independent predictor of worse stroke outcomes2. The rate of stroke doubles every decade after the age of 55 3 and the burden of this disease on public health is projected to grow with the increasing life expectancy. Many patients are not candidates for tissue plasminogen activator (tPA, the only FDA approved treatment for ischemic stroke) therapy due to the short approved time window (3 hours) or contraindications to treatment. Despite the development of numerous promising agents in preclinical models, bench to bed translation of therapies is a challenge for stroke researchers. This may be secondary, in part, by the almost exclusive use of young animals which may not mimic the hemodynamic and inflammatory milieu of the aged brain.
We have shown that aged male mice have smaller infarct volumes but worse functional recovery when compared with young mice 4–6, which is consistent with clinical data 7. However, the underlying mechanism for this discrepancy in histological and functional outcome is unclear. Aging causes several morphological and pathological changes in the vascular bed, such as atherosclerosis, small vessel disease 8 and altered cerebrovascular reserve 9, etc. Therefore, we hypothesized that structural differences in the microvasculature in the aged or differences in intra ischemic perfusion may be responsible for the smaller infarct volumes seen in aged animals. In the present study, we used Laser Speckle Flowmetry (LSF) to measure CBF before and after MCAO in both young and aged mice. Unlike the traditional Laser Doppler Flowmetry (LDF), LSF provides the spatial and temporal pattern of CBF in the brain10. Therefore, perfusion of the brain through collaterals can also be visualized and quantified. The high resolution images and flux traces allowed efficient intraoperative monitoring of the CBF and its dynamics in young and aged mice. FITC-dextran, CD31, and IgG staining were also used to evaluate differences in the microvasculature and extravasation of blood brain barrier (BBB) in the young and aged brains after stroke.
Materials and Methods
Experimental animals
Young (8–12 weeks) and aged (18–20 months) C57BL/6 male mice were purchased from National Institute of Aging colonies. The mice were housed in sawdust bedding cages and had access to chow and water ad libitium. This study was conducted in accordance with the National Institute of Health guidelines for the care and use of animals in research and under protocols approved by the Center for Lab Animal Care at the University of Connecticut Health Center.
Focal cerebral ischemic model
Focal transient cerebral ischemia was induced by 60-minute middle cerebral artery occlusion (MCAO) under isoflurane anesthesia as described previously11. 0.21 mm and 0.23 mm silicone coated sutures were utilized in young and aged mice respectively to achieve occlusion4. Rectal temperature was maintained at approximately 37°C during surgery with an automated temperature control feedback system. Animals that did not show any decrease in CBF by LSF after suture insertion were excluded from the study.
Laser Speckle Flowmetry
Laser Speckle Perfusion imaging of the brain was performed using moorFLPI Full-Field Laser Perfusion Imager (Moor Instruments, Devon, UK) according to manufacturer’s instructions and as described previously10. Briefly, a midline scalp incision was made to an isoflurane-anaesthetized mouse and the skull was exposed; the CCD (charge coupled device) camera of LSF was installed 30 cm above the skull using an articulating arm. Two identical rectangular regions of interest (ROI) were selected on each of the two hemispheres (Figure 1A). The imaging was set up at a display rate of 25 Hz, time constant of 1 second and a camera exposure time of 4 milliseconds. A 2 minute baseline CBF Flux was recorded before induction of ischemia. MCAO surgery was performed and live image and flux recordings were made throughout the intra-ischemic and post-reperfusion periods. Data were expressed as mean CBF flux of 2 minutes of pre-ischemic period, 50 minutes of intra-ischemic period and 2 minutes of post-reperfusion period following 10-minute reperfusion. Neurological deficit scores (NDS) was recorded 24 hours after MCAO 11: 0, no deficit; 1, forelimb weakness and torso turning to the ipsilateral side when held by tail; 2, circling to affected side; 3, unable to bear weight on affected side; 4, no spontaneous locomotor activity or barrel rolling.
Figure 1.
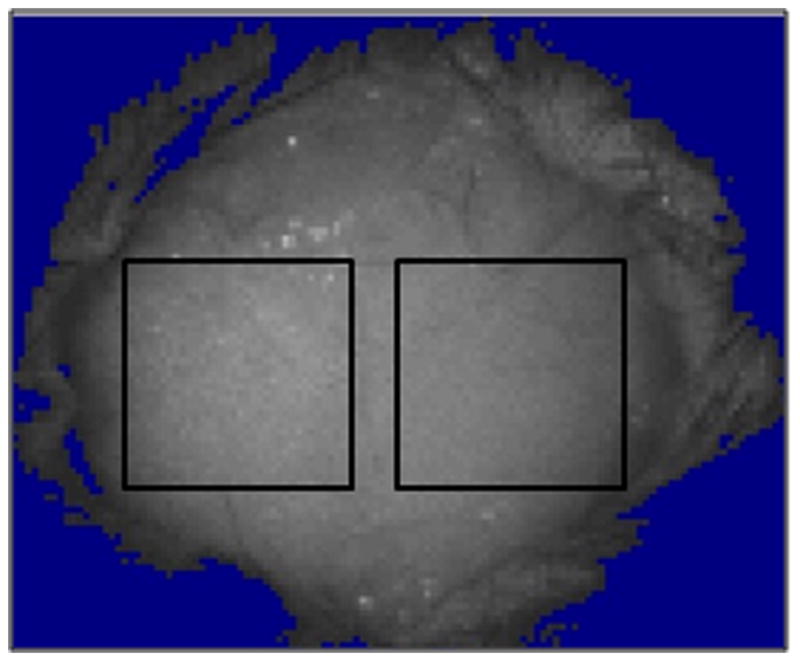
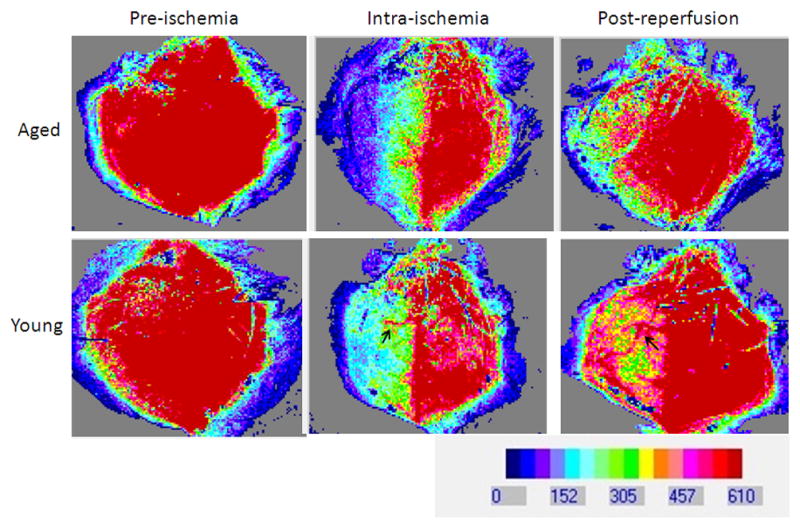
LSF images in the brain after stroke. (A). The two rectangular boxes indicate regions of interest (ROI) selected for measurement on the exposed skull of the mice. (B). LSF flux images (perfusion images) showing CBF pre-ischemia, intra-ischemia and post-reperfusion in young versus aged mice. No difference in CBF was noted in young vs. aged animals at the baseline (pre-ischemia), intra-ischemically and post-reperfusion. Post MCAO the ipsilateral side (right side) shows a reduction in CBF and post reperfusion the CBF is restored. The arrow in Young/Intra-ischemia indicates occluded blood vessel. The arrow in Young/Post-reperfusion shows release of obstruction and blood flow through that vessel. Red color, high CBF; blue color, low CBF.
Terminal histopathology and IgG staining
One cohort of mice was sacrificed at 24 hours after MCAO and a second cohort was sacrificed at a 72 hours endpoint. The brains were perfused with cold PBS followed by 4% paraformaldehyde. The brains were then post-fixed in paraformaldehyde for 24 hours and placed in cyroprotectant (30% sucrose), frozen and then sliced into 30-μm free-floating sections on a freezing microtome. Every eighth section was stained with cresyl violet stain to evaluate ischemic cell damage, as described previously12.
IgG staining shows blood brain barrier compromise13. Brain sections were stained with IgG, as described previously 13. Briefly, first endogenous peroxidase activity was blocked using 1% H2O2 and then incubated with biotinylated antimouse antibody (Vector Laboratories) for 30 minutes, rinsed in PBS, and incubated in Vectastain ABC reagent for 30 minutes followed by 1minute hemotoxylin counterstaining. After rinsing in PBS, the reaction product was visualized using DAB staining 14 and images were captured using the MicroPublisher 5.0 RTV (Q Imaging) brightfield microscope with the Metamorph software (Universal Imaging Corporation). Sections were subsequently quantified by a blinded observer using SigmaScan Pro (version 5.0) image measurement software. The area of DAB signal on the ipsilateral hemisphere was measured and IgG extravasation was calculated as the ratio of DAB signal/Total hemispheric area.
FITC- dextran staining
150 kDa FITC dextran (Sigma) was injected through the femoral vein of mice at 24 hours of MCAO. The mice were then sacrificed by cervical dislocation (after 2 minutes of injection) and the brains were harvested and flash frozen in 2-methy butane on dry ice. Frozen brains were then embedded in OCT embedding solution and cut into 10 μm sections on a cryostat at −19° C and mounted on charged slides 15. Every eighth section was cover slipped and used to visualize and quantify FITC dextran staining.
Immunohistochemistry
Immunohistochemical staining of frozen fixed sections (30 μm) was performed as described previously 6, 16, 17. Briefly, brain slices were mounted onto gelatin-coated slides, allowed to air dry and then blocked in 0.1 M phosphate buffer (PB) with 0.3% TritonX-100 (sigma) and 10% donkey serum (PBTDS) for an hour. Primary antibody (CD31, Sigma, 1:500) was added overnight. After washes, the sections were incubated with secondary antibody (1:1000) and 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI, 1:1000, Invitrogen, Carlsbad, CA). Secondary antibody (1:1000, goat anti- conjugated to Alexa-488) was removed with three consecutive washes in PBTDS, 0.1 M PB, and 0.05 M PB respectively.
Fluorescent microscopy
FITC dextran and CD31 images were acquired with a Zeiss Axiovert 200M microscope (Carl Zeiss, Oberkochen, Germany) using a X-Cite 120Q fluorescence illumination system (Lumen Dynamics Group Inc., Mississauga, ON, Canada) and Zeiss image acquisition software (Zeiss LSM 510).
Brain slices were taken at the same distance from bregma (0.5 mm anterior to bregma) and three 20× fields/animal were analyzed by a blinded observer in the penumbral area of the infarct. Microvascular density was evaluated by calculating the integrated pixel density of the images using MacBiophotonics ImageJ software. The average of the integrated pixel density of 3 fields of view (20x) was used for analysis.
Statistical Analysis
Flux data is presented as Mean ± SEM. CBF flux % was calculated by normalizing the baseline pre-ischemic flux to 100% and expressing the intra-ischemic and post-reperfusion flux as a percentage of the baseline. Two-way ANOVA with factors of aging (young and aged) and stroke (pre stroke, intra-ischemic and post-reperfusion) was used. The ordinal data (neurological score) is expressed as Median (interquartile) and was analyzed using Mann Whitney U test on SPSS software (v.20). Integrated pixel density and IgG immunoreactivity was analyzed by t-test with unequal variance. p<0.05 was considered statistically significant.
Results
CBF Flux in the brain after stroke
LSF was performed in both young and aged mice subjected to 60 min MCAO (Figure 1 A&B). One mouse that did not show ischemia by LSF was excluded, and the mortality was 5% by the end of this experiment. There was no significant effect of aging on the CBF flux in the ipsilateral hemisphere (Figure 2A; p=0.4; n = 7/young group, n = 8/aged group), indicating there was no difference in CBF in young versus aged mice at the baseline (pre-stroke measurements), during ischemia and post-reperfusion. Not surprisingly, there was a significant main effect of stroke on both the CBF flux [F (2, 45) = 50.4; p < 0.001] and CBF flux % [F (2, 45) = 62.2; p < 0.001] in young and aged mice (Figure 2 A&B). Intra-ischemically, there was a significant decrease in CBF flux to 33.8±1.9% (young) and 33.4±6.6% (aged) from the baseline. No collateral vascular flow specific to the aged was seen on the flux images. The CBF returned to 73±6.4% in young and 64.5±9.5% in aged mice 10 minutes after reperfusion.
Figure 2.
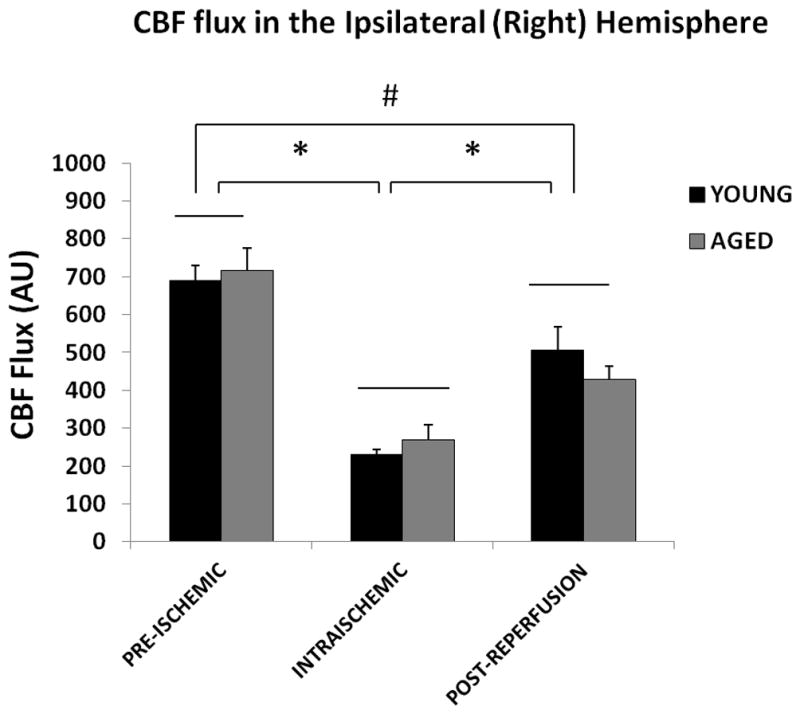
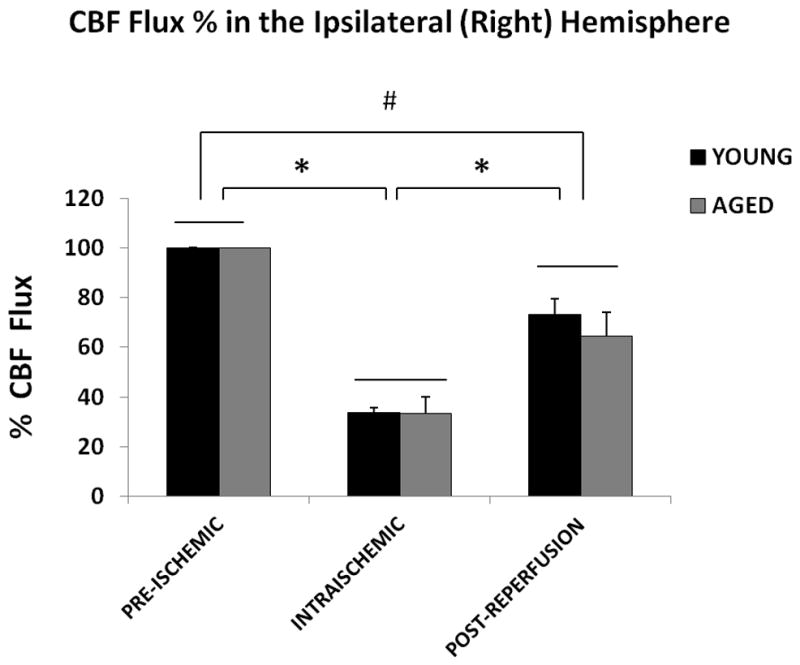
CBF flux in the ipsilateral hemisphere. (A) Flux in young vs. aged mice in the ipsilateral (right) hemisphere. No significant difference was seen in the CBF flux in young vs. aged mice pre-ischemically, intraischemically or post-reperfusion. The CBF flux significantly decreased after MCAO, and was recovered after reperfusion, *p<0.05. (B) CBF Flux % in young vs. aged mice in the ipsilateral (right) hemisphere. No significant difference was seen in the CBF flux in young vs. aged mice pre-ischemically, intra-ischemically or post-reperfusion. The CBF flux% significantly decreases after MCAO, and was recovered after reperfusion, *p<0.05.
Similar to the ipsilateral hemisphere, no significant difference in perfusion (CBF flux) was seen in young vs. aged mice at baseline, during MCAO and post-reperfusion in the contralateral hemisphere (Figure 3A&B; p=0.6). A significant effect of stroke on the CBF flux was observed [F (2, 45) = 6.2; p < 0.001], as the perfusion during MCAO in both the young (79.6±4.4%) and aged (79.9±6.7%) mice was significantly decreased from the baseline.
Figure 3. CBF flux in the contralateral hemisphere.
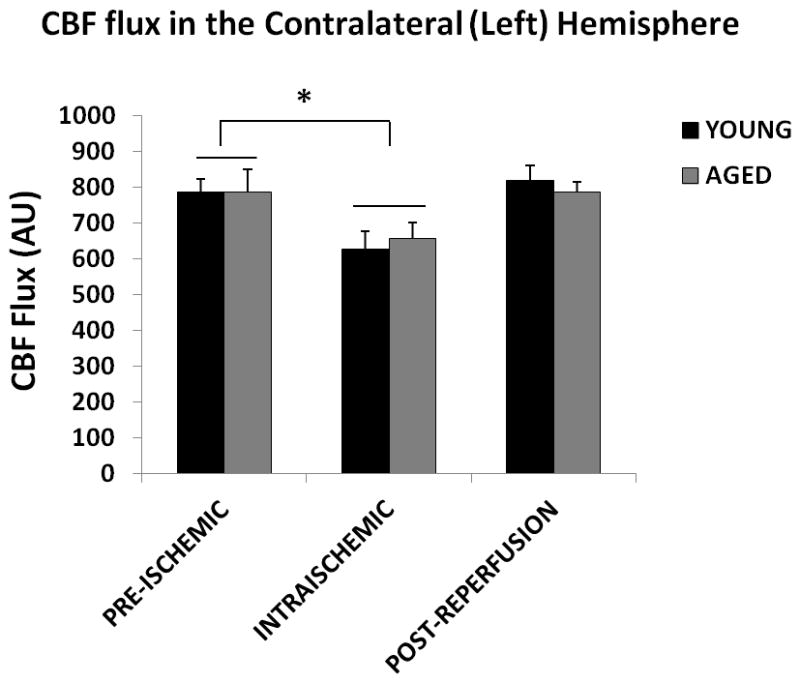
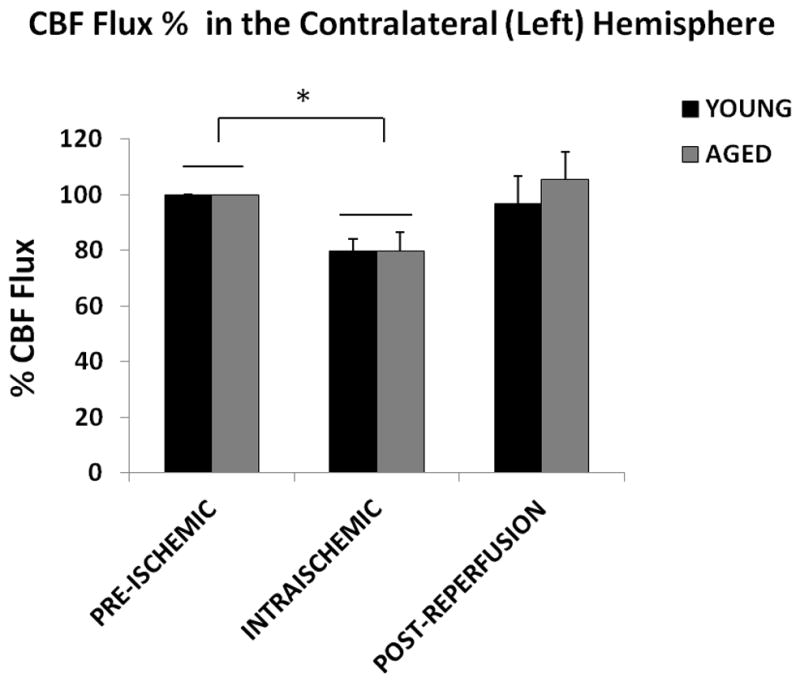
(A) Flux in young vs. aged mice in the contralateral (left) hemisphere. No significant difference was seen in the CBF flux in young vs. aged mice in the contralateral hemisphere pre-ischemically, intra-ischemically or post-reperfusion. The CBF flux significantly decreases after MCAO, *p<0.05. (B) Flux % in young vs. aged mice in the contralateral (left) hemisphere. No significant difference was seen in the CBF flux in young vs. aged mice in the contralateral hemisphere, pre-ischemically, intra-ischemically or post-reperfusion. The CBF flux% significantly decreased after MCAO, *p<0.05.
Neurological scores are worse in the aged mice
The neurological deficit was scored 24 and 72 hours after stroke. At 24 hours of stroke, aged mice had significantly worse neurological scores, 4(0) vs. 1(0.5) in young mice, p<0.05. There was no significant difference in the weight loss between young (5.7±1.5%) and aged (6.8±1.3 %) mice at 24 hours after MCAO. The same pattern was also seen in 72-hour cohort.
FITC dextran and CD31 show no difference in micro vascular density
To confirm the LSF data that there was no difference in CBF flux between young and aged mice after stroke, we further examined the integrity of microvasculature in both cohorts. FITC-dextran staining found dextran localized along the vessels; there was no significant difference in dextran integrated pix density between the young and aged cohorts (Figure 4 B&C; p<0.05; 9 images from 3 brains were analyzed). We further assessed the microvasculature using CD31 staining. The integrated vessel density was not significantly different in young vs. aged mice (Figure 5 A&B; p<0.05; 9 images from 3 brains were analyzed). This suggests that structural differences pertaining to vessel density do not exist in young vs. aged mice.
Figure 4.
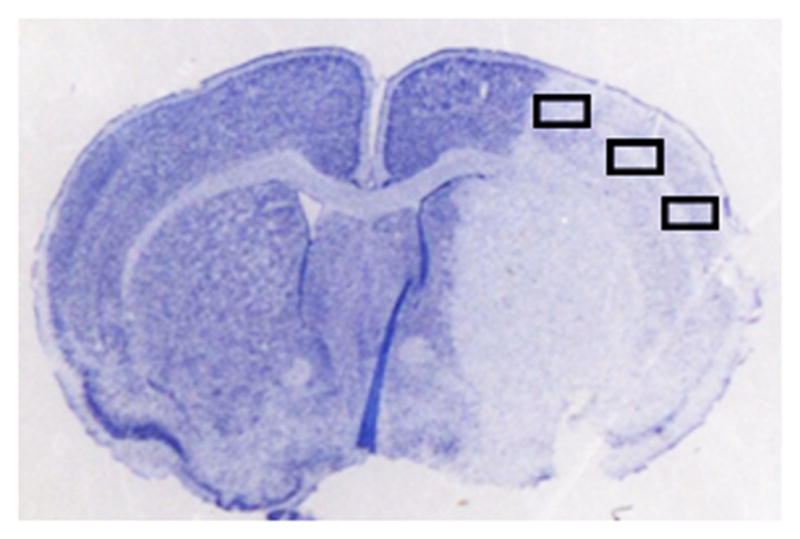
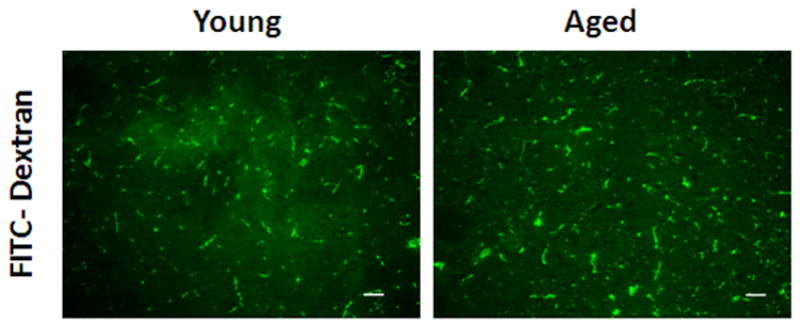
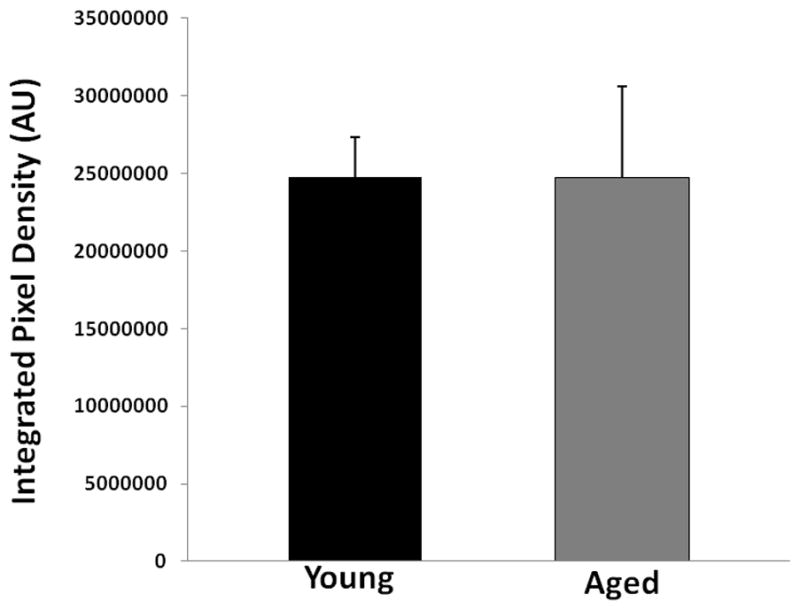
FITC-dextran (150kd) staining of young vs. aged mice 24 hours after MCAO. (A) A representative TTC picture showing all IHC images were taken in the boxed areas. (B) Representative FITC-dextran staining pictures (10x). Scale bar = 50 μm. (C) Integrated vascular density in young vs. aged mouse brain sections 24 hours after MCAO. No significant difference in the integrated pixel density was seen in young vs. aged mice.
Figure 5.
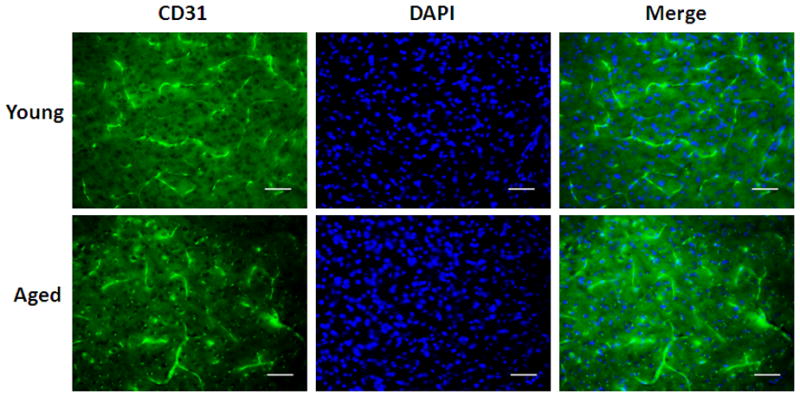
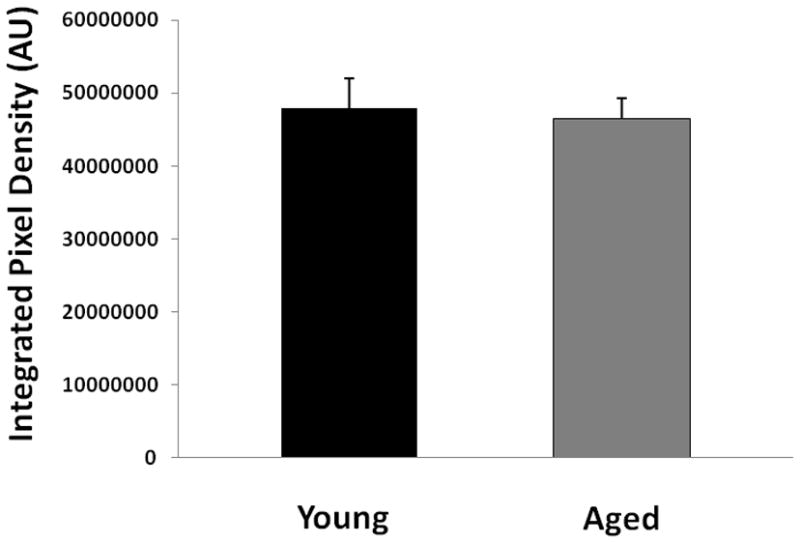
CD31 staining of young vs. aged mice 24 hours after MCAO. (A) Representative FITC-dextran staining pictures (20x). Scale bar = 50 μm. (B) Integrated vascular density in young vs. aged mouse brain sections 24 hours after MCAO. There was no significant difference in the integrated pixel density of young vs. aged mice.
Blood Brain Barrier breakdown is higher in young mice
Since we found no difference in the perfusion but worse neurological scores in the aged mice, we wanted to explore the integrity of the BBB in these mice using IgG staining. Figure 6A shows representative coronal brain sections of cresyl violet staining. Figure 6B shows IgG staining in mice sacrificed at 24 hours and 72 hours of MCAO respectively (n=5 in aged and n=6 in young group). Significantly higher IgG immunoreactivity was seen in young vs. aged mice post stroke, indicating higher blood brain barrier leakage in young brains, p<0.05 (Figure 6C, D).
Figure 6.
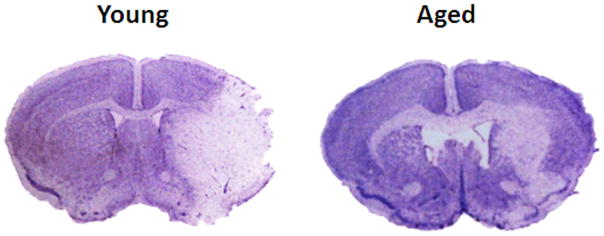
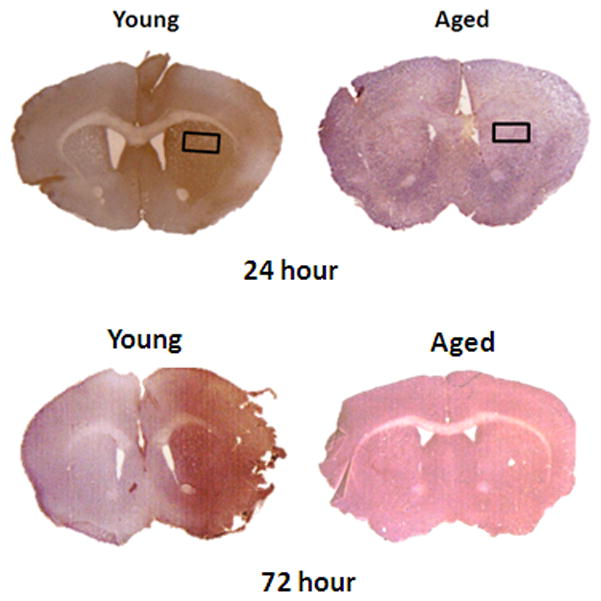
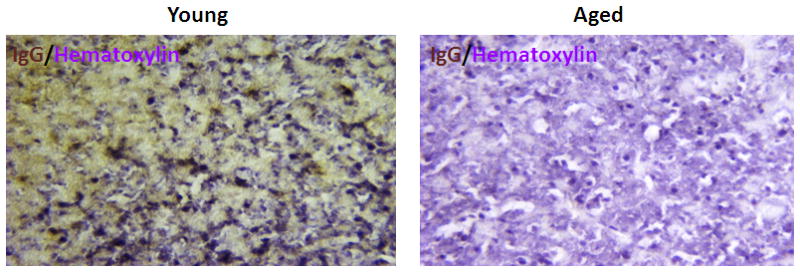
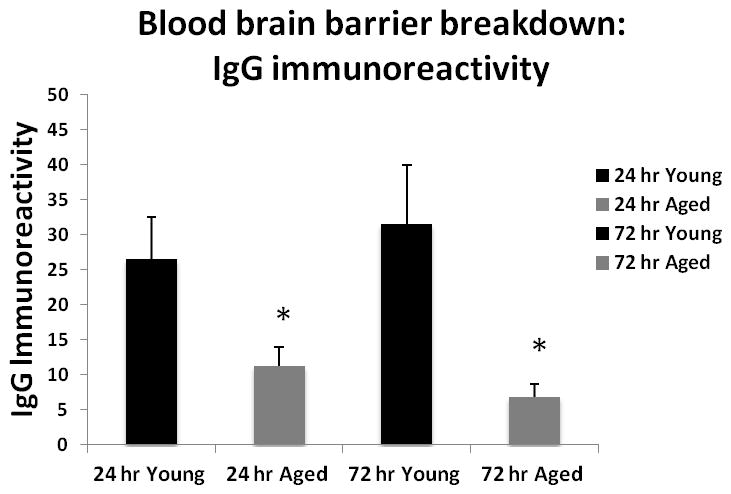
Cresyl violet and IgG staining (Hematoxylin counterstain) in young vs. aged brain sections 24 hrs after MCAO. (A) Representative CV staining pictures showing young brains had larger infarct volumes (white) than aged brains. (B) Representative IgG staining pictures showing young brains had higher IgG immunoreactivity (brown) than aged brains at 24 and 72 hours endpoint. (C) Microscopic images (20x) of IgG staining in young vs. aged brains. Pictures are taken from the area marked with a box in (B). Brown shows the IgG staining. Blue is the counterstain with hematoxylin. (D) Quantification of IgG immunoreactivity shown in 6B, 6C. Young brain shows higher IgG immunoreactivity (brown) than the aged at both 24 and 72 hours after MCAO, *p<0.05 vs. young group (n=6 young, n=5 aged).
Discussion
This study is the first to use LSF to monitor CBF changes in the brains of aged mice peri-ischemia. Several important novel findings were found. First, by the functional analysis of CBF with LSF, we established that the different stroke phenotypes between young and aged animals seen in this study and previous reports6, 18 are not due to different levels of ischemia in young vs. aged brains. Secondly, the structural analysis of CBF with FITC-dextran and CD31 staining also revealed that the cerebral microvascular density is not different in young vs. aged animals after MCAO. Third, our LSF data demonstrated that the unilateral MCAO not only induces infarction in the ipsilateral hemisphere, but also caused CBF reduction in the contralateral hemisphere, indicating a widespread and diffuse change that occurs throughout the brain and vasculature even in focal stroke models. Finally, we found that aged male mice have less IgG extravasation at both the acute and subacute stage of stroke as compared to the young animals, suggesting that there was less BBB breakdown in the aged ischemic brains.
It is increasingly accepted that modeling stroke in aged animals more appropriately mimics stroke patients. However, stroke studies in aged animals are often not performed due to the high cost of aging animals and technical expertise required for surgeries. The few studies available in literature have shown varying results in the histological outcomes in young vs. aged animal4, 6, 19–23. There have been reports of increased 22, 24, equal 23, 25 or decreased infarct volumes 4, 19–21 in aged vs. young animals. The discrepancies may be due to the difference in animal strains, animal sexes, or ischemic models used in the studies. Several clinical studies 7, 26 demonstrated age negatively correlated with the size of either the ischemic lesion or the penumbral area in stroke patients, which is consistent with the results from our studies. Despite discrepancies in histological outcomes, there is agreement in most preclinical and clinical studies that functional (behavioral) stroke outcomes are worse in aged cohorts2, 6, 22, 27. The underlying mechanism remains elusive and may be due to age related co-morbidities, an increased systemic immune response 28, autonomic or cardiac instability 29 and decreased neurogenesis/angiogenesis 8, all of which have been well documented in aged animals.
Aging is associated with structural alterations in the vessel wall (intima and smooth muscle cells), which leads to arterial stiffening, endothelial dysfunction, atherosclerosis and calcification of vessels 30–32. Therefore, it was physiologically plausible that there may be a structural difference in the cerebral vasculature and also a functional difference in the cerebral perfusion either at the baseline and/or in the setting of an ischemic injury, leading to smaller infarcts in the aged. However, both functional and structural analysis of CBF perfusion performed in the present study did not support this hypothesis. We did not see differences in the baseline, intra-ischemic and post-reperfusion CBF between the young and aged cohorts, consistent with clinical studies that showed CBF did not decline with age in the healthy individuals 33 and that there were no differences in CBF in young and aged acute stroke patients 7. Thus, it appears that age related changes in the vascular bed do not significantly change the perfusion of the brain territories before or after stroke, and neither does the aged brain appear to have enhanced collateral mediated reperfusion intra-ischemically, suggesting other molecular mechanisms downstream to ischemia instead of CBF changes may be involved in mediating the age dependent stroke injury 5, 16. Interestingly, our LSF data revealed that CBF in the contralateral hemisphere also significantly dropped from the baseline after stroke, which exemplifies the fact that stroke is a systemic disease and that the ischemic challenge may be sensed throughout the vasculature. Systemic changes in distant organs (e.g. the spleen) induced by stroke have been increasingly reported 34,35 and are most likely mediated by activated molecules or metabolites released from the ischemic brain tissue into the circulation.
The amount of plasma protein IgG leakage correlated with infarct volumes. Less IgG extravasation was seen in the aged brain indicating less BBB breakdown, consistent with our previous finding that evans blue extravasation was significantly less in aging vs. young ischemic brains4. It is interesting that despite similar intra ischemic reductions in CBF, the BBB integrity was less compromised in the aged brain. Expression of connexin 43, a major gap junction protein and a key component of BBB, was reduced in young mice but preserved in aging brains after ischemic stroke18. Although aging has deleterious effect on BBB function under normal conditions36 young brains may still have more robust BBB breakdown after ischemia due to the enhanced inflammatory responses compared to aged brains 20. However, whether the relatively preserved BBB integrity in the aged brain is a result of less ischemic injury or vice versa, remains unknown. Experimental studies 37, 38 found inhibition of matrix metalloproteinase-9 (MMP-9), a proteolytic enzyme that degrades the BBB, is neuroprotective by reducing infarct size, suggesting BBB integrity is important in limiting the ischemic lesion. The possibility could not be excluded that the less injured BBB in aged ischemic brains may be causative to the smaller infarct compared to young brains in the present study. Future studies are needed that examine age related BBB changes following ischemia.
There are several limitations to this work that need to be recognized when interpreting our data. We only utilized LSF for the functional analysis of CBF, as LSF allows us to monitor the CBF change intra-ischemically in a live animal. 14C-iodoantipyrine autoradiography (14C-IAP) is a more accurate and quantitative method to examine brain tissue reperfusion after ischemia39. However, we did not elect to do this because 14C-IAP is a terminal study and cannot be used to monitor CBF in real time pre-ischemic, intra-ischemic and also post-reperfusion in the same animal. Moreover, LSF does not inform us about CBF differences in subcortical grey and white matter. IAP would be more appropriate for such an assessment. IAP, Arterial spin labeling (ASL), diffusion tensor imaging (DTI) are the techniques which can be used in future studies to examine the association between cortical and subcortical white matter CBF 40. Another caveat is that we only focused on the acute stage of MCAO for structural analysis of CBF; whether microvascular density post-ischemia changes overtime to cause a difference between young and aged animals needs further investigation. All the animals involved in this study were males and we did not examine whether there is a difference in CBF changes between young and aged female animals. Sex differences in age-related CBF changes after ischemia could exist as females exhibit different stroke phenotypes than their male counterparts at either young or old age41 which is one of the on-going projects by our research group.
In conclusion, young and aged animals exhibit equivalent levels of CBF throughout ischemia, and the age-specific stroke phenotype appears to be unrelated to CBF/perfusion changes in stroke. Microvascular density in the ischemic brain was not different between young and aged animals. Aged brains have less IgG immunoreactivity after MCAO than the young group, which is related to the smaller infarct size seen in aged brains. Other molecular mechanisms that are unrelated to acute CBF changes may underlie age-specific stroke phenotypes.
Acknowledgments
Sources of Funding: This work was supported by American Heart Association-grant number 11PRE7440068 to BM; grant number 12SDG9030000 to FL and the NIH/NINDS grants NS050505 and NS055215 to LDM.
Joshua Crapser for help in slicing brain sections.
Footnotes
Conflict of Interest: The authors declare that there are no conflicts of interest.
References
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. Heart disease and stroke statistics--2008 update: A report from the american heart association statistics committee and stroke statistics subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Knoflach M, Matosevic B, Rucker M, Furtner M, Mair A, Wille G, et al. Functional recovery after ischemic stroke--a matter of age: Data from the austrian stroke unit registry. Neurology. 2012;78:279–285. doi: 10.1212/WNL.0b013e31824367ab. [DOI] [PubMed] [Google Scholar]
- 3.Rojas JI, Zurru MC, Romano M, Patrucco L, Cristiano E. Acute ischemic stroke and transient ischemic attack in the very old--risk factor profile and stroke subtype between patients older than 80 years and patients aged less than 80 years. European journal of neurology: the official journal of the European Federation of Neurological Societies. 2007;14:895–899. doi: 10.1111/j.1468-1331.2007.01841.x. [DOI] [PubMed] [Google Scholar]
- 4.Yuan M, Siegel C, Zeng Z, Li J, Liu F, McCullough LD. Sex differences in the response to activation of the poly (adp-ribose) polymerase pathway after experimental stroke. Experimental neurology. 2009;217:210–218. doi: 10.1016/j.expneurol.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu F, Benashski SE, Persky R, Xu Y, Li J, McCullough LD. Age-related changes in amp-activated protein kinase after stroke. Age. 2012;34:157–168. doi: 10.1007/s11357-011-9214-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manwani B, Liu F, Xu Y, Persky R, Li J, McCullough LD. Functional recovery in aging mice after experimental stroke. Brain, behavior, and immunity. 2011;25:1689–1700. doi: 10.1016/j.bbi.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agarwal S, Scoffings DJ, Jones PS, Marrapu ST, Barry PJ, O’Brien EW, et al. Interaction of age with the ischaemic penumbra, leptomeningeal collateral circulation and haemodynamic variables in acutestroke: A pilot study. J Neurol Neurosurg Psychiatry. 2013;84:271–6. doi: 10.1136/jnnp-2012-303258. [DOI] [PubMed] [Google Scholar]
- 8.Petcu EB, Smith RA, Miroiu RI, Opris MM. Angiogenesis in old-aged subjects after ischemic stroke: A cautionary note for investigators. Journal of angiogenesis research. 2010;2:26. doi: 10.1186/2040-2384-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta A, Nair S, Schweitzer AD, Kishore S, Johnson CE, Comunale JP, et al. Neuroimaging of cerebrovascular disease in the aging brain. Aging and disease. 2012;3:414–425. [PMC free article] [PubMed] [Google Scholar]
- 10.Dunn AK, Bolay H, Moskowitz MA, Boas DA. Dynamic imaging of cerebral blood flow using laser speckle. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2001;21:195–201. doi: 10.1097/00004647-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 11.McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD. Ischemic nitric oxide and poly (adp-ribose) polymerase-1 in cerebral ischemia: Male toxicity, female protection. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2005;25:502–512. doi: 10.1038/sj.jcbfm.9600059. [DOI] [PubMed] [Google Scholar]
- 12.Liu F, Benashski SE, Xu Y, Siegel M, McCullough LD. Effects of chronic and acute oestrogen replacement therapy in aged animals after experimental stroke. Journal of neuroendocrinology. 2012;24:319–330. doi: 10.1111/j.1365-2826.2011.02248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bake S, Friedman JA, Sohrabji F. Reproductive age-related changes in the blood brain barrier: Expression of igg and tight junction proteins. Microvascular research. 2009;78:413–424. doi: 10.1016/j.mvr.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richmon JD, Fukuda K, Maida N, Sato M, Bergeron M, Sharp FR, et al. Induction of heme oxygenase-1 after hyperosmotic opening of the blood-brain barrier. Brain research. 1998;780:108–118. doi: 10.1016/s0006-8993(97)01314-0. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann A, Bredno J, Wendland M, Derugin N, Ohara P, Wintermark M. High and low molecular weight fluorescein isothiocyanate (fitc)-dextrans to assess blood-brain barrier disruption: Technical considerations. Translational stroke research. 2011;2:106–111. doi: 10.1007/s12975-010-0049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu F, Akella P, Benashski SE, Xu Y, McCullough LD. Expression of na-k-cl cotransporter and edema formation are age dependent after ischemic stroke. Experimental neurology. 2010;224:356–361. doi: 10.1016/j.expneurol.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manwani B, McCullough LD. Sexual dimorphism in ischemic stroke: Lessons from the laboratory. Women’s Health. 2011;7:319–339. doi: 10.2217/whe.11.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu F, Yuan R, Benashski SE, McCullough LD. Changes in experimental stroke outcome across the life span. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2009;29:792–802. doi: 10.1038/jcbfm.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shapira S, Sapir M, Wengier A, Grauer E, Kadar T. Aging has a complex effect on a rat model of ischemic stroke. Brain research. 2002;925:148–158. doi: 10.1016/s0006-8993(01)03270-x. [DOI] [PubMed] [Google Scholar]
- 20.Sieber MW, Claus RA, Witte OW, Frahm C. Attenuated inflammatory response in aged mice brains following stroke. PloS one. 2011;6:e26288. doi: 10.1371/journal.pone.0026288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Correa F, Gauberti M, Parcq J, Macrez R, Hommet Y, Obiang P, et al. Tissue plasminogen activator prevents white matter damage following stroke. The Journal of experimental medicine. 2011;208:1229–1242. doi: 10.1084/jem.20101880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DiNapoli VA, Huber JD, Houser K, Li X, Rosen CL. Early disruptions of the blood-brain barrier may contribute to exacerbated neuronal damage and prolonged functional recovery following stroke in aged rats. Neurobiology of aging. 2008;29:753–764. doi: 10.1016/j.neurobiolaging.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang RY, Wang PS, Yang YR. Effect of age in rats following middle cerebral artery occlusion. Gerontology. 2003;49:27–32. doi: 10.1159/000066505. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, Sun FY. Age-related decrease of striatal neurogenesis is associated with apoptosis of neural precursors and newborn neurons in rat brain after ischemia. Brain research. 2007;1166:9–19. doi: 10.1016/j.brainres.2007.06.043. [DOI] [PubMed] [Google Scholar]
- 25.Won SJ, Xie L, Kim SH, Tang H, Wang Y, Mao X, et al. Influence of age on the response to fibroblast growth factor-2 treatment in a rat model of stroke. Brain research. 2006;1123:237–244. doi: 10.1016/j.brainres.2006.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roussel BD, Macrez R, Jullienne A, Agin V, Maubert E, Dauphinot L, et al. Age and albumin d site-binding protein control tissue plasminogen activator levels: Neurotoxic impact. Brain: a journal of neurology. 2009;132:2219–2230. doi: 10.1093/brain/awp162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ford GA, Ahmed N, Azevedo E, Grond M, Larrue V, Lindsberg PJ, et al. Intravenous alteplase for stroke in those older than 80 years old. Stroke; a journal of cerebral circulation. 2010;41:2568–2574. doi: 10.1161/STROKEAHA.110.581884. [DOI] [PubMed] [Google Scholar]
- 28.Machado MC, Coelho AM, Carneiro D’Albuquerque LA, Jancar S. Effect of ageing on systemic inflammatory response in acute pancreatitis. International journal of inflammation. 2012;2012:270319. doi: 10.1155/2012/270319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esler MD, Thompson JM, Kaye DM, Turner AG, Jennings GL, Cox HS, et al. Effects of aging on the responsiveness of the human cardiac sympathetic nerves to stressors. Circulation. 1995;91:351–358. doi: 10.1161/01.cir.91.2.351. [DOI] [PubMed] [Google Scholar]
- 30.Lakatta EG. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part iii: Cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–497. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 31.Herrera MD, Mingorance C, Rodriguez-Rodriguez R, Alvarez de Sotomayor M. Endothelial dysfunction and aging: An update. Ageing research reviews. 2010;9:142–152. doi: 10.1016/j.arr.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Cau SB, Carneiro FS, Tostes RC. Differential modulation of nitric oxide synthases in aging: Therapeutic opportunities. Frontiers in physiology. 2012;3:218. doi: 10.3389/fphys.2012.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meltzer CC, Cantwell MN, Greer PJ, Ben-Eliezer D, Smith G, Frank G, et al. Does cerebral blood flow decline in healthy aging? A pet study with partial-volume correction. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2000;41:1842–1848. [PubMed] [Google Scholar]
- 34.Gendron A, Teitelbaum J, Cossette C, Nuara S, Dumont M, Geadah D, et al. Temporal effects of left versus right middle cerebral artery occlusion on spleen lymphocyte subsets and mitogenic response in wistar rats. Brain research. 2002;955:85–97. doi: 10.1016/s0006-8993(02)03368-1. [DOI] [PubMed] [Google Scholar]
- 35.Offner H, Vandenbark AA, Hurn PD. Effect of experimental stroke on peripheral immunity: Cns ischemia induces profound immunosuppression. Neuroscience. 2009;158:1098–1111. doi: 10.1016/j.neuroscience.2008.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farrall AJ, Wardlaw JM. Blood-brain barrier: Ageing and microvascular disease--systematic review and meta-analysis. Neurobiology of aging. 2009;30:337–352. doi: 10.1016/j.neurobiolaging.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 37.Hu Q, Chen C, Khatibi NH, Li L, Yang L, Wang K, et al. Lentivirus-mediated transfer of mmp-9 shrna provides neuroprotection following focal ischemic brain injury in rats. Brain research. 2011;1367:347–359. doi: 10.1016/j.brainres.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Romanic AM, White RF, Arleth AJ, Ohlstein EH, Barone FC. Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: Inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke; a journal of cerebral circulation. 1998;29:1020–1030. doi: 10.1161/01.str.29.5.1020. [DOI] [PubMed] [Google Scholar]
- 39.Chen J, Graham SH, Zhu RL, Simon RP. Stress proteins and tolerance to focal cerebral ischemia. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 1996;16:566–577. doi: 10.1097/00004647-199607000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Chen JJ, Rosas HD, Salat DH. The relationship between cortical blood flow and sub-cortical white-matter health across the adult age span. PloS one. 2013;8:e56733. doi: 10.1371/journal.pone.0056733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manwani B, McCullough LD. Sexual dimorphism in ischemic stroke: Lessons from the laboratory. Women’s health. 2011;7:319–339. doi: 10.2217/whe.11.22. [DOI] [PMC free article] [PubMed] [Google Scholar]


