Abstract
Placental malaria is a serious problem in sub-Saharan Africa. Young women are particular susceptible to contracting this form of malaria during their first or second pregnancy despite previously acquired immunity from past infections. Placental malaria is caused by Plasmodium falciparum parasites expressing VAR2CSA on the erythrocyte surface. This protein adheres to a low-sulfated chondroitin sulfate-A found in placental tissue causing great harm to both mother and developing fetus. In rare cases, the localization of infected erythrocytes to the placenta can even result in the vertical transmission of malaria. In an effort to better understand this infection, chondroitin sulfate was isolated from the cotyledon part of the placenta, which should be accessible for parasite adhesion, as well as two non-accessible parts of the placenta to serve as controls. The placental chondroitin sulfate structures and their VAR2CSA binding were characterized. All portions of human placenta contained sufficient amounts of the appropriate low-sulfated chondroitin sulfate-A to display high-affinity binding to a recombinant truncated VAR2CSA construct, as determined using surface plasmon resonance. The cotyledon is the only placental tissue accessible to parasites in the bloodstream, suggesting it is the primary receptor for parasite infected red blood cells.
Keywords: placental malaria, VAR2CSA, surface plasmon resonance, PfEMP1, chondroitin sulfate, disaccharide analysis
Introduction
Malaria is a serious health concern, endemic to 140 countries around the world. In 2012, the World Health Organization estimates there were approximately 219 million cases of malaria, resulting in 660,000 deaths.1 Malaria is caused by infection by any of five Plasmodium species; P. falciparum, P. vivax, P. ovale, P. malariae, or P. knowlesi. Infection by P. falciparum is the most deadly and predominates in Africa, where 90% of malaria deaths occur.1 What makes the P. falciparum parasite especially virulent is its unique ability to insert proteins, functioning as adhesins, into the membrane of the infected erythrocyte. These adhesins, called P. falciparum erythrocyte membrane protein 1 (PfEMP1) proteins, bind various receptors in the host microvasculature allowing the infected erythrocytes to sequester and avoid clearance in the spleen.2 People living in endemic regions acquire protective immunity to malaria as a function of age.3 Clinical immunity is correlated with the buildup of antibodies capable of inhibiting sequestration, by blocking the interaction between the expressed PfEMP1 proteins and its host receptors.4 Pregnant women are especially susceptible to infection, despite previously acquired immunity. There are numerous maternal and fetal complications associated with malaria in pregnant women including severe anemia, pulmonary edema, kidney failure, pre-eclampsia, low-birth weight, premature delivery, miscarriage, and death.5–10 Placental malaria is caused by a subgroup of parasites expressing a distinct PfEMP1 protein, called VAR2CSA, that enables the infected erythrocyte to adhere to chondroitin sulfate-A in the placenta.11–13 Immunity to placental malaria is developed over successive pregnancies and is correlated with the buildup of anti-VAR2CSA antibodies capable of blocking the VAR2CSA-chondroitin sulfate-A interaction.14–15 This supports the use of VAR2CSA in a vaccine against P. falciparum.
It has been shown that infected erythrocytes selected for placental binding adhere preferentially to a low sulfated form of chondroitin sulfate-A (chondroitin-4-sulfate) in the intervillious spaces of the placental.16 This region of the placenta is found in the cotyledons of the decidua basalis or the maternal side of the organ.17 It has further been suggested that the minimal structural requirement within this type of chondroitin sulfate-A is a dodecasaccharide sequence containing two to three 4-O-sulfated disaccharide units.18 VAR2CSA is a large protein (350 kDa) consisting of six Duffy binding-like domains (three DBLX followed by three DBL∈ domains), a cysteine-rich inter-domain region (termed CIDRPAM) between DBL2X and DBL3X, and a number of interdomains.19,20 The minimal chondroitin sulfate-A binding region in VAR2CSA is the ID1-DBL2x region of the protein with an additional 93 amino acids on the C-terminus.20,21
In this study we isolated the GAGs from the placenta cotyledon, chorionic plate (barrier joining maternal and fetal organ portions), and umbilical cord (Figure 1) for characterization. Chondroitin sulfates isolated from these tissues were characterized by disaccharide analysis and then used for surface plasmon resonance analysis to measure the binding to a VAR2CSA construct including the minimal chondroitin sulfate-A binding domain.
Figure 1.
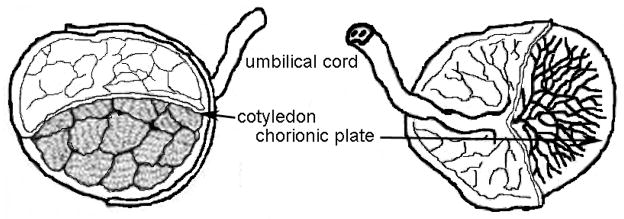
Drawing of human placenta with sections analyzed labeled as umbilical cord, chorionic plate and cotyledon.
Materials and methods
Materials
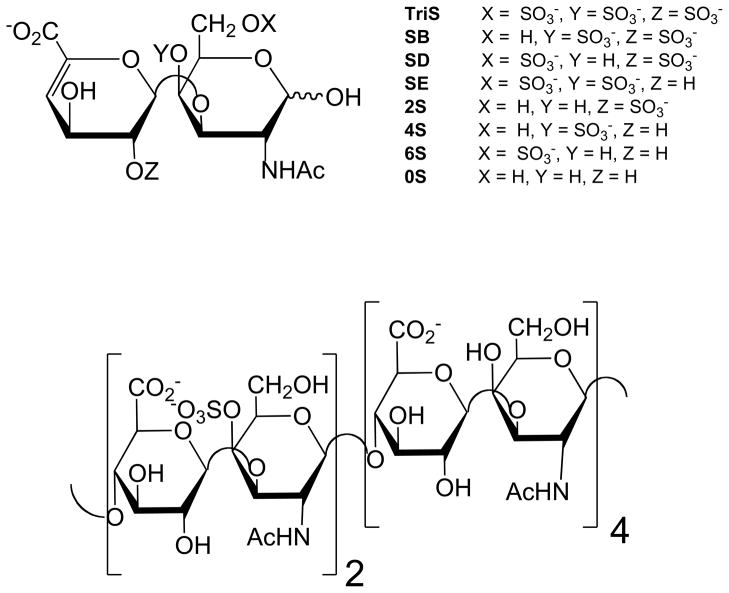
Three whole human placentas with umbilical cord still attached were obtained from Cardinal Biologicals (Tyler, Texas). Actinase E was from Kaken Biochemicals (Tokyo, Japan). 3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), and urea were from Sigma Chemical Company (St. Louis, MO). Vivapure Q Mini H columns were from Sartorius Stedium Biotech (Goettingen, Germany). Amicon ultracentrifugal filters (3000 molecular weight cut-off) and 0.22 μm stericup filters were from Millipore (Billerica, MA). Unsaturated disaccharides standards of chondroitin sulfate (0S, ΔUA-GalNAc; 4S, ΔUA-GalNAc4S; 6S, ΔUAGalNAc6S; 2S, ΔUA2S-GalNAc; 2S4S or SB, ΔUA2SGalNAc4S; 2S6S or SD, ΔUA2S-GalNAc6S; 4S6S or SE, ΔUA-GalNAc4S6S; and TriS, ΔUA2SGalNAc4S6S) (Figure 2), and Chondroitin lyases ABC and ACII were from Associates of Cape Cod, Inc. (East Falmouth, MA). Unsaturated disaccharides standards of heparan sulfate (0S, ΔUA-GlcNAc; NS, ΔUA-GlcNS; 6S, ΔUA-GlcNAc6S; 2S, ΔUA2S-GlcNAc; 2SNS, ΔUA2S-GlcNS; NS6S, ΔUA-GlcNS6S; 2S6S, ΔUA2SGlcNAc6S; and TriS, ΔUA2S-GlcNS6S) were purchased from Seikagaku Corporation (Japan). Expression and purification of the recombinant heparin lyase I, II, and III from Flavobacterium heparinum were performed using previously established protocols.22–24 All other chemicals were of reagent grade.
Figure 2.
Common chondroitin disaccharides formed through chondroitin lyase treatment (top) and chondroitin sulfate undersulfated dodecasaccharide sequence (bottom) proposed to bind to infected erythrocytes.18
Extraction of glycosaminoglycans from placenta tissue
Tissue samples were stored at 4 °C until free of ice. Excess blood was washed from the tissue using chilled phosphate buffered saline. The whole placenta was dissected based on the three regions of the organ present, the cotyledon, the chorionic plate, and the umbilical cord.17
Samples from each region were lyophilized. Completely dry samples were ground into a fine powder. Tissue was defatted using a series of chloroform and methanol washes using 2:1, 1:1, 1:2 v/v ratios. Washes were carried out overnight using a stir-plate in a fume hood. The defatted sample was resuspended using the minimal volume of water and proteolyzed using 1% actinase E (20 mg/mL) at 55°C. After proteolysis, dry urea and CHAPS were combined with the mixture to form a solution of 8 M urea and 2 wt% CHAPS. After the mixture equilibrated it was centrifuged to remove solid residue. The supernatant was then passed through a 0.22 μm filter. GAGs were isolated using Vivapure Q Maxi H spin columns. Columns were equilibrated with 3 mL 8 M urea with 2% CHAPS. Samples were loaded onto the column at 500 × g and then washed, first with 5 mL 8 M urea with 2% CHAPS, then five-times with 5 mL 200 mM NaCl. GAGs were then released from the spin columns by washing three-times with 1 mL 16% NaCl. Using an 80 vol% methanol, the GAGs were precipitated from the 16% NaCl solution. The white precipitate was recovered using centrifugation and resuspended in 1 mL water for further analysis. The amount of GAG isolated from each region was determined using a carbazole assay.24
Isolation of chondroitin sulfate for gel permeation chromatography and SPR analysis
Intact chondroitin sulfate samples were isolated from the whole GAG samples by subjecting the sample to 10 mU of heparin lyases I, II, and III for 10 h at 37°C.25 Heparin lyases were then denatured by placing samples in 100°C water bath for 10 min. Samples were centrifuged at 10,000 g for 5 min to pellet denatured enzyme. Supernatant was purified using 3000 molecular weight cut-off spin column. Retentate contained intact chondroitin sulfate, which was lyophilized and kept for molecular weight analysis and for use in SPR analysis and disaccharide analysis. Permeate, containing heparan sulfate disaccharides, was kept for use in disaccharide analysis.
Disaccharide analysis by ultraperformance liquid chromatography-mass spectrometry
Chondroitin sulfate disaccharides were prepared by depolymerizing each chondroitin sulfate sample with chondroitinases ABC and ACII.25 Heparan sulfate disaccharides, chondroitin sulfate disaccharides and heparan sulfate disaccharide standards and chondroitin sulfate disaccharide standards were labeled with 2-aminoacridone (AMAC)26 and analyzed by liquid chromatography-mass spectrometry using a previously described method.25
Average molecular weight calculation using gel permeation high performance liquid chromatography
Gel permeation high performance liquid chromatography was performed on the intact chondroitin sulfate samples using a 30 cm × 7.8 mm TSK-GEL G4000PWxl size exclusion column, a Shimadzu LC-10Ai pump, Shimadzu CBM-20A controller, and a Shimadzu RID-10A refractive index detector. The mobile phase was 0.01 M NaNO3 at a flow rate of 0.6 mL/min. The column was maintained at 40 °C and the sample volume injected was 20 μL (5 mg/mL). A calibration curve was created using 30.6 kDa, 43.8 kDa, 78.7 kDa, 130.2 kDa hyaluronan molecular weight markers (Hyalose LLC, Oklahoma City, OK). All gel permeation high performance liquid chromatography chromatograms were recorded with LC Solution Version 1.25 software and analyzed with the “GPC Postrun” function.27
Preparation of the chondroitin sulfate sensor chip
BIAcore 3000 (GE Healthcare, Uppsala, Sweden) was used to measure the interaction between the DBL3x region of VAR2CSA and chondroitin sulfate isolate from the cotyledon, chorionic plate, and umbilical cord. These regions were chosen based on their expected proximity to iRBCs. Chondroitin sulfates were isolated from different regions of the placenta from which heparan sulfate had been removed using heparinases, were biotinylated with NHS-PEG4-biotin (Thermo Scientific, Rockford, IL). Dry chondroitin sulfate samples were dissolved in PBS, pH 7.2 and combined with 20-mmol excess of fresh NHS-PEG4-Biotin in ultrapure water. Incubation of the reaction for 30 min at room temperature was followed by purification with 3000 molecular weight cut-off spin columns and lyophilization. Biotinylated chondroitin sulfate samples were immobilized on strepaviden chip using the manufacturer’s protocol. Briefly, 20 μL of biotinylated chondroitin sulfate sample from the cotyledon, chorionic plate, and umbilical cord dissolved in HBS-EP (10 mM 4-(2-hydroxyethyl-1-piperazineethanesulfonic acid (HEPES), 150 mM sodium chloride, 3 nM ethylenediaminetetraacetic acid (EDTA), 0.005% polysorbate P20, pH 7.4) running buffer was injected over flow cell 2, flow cell 3, and flow cell 4, respectively, of the streptavidin chip at a flow rate of 10 μL/min. Successful immobilization was indicated by a change in resonance units greater than or equal to 100 resonance units after injection. Flow cell 1 was prepared as a control cell by a 1 min injection of a saturated solution of biotin.
Measurement of interaction between VAR2CSA and regional placental chondroitin sulfate
The MP907 construct of VAR2CSA, prepared by a method previously described by Clausen and Salanti,20 is a construct that contains the minimal chondroitin sulfate-A binding region. The protein construct was diluted in HBS-EP running buffer to 250 nM, 125 nM, 63 nM, 32 nM, and 16 nM. Each dilution was injected (90 μL) at a flow rate of 30 μL/min, followed by a 3 min dissociation period of running buffer flowing over the sensor. To ensure complete regeneration, 30 μL of 2 M NaCl was injected before the next sample injection.
Results and discussion
GAG isolation and chondroitin sulfate purification from three regions of placenta
To isolate GAGs from three regions of placenta tissues (cotyledon, chorionic plate, and umbilical cord), we used a simple four-step procedure involving: 1. Defatting; 2. protease digestion; 3. strong-anion-exchange chromatography on a spin column; and 4. methanol precipitation. This procedure had been previously established in our laboratory for the quantitative isolation of heparin from human plasma.28 The total GAG isolated from each sample was subject to structural and biological activity analysis. Chondroitin sulfate from each sample was further purified from the total GAG by selective polysaccharide digestion using heparin lyases.
Calculated molecular weights of isolated chondroitin sulfate
GPC analysis of chondroitin sulfate indicates average molecular weights from 36 kDa to over 60 kDa (Table 1). The average weighted molecular weight of the samples collected was 49.4 kDa. Chondroitin sulfate, especially that associated with blood vessels, can range between 20–80 kDa.29,30 A previous placental glycosaminoglycan recorded average relative molecular weight (Mr) of GAGs isolated to range from 46 kDa to 60 kDa.16
Table 1.
Average calculated chondroitin sulfate molecular weight of the placenta by region.
| Sample | MN (kDa) | MW (kDa) | Polydispersity |
|---|---|---|---|
| Cotyledon | 45.2 ± 12.9 | 49.1 ± 13.8 | 1.09 ± 0.07 |
| Chorionic Plate | 41.7 ± 5.2 | 45.3 ± 7.7 | 1.11 ± 0.04 |
| Umbilical Cord | 46.7 ± 3.4 | 54.0 ± 2.8 | 1.16 ± 0.02 |
|
| |||
| Overall Average | 44.1 ± 6.9 | 49.4 ± 7.6 | 1.13 ± 0.06 |
Disaccharide analysis
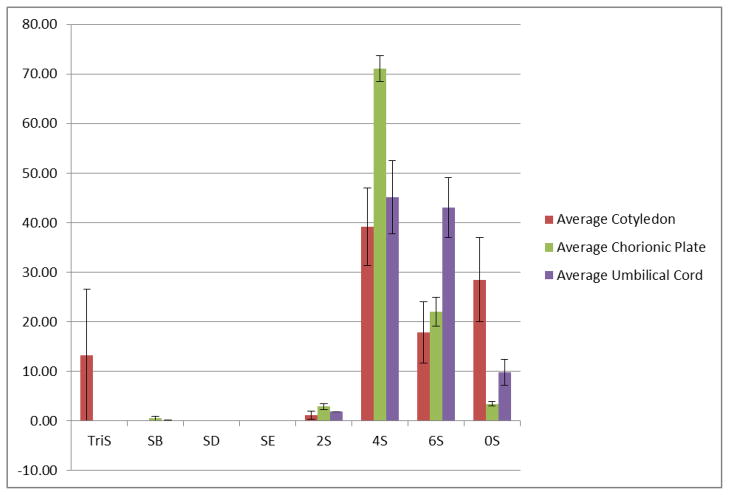
Ultraperformance liquid chromatography-mass spectrometric analysis of the resulting disaccharides was carried out using the AMAC-conjugated protocol to examine all 17 potential disaccharides represented by the standards. Disaccharide analysis affords the relative amounts of each disaccharide and also allows us to calculate the ratio of heparan sulfate disaccharides to chondroitin sulfate disaccharides (Table 2). Chondroitin sulfate is the dominant GAG component in all three umbilical cord samples with chondroitin sulfate to heparan sulfate ratios of 44:1, 23:1, and 21:1. Cotyledons exhibit the lowest ratios (5:1, 4:1, and 3:1) while the chorionic plate varied considerably from sample to sample (9:1, 13:1, and 24:1). The analysis becomes more meaningful when examining the normalized average chondroitin sulfate composition based on regions of the placenta (Figure 3). From this perspective the low-sulfation content of placental chondroitin sulfate is apparent. Over two-thirds of chondroitin sulfate found in the chorionic plate is chondroitin sulfate-A, the major form recognized substrate of VAR2CSA. The umbilical cord contains equivalent fractions of CS4S and CS6S. Meanwhile the cotyledons are composed of a lower percentage of CS4S but a much higher percentage of CS0S. Chondroitin 4-O-sulfate and unsulfated chondroitin are the two most recognized substrates for erythrocyte membrane protein binding. Based on these populations we would predict that cotyledon chondroitin sulfate should show the strongest binding to erythrocyte membrane protein.
Table 2.
Total relative disaccharide composition of three placental samples 1, 2, and 3 from various regions of the placenta.
| Samples | Heparan sulfate disaccharides | Chondroitin sulfate disaccharides | 0SHA | Ratio of chondroitin sulfate to heparan sulfate | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tri S | NS6S | NS2S | NS | 2S6S | 6S | 2S | 0S | Tri S | SB | SD | SE | 2S | 4S | 6S | 0S | |||
| 1 Cotyledon | 0.2 | 0.4 | 0.5 | 5.1 | - | 0.3 | 1.3 | 14.3 | 18.2 | - | - | - | 0.2 | 12.7 | 2.9 | 11.7 | 32.1 | 5:1 |
| 1 Chorionic plate | 0.1 | 0.3 | 0.7 | 3.7 | - | 0.3 | 0.8 | 3.4 | - | 0.2 | - | - | 2.0 | 55.1 | 22.8 | 2.8 | 7.8 | 9:1 |
| 1 Umbilical Cord | - | - | 0.1 | 0.5 | - | 0.1 | 1.0 | 0.5 | - | 0.3 | - | - | 1.8 | 56.9 | 29.8 | 7.4 | 1.5 | 44:1 |
| 2 Cotyledon | 0.1 | 0.2 | 0.3 | 3.9 | - | 0.2 | 0.1 | 7.7 | - | - | - | - | 0.1 | 19.0 | 10.6 | 23.8 | 33.9 | 4:1 |
| 2 Chorionic plate | 0.2 | 0.2 | 0.4 | 2.8 | - | 0.2 | 0.5 | 2.5 | - | 1.3 | - | - | 2.1 | 67.5 | 19.6 | 4.0 | 3.4 | 13:1 |
| 2 Umbilical Cord | - | - | 0.2 | 1.2 | - | 0.1 | 1.4 | 0.9 | - | 0.2 | - | - | 1.5 | 36.2 | 41.5 | 5.7 | 7.4 | 23:1 |
| 3 Cotyledon | 0.4 | 0.3 | 0.5 | 3.7 | 0.4 | - | 0.6 | 14.0 | - | - | - | - | 1.7 | 32.7 | 16.5 | 9.3 | 19.8 | 3:1 |
| 3 Chorionic Plate | 0.1 | 0.1 | 0.2 | 1.1 | 0.2 | - | 0.5 | 1.8 | - | - | - | - | 3.8 | 70.8 | 16.7 | 2.5 | 2.0 | 24:1 |
| 3 Umbilical Cord | - | - | 0.3 | 1.1 | 0.1 | - | 1.2 | 1.4 | - | - | - | - | 1.6 | 29.7 | 43.1 | 13.1 | 8.9 | 21:1 |
Figure 3.
Normalized average chondroitin sulfate disaccharide composition based on data in Table 2. Standard deviation represents variation between placenta samples.
Surface plasmon resonance (SPR) analysis
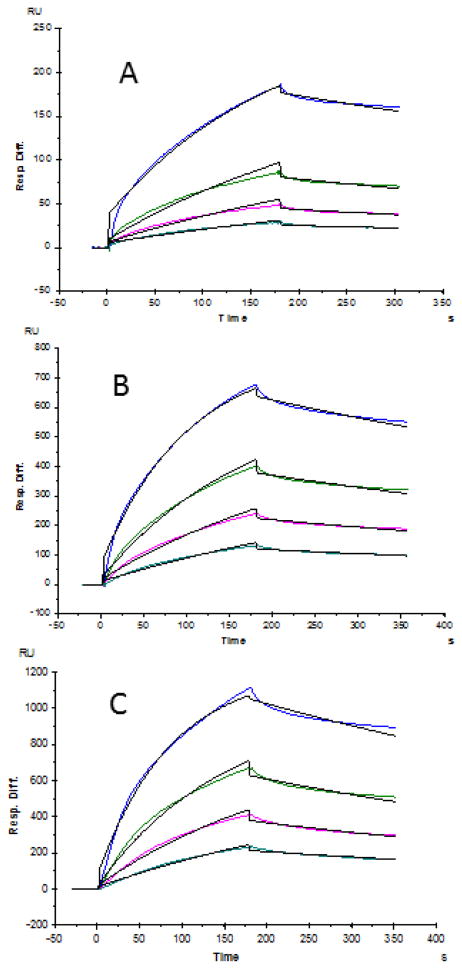
SPR binding analysis was carried out to measure the interaction between the isolated placenta chondroitin sulfate samples and VAR2CSA (MP907). Different concentrations of MP907 were injected over a sensor chip containing cotyledon, chorionic plate, and umbilical cord chondroitin sulfate. Sensorgrams were processed using Biaevaluation 4.0.1 software (Figure 4). Kinetics and binding affinity (on/off-rates and dissociation constants) was calculated by applying 1:1 Langmuir binding in accordance with past experiments.20,31 Based on the binding kinetic data (Table 3), the chondroitin sulfate from chorionic plate, and umbilical cord showed slightly higher affinity (lower KD) to VAR2CSA protein than the chondroitin sulfate from cotyledon.
Figure 4.
SPR sensorgrams of isolated chondroitin sulfate (from different regions of placenta) and MP907 interaction. A: Cotyledon; B: Chorionic Plate; C: Umbilical Cord. Concentrations of MP907 (from top to bottom): 125, 63, 32, and 16 nM, respectively. The black curves are the fitting curves. SPR experiments relied on duplicate injections performed after the regeneration of the chip surface. Standard errors were calculated from the global fitting of different protein concentrations and are presented in Table 2.
Table 3.
Summary of kinetic data of regional isolated chondroitin sulfate with MP907 VAR2CSA construct.
| Interaction | kon (M−1s−1) | koff (s−1) | KD (M) |
|---|---|---|---|
| Cotyledon CS-MP907 | 3.5 × 104 (± 1.6 × 103) | 1.3 × 10−3 (± 6.2 × 10−5) | 3.7 × 10−8 |
| Chorionic Plate CS-MP907 | 7.1 × 104 (± 1.1 × 103) | 1.1 × 10−3 (±2.4 × 10−5) | 1.6 × 10−8 |
| Umbilical Cord CS-MP907 | 8.2 × 104 (± 1.1 × 103) | 1.4 × 10−3 (± 2.9 × 10−5) | 1.7 × 10−8 |
± standard errors were from the global fitting of different protein concentrations
Conclusions
Our results confirmed findings of many other studies on placental chondroitin sulfate interaction with P. falciparum infected erythrocytes.16,18,20 Differences in characterization are associated with differences in analytical method and detection sensitivity. In all analyses, cotyledon chondroitin sulfate displays the presence of predominantly 4S and 0S disaccharides. The observation of 6S disaccharide and other minor disaccharides in the current study may be attributable to the inclusion of GAGs from the fibrous scaffold of the lobes and/or the increased sensitivity of the ultraperformance liquid chromatography-mass spectrometric method used. The other three regions examined also had substantial fractions of 4S disaccharides. However they did not possess the same level of unsulfated disaccharide. The impact of this difference was next assessed using SPR. Cotyledon, chorionic plate, and umbilical cord GAGs all initiated a binding of the VAR2CSA construct MP907. All three placental tissues showed similar tight binding, with only a ~2-fold range in binding affinities. Despite slightly weaker binding to the cotyledon chondroitin sulfate, the maternal blood flow with the circulating infected erythrocytes only have access to this part of the placenta.
Future work will focus on the prevention or treatment of malaria. There are several possible approaches. It is established that once a mother produces antibodies preventing VAR2CSA binding the symptoms of placental malaria are significantly reduced resulting in healthier, heavier neonates than those born to infected mothers without antibodies.12,32 This suggest that one of the several conserved domains of VAR2CSA could be a potential vaccine candidate. Since VAR2CSA is a very large protein current opinion is to use a construct of VAR2CSA that contains the minimal strong chondroitin sulfate-A binding domain.20 This region enables the strongest binding of erythrocytes and therefore is more desirable to block infected erythrocytes from the endothelial membrane.
Another treatment approach focuses on the GAG to keep infected erythrocytes in circulation resulting in their destruction by the spleen. A chondroitin sulfate-A oligosaccharide or polysaccharide might be used to bind to infected erythrocytes so that they cannot adhere to endothelium. Such a therapeutic target must be a stronger binder than the low-sulfated placental chondroitin sulfate A for this approach to be effective and must also stay in the circulation long enough to go through several blood-phase reproduction cycles. One advantage of using a GAG therapeutic is that many anti-malarials can produce intense side effects and GAGs generally are better tolerated. The development of a therapeutic chondroitin sulfate-oligomer would require more extensive kinetic studies.
Although malaria and other mosquito-borne illnesses are a serious global health concern, the areas that bear the brunt of mortalities are severely impoverished. Malaria treatment is not economically attractive option for drug companies. The information gathered from characterization of substrates and ligand kinetics will be integral in developing cost-effective treatments to lessen the severity or eliminate placental malaria.
Acknowledgments
This work was supported by grants from the funded by the National Institutes of Health HL101721 and HL096972.
Abbreviations
- AMAC
2-aminoacridone
- CHAPS
3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate
- EDTA
ethylenediaminetetraacetic acid
- GAG
glycosaminoglycan
- HEPES
4-(2-hydroxyethyl-1-piperazineethanesulfonic acid
- pfEMP
Plasmodium falciparum erythrocyte membrane protein
- SPR
surface plasmon resonance
- VAR2CSA
a PfEMP1 protein
References
- 1.World Health Organization. World Malaria Report. Geneva, Switzerland: 2012. pp. 1–2. [Google Scholar]
- 2.Kraemer SM, Smith JD. A family affair: var genes, PfEMP1 binding, and malaria disease. Curr Opin Microbiol. 2006;9:374–380. doi: 10.1016/j.mib.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Doolan DL, Dobaño C, Baird JK. Acquired immunity to malaria. Clin Microbiol Rev. 2009;22:13–36. doi: 10.1128/CMR.00025-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hviid L. Naturally acquired immunity to Plasmodium falciparum malaria in Africa. Acta Trop. 2005;95:270–275. doi: 10.1016/j.actatropica.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Menendez C. Malaria during pregnancy: a priority area of malaria research and control. Parasitol Today. 1995;11:178–183. doi: 10.1016/0169-4758(95)80151-0. [DOI] [PubMed] [Google Scholar]
- 6.McGregor IA. Thoughts on malaria in pregnancy with consideration of some factors which influence remedial strategies. Parassitologia. 1987;29:153–163. [PubMed] [Google Scholar]
- 7.Shulman CE, Graham WJ, Jilo H, Lowe BS, New L, Obiero J, Snow RW, Marsh K. Malaria is an important cause of anaemia in primigravidae: evidence from a district hospital in coastal Kenya. Trans Royal Soc Trop Med Hyg. 1996;90:535–539. doi: 10.1016/s0035-9203(96)90312-0. [DOI] [PubMed] [Google Scholar]
- 8.Steketee RW, Wirima JJ, Hightower AW, Slutsker L, Heymann DL, Breman JG. The effect of malaria and malaria prevention in pregnancy on offspring birthweight, prematurity, and intrauterine growth retardation in rural Malawi. Am J Trop Med Hyg. 1996;55:33–41. doi: 10.4269/ajtmh.1996.55.33. [DOI] [PubMed] [Google Scholar]
- 9.Menendez CO. The Impact of Placental Malaria on Gestational Age and Birth Weight. J Infect Diseases. 2000;181:1740. doi: 10.1086/315449. [DOI] [PubMed] [Google Scholar]
- 10.Shulman CE, Marshall T, Dorman EK, Bulmer JN, Cutts F, Peshu N, Marsh K. Malaria in pregnancy: adverse effects on haemoglobin levels and birthweight in primigravidae and multigravidae. Trop Med Internat Health. 2001;6:770–778. doi: 10.1046/j.1365-3156.2001.00786.x. [DOI] [PubMed] [Google Scholar]
- 11.Walter PR, Garin Y, Blot P. Placental pathologic changes in malaria. A histologic and ultrastructural study. Am J Pathol. 1982;109:330–342. [PMC free article] [PubMed] [Google Scholar]
- 12.Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272:1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 13.Salanti A, Staalsoe T, Lavstsen T, Jensen AT, Sowa MP, Arnot DE, Hviid L, Theander TG. Selective up-regulation of a single distinctly structured var gene in chondroitin sulfate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol Microbiol. 2003;49:179–191. doi: 10.1046/j.1365-2958.2003.03570.x. [DOI] [PubMed] [Google Scholar]
- 14.Salanti A, Dahlbäck M, Turner L, Nielsen MA, Barfod L, Magistrado P, Jensen AT, Lavstsen T, Ofori MF, Marsh K, Hviid L, Theander TG. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J Exp Med. 2004;200:1197–1203. doi: 10.1084/jem.20041579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brabin BJ. An analysis of malaria in pregnancy in Africa. Bull World Health Organ. 1983;61:1005–1016. [PMC free article] [PubMed] [Google Scholar]
- 16.Achur RN, Valiyaveettil M, Alkhalil A, Ockenhouse CF, Gowda DC. Characterization of proteoglycans of human placenta and identification of unique chondroitin sulfate proteoglycans of the intervillous spaces that mediate the adherence of Plasmodium falciparum-infected erythrocytes to the placenta. J Biol Chem. 2000;275:40344–40356. doi: 10.1074/jbc.M006398200. [DOI] [PubMed] [Google Scholar]
- 17.Gray H. In: Gray’s Anatomy. 38. Williams PL, Bannister LH, Berry MM, Collins P, Dyson M, Dussek JE, Ferguson MWJ, editors. Pearson Professional Limited; London: 1995. pp. 166–173. [Google Scholar]
- 18.Achur RN, Kakizaki I, Goel S, Kojima K, Madhunapantula SV, Goyal A, Ohta M, Kumar S, Takagaki K, Gowda DC. Structural interactions in chondroitin 4-sulfate mediated adherence of Plasmodium falciparum infected erythrocytes in human placenta during pregnancy-associated malaria. Biochemistry. 2008;47:12635–12643. doi: 10.1021/bi801643m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andersen P, Nielsen MA, Resende M, Rask TS, Dahlbäck M, Theander T, Lund O, Salanti A. Structural insight into epitopes in the pregnancy-associated malaria protein VAR2CSA. PLoS Pathog. 2008;4:e42. doi: 10.1371/journal.ppat.0040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salanti A, Staalsoe T, Lavstsen T, Jensen AT, Sowa MP, Arnot DE, Hviid L, Theander TG. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol Microbiol. 2003;49:179–191. doi: 10.1046/j.1365-2958.2003.03570.x. [DOI] [PubMed] [Google Scholar]
- 20.Clausen TM, Christoffersen S, Dahlbäck M, Langkilde AE, Jensen KE, Resende M, Agerbæk MØ, Andersen D, Berisha B, Ditlev SB, Pinto VV, Nielsen MA, Theander TG, Larsen S, Salanti A. Structural and functional insight into how the Plasmodium falciparum VAR2CSA protein mediates binding to chondroitin sulfate A in placental malaria. J Biol Chem. 2012;287:23332–23345. doi: 10.1074/jbc.M112.348839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srivastava A, Gangnard S, Dechavanne S, Amirat F, Lewit Bentley A, Bentley GA, Gamain B. Var2CSA minimal CSA binding region is located within the N-terminal region. PloS one. 2011;6:e20270. doi: 10.1371/journal.pone.0020270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshida E, Arakawa S, Matsunaga T, Toriumi S, Tokuyama S, Morikawa K, Tahara Y. Cloning, Sequencing, and Expression of the Gene from Bacillus circulans That Codes for a Heparinase That Degrades Both Heparin and Heparan Sulfate. Biosci Biotechnol Biochem. 2002;66:1873–1879. doi: 10.1271/bbb.66.1873. [DOI] [PubMed] [Google Scholar]
- 23.Shaya D, Tocilj A, Li Y, Myette J, Venkataraman G, Sasisekharan R, Cygler M. Crystal structure of heparinase II from Pedobacter heparinus and its complex with a disaccharide product. J Biol Chem. 2006;281:15525–15535. doi: 10.1074/jbc.M512055200. [DOI] [PubMed] [Google Scholar]
- 24.Godavarti R, Davis M, Venkataraman G, Cooney C, Langer R, Sasisekharan R. Heparinase III from Flavobacterium heparinum: cloning and recombinant expression in Escherichia coli. Biochem Biophys Res Commun. 1996;225:751–758. doi: 10.1006/bbrc.1996.1246. [DOI] [PubMed] [Google Scholar]
- 24.Bitter T, Muir HM. A modified uronic acid carbazole reaction. Anal Biochem. 1962;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- 25.Yang B, Chang Y, Weyers AM, Sterner E, Linhardt RJ. Disaccharide analysis of glycosaminoglycan mixtures by ultra-high-performance liquid chromatography-mass spectrometry. J Chromatogr A. 2012;1225:91–98. doi: 10.1016/j.chroma.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitagawa H, Kinoshita A, Sugahara K. Microanalysis of glycosaminoglycan-derived disaccharides labeled with the fluorophore 2-aminoacridone by capillary electrophoresis and high-performance liquid chromatography. Anal Biochem. 1995;232:114–21. doi: 10.1006/abio.1995.9952. [DOI] [PubMed] [Google Scholar]
- 27.Higashi K, Ly M, Wang Z, Masuko S, Bhaskar U, Sterner E, Zhang F, Toida T, Dordick JS, Linhardt RJ. Controlled Photochemical Depolymerization of K5 Heparosan, a Bioengineered Heparin Precursor. Carbohydr Polym. 2011;86:1365–1370. doi: 10.1016/j.carbpol.2011.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang F, Sun P, Munoz E, Chi L, Sakai S, Toida T, Zhang H, Mousa S, Linhardt RJ. Microscale Isolation and Analysis of Heparin from Plasma using an Anion Exchange Spin Column. Anal Biochem. 2006;353:284–286. doi: 10.1016/j.ab.2006.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blix G, Snellman O. On Chondroitin Sulfuric Acid and Hyaluronic Acid. Arkiv Kemi Mineralogi Och Geologi. 1945;19:1–19. [Google Scholar]
- 30.Alves CS, Mourão P. A Interaction of high molecular weight chondroitin sulfate from human aorta with plasma low density lipoproteins. Atheroscler. 1988;73:113–124. doi: 10.1016/0021-9150(88)90032-9. [DOI] [PubMed] [Google Scholar]
- 31.Dahlbäck M, Jørgensen LM, Nielsen MA, Clausen TM, Ditlev SB, Resende M, Pinto VV, Arnot DE, Theander TG, Salanti A. The chondroitin sulfate A-binding site of the VAR2CSA protein involves multiple N-terminal domains. J Biol Chem. 2011;286:15908–15917. doi: 10.1074/jbc.M110.191510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duffy PE, Fried M. Antibodies That Inhibit Plasmodium falciparum Adhesion to Chondroitin Sulfate A Are Associated with Increased Birth Weight and the Gestational Age of Newborns. Infect Immun. 2003;71:6620–6623. doi: 10.1128/IAI.71.11.6620-6623.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]