Abstract
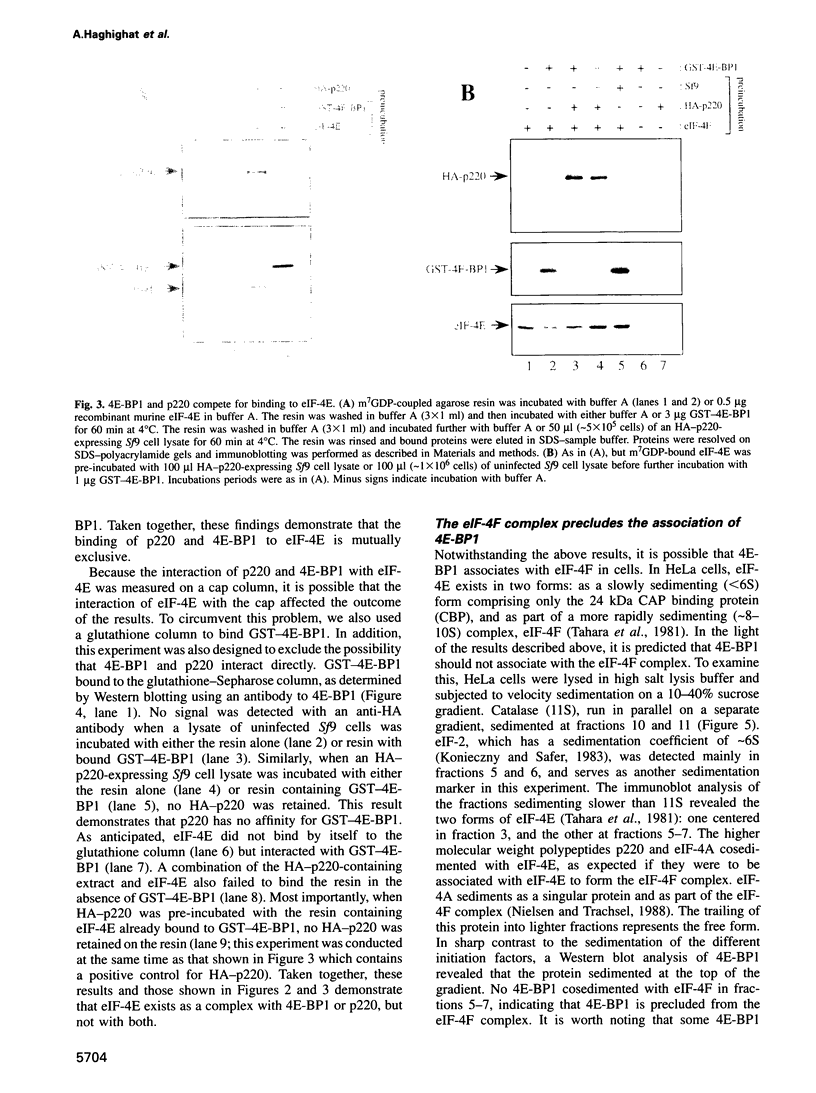
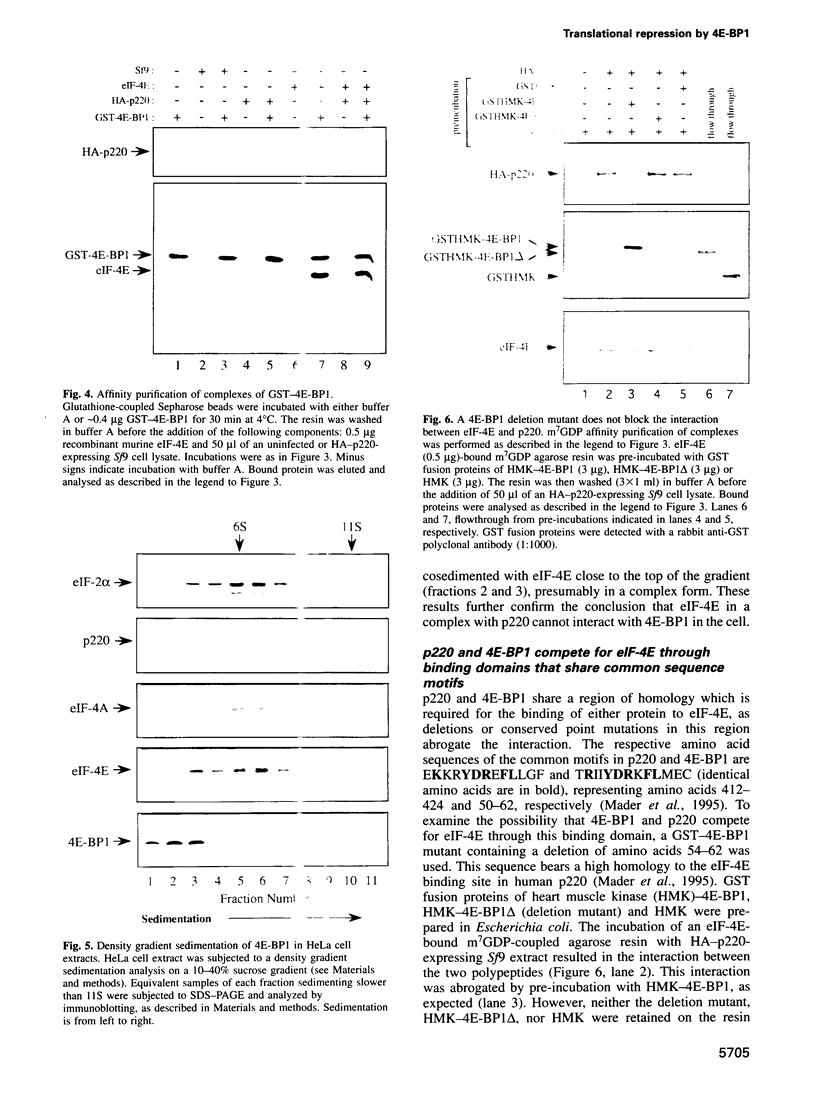
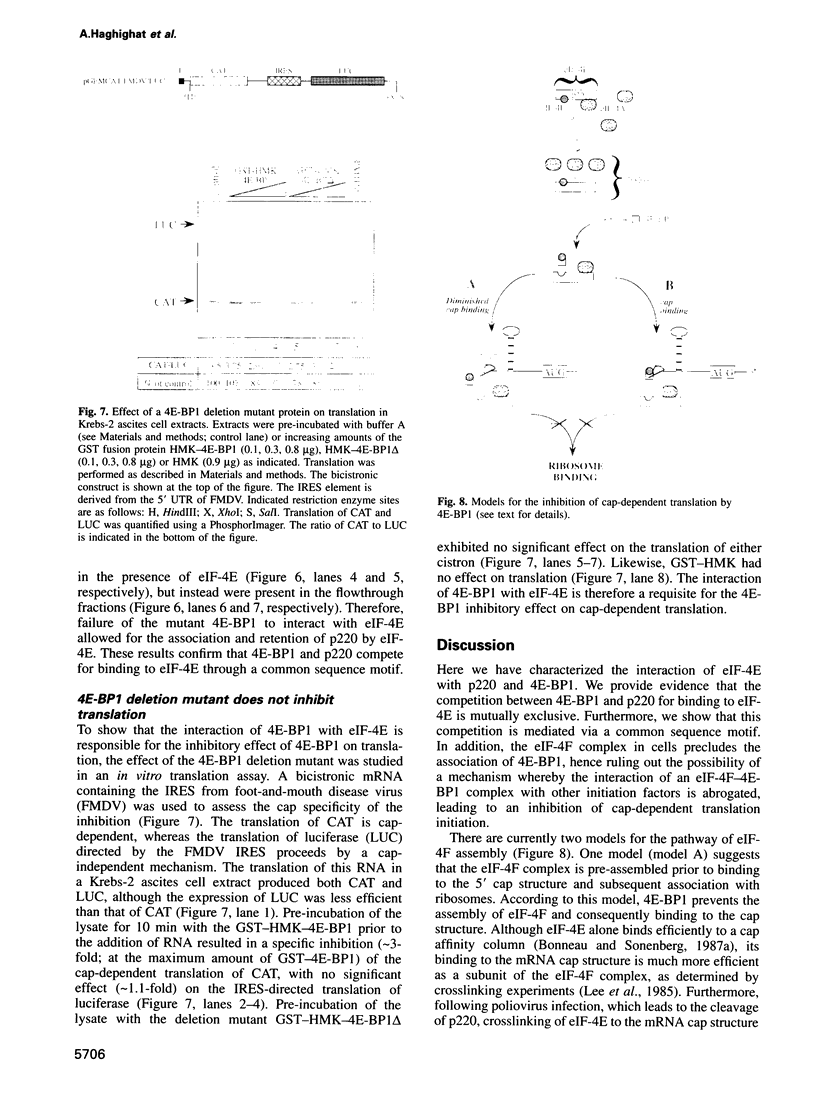
An important aspect of the regulation of gene expression is the modulation of translation rates in response to growth factors, hormones and mitogens. Most of this control is at the level of translation initiation. Recent studies have implicated the MAP kinase pathway in the regulation of translation by insulin and growth factors. MAP kinase phosphorylates a repressor of translation initiation [4E-binding protein (BP) 1] that binds to the mRNA 5' cap binding protein eukaryotic initiation factor (eIF)-4E and inhibits cap-dependent translation. Phosphorylation of the repressor decreases its affinity for eIF-4E, and thus relieves translational inhibition. eIF-4E forms a complex with two other polypeptides, eIF-4A and p220, that promote 40S ribosome binding to mRNA. Here, we have studied the mechanism by which 4E-BP1 inhibits translation. We show that 4E-BP1 inhibits 48S pre-initiation complex formation. Furthermore, we demonstrate that 4E-BP1 competes with p220 for binding to eIF-4E. Mutants of 4E-BP1 that are deficient in their binding to eIF-4E do not inhibit the interaction between p220 and eIF-4E, and do not repress translation. Thus, translational control by growth factors, insulin and mitogens is affected by changes in the relative affinities of 4E-BP1 and p220 for eIF-4E.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony D. D., Merrick W. C. Eukaryotic initiation factor (eIF)-4F. Implications for a role in internal initiation of translation. J Biol Chem. 1991 Jun 5;266(16):10218–10226. [PubMed] [Google Scholar]
- Bonneau A. M., Sonenberg N. Involvement of the 24-kDa cap-binding protein in regulation of protein synthesis in mitosis. J Biol Chem. 1987 Aug 15;262(23):11134–11139. [PubMed] [Google Scholar]
- Bonneau A. M., Sonenberg N. Proteolysis of the p220 component of the cap-binding protein complex is not sufficient for complete inhibition of host cell protein synthesis after poliovirus infection. J Virol. 1987 Apr;61(4):986–991. doi: 10.1128/jvi.61.4.986-991.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu X., Haas D. W., Hagedorn C. H. Novel phosphorylation sites of eukaryotic initiation factor-4F and evidence that phosphorylation stabilizes interactions of the p25 and p220 subunits. J Biol Chem. 1993 Mar 5;268(7):4975–4978. [PubMed] [Google Scholar]
- De Benedetti A., Rhoads R. E. Overexpression of eukaryotic protein synthesis initiation factor 4E in HeLa cells results in aberrant growth and morphology. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8212–8216. doi: 10.1073/pnas.87.21.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan R., Milburn S. C., Hershey J. W. Regulated phosphorylation and low abundance of HeLa cell initiation factor eIF-4F suggest a role in translational control. Heat shock effects on eIF-4F. J Biol Chem. 1987 Jan 5;262(1):380–388. [PubMed] [Google Scholar]
- Edery I., Altmann M., Sonenberg N. High-level synthesis in Escherichia coli of functional cap-binding eukaryotic initiation factor eIF-4E and affinity purification using a simplified cap-analog resin. Gene. 1988 Dec 30;74(2):517–525. doi: 10.1016/0378-1119(88)90184-9. [DOI] [PubMed] [Google Scholar]
- Edery I., Hümbelin M., Darveau A., Lee K. A., Milburn S., Hershey J. W., Trachsel H., Sonenberg N. Involvement of eukaryotic initiation factor 4A in the cap recognition process. J Biol Chem. 1983 Sep 25;258(18):11398–11403. [PubMed] [Google Scholar]
- Feigenblum D., Schneider R. J. Modification of eukaryotic initiation factor 4F during infection by influenza virus. J Virol. 1993 Jun;67(6):3027–3035. doi: 10.1128/jvi.67.6.3027-3035.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederickson R. M., Montine K. S., Sonenberg N. Phosphorylation of eukaryotic translation initiation factor 4E is increased in Src-transformed cell lines. Mol Cell Biol. 1991 May;11(5):2896–2900. doi: 10.1128/mcb.11.5.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie D. R., Traugh J. A. Serum and insulin regulate cap function in 3T3-L1 cells. J Biol Chem. 1994 Mar 11;269(10):7174–7179. [PubMed] [Google Scholar]
- Grifo J. A., Tahara S. M., Morgan M. A., Shatkin A. J., Merrick W. C. New initiation factor activity required for globin mRNA translation. J Biol Chem. 1983 May 10;258(9):5804–5810. [PubMed] [Google Scholar]
- Hiremath L. S., Webb N. R., Rhoads R. E. Immunological detection of the messenger RNA cap-binding protein. J Biol Chem. 1985 Jul 5;260(13):7843–7849. [PubMed] [Google Scholar]
- Hu C., Pang S., Kong X., Velleca M., Lawrence J. C., Jr Molecular cloning and tissue distribution of PHAS-I, an intracellular target for insulin and growth factors. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3730–3734. doi: 10.1073/pnas.91.9.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. T., Schneider R. J. Adenovirus inhibition of cellular protein synthesis involves inactivation of cap-binding protein. Cell. 1991 Apr 19;65(2):271–280. doi: 10.1016/0092-8674(91)90161-q. [DOI] [PubMed] [Google Scholar]
- Jang S. K., Kräusslich H. G., Nicklin M. J., Duke G. M., Palmenberg A. C., Wimmer E. A segment of the 5' nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J Virol. 1988 Aug;62(8):2636–2643. doi: 10.1128/jvi.62.8.2636-2643.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo M., Dever T. E., Merrick W. C., Sonenberg N. RNA unwinding in translation: assembly of helicase complex intermediates comprising eukaryotic initiation factors eIF-4F and eIF-4B. Mol Cell Biol. 1991 Dec;11(12):5992–5997. doi: 10.1128/mcb.11.12.5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis D. L., Summers M. D. Glycosylation and secretion of human tissue plasminogen activator in recombinant baculovirus-infected insect cells. Mol Cell Biol. 1989 Jan;9(1):214–223. doi: 10.1128/mcb.9.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. W., Attia J., Richardson C. D., Roder J. C., Dunn R. J. Synthesis of soluble myelin-associated glycoprotein in insect and mammalian cells. Gene. 1989 Apr 30;77(2):287–296. doi: 10.1016/0378-1119(89)90076-0. [DOI] [PubMed] [Google Scholar]
- Joshi B., Yan R., Rhoads R. E. In vitro synthesis of human protein synthesis initiation factor 4 gamma and its localization on 43 and 48 S initiation complexes. J Biol Chem. 1994 Jan 21;269(3):2048–2055. [PubMed] [Google Scholar]
- Klein P. S., Melton D. A. Induction of mesoderm in Xenopus laevis embryos by translation initiation factor 4E. Science. 1994 Aug 5;265(5173):803–806. doi: 10.1126/science.8047887. [DOI] [PubMed] [Google Scholar]
- Konieczny A., Safer B. Purification of the eukaryotic initiation factor 2-eukaryotic initiation factor 2B complex and characterization of its guanine nucleotide exchange activity during protein synthesis initiation. J Biol Chem. 1983 Mar 10;258(5):3402–3408. [PubMed] [Google Scholar]
- Lazaris-Karatzas A., Montine K. S., Sonenberg N. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5' cap. Nature. 1990 Jun 7;345(6275):544–547. doi: 10.1038/345544a0. [DOI] [PubMed] [Google Scholar]
- Lazaris-Karatzas A., Sonenberg N. The mRNA 5' cap-binding protein, eIF-4E, cooperates with v-myc or E1A in the transformation of primary rodent fibroblasts. Mol Cell Biol. 1992 Mar;12(3):1234–1238. doi: 10.1128/mcb.12.3.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. A., Edery I., Sonenberg N. Isolation and structural characterization of cap-binding proteins from poliovirus-infected HeLa cells. J Virol. 1985 May;54(2):515–524. doi: 10.1128/jvi.54.2.515-524.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. A., Sonenberg N. Inactivation of cap-binding proteins accompanies the shut-off of host protein synthesis by poliovirus. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3447–3451. doi: 10.1073/pnas.79.11.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. A., Kong X., Haystead T. A., Pause A., Belsham G., Sonenberg N., Lawrence J. C., Jr PHAS-I as a link between mitogen-activated protein kinase and translation initiation. Science. 1994 Oct 28;266(5185):653–656. doi: 10.1126/science.7939721. [DOI] [PubMed] [Google Scholar]
- Mader S., Lee H., Pause A., Sonenberg N. The translation initiation factor eIF-4E binds to a common motif shared by the translation factor eIF-4 gamma and the translational repressors 4E-binding proteins. Mol Cell Biol. 1995 Sep;15(9):4990–4997. doi: 10.1128/mcb.15.9.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzella J. M., Rychlik W., Rhoads R. E., Hershey J. W., Blackshear P. J. Insulin induction of ornithine decarboxylase. Importance of mRNA secondary structure and phosphorylation of eucaryotic initiation factors eIF-4B and eIF-4E. J Biol Chem. 1991 Feb 5;266(4):2383–2389. [PubMed] [Google Scholar]
- Minich W. B., Balasta M. L., Goss D. J., Rhoads R. E. Chromatographic resolution of in vivo phosphorylated and nonphosphorylated eukaryotic translation initiation factor eIF-4E: increased cap affinity of the phosphorylated form. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7668–7672. doi: 10.1073/pnas.91.16.7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley S. J., Traugh J. A. Differential stimulation of phosphorylation of initiation factors eIF-4F, eIF-4B, eIF-3, and ribosomal protein S6 by insulin and phorbol esters. J Biol Chem. 1990 Jun 25;265(18):10611–10616. [PubMed] [Google Scholar]
- Morley S. J., Traugh J. A. Phorbol esters stimulate phosphorylation of eukaryotic initiation factors 3, 4B, and 4F. J Biol Chem. 1989 Feb 15;264(5):2401–2404. [PubMed] [Google Scholar]
- Nielsen P. J., Trachsel H. The mouse protein synthesis initiation factor 4A gene family includes two related functional genes which are differentially expressed. EMBO J. 1988 Jul;7(7):2097–2105. doi: 10.1002/j.1460-2075.1988.tb03049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
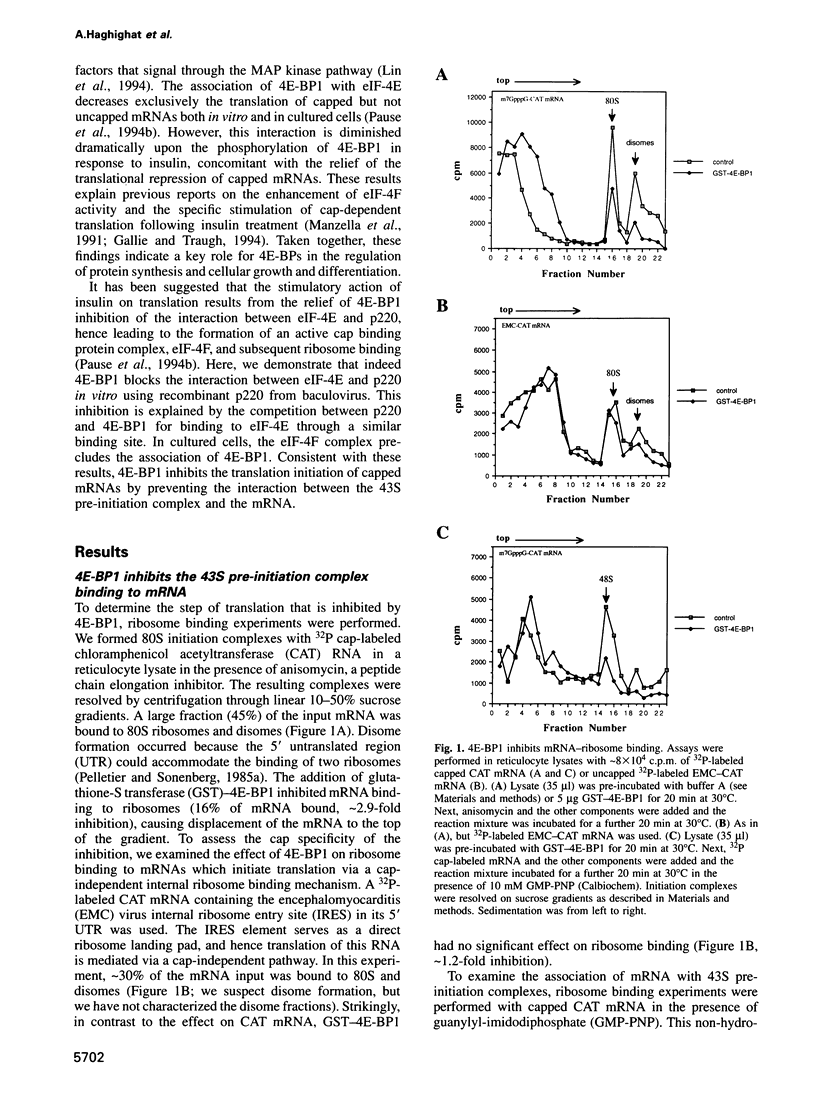
- Pause A., Belsham G. J., Gingras A. C., Donzé O., Lin T. A., Lawrence J. C., Jr, Sonenberg N. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5'-cap function. Nature. 1994 Oct 27;371(6500):762–767. doi: 10.1038/371762a0. [DOI] [PubMed] [Google Scholar]
- Pause A., Méthot N., Svitkin Y., Merrick W. C., Sonenberg N. Dominant negative mutants of mammalian translation initiation factor eIF-4A define a critical role for eIF-4F in cap-dependent and cap-independent initiation of translation. EMBO J. 1994 Mar 1;13(5):1205–1215. doi: 10.1002/j.1460-2075.1994.tb06370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Insertion mutagenesis to increase secondary structure within the 5' noncoding region of a eukaryotic mRNA reduces translational efficiency. Cell. 1985 Mar;40(3):515–526. doi: 10.1016/0092-8674(85)90200-4. [DOI] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988 Jul 28;334(6180):320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Photochemical cross-linking of cap binding proteins to eucaryotic mRNAs: effect of mRNA 5' secondary structure. Mol Cell Biol. 1985 Nov;5(11):3222–3230. doi: 10.1128/mcb.5.11.3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possee R. D. Cell-surface expression of influenza virus haemagglutinin in insect cells using a baculovirus vector. Virus Res. 1986 Jul;5(1):43–59. doi: 10.1016/0168-1702(86)90064-x. [DOI] [PubMed] [Google Scholar]
- Ray B. K., Lawson T. G., Kramer J. C., Cladaras M. H., Grifo J. A., Abramson R. D., Merrick W. C., Thach R. E. ATP-dependent unwinding of messenger RNA structure by eukaryotic initiation factors. J Biol Chem. 1985 Jun 25;260(12):7651–7658. [PubMed] [Google Scholar]
- Rosenwald I. B., Lazaris-Karatzas A., Sonenberg N., Schmidt E. V. Elevated levels of cyclin D1 protein in response to increased expression of eukaryotic initiation factor 4E. Mol Cell Biol. 1993 Dec;13(12):7358–7363. doi: 10.1128/mcb.13.12.7358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen F., Edery I., Meerovitch K., Dever T. E., Merrick W. C., Sonenberg N. Bidirectional RNA helicase activity of eucaryotic translation initiation factors 4A and 4F. Mol Cell Biol. 1990 Mar;10(3):1134–1144. doi: 10.1128/mcb.10.3.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen F., Sonenberg N. Identification of nuclear cap specific proteins in HeLa cells. Nucleic Acids Res. 1987 Aug 25;15(16):6489–6500. doi: 10.1093/nar/15.16.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shantz L. M., Pegg A. E. Overproduction of ornithine decarboxylase caused by relief of translational repression is associated with neoplastic transformation. Cancer Res. 1994 May 1;54(9):2313–2316. [PubMed] [Google Scholar]
- Shatkin A. J. mRNA cap binding proteins: essential factors for initiating translation. Cell. 1985 Feb;40(2):223–224. doi: 10.1016/0092-8674(85)90132-1. [DOI] [PubMed] [Google Scholar]
- Smith M. R., Jaramillo M., Liu Y. L., Dever T. E., Merrick W. C., Kung H. F., Sonenberg N. Translation initiation factors induce DNA synthesis and transform NIH 3T3 cells. New Biol. 1990 Jul;2(7):648–654. [PubMed] [Google Scholar]
- Sonenberg N. Cap-binding proteins of eukaryotic messenger RNA: functions in initiation and control of translation. Prog Nucleic Acid Res Mol Biol. 1988;35:173–207. doi: 10.1016/s0079-6603(08)60614-5. [DOI] [PubMed] [Google Scholar]
- Sonenberg N., Morgan M. A., Merrick W. C., Shatkin A. J. A polypeptide in eukaryotic initiation factors that crosslinks specifically to the 5'-terminal cap in mRNA. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4843–4847. doi: 10.1073/pnas.75.10.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N. Regulation of translation by poliovirus. Adv Virus Res. 1987;33:175–204. doi: 10.1016/s0065-3527(08)60318-8. [DOI] [PubMed] [Google Scholar]
- Svitkin Y. V., Agol V. I. Complete translation of encephalomyocarditis virus RNA and faithful cleavage of virus-specific proteins in a cell-free system from Krebs-2 cells. FEBS Lett. 1978 Mar 1;87(1):7–11. doi: 10.1016/0014-5793(78)80121-5. [DOI] [PubMed] [Google Scholar]
- Tahara S. M., Morgan M. A., Shatkin A. J. Two forms of purified m7G-cap binding protein with different effects on capped mRNA translation in extracts of uninfected and poliovirus-infected HeLa cells. J Biol Chem. 1981 Aug 10;256(15):7691–7694. [PubMed] [Google Scholar]
- Vialard J., Lalumière M., Vernet T., Briedis D., Alkhatib G., Henning D., Levin D., Richardson C. Synthesis of the membrane fusion and hemagglutinin proteins of measles virus, using a novel baculovirus vector containing the beta-galactosidase gene. J Virol. 1990 Jan;64(1):37–50. doi: 10.1128/jvi.64.1.37-50.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R., Rychlik W., Etchison D., Rhoads R. E. Amino acid sequence of the human protein synthesis initiation factor eIF-4 gamma. J Biol Chem. 1992 Nov 15;267(32):23226–23231. [PubMed] [Google Scholar]