Abstract
Background
Essential tremor (ET) is typically measured in the clinic with subjective tremor rating scales which require the presence of a clinician for scoring and are not appropriate for measuring severity throughout the day. Motion sensors can accurately rate tremor severity during a set of predefined tasks in a laboratory.
Methods
We evaluated the ability of motion sensors to quantify tremor during unconstrained activities at home. 20 ET subjects wore a wireless sensor continuously for up to 10 hours daily on two days and completed hourly standardized tremor assessments involving pre-defined tasks. Mathematical models were used to predict tremor rating scores from the sensor data.
Results
At home tremor scores from hourly standardized assessments correlated with at home tremor scores estimated during unconstrained activities immediately following the standardized assessments. The hourly standardized assessments did not significantly fluctuate throughout the day, while fluctuations in the continuous assessments tended to follow changes in voluntary activity level. Both types of tremor ratings (standardized and continuous) showed high day-to-day test-retest reliability with intraclass correlation coefficients ranging from 0.67 to 0.90 for continuous ratings and 0.77 to 0.95 for standardized ratings.
Conclusions
Results demonstrate the feasibility of continuous monitoring of tremor severity at home, which should provide clinicians with a measure of the temporal pattern of tremor in the context of daily life and serve as a useful tool for the evaluation of novel anti-tremor medications in clinical trials.
Keywords: Essential tremor, ambulatory monitoring, Kinesia, accelerometer, gyroscope
Introduction
Essential tremor (ET), characterized primarily by postural and kinetic tremor of the limbs, has a negative impact on quality of life, as it affects activities of daily living (ADL) and has psychological effects associated with tremor exacerbation in public [1,2]. Typical activities prominently affected by ET are handwriting, eating, dressing and self-care. In moderate to severe cases of ET, pharmaceutical interventions or deep brain stimulation are often needed to optimize quality of life. In order to monitor effects of treatment, it is important to accurately quantify motor function and disability associated with ET.
Currently, rating scales such as the Tremor Research Group Essential Tremor Rating Assessment Scale (TETRAS) [3], Washington Heights-Inwood Genetic Study of Essential Tremor (WHIGET) tremor rating scale (wTRS) [4], and Fahn-Tolosa-Marin tremor rating scale [5] are used to evaluate ET during a clinical examination. Each rates tremor on a subjective, qualitative 0–4 scale. While these rating scales are useful tools for clinicians treating patients with ET and are often used as an outcome measure for clinical drug trials, they require the presence of a clinician for scoring, are subject to clinical judgment and bias, and cannot be used practically for monitoring fluctuations in tremor throughout the day, especially in a patient’s home environment. Even if these ratings provide an accurate momentary assessment, they are only a snapshot of tremor during the clinical visit. Indeed, quantitative assessments every 2 hours for 6 hours have found a maximal 23% absolute variation in tremor amplitude during this period [6]. This fluctuation in amplitude has to be considered when designing trials to assess therapeutic interventions. There is, therefore, a need to provide quantitative data on ET patients in their home environment during routine activities. Given current limitations in the understanding of how most ET therapies affect tremor throughout the day, such a system could allow clinicians to better discriminate which medications and doses are most effective for patients in their natural environment. The opportunity for home monitoring may also expand care to patients who are reluctant or unable to travel for research or numerous clinical visits for evaluation if the patients do not live near a movement disorders center or have significant mobility impairments.
Previously, accelerometers, gyroscopes, and EMG have been used to obtain quantitative measurements of tremor in both Parkinson’s disease (PD) [7–10] and ET [11,12]. The motion sensing system used in the present study previously quantified PD tremor during standardized clinical exams, with high correlations to the Unified Parkinson’s Disease Rating Scale [7,13]. More recently we demonstrated that this sensor system could be used to quantify tremor in patients with ET during simulated ADL performed in a laboratory setting [11]. Algorithms were developed and validated for using motion data to rate tremor severity during standardized motors tasks and ADL. The goals of this study were to evaluate the system during the performance of unconstrained activities in the home environment and to study the associations between unconstrained tremor recordings and those obtained during standardized tasks that are similar to those used in the office setting.
Methods
Twenty adults (11 male, 9 female; age 48–85 years; disease duration, 2–60 years) with ET were recruited. Subject medication use for treatment of ET-related symptoms was recorded, but not altered during participation in this study. Five subjects were not on ET-related medication. The remaining 15 subjects were on one or more drugs for management of their symptoms, with propranolol (11/15) and primidone (5/15) being the most common. All clinical testing was completed at Baylor College of Medicine and Rush University Medical Center under the purview of their respective institutional review boards and in accordance with the Declaration of Helsinki [14]. All subjects provided informed consent prior to their participation in the study.
Each subject underwent an initial training session during which the components and operation of the system were described. The subjects were then sent home with a modified motion sensor-based home monitoring system (Kinesia HomeView™, Great Lakes NeuroTechnologies, Cleveland, OH) and were monitored for two days. The system included a wireless motion sensor unit (Supplementary Figure 1) and a tablet PC. At the start of data collection each day, subjects wore the wireless motion sensor unit on the base of the index finger of his/her more affected hand. The tablet PC then guided the subject through a standardized tremor assessment consisting of three pre-defined tasks to evaluate rest, postural, and kinetic tremor for 15 seconds each. These tasks included having the subjects place their hands in their laps (rest tremor), hold their arms extended horizontally (postural tremor), and repeatedly reach out and touch their noses (kinetic tremor). After completion of this assessment, subjects went about their normal activities while continuing to wear the sensor. At one hour intervals, the subjects returned to the tablet PC and were prompted to repeat the standardized tremor assessment. Subjects were instructed to repeat this hourly cycle of a standardized tremor assessment followed by unconstrained activities for ten hours each of the two days. To minimize the burden placed on subjects by the protocol and interference with their normal routines, the start time of home tremor evaluation was not regulated.
Kinematic data recorded at each of the hourly standardized assessments were processed into 0–4 scores corresponding to the amplitude severity of rest, postural, and kinetic tremor using previously validated algorithms. These algorithms have been shown to output scores highly correlated with clinician UPDRS and wTRS ratings [7,11,13]. A repeated-measures ANOVA with a Greenhouse-Geisser correction for sphericity was used to determine if tremor severity, as quantified by the tremor scores from the standardized assessments, fluctuated across time. An additional multiple regression model was developed to rate tremor severity during unconstrained activities on a continuous basis (i.e., every 12 seconds), generating a “continuous” waveform throughout the day. The continuous waveform was low pass filtered with a 5-minute sliding median filter, with one minute of overlap between consecutive windows. Further details of the algorithm development and validation are available in the online Supplementary Material.
The test re-test reliability of each assessment type was calculated as the intraclass correlation (ICC) between tremor ratings from days 1 and 2. For the hourly assessments, the average severity scores were taken across time for rest, postural, and kinetic tremor, respectively, for each day. These averages were then used to calculate the day-to-day ICCs. For continuous ratings, the percentage of time during movement in each tremor category (i.e., 0, 1, 2, 3, and 4) was calculated for each day and subsequently used to calculate the ICCs between days 1 and 2.
Results
Compliance
The twenty subjects were instructed to wear the sensors for ten hours on each of two separate days (40 subject-days total); however, the actual duration of monitoring varied across subjects. Consistent with our previous findings in patients with Parkinson’s disease [15], the ET subjects in this study accurately and consistently performed the standardized motor tasks in the home. The motion sensor was worn for multiple hours in 39 of the 40 subject-days evaluated. Eighty-percent of subject-days (32/40) included the subject wearing the motion sensor for at least eight hours. Only five out of the 40 subject-days were comprised of six or fewer hours of wear.
Hourly Standardized Tremor Assessments
Table 1 summarizes the intraday mean and intraday range across the 39 subject-days in this study and the ICCs between day 1 and 2 for each type of tremor. Kinetic tremor was, on average, more severe than rest or postural tremor, consistent with the typical phenomenology of ET. No statistically significant differences (repeated measures ANOVA, p > 0.13) were detected across time for rest, postural, or kinetic tremor, indicating that these subjects did not exhibit significant fluctuations throughout the day.
Table 1.
Summary tremor statistics for hourly standardized tremor assessments. Average and standard deviation are given for the intraday mean and range of the severity scores. Day-to-day ICCs are given as a measure of test-retest reliability.
| Intraday Mean | Intraday Range | ICC | |
|---|---|---|---|
| Rest Tremor | 0.85 ± 0.52 | 0.94 ± 0.41 | 0.77 |
| Postural Tremor | 0.96 ± 0.59 | 0.80 ± 0.50 | 0.91 |
| Kinetic Tremor | 1.97 ± 0.47 | 0.50 ± 0.20 | 0.95 |
Continuous Tremor Ratings
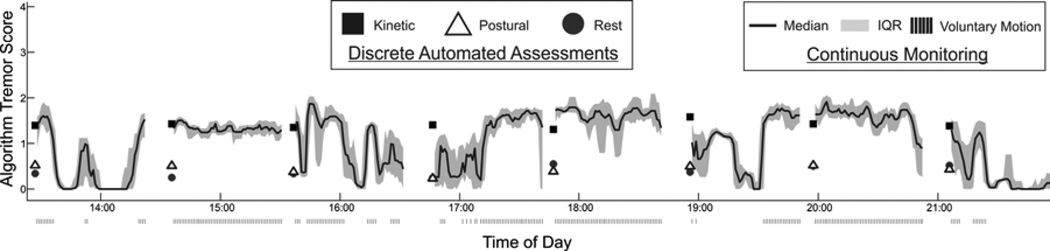
An example of this continuous waveform for one subject, along with the scores for kinetic, postural, and rest tremor generated during the hourly standardized tremor assessments is shown in Figure 1. As expected given the typical appearance of ET, the tremor ratings tended to increase during voluntary motions (indicated by the tick marks at the bottom of the panel). The standardized tremor assessments were immediately followed by voluntary unconstrained activity. As a result, most segments of the continuous waveform have an initial value that is approximately equal to the preceding kinetic tremor score (square marker). When voluntary motion was not detected immediately following the standardized assessment (e.g., at the assessment just before 17:00), the initial value is closer to the rest tremor score (circle marker). The accuracy of the continuous tremor waveforms was assessed by comparing the scores predicted during voluntary motion in the five minute interval immediately following the standardized assessments to the standardized kinetic tremor scores. The continuous scores and standardized scores were generally consistent, with a strong correlation and low error (see Supplementary Results).
Figure 1.
Continuous tremor scoring for an 8-hour period. The line represents continuous ratings during unknown activities and the markers represent ratings during pre-defined tasks performed at the standardized assessments. Gaps in the waveform reflect the subject changing motion sensor units.
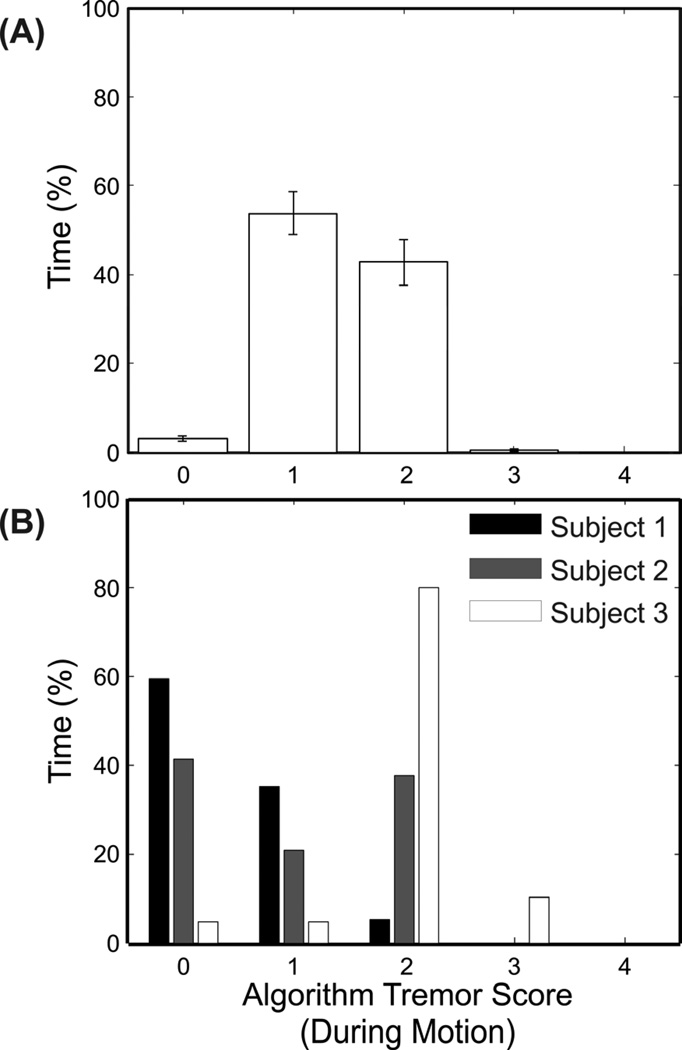
For clinical management of symptoms and evaluation of pharmaceutical agents in clinical trials, a graphical representation of the percentage of time at different levels of tremor severity (Figure 2), generated by assigning the continuous scores into appropriate tremor categories, is a potentially useful tool. Since tremor appeared to be movement-dependent, only periods during which voluntary movement was detected were included. Figure 2A shows the mean and standard error in each category across all subject-days. The distributions for three individual subjects are shown in Figure 2B. Subject 1’s tremor was rated by the algorithms as 0 for approximately 60% of the time, while the distribution of subject 2’s tremor ratings was more evenly distributed between 0 and 2. Subject 3’s tremor was more marked, rated as 2 for 80% of the time and 3 for 10% of the time. The reliability of the amount of time at each level of tremor severity was evaluated by calculating the ICCs across day 1 and day 2. The ICCs for levels 0, 1, 2, and 3 were 0.78, 0.90, 0.88, and 0.67, respectively. The most severe category (4) was excluded because the scoring algorithm did not detect tremor of this severity.
Figure 2.
Percentage of time at different tremor severities when voluntary motion was detected. Shown are (A) the average times across all subject-days, and (B) the times during a single day for three individual subjects. The error bars represent standard errors.
Discussion
The goal of this study was to evaluate the feasibility of using a motion sensor system for monitoring ET patients in their home environment. The temporal resolution provided by such a system is much richer than what can be captured during the typical clinical appointment and will provide clinicians with a more complete temporal picture of a patient’s tremor as experienced on a daily basis. Subjects were able to learn the use of the apparatus with a single training session and to don and wear the motion sensor throughout the day as data was collected to quantify tremor severity during routine activities of daily living.
The hourly standardized assessments involving pre-defined motions did not demonstrate statistically significant temporal variability in tremor severity, with average intra-day ranges of 0.5 to 1 on a 0–4 scale (Table 1). It is worth noting that the lack of significant variability may have been affected by the small number of recruited subjects with severe tremor, as these patients are likely to have a wider range of symptom severities throughout the day. Since medication regimens were not altered and the exact timing of medication doses was not monitored during this study, we cannot draw conclusions related to changes in medication effects on the underlying disease state based on these data. This finding is, however, consistent with other recent clinical studies evaluating amplitude fluctuations in ET [6]. Fluctuations observed during continuous monitoring throughout the day (Figure 1) may have been due to changes in activity level rather than medication or disease state since tremor is primarily present during voluntary movements in ET.
We demonstrated that finger-worn motion sensors can be used to objectively categorize the severity and duration of tremor throughout the day during unconstrained activities (Figures 1 and 2) on a continuous basis. This has several potential advantages over the current standards for both the optimization of medication regimen in a clinical setting and clinical drug trials. Despite the fact that the activities subjects performed throughout the day were unknown, this characterization of tremor severity and duration was shown to have high test-retest reliability, with ICCs falling in a range that is comparable to what has been demonstrated during known, standardized tasks [6,16]. It is possible that the paucity of subjects with severe tremor may have resulted in a biased estimate of the ICCs, as these individuals might be expected to have greater symptom variability during the day, resulting in lower test-retest reliability. Since the continuous assessments generally agreed with the hourly standardized assessments (Supplementary Material), the hourly assessments at the tablet PC can be eliminated when using continuous monitoring in clinical practice. Allowing patients to simply don the motion sensor and go about their normal routines without having to return to the computer for standardized tests may reduce the burden associated with home monitoring and eliminate compliance problems related to the need to interrupt daily life to perform these tests. In this study, the size of the sensor unit may have interfered with some activities of daily living. Current work is underway to reduce the unit into a more ergonomic device and to update the electronics to extend wear-time to a full day. A simulation of various duty cycles that would enable use throughout an entire day (Supplementary Material) showed that there would be little information loss when operating the sensor in a pattern of 12 seconds on followed by 12 seconds off (i.e., a 50% duty cycle). With the updated hardware, reducing the sampling rate to 64 Hz and using a 50% duty cycle would enable the motion sensor to operate for 16 hours before filling up its memory or needing to be recharged, which should be sufficient for characterizing symptoms throughout the day.
By capturing tremor during the activities that impact quality of life most, an objective motion sensor-based system has implications both in primary care settings and in clinical trials. In a primary care setting, medication type and dosages may be optimized by considering total tremor throughout the day and its temporal pattern, rather than just what is observed during the clinical visit. Future work is planned to determine if the additional information gained from such a tool could improve patient outcomes relative to traditional clinic assessments alone. For clinical trials, it is important to determine how long a putative therapy remains effective during the day for medication adjustments. Motor fluctuations are an established phenomenon in Parkinson’s disease, where patients exhibit large changes in symptom severity as their medication takes effect and wears off [17]. Although statistically significant fluctuations were not detected in the current study, it is possible that future anti-tremor agents may induce variability in symptoms across the dose cycle and it will be important to capture such fluctuations for optimal drug treatment. In addition to quantifying total tremor throughout the day, the temporal pattern captured by a motion sensor system could be an important variable to monitor to demonstrate efficacy of new drugs being evaluated in clinical trials.
Supplementary Material
Acknowledgement
This work was supported by grant number 2R44AG034708-02A1 from the National Institute on Aging. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH/NIA.
CL Pulliam received compensation from Great Lakes NeuroTechnologies for employment.
SR Eichenseer’s institution has received research support from the Parkinson’s Disease Foundation.
CG Goetz has received compensation from AOP Orphan, Addex Pharma, Advanced Studies of Medicine, Boston Scientific, CHDI, Health Advances, ICON Clinical Research, Ingenix (i3 Research), National Institutes of Health, Neurocrine, Oxford Biomedica, Synthonics for consulting services and honoraria; from MDS, AAN, U of Miami, U of Pennsylvania, U of Montreal, and the Neurological Society for editorial services; from Oxford University Press, Elsevier Publishers, Wolters Kluwer Health- Lippincott, Wilkins and Williams for royalties; from the Michael J. Fox Foundation and the NIH for research support. Dr. Goetz directs the Rush Parkinson’s Disease Research Center that receives support from the Parkinson’s Disease Foundation. He directs the translation program for the MDS-UPDRS and UDysRS and receives funds from the Movement Disorders Society for this effort.
CB Hunter received compensation from Lundbeck Inc. for consulting services.
J Jankovic received compensation from Allergan, Inc, Chelsea Therapeutics, EMG Serono, Merz Pharmaceuticals, Lundbeck Inc., and Teva for consulting services; from Medlink: Neurology in Clinical Practice for Serving editorial services; from Allergan Inc, Allon Therapeutics, Ceregene Inc, Chelsea Therapeutics, Diana Helis Henry Medical Research Foundation, EMG Serono, Huntington’s Disease Society of America, Huntington Study Group, Impax Pharmaceuticals, Inpsen Limited, and Lun for research support.
DE Vaillancourt received compensation from NIH, Bachmann-Straus Foundation, and Tyler’s Hope Foundation for research support; from UT Southwestern Medical Center, University of Illinois, and Great Lakes NeuroTechnologies for consulting services. He is a co-founder and manager of Neuroimaging Solutions, LLC.
JP Giuffrida received compensation from Great Lakes NeuroTechnologies for employment.
DA Heldman received compensation from Great Lakes NeuroTechnologies for employment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Full Financial Disclosures
O Waln has nothing to disclose.
References
- 1.Lorenz D, Schwieger D, Moises H, Deuschl G. Quality of life and personality in essential tremor patients. Mov Disord. 2006 Aug;21:1114–1118. doi: 10.1002/mds.20884. [DOI] [PubMed] [Google Scholar]
- 2.Hariz G-M, Blomstedt P, Koskinen L-OD. Long-term effect of deep brain stimulation for essential tremor on activities of daily living and health-related quality of life. Acta Neurol Scand. 2008 Jun 26;118:387–394. doi: 10.1111/j.1600-0404.2008.01065.x. [DOI] [PubMed] [Google Scholar]
- 3.Elble R, Comella C, Fahn S, Hallett M, Jankovic J, Juncos JL, et al. Reliability of a new scale for essential tremor. Movement Disorders. 2012;27:1567–1569. doi: 10.1002/mds.25162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Louis ED, Ottman R, Ford B, Pullman S, Martinez M, Fahn S, et al. The Washington Heights-Inwood Genetic Study of Essential Tremor: methodologic issues in essential-tremor research. Neuroepidemiology. 1997;16:124–133. doi: 10.1159/000109681. [DOI] [PubMed] [Google Scholar]
- 5.Fahn S, Tolosa E, Marin C. Clinical Rating Scale for Tremor. In: Jankovic, Tolosa, editors. Parkinson’s disease and movement disorders. 2nd ed. Baltimore: Williams and Wilkins; 1993. pp. 271–280. [Google Scholar]
- 6.Mostile G, Fekete R, Giuffrida JP, Yaltho T, Davidson A, Nicoletti A, et al. Amplitude fluctuations in essential tremor. Parkinsonism Relat. Disord. 2012;18:859–863. doi: 10.1016/j.parkreldis.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 7.Giuffrida JP, Riley D, Maddux B, Heldman DA. Clinically deployable Kinesia technology for automated tremor assessment. Mov Disord. 2009;24:723–730. doi: 10.1002/mds.22445. [DOI] [PubMed] [Google Scholar]
- 8.Norman KE, Edwards R, Beuter A. The measurement of tremor using a velocity transducer: comparison to simultaneous recordings using transducers of displacement, acceleration and muscle activity. Journal of Neuroscience Methods. 1999 Oct 15;92:41–54. doi: 10.1016/s0165-0270(99)00091-6. [DOI] [PubMed] [Google Scholar]
- 9.Van Someren EJW, Pticek MD, Speelman JD, Schuurman PR, Esselink R, Swaab DF. New actigraph for long-term tremor recording. Mov Disord. 2006;21:1136–1143. doi: 10.1002/mds.20900. [DOI] [PubMed] [Google Scholar]
- 10.Sturman MM, Vaillancourt DE, Metman LV, Bakay RAE, Corcos DM. Effects of subthalamic nucleus stimulation and medication on resting and postural tremor in Parkinson’s disease. Brain. 2004 Sep 1;127:2131–2143. doi: 10.1093/brain/awh237. [DOI] [PubMed] [Google Scholar]
- 11.Heldman DA, Jankovic J, Vaillancourt DE, Prodoehl J, Elble RJ, Giuffrida JP. Essential tremor quantification during activities of daily living. Parkinsonism & related disorders. 2011;17:537–542. doi: 10.1016/j.parkreldis.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaillancourt DE, Sturman MM, Verhagen Metman L, Bakay RAE, Corcos DM. Deep brain stimulation of the VIM thalamic nucleus modifies several features of essential tremor. Neurology. 2003;61:919–925. doi: 10.1212/01.wnl.0000086371.78447.d2. [DOI] [PubMed] [Google Scholar]
- 13.Mostile G, Giuffrida JP, Adam OR, Davidson A, Jankovic J. Correlation between Kinesia system assessments and clinical tremor scores in patients with essential tremor. Mov Disord. 2010;25:1938–1943. doi: 10.1002/mds.23201. [DOI] [PubMed] [Google Scholar]
- 14.World Medical Association. Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects [Internet] [cited 2013 May 20];2008 doi: 10.1001/jama.2013.281053. Available from: http://www.wma.net/en/30publications/10policies/b3/index.html. [DOI] [PubMed]
- 15.Mera TO, Heldman DA, Espay AJ, Payne M, Giuffrida JP. Feasibility of home-based automated Parkinson’s disease motor assessment. J Neurosci Meth. 2012 Jan 15;203(1):152–156. doi: 10.1016/j.jneumeth.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heldman DA, Espay A, LeWitt P, Giuffrida J. Test-Retest Reliability of a Parkinson’s Disease Monitoring System. Neurology. 2013;80 S03.003. [Google Scholar]
- 17.Weiner WJ. Motor fluctuations in Parkinson’s disease. Rev Neurol Dis. 2006;3:101–108. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.