Abstract
Introduction:
Testosterone replacement therapy (TRT) for male hypogonadism is rapidly gaining popularity and acceptance. Options include gels, injections, and implantable subcutaneous pellets.
Aims:
To determine rates of patientsatisfaction and reasons forpatient preferences in hypogonadal men on TRT.
Main Outcome Measures:
Patient satisfaction responses obtained via anonymous survey.
Methods:
An anonymous, prospectivesurvey was distributed to men presenting for TRT at an academic urology clinic. The survey was organized into multiple domains including patient satisfaction and treatment motivation.
Results:
Average patient age was 49±0.7years (n=382). Injectable testosterone was chosen by 53%, gel-based regimens by 31%, and pellets by 17%. Overall, 70% of patients were satisfied with their TRT and 14% reported dis-satisfaction. Satisfaction rates were similar between gels (68%), injections (73%), and implantable pellets (70%). Doctor recommendation was the sole significant reason forpatients preferring gel-based TRT (66% vs. 37% injection users vs. 31% pellet users). Injectable TRT was favored due to lower cost (35% vs. 21% gel users vs. 19% pellet users). Pellets were favored forease of use (64% vs. 44% injection users vs. 43% gel users) and convenience (58% vs. 26% injection users vs. 19% gel users).Pellets had increased rates of satisfaction within the first 12 months. Improvements in concentration and moodoccurred at higher percentagesin satisfied patients.
Conclusions:
Patients are satisfied with TRT.Lower costs are important to patients on injections. Convenience and ease of use are central in choosing pellet therapy. Men on TRT should be questioned about mood and concentrationsince these factors exhibited the greatest improvements in satisfied patients.
Keywords: Testosterone replacement therapy, patient satisfaction, patient preferences, male hypogonadism
INTRODUCTION
Typified by low serum testosterone levels and pervasive, non-specific symptoms of diminished libido, fatigue, poor concentration, erectile dysfunction, lack of concentration, and depressed mood, male hypogonadism is currently characterized as a male health epidemic 1-5. Indeed, idiopathic age-related declines in testosterone affects a range between 5% and nearly 40% of men2, 6 with recognized associations between low levels of testosterone and obesity, diabetes, metabolic syndrome, dyslipidemia, osteoporosis, cardiovascular disease as well as all-cause mortality 7-17.
Testosterone replacement therapy (TRT)unequivocally increases levels of serum testosterone 18-26. In males,prepubertal-onset hypogonadotropic hypogonadism, can also gradually lead to the development of secondary sexual characteristics 27. Additionally, clinicians note improvements in quality of life 28-32 as well as body weight and waist circumference occur33, 34. In recent years, increased awareness of male hypogonadism and the benefits of TRT, partially driven by the media and pharmaceutical marketing strategies, have led more men to seek diagnosis and treatment. In a recent study examining prescribing patterns in the United States, 2.91% of men aged greater than 40 years, and 3.75% of men greater than 60 years of age, were prescribed some form of TRT 35. Indeed, the current popularity of TRT is evidenced by the fact that testosterone prescriptions have increased by over 170% since 2007 and 500% since 19936, 36, 37.
Presently, several American Food and Drug Administration (FDA)-approved methods of TRT exist including transdermal gel formulations, intramuscular injectables, and subcutaneous testosterone pellets. Each modality has its own advantages based upon their method of administration, pharmacokinetic, economic, and safety profiles. For example, while injectable TRTis traditionally cheaper than other methods,itis associated with increased variability in testosterone levels over time24. Specifically, following injection of 200 mg intramuscular testosterone cypionate, a threefold increase in serum testosterone is observed between days 2-5 with basal levels returning by day 13-14 24.On the contrary, gel formulations provide more stable testosterone levels38 but tend to be more costly and are possibly transferred to others via skin-to-skin contact39. Implantable subcutaneous pellets (Testopel; Auxilium Pharmaceuticals, Malvern, PA) have the advantage of only requiring treatment every three to four months, but are relatively expensive and negatethe ability to control a patients exogenous testosterone dose within this window of time21, 25. The testosterone formulations discussed within this study are limited to those available only in the United States. Other options for treatment in hypogonadal men include anti-estrogens, selective estrogen receptor modulators as well as human chorionic gonadotropin40.
In spite of the rapidly increasing diagnosis of hypogonadism and the acceptance of TRTwithin the medical community and general population 21, 24, 25, 41, the factors contributing to patient preferences and satisfaction with TRThave yet to be described. As such, the current study was devised with the goal of giving physicians a perspective into the reasons why patients in the United States choose specific methodsof TRT and how this choice impacts patient satisfaction.
METHODS
Following approval by the Institutional Review Board (IRB),an anonymous, prospective survey was distributed to all patients with a diagnosis of “hypogonadism” listed as the reason for their follow-up clinic appointment. The survey was self-administered in aphysician’s officewaiting room. The physician is a high-volume, academic urologist with a specialization in Andrology located in the United States. As such, all testosterone formulations prescribed, and discussed within the manuscript, are limited to those available in America. Written instructions were provided explaining the purpose of the questionnaire and emphasizing its confidential and anonymous nature. A private and secure drop box was located in the clinic waiting room to assure patients that no physician would have access to the questionnaire prior to the appointment. The box was locked and emptied daily. The surveys were stored in a secured cabinet with the collected data encrypted on password-protected computers in accordance with IRB policy.
Patients were excluded from analysis if their surveys contained multiple incomplete or conflicting responses and if the respondents indicated that they were not currently using TRT for hypogonadism at the time of survey completion. In some instances, patients documented only certain parameters (i.e. height but not weight). When this occurred, resultswere compiled using only those patients where complete data were obtained. Within the tables, the number of patients included is listed in parenthesis. For parameterssuch as for body mass index (BMI) where values were dependent on the patient responding to two questions (i.e. height and weight), the value was not calculated if a patient neglected to answer one of the necessary components.
The survey was designed to assess all patients presenting for TRT and included basic patient demographics such as age, sexual orientation, marital status, level of education, and income. Further questions examined the patient’s current modality of TRT and specifically focused on the domains of self-worth, embarrassment, satisfaction and motivation. The majority of the questions produced single-answer responses via multiple choice or yes/no answers. Several questionsgave patients the option to provide additional written details. Assessment of energy, libido, mood, muscle mass, and concentration were conducted via self-report. Patients were given a chart and asked to check the most-representative section. The question was phrased as: “Consider the following while on your current testosterone supplement” and the aforementioned aspects were assessed within the context of “No improvement”, “Some improvement” and “Lots of improvement”. Data were stratified according to patients’ self-reports of the modality of TRT currently prescribed.
Data were tabulated and organized using Microsoft Excel (Microsoft, Redmond, WA). Prism 6 and QuickCalcs(GraphPad Software Inc.; La Jolla, CA) were used to perform Chi-square testing, Yates correction, Fisher’s exact test and linear regression analysis. A p-value of ≤0.05 was considered statistically significant and all values were reported as mean ± SEM unless otherwise noted.
RESULTS
A total of 382 completed surveys were collected and available for analysis. The average patient age was 49.2±0.67years (Table 1). Overall, the patient cohort had an overweight BMI with the majorityself-described as college-educated, married, heterosexual and childless with annual income greater than $75,000(Table 1).
Table 1.
Patient Demographics.
| Patient Cohort | |
|---|---|
| Total number (n) | 382 |
| Age at time of evaluation (years±SEM) | 49.2±0.67 (n=382) |
| Weight (kg±SEM) | 95.9±0.82 (n=373) |
| Height (cm±SEM) | 180.5±0.37 (n=377) |
| BMI (kg/m2) | 29.4±0.22 (n=373) |
|
Sexual Orientation (n=382):
Heterosexual Homosexual Bisexual |
96.3% (n=368) 3.4% (n=13) 0.3% (n=1) |
|
Marital Status (n=381):
Single Married Divorced Living with partner |
15.5% (n=58) 70.8% (n=270) 8.7% (n=33) 5% (n=19) |
|
Number of children at home (n=382):
0 1-2 3-5 >6 |
57.9% (n=221) 35.9% (n=137) 5.7% (n=22) 0.5% (n=2) |
|
Current Annual Income (n=364):
<$25,000 $25,000-$50,000 $50,001-$75,000 $75,001-$100,000 $100,001-$150,000 $150,000-$200,000 >$200,001 |
3.3% (n=12) 4.1% (n=15) 15.9% (n=58) 21.4% (n=78) 20.1% (n=73) 15.7% (n=57) 19.5% (n=71) |
|
Highest level of education (n=380):
Grade School High School Some College/University College/University Graduate Level |
0.8% (n=3) 7.9% (n=30) 23.7% (n=90) 41.8% (n=159) 25.8% (n=98) |
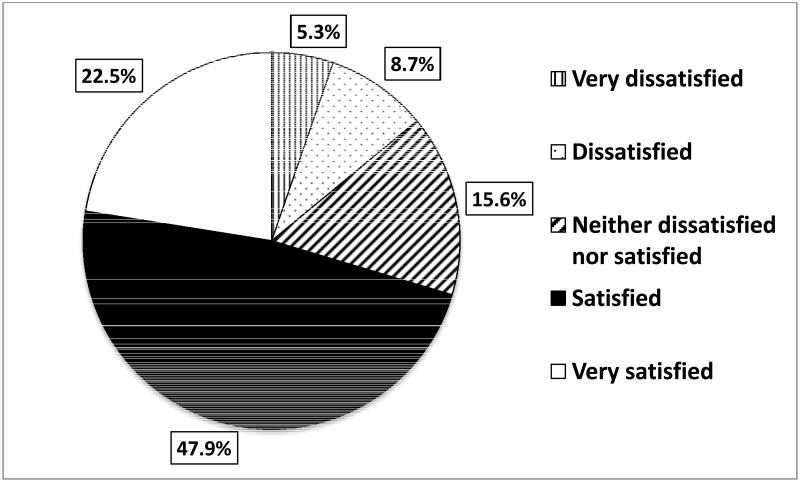
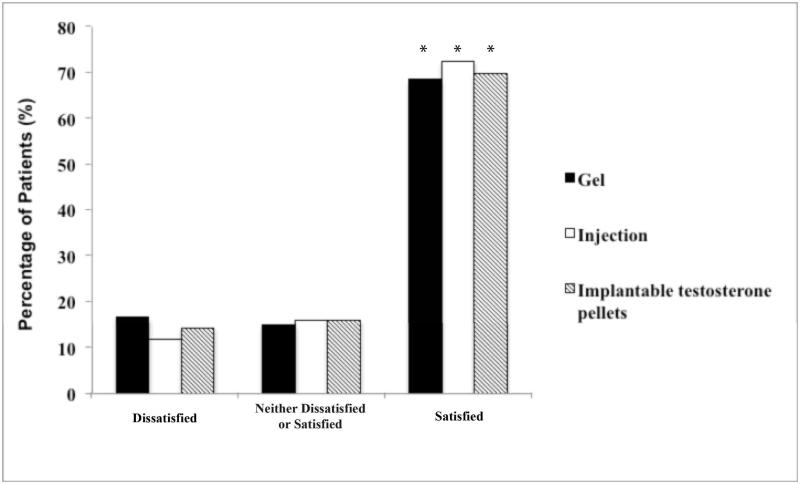
Of the patients who indicated that they were currently on TRT, injectable testosterone formulations were used by 52.5% (n=196), gel-based regimens by 30.6% (n=114),and implantable testosterone pellets by 16.9% (n=63). Overall, 70.4% of patients were satisfied with their current TRT, while 14.0% were dissatisfied (Figure 1A). When stratified by the modality of TRT, satisfaction rates were similar between those using gels (68.4%), injections (72.5%) and implantable pellets (69.8%)(Figure 1B). The overallnumber of satisfied patients was significantly greater than those who were in the ‘dissatisfied’ group (gel: p<0.0001, injection: p<0.0001, pellets:p=0.0005) (Figure 1B).
Figure 1A.
Patients on TRT for hypogonadism are highly satisfied overall.
Figure 1B.
Patients are satisfied regardless of the modality of TRT employed (*, p<0.05 for all modalities of TRT when compared to patients who were ‘Dissatisfied’ and ‘Neither Dissatisfied or Satisfied’)
Gel-based regimens were preferred primarily due to ‘Doctor Recommendation’(65.7%, Table 2). This was significant compared to both injections (37.1%, p<0.0001) and pellet-based TRT (31.3%, p<0.0001) (Table 2). Cost was a statistically significant reason for a patient’s preference for injectable therapy (34.5%) over gel- (21.3%, p=0.023) and pellet- (18.8%, p=0.0264) based approaches (Table 2).Pellet users rated ‘ease of use’ as the most important reason for their choices (64.1%). This was significantly different from both the injection (43.8%, p=0.0077) and gel (42.6%, p=0.0103) subgroups (Table 2). Similarly, convenience was rated as a reason for preference in 57.8% of pellet-based regimens. This was significantly higher than gel- (19.4%, p<0.0001) and injection- (26.3%, p<0.0001) based TRT.
Table 2.
Patient satisfaction 17 with TRT is partially due to factors that are unique to the chosen 18 modality.
| Reasons for Preference | Gel (n=108) | Injection (n=194) | Pellet (n=64) |
|---|---|---|---|
| Cost | 21.3% (n=23) * | 34.5% (n=67) $,# | 18.8% (n=12) * |
| Ease of use | 42.6% (n=46) # | 43.8% (n=85) # | 64.1% (n=41) *,$ |
| Ability to raise testosterone levels | 26.9% (n=29) * | 42.8% (n=83) $ | 34.4% (n=22) |
| Improvement in symptoms | 21.3% (n=23) * | 41.2% (n=80) $ | 32.8% (n=21) |
| Doctor recommendation | 65.7% (n=71) #,* | 37.1% (n=72) $ | 31.3% (n=20) $ |
| Concern for transference | 0.1% (n=1) *,# | 28.4% (n=55) $ | 37.5% (n=24) $ |
| Convenience | 19.4% (n=21) # | 26.3% (n=51) # | 57.8% (n=37) *,$ |
A significance of p<0.05 is shown between groups and are depicted as:
vs. injection,
vs. Gel,
vs. Pellets.
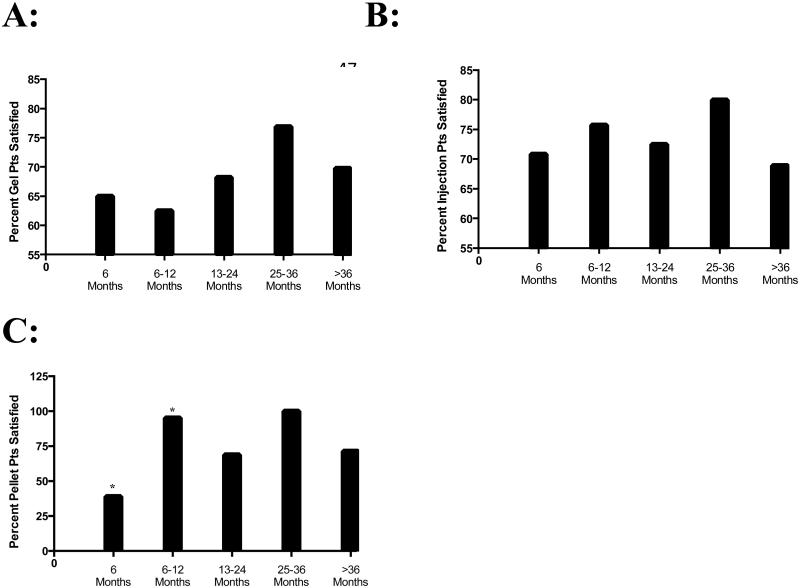
Overallpatient satisfactionand dissatisfaction did not change with increased duration of TRT (Table 3). When the rates within each discreet time-block were compared, patients on pellet-based therapy were significantly less satisfied than those patients on injections (p=0.02; Table 3) when first beginning therapy (<6 months). Satisfaction rates achieved a plateau between the two modalities after this initial change (Table 3). In the 6-12 month time block, patients on pellet-based TRT were significantly more satisfied compared to gel-based TRT (p=0.03; Table 3). Again, these rates of satisfaction reached a plateau after this point. The rates of patient dissatisfaction were not significantly different at any time point or across any modality of TRT (Table 3). When subdivided based upon TRT modality, the most significant change occurred in the group of patients on pellet-based therapy between <6 months and 6-12 months (p=0.0003; Figure 2). The other modalities of TRT did not exhibit any significant changes over time.
Table 3.
Patient satisfaction and dissatisfaction with various modalities of TRT. When each distinct time-point was considered, significance was noted in the rates of satisfaction in patients on Pellets compared to Injections at <6 months as well as between the Gel and Pellet patients at the 6-12 months time point. Rates of dis-satisfaction were similar between all modalities.
| <6 months | 6-12 months | 13-24 months | 25-36 months | >36 months | |
|---|---|---|---|---|---|
|
| |||||
|
Overall Satisfied
Patients (n=264) |
62.8% (n=54/86) | 78.4% (n=58/74) | 70.8% (n=63/89) | 79.3% (n=23/29) | 69.5% (n=66/95) |
|
| |||||
|
Satisfaction based
on TRT: |
|||||
| Gel Satisfied | 65.0% (n=13/20 | 62.5% (n=10/16)# | 68.2% (n=15/22) | 76.9% (n=10/13) | 69.8% (n=30/43) |
| Gel Dissatisfied | 5% (n=1/20) | 18.8% (n=3/16) | 22.7% (n=5/22) | 15.4% (n=2/13) | 18.6% (n=8/43) |
| Pellet Satisfied | 38.9% (n=7/18)* | 95.2% (n=20/21)$ | 68.8% (n=11/16) | 100% (n=1/1) | 71.4% (n=5/7) |
| Pellet Dissatisfied | 16.7% (n=3/18) | 4.8% (n=1/21) | 25% (n=4/16) | 0% (n=0/1) | 14.3% (n=1/7) |
| Injection Satisfied | 70.8% (n=34/48)# | 75.7% (n=28/37) | 72.5% (n=37/51) | 80.0% (n=12/15) | 68.9% (n=31/45) |
| Injection Dissatisfied | 6.3% (n=3/48) | 16.2% (n=6/37) | 13.7% (n=7/51) | 6.7% (n=1/15) | 13.3% (n=6/45) |
A significance of p<0.05 was depicted as:
vs. injection,
vs. Gel,
vs. Pellets.
Figure 2. Changes in patient satisfaction over time.
Patients on gel- (Panel A) and injection (Panel B)-based TRT regiments exhibited consistent trends of patient satisfaction over time. Patients on pellet (Panel C)–based TRT exhibited a significant (*, p=0.0003) improvement from 6 to 6-12 months followed by a plateau in their rates of satisfaction over a 36-month period.
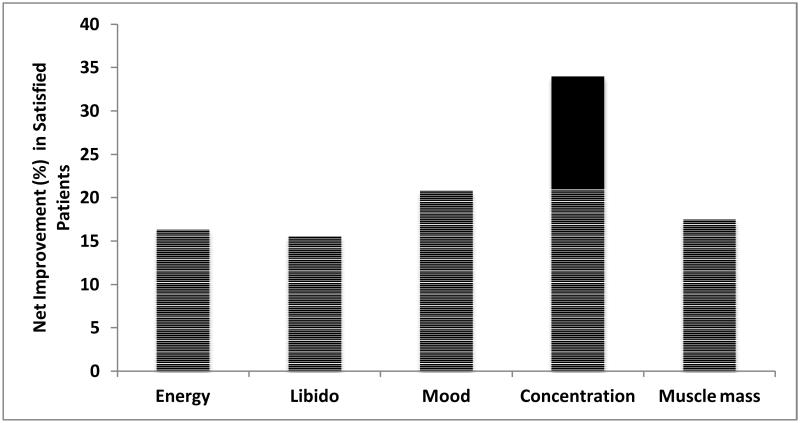
When factors contributing to patient satisfaction with TRT were examined, satisfied patients exhibited higher rates of improvement in all domains assessed (Figure 3). Specifically, satisfied patients (n=266) reported statistically significant improvements in comparison to dissatisfied patients (n=53) in energy (95.5% vs. 79.2%, p<0.001), libido (92.9% vs. 77.4%, p<0.01), mood (88.7% vs. 67.9%, p<0.001), concentration (77.4% vs. 43.4%, p<0.0001), and muscle mass (74.1% vs. 56.6%, p<0.05). When the net difference was calculated, the percentage of patients reporting improvement in the domains of mood and concentration were the greatest (20.8% and 34% respectively; Figure 3).
Figure 3.
61 Satisfied patients exhibited the greatest improvements in mood and concentration 62 compared to dissatisfied patients
DISCUSSION
Population-based estimates have identified the prevalence of hypogonadism within the North American population to lie within the 12 to 37% range 6, 42. Recent studies have noted that in men 40 years of age or greater, the use of TRT has increased from 0.81% in 2001 to 2.91% in 2011 35. Furthermore, the discovery of links between testosterone and numerous diseases states such as atherosclerosis and metabolic syndrome 7, 15-17, 43, 44, will no doubt contribute to increased TRT prescriptions in the future35.
Numerous methods of TRT are available; however, very few studies 25currently exist to document the reasons why patients choose certain modalities and whether or not they are satisfied with their choices. Very recently, Smith et al.25 examined the factors influencing patient decisions to initiate and discontinue subcutaneous testosterone pellets. From that work, it can be inferred that the 28.3% of men who stopped using pellets were dissatisfied 25. When this subgroup was looked at in more detail, 50% of the patients cited cost as the primary reason for discontinuation while pain at the time of procedure (18.7%) or persistent pain following implantation (21.8%) were also mentioned. These findings echo those in the current study where cost was a significant reason for a patient’s preference for injectable therapy over gel- and pellet-based approaches. Similarly, pellet users in this cohort rated ‘ease of use’ as the most important reason for their choices. Of the 382 men surveyed in this study, 70.4% reportedgood overall rates of satisfaction. Similar satisfaction rates werefound between the different formulations (i.e. gels, injectables and pellets) suggesting that the vast majority of hypogonadal patients on TRT are happy andexperience symptomatic improvementswhile on TRT.
A recent study noted that 18.63% of first incident users in the United States filled only one prescription; suggesting that this portion of men failed to see any significant improvements35. It is thus tempting to speculate that the patients we found to be ‘very dissatisfied’ (5.3%) and ‘dissatisfied’ (8.7%) were actually captured at the time of their early follow-up appointments. Physicians should thus question patients during their early follow-up visits to ascertain levels of satisfaction.If ‘dissatisfied’, the physician should direct the patient to a more appropriate method of TRT based on individual patient goals; leading to improved symptom control and better rates of satisfaction with fewer dropouts.Other reasons for patient dissatisfaction may lie within the realms of patient physiology and expectations. Previous work has shown that while total serum and free testosterone was highly reproducible within individuals, the differences varied markedly between men 45. Accordingly, not all individuals perceive symptoms at the same levels of serum testosterone. Furthermore, differenthypogonadal symptoms mayimprove with varying degrees of rapidity following TRT 46. As such, the rates of patient dissatisfaction observed in the current study may be affected by an individual patients desire for symptom improvement in a particular symptom domain. For example, since the effects on sexual interest appear 3 weeks after TRT but the changes in erections/ejaculation may require up to 6 months 46, patients who desire improvement in the latter may be dissatisfied if it does not occur as promptly as improvements in other symptoms.
Testosterone has been used to treat hypogonadism since the 1930s witha multitude of safe and effective TRT options available47. While direct absorptive transdermal skin patch products exist (i.e. Androderm, Watson Pharmceuticals Inc.), local irritation has proven to be a detriment 48. Since no oral modalities are currently approved for use in the United States49, gels, injectables and implantable pellets represent the currently available forms of TRT.At present, testosterone gels have the highest rate of overall prescription35 but are also known to have high rates of patient drop-out 50. Gel-based TRTshave been specifically shown to improve sexual dysfunction 51and sub-threshold depression in men with hypogonadism 52. In the current study, gels were used by 30.6% of patients. Work by Schoenfeldet al. 50 has found that adherence to topical TRT is traditionally low and that by six months post prescription, only 37.4% of men continue on the medication. This number dropped further to 15.4% after 12 months 50. The reasons for the high discontinuation rates with gels are unknown; however, in our experience, the rates of satisfaction did not vary significantly according to the modality of TRT employed (Figure 1B).
This is less than the 52.5% of patients using injections but greater than the 16.9% using subcutaneous pellets. This is in contrast to the prescribing trends observed using data from the nations largest commercial American health insurance populations 35. It does correspond, however, to the numbers observed in other studies evaluating TRT 18. Indeed, prescribing patterns from American employment-based commercial insurance plans 35, cannot be directly extrapolated to general population cohorts, such as the ones seen in the current study, especially in light of our findings that cost plays a significant factor in patients usage of injectable-based TRT. Furthermore, the findings discussed within this study are limited to those available in the United States. Other types of TRT not available in America, such as long acting testosterone undecanoate, have also shown excellent rates of compliance and improvements in the intercourse and overall satisfaction domains of the International Index of Erectile Function 53 as well as consistent losses in body weight, waist circumference and body mass index 54, 55.
The current study also found that, in our population cohort, patient preference for gels was largely driven by physician recommendation (Table 2). This was significantly different when compared to injections and pellets. Potential explanations for this may include factors such as ease of use (42.6%) and effectiveness (26.9%). Traditionally, in our practice, we offer gel-based therapies to older; hypogonadal men who have no young children at home, thus decreasing the risk of transference. Since the average age of our cohort was younger (49.2 years) and a large proportion (35.9%) had young children at home, this may have also influenced our findings in that gel-based TRT may not have been the recommended option.
One limitation that deserves consideration is that this study did not discriminate between the different types of gelscurrently available. This is important in that previous studies have shown that different gels can have different efficacies23. Indeed, Groberet al.23found that 20% of men had a suboptimal clinical or biochemical responses to theirinitial alcohol-based gel selection23. The authors attributed this to the pentadecalactone emollient that is specific to Testim22, 23 and noted that total and free serum testosterone levels increased after switching from Androgel to Testim but not from Testim to Androgel23. Unfortunately, our study did not capture those men who were testosterone gel non-responders. Given theanonymous nature of the survey, testosterone levels could not be compared. It is therefore possible that unsatisfied patientsmight have been part of the testosterone‘non-responder’ sub-group. A further limitation includes the patient population surveyed. They are highly educated with high incomes (Table 1) and as such, those patient preferences tend to predominate in our patient cohort.
Subcutaneous implantable pellets were used by 16.9% of our patient population. The majority of these patients cited ease of use (64.1%) and convenience (57.8%) as the major reasons for their satisfaction with the product (Table 2).Previous work showed that the majority of patients on pellet-based therapy are typically switched from either gel-, or injectable-based therapies25 but no studies exist documenting satisfaction. The majority of the research on pellet-based TRT has focused on detailing the pharmacokinetics of the delivery system.In one such study, Pastuszaket al.21 found that men with a body mass index (BMI) >25 kg/m2 attained lower testosterone peaks with slower decay pharmacokinetics than men with BMI <25 kg/m2 and those with 10-12 pellets implanted exhibited higher levels of estradiol 21.Another study by McCullough et al. 20identified that implantation of greater than six pellets resulted in therapeutic testosterone levels (said to be >300 mg) at 1 month. Patients were then able to maintain this acceptable range for approximately 4-6 months20. Higher pellet levels achieved higher and more consistent levels of testosterone 20. Since our study did not stratify patients based on the number of implanted pellets or BMI, it is possible that these factors may have also affected our findings. Furthermore, a previous study by Moiseyet al. 56 investigated the kinetics of testosterone following intramuscular injection of testosterone undecanoate. The authors found that body size (i.e. body weight, BMI and body surface area) negatively correlated with total serum testosterone levels; suggesting that men of increased body weight required more testosterone to achieve similar levels 56. While BMI was recorded in our study population, we did not stratify patient satisfaction results on this basis but future work could easily be conducted to address this issue.
The majority of the patients in the current study were treated with injection-based TRT (52.5%). Interestingly, the main reasons for patient satisfaction on injectable-based TRT were not as clearly defined as those patients on either gel- or pellet-based TRT (Table 2). One factor that was identified as a significant advantage compared to the other modalities of TRT was the cheaper cost of injectable-based TRT(Table 2). Other factors that may contribute include the variability of dosages. For example, one study detailed the use of 100 mg per week at intervals of 1-3 weeks based on patient preference or response 18. In the current study, doses of 200 mg per week were routinely used in the majority of patients. Another aspect to consider is the wide variation of circulating androgen levels resulting from injectable-based TRT. Indeed, as documented by Nankin24, bi-weekly injections of 200 mg testosterone cypionate resulted in rapid highs on days two to five post-injection followed by a prompt return to baseline by day 13-14. As such, this peak and valley cycling of serum testosterone levels may contribute to a patient’s sense ofwell-being and satisfaction; a factor that should be addressed in future studies.
The current study also revealed that satisfied patients report improvements in the domains of energy, libido, concentration, mood, and muscle mass at a significantly higher rate than patients who are dissatisfied. Concentration and mood appear to be the biggest determinants of patient satisfaction, as these two parameters exhibited the largest percentage of improvement among satisfied patients in comparison to dissatisfied patients. Therefore, it is highly relevant to assess improvement in these two domains during follow-up visits of patients on TRT, especially in dissatisfied patients.
CONCLUSIONS
Hypogonadal patients on TRT have high rates of satisfaction (~70%) that is independent of the modality employed. Gel and pellet-based TRT may befavorable for long-term therapy given the correlation between patient satisfaction and increased duration of treatment. Improvements in mood and concentration are highly important factors in satisfied patients and clinical follow-up should specifically inquire about these symptomatic improvements.
ACKNOWLEDGEMENTS
J.R. Kovac is a scholar supported by a Male Reproductive Health Research (MRHR) Career Development Physician-Scientist Award (K12, HD073917-01) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Program awarded to Dolores J. Lamb (DJL). DJL is supported by NIH grants P01HD36289 from the Eunice Kennedy Shriver NICHD and 1R01DK078121 from the National Institute of Kidney and Digestive Diseases. The authors would like to thankMarshall Gonzalez(PA-C) for assistance with data collection and analysis.
Abbreviations
- BMI
Body mass index
- ED
erectile dysfunction
- TRT
testosterone replacement therapy
Footnotes
Conflict of Interest: none
REFERENCES
- 1.Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in adult men with androgen deficiency syndromes: an endocrine society clinical practice guideline. The Journal of clinical endocrinology and metabolism. 2006;91:1995–2010. doi: 10.1210/jc.2005-2847. [DOI] [PubMed] [Google Scholar]
- 2.Wu FC, Tajar A, Beynon JM, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. The New England journal of medicine. 2010;363:123–35. doi: 10.1056/NEJMoa0911101. [DOI] [PubMed] [Google Scholar]
- 3.Traish AM, Miner MM, Morgentaler A, Zitzmann M. Testosterone deficiency. The American journal of medicine. 2011;124:578–87. doi: 10.1016/j.amjmed.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 4.Moskovic DJ, Araujo AB, Lipshultz LI, Khera M. The 20-year public health impact and direct cost of testosterone deficiency in U.S. men. The journal of sexual medicine. 2013;10:562–9. doi: 10.1111/j.1743-6109.2012.02944.x. [DOI] [PubMed] [Google Scholar]
- 5.Buvat J, Maggi M, Guay A, Torres LO. Testosterone deficiency in men: systematic review and standard operating procedures for diagnosis and treatment. The journal of sexual medicine. 2013;10:245–84. doi: 10.1111/j.1743-6109.2012.02783.x. [DOI] [PubMed] [Google Scholar]
- 6.Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. International journal of clinical practice. 2006;60:762–9. doi: 10.1111/j.1742-1241.2006.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maggio M, Basaria S. Welcoming low testosterone as a cardiovascular risk factor. International journal of impotence research. 2009;21:261–4. doi: 10.1038/ijir.2009.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giannoulis MG, Martin FC, Nair KS, Umpleby AM, Sonksen P. Hormone replacement therapy and physical function in healthy older men. Time to talk hormones? Endocrine reviews. 2012;33:314–77. doi: 10.1210/er.2012-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocrine reviews. 2005;26:833–76. doi: 10.1210/er.2004-0013. [DOI] [PubMed] [Google Scholar]
- 10.Zitzmann M, Faber S, Nieschlag E. Association of specific symptoms and metabolic risks with serum testosterone in older men. The Journal of clinical endocrinology and metabolism. 2006;91:4335–43. doi: 10.1210/jc.2006-0401. [DOI] [PubMed] [Google Scholar]
- 11.Kupelian V, Page ST, Araujo AB, Travison TG, Bremner WJ, McKinlay JB. Low sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in nonobese men. The Journal of clinical endocrinology and metabolism. 2006;91:843–50. doi: 10.1210/jc.2005-1326. [DOI] [PubMed] [Google Scholar]
- 12.Agledahl I, Skjaerpe PA, Hansen JB, Svartberg J. Low serum testosterone in men is inversely associated with non-fasting serum triglycerides: the Tromso study. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2008;18:256–62. doi: 10.1016/j.numecd.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 13.Hak AE, Witteman JC, de Jong FH, Geerlings MI, Hofman A, Pols HA. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam study. The Journal of clinical endocrinology and metabolism. 2002;87:3632–9. doi: 10.1210/jcem.87.8.8762. [DOI] [PubMed] [Google Scholar]
- 14.Giagulli VA, Kaufman JM, Vermeulen A. Pathogenesis of the decreased androgen levels in obese men. The Journal of clinical endocrinology and metabolism. 1994;79:997–1000. doi: 10.1210/jcem.79.4.7962311. [DOI] [PubMed] [Google Scholar]
- 15.Corona G, Rastrelli G, Balercia G, Sforza A, Forti G, Maggi M. Testosterone and cardiovascular risk in patients with erectile dysfunction. Journal of endocrinological investigation. 2012;35:809–16. doi: 10.3275/8063. [DOI] [PubMed] [Google Scholar]
- 16.Corona G, Rastrelli G, Vignozzi L, Mannucci E, Maggi M. Testosterone, cardiovascular disease and the metabolic syndrome. Best practice & research Clinical endocrinology & metabolism. 2011;25:337–53. doi: 10.1016/j.beem.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Corona G, Rastrelli G, Morelli A, Vignozzi L, Mannucci E, Maggi M. Hypogonadism and metabolic syndrome. Journal of Endocrinology Investigation. 2011;34:557–67. doi: 10.3275/7806. [DOI] [PubMed] [Google Scholar]
- 18.Rhoden EL, Morgentaler A. Symptomatic response rates to testosterone therapy and the likelihood of completing 12 months of therapy in clinical practice. The journal of sexual medicine. 2010;7:277–83. doi: 10.1111/j.1743-6109.2009.01544.x. [DOI] [PubMed] [Google Scholar]
- 19.von Eckardstein S, Nieschlag E. Treatment of male hypogonadism with testosterone undecanoate injected at extended intervals of 12 weeks: a phase II study. Journal of andrology. 2002;23:419–25. [PubMed] [Google Scholar]
- 20.McCullough AR, Khera M, Goldstein I, Hellstrom WJ, Morgentaler A, Levine LA. A multi-institutional observational study of testosterone levels after testosterone pellet (Testopel((R))) insertion. The journal of sexual medicine. 2012;9:594–601. doi: 10.1111/j.1743-6109.2011.02570.x. [DOI] [PubMed] [Google Scholar]
- 21.Pastuszak AW, Mittakanti H, Liu JS, Gomez L, Lipshultz LI, Khera M. Pharmacokinetic evaluation and dosing of subcutaneous testosterone pellets. Journal of andrology. 2012;33:927–37. doi: 10.2164/jandrol.111.016295. [DOI] [PubMed] [Google Scholar]
- 22.Marbury T, Hamill E, Bachand R, Sebree T, Smith T. Evaluation of the pharmacokinetic profiles of the new testosterone topical gel formulation, Testim, compared to AndroGel. Biopharmaceutics & drug disposition. 2003;24:115–20. doi: 10.1002/bdd.345. [DOI] [PubMed] [Google Scholar]
- 23.Grober ED, Khera M, Soni SD, Espinoza MG, Lipshultz LI. Efficacy of changing testosterone gel preparations (Androgel or Testim) among suboptimally responsive hypogonadal men. International journal of impotence research. 2008;20:213–7. doi: 10.1038/sj.ijir.3901618. [DOI] [PubMed] [Google Scholar]
- 24.Nankin HR. Hormone kinetics after intramuscular testosterone cypionate. Fertility and sterility. 1987;47:1004–9. [PubMed] [Google Scholar]
- 25.Smith RP, Khanna A, Coward RM, et al. Factors Influencing Patient Decisions to Initiate and Discontinue Subcutaneous Testosterone Pellets (Testopel®) for Treatment of Hypogonadism. Journal of Sexual Medicine. 2013 doi: 10.1111/jsm.12226. [DOI] [PubMed] [Google Scholar]
- 26.Smith RP, Coward RM, Kovac JR, Lipshultz LI. The evidence for seasonal variations of testosterone in men. Maturitas. 2013 doi: 10.1016/j.maturitas.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Giagulli VA, Triggiani V, Carbone MD, et al. The role of long-acting parenteral testosterone undecanoate compound in the induction of secondary sexual characteristics in males with hypogonadotropic hypogonadism. The journal of sexual medicine. 2011;8:3471–8. doi: 10.1111/j.1743-6109.2011.02497.x. [DOI] [PubMed] [Google Scholar]
- 28.Snyder PJ, Peachey H, Berlin JA, et al. Effects of testosterone replacement in hypogonadal men. The Journal of clinical endocrinology and metabolism. 2000;85:2670–7. doi: 10.1210/jcem.85.8.6731. [DOI] [PubMed] [Google Scholar]
- 29.Wang C, Cunningham G, Dobs A, et al. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. The Journal of clinical endocrinology and metabolism. 2004;89:2085–98. doi: 10.1210/jc.2003-032006. [DOI] [PubMed] [Google Scholar]
- 30.Yassin AA, Saad F. Improvement of sexual function in men with late-onset hypogonadism treated with testosterone only. The journal of sexual medicine. 2007;4:497–501. doi: 10.1111/j.1743-6109.2007.00442.x. [DOI] [PubMed] [Google Scholar]
- 31.Wang C, Alexander G, Berman N, et al. Testosterone replacement therapy improves mood in hypogonadal men--a clinical research center study. The Journal of clinical endocrinology and metabolism. 1996;81:3578–83. doi: 10.1210/jcem.81.10.8855804. [DOI] [PubMed] [Google Scholar]
- 32.Moncada I. Testosterone and men's quality of life. The aging male : the official journal of the International Society for the Study of the Aging Male. 2006;9:189–93. doi: 10.1080/13685530601003180. [DOI] [PubMed] [Google Scholar]
- 33.Heufelder AE, Saad F, Bunck MC, Gooren L. Fifty-two-week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. Journal of andrology. 2009;30:726–33. doi: 10.2164/jandrol.108.007005. [DOI] [PubMed] [Google Scholar]
- 34.Haider A, Gooren LJ, Padungtod P, Saad F. Improvement of the metabolic syndrome and of non-alcoholic liver steatosis upon treatment of hypogonadal elderly men with parenteral testosterone undecanoate. Experimental and clinical endocrinology & diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association. 2010;118:167–71. doi: 10.1055/s-0029-1202774. [DOI] [PubMed] [Google Scholar]
- 35.Baillargeon J, Urban RJ, Ottenbacher KJ, Pierson KS, Goodwin JS. Trends in Androgen Prescribing in the United States, 2001 to 2011. JAMA Internal Medicine. 2013 doi: 10.1001/jamainternmed.2013.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nigro N, Christ-Crain M. Testosterone treatment in the aging male: myth or reality? Swiss medical weekly. 2012;142:w13539. doi: 10.4414/smw.2012.13539. [DOI] [PubMed] [Google Scholar]
- 37.Bassil N, Alkaade S, Morley JE. The benefits and risks of testosterone replacement therapy: a review. Therapeutics and clinical risk management. 2009;5:427–48. doi: 10.2147/tcrm.s3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swerdloff RS, Wang C, Cunningham G, et al. Long-term pharmacokinetics of transdermal testosterone gel in hypogonadal men. The Journal of clinical endocrinology and metabolism. 2000;85:4500–10. doi: 10.1210/jcem.85.12.7045. [DOI] [PubMed] [Google Scholar]
- 39.de Ronde W. Hyperandrogenism after transfer of topical testosterone gel: case report and review of published and unpublished studies. Hum Reprod. 2009;24:425–8. doi: 10.1093/humrep/den372. [DOI] [PubMed] [Google Scholar]
- 40.Corona G, Rastrelli G, Vignozzi L, Maggi M. Emerging medication for the treatment of male hypogonadism. Expert opinion on emerging drugs. 2012;17:239–59. doi: 10.1517/14728214.2012.683411. [DOI] [PubMed] [Google Scholar]
- 41.McNicholas TA, Dean JD, Mulder H, Carnegie C, Jones NA. A novel testosterone gel formulation normalizes androgen levels in hypogonadal men, with improvements in body composition and sexual function. BJU international. 2003;91:69–74. doi: 10.1046/j.1464-410x.2003.04016.x. [DOI] [PubMed] [Google Scholar]
- 42.Araujo AB, O'Donnell AB, Brambilla DJ, et al. Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from the Massachusetts Male Aging Study. The Journal of clinical endocrinology and metabolism. 2004;89:5920–6. doi: 10.1210/jc.2003-031719. [DOI] [PubMed] [Google Scholar]
- 43.Corona G, Rastrelli G, Monami M, et al. Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. European journal of endocrinology / European Federation of Endocrine Societies. 2011;165:687–701. doi: 10.1530/EJE-11-0447. [DOI] [PubMed] [Google Scholar]
- 44.Corona G, Monami M, Rastrelli G, et al. Type 2 diabetes mellitus and testosterone: a meta-analysis study. International journal of andrology. 2011;34:528–40. doi: 10.1111/j.1365-2605.2010.01117.x. [DOI] [PubMed] [Google Scholar]
- 45.Kelleher S, Conway AJ, Handelsman DJ. Blood testosterone threshold for androgen deficiency symptoms. The Journal of clinical endocrinology and metabolism. 2004;89:3813–7. doi: 10.1210/jc.2004-0143. [DOI] [PubMed] [Google Scholar]
- 46.Saad F, Aversa A, Isidori AM, Zafalon L, Zitzmann M, Gooren L. Onset of effects of testosterone treatment and time span until maximum effects are achieved. European journal of endocrinology / European Federation of Endocrine Societies. 2011;165:675–85. doi: 10.1530/EJE-11-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Freeman ER, Bloom DA, McGuire EJ. A brief history of testosterone. The Journal of urology. 2001;165:371–3. doi: 10.1097/00005392-200102000-00004. [DOI] [PubMed] [Google Scholar]
- 48.McGriff NJ, Csako G, Kabbani M, Diep L, Chrousos GP, Pucino F. Treatment options for a patient experiencing pruritic rash associated with transdermal testosterone: a review of the literature. Pharmacotherapy. 2001;21:1425–35. doi: 10.1592/phco.21.17.1425.34428. [DOI] [PubMed] [Google Scholar]
- 49.Edelstein D, Sivanandy M, Shahani S, Basaria S. The latest options and future agents for treating male hypogonadism. Expert opinion on pharmacotherapy. 2007;8:2991–3008. doi: 10.1517/14656566.8.17.2991. [DOI] [PubMed] [Google Scholar]
- 50.Schoenfeld MJ, Shortridge E, Cui Z, Muram D. Medication adherence and treatment patterns for hypogonadal patients treated with topical testosterone therapy: a retrospective medical claims analysis. The journal of sexual medicine. 2013;10:1401–9. doi: 10.1111/jsm.12114. [DOI] [PubMed] [Google Scholar]
- 51.Chiang HS, Cho SL, Lin YC, Hwang TI. Testosterone gel monotherapy improves sexual function of hypogonadal men mainly through restoring erection: evaluation by IIEF score. Urology. 2009;73:762–6. doi: 10.1016/j.urology.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 52.Shores MM, Kivlahan DR, Sadak TI, Li EJ, Matsumoto AM. A randomized, double-blind, placebo-controlled study of testosterone treatment in hypogonadal older men with subthreshold depression (dysthymia or minor depression) The Journal of clinical psychiatry. 2009;70:1009–16. doi: 10.4088/jcp.08m04478. [DOI] [PubMed] [Google Scholar]
- 53.Hackett G, Cole N, Bhartia M, Kennedy D, Raju J, Wilkinson P. Testosterone replacement therapy with long-acting testosterone undecanoate improves sexual function and quality-of-life parameters vs. placebo in a population of men with type 2 diabetes. The journal of sexual medicine. 2013;10:1612–27. doi: 10.1111/jsm.12146. [DOI] [PubMed] [Google Scholar]
- 54.Yassin AA, Doros G. Testosterone therapy in hypogonadal men results in sustained and clinically meaningful weight loss. Clinical Obesity. 2013;3:73–83. doi: 10.1111/cob.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saad F, Haider A, Doros G, Traish A. Long-term treatment of hypogonadal men with testosterone produces substantial and sustained weight loss. Obesity. 2013 doi: 10.1002/oby.20407. [DOI] [PubMed] [Google Scholar]
- 56.Moisey R, Swinburne J, Orme S. Serum testosterone and bioavailable testosterone correlate with age and body size in hypogonadal men treated with testosterone undecanoate (1000 mg IM--Nebido) Clinical endocrinology. 2008;69:642–7. doi: 10.1111/j.1365-2265.2008.03251.x. [DOI] [PubMed] [Google Scholar]