Abstract
Objective
To describe the clinical and imaging characteristics of a new lymphatic disorder with a unique histological pattern and poor prognosis.
Study design
An observational, retrospective study identified and characterized 20 patients with distinct lymphatic histopathology referred to the Vascular Anomalies Center at Boston Children’s Hospital between 1995 and 2011.
Results
The median age at onset was 6.5 years (range, birth-44 years). Clinical and radiologic findings suggested a generalized process. The most common presentations were respiratory symptoms (50%), hemostatic abnormalities (50%), and an enlarging, palpable mass (35%). All patients had mediastinal involvement; 19 patients developed pericardial (70%) and/or pleural effusions (85%). Extrathoracic disease manifested in bone and spleen and less frequently in abdominal viscera, peritoneum, integument, and extremities. Despite aggressive procedural and medical therapies, the 5-year survival was 51%, and the overall survival was 34%. Mean interval between diagnosis and death was 2.75 years (range, 1 to 6.5 years).
Conclusions
We describe a clinicopathologically distinct lymphatic anomaly. We propose the term kaposiform lymphangiomatosis (KLA) because of characteristic clusters or sheets of spindled lymphatic endothelial cells accompanying malformed lymphatic channels. The intrathoracic component is most commonly implicated in morbidity and mortality; however, extra-thoracic disease is frequent, indicating that KLA is not restricted to pulmonary lymphatics. The mortality rate of KLA is high despite aggressive multi-modal therapy.
Keywords: vascular anomaly, lymphatic malformation, hemorrhagic effusion, pulmonary, lymphangioma, lymphangiectasia
The lymphatic vascular system is fundamental to interstitial circulation and immunity. Focal and generalized anomalies in the structure and function of lymphatic vasculature cause major morbidity through edema, effusion and infection.1–3 Complications vary by anatomic location and age at presentation as illustrated by intrathoracic lymphatic anomalies that range from fetal imaging abnormalities to acute or chronic pulmonary insufficiency in older children and young adults.4–6 Intrathoracic lymphatic anomalies can be confined to the thoracic cavity causing effusions, lymphatic reflux into air spaces, interstitial disease and/or dilation of mediastinal and bronchopulmonary lymphatics5–7 or be combined with systemic manifestations (splenic and osseus lesions, lymphedema, cutaneous changes and chylous leak) as is observed with generalized lymphatic anomaly (GLA).4, 5, 8 Microscopically, GLA is characterized by an anastomotic pattern of variably sized, thin walled lymphatic channels lined by flattened endothelial cells. The presence of spindled endothelial cells amidst the background of malformed lymphatic channels is not typical of GLA and, though reported in the pulmonary literature, has not been delineated as a separate entity.4, 6, 9
We describe a novel subtype of generalized lymphatic anomaly, which is distinguished by histopathology, and highlight the presenting signs/symptoms, organ system involvement, clinical course, response to therapy, and outcome.
METHODS
Patients referred to our Vascular Anomalies Center from 1995 to 2011 with various types of lymphatic anomalies were reviewed at an interdisciplinary conference. Twenty patients had a generalized lymphatic anomaly with novel histopathology. The histological hallmark was clusters or sheets of “kaposiform” hemosiderotic, spindled lymphatic endothelial cells oriented in parallel fashion amidst abnormal and dilated lymphatic channels. These spindled cells were immunoreactive for lymphatic markers (D2-40, Lyve-1, and Prox-1). Mitoses and cellular atypia were rare. Interspersed red blood cells and hemorrhage were also frequently observed. Our Committee on Clinical Investigation approved a retrospective, observational study of these patients. No patients/guardians declined participation. Nine patients/guardians were successfully contacted and consented to a structured telephone interview and release of medical records to gather data missing from the initial referral. All patients had their medical records reviewed with attention to clinical history, laboratory data, and imaging reports.
RESULTS
All patients had a distinctive histopathological pattern10, characteristic imaging and poor prognosis (Figure 1). They were often referred with a diagnosis of “lymphangiomatosis.” Thoracic disease often manifested as pleural and/or pericardial effusions. High-resolution CT demonstrated interlobular septal thickening due to dilated lymphatic channels usually with a prominent soft tissue component in the mediastinal, paraspinal, or retroperitoneal region. On MRI this soft tissue component was heterogeneous and infiltrative in appearance with hyperintensity on fluid-weighted sequences. Numerous hypoechoic, round foci were identified in the spleen on sonography. Involvement of bony elements, evidenced by lucent lesions with cortical sparing, was also regularly observed on CT or plain radiographs.
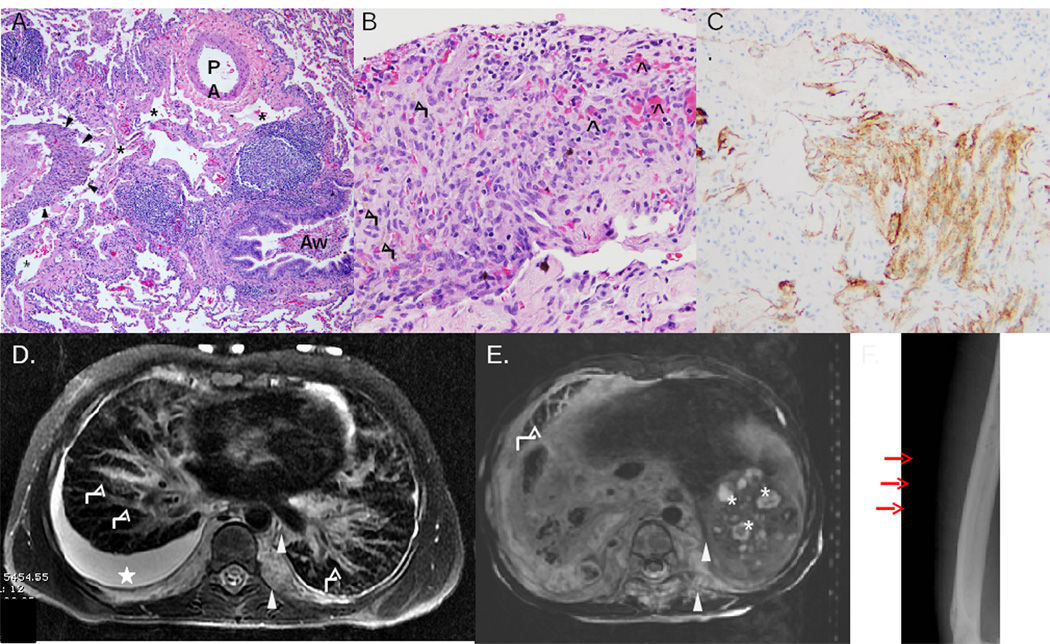
Figure 1.
KLA histopathology (A-C) and radiologic (D-F) features of KLA. A, Pulmonary parenchyma with dilated lymphatic channels (asterisks), accompanying airway (Aw), pulmonary artery (PA), and focus of spindled cells (H&E stain). B, Focus of spindled cells some with cytoplasmic hemosiderin granules (black arrows); interspersed red blood cells present (black open arrow heads) (H&E stain). C, Spindled lymphatic endothelial cells immunopositive for lymphatic marker D2-40. D, MRI of chest. T2 axial thoracic image demonstrates pleural effusion (star), pulmonary interlobular septal thickening (white arrows), and retroperitoneal soft tissue mass (arrow heads). E, MRI of abdomen. T2 axial abdominal image shows heterogeneous, infiltrative, hyperintense retroperitoneal soft tissue mass (arrow heads), splenic cysts (asterisks), and pulmonary interlobular septal thickening (white arrow). F, Plain film illustrates multiple lucent bony lesions of the humerus (red arrows).
The median age at onset of signs and symptoms was 6.5 years (range, birth-44 years); however, the median age at diagnosis of a lymphatic anomaly was 8.5 years (Figure 2, A). Several factors contributed to this interval, such as limited access to medical care and nonspecific findings including thrombocytopenia with splenomegaly and indolent respiratory symptoms. There were 13 males and 7 females.
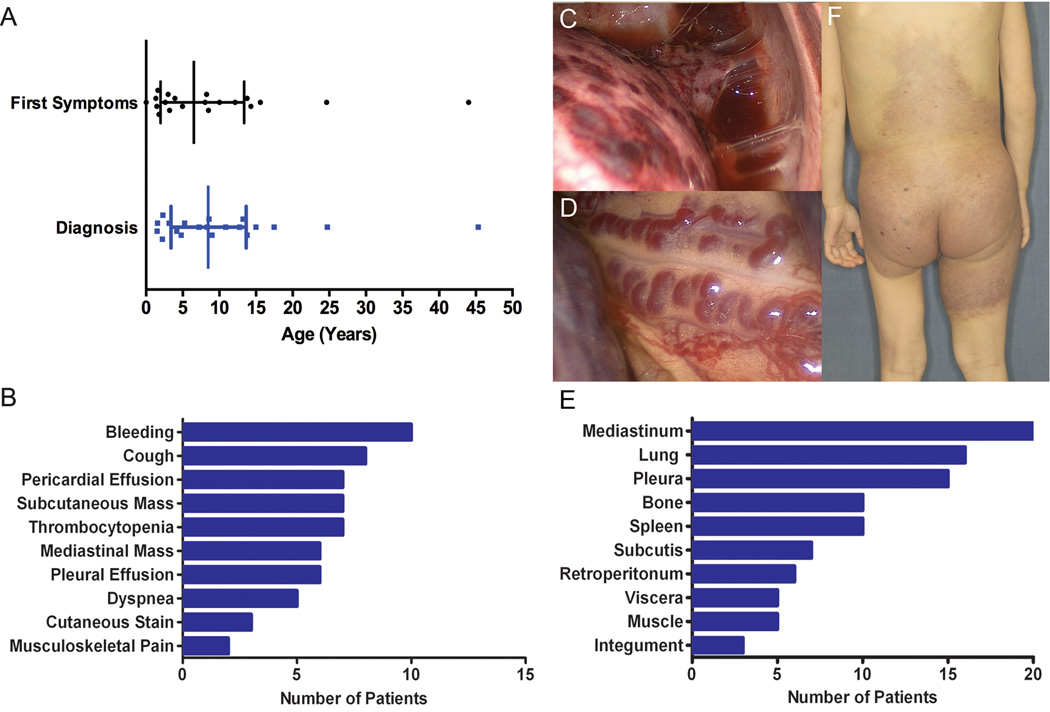
Figure 2.
Clinical Phenotype of KLA. A, Patient age at initial symptoms compared with age at diagnosis of lymphatic anomaly. Median age with interquartile range indicated; mean interval between onset of symptoms and diagnosis of lymphatic anomaly was 2 years. B, Frequency of presenting features. C, Hemorrhagic plaques on thoracic visceral pleura. D, Dilated tortuous blood vessels coursing the surface of the mediastinum. E, Frequency of involvement of anatomical sites. F, Cutaneous manifestations of KLA with red-purple discoloration and scattered lymphatic vesicles.
Presenting features included respiratory symptoms (50%), bleeding (50%), and subcutaneous mass (35%) (Figure 2, B). The most common respiratory complaints were cough and dyspnea. Five patients had an acute onset of cough and fever or dyspnea. Non-specific respiratory problems developed in five patients and worsened over an average of 6.4 months (range, 1–12 months); parents recalled shortness of breath with exertion or decreased exercise tolerance. Three patients were diagnosed with asthma but failed to improve on inhaled albuterol and corticosteroid prompting diagnostic imaging. Among the 10 patients with respiratory symptoms at presentation, four had pericardial effusion, three had pleural effusion, and two had both. All patients required drainage of effusion(s) for symptomatic relief. Of patients who did not have respiratory symptoms at presentation, one had a small pleural effusion and another had a pericardial effusion requiring intervention. Independent of location, drained fluid was variably milky, serosanguinous, or hemorrhagic. Volume of drained pericardial fluid ranged from 400 ml to 1500 ml and pleural fluid from 1000 ml to 3000 ml.
Bleeding was a presenting feature in 10 patients: epistaxis, scleral hemorrhage, ecchymosis, vaginal bleeding, epidural hematoma, and hemorrhagic effusion. Coagulation studies were not available for these patients, but video-assisted thoracoscopy of several patients revealed dilated tortuous blood vessels and hemorrhagic plaques on the visceral pleura (Figure 2, C and D). Six patients also had thrombocytopenia (mean, 78,000 platelets/µl). Two patients were followed for thrombocytopenia with splenomegaly for several months prior to progression of symptoms.
The third most common presenting sign was a discrete, soft, and nontender mass. Three lesions (50%) arose on the flank; others involved the scapula, posterior neck, and anterior chest wall. Although their lesions were not associated with pain or functional limitation, three patients had resection. Histopathology was reported at outside institutions as “lymphaticovenous malformation”, “lymphangioma”, and “lymphangiohemangioma”. These lesions did not recur locally and cutaneous lesions did not develop.
Anatomic Distribution
Radiologic imaging modality and frequency varied for each patient, making it difficult to review progression systematically. The most common regions involved were thoracic cavity (100%), bone (50%), and spleen (50%) (Figure 2, E). All patients had mediastinal disease by cross-sectional imaging. Microcystic lymphatic anomaly involving the pleura and lung parenchyma was noted in 80% of patients. Pulmonary involvement was asymmetric in one-half of the patients without left or right predilection. Bony lesions were lytic, typically with cortical sparing, and were observed most commonly in the vertebral bodies (70%). Frequently multiple non-contiguous bones were involved including: femur, humerus, ribs, and pelvis. Splenic involvement was largely an incidental finding by imaging typically with multi-focal hypoechoic cystic lesions.
Evolution of Disease
All but two patients developed respiratory symptoms, at an average onset of 1.1 years from presentation. Ultimately, 19 of 20 patients developed pericardial (70%) and/or pleural effusions (85%), and all but one required drainage. Seven patients had thrombocytopenia prior to initiation of therapy; the mean nadir platelet count was 44,000/µl (range, 28,000 – 80,000/µl). Again coagulation panels were not routinely reported; however, three patients with thrombocytopenia and bleeding symptoms were found to have prolonged prothrombin time or INR, elevated D-dimer (>8000 ng/ml), and low fibrinogen (<100 mg/dl). Splenectomy was performed in three patients because of thrombocytopenia. Two responded with normalization of platelets, and one showed no improvement. Progressive cutaneous and subcutaneous changes evolved over several years in two patients from small, soft, subcutaneous masses to tense edema of the affected hip and lower extremity with overlying violaceous discoloration and lymphatic blebs (Figure 2, F). These changes were associated with painful ambulation and restricted function.
Therapeutic Interventions
Patients received a combination of procedural and medical therapies (Table). Only two patients had attempted resection of a mediastinal mass, and both had post-operative decompensation within 48 hours. One developed bilateral pleural effusions requiring chest tubes and ongoing drainage for three months before slowly improving; the other had respiratory failure with chylous drainage requiring extracorporal membrane oxygenation and died six weeks later. Eight patients had a pericardial window; others required multiple pleural/pericardiocentesis or a pericardial drain due to rapid reaccumulation of fluid. Daily pericardial drain output greater than one liter per day was common. Likewise, patients with pleural effusion(s) required placement of chest tubes that often drained up to three liters per day for several weeks. Fluid drained at time of reaccumulation was noted to be hemorrhagic (n=6), serosanginous (n=4), or milky (n=2). Nine patients underwent pleurodesis with bleomycin or talc to decrease accumulation of pleural effusions; however, no sustained improvement was seen. Four patients had ligation of the thoracic duct with transient improvement in pleural effusion after several weeks, including removal of the chest tube in one patient.
Table I.
Qualitative efficacy of therapeutic interventions
| Therapy | Sustained Improvement (>6 months) |
Acute Improvement |
Total Patients Treated |
Range (Procedure Number/ Treatment Duration) |
|---|---|---|---|---|
| Vincristine | 5 | 5 | 9 | 0.5–72 months |
| Sirolimus | 4 | 4 | 5 | 5 days–18 months |
| Interferon–α | 2 | 2 | 5 | 9–24 months |
| Sclerotherapy | 2 | 2 | 3 | 1–2 |
| Pericardial window | 1 | 4 | 8 | 1–2 |
| Corticosteroids | 1 | 3 | 7 | 1–12 months |
| Doxcycline | 1 | 1 | 1 | 17 months |
| Splenectomy | 1 | 2 | 3 | 1 |
| Thalidomide | 1 | 1 | 4 | 1–24 months |
| Chest tube | 0 | 13 | 13 | 4–16 weeks |
| Pericardiocentesis | 0 | 9 | 11 | 2–22 |
| Pericardial drain | 0 | 6 | 8 | 1–4 weeks |
| Pleurocentesis | 0 | 5 | 5 | 1–4 |
| Pleurodesis | 0 | 4 | 9 | 1–2 |
| Thoracic duct ligation | 0 | 3 | 4 | 1 |
| Aminocaproic acid | 0 | 2 | 3 | 1–24 months |
| Octreotide | 0 | 1 | 3 | 1–2 months |
| Thoracic duct reimplantation | 0 | 1 | 1 | 1 |
| Mediastinal mass excision | 0 | 0 | 2 | 1 |
Multiple medical therapies were also tried, vincristine, sirolimus, interferon, thalidomide, and doxycycline, usually in conjunction with the interventions noted above. For most patients signs and symptoms were temporized by pharmacotherapy. Patients who survived more than one year after completion of treatment had clinical improvement with sirolimus (n=3), vincristine (n=1) or interferon (n=1).
Outcomes
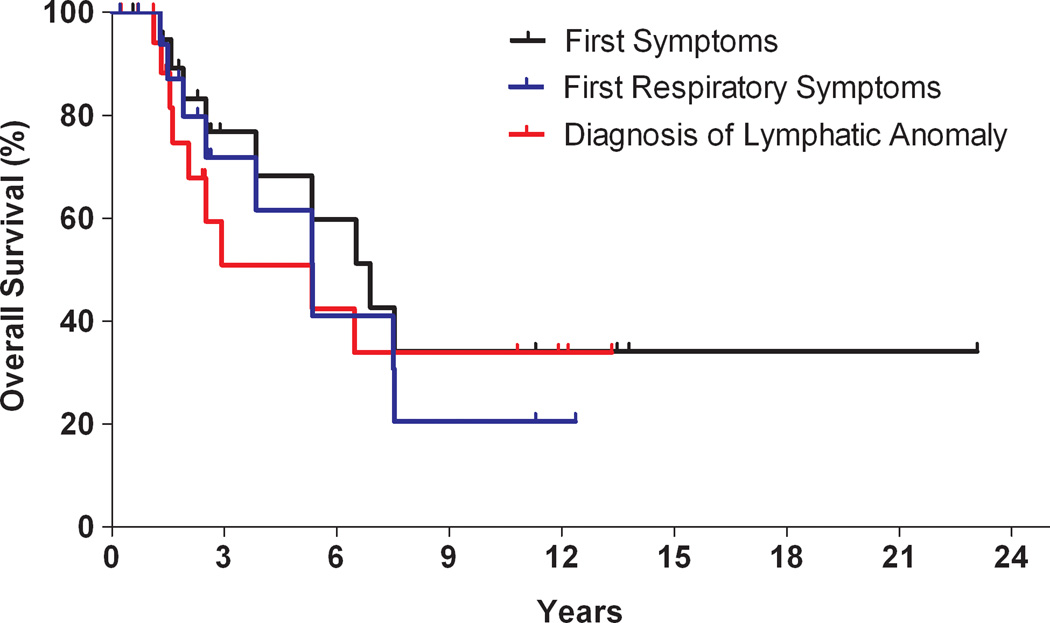
The 5-year survival for KLA was 51%; overall survival was 34% (Figure 3). The cause of death in most instances was cardio-respiratory failure. Mean interval between diagnosis and death was 2.75 years (range, 1.1 to 6.5 years). Outcome for survivors varied; median follow-up was 2.5 years. Three patients continue on pharmacotherapy. Two patients, off treatment for more than 10 years, reported chronic musculoskeletal pain as a prominent, long-term issue. Only one of these patients had major respiratory symptoms; the other had cutaneous staining and functional limitation in the affected extremity prior to therapy. One patient, who presented as an adult, never required intervention and denied any problems.
Figure 3.
Kaplan-Meier curve. Overall survival of patients compared from three time points: emergence of first symptoms (black), development of first respiratory symptoms (blue), and from time of lymphatic anomaly diagnosis (red).
DISCUSSION
We describe clinical features of a distinct, generalized lymphatic anomaly that we term kaposiform lymphangiomatosis (KLA) given foci of “kaposiform” spindled lymphatic endothelial cells and the progressive nature of the anomaly. Intrathoracic disease with worsening respiratory symptoms and hemorrhagic effusions are hallmarks of KLA. Therapies are largely temporizing.
Spindled cells within anomalous pulmonary lymphatic channels have been noted previously,4, 6, 9 but such lesions have not been designated as a specific entity. It is known that kaposiform hemangioendothelioma (KHE) is associated with abnormal lymphatic channels11–14 and occasionally with lymphangiomatosis.13 The spindled cell component in KLA is often arranged in parallel fashion as dispersed, poorly marginated clusters or anastomosing strands/sheets, whereas in KHE the growth occurs primarily as more defined, rounded, confluent nodules with glomeruloid foci and microthrombi.13
Despite the histological similarity between KHE and KLA, the clinical and imaging features are distinctive. KHE-spectrum lesions are unifocal vascular tumors, except in rare reports of multifocal disease. KHE typically presents in early infancy with a characteristic purpuric, cutaneous lesion.15 In contrast, most patients with KLA present in childhood with respiratory or bleeding concerns; cutaneous involvement is rare. Furthermore, KHE often responds to medical therapy with cessation of growth and normalization of hematologic measures,16–18 unlike the refractory behavior of KLA. Some patients with KLA manifest thrombocytopenia, hypofibrinogenemia and prolonged PT and/or aPTT. This pattern is similar to Kasabach-Merritt phenomenon (KMP) seen in KHE-spectrum lesions; however, the thrombocytopenia in KLA is generally less severe. The degree of thrombocytopenia in KLA is not explained by splenomegaly and is insufficient to cause profound bleeding. We speculate that normal platelets are adherent to abnormal lymphatic endothelium, as has been described in KHE.19, 20 By imaging KHE is a unifocal, infiltrative, enhancing mass frequently associated with stranding of subcutaneous tissues, and KLA is diffuse, multifocal and often involves the mediastinum and multiple bones, rare locations for KHE.15
Imaging and presenting features of KLA overlap with generalized lymphatic anomaly (GLA), also called “thoracic lymphangiomatosis”6 or “diffuse lymphangiomatosis”.5, 7 The bony changes and sites of involvement in KLA are similar to those in GLA.8 Likewise, both intra- and extrathoracic disease has been described in GLA including pleural and pericardial effusions and cystic lesions of the spleen. These similarities hint that KLA can arise from GLA; however, such a transition has not been observed. On considering a diagnosis of GLA, suspicion for KLA should arise if there is hemorrhagic effusion, extensive retroperitoneal or mediastinal involvement, a deteriorating clinical course, or associated hematologic abnormalities. Biopsy is necessary to confirm KLA.
The current classification schema for vascular anomalies distinguishes between tumors (neoplasia) and malformations (dysmorphogenesis).21 KLA exhibits features of both categories. The progression of intrathoracic effusions and bony changes, intralesional hemorrhage and hemosiderosis related to loss of vascular integrity, and onset in childhood suggest a more aggressive and invasive nature. On the other hand, the bland histological appearance of KLA and absence of prominent mitoses and cellular atypia favor classification as a malformation. The spindled lymphatic endothelial cell component, although a defining feature, has an unclear role in the pathogenesis and evolution of KLA.
This study has several limitations. Because information was gathered retrospectively, uniform data was not available for every patient. Primary data were largely collected through referrals, potentially influencing accuracy and quantity. Additionally, patients diagnosed with KLA were captured over more than a decade. Few patients in our cohort presented in late adolescence or adulthood, which may reflect an important characteristic of KLA or a referral bias to our pediatric center.
KLA is distinctive in histopathology, clinical course, response to therapy, and mortality rate. Awareness of KLA is essential for accurate diagnosis and therapeutic considerations. The diffuse, infiltrative nature of KLA and the severe, prolonged chylous leak observed in patients with manipulation of their lesion makes attempt at resection of thoracic components ill-advised. Procedural interventions such as pleural and pericardial drainage, pleurodesis, sclerotherapy and thoracic duct ligation can provide transient improvement and serve as a bridge to medical therapy. As has been reported for other vascular anomalies, several agents including vincristine, interferon-α, and sirolimus have shown some promise in treatment of KLA, but no reproducible long-term efficacy has been observed. We expect that awareness of KLA will lead to increased diagnosis and earlier therapy, hopefully yielding improved outcomes. Further prospective studies to risk-stratify lymphatic diseases and optimize management for KLA are urgently needed.
Acknowledgments
Supported by American Society of Hematology Trainee Research Grant, the National Institutes of Health’s Heart, Lung, and Blood Institute (NIH NLBI) (T32 HL007574 [to S.C.] andK08 HL089509 [to C.T.]), and Lymphatic Malformation Institute (to C.T.).
Abbreviations
- KLA
kaposiform lymphangiomatosis
- GLA
generalized lymphatic anomaly
- KHE
kaposiform hemangioendothelioma
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
REFERENCES
- 1.Detaille T, Joomye R, Barrea C, Clapuyt P, Boon LM, Clement de Clety S. Acute life-threatening presentation of unknown lymphatic malformation. Am J Emerg Med. 2010;28:1062 e1–1062 e3. doi: 10.1016/j.ajem.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 2.Greene AK, Burrows PE, Smith L, Mulliken JB. Periorbital lymphatic malformation: clinical course and management in 42 patients. Plast Reconstr Surg. 2005;115:22–30. [PubMed] [Google Scholar]
- 3.Hogeling M, Adams S, Law J, Wargon O. Lymphatic malformations: clinical course and management in 64 cases. Australas J Dermatol. 2011;52:186–190. doi: 10.1111/j.1440-0960.2011.00777.x. [DOI] [PubMed] [Google Scholar]
- 4.Faul JL, Berry GJ, Colby TV, Ruoss SJ, Walter MB, Rosen GD, et al. Thoracic lymphangiomas, lymphangiectasis, lymphangiomatosis, and lymphatic dysplasia syndrome. Am J Respir Crit Care Med. 2000;161:1037–1046. doi: 10.1164/ajrccm.161.3.9904056. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez OA, Kjellin I, Zuppan CW. Thoracic lymphangiomatosis in a child. J Pediatr Hematol Oncol. 2004;26:136–141. doi: 10.1097/00043426-200402000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Tazelaar HD, Kerr D, Yousem SA, Saldana MJ, Langston C, Colby TV. Diffuse pulmonary lymphangiomatosis. Hum Pathol. 1993;24:1313–1322. doi: 10.1016/0046-8177(93)90265-i. [DOI] [PubMed] [Google Scholar]
- 7.Satria MN, Pacheco-Rodriguez G, Moss J. Pulmonary lymphangiomatosis. Lymphat Res Biol. 2011;9:191–193. doi: 10.1089/lrb.2011.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lala S, Mulliken JB, Alomari AI, Fishman SJ, Kozakewich HP, Chaudry G. Gorham-Stout disease and generalized lymphatic anomaly-clinical, radiologic, and histologic differentiation. Skeletal Radiol. 2013;42:917–924. doi: 10.1007/s00256-012-1565-4. [DOI] [PubMed] [Google Scholar]
- 9.Boland JM, Tazelaar HD, Colby TV, Leslie KO, Hartman TE, Yi ES. Diffuse pulmonary lymphatic disease presenting as interstitial lung disease in adulthood: report of 3 cases. Am J Surg Pathol. 2012;36:1548–1554. doi: 10.1097/PAS.0b013e31825eae67. [DOI] [PubMed] [Google Scholar]
- 10.Debelenko L, Marler J, Perez-Atayde A, Fishman S, Mulliken J, Burrows P, et al. Kaposiform Lymphangiomatosis (KLA): An aggressive variant of Lymphangiomatosis [ABSTRACT] Lab Invest. 2004;84:269. [Google Scholar]
- 11.Debelenko LV, Perez-Atayde AR, Mulliken JB, Liang MG, Archibald TH, Kozakewich HP. D2-40 immunohistochemical analysis of pediatric vascular tumors reveals positivity in kaposiform hemangioendothelioma. Mod Pathol. 2005;18:1454–1460. doi: 10.1038/modpathol.3800444. [DOI] [PubMed] [Google Scholar]
- 12.Le Huu AR, Jokinen CH, Rubin BP, Mihm MC, Weiss SW, North PE, et al. Expression of prox1, lymphatic endothelial nuclear transcription factor, in Kaposiform hemangioendothelioma and tufted angioma. Am J Surg Pathol. 2010;34:1563–1573. doi: 10.1097/PAS.0b013e3181f6076f. [DOI] [PubMed] [Google Scholar]
- 13.Lyons LL, North PE, Mac-Moune Lai F, Stoler MH, Folpe AL, Weiss SW. Kaposiform hemangioendothelioma: a study of 33 cases emphasizing its pathologic, immunophenotypic, and biologic uniqueness from juvenile hemangioma. Am J Surg Pathol. 2004;28:559–568. doi: 10.1097/00000478-200405000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Zukerberg LR, Nickoloff BJ, Weiss SW. Kaposiform hemangioendothelioma of infancy and childhood. An aggressive neoplasm associated with Kasabach-Merritt syndrome and lymphangiomatosis. Am J Surg Pathol. 1993;17:321–328. doi: 10.1097/00000478-199304000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Croteau SE, Liang MG, Kozakewich HP, Alomari AI, Fishman SJ, Mulliken JB, et al. Kaposiform Hemangioendothelioma: Atypical Features and Risks of Kasabach-Merritt Phenomenon in 107 Referrals. J Pediatr. 2013;162:142–147. doi: 10.1016/j.jpeds.2012.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haisley-Royster C, Enjolras O, Frieden IJ, Garzon M, Lee M, Oranje A, et al. Kasabach-merritt phenomenon: a retrospective study of treatment with vincristine. J Pediatr Hematol Oncol. 2002;24:459–462. doi: 10.1097/00043426-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Hammill AM, Wentzel M, Gupta A, Nelson S, Lucky A, Elluru R, et al. Sirolimus for the treatment of complicated vascular anomalies in children. Pediatr Blood Cancer. 2011;57:1018–1024. doi: 10.1002/pbc.23124. [DOI] [PubMed] [Google Scholar]
- 18.Enjolras O, Mulliken JB, Wassef M, Frieden IJ, Rieu PN, Burrows PE, et al. Residual lesions after Kasabach-Merritt phenomenon in 41 patients. J Am Acad Dermatol. 2000;42:225–235. doi: 10.1016/s0190-9622(00)90130-0. [DOI] [PubMed] [Google Scholar]
- 19.Shulkin BL, Argenta LC, Cho KJ, Castle VP. Kasabach-Merritt syndrome: treatment with epsilon-aminocaproic acid and assessment by indium 111 platelet scintigraphy. J Pediatr. 1990;117:746–749. doi: 10.1016/s0022-3476(05)83334-7. [DOI] [PubMed] [Google Scholar]
- 20.Sondel PM, Ritter MW, Wilson DG, Lieberman LM. Use of 111In platelet scans in the detection and treatment of Kasabach-Merritt syndrome. J Pediatr. 1984;104:87–89. doi: 10.1016/s0022-3476(84)80597-1. [DOI] [PubMed] [Google Scholar]
- 21.Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg. 1982;69:412–422. doi: 10.1097/00006534-198203000-00002. [DOI] [PubMed] [Google Scholar]