Abstract
BACKGROUND
Small animal models of ischemic left ventricular (LV) dysfunction are important for the pre-clinical optimization of stem cell therapy. We hypothesized that temporal changes in LV function and regional perfusion after cell therapy can be assessed in mice using echocardiographic imaging.
METHODS
Wild-type mice (n=25) were studied 7 and 28 days after permanent ligation of the left anterior descending artery. Animals were randomized to receive closed-chest ultrasound-guided intramyocardial delivery of saline (n=13) or 5×105 multipotential adult progenitor cells (MAPC) (n=12) at day 7. Left ventricular end-diastolic (LVEDV) and end-systolic (LVESV) volume, left ventricular ejection fraction (LVEF), and stroke volume were measured by high-frequency echocardiography. Multiplanar assessment of perfusion and defect area size were made by myocardial contrast echocardiography (MCE).
RESULTS
Between day 7 and 28, MAPC-treated animals had a 40–50% reduction in defect size (p<0.001) and a 20–30% increase in total perfusion (p<0.01). Perfusion did not change in non-treated controls. Both LVEDV and LVESV increased between day 7 and 28 in both groups, however LVESV increased to a lesser degree in MAPC-treated versus control mice (+4.2±7.9 vs +19.2±22.0 μL, p<0.05). LVEF increased in the MAPC-treated mice and decreased in control mice (+3.0±4.3 vs −5.6±5.9 %, p<0.01). There was a significant linear relation between the change in LVEF and the change in either defect area size or total perfusion.
CONCLUSIONS
High-frequency echocardiography and MCE in murine models of ischemic LV dysfunction can be used to assess the response to stem cell therapy and to characterize the relationship between spatial flow, ventricular function and ventricular remodeling.
The number of patients in the United States with symptomatic ischemic left ventricular (LV) dysfunction who are not eligible for surgical or percutaneous revascularization therapy because of comorbidity or diffuse pattern of disease is growing.1–3 Pro-angiogenic stem cell therapy is a potential option for preserving myocardial viability and reducing debilitating angina and heart failure symptoms. Pro-angiogenic effects may also be important in stem cell-mediated myocardial regeneration.4,5 Although clinical trials with cell therapy for ischemic LV dysfunction have been completed, there are many unanswered questions with regards to the most appropriate cell type(s), route of administration, dose, and even mechanism of action. Accordingly, methods for spatial and temporal characterization of left ventricular (LV) function and regional perfusion are important for pre-clinical and clinical evaluation of these unsolved issues as well as defining the relationship between flow and functional recovery with cell therapy.
Small animal models of ischemic LV dysfunction have been developed and provide a relatively inexpensive and high-throughput platform to test new angiogenic or regenerative therapies. We hypothesized that quantitative myocardial contrast echocardiography (MCE) and 2-D and Doppler echocardiographic methods for evaluating LV function beyond fractional shortening could be used to assess the impact of stem cell therapy in a murine model of chronic ischemic LV dysfunction. To test this hypothesis, mice with chronic coronary artery occlusion and moderate to severely reduced LV systolic function were treated with intramyocardial injection of xenogenic multipotential adult progenitor cells (MAPC) which have been shown to improve LV function after myocardial infarction and have also been shown to increase perfusion in ischemic muscle through pro-angiogenic paracrine effects.6–9
METHODS
Animal Preparation and Murine Model of Ischemic LV Dysfunction
The study was approved by the Animal Care and Use Committee at Oregon Health & Science University. Ischemic LV dysfunction was produced by permanent coronary occlusion in 25 C57Bl/6 mice (Jackson Laboratories) 8–10 wks of age for echocardiographic and histologic studies; and in 4 athymic Crl:CD1-Foxn1nu mice with spontaneous albinism (Charles River) for in vivo optical imaging experiments. Mice were anesthetized with inhaled isoflurane (1.0–1.5%), intubated, and placed on positive pressure ventilation. A left lateral thoracotomy was performed using aseptic technique to expose the anterior myocardial wall and a suture was placed around the mid left anterior descending (LAD) coronary artery. ECG was monitored to confirm myocardial injury evidenced by ST elevation. The chest wall was then closed with interrupted sutures, and the endotracheal tube was removed after voluntary respiration was confirmed. Mice were then recovered and buprenorphine HCL (0.2 mg/kg IM) was administered for analgesia. Ten non-ischemic control C57Bl/6 mice were also studied at 8–10 wks of age. For all subsequent imaging studies, mice were anesthetized with inhaled isoflurane (1.0–1.5%) and HR was 450–530 beats per minute. For MCE studies, a catheter was placed in a jugular vein for administration of microbubbles. All follow-up echocardiographic imaging was completed in <30 min.
Cell Therapy
Rat MAPC (Athersys Inc., Cleveland OH) were isolated and expanded as previously decribed, and stored in liquid nitrogen until the day of use.10 These cells are positive for CD29, CD49c, CD90; negative for CD34, CD45, and CD106.10,11 Cell viability on the day of use was assessed by trypan blue exclusion. At day 7 after LAD ligation, mice were anesthetized with inhaled isoflurane. Either MAPC (5×105 suspended in 20 μL of saline) (n=12) or saline alone (n=13) was injected into the anterior-lateral aspect of infarct border zone. Intramyocardial injection were performed percutaneously using a 30 G needle and high frequency (40 MHz) ultrasound guidance (Vevo 770, VisualSonics Inc. Toronto, Canada) and a 3-D micro-positioning system (VisualSonics, Inc.). Intramyocardial delivery was confirmed by distortion of myocardial speckle and acute increase in wall thickness by real-time imaging during injection. Animals were not studied if there was evidence for focal pericardial/mediastinal injection evidence by extracardiac fluid accumulation.
Cell Localization and Survival
In vivo optical imaging was performed to assess MAPC location and survival and function post-injection in athymic Crl:CD1-Foxn1nu mice (n=4). For these experiments, MAPC were stably transfected with the firefly luciferase gene using a lentivirus vector. The number of cells injected 7 days after LAD ligation was increased to 1×106 in order to produce robust luciferase-generated photon activity through the chest wall. At days 1, 2, 3, and 7 after MAPC injection, mice were anesthetized with inhaled isofluorane and d-luciferin (150 μg/g, I.P.) was administered. Optical imaging (IVIS Spectrum, Caliper Life Sciences, Hopkinton, MA) was performed 10 min after luciferin injection using medium binning. Luciferase activity was expressed as photons/s/cm2.
LV Function by Echocardiography
Echocardiography for LV function was performed in non-ischemic control mice and in post-infarction mice at day 7 after coronary ligation (prior to MAPC or saline injection), and at day 28 (21 days after injection). High-frequency fundamental imaging (Vevo 770, Visualsonics Inc.) was performed at 25 to 40 MHz depending on the echocardiographic data that were acquired. Mice were sedated with inhaled isoflurane (1.0–1.5%). Images were obtained in the parasternal long-axis plane and three equally-spaced parasternal short-axis planes 1 mm apart representing the basal, mid-papillary, and apical levels. Image registration to ensure similar imaging planes for day 7 and 28 was based on both anatomic features (papillary muscles and trabecular features) as well as distance from the mitral valve plane. The LV cross-sectional area was measured at end-diastole and end-systole for each of the short-axis image acquisitions. Left ventricular end-diastolic volume (LVEDV) and end-systolic volume (LVESV) were calculated by summation of interpolated short-axis area measurements. Left ventricular ejection fraction (LVEF) was calculated by: (LVEDV−LVESV)/LVEDV. Stroke volume was determined by two methods: (1) the difference in LVESV and LVEDV; and (2) and the product of left ventricle outflow tract (LVOT) area and time-velocity integral on pulsed-wave Doppler. For the latter LVOT pulsed-wave Doppler was obtained from a high parasternal long-axis view with heel-toe angulation to provide a similar angle of incidence between animals and within the range for automated angle correction.
Myocardial Perfusion Imaging
Regional myocardial perfusion and spatial extent of perfusion was assessed by MCE on the same days that LV function was assessed by 2-D echocardiography.12 Lipid-shelled decaflourobutane microbubbles were prepared by sonication of an aqueous lipid dispersion of polyoxyethylene-40-stearate and distearoyl phosphatidylcholine saturated with decafluorobutane gas. Microbubble concentration was measured by electrozone sensing (Multisizer III, Beckman Coulter). Myocardial perfusion imaging was performed with a linear-array transducer (15L8) interfaced with an ultrasound system (Sequoia, Siemens Medical Systems, Mountain View, CA). Short-axis images at same mid-ventricular and apical level as functional imaging were obtained, the position for which was guided by 14 MHz fundamental imaging and a micropositioning imaging state. For perfusion imaging a multipulse algorithm using phase-inversion and amplitude-modulation was used to detect the nonlinear fundamental component of the microbubble signal at a frequency of 7 MHz. Imaging was performed at a mechanical index of 0.16 and a dynamic range of 55 dB. ECG-triggered end-systolic frames were acquired during continuous intravenous infusion of microbubbles at 5×105 min−1. Images were acquired for 15 cardiac cycles after a 5-frame destructive pulse sequence at a mechanical index 0.97. Quantification of myocardial perfusion was performed from regions-of-interest placed over the entire short-axis area. Time-intensity data were fit to the function: y = A(1−e−βt) whereby A is plateau intensity representing relative microvascular blood volume (MBV), β is the rate constant reflecting microvascular flux rate, and the product of A and β is an index of myocardial blood flow (MBF).13 Color-coded parametric images of MBV (images at the completion of the replenishment sequence) were used to delineate the region devoid of perfusion.14 Interobserver variation of blinded measurements for the region devoid of perfusion was 7±4 %. The percent change in non-perfused area from baseline to follow-up was calculated by averaging data for the mid-ventricular and apical planes.
Microsphere Validation of Spatial Perfusion Assessment
Microsphere evaluation of regional perfusion on day 28 was performed in 8 mice undergoing LAD ligation (n=4 each for MAPC and sham saline injection groups). After completion of MCE, 8×105 fluorescent microspheres (15 μm Dye-Trak, Triton Technology, Inc., San Diego, CA) were injected into left ventricle using high-frequency ultrasound guidance. Several minutes later, hearts were excised, rinsed in PBS, and sectioned into 1-mm thickness short-axis slices. Fluorescent epi-illumination microscopy (×20 objective) with 460–500 nm excitation filters was performed. Digital planimetry was used to determine the region void of fluorescent microspheres on both the basal and apical surfaces of each slice which were averaged and expressed as a ratio to the total LV short-axis area. Analysis was performed for the slices where ventricular geometry matched the MCE imaging planes. Interobserver variation of blinded measurements was 6±2 %.
Statistical Analysis
Data were analyzed on SPSS (version 16.0). All echocardiographic data were normally distributed and expressed as mean (±SD) unless otherwise stated. Temporal changes in luciferase data were analyzed with one-way ANOVA and tests for non-linear trends. Echocardiographic perfusion and function data were analyzed with paired Student’s t-tests (two-sided) for temporal differences within a treatment group, or unpaired Students t-tests for cohort-related differences at the different study intervals and Bonferroni correction for multiple comparisons when comparing the two treatment groups to non-ischemic control animals. Correlations were made using least squares linear regression analysis. Differences in proportions for risk area size were analyzed with a chi-square test (χ2). Differences were considered significant at p<0.05.
RESULTS
Intramyocardial Stem Cell Survival
On ultrasound guided percutaneous injection of MAPC, success of intramyocardial injection of cells into the anterolateral portion of the infarct was confirmed by mild tissue distortion during injection. In vivo optical imaging of luciferase-transfected MAPC indicated that the cells remained primarily at the site of injection in the myocardium (Figure 1). There was either rapid loss of cells or of cell function over the first week manifest by a 500-fold reduction in luciferase signal.
Figure 1.
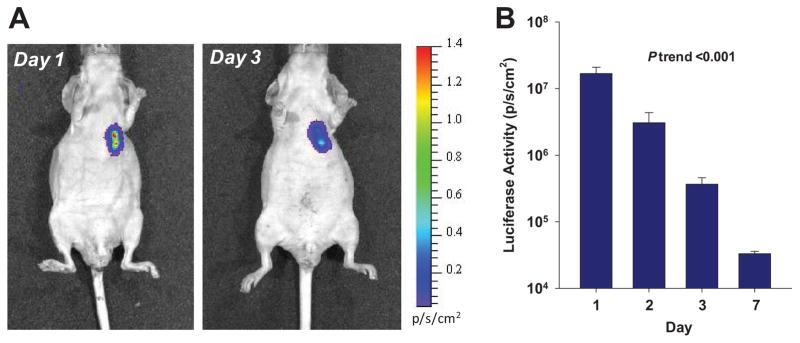
(A) Examples of optical imaging of luciferase-transfected MAPC at day 1 and 3 illustrating location and decay of luciferase signal. (B) Mean (±SD) luciferase signal on a logarithmic scale from a region-of-interest placed over the chest. ANOVA p<0.01.
Myocardial Perfusion
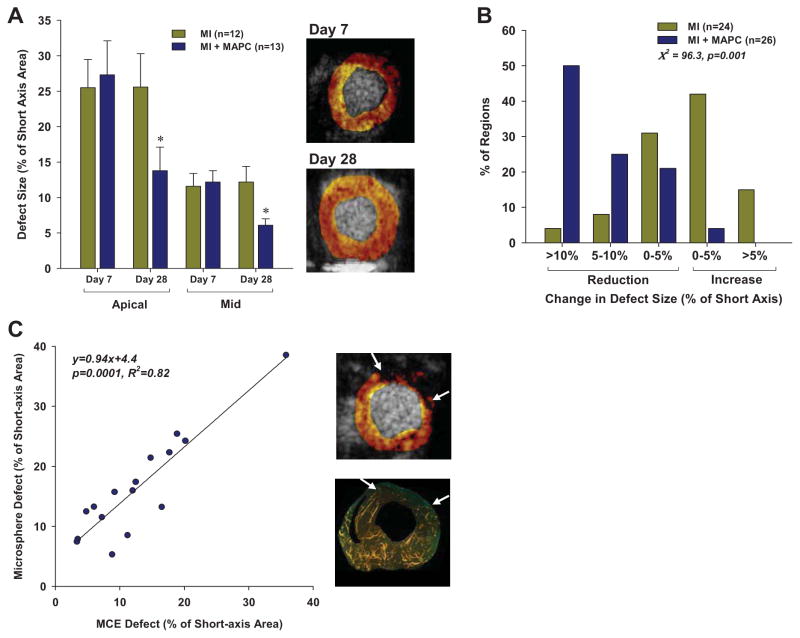
Myocardial perfusion was assessed by MCE in both an apical and mid-left ventricular short-axis location which encompassed regions with akinesis on day 7 after LAD ligation in all animals. The apical location was mid-way between the mid ventricle and the true apex which was not imaged since it was entirely akinetic in all animals and lacked any evidence for myocardial perfusion. On day 7 (prior to MAPC injection), the region that was void of myocardial perfusion was larger for the apical than the mid ventricular plane (26±15 vs 12±6 %, p=0.0001) (Figure 2A). There was no difference in baseline defect size at day 7 between the MAPC-treated and sham-treated post-infarct groups. At day 28 (21 days after injection), there was a significant reduction in perfusion defect size only for MAPC-treated mice. In these mice, there was a similar 50% relative reduction in defect size for the apical and mid-ventricular regions. The majority of MAPC-treated segments had a reduction in perfusion defect size which was not the case with sham-treated mice (Figure 2B). Validation of MCE defect size by fluorescent microsphere injection was performed in 8 mice at day 28 which demonstrated a good agreement in the spatial extent of the defect size for the two techniques (Figure 2C).
Figure 2.
(A) Mean (±SEM) perfusion defect size by MCE expressed as a percentage of the short-axis area in the apical and mid-ventricular regions at day 7 and 28. *p<0.001 vs day 7 and p<0.05 versus MI group. Examples of the mid-ventricular defect area on MCE at day 7 and 28 from the mouse with the largest change in defect size are illustrated at the right. (B) Histogram illustrating the percent of short-axis slices that underwent different patterns of defect area change between day 7 and 28. Data for apical and mid-ventricular slices have been combined. (C) Relation between defect size (arrows) measured on MCE and on fluorescent microsphere examination at day 28. An example of defect size for each technique from a single animal is illustrated at the right.
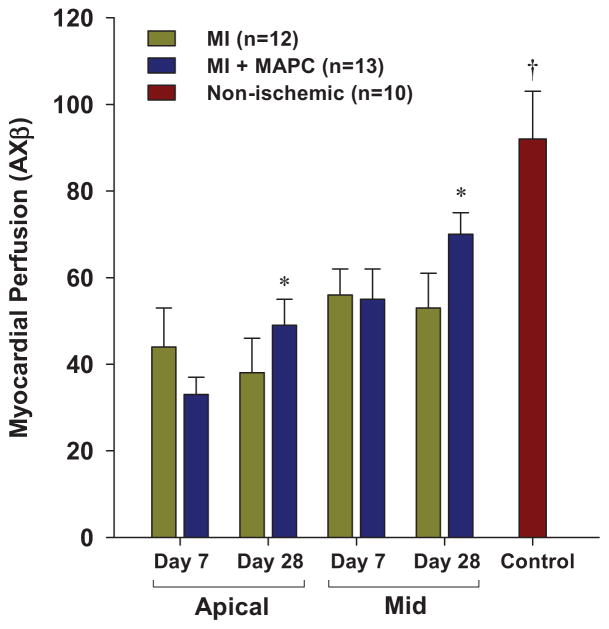
Quantitative transmural myocardial perfusion for the entire apical and mid-ventricular short-axis regions at day 7 in mice undergoing LAD ligation was reduced compared to non-ischemic control mice (Figure 3). At day 7, myocardial perfusion in the apical region was slightly lower for the MAPC-treated mice, although this did not reach statistical significance. Between day 7 and 28, myocardial perfusion increased significantly in the MAPC-treated mice and did not change in the non-treated mice. However, differences in perfusion between the treatment groups at day 28 did not reach statistical significance.
Figure 3.
Mean (±SEM) myocardial perfusion on MCE measured from the entire apical or mid-left ventricular short axis regions. Data from the mid-ventricle from non-ischemic control animals are illustrated for comparison. *p<0.01 versus day 7 data by paired analysis (p-value not significant versus MI data). †p<0.05 versus all post-MI groups.
Left Ventricular Function
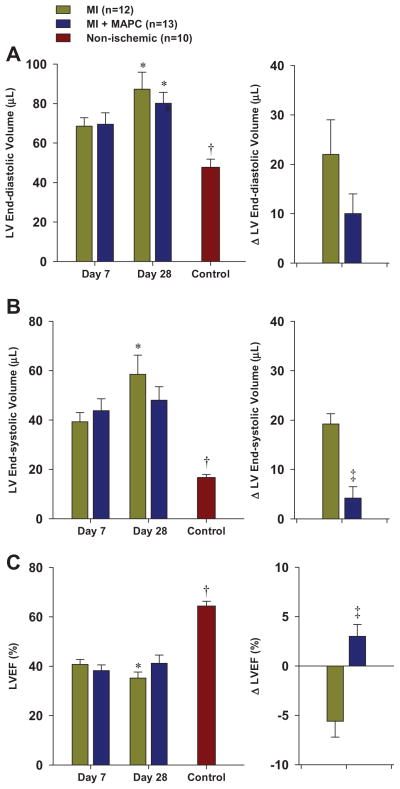
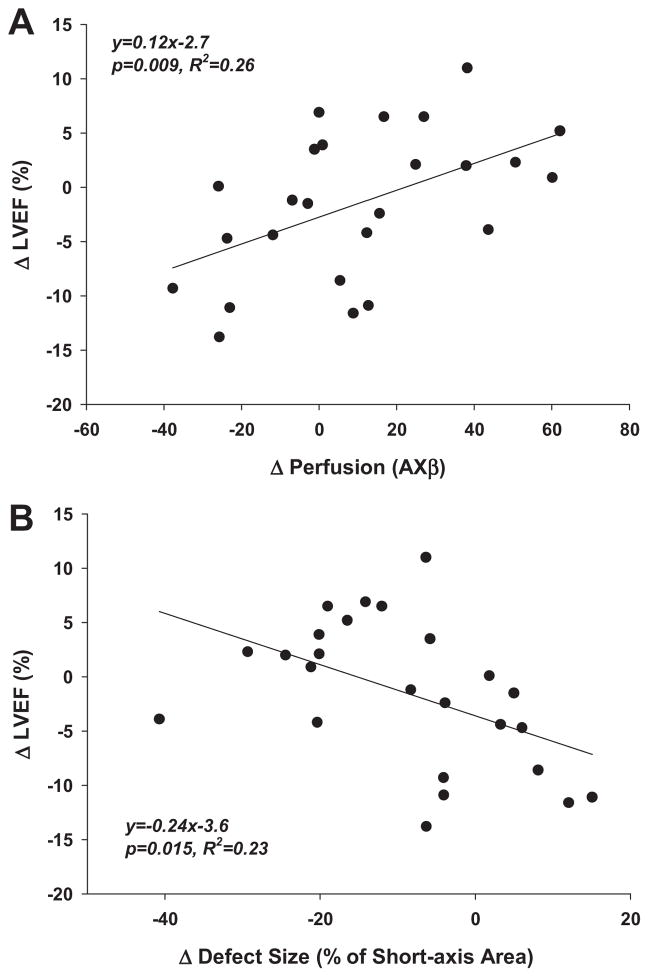
Left ventricular volumes at end-systole and end-diastole were significantly larger and LVEF was significantly lower in mice undergoing LAD ligation compared to normal control mice (Figure 4). At day 7 after LAD ligation there were no significant differences in LVEDV, LVESV and LVEF according to treatment group. The LVEDV significantly increased between day 7 and 28 in both treatment groups, although the relative increase tended to be less in the MAPC-treated mice. The LVESV significantly increased between day 7 and 28 only in untreated mice. Accordingly, the LVEF decreased in untreated mice whereas the LVEF tended to increase in MAPC-treated mice. There was a modest linear relation between the change in LVEF and the change in perfusion (averaged for mid and apical planes) between day 7 and 28; and a modest inverse relation between change in LVEF and the change in the perfusion defect size summed for the mid and apical planes (Figure 5).
Figure 4.
(A) Mean (±SEM) LV end-diastolic volume and change in LV end-diastolic volume between day 7 and 28 from post-MI mice and non-ischemic control mice. (B) Mean (±SEM) LV end-systolic volume and change in LV end-systolic volume. (C) Mean (±SEM) LVEF and change in LVEF. *p<0.01 vs day 7 data. †p<0.01 vs all day 7 data. ‡p,0.05 vs MI group.
Figure 5.
(A) Correlation between change in MCE-derived perfusion for the entire short axis from day 7 to 28 and absolute change in LVEF. Perfusion data are averaged from the apical and mid-ventricular planes. (B) Correlation between change in MCE-derived perfusion defect size from day 7 to 28 and absolute change in LVEF. Defect size data represent summed area change from the apical and mid-ventricular planes.
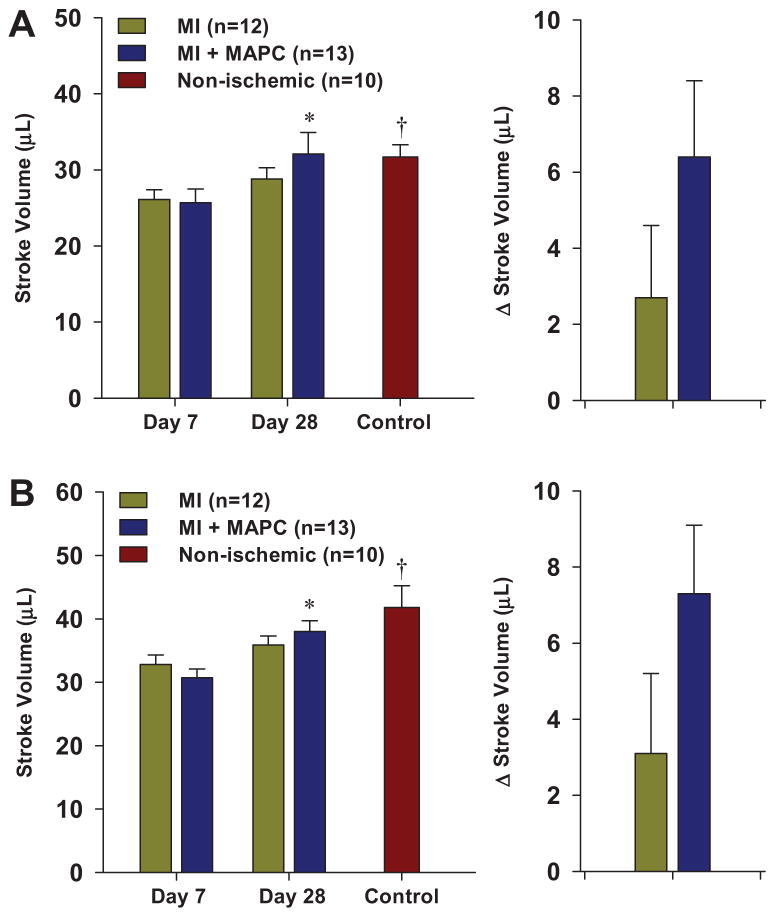
Stroke volume was measured by both volumetric technique as well as the product of LVOT area and Doppler time-velocity integral (Figure 6). For both techniques, stroke volume in normal control mice was higher than both post-infarction groups only at the day 7 interval. Stroke volumes increased between day 7 and 28, but this reached statistical significance only for the MAPC-treated mice.
Figure 6.
Mean (±SEM) LV stroke volume and change in stroke volume between day 7 and 28. Data were calculated by either: (A) difference in LV end-diastolic and end-systolic volume, or (B) Product of LVOT area and Doppler time-velocity integral. *p<0.01 vs day 7 data. †p<0.01 vs all day 7 data.
DISCUSSION
In this study we have demonstrated that echocardiographic assessment of perfusion and left ventricular function can be used to evaluate the effects of stem cell therapy in a murine model of ischemic LV dysfunction. Improvements in total myocardial perfusion and the spatial distribution of perfusion produced by intramyocardial injection of MAPC were able to be quantified by MCE. The improvement in myocardial perfusion in MAPC-treated mice was associated with less disadvantageous ventricular remodeling and an improvement in LVEF.
Pro-angiogenic stem cell therapy is a promising approach for treating severe angina and other ischemic symptoms in patients who are not candidates for traditional revascularization techniques. The ability to influence vascular remodeling is an important consideration for endogenous myocardial repair. For example, stem-cell mediated improvements in microvascular blood flow in patients with recent MI or with viable but chronically dysfunctional myocardium has been shown to also improve LVEF, reduce LVESV and improve functional status.15 It is also likely that the success of “regenerative” cell therapy to repopulate the myocellular content of the injured heart is influenced by the reparative environment including the status of regional perfusion. Imaging techniques that are able to spatially and temporally assess regional perfusion and left ventricular performance are of paramount importance for studying and optimizing stem cell therapy.
Increasingly, the scientific community has relied on pre-clinical animal models of cardiovascular disease, including murine models, to test the efficacy and off-target effects of new therapies. Because of the overwhelming clinical need, stem cells for promoting vascular remodeling have been studied prior to complete pre-clinical characterization of the effects of dose, timing, method of administration, and the influence of perfusion status on cell survival and function. The importance of this issue is underscored by the heterogeneity in clinical trial outcomes and even negative results in some clinical studies where stem cell therapy was used in ischemic LV dysfunction.16–18 Advanced imaging techniques are useful for addressing these knowledge gaps and optimally would involve techniques that (a) can simultaneously evaluate the effects of cell therapy on flow and function, (b) are widely available to researchers, (c) are repeatable to provide information over time, and (d) can be easily translated from animal models to clinical studies.
Myocardial contrast echocardiography has been used to non-invasively quantify regional perfusion in small and large animal models as well as humans with ischemic LV dysfunction,14,19–23 and has been applied in large animal models to study growth factor-mediated angiogenesis.24 MCE has been used to evaluate myocardial perfusion in pigs with recent MI treated with either intramuscular injection of bone marrow-derived stem cells or intravenous administration of autologous mesenchymal stem cells.25,26 In this study we demonstrated for the first time that changes in myocardial perfusion after stem cell therapy can be assessed with MCE in a murine model of infarction and correlate with improvements in function.
In our study we evaluated the pro-angiogenic effect of MAPCs. These cells were chosen because they contain a wide variety of pro-angiogenic compounds,7,27 have proven efficacy in increasing vascular density in murine models of myocardial infarction and muscle ischemia,6,7,9 and are currently in clinical trials in patients.28 Although there is controversy regarding the ability of MAPCs to differentiate into multiple cell types, there is overwhelming evidence that these cells trigger vascular remodeling. Our previous studies have demonstrated that accelerated recovery of perfusion with MAPC in ischemic limbs occurs primarily through paracrine signaling and recruitment of other pro-arteriogenic cells.7
Perfusion was evaluated in two short-axis slices that showed perfusion defects one week after LAD ligation. We avoided both the basal region which typically lacked infarction, and the true apex where circumferential akinesis or dyskinesis was present in most animals. The presence of tissue perfusion was determined by the spatial extent of contrast signal late after a high-MI pulse sequence when full microbubble replenishment had occurred. With this approach, MCE information on the spatial extent of infarction correlates well with that derived from single-photon emission computed tomography as well as delayed gadolinium enhancement on cardiac magnetic resonance.21,29 MAPCs were delivered by percutaneous intramuscular injection and a borderzone region was targeted because of our previous observation that accelerated recovery of perfusion occurs primarily through remodeling of the existing arteriolar circulation.7 Consistent with this previous study, optical imaging indicated that few of the transplanted cells survive by 7 days after injection, suggesting that persistent paracrine effects rather than engraftment was the dominant mechanism for vascular remodeling.
Compared to untreated controls, mice treated with MAPC had greater recovery of perfusion and greater reduction in defect size, defined by the area completely lacking perfusion, although there was considerable heterogeneity in the response. The MAPC-treated animals also had less adverse remodeling between day 7 and 28, indicated by a smaller increase in LVEDV and LVESV. In the MAPC-treated group, the increase in LVESV was smaller than the increase in LVEDV resulting in an increase in the calculated LVEF. The change LVEF was linearly related to the change in perfusion and inversely to the change in perfusion defect size. These correlations were, however, only modest probably because: (1) functional data were based on global LV evaluation whereas myocardial perfusion was assessed only in two short-axis planes, and (2) data analysis was not controlled for the baseline total infarct size. Nonetheless, these data indicate that despite the spatial resolution limitations in small animal models of disease, the interplay between perfusion, LV function, and LV geometry in cell therapy can be characterized.
Our results raise important mechanistic questions regarding the relation between flow and function after stem cell therapy for ischemic LV dysfunction. Since MAPC were injected 7 days after infarction, we do not believe that the pro-angiogenic effects resulted in greater myocardial salvage. It is possible that improved perfusion at the infarct borders in MAPC-treated animals permitted endogenous myocellular regeneration in this area. There is a much more likely explanation. Although defect size on MCE was defined as the region that completely lacked perfusion, it is quite likely that borderzone regions that were viable, hypoperfused, and not quantified as part of the defect were benefited by MAPC-mediated vascular remodeling resulting in improved function. We do not, however, have confidence that the contrast-specific MCE imaging algorithm used in this study can spatially resolve subtle changes in flow in borderzone regions and, hence, used a defect size (complete absence of perfusion) as an indicator for an angiogenic response.
There are several other limitations in this study. With regards to the cells used, we did not evaluate dose response and used xenogenic cell therapy. We also imaged used several key short-axis images for MCE rather than attempting to quantify total perfusion defect mass. Our decision to use several short-axis frames was based on difficulties in evaluating the true apex which in mice becomes aneurysmal, very thin, and entirely lacks perfusion after LAD ligation. Although we used same-plane microsphere technique to validate perfusion defect area, we did not perform any complementary methods to validate LV function and geometry data. However, it is reassuring that the echocardiographic values for LV volumes, LVEF, and stroke volume in normal control mice and post-LAD ligation mice are in line with those obtained by murine cardiac magnetic resonance imaging.30 It should be noted, however, that more advanced methods for evaluating function, such as with regional speckle-based strain analysis, was not performed. Control animals did not undergo sham operation since no perfusion or functional abnormalities have been found in previous studies.12,31 Finally, our differences in LV function are small and of unknown clinical significance.
In summary, we have demonstrated that echocardiographic imaging of perfusion and function in mice can provide important information on temporal changes in myocardial blood flow and left ventricular function in response to intramyocardial injection of stem cells in the non-acute phase of myocardial infarction. These results are likely to provide justification for applying these techniques for pre-clinical testing of cell therapy.
Supplementary Material
Acknowledgments
The authors are grateful for assistance from Juliana M. Woda, Ph.D. and Anthony Ting, Ph.D., Athersys Inc., Cleveland OH, for providing cells and guidance with regards to the use of MAPC.
Dr. Davidson was supported by a Ruth L. Kirschstein National Research Service Award (T32-HL094294) from the National Institutes of Health and by a Clinical Research Program Award from the American Heart Association. Dr. Lindner is supported by grants R01-HL-078610, RC1-HL-100659, and R01-HL111969 from the National Institutes of Health.
Footnotes
DISCLOSURES
The cells for these studies were provided free of charge from Athersys, Inc; Cleveland OH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mukherjee D, Bhatt DL, Roe MT, Patel V, Ellis SG. Direct myocardial revascularization and angiogenesis--how many patients might be eligible? Am J Cardiol. 1999;84:598–600. A8. doi: 10.1016/s0002-9149(99)00387-2. [DOI] [PubMed] [Google Scholar]
- 2.Mannheimer C, Camici P, Chester MR, Collins A, DeJongste M, Eliasson T, et al. The problem of chronic refractory angina; report from the esc joint study group on the treatment of refractory angina. Eur Heart J. 2002;23:355–70. doi: 10.1053/euhj.2001.2706. [DOI] [PubMed] [Google Scholar]
- 3.Williams B, Menon M, Satran D, Hayward D, Hodges JS, Burke MN, et al. Patients with coronary artery disease not amenable to traditional revascularization: Prevalence and 3-year mortality. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2010;75:886–91. doi: 10.1002/ccd.22431. [DOI] [PubMed] [Google Scholar]
- 4.Chachques JC, Duarte F, Cattadori B, Shafy A, Lila N, Chatellier G, et al. Angiogenic growth factors and/or cellular therapy for myocardial regeneration: A comparative study. J Thorac Cardiovasc Surg. 2004;128:245–53. doi: 10.1016/j.jtcvs.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Lautamaki R, Terrovitis J, Bonios M, Yu J, Tsui BM, Abraham MR, et al. Perfusion defect size predicts engraftment but not early retention of intra-myocardially injected cardiosphere-derived cells after acute myocardial infarction. Basic research in cardiology. 2011;106:1379–86. doi: 10.1007/s00395-011-0197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van’t Hof W, Mal N, Huang Y, Zhang M, Popovic Z, Forudi F, et al. Direct delivery of syngeneic and allogeneic large-scale expanded multipotent adult progenitor cells improves cardiac function after myocardial infarct. Cytotherapy. 2007;9:477–87. doi: 10.1080/14653240701452065. [DOI] [PubMed] [Google Scholar]
- 7.Ryu JC, Davidson BP, Xie A, Qi Y, Zha D, Belcik JT, et al. Molecular imaging of the paracrine proangiogenic effects of progenitor cell therapy in limb ischemia. Circulation. 2013;127:710–9. doi: 10.1161/CIRCULATIONAHA.112.116103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medicetty S, Wiktor D, Lehman N, Raber A, Popovic ZB, Deans R, et al. Percutaneous adventitial delivery of allogeneic bone marrow-derived stem cells via infarct-related artery improves long-term ventricular function in acute myocardial infarction. Cell Transplant. 2012;21:1109–20. doi: 10.3727/096368911X603657. [DOI] [PubMed] [Google Scholar]
- 9.Aranguren XL, McCue JD, Hendrickx B, Zhu XH, Du F, Chen E, et al. Multipotent adult progenitor cells sustain function of ischemic limbs in mice. J Clin Invest. 2008;118:505–14. doi: 10.1172/JCI31153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boozer S, Lehman N, Lakshmipathy U, Love B, Raber A, Maitra A, et al. Global characterization and genomic stability of human multistem, a multipotent adult progenitor cell. J Stem Cells. 2009;4:17–28. [PubMed] [Google Scholar]
- 11.Lehman N, Cutrone R, Raber A, Perry R, Van’t Hof W, Deans R, et al. Development of a surrogate angiogenic potency assay for clinical-grade stem cell production. Cytotherapy. 2012;14:994–1004. doi: 10.3109/14653249.2012.688945. [DOI] [PubMed] [Google Scholar]
- 12.Davidson BP, Kaufmann BA, Belcik JT, Xie A, Qi Y, Lindner JR. Detection of antecedent myocardial ischemia with multiselectin molecular imaging. J Am Coll Cardiol. 2012;60:1690–7. doi: 10.1016/j.jacc.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Basis for detection of stenosis using venous administration of microbubbles during myocardial contrast echocardiography: Bolus or continuous infusion? J Am Coll Cardiol. 1998;32:252–60. doi: 10.1016/s0735-1097(98)00212-5. [DOI] [PubMed] [Google Scholar]
- 14.Coggins MP, Sklenar J, Le DE, Wei K, Lindner JR, Kaul S. Noninvasive prediction of ultimate infarct size at the time of acute coronary occlusion based on the extent and magnitude of collateral-derived myocardial blood flow. Circulation. 2001;104:2471–7. doi: 10.1161/hc4501.098954. [DOI] [PubMed] [Google Scholar]
- 15.Kumar AH, Caplice NM. Clinical potential of adult vascular progenitor cells. Arterioscler Thromb Vasc Biol. 2010;30:1080–7. doi: 10.1161/ATVBAHA.109.198895. [DOI] [PubMed] [Google Scholar]
- 16.Grajek S, Popiel M, Gil L, Breborowicz P, Lesiak M, Czepczynski R, et al. Influence of bone marrow stem cells on left ventricle perfusion and ejection fraction in patients with acute myocardial infarction of anterior wall: Randomized clinical trial: Impact of bone marrow stem cell intracoronary infusion on improvement of microcirculation. Eur Heart J. 2010;31:691–702. doi: 10.1093/eurheartj/ehp536. [DOI] [PubMed] [Google Scholar]
- 17.Meyer GP, Wollert KC, Lotz J, Pirr J, Rager U, Lippolt P, et al. Intracoronary bone marrow cell transfer after myocardial infarction: 5-year follow-up from the randomized-controlled boost trial. Eur Heart J. 2009;30:2978–84. doi: 10.1093/eurheartj/ehp374. [DOI] [PubMed] [Google Scholar]
- 18.Perin EC, Willerson JT, Pepine CJ, Henry TD, Ellis SG, Zhao DX, et al. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: The focus-cctrn trial. JAMA. 2012;307:1717–26. doi: 10.1001/jama.2012.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lepper W, Belcik T, Wei K, Lindner JR, Sklenar J, Kaul S. Myocardial contrast echocardiography. Circulation. 2004;109:3132–5. doi: 10.1161/01.CIR.0000132613.53542.E9. [DOI] [PubMed] [Google Scholar]
- 20.Lafitte S, Higashiyama A, Masugata H, Peters B, Strachan M, Kwan OL, et al. Contrast echocardiography can assess risk area and infarct size during coronary occlusion and reperfusion: Experimental validation. J Am Coll Cardiol. 2002;39:1546–54. doi: 10.1016/s0735-1097(02)01771-0. [DOI] [PubMed] [Google Scholar]
- 21.Lindner JR, Villanueva FS, Dent JM, Wei K, Sklenar J, Kaul S. Assessment of resting perfusion with myocardial contrast echocardiography: Theoretical and practical considerations. Am Heart J. 2000;139:231–40. [PubMed] [Google Scholar]
- 22.Scherrer-Crosbie M, Steudel W, Ullrich R, Hunziker PR, Liel-Cohen N, Newell J, et al. Echocardiographic determination of risk area size in a murine model of myocardial ischemia. Am J Physiol. 1999;277:H986–92. doi: 10.1152/ajpheart.1999.277.3.H986. [DOI] [PubMed] [Google Scholar]
- 23.Raher MJ, Thibault H, Poh KK, Liu R, Halpern EF, Derumeaux G, et al. In vivo characterization of murine myocardial perfusion with myocardial contrast echocardiography: Validation and application in nitric oxide synthase 3 deficient mice. Circulation. 2007;116:1250–7. doi: 10.1161/CIRCULATIONAHA.107.707737. [DOI] [PubMed] [Google Scholar]
- 24.Villanueva FS, Abraham JA, Schreiner GF, Csikari M, Fischer D, Mills JD, et al. Myocardial contrast echocardiography can be used to assess the microvascular response to vascular endothelial growth factor-121. Circulation. 2002;105:759–65. doi: 10.1161/hc0602.103634. [DOI] [PubMed] [Google Scholar]
- 25.Wolf D, Reinhard A, Krause U, Seckinger A, Katus HA, Kuecherer H, et al. Stem cell therapy improves myocardial perfusion and cardiac synchronicity: New application for echocardiography. J Am Soc Echocardiogr. 2007;20:512–20. doi: 10.1016/j.echo.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Fujii H, Tomita S, Nakatani T, Fukuhara S, Hanatani A, Ohtsu Y, et al. A novel application of myocardial contrast echocardiography to evaluate angiogenesis by autologous bone marrow cell transplantation in chronic ischemic pig model. J Am Coll Cardiol. 2004;43:1299–305. doi: 10.1016/j.jacc.2003.09.061. [DOI] [PubMed] [Google Scholar]
- 27.Wragg A, Mellad JA, Beltran LE, Konoplyannikov M, San H, Boozer S, et al. Vegfr1/cxcr4-positive progenitor cells modulate local inflammation and augment tissue perfusion by a sdf-1-dependent mechanism. J Mol Med (Berl) 2008;86:1221–32. doi: 10.1007/s00109-008-0390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Penn MS, Ellis S, Gandhi S, Greenbaum A, Hodes Z, Mendelsohn FO, et al. Adventitial delivery of an allogeneic bone marrow-derived adherent stem cell in acute myocardial infarction: Phase i clinical study. Circ Res. 2012;110:304–11. doi: 10.1161/CIRCRESAHA.111.253427. [DOI] [PubMed] [Google Scholar]
- 29.Hayat SA, Janardhanan R, Moon JC, Pennell DJ, Senior R. Comparison between myocardial contrast echocardiography and single-photon emission computed tomography for predicting transmurality of acute myocardial infarction. Am J Cardiol. 2006;97:1718–21. doi: 10.1016/j.amjcard.2005.12.070. [DOI] [PubMed] [Google Scholar]
- 30.Ross AJ, Yang Z, Berr SS, Gilson WD, Petersen WC, Oshinski JN, et al. Serial mri evaluation of cardiac structure and function in mice after reperfused myocardial infarction. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2002;47:1158–68. doi: 10.1002/mrm.10166. [DOI] [PubMed] [Google Scholar]
- 31.Kaufmann BA, Lewis C, Xie A, Mirza-Mohd A, Lindner JR. Detection of recent myocardial ischaemia by molecular imaging of p-selectin with targeted contrast echocardiography. Eur Heart J. 2007;28:2011–7. doi: 10.1093/eurheartj/ehm176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.