Abstract
Background and Purpose
It remains unclear whether transient global amnesia (TGA) represents an arterial insult that heralds ischemic stroke. We therefore examined stroke risk after TGA in a population-based cohort.
Methods
After performing chart review at our institution to validate the ICD-9-CM diagnosis code for TGA, we used administrative claims data to identify all patients discharged from nonfederal California emergency departments or acute care hospitals between 2005 through 2010 with a primary discharge diagnosis of TGA. Patients with a primary discharge diagnosis of migraine, seizure, or transient ischemic attack (TIA) were included as controls. Kaplan-Meier statistics were used to calculate rates of ischemic stroke, and Cox proportional hazards analyses were used to compare stroke risk among the four exposure groups while controlling for traditional stroke risk factors.
Results
ICD-9-CM code 437.7 had a sensitivity of 86% and a specificity of 95% for TGA. The cumulative 1-year rate of stroke was 0.54% (95% confidence interval [CI], 0.36–0.81%) after TGA, 0.22% (95% CI, 0.20–0.25%) after migraine, 0.90% (95% CI, 0.83–0.97%) after seizure, and 4.72% (95% CI, 4.60–4.85%) after TIA. After adjustment for demographic characteristics and stroke risk factors, TGA was not associated with stroke risk when compared to migraine (hazard ratio [HR], 0.82; 95% CI, 0.61–1.10). The likelihood of stroke after TGA was lower than after seizure (HR, 0.57; 95% CI, 0.44–0.76) or TIA (HR, 0.27; 95% CI, 0.20–0.35).
Conclusions
Compared to patients diagnosed with migraine or seizure, patients diagnosed with TGA do not appear to face a heightened risk of stroke.
Keywords: Transient global amnesia, stroke, risk factors for stroke
Introduction
Transient global amnesia (TGA) is characterized by a sudden deficit of anterograde and retrograde memory that usually lasts for a few hours and is not accompanied by other focal neurological symptoms or signs.1 Fisher and Adams introduced the term in 1964,1 although Bender and separately Guyotat and Courjon had initially described a syndrome consistent with TGA in 1956.2, 3 The incidence of TGA ranges from 3 to 8 per 100,000 people per year.4–6 Most episodes (75%) occur in people between 50 and 70 years of age, and TGA rarely occurs in patients younger than 40 years of age.7
Although neurologists generally view TGA as a benign entity, its exact prognosis remains unclear. While many studies have supported the benign nature of TGA,6, 8–17 others have suggested that TGA may be a vascular prelude18 that confers the same risk of stroke as transient ischemic attack (TIA).19–22 Many of these studies were done decades ago, and the diagnosis of vascular events such as stroke or TIA has improved since then due to better imaging and clearer definitions.23 Furthermore, the majority of studies on the outcome of TGA have had low statistical power, with an average of approximately 70 patients among the studies cited above and a maximum sample size of 236. It is important to clearly resolve whether TGA is a benign entity or a stroke risk factor, because uncertainty about the etiology and prognosis of TGA drives potentially unnecessary stroke evaluations, especially when vascular risk factors are present.7 Therefore, to build on prior studies and confirm that TGA confers a low risk of subsequent stroke, we performed a retrospective cohort study using statewide administrative claims data from across California.
Methods
Design
We examined the risk of ischemic stroke after a diagnosis of TGA using statewide administrative claims data collected by the California Office for Statewide Healthcare Planning and Development. All nonfederal acute care emergency departments (EDs) and hospitals in California use standardized methods to collect and electronically submit discharge data about all patient visits and hospitalizations.24 These data are checked for inconsistent or invalid elements and codes before being provided in a deidentified format to the Agency for Healthcare Research and Quality’s Healthcare Cost and Utilization Project.25 An anonymous, patient-specific record number allows longitudinal tracking of patients across visits.26
Validation of the TGA Diagnosis Code
Since the accuracy of the International Classification of Diseases, 9th Edition, Clinical Modification (ICD-9-CM) code for TGA (437.7) has not been validated, we first performed a retrospective chart review at our institution to determine its sensitivity and specificity. To do so, we identified 25 ED visits or hospitalizations from 2008 through 2012 with a primary ICD-9-CM discharge diagnosis of 437.7, as well as 25 discharges with other primary cerebrovascular diagnosis codes (e.g., TIA) and no TGA code. A single board-certified neurologist (A.M.) reviewed the medical records from these 50 encounters while blinded to their ICD-9-CM diagnoses, and adjudicated cases of TGA based on its standard definition as the sudden onset of anterograde and retrograde memory lasting <24 hours and unaccompanied by other neurological deficits.7 Using his clinical determination of the primary diagnosis as the gold standard, we calculated the sensitivity and specificity of ICD-9-CM code 437.7 for TGA.
Patient Population
After validating the TGA diagnosis code, we used it to identify a cohort of all patients discharged from a nonfederal California ED or acute care hospital between 2005 through 2010 with a primary discharge diagnosis of TGA.
As control groups, we identified all patients discharged from a nonfederal California ED or acute care hospital from 2005 through 2010 with a primary discharge diagnosis of migraine (ICD-9-CM code 346.x), seizure (345.x), or TIA (435.x). We selected migraine and seizure as negative controls given that these are paroxysmal neurological complaints referable to the central nervous system and are not typically treated as stroke heralds, and we chose TIA as a positive control given its well-established risk of subsequent stroke.27
In all four groups, we excluded patients with any concurrent secondary diagnoses of ischemic stroke (ICD-9-CM code 433.x1, 434.x1, or 436), intracerebral hemorrhage (431), or subarachnoid hemorrhage (430) at baseline. Furthermore, to focus our analysis on incident cases of stroke, we excluded patients with any diagnoses of ischemic stroke at visits prior to baseline. Lastly, to maximize our ability to ascertain follow-up events, we excluded patients who were not California residents at baseline.
Measurements
The primary outcome was any hospital discharge diagnosis of ischemic stroke (ICD-9-CM code 433.x1, 434.x1, or 436) without concurrent codes for rehabilitation (V57), trauma (800–804 or 850–854), intracerebral hemorrhage (431), or subarachnoid hemorrhage (430). This code schema has been validated to have a sensitivity of 86% and a specificity of 95% when compared with detailed medical record review.28 Additionally, we excluded patients after their first stroke hospitalization, a strategy that has been shown to create stroke samples comprising 88% incident cases.28
Our primary exposures were time-varying covariates for TGA, migraine, seizure, and TIA. To control for potential confounders between our exposures and the outcome of ischemic stroke, we used secondary ED or hospital discharge ICD-9-CM diagnosis codes and the Healthcare Cost and Utilization Project’s Clinical Classification Software algorithm to identify the following traditional stroke risk factors: hypertension, diabetes, coronary heart disease, congestive heart failure, chronic kidney disease, chronic obstructive pulmonary disease, peripheral vascular disease, and atrial fibrillation.29
Statistical Analysis
We used standard descriptive statistics to report and compare proportions with exact binomial confidence intervals (CI) and means with standard deviations (SD). Kaplan Meier survival statistics were used to calculate cumulative rates of stroke. Patients entered observation at the first visit that resulted in a discharge diagnosis of TGA, migraine, seizure, or TIA, and were censored at the time of stroke, in-hospital death, or December 31, 2011. Patients were allowed multiple concurrent exposures (e.g., a patient discharged with TGA might at a later encounter also receive a diagnosis of migraine), except that because TIA is a well-established herald of stroke,30 patients with TGA, migraine, and seizure had these time-varying covariates set to zero if they developed a TIA. Since we only considered exposures occurring through the end of 2010, the data from 2011 ensured at least 1 year of follow-up for all patients. Multivariable Cox proportional hazards models were used to examine the association between our exposures and outcome while controlling for potential confounders. Since our goal was to isolate the relationship between our exposures and outcome, all covariates were left in the model regardless of statistical significance. We performed tests of interaction to explore the effects of concurrent exposures (e.g., patients with both TGA and migraine).
To assess the robustness of our results in the face of changes to the structure of our baseline analyses, we additionally performed stratified analyses that defined exposures based on ED discharges only versus hospital discharges only. Furthermore, we performed sensitivity analyses using a more sensitive definition of ischemic stroke that did not exclude patients with concurrent rehabilitation, trauma, or hemorrhage codes.
All analyses were performed using Stata MP (Version 12, College Station, TX). The threshold of statistical significance was α = 0.05. Our institutional review board certified our analysis of the publicly available and deidentified California dataset as exempt from review, and separately approved the validation of ICD-9-CM codes via retrospective chart review at our institution.
Results
Validation of ICD-9-CM code 437.7 via medical record review indicated that this code has a sensitivity of 86% (95% CI, 67–96%) and a specificity of 95% (95% CI, 77–100%) for TGA.
Across California between 2005 through 2010, we identified 4,299 eligible patients with TGA, 170,400 with migraine, 71,087 with seizure, and 115,105 with TIA. At baseline, patients with TGA were older than those with migraine or seizure and younger than those with TIA (Table 1). Their burden of cardiovascular comorbidities and risk factors was much lower than those with TIA, generally similar to those with seizure, and much higher than those with migraine (Table 1).
Table 1.
Baseline Characteristics of Patients with TGA Compared to Negative Controls (Migraine and Seizure) and Positive Controls (TIA)a.
| Characteristic | TGA (N = 4,299) | Migraine (N = 170,400) | Seizure (N = 71,087) | TIA (N = 115,105) |
|---|---|---|---|---|
| Age, mean (SD), y | 65 (13) | 38 (12) | 44 (16) | 71 (15) |
| Female | 2,239 (52.1) | 140,120 (82.2) | 32,695 (46.0) | 65,067 (56.5) |
| Race: | ||||
| White | 3,202 (79.4) | 92,705 (58.0) | 37,328 (55.5) | 73,869 (67.8) |
| Black | 94 (2.3) | 16,643 (10.4) | 10,011 (14.9) | 8,113 (7.4) |
| Hispanic | 367 (9.1) | 41,047 (25.7) | 16,016 (23.8) | 18,664 (17.1) |
| Other | 368 (9.1) | 9,315 (5.8) | 3,946 (5.9) | 8,265 (7.6) |
| Payment source: | ||||
| Medicare | 1,752 (40.8) | 13,905 (8.2) | 20,559 (28.9) | 73,924 (64.2) |
| Medicaid | 81 (1.9) | 35,371 (20.8) | 16,571 (23.3) | 7,066 (6.1) |
| Private | 2,184 (50.8) | 89,105 (52.3) | 18,414 (25.9) | 27,581 (24.0) |
| Self-pay | 184 (4.3) | 23,036 (13.5) | 10,934 (15.4) | 3,571 (3.1) |
| Other | 97 (2.3) | 8,929 (5.2) | 4,597 (6.5) | 2,938 (2.6) |
| Hypertension | 1,792 (41.7) | 16,740 (9.8) | 15,424 (21.7) | 72,206 (62.7) |
| Diabetes | 380 (8.8) | 6,243 (3.7) | 7,473 (10.5) | 28,160 (24.5) |
| CHD | 314 (7.3) | 1,227 (0.7) | 3,279 (4.6) | 21,242 (18.5) |
| Atrial fibrillation | 172 (4.0) | 376 (0.2) | 1,599 (2.2) | 12,301 10.7) |
| CHF | 53 (1.2) | 379 (0.2) | 1,581 (2.2) | 7,514 (6.5) |
| CKD | 71 (1.7) | 609 (0.4) | 2,304 (3.2) | 7,004 (6.1) |
| COPD | 73 (1.7) | 1,158 (0.7) | 2,376 (3.3) | 7,534 (6.5) |
| PVD | 56 (1.3) | 181 (0.1) | 664 (0.9) | 4,619 (4.0) |
Abbreviations: CHD, coronary heart disease; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; PVD, peripheral vascular disease; SD, standard deviation; TGA, transient global amnesia; TIA, transient ischemic attack.
Data are presented as Number (%) of participants unless otherwise specified.
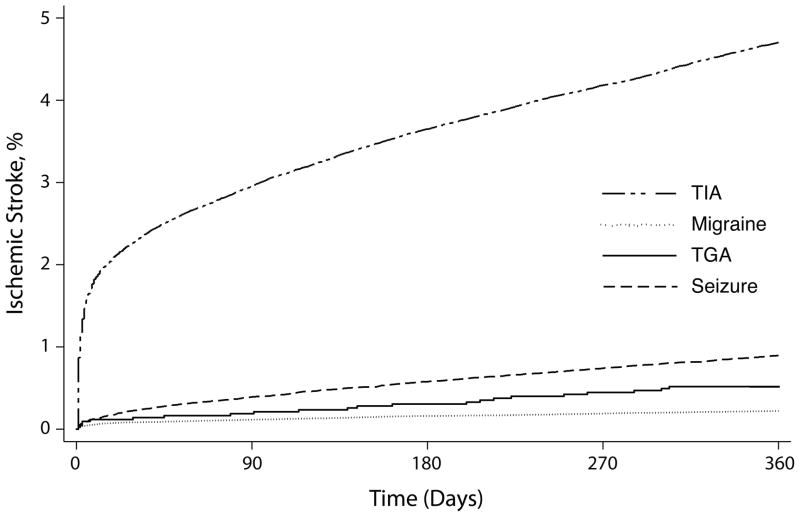
The cumulative 1-year rate of ischemic stroke after TGA was 0.54% (95% CI, 0.36–0.81%), compared to 0.22% (95% CI, 0.20–0.25%) after migraine, 0.90% (95% CI, 0.83–0.97%) after seizure, and 4.72% (95% CI, 4.60–4.85%) after TIA (Figure). Cumulative 5-year rates of ischemic stroke were 2.44% (95% CI, 1.86–3.21%) after TGA, 0.86% (95% CI, 0.80–0.91%) after migraine, 2.90% (95% CI, 2.72–3.10%) after seizure, and 12.23% (95% CI, 12.00–12.46%) after TIA.
Figure.
Cumulative rates of ischemic stroke after diagnoses of transient global amnesia (TGA), compared to diagnoses of migraine, seizure, or transient ischemic attack (TIA).
After adjustment for demographic characteristics and traditional stroke risk factors (Table 2), TGA was not associated with stroke when compared to migraine (hazard ratio [HR], 0.82; 95% CI, 0.61–1.10). The likelihood of stroke after TGA was lower than after seizure (HR, 0.57; 95% CI, 0.44–0.76) or TIA (HR, 0.27; 95% CI, 0.20–0.35). We found no significant evidence of interaction between TGA and the control exposures.
Table 2.
Baseline Characteristics of Patients, Stratified by the Occurrence of Ischemic Strokea.
| Characteristic | Stroke (N = 11,674) | No Stroke (N = 349,217) |
|---|---|---|
| Age, mean (SD), y | 71.4 (14.7) | 50.1 (20.2) |
| Female | 6,699 (57.4) | 233,422 (66.8) |
| Race: | ||
| White | 7,185 (65.0) | 199,919 (60.8) |
| Black | 1,225 (11.1) | 33,636 (10.2) |
| Hispanic | 1,896 (17.2) | 74,198 (22.6) |
| Other | 741 (6.7) | 21,153 (6.4) |
| Payment source: | ||
| Medicare | 7,865 (67.4) | 102,275 (29.3) |
| Medicaid | 1,045 (8.9) | 58,044 (16.6) |
| Private | 2,049 (17.6) | 135,235 (38.7) |
| Self-pay | 419 (3.6) | 37,306 (10.7) |
| Other | 293 (2.5) | 16,268 (4.7) |
| Hypertension | 7,231 (61.9) | 98,931 (28.3) |
| Diabetes | 3,250 (27.8) | 39,006 (11.2) |
| CHD | 2,305 (19.7) | 23,757 (6.8) |
| Atrial fibrillation | 1,518 (13.0) | 12,930 (3.7) |
| CHF | 921 (7.9) | 8,606 (2.5) |
| CKD | 800 (6.9) | 9,188 (2.6) |
| COPD | 759 (6.5) | 10,382 (3.0) |
| PVD | 475 (4.1) | 5,045 (1.4) |
Abbreviations: CHD, coronary heart disease; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; PVD, peripheral vascular disease; SD, standard deviation.
Data are presented as Number (%) of participants unless otherwise specified. P < 0.001 for all comparisons.
The results of our multivariable analyses did not substantially change when we stratified exposures by ED visits only or hospitalizations only, or when we performed sensitivity analyses using a more sensitive definition of ischemic stroke.
Discussion
In a large and demographically heterogeneous population, we found that patients discharged from an ED or hospital with a primary diagnosis of TGA faced a low risk of subsequent ischemic stroke. Their risk was similar to the risk of stroke after a diagnosis of migraine, lower than the risk after presentation with seizure, and much lower than the risk after TIA.
Our results are consistent with several smaller studies suggesting that TGA is benign in terms of future vascular events,6, 8–17 and our results do not support other studies suggesting that TGA heralds stroke in a similar fashion as TIA.19–22 Furthermore, our findings do not support the proposed etiological explanation of TGA as a form of arterial ischemia.18 Our findings are not inconsistent with alternative and still unproven explanations of TGA as a manifestation of venous insufficiency31 or migrainous phenomena,15 but our epidemiological analysis cannot further inform the debate about the exact etiology of TGA. From a practical standpoint, our study suggests that patients with classical presentations of TGA do not require urgent evaluation or any specific intensification of stroke risk factor treatment, even if they have vascular comorbidities.
The results of our study should be interpreted in light of several limitations. First, our analysis relied on administrative data and ICD-9-CM diagnosis codes without detailed clinical data, and therefore may be susceptible to incorrect or biased ascertainment of TGA cases; we also lacked access to ICD-10 codes, which are likely to offer advantages such as greater specificity.32 This concern is somewhat mitigated by our validation of the TGA ICD-9-CM code via detailed chart review, whose results indicate that cases identified on the basis of this diagnosis code are likely to be representative of patients evaluated in an ED or hospital for TGA. Second, our use of administrative data may have led to under-ascertainment of strokes. However, the rate of stroke after TIA in our sample is broadly consistent with rates in recent population-based prospective studies,33 suggesting adequate capture of incident strokes in our study. Furthermore, even if our overall ascertainment of strokes were insensitive, there is no obvious reason to suspect differential misclassification of strokes in patients with TGA versus migraine or seizure, which supports our finding that TGA is not a risk factor for stroke compared to these negative controls. Third, this was a hospital-based study in that we lacked access to ambulatory care visits, and therefore our results cannot be generalized to patients presenting with TGA in such settings. However, this would have biased our study in a conservative direction, since patients diagnosed with TGA in the ambulatory setting would most likely have been less severely ill than those who presented to the hospital, and therefore be unlikely to face a higher risk of stroke.
In summary, patients with TGA do not appear to face a heightened risk of ischemic stroke compared to patients diagnosed with migraine or seizure. Our findings may be helpful in reassuring patients with this dramatic and distressing syndrome that their prognosis is good, and in reassuring clinicians that these patients do not need extensive testing or specific therapy if the clinical evaluation is consistent with TGA.
Acknowledgments
Sources of funding: Babak B. Navi receives research funding from the NIH (KL2TR000458, U01S062835). Hooman Kamel receives research funding from the NIH (K23NS082367).
Footnotes
Conflicts of Interest/Disclosures: None.
References
- 1.Fisher CM, Adams RD. Transient global amnesia. Acta Neurol Scand Suppl. 1964;40(suppl 9):1–83. [PubMed] [Google Scholar]
- 2.Bender MB. Syndrome of isolated episode of confusion with amnesia. J Hillside Hosp. 1956;5:212–215. [Google Scholar]
- 3.Guyotat M, Courjon J. Les ictus amnesiques. J Med Lyon. 1956;37:697–701. [PubMed] [Google Scholar]
- 4.Lauria G, Gentile M, Fassetta G, Casetta I, Caneve G. Incidence of transient global amnesia in the Belluno province, Italy: 1985 through 1995. Results of a community-based study. Acta Neurol Scand. 1997;95:303–310. doi: 10.1111/j.1600-0404.1997.tb00215.x. [DOI] [PubMed] [Google Scholar]
- 5.Miller JW, Petersen RC, Metter EJ, Millikan CH, Yanagihara T. Transient global amnesia: clinical characteristics and prognosis. Neurology. 1987;37:733–737. doi: 10.1212/wnl.37.5.733. [DOI] [PubMed] [Google Scholar]
- 6.Hodges JR, Warlow CP. The aetiology of transient global amnesia. A case-control study of 114 cases with prospective follow-up. Brain. 1990;113:639–657. doi: 10.1093/brain/113.3.639. [DOI] [PubMed] [Google Scholar]
- 7.Bartsch T, Deuschl G. Transient global amnesia: functional anatomy and clinical implications. Lancet Neurol. 2010;9:205–214. doi: 10.1016/S1474-4422(09)70344-8. [DOI] [PubMed] [Google Scholar]
- 8.Pantoni L, Bertini E, Lamassa M, Pracucci G, Inzitari D. Clinical features, risk factors, and prognosis in transient global amnesia: a follow-up study. Eur J Neurol. 2005;12:350–356. doi: 10.1111/j.1468-1331.2004.00982.x. [DOI] [PubMed] [Google Scholar]
- 9.Hinge HH, Jensen TS, Kjaer M, Marquardsen J, de Fine Olivarius B. The prognosis of transient global amnesia. Results of a multicenter study. Arch Neurol. 1986;43:673–676. doi: 10.1001/archneur.1986.00520070031013. [DOI] [PubMed] [Google Scholar]
- 10.Guidotti M, Anzalone N, Morabito A, Landi G. A case-control study of transient global amnesia. J Neurol Neurosurg Psychiatry. 1989;52:320–323. doi: 10.1136/jnnp.52.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shuping JR, Rollinson RD, Toole JF. Transient global amnesia. Ann Neurol. 1980;7:281–285. doi: 10.1002/ana.410070313. [DOI] [PubMed] [Google Scholar]
- 12.Gandolfo C, Caponnetto C, Conti M, Dagnino N, Del Sette M, Primavera A. Prognosis of transient global amnesia: a long-term follow-up study. Eur Neurol. 1992;32:52–57. doi: 10.1159/000116787. [DOI] [PubMed] [Google Scholar]
- 13.Chen ST, Tang LM, Hsu WC, Lee TH, Ro LS, Wu YR. Clinical features, vascular risk factors, and prognosis for transient global amnesia in Chinese patients. J Stroke Cerebrovasc Dis. 1999;8:295–299. doi: 10.1016/s1052-3057(99)80003-2. [DOI] [PubMed] [Google Scholar]
- 14.Zorzon M, Antonutti L, Mase G, Biasutti E, Vitrani B, Cazzato G. Transient global amnesia and transient ischemic attack. Natural history, vascular risk factors, and associated conditions. Stroke. 1995;26:1536–1542. doi: 10.1161/01.str.26.9.1536. [DOI] [PubMed] [Google Scholar]
- 15.Quinette P, Guillery-Girard B, Dayan J, de la Sayette V, Marquis S, Viader F, et al. What does transient global amnesia really mean? Review of the literature and thorough study of 142 cases. Brain. 2006;129:1640–1658. doi: 10.1093/brain/awl105. [DOI] [PubMed] [Google Scholar]
- 16.Toledo M, Pujadas F, Grive E, Alvarez-Sabin J, Quintana M, Rovira A. Lack of evidence for arterial ischemia in transient global amnesia. Stroke. 2008;39:476–479. doi: 10.1161/STROKEAHA.107.498303. [DOI] [PubMed] [Google Scholar]
- 17.Nausieda PA, Sherman IV. Long-term prognosis in transient global amnesia. JAMA. 1979;241:392–393. [PubMed] [Google Scholar]
- 18.Winbeck K, Etgen T, von Einsiedel HG, Rottinger M, Sander D. DWI in transient global amnesia and TIA: proposal for an ischaemic origin of TGA. J Neurol Neurosurg Psychiatry. 2005;76:438–441. doi: 10.1136/jnnp.2004.042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jensen TS, de Fine Olivarius B. Transient global amnesia -- its clinical and pathophysiological basis and prognosis. Acta Neurol Scand. 1981;63:220–230. doi: 10.1111/j.1600-0404.1981.tb00775.x. [DOI] [PubMed] [Google Scholar]
- 20.Jensen TS, De Fine Olivarius B. Transient global amnesia as a manifestation of transient cerebral ischemia. Acta Neurol Scand. 1980;61:115–124. doi: 10.1111/j.1600-0404.1980.tb01473.x. [DOI] [PubMed] [Google Scholar]
- 21.Mathew NT, Meyer JS. Pathogenesis and natural history of transient global amnesia. Stroke. 1974;5:303–311. doi: 10.1161/01.str.5.3.303. [DOI] [PubMed] [Google Scholar]
- 22.Cattaino G, Querin F, Pomes A, Piazza P. Transient global amnesia. Acta Neurol Scand. 1984;70:385–390. doi: 10.1111/j.1600-0404.1984.tb00842.x. [DOI] [PubMed] [Google Scholar]
- 23.Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–2089. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. [Accessed October 21, 2013];California Office of Statewide Healthcare Planning and Development. http://www.oshpd.ca.gov/
- 25.Agency for Healthcare Research and Quality. [Accessed October 21, 2013];Healthcare Cost and Utilization Project. http://hcupnet.ahrq.gov.
- 26.Agency for Healthcare Research and Quality. [Accessed October 21, 2013];HCUP methods series: methodological issues when studying readmissions and revisits using hospital administrative data. http://www.hcup-us.ahrq.gov/reports/methods/2011_01.pdf.
- 27.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics, 2013 update. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tirschwell DL, Longstreth WT., Jr Validating administrative data in stroke research. Stroke. 2002;33:2465–2470. doi: 10.1161/01.str.0000032240.28636.bd. [DOI] [PubMed] [Google Scholar]
- 29.Agency for Healthcare Research and Quality. [Accessed October 21, 2013];Clinical classification software for ICD-9-CM. http://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp.
- 30.Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA. 2000;284:2901–2906. doi: 10.1001/jama.284.22.2901. [DOI] [PubMed] [Google Scholar]
- 31.Chung CP, Hsu HY, Chao AC, Chang FC, Sheng WY, Hu HH. Detection of intracranial venous reflux in patients of transient global amnesia. Neurology. 2006;66:1873–1877. doi: 10.1212/01.wnl.0000219620.69618.9d. [DOI] [PubMed] [Google Scholar]
- 32.RAND Corporation. [Accessed October 21, 2013];The costs and benefits of moving to the ICD-10 code sets. http://www.rand.org/content/dam/rand/pubs/technical_reports/2004/RAND_TR132.pdf.
- 33.Paul NL, Simoni M, Chandratheva A, Rothwell PM. Population-based study of capsular warning syndrome and prognosis after early recurrent TIA. Neurology. 2012;79:1356–1362. doi: 10.1212/WNL.0b013e31826c1af8. [DOI] [PMC free article] [PubMed] [Google Scholar]