Abstract
Objective
To determine the prevalence of feeding dysfunction in children with single ventricle defects and identify associated risk factors.
Study design
Patients aged 2–6 years with single ventricle physiology presenting for routine cardiology follow-up at the Children’s Hospital of Wisconsin were prospectively identified. Parents of the patients completed 2 validated instruments for assessment of feeding dysfunction. Chart review was performed to retrospectively obtain demographic and diagnostic data.
Results
Instruments were completed for 56 patients; median age was 39 months. Overall, 28 (50%) patients had some form of feeding dysfunction. Compared with a normal reference population, patients with single ventricle had statistically-significant differences in dysfunctional food manipulation (p<0.001), mealtime aggression (p=0.002), choking/gagging/vomiting (p<0.001), resistance to eating (p<0.001) and parental aversion to mealtime (p<0.001). Weight and height for age z-scores were significantly lower in subjects with feeding dysfunction (−0.84 vs. −0.33; p<0.05 and −1.46 vs. −0.56; p=0.001 respectively). Multivariable analysis identified current gastrostomy tube use (p=0.02) and a single parent household (p=0.01) as risk factors for feeding dysfunction.
Conclusion
Feeding dysfunction is common in children with single ventricle defects, occurring in 50% of our cohort. Feeding dysfunction is associated with worse growth measures. Current gastrostomy tube use and a single parent household were identified as independent risk factors for feeding dysfunction.
Keywords: Univentricular, growth, nutrition
Patients with single ventricle require staged palliation to create a passive flow circuit to the lungs. As survival has improved, it has become evident that the effects of single ventricle physiology and the palliations required are seen in many organ systems. There are known neurologic consequences that manifest as lower IQ and a higher prevalence of attention deficits(2,3). There is also a high risk for restrictive lung disease in older patients(4). Problems with nutrition and the gastrointestinal system, however, are nearly universal and occur throughout the palliative stages (5–7). These include an increased risk of slow growth and feeding disorders(5,8). Of all cardiac defects, patients with single ventricle lesions most frequently manifest feeding problems both at time of initial discharge and at 2 year follow up(5,9).
Studies have shown that up to 89% of patients with single ventricle hearts failed to meet Centers for Disease Control and Prevention standards for adequate growth and 50% were considered severely underweight at admission for S2P(10,11). When compared with transposition of the great arteries, a heart defect that similarly requires neonatal heart surgery but results in a biventricular heart, those with HLHS had a longer duration to achieve goal feeding levels after surgery and demonstrated slower weight gain at every interval measured up to 1 year of age despite both groups starting with comparable anthropometrics(12).
Suboptimal growth continues in older patients with single ventricle despite surgical palliation and targeted interventions. The cause of these long term growth problems remains unknown. The abnormal physiology has frequently been implicated. Caregiver nutrition concerns may negatively affect caregiver-child interactions around feeding and might exacerbate this feeding dysfunction. We sought to compare the prevalence of feeding dysfunction in patients with single ventricle aged 2 to 6 years of age with the known prevalence in the general population and identify risk factors for feeding dysfunction in these patients.
METHODS
After approval from the Children’s Hospital of Wisconsin institutional review board, patienrs with single ventricle were recruited sequentially from the cardiology clinic, the catheterization lab, at the time of standard pre-surgical catheterization, or at the time of hospital admission for elective surgery at the Children’s Hospital of Wisconsin over a 6 month period from April 2012 through September 2012. Patients were approached if they were between 2 and 6 years of age at study initiation and had completed S2P prior to 2 years of age. This age range was selected because it was the range used in the validation study of the Mealtime Behavior Questionnaire (MBQ). Patients were excluded if they had a congenital gastroesophageal malformation that required surgical repair or were enrolled in the Single Ventricle Reconstruction Trial. Additionally, as the instruments have only been validated in English, parents unable to complete them as such were excluded. A caregiver completed the MBQ, the About Your Child’s Eating (AYCE), and a brief demographic questionnaire at the time of consent. Diagnostic and anthropometric data were collected retrospectively by chart review on all consented patients. Study data were collected and managed using REDCap electronic data capture tools at the Medical College of Wisconsin(19).
The MBQ is a 33 question Likert scale instrument with 4 sub-categories of feeding dysfunction. These sub-categories are distraction/avoidance, food manipulation, mealtime aggression and choking/gagging/vomiting. The AYCE is a 25 question Likert scale instrument with 3 subcategories. These sub-categories are child resistance to eating, non-positive mealtime environment and parental aversion to mealtime. Cut-off values for each sub-category have been determined by validation studies. Any positive sub-category was defined as being positive for feeding dysfunction.
Statistical Analyses
Descriptive data are presented as median with range or mean ± standard deviation. We sought to determine the prevalence of feeding dysfunction in patients with single ventricle and compare the frequency of these subcategories with those in the general population established by validation studies for the assessment tools. This was performed using Fisher exact test. Weight for age z-score (WAZ) and height for age z-score (HAZ) were calculated using the World Health Organization Anthro and AnthroPlus applications. These continuous variables were compared between groups using Student t test. Risk factors for feeding dysfunction were evaluated using univariate analysis with Fisher exact test and multivariable analysis with logistic regression. Patient characteristics including race/ethnicity, diagnosis and birth WAZ and HAZ, as well as suspected risk factors for feeding problems including history of stage 1 palliation (S1P), vocal cord injury and Gastrostomy (G) tube status as well as social factors including household income and parental involvement were entered into the model with backward selection. Odds ratios are presented with 95% confidence intervals. Statistical analysis was performed using SAS OnDemand (SAS Institute, Cary, NC).
RESULTS
A total of 56 patients were enrolled and completed the instruments. Baseline characteristics of the cohort can be seen in Table I (available at www.jpeds.com). Median age was 39 (24–74) months. Median WAZ and HAZ at the time of enrollment were −0.6 (−3.7–1.5) and −1.1 (−3.8–1.4) respectively. Twenty-eight patients had feeding dysfunction for a prevalence of 50%. Thirteen patients were positive for one sub-type of feeding dysfunction, 3 were positive for 2 sub-types, 8 were positive for 3 sub-types and 4 were positive for >3 sub-types. Comparison between the group with feeding dysfunction and the group without can be seen in Table II with G tube use being associated with feeding dysfunction in this univariate analysis. Multivariable analysis showed current G tube use (p = 0.02) and a single parent home (p = 0.01) were associated with feeding dysfunction. Odds ratios and 95% confidence intervals for all factors used in multivariable analysis can be seen in Table III (available at www.jpeds.com). Significant factors not associated with feeding dysfunction were a diagnosis of HLHS (p=0.53), whether a S1P was required (p=0.43), birth WAZ and HAZ (p=0.74 and p=0.23 respectively) and a history of vocal cord injury (p=0.60).
Table 1.
Demographics of the single ventricle cohort
| Male, n (%) | 34 (61) | |
|
| ||
| Race/Ethnicity, n (%) | ||
| White | 41 (74) | |
| Black/African American | 8 (14) | |
| Hispanic | 4 (7) | |
| Other | 3 (6) | |
|
| ||
| Diagnosis, n (%) | ||
| Hypoplastic left heart syndrome | 19 (34) | |
| Double outlet right ventricle | 11 (20) | |
| Double inlet left ventricle | 8 (14) | |
| Tricuspid atresia | 8 (14) | |
| Unbalanced atrioventricular septal defect | 5 (9) | |
| Pulmonary atresia with intact ventricular septum | 2 (4) | |
| Other | 3 (5) | |
|
| ||
| Stage of palliation, n (%) | ||
| Stage 2 (Cavopulmonary anastamosis) | 39 (70) | |
| Stage 3 (Fontan) | 17 (30) | |
Demographic data for the study population as number and percent of total
Table 2.
Comparison of feeding dysfunction and non-feeding dysfunction groups by univariate analysis
| No feeding dysfunction | Feeding dysfunction | P value | |
|---|---|---|---|
|
| |||
| Male, n (%) | 17 (61) | 17 (61) | 1 |
|
| |||
| Diagnosis of HLHS, n (%) | 7 (25) | 12 (43) | 0.26 |
|
| |||
| Completed S3P, n (%) | 11 (39) | 6 (21) | 0.24 |
|
| |||
| S1P required, n (%) | 12 (43) | 14 (50) | 0.79 |
|
| |||
| G tube use, n (%) | 0.02 | ||
| Never | 18 (55) | 15 (45) | |
| Former | 8 (73) | 3 (27) | |
| Current | 2 (17) | 10 (83) | |
|
| |||
| Vocal cord injury, n (%) | 8 (29) | 9 (32) | 1 |
|
| |||
| Lower household income, n (%) | 15 (54) | 16 (59) | 0.79 |
|
| |||
| Single parent home, n (%) | 4 (14) | 11 (39) | 0.03 |
HLHS – hypoplastic left heart syndrome, S3P – stage 3 palliation, S1P – stage 1 palliation. P values obtained by Fisher’s exact test.
Table 3.
Odds ratios and 95% confidence intervals for potential risk factors for feeding dysfunction in multivariable analysis
| Odds Ratio(95% CI) | p value | |
|---|---|---|
| G tube use (versus Never) | ||
| Former | 0.4 (0.1–2.1) | 0.29 |
| Current | 8.6 (1.5–48.7) | 0.02 |
| Single parent household | 5.7 (1.4–23) | 0.01 |
| Fontan not completed | 3.0 (0.7–14.1) | 0.16 |
| HLHS | 4.8 (0.4–62.3) | 0.23 |
| S1P performed | 0.3 (0.1–2.9) | 0.27 |
| Birth HAZ | 0.8 (0.4–1.5) | 0.44 |
| Male gender | 0.6 (0.2–2.7) | 0.53 |
| Vocal cord injury | 1.7 (0.3–10.6) | 0.57 |
| Household income <$45,000 | 1.4 (0.3–7.3) | 0.67 |
| Nonwhite race | 1.5 (0.3–8.4) | 0.68 |
| Birth WAZ | 0.9 (0.3–2.2) | 0.74 |
HLHS – hypoplastic left heart syndrome, S1P – stage 1 palliation, HAZ – height for age z-score, WAZ – weight for age z-score. P values obtained by logistic regression
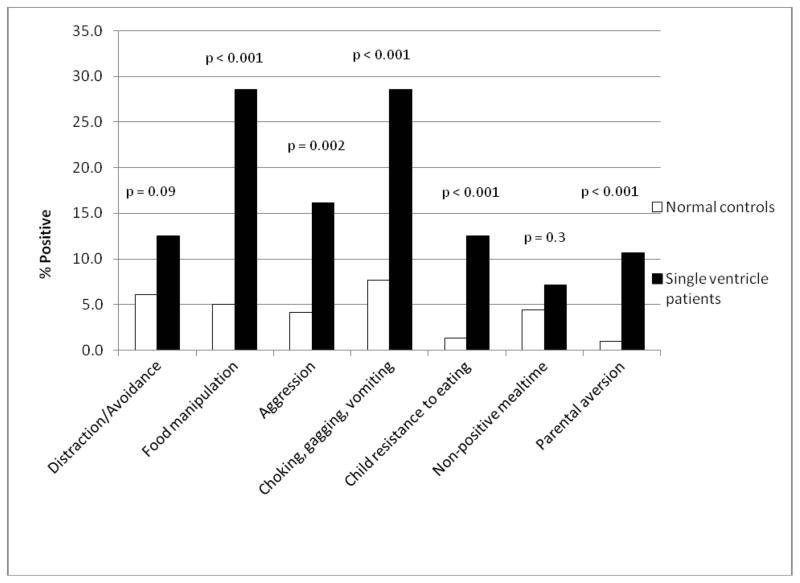
Comparison by type of dysfunction was made with a normal population consisting of 355 children for the MBQ and 384 children for the AYCE (Figure 1). There was significantly more food manipulation (29% vs. 5%, p<0.001), mealtime aggression (16% vs. 4%, p=0.002), choking/gagging/vomiting (29% vs. 8%, p<0.001), child resistance to eating (13% vs. 1%, p<0.001) and parental aversion to mealtime (11% vs. 1%, p<0.001) in the single ventricle cohort.
Figure 1. Comparison of feeding dysfunction subtypes between single ventricle patients and normal controls.
Comparison of the frequency of feeding dysfunction subtypes in patients with single ventricle and normal controls based on the Mealtime behavior questionnaire and About Your Child’s Eating questionnaire
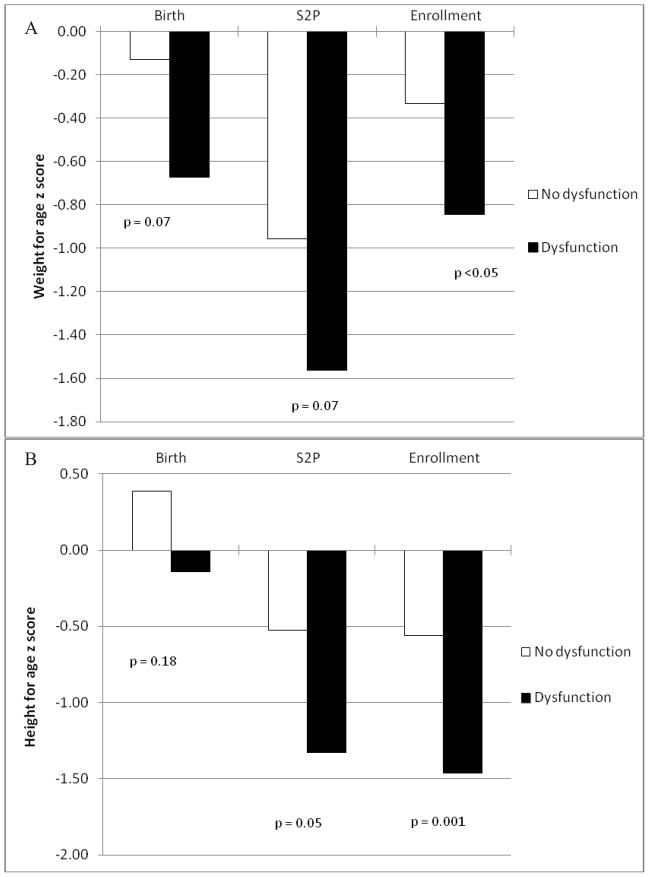
Weight and height for age z scores were compared between the patients with and without dysfunction at 3 time points, birth, at S2P and at study enrollment as seen in Figure 2. At birth and S2P, the group with feeding dysfunction had a lower WAZ although this did not reach statistical significance in either (p=0.07) but by enrollment the difference was significant (p<0.05). This was also seen in the height for age z scores which were similar at birth (p=0.18) but were lower in the feeding dysfunction group at S2P (p=0.05) and were even more divergent at time of study enrollment (p=0.001).
Figure 2. Comparison of growth between single ventricle patients with and without feeding dysfunction.
Comparison of A, weight for age z scores and B, height for age z scores at birth, stage 2 palliation and study enrollment between the groups with and without feeding dysfunction.
DISCUSSION
Poor oral feeding and growth are extremely common in patients with single ventricle after S1P (12,13,21–23). Feeding difficulties after S1P resulted in additional hospitalization time with an average of over 6 days of additional hospitalization for those with feeding problems(8). Nutritional status also has significant implications between surgeries, with feeding difficulties being associated with mortality between S1P and S2P and also associated with altering the timing of surgeries. Those having more feeding and growth problems underwent an earlier S2P(24). Additionally, those undergoing S2P with a lower WAZ had longer post-operative hospitalization following surgery(10).
Early feeding problems are generally believed to improve after S2P with catch up growth as the single ventricle is unloaded and heart failure reduced. Although some growth potential may be lost the cardiac etiology of poor growth and feeding is significantly improved. Still, many patients continue to struggle with feeding disorder and poor growth. Vogt et al reported a failure to achieve adequate caloric intake requiring feeding tube supplementation in 18% of patients following S2P and 5% following Fontan completion(13). Our data are similar with 22% requiring continued G tube supplementation at a median of 36 months after S2P.
Numerous strategies have been employed to improve growth and intake for patients with single ventricle. At the time of initial discharge home from S1P, between 25 and 75% of patients with single ventricle require some form of feeding tube supplementation(8,10,11). Many institutions have also adopted home surveillance programs during the interstage period, from discharge from S1P to readmission for S2P. These generally include discharge home with an infant scale, to measure daily weights as well as daily intake records. Parents are advised to seek medical care for their child for a weight loss of 30g in 24 hours or failure to gain 20g in 3 days. This program significantly reduces the mortality during the interstage period, identifying residual or recurrent lesions, illnesses and feeding difficulties which are associated with interstage mortality(24).
Although interstage monitoring programs are important, they place the responsibility for growth and monitoring on the parents, which is likely to increase stress. It has been shown that high maternal promotion of intake in healthy children preceded and was correlated with worse growth(26). It is very likely there is high promotion of intake in patients with single ventricle early in life to ensure adequate growth and avoid further hospitalization. Mealtime aggression and resistance to eating were common in our cohort and this could be a response to high promotion of intake.
The major limitation of this study is, as a cohort study with cross sectional instrument administration, there is no indication of timing. It may be that those with worse growth at S2P develop feeding dysfunction or that feeding dysfunction early is responsible for the growth difference seen. Furthermore, these instruments are only validated for patients older than 2 years but many risk factors may occur before this age. For example, data on reflux are unavailable as most of these patients were treated with acid suppression and it is not possible to determine who truly had the diagnosis and who was empirically treated. Additionally, we do not routinely investigate for vocal cord injury, reserving laryngoscopy for those noted to have a weak cry or concern for aspiration by speech pathologists. This may underestimate the true prevalence. Although current G tube use was significantly associated with feeding dysfunction, it may be that it is duration of G tube supplementation for which data were limited. Another limitation was that speakers of a foreign language were eliminated. Even though this was a relatively low number of patients it reduces generalization of the results.
Feeding dysfunction is very common in patients with single ventricle 2–6 years of age. Prevention and management of feeding dysfunction will likely require coordinated care by multiple health care providers.
Acknowledgments
Supported in part by the Clinical & Translational Science Institute of Southeast Wisconsin, National Institutes of Health (NIH UL1RR031973). The authors declare no conflicts of interest.
Abbreviations
- AYCE
About Your Child’s Eating
- G
Gastrostomy
- HAZ
Height for age z-score
- HLHS
Hypoplastic left heart syndrome
- MBQ
Mealtime Behavior Questionnaire
- S1P
Stage 1 palliation
- S2P
Stage 2 palliation
- S3P
Stage 3 palliation
- WAZ
Weight for age z-score
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jonas RA, DiNardo JA. Comprehensive surgical management of congenital heart disease. London; New York: Arnold; Distributed in the United States of America by Oxford University Press; 2004. [Google Scholar]
- 2.Sarajuuri A, Jokinen E, Puosi R, Eronen M, Mildh L, Mattila I, et al. Neurodevelopmental and neuroradiologic outcomes in patients with univentricular heart aged 5 to 7 years: related risk factor analysis. J Thorac Cardiovasc Surg. 2007 Jun;133:1524–1532. doi: 10.1016/j.jtcvs.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 3.Sarajuuri A, Jokinen E, Mildh L, Tujulin AM, Mattila I, Valanne L, et al. Neurodevelopmental burden at age 5 years in patients with univentricular heart. Pediatrics. 2012 Dec;130:e1636–46. doi: 10.1542/peds.2012-0486. [DOI] [PubMed] [Google Scholar]
- 4.Ginde S, Bartz PJ, Hill GD, Danduran MJ, Biller J, Sowinski J, et al. Restrictive Lung Disease is an Independent Predictor of Exercise Intolerance in the Adult with Congenital Heart Disease. Congenit Heart Dis. 2012 Oct 18; doi: 10.1111/chd.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maurer I, Latal B, Geissmann H, Knirsch W, Bauersfeld U, Balmer C. Prevalence and predictors of later feeding disorders in children who underwent neonatal cardiac surgery for congenital heart disease. Cardiol Young. 2011 Jun;21:303–309. doi: 10.1017/S1047951110001976. [DOI] [PubMed] [Google Scholar]
- 6.Varan B, Tokel K, Yilmaz G. Malnutrition and growth failure in cyanotic and acyanotic congenital heart disease with and without pulmonary hypertension. Arch Dis Child. 1999 Jul;81:49–52. doi: 10.1136/adc.81.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ginde S, Hohenwalter MD, Foley WD, Sowinski J, Bartz PJ, Venkatapuram S, et al. Noninvasive assessment of liver fibrosis in adult patients following the Fontan procedure. Congenit Heart Dis. 2012 May-Jun;7:235–242. doi: 10.1111/j.1747-0803.2012.00632.x. [DOI] [PubMed] [Google Scholar]
- 8.Jeffries HE, Wells WJ, Starnes VA, Wetzel RC, Moromisato DY. Gastrointestinal morbidity after Norwood palliation for hypoplastic left heart syndrome. Ann Thorac Surg. 2006 Mar;81:982–987. doi: 10.1016/j.athoracsur.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Natarajan G, Reddy Anne S, Aggarwal S. Enteral feeding of neonates with congenital heart disease. Neonatology. 2010;98:330–336. doi: 10.1159/000285706. [DOI] [PubMed] [Google Scholar]
- 10.Anderson JB, Beekman RH, 3rd, Border WL, Kalkwarf HJ, Khoury PR, Uzark K, et al. Lower weight-for-age z score adversely affects hospital length of stay after the bidirectional Glenn procedure in 100 infants with a single ventricle. J Thorac Cardiovasc Surg. 2009 Aug;138:397–404. e1. doi: 10.1016/j.jtcvs.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 11.Kelleher DK, Laussen P, Teixeira-Pinto A, Duggan C. Growth and correlates of nutritional status among infants with hypoplastic left heart syndrome (HLHS) after stage 1 Norwood procedure. Nutrition. 2006 Mar;22:237–244. doi: 10.1016/j.nut.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Davis D, Davis S, Cotman K, Worley S, Londrico D, Kenny D, et al. Feeding difficulties and growth delay in children with hypoplastic left heart syndrome versus d-transposition of the great arteries. Pediatr Cardiol. 2008 Mar;29:328–333. doi: 10.1007/s00246-007-9027-9. [DOI] [PubMed] [Google Scholar]
- 13.Vogt KN, Manlhiot C, Van Arsdell G, Russell JL, Mital S, McCrindle BW. Somatic growth in children with single ventricle physiology impact of physiologic state. J Am Coll Cardiol. 2007 Nov 6;50:1876–1883. doi: 10.1016/j.jacc.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 14.Hasan BS, Bendaly EA, Alexy RD, Ebenroth ES, Hurwitz RA, Batra AS. Somatic growth after fontan and mustard palliation. Congenit Heart Dis. 2008 Sep-Oct;3:330–335. doi: 10.1111/j.1747-0803.2008.00206.x. [DOI] [PubMed] [Google Scholar]
- 15.Cohen MI, Bush DM, Ferry RJ, Jr, Spray TL, Moshang T, Jr, Wernovsky G, et al. Somatic growth failure after the Fontan operation. Cardiol Young. 2000 Sep;10:447–457. doi: 10.1017/s1047951100008118. [DOI] [PubMed] [Google Scholar]
- 16.Srinivasan C, Jaquiss RD, Morrow WR, Frazier EA, Martin D, Imamura M, et al. Impact of staged palliation on somatic growth in patients with hypoplastic left heart syndrome. Congenit Heart Dis. 2010 Nov-Dec;5:546–551. doi: 10.1111/j.1747-0803.2010.00457.x. [DOI] [PubMed] [Google Scholar]
- 17.Davies WH, Ackerman LK, Davies CM, Vannatta K, Noll RB. About Your Child’s Eating: factor structure and psychometric properties of a feeding relationship measure. Eat Behav. 2007 Dec;8:457–463. doi: 10.1016/j.eatbeh.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Berlin KS, Davies WH, Silverman AH, Rudolph CD. Assessing family-based feeding strategies, strengths, and mealtime structure with the Feeding Strategies Questionnaire. J Pediatr Psychol. 2011 Jun;36:586–595. doi: 10.1093/jpepsy/jsp107. [DOI] [PubMed] [Google Scholar]
- 19.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 Apr;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedersen SD, Parsons HG, Dewey D. Stress levels experienced by the parents of enterally fed children. Child Care Health Dev. 2004 Sep;30:507–513. doi: 10.1111/j.1365-2214.2004.00437.x. [DOI] [PubMed] [Google Scholar]
- 21.Williams RV, Zak V, Ravishankar C, Altmann K, Anderson J, Atz AM, et al. Factors affecting growth in infants with single ventricle physiology: a report from the pediatric heart network infant single ventricle trial. J Pediatr. 2011 Dec;159:1017–1022. e2. doi: 10.1016/j.jpeds.2011.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson JB, Beekman RH, 3rd, Eghtesady P, Kalkwarf HJ, Uzark K, Kehl JE, et al. Predictors of poor weight gain in infants with a single ventricle. J Pediatr. 2010 Sep;157:407–13. 413.e1. doi: 10.1016/j.jpeds.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 23.Hehir DA, Rudd N, Slicker J, Mussatto KA, Simpson P, Li SH, et al. Normal Interstage Growth After the Norwood Operation Associated With Interstage Home Monitoring. Pediatr Cardiol. 2012 Apr 20; doi: 10.1007/s00246-012-0320-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghanayem NS, Hoffman GM, Mussatto KA, Cava JR, Frommelt PC, Rudd NA, et al. Home surveillance program prevents interstage mortality after the Norwood procedure. J Thorac Cardiovasc Surg. 2003 Nov;126:1367–1377. doi: 10.1016/s0022-5223(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 25.Mukkada VA, Haas A, Maune NC, Capocelli KE, Henry M, Gilman N, et al. Feeding dysfunction in children with eosinophilic gastrointestinal diseases. Pediatrics. 2010 Sep;126:e672–7. doi: 10.1542/peds.2009-2227. [DOI] [PubMed] [Google Scholar]
- 26.Wright CM, Parkinson KN, Drewett RF. How does maternal and child feeding behavior relate to weight gain and failure to thrive? Data from a prospective birth cohort. Pediatrics. 2006 Apr;117:1262–1269. doi: 10.1542/peds.2005-1215. [DOI] [PubMed] [Google Scholar]
- 27.Silverman AH, Kirby M, Clifford LM, Fischer E, Berlin KS, Rudolph CD, et al. Nutritional and psychosocial outcomes of gastrostomy tube-dependent children completing an intensive inpatient behavioral treatment program. J Pediatr Gastroenterol Nutr. doi: 10.1097/MPG.0b013e3182a027a3. in press. [DOI] [PubMed] [Google Scholar]
- 28.Medical Home Initiatives for Children With Special Needs Project Advisory Committee. American Academy of Pediatrics. The medical home. Pediatrics. 2002 Jul;110:184–186. [Google Scholar]