Abstract
The prevalence of obesity is high resulting from chronic imbalances between energy intake and expenditure. On the expenditure side, regular exercise is associated with health benefits, including enhanced brain function. The benefits of exercise are not immediate and require persistence to be realized. Brain regions associated with health-related decisions, such as whether or not to exercise or controlling the impulse to engage in immediately rewarding activities (e.g., sedentary behavior), include reward processing and cognitive control regions. A 9 month aerobic exercise study will be conducted in 180 sedentary adults (n = 90 healthy weight [BMI= 18.5 to 26.0 kg/m2]; n = 90 obese [BMI=29.0 to 41.0 kg/m2) to examine the brain processes underlying reward processing and impulse control that may affect adherence in a new exercise regimen. The primary aim is to use functional magnetic resonance imaging (fMRI) to examine reward processing and impulse control among participants that adhere (exercise >80% of sessions) and those that do not adhere to a nine-month exercise intervention with secondary analyses comparing sedentary obese and sedentary healthy weight participants. Our results will provide valuable information characterizing brain activation underlying reward processing and impulse control in sedentary obese and healthy weight individuals. In addition, our results may identify brain activation predictors of adherence and success in the exercise program along with measuring the effects of exercise and improved fitness on brain activation.
Keywords: Exercise Adherence, Functional Magnetic Resonance Imaging, Obesity
Introduction
Exercise is recommended for weight management by virtually all public health organizations [1–4]. Adherence to an exercise routine is one of the strongest differentiators between those who are able to maintain previously lost weight and those who regain weight [5–10]. However, approximately 50% of adults who initiate an exercise program drop out during the first 6 to 12 months; a figure which has remained constant since publication of the first studies on exercise adherence over 25 years ago [11–14].
Impulsivity appears to play a role in health-related behaviors (e.g., exercise) such that individuals who prefer immediately available small rewards over delayed larger rewards have difficulty making health promoting decisions that may be most beneficial in the future [15–18]. Obesity has been associated with increased levels of impulsivity, which is believed to influence unhealthy eating behavior due to the inherently reinforcing properties of food [19–21]. Obesity has also been associated with increased impulsivity and reward sensitivity [22], and measures of impulsivity have been shown to predict eating volume following fasting in obese participants [19, 23]. In addition to eating behavior, evidence suggests that impulsivity plays a role in exercise adherence [24, 25].
Impulsivity likely influences daily decision-making, impacting exercise behavior because sedentary activities are more immediately reinforcing than physical activities, especially among the obese [26, 27]. The greater reinforcing value of sedentary activities compared to exercise may be particularly true among obese individuals because they are more likely to experience physical exertion as unpleasant. Although acute exercise typically improves affect (short-term feeling states including aspects of hedonic tone, e.g. pleasure vs. displeasure, and level of energy) an individual must be willing to endure exertion and potential negative affect during the exercise bout before these benefits are felt. Affect during exercise worsens with increasing intensity, and most sedentary obese individuals are inaccurate in regulating exercise intensity [28]. For example, obese women have been shown to reach relative exercise intensities much higher than expected [29] and are thus more likely to experience heightened feelings of displeasure during exercise compared to normal weight sedentary women [30]. In addition, obese sedentary adults often have more negative cognitions about exercise (e.g., lower self-efficacy; [31]), which are known to moderate the exercise-affect relationship and heighten feelings of displeasure [32, 33].
Little is known regarding underlying neural mechanisms associated with exercise adherence and the relationship between the neural systems of reward and exercise adherence. Furthermore, the degree to which the ability to delay gratification is reversible in obesity is also not known. We hypothesize that brain processes, underlying reward processing, and impulse control contribute to obesity and to adherence in a new exercise regimen. We propose to scan sedentary obese and sedentary healthy weight (HW) participants with two Functional Magnetic Resonance Imaging (fMRI) tasks before and after a nine-month exercise intervention. The first fMRI task will focus on brain responses that will provide insight about the relationship between general processing of reward/punishment stimuli and exercise adherence. In this Reward Prediction paradigm, participants are scanned as they anticipate and receive predicted and unpredicted monetary rewards and punishments. The second task will focus on brain responses during impulsive decision-making (i.e., delay discounting) that will provide insight about the relationship between immediate compared to long-term decision-making (i.e., choosing a smaller monetary reward immediately versus a larger monetary reward later) and exercise adherence. Together, these tasks will characterize brain activation underlying reward processing and impulse control in individuals that adhere to exercise verses those that do not, examine differences between obese and HW individuals, identify brain activation predictors of adherence and success in the exercise program and evaluate the effects of exercise and improved fitness on brain activation. Findings from this study will have significant implications for understanding brain processing mechanisms contributing to exercise adherence, and may ultimately lead to more effective interventions.
Methods
Study Overview
We will conduct a 9 month exercise trial with fMRI scans completed at baseline and after 9 months of exercise. We will study a sample of (n = 180) healthy, HW (BMI = 18.5 to 26.0 kg/m2) and obese (BMI = 29.0 to 41.0 kg/m2), sedentary, adult men and women (age 18–50 years). Exercise will consist of a progressive exercise prescription program that will increase in intensity and duration with a target of moderate intensity walking or jogging (75% maximal heart rate), 50 minutes/session, 5 days/week. Participants will be encouraged to attend a 2–3 exercise sessions/week in one of three private exercise rooms at different locations (on campus and off campus) with the remaining 2–3 unsupervised exercise sessions verified through downloadable heart rate monitors. Participants will be encouraged to maintain an ad libitum diet. The following variables will be assessed: height, weight, body composition (dual energy x-ray absorptiometry [DEXA], waist circumference), fitness (sub-maximal treadmill test), dietary intake (3-day food record). Reward prediction and delay discounting will be assessed using fMRI paradigms described below. The goal of our project is to identify brain function predictors of successful exercise adherence, identify differences between normal weight and obese adults, and monitor changes in brain function as a result of exercise training (Figure 1).
Figure 1.
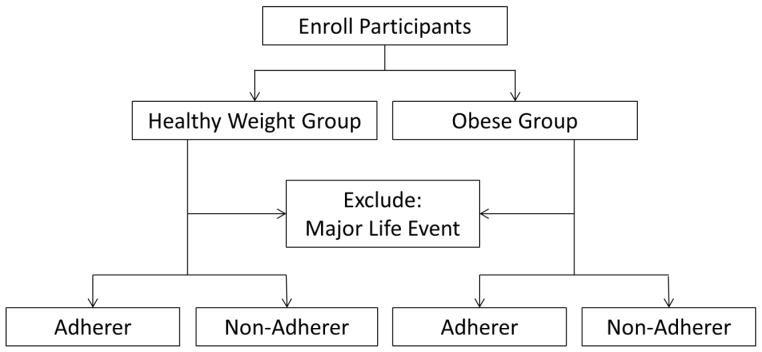
Proposed participant enrollment and flow diagram
Design Justification
All participants will be asked to follow a progressive exercise prescription of moderate intensity walking or jogging (75% maximal heart rate) for 50 min/session on 5 days/wk for 9 months. An exercise prescription of 250 minutes/week of moderate intensity exercise was selected based on the 2009 American College of Sports Medicine (ACSM) recommendation for exercise for weight loss and prevention of weight regain in adults. ACSM suggests that 200–300 min/wk of moderate intensity activity (3–6 METs) may provide long term weight loss [4]. In addition, findings from our recently completed exercise trial where the energy expenditure of exercise was assessed monthly by indirect calorimetry and adjusted to maintain adherence with the exercise prescription, supports these duration and intensity guidelines for weight loss [34]. In this study, overweight participants (BMI >25 to < 40 kg/m2) randomized to 400 kcal/session of moderate intensity (70% to 80% maximal heart rate) laboratory supervised exercise, 5 days/wk for 10 months expended an average of 402 ± 6 kcal/session over 31 ± 6 (males) to 48 ± 7 (females) min/session and achieved ~4.3% weight loss from baseline [34]. Furthermore, in our completed 16 month exercise trial we observed no significant changes in cardiovascular fitness or body weight after 9 months of exercise [35]. Therefore we are confident that the 9 month exercise prescription of 250 min/wk at 75% maximal heart rate will be sufficient to produce weight loss in our obese participants.
Participants
Inclusion
Individuals are eligible if they meet the following criteria. Adults aged 18 to 50 years, an age group which may be likely to consider exercise as a mode of weight management. The age range is set at 18–50 to minimize variability due to development (less than age 18) or effects of aging (greater than age 50). We will recruit an equal number of healthy weight (BMI = 18.5 to 26.0 kg/m2) and obese (BMI = 29.0 to 41.0 kg/m2) men and women, who declare they do not plan to move out of the Greater Kansas City area for at least 1 year, and are willing to exercise. Prior to participation individuals will be required to provide written approval from a licensed physician that they are healthy enough to participate in a program of moderate physical activity. All participants must be sedentary except for casual recreation such as softball, bowling, etc. This will be determined by Dr. Washburn as less than 500 kcal per week of planned activity as estimated by the Minnesota Leisure Time Physical Activity Questionnaire [36].
We chose not to study individuals with a BMI > 41 kg/m2 as they are less likely to meet inclusion criteria due to comorbid risks (e.g., heart disease, etc.) and may encounter difficulties with physical activity. Furthermore, there are size limitations for the fMRI scanner and in our experience, individuals with BMI > 41 kg/m2 are difficult to fit deeply enough into the scanner.
Exclusion
Individuals will be ineligible if they report any of the following or at the discretion of the principal investigator: Color-blindness, left-handedness, any diagnosed neurological disorder or conditions that preclude MRI scanning (e.g., metal in body). Participation in a research project involving weight loss or exercise in the previous 6 months, as these proximal experiences may impact the results of this study. Subjects who smoke, use medications that affect metabolism (i.e., thyroid, beta blockers), use medications that affect appetite, exhibit symptoms of alcohol abuse/dependence (binge drinkers [males ≥ 5 drinks and females ≥ 4 drinks on one occasion in the past 30 days]: Health Risk Behaviors of Kansans, 2002 [37], or cannot exercise. Participants will be excluded by study personnel following interview if they exhibit eating disorders (i.e., score >20 on the Eating Attitudes Test [38], binge eating (i.e., score > 27 on the Binge Eating Scale—BES)[39], depression (i.e., score >16 on the Center for Epidemiological Studies Depression Scale) [40] or drug addiction (medical history). Participants will be excluded if they have any metabolic disease that would affect energy balance (e.g. diabetes mellitus or hypothyroidism). Individuals are excluded if they report being pregnant during the previous 6 months, are lactating, or plan pregnancy within 12 months. Individuals who are not weight stable (± 4.5 kg) within the previous year. Participants with serious medical risks such as type 1-diabetes, cancer, recent cardiac event (e.g., heart attack, angioplasty, etc.) will be excluded. Participants will be excluded if high blood pressure is reported during screening or measured at baseline. If a participant experiences claustrophobia in the fMRI, the testing session will end and the participant will be ineligible. Participants will be excluded if their general IQ, as measured at the baseline MRI appointment via the WASI matrix reasoning and vocabulary sub-tests [41] is less than 80. Individuals will be excluded if they report radiation exposure from CT, PET, fluoroscopic or nuclear medicine studies within the previous year, or other radiation exposure at the discretion of the principal investigator.
Recruitment
We will recruit 180 individuals across 5 years. Our center currently recruits for all of our projects using newspaper/online advertising, email list serves, public service messages, media contacts, and word of mouth. All forms of advertising will have a dedicated study phone, email address, and our Research Center web site. There are 2.3 million individuals in the Greater Kansas City Region, 62% are between the ages of 18–65, and minorities comprise 20% of the population (US Census Bureau). We will include minorities to total at least 20% of the participants for this study. We have chosen 180 participants to allow for post enrollment attrition. We have proposed the 25% attrition level, to account for individuals who are not present for the final fMRI scanning session, for reasons not specifically related to exercise adherence (e.g., moving out of town, becoming pregnant, jury duty, etc.). We believe this is a reasonable, conservative estimate, which will ensure adequate statistical power. Participants who drop out of the program for adherence-related reasons as determined by interview, and who have completed less than 80% of sessions, will be assigned to the “non-adherer” group and every attempt will be made to get them to return for the Post-Exercise fMRI scanning session.
Participant Matching
Participant enrollment will be monitored to ensure participants in the HW and Obese groups are matched (p > 0.05) for age, gender, education level, race/ethnicity.
Exercise program overview
Participants will be initially sedentary, therefore both intensity and duration of exercise will be progressed to reach the goal of 50 min/day of moderate intensity walking or jogging (75% maximal heart rate) by the end of month 3 and maintain this for the remainder of the 9 month study (Table 1. Exercise Progression). Exercise may be intermittent with minimum bout duration of 10 minutes. Walking/jogging activity is proposed as the primary activity mode to standardize the mode of exercise. Alternate activities of biking, swimming, or elliptical trainer, etc. may be substituted for a maximum of 20% of the total exercise sessions (1 out of 5 days). Participants will be encouraged to perform up to 60% (3 days) with a minimum of 40% (2 days) of exercise sessions under supervision by study staff in a private exercise facility. The participants’ exercise heart rate range (± 4 beats/minute) will be calculated from their age predicted maximum heart rate.
Table 1.
Nine month exercise progression
| Week of intervention | Intensity (% HR max) | Session Duration (min) |
|---|---|---|
| 1 | 65% | 20 |
| 2 | 65% | 25 |
| 3 | 65% | 30 |
| 4 | 65% | 35 |
| 5 | 65% | 35 |
| 6 | 70% | 35 |
| 7 | 70% | 40 |
| 8 | 70% | 40 |
| 9 | 70% | 45 |
| 10 | 70% | 45 |
| 11 | 75% | 45 |
| 12–40 | 75% | 50 |
Note. % HR max = percent of heart rate maximum; min = minutes.
Exercise education sessions
To help prevent injuries and to assist participants with the transition from sedentary behavior to regular exercise, participants will be required to attend 4 one-on-one educational sessions, once each week during the first 4 weeks of the exercise program held in conjunction with their supervised exercise session. These lessons, conducted by our research staff will provide information on reducing barriers to exercise, proper warm-up, stretching, clothing/footwear and exercising during heat/cold. We have successfully employed this strategy for over 25 years in sedentary overweight individuals participating in our on-going weight control research program.
Exercise Adherence
Adherence will be defined as completing ≥ 80% of the target 250 min/wk at the target intensity as verified by downloadable heart rate monitor to be worn during each exercise session (RS 400; Polar Electro Inc, Woodbury, NY). Individuals will be considered compliant if the average heart rate is ± 4 beats/minute and duration is ± 5 minutes of the target. Participants will be advised not to exceed the targets for both exercise duration and intensity. Heart rate monitors will be programmed to collect heart rate averaged over 1-minute intervals. Prior to performing weekly supervised exercise sessions the research assistant will download all past exercise data stored on the monitor using the Polar Precision Performance Software. The Polar RS 400 has the ability to store up to 99 hours of heart rate data collected at 1-min intervals. Since participants are encouraged to perform 2 supervised exercise sessions/week, we do not anticipate storing more than 10 sessions (about 9 hours) on the monitor prior to a download. A 25% allowance for attrition is built into our estimates of retention in order to account for participants who might not be available for the Post-Exercise Scan for reasons not directly tied to adherence (e.g., moving, injury, major change in employment, unexplained loss to follow-up). Reasons for non-adherence/attrition will be assessed based on semi-structured interviews, conducted by study personnel trained in behavioral psychology. The research team will develop specific questions to explore adherence issues, such as lack of motivation, self-efficacy for exercise, enjoyment of exercise, outcome expectations, etc. All interviews will be tape recorded and transcribed verbatim. Two independent investigators will read the transcripts and code reasons of non-adherence. A third investigator will cross-check codes and the team will meet to discuss discrepancies as recommended by Strauss and Corbin (1998) [42]. Disagreements regarding coding of the reasons for non-adherence will be resolved through discussion until the group has achieved mutual consensus. The ATLAS.ti5.0 (Scientific Software Development) will be used to assist in coding, storage, and retrieval of data. Participants who drop out of the program for adherence-related reasons and who have completed less than 80% of sessions, will be assigned to the “non-adherer” group and every attempt will be made to get them to return for the Post-Exercise scanning session.
fMRI Paradigm Overview
Functional Neuroimaging Studies of Reward Processing and Impulse Control
There is mounting evidence from behavioral studies that reward processing and impulse control play some role in overeating and sedentary behavioral choices, yet little is known regarding brain systems underlying these processes in obesity. Functional neuroimaging techniques, such as functional magnetic resonance imaging (fMRI), provide non-invasive measures of brain function. Recent functional neuroimaging studies examining brain activation during reward processing and impulse control in healthy adult populations support central roles for components of the limbic system, particularly ventral striatum (nucleus accumbens), amygdala, and the hippocampal formation. In addition, cortical regions in the frontal lobe connected to the limbic system, referred to as paralimbic cortex, have also been implicated. For example, the orbitofrontal cortex (OFC), medial prefrontal cortex (MPFC), and anterior cingulate cortex (ACC) have been implicated in various aspects of the processing of reward and making decisions based upon the rewarding aspects of stimuli.
Reward processing involves the evaluation of whether a stimulus, action, or decision is associated with a positive or negative outcome. Striatal regions show greater fMRI activation to the occurrence of good outcomes, such as winning money, compared to bad outcomes, such as losing money [43]. The striatum also shows sustained activation to rewards and a sharp decrease in activation to punishments [44, 45], indicating its role in appetitive motivation. The ventral striatum responds to the presence of a reward regardless of its magnitude indicating a role in categorical distinctions between good and bad options. Predictability of rewards plays a role in increased activation of the ventral striatum and OFC in humans especially when unpredicted rewards are delivered [46]. These increases in activity have been attributed to the projection of dopamine from the ventral tegmental area (VTA) to the activated reward processing regions in ventral striatum and ventral PFC. Abler et al. (2006) used fMRI to study reward sensitivity using a reward prediction paradigm in which participants were scanned while monetary rewards and punishments were predicted and then received [47]. fMRI signal in the nucleus accumbens increased linearly with probability of reward in the expectation phase and error during the delivery phase. Activity in this reward processing region was also positively correlated with participant self-report measures of sensation and novelty seeking.
Impulsive decision making can be described as a preference for immediate smaller rewards over delayed larger rewards, in which reward value is discounted when there is a delay in receiving the reward [48]. The delay discounting paradigm is a widely used index of impulsivity in which people choose immediate vs. delayed rewards. McClure et al. (2004) used fMRI to evaluate neural systems underlying delay discounting [49]. They found that limbic and paralimbic reward processing regions were activated by decisions involving immediate reward, while lateral prefrontal cortex was activated when choices involved a delayed reward. Thus, more impulsive decisions were associated with activation of reward processing regions, while the prefrontal cortex controlled less impulsive decisions. Striatal activations have also been noted in other studies when participants choose immediate rewards (e.g., Wittman et al. 2007 [50]). Furthermore, striatal and medial prefrontal regions positively correlate with the magnitude of future rewards, whereas lateral prefrontal regions negatively correlate with the length of the delay in receiving future rewards [51].
Reward Prediction
The reward prediction task is based on Martin et al [52–56]. The fMRI reward prediction task contains predicted and unpredicted rewards and punishments. In this paradigm, participants will be presented with images that predict the delivery of a monetary reward or punishment with about 75% probability. A sample trial is illustrated in Figure 2. The predictor is presented for 1650 ms and followed by a fixation cross jittered for 850–8350 ms, which serves as the prediction phase of the trial. While the cue is on the screen participants indicate with a keypress whether the cue predicts a reward or a punishment. Participants receive feedback for 1650 ms indicating how much they won or lost on the current trial and what their total is for the current block of trials following the prediction phase. Delays ranging from 0–12 seconds are inserted between trials to jitter trial presentation. The optimal stimulus and delay timing will be determined using AFNI’s RSFgen. Rewards (+$1) and punishments (−$1) will be delivered so that 75% of the trials will yield the outcome predicted whereas 25% of the trials will result in an unexpected outcome. In addition, participants will be rewarded for responding quickly and penalized for responding slowly. Therefore participants must respond as quickly and accurately as possible to maximize their overall total on each block of trials. Reaction time cutoffs for receiving high reward values will be determined on an individual basis based on pre-testing out of the scanner. These values will be set so that participants receive high rewards on about 50% of the trials. In total the MRI experiment should take approximately 40 minutes to complete. Participants will begin with $12.50 in their bankroll at the beginning of each experimental block. Overall participants will be able to earn between $0–25 extra during the reward prediction task. On average participants should earn about $17.50 if they respond with 95% accuracy. Participants will receive these rewards in addition to the reimbursement they will receive for their time per IRB approved protocol.
Figure 2.
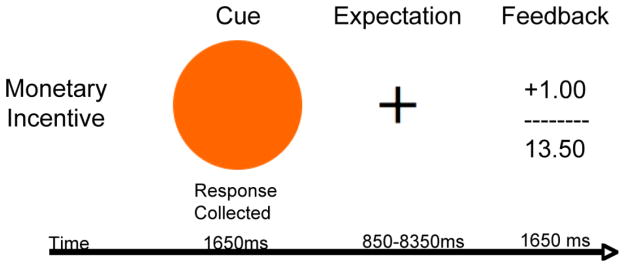
Reward Prediction: A circle is presented that indicates whether participants are most likely to win money or loss money and participants are told what color of circle predicts wins and losses. Participants are instructed to press a button if the circle predicts a win and then receive feedback about how much money is earned on the current trial and their total for the current block of trials.
The out-of-scanner practice will ensure that subjects fully understand the instructions and can complete the task. Subjects will be allowed to practice up to 3 times and must achieve 80% or they will be ineligible for the study and the testing will end.
Delay Discounting
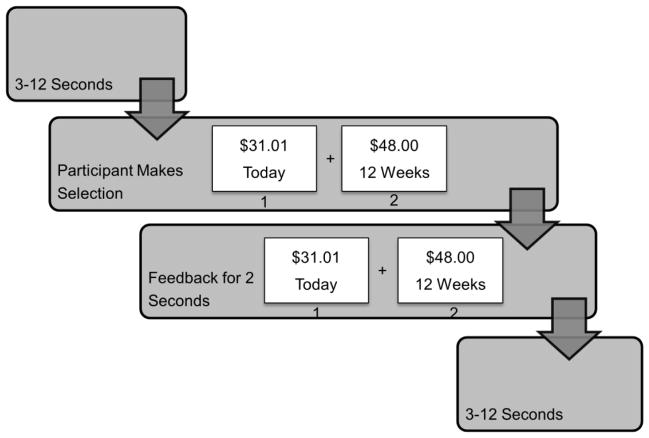
The delay discounting paradigm is based on the observation that the relative values of rewards are “discounted” based on the anticipated delay of delivery – less value is placed on rewards with greater anticipated delays. Our fMRI study is modeled after McClure et al. (2004). In this task (Figure 3), participants are instructed to choose between two monetary reward options: 1) A smaller reward delivered immediately, or 2) a larger reward delivered after a delay (of hours to days to weeks).
Figure 3.
Delay Discounting: Participants are presented with a choice between a smaller immediately available reward and a larger delayed reward. Participants are given as long as they need to make their decision. Once they make a decision the decision is highlighted before moving on to the next trial.
Immediately before the MRI session participants will complete a 5–10 minute out-of-scanner practice session to familiarize themselves with the Delay Discounting Task. In addition, the practice is based on Wittman et al (2007) and will assess participants’ “switchpoints” (the value where participants switch their preference from the larger delayed reward to the smaller immediate reward) [50]. These values will then be entered into an individually tailored version of the fMRI experiment, which is designed to yield a similar number of trials on which the subject prefers the larger delayed reward and the smaller early reward among groups of subjects that are expected to show differences in impulsive decision-making. The order of task presentation in the scanner will be counterbalanced across subjects to minimize order effects.
Outcomes Measures
The fMRI assessments will take place at baseline and 9 months. Anthropometric, fitness, and dietary assessments will be completed at baseline, 4 and 9 months. All assessments will take place within two weeks of each other at each time point, and within two weeks of the fMRI appointment.
fMRI methods
Image Acquisition
Scanning is performed at the University of Kansas Medical Center Hoglund Brain Imaging Center (HBIC) on a 3 Tesla Siemens Skyra scanner (Siemens, Erlangen, Germany). T1-weighted anatomic images are acquired with 3D MPRAGE sequence (TR/TE = 2300/2.01 ms, flip angle = 9°, FOV = 256 mm, matrix = 256 × 256, slice thickness = 1 mm). This scan will be used for slice localization for the functional scans, Talairach transformation, and coregistration with fMRI data. Scanning parameters for the Reward Prediction Task will be gradient echo blood oxygen level dependent (BOLD) scans will be acquired in 43 contiguous slices at a 40° angle to the AC-PC line (repetition time/echo time [TR/TE] = 2500/25 ms, flip angle = 90°, field of view [FOV] = 232 mm, matrix = 80 × 80, slice thickness = 3 mm, in-plane resolution = 2.9 × 2.9 mm). Scanning parameters for the Delay Discounting Task will include gradient echo blood oxygen level dependent (BOLD) scans that will be acquired in 35 contiguous slices at a 40° angle to the AC-PC line (repetition time/echo time [TR/TE] = 2000/25 ms, flip angle = 90°, field of view [FOV] = 232 mm, matrix = 80 × 80, slice thickness = 3 mm, in-plane resolution = 2.9 × 2.9 mm). All functional scans will be acquired at a 40° angle to the AC-PC line to optimize signal in the orbitofrontal cortex by minimizing susceptibility artifact. Based on recommendations by Deichmann et al [57], all participants will be positioned in the scanner so that the angle of the AC-PC plane is between 17° and 22° in scanner coordinate space. This angle will be verified with a localization scan. In addition to minimizing susceptibility artifact, this procedure acts to standardize head positioning between subject groups of widely divergent size (lean and obese).
Arterial Spin Labeling
In order to evaluate potential changes in the hemodynamic response arising from nonspecific cardiovascular changes, we will use arterial spin labeling to estimate mean cerebral blood flow. During each MR imaging session, one arterial spin labeling sequence (FOV=240mm×240mm, base resolution=64mm×64mm, slice thickness/gap/number of slices=8.0mm/2.0mm/9, TR=2500, TE=12, FA=90) will be collected to estimate mean cerebral blood flow. The Arterial Spin Labeling procedure should take approximately 5 minutes.
Resting state scan
One of the functional paradigms will be a resting state scan to examine the default mode network functional connectivity in obese and healthy weight participants. Image acquisition will occur under the same parameters as the reward prediction task. We will compare Adherers to Non-Adherers in the Baseline scan. Among Adherers, we hypothesize comparatively increased functional connectivity between lateral pre-frontal cortex and limbic regions, especially ventral striatum.
Anthropometric
Body weight/height and body composition
Anthropometric measures will be obtained from participants in the morning (between 7am and 10am) following a 12-hour fast at baseline, 4 and 9 months. Specifically, body weight will be assessed using a digital scale accurate to ± 0.1 kg (Befour Inc Model #PS6600, Saukville, WI). Height, weight, waist circumference and blood pressure measures will be obtained in standard hospital gown after attempting to void. Height will be measured using a stadiometer (Model PE-WM-60-84, Perspective Enterprises, Portage, MI) and BMI (kg/m2) will be calculated. Two waist circumference measures (within 2cm), using the procedures of Lohman et al. 1988 [58], will be averaged.
Fat-free mass, fat mass and % body fat will be assessed by DEXA (Prodigy Advance Plus, GE, Madison, WI) at baseline, 4 and 9 months. DEXA uses low level x-rays to determine body mass differences. Women will undergo pregnancy testing prior to each DEXA test and will be excluded if pregnant. Participants will wear a hospital gown during DEXA scans to standardize clothing. We will also use DEXA to estimate visceral adipose using existing equations [59, 60]. Skeletal muscle mass from DEXA derived appendicular lean soft tissue mass will be calculated using the equation of Kim et al. 2004 [61]. DEXA measures will allow us to compare body composition responses and any differences between men and women.
Fitness
Sub-maximal Treadmill Test
Participants will undergo a sub-maximal treadmill test. Maximal oxygen consumption will be estimated from the sub-maximal assessment to assess baseline and changes in estimated maximal oxygen consumption. Participants will walk at a speed of 3.0 mph beginning at 0% grade. The grade will be increased 1% at 1-minute intervals until target heart rate is achieved. Heart rates will be collected continuously with a multiple channel electrocardiograph. Expired air will be measured for oxygen and carbon dioxide at 20-second intervals using a ParvoMedics TrueOne 2400 indirect calorimetry system. The system will be calibrated before each test according to the specifications of the manufacturer (ParvoMedics, Inc Sandy, Utah).
Energy and Macronutrient Intake
Measurement of Energy Intake
Participants will be asked not to alter their dietary intake during the intervention. Dietary intake will be documented from 3-day food records (2 weekdays, 1 weekend day). The nutrient content of the record data will be determined using Nutrition Data System for Research (NDS-R, University of Minnesota, Minneapolis, MN). We do not anticipate changes in dietary total energy intake or macronutrient composition based on results of our previous exercise studies [62, 63] and recent systematic review [64]. The following quality control measures will be employed to minimize errors: 1) All participants will be provided a pamphlet (How to record your food intake) and trained on how to completely and accurately maintain a food record; 2) All 3-day records will be reviewed for clarification with the participants by the nutrition research staff; 3) All staff will be trained and verified as reliable prior to allowing them to enter dietary record data into NDS-R; 4) A uniform coding manual will be developed for entry of items into NDS-R; 5) All data will be checked by nutrition staff for quality assurance.
Data Management
Data will be categorized and entered into separate tables within a secure and relational SQL database. For example, daily records of PA will be in one table, while body weights will be in another table, blood pressure another table, etc., all linked by participant number. At logical time points, the data will be checked for outliers and normalcy. Questionable data (e.g., >3 standard deviations from the mean) will be re-checked for accuracy and re-entered if necessary.
Analysis plan and statistical power
Analysis plan
The planned data analyses are built around the Specific Aims. The first aim is to characterize brain activation underlying reward processing and impulse control in obese and healthy weight individuals at baseline.
Aim 1
We will use a reward prediction and delay discounting paradigms to examine neural systems underlying reward processing in obese participants in comparison to HW controls. We hypothesize that the obese group will show greater activation, compared to the healthy weight group, in limbic and paralimbic brain regions, during reward prediction and delivery and when making immediately reinforcing choices. The HW group will show greater activation, compared to the obese group, in lateral prefrontal regions when making delayed reinforcement choices. Hypotheses will be tested with common fMRI analysis software in Group (Obese, HW) x Valence (Reward, Punishment) and Group x Choice Type (Immediate Reward, Delayed Reward) repeated measures ANOVA, separately for prediction and delivery. We expect to find a significant Group x Valence interaction in both conditions. A priori regions include the amygdala, hippocampal formation, striatum (dorsal and ventral), OFC, MFC and ACC for immediate rewards, and lateral prefrontal cortex (dorsal and ventral) for delayed rewards. While the a priori hypotheses are focused on predictions of reward > punishment, we will also examine statistical maps in post-hoc analyses for areas in which punishment trials are greater than reward trials.
We will identify behavioral correlates of these activation differences. We hypothesize that areas of increased activation in limbic and paralimbic brain regions will be significantly correlated with self-report measures of cognitive and behavioral impulsivity (BIS-11 and BIS/BAS) in the obese group when examining immediate rewards, and negatively correlated with lateral prefrontal regions when examining delayed rewards. We will compute Pearson’s correlations between percent signal change (Reward vs. Punishment) and (Reward vs. Baseline) in the maximum voxel of the six a priori ROIs, bilaterally, and BIS-11 Total score, Behavioral Inhibition score of the BIS/BAS and Behavioral Activation score of the BIS/BAS. If the distribution is skewed for any of the scores, we will compute the Spearman’s correlation coefficients instead.
Our second aim is to identify brain activation predictors, from the baseline session, of adherence and success in the exercise program.
Aim 2
Identify fMRI activation differences in the Baseline Session between groups that ultimately adhere to the exercise program (adherers; complete ≥ 80% of sessions) or do not adhere to the exercise program (non-adherers; complete < 80% of sessions) along with continuous measures of adherence to the exercise program, including number of sessions completed, change in estimated VO2 Max, change in BMI, and change in % fat mass as measured by DEXA. We hypothesize that non-adherers with show greater activation, compared to adherers, in limbic and paralimbic brain regions, on both the reward prediction paradigm during reward prediction and delivery and the delay discounting paradigm when making immediately reinforcing choices. Adherers will show increased activation in lateral prefrontal regions when making delayed reinforcement choices. Hypotheses will be tested with fMRI analysis software in Outcome Group (Adherers, Non-adherers) x Valence (Reward, Punishment) during prediction and delivery and Outcome Group x Choice Type (Immediate Reward, Delayed Reward) repeated measures ANOVA, separately in obese and HW groups. We expect to find a significant Outcome Group x Valence interaction and Outcome Group x Choice Type interaction in both obese and HW groups. A priori regions include the amygdala, hippocampal formation, striatum (dorsal and ventral), OFC, MFC and ACC for immediate rewards, and lateral prefrontal cortex (dorsal and ventral) for delayed rewards.
Furthermore, on the reward prediction and delay discounting paradigms, we hypothesize that fMRI measures of activation in limbic and paralimbic regions will be negatively correlated with continuous measures of adherence. The primary outcome measure for this aim is number of sessions successfully completed between study entry and exit from the 9-month exercise program, across all obese and healthy weight participants. We will compute Pearson’s correlations (if the assumption of normality is violated then the Spearman correlations will be calculated) between number of sessions successfully completed and our main brain activation variables, % signal change (Reward vs. Punishment) from the 6 ROIs bilaterally.
In exploratory analyses, we will also examine other dependent variables that might better capture adherence, including change in VO2 Max, change in weight, and change in % fat mass as measured by DEXA. Further, we will model number of sessions completed by a multiple linear regression model using the Baseline Session % signal changes at the ROIs as predictors. We expect the model will contain fewer predictors (less than 12) as some predictors will be dropped out due to collinearity or non-significance, and can be used to study whether the cut-point of 80% sessions successfully completed is appropriate (e.g. dichotomize number of sessions at different proportions – e.g., 75%, 70%, and 65% – and construct ROC curves by logistic regression to determine which cut-point provides best sensitivity and specificity; i.e., the largest area under the ROC curve).
Note that we have designed the study to be adequately powered in the smallest subgroup (non-adherers) at the Post-Exercise scanning session. However, participants who drop out of the program for adherence-related reasons and who have completed less than 80% of sessions, will be assigned to the “non-adherer” group and every attempt will be made to get them to return for the Post-Exercise scanning session. Thus, it is likely that the final sample will be larger in the non-adhering obese and HW groups.
Our third aim is to identify the beneficial effects of exercise, weight loss and increased fitness on brain activation using fMRI data from baseline and 9 month scans.
Aim 3
For the reward prediction paradigm, adherers will show decreased activation to reward prediction and delivery from the Baseline to Post-Exercise sessions, compared to non-adherers, in limbic and paralimbic brain regions. This should be most pronounced on reward delivery, consistent with decreased impulsivity in the adherers. For the delay discounting paradigm, we predict that adherers will show greater increases in delayed reinforcement choices and greater increases in activation, from the Baseline to Post-Exercise sessions, in lateral prefrontal regions. Hypotheses will be tested in Session (Baseline, Post-Exercise) x Valence ANOVA and with Session x Choice Type ANOVA, separately in the four groups (Obese Adherers, Obese Non-adherers, HW Adherers, HW Non-adherers) in initial voxelwise analyses with fMRI analysis software. We will first inspect activation maps with thresholds set at p<.01 (corrected) to identify group differences, as described in Research Design and Methods. We will follow-up with ROI analyses in the six a priori regions, bilaterally by calculating % signal change (Reward vs. Punishment), for each group and session, in the maximum voxel and computing the changes in activation (% signal change at Post-Exercise session minus % signal change at Baseline). The changes in activation of the 2 obese and 2 healthy control groups will be compared by ANOVA at each ROI. We will also examine correlations (Pearson or Spearman) between % signal change from the ROIs and increased fitness as measured in changes in VO2 Max.
Power and sample size
Effect sizes have been calculated from our pilot data, supporting Aims 1 and 2, in which we expect that at the Baseline Session, the HW group will show greater activation compared to the obese group (Aim 1), and the non-adherers to the 9-week intervention will show greater activation compared to the adherers (Aim 2), in limbic and paralimbic brain regions, during reward expectation and delivery phases.
For Aim 1, our pilot data suggest the effect sizes for the reward-vs-punishment brain activation between the Obese and the HW groups are 1.5, 1.8, 2.0, 1.9, 1.2, 1.6, 0.9, 1.3, and 1.4 in regions of L- and R-OFC, L- and R-PFC, ACC, L- and R-Striatum, and L- and R-Temporal, respectively, during the expectation phase. The corresponding effects sizes during the delivery phase are 1.5, 1.3, 1.5, 1.9, 1.5, 1.3, 1.4, 1.8, and 1.7. For each aim, we will conduct 6 ROI comparisons/hemisphere × 2 hemispheres × 2 phases = 24 tests. Considering the multiplicity issue, we plan to control the false discovery rate at level of 0.05. If the true effect sizes are similar to what pilot data suggest, the minimal effect size will be 0.9 for the obese-vs-HW comparisons. Thus, the proposed 90 obese and 90 HW participants will provide statistical power greater than 99% for this comparison at R-Striatum during the expectation phase based on the 1-sided 2-sample t-test at significance level 0.05/24 = 0.002, and even greater power for each of the other 23 − 2 = 21 comparisons with greater effect sizes according to the Benjamini-Hochberg procedure.
For the correlations between self-reported impulsivity and brain activation, the 90 OB participants will provide 80% power to detect a correlation ranging from 0.29 to 0.42 based on 2-sided t-tests at the adjusted significance level (the Benjamini-Hochberg procedure) ranging from 0.05 to 0.05/72 = 0.0007 (12 ROI’s per phase × 2 phases × 3 cognitive/behavioral scores = 72) for the reward paradigm.
For Aim 2, our pilot data suggest the effect sizes for the reward-vs-punishment brain activation between the non-adherers and the adherers at the aforementioned ROI’s are respectively 4.3, 4.0, 2.6, 3.9, 1.4, 2.6, 1.8, 2.6, and 2.8 during the expectation phase, and are 3.5, 2.8, 3.4, 4.7, 3.2, 4.3, 3.1, 3.2, and 2.8 during the delivery phase. Assuming a conservative 25% attrition rate in the 90 obese and 90 HW participants who might not be available for the final scanning session, we expect 70% will complete 80% of sessions and 30% won’t, which are equal to 94 adherers and 41 non-adherers participants, respectively. This sample size will provide greater than 99% power for this comparison at the ACC during the expectation phase based on 1-sided 2-sample t-test at significance level 0.05/24 = 0.002, and even greater power for each of the other 23 − 6 (number of comparisons with directions opposite to the hypothesis) = 17 comparisons with greater effect sizes. If the attrition rate remains the same, statistical power will be greater if the ratio of non-adherers vs. adherers approaches 1:1 but will be lower if the ratio departs from 1:1. In the latter case, if the ratio is 19:1, or 128 vs. 7 participants, we will still have 74% power for the each of the 1 + 17 = 18 comparisons discussed above; however, we do not anticipate the ratio will be so extreme based on previous experience with exercise programs.
Anticipated Problems and Challenges
We have considered several potential problems and challenges.
1. Potential data analysis challenges
The groups may not segregate as predicted into adherers and non-adherers. The expectations are made based on previous work from the Donnelly Lab, using the same exercise program. It is still possible, however, that the numbers will not come out as expected. Accordingly, the adherence data will be treated as both categorical and continuous in the planned data analyses. In addition, we plan to use multiple linear regression modeling to evaluate the distribution of the data and determine whether other cut-offs for adherence might better fit our data. These analyses are detailed in the Data Analysis section (Specific Aim 2.2).
2. Potential negative results for longitudinal arm of the study
We do not have longitudinal fMRI data to establish the outcome of exercise on brain function yet. However, the outcome of the post-exercise analyses will be of interest regardless of outcome, whether brain function changes or not. Either outcome answers an important scientific question and we have adequate statistical power to prove a negative result.
3. Other potential exercise interventions
There are many potential choices in exercise programs that might produce changes in weight loss, fitness and health benefits (e.g., positive metabolic and physiologic changes). We chose an exercise program with which we have a history of success and necessary data to generate realistic expected outcomes. Additionally, it is a program that is frequently used in other studies and, thus, provides comparisons.
4. Potential non-specific effects on the hemodynamic response arising from vascular changes
Previous research suggests that fitness has beneficial effects on specific brain networks, at least in the context of specific cognitive activation paradigms. There may also be non-specific effects on the hemodynamic response, however, arising from cardiovascular changes. We will conduct arterial spin labeling (ASL-MRI) scanning and analyze data from the primary ROIs and also two control regions (motor cortex and visual cortex).
5. BMI constraints for MRI
Limitations in the size of the bore of the MR magnet mean that we must limit our obese group to a BMI < 41 and cannot, therefore study morbid obesity. This constraint applies equally to all MRI studies and does not reduce the advantages fMRI has over other imaging methods, such as PET (e.g., increased spatial and temporal resolution, no exposure to ionizing radiation). In addition, moderate obesity is still associated with negative health consequences and the same individuals, left untreated, may ultimately progress to morbid obesity.
6. No non-aerobic exercise control group
Other studies examining the effects of cardiovascular fitness on brain function have included a non-aerobic exercise control group to control for the effects of paradigm repetition and other non-specific effects. The focus of designing our study was first to ensure adequate statistical power for the prediction Aims. We do not have a non-aerobic exercise control group; however, we will compare adherers to non-adherers. Both groups will undergo two scanning sessions and will have some degree of interaction with investigators and staff, but the non-adherers should have less cardiovascular fitness than adherers.
Discussion
Obesity is a complex problem in the United States and other developed countries with significant public health consequences. Regular exercise has important health benefits; however, many individuals have difficulty maintaining adequate levels of exercise. This is particularly problematic in the obese. Initial studies suggest that reward processing and impulse control are potentially associated with difficulties in adhering to exercise programs. However, no direct studies have been reported and little is known regarding underlying neural mechanisms. Furthermore, the degree to which these reward processing and impulse control characteristics are influenced by exercise is also not known.
This project will characterize brain activation underlying reward processing and impulse control in obese and HW individuals, identify brain activation predictors of adherence and success in the exercise program and measuring the effects of exercise and improved fitness on brain activation. To do this, we will conduct reward prediction and delay discounting fMRI paradigms in sedentary obese and sedentary healthy weight participants before and after a nine-month exercise intervention.
Findings from this study will have significant implications for understanding mechanisms contributing to obesity and exercise, and may ultimately lead to more effective interventions. For instance functional neuroimaging may provide support and insight for new interventions designed to specifically change activity in target areas of the brain in order to facilitate more adaptive behaviors that are less contingent on immediate reward.
Acknowledgments
Funding: National Institutes of Health (R01 - DK64972).
The Hoglund Brain Imaging Center is supported by a generous gift from Forrest and Sally Hoglund and funding from the National Institutes of Health (P30 HD002528, S10 RR29577, UL1 TR000001, and P30 AG035982). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Abbreviations
- BMI
Body Mass Index
- DEXA
Dual Energy X-ray Absorptiometry
- fMRI
Functional Magnetic Resonance Imaging
- HW
Healthy Weight
- NDS-R
Nutrition Data System for Research
- MPA
Moderate Physical Activity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.U.S. Department of Health and Human Services, Physical Activity Advisory Committee. Physical Activity Advisory Committee Report, 2008. Washington D.C: U.S. Department of Health and Human Services; 2008. [Google Scholar]
- 2.Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273:402–7. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 3.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–34. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 4.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459–71. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 5.Leser MS, Yanovski SZ, Yanovski JA. A low-fat intake and greater activity level are associated with lower weight regain 3 years after completing a very-low-calorie diet. J Am Diet Assoc. 2002;102:1252–6. doi: 10.1016/s0002-8223(02)90277-4. [DOI] [PubMed] [Google Scholar]
- 6.Fogelholm M, Kukkonen-Harjula K, Oja P. Eating control and physical activity as determinants of short-term weight maintenance after a very-low-calorie diet among obese women. Int J Obes Relat Metab Disord. 1999;23:203–10. doi: 10.1038/sj.ijo.0800825. [DOI] [PubMed] [Google Scholar]
- 7.Schoeller DA, Shay K, Kushner RF. How much physical activity is needed to minimize weight gain in previously obese women? Am J Clin Nutr. 1997;66:551–6. doi: 10.1093/ajcn/66.3.551. [DOI] [PubMed] [Google Scholar]
- 8.Kayman S, Bruvold W, Stern JS. Maintenance and relapse after weight loss in women: behavioral aspects. Am J Clin Nutr. 1990;52:800–7. doi: 10.1093/ajcn/52.5.800. [DOI] [PubMed] [Google Scholar]
- 9.Crawford D, Jeffery RW, French SA. Can anyone successfully control their weight? Findings of a three year community-based study of men and women. Int J Obes. 2000;24(9):1107–10. doi: 10.1038/sj.ijo.0801374. [DOI] [PubMed] [Google Scholar]
- 10.Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr. 2001;21:323–41. doi: 10.1146/annurev.nutr.21.1.323. [DOI] [PubMed] [Google Scholar]
- 11.Dishman RK. Compliance/adherence in health-related exercise. Health Psychology. 1982;1:237–67. [Google Scholar]
- 12.Ward A, Morgan WP. Adherence patterns of healthy men and women enrolled in an adult exercise program. Journal of Cardiac Rehabilitation. 1984:143–52. [Google Scholar]
- 13.Dishman RK. The problem of exercise adherence: Fighting sloth in nations with market economies. Quest. 2001;53:279–94. [Google Scholar]
- 14.Dishman RK, Washburn RA, Heath GW. Physical Activity Epidemiology. Champaign, IL: Human Kinetics Publishers; 2004. [Google Scholar]
- 15.Bickel WK, Jarmolowicz DP, Mueller ET, Koffarnus MN, Gatchalian KM. Excessive discounting of delayed reinforcers as a trans-disease process contributing to addiction and other disease-related vulnerabilities: emerging evidence. Pharmacology & therapeutics. 2012;134:287–97. doi: 10.1016/j.pharmthera.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Appelhans BM, Waring ME, Schneider KL, Pagoto SL, DeBiasse MA, Whited MC, et al. Delay discounting and intake of ready-to-eat and away-from-home foods in overweight and obese women. Appetite. 2012 doi: 10.1016/j.appet.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garza KB, Harris CV, Bolding MS. Examination of value of the future and health beliefs to explain dietary and physical activity behaviors. Research in Social and Administrative Pharmacy. 2012 doi: 10.1016/j.sapharm.2012.12.001. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Melanko S, Larkin KT. Preference for immediate reinforcement over delayed reinforcement: relation between delay discounting and health behavior. Journal of behavioral medicine. 2013;36:34–43. doi: 10.1007/s10865-012-9399-z. [DOI] [PubMed] [Google Scholar]
- 19.Davis C, Patte K, Levitan R, Reid C, Tweed S, Curtis C. From motivation to behaviour: a model of reward sensitivity, overeating, and food preferences in the risk profile for obesity. Appetite. 2007;48:12–9. doi: 10.1016/j.appet.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Guerrieri R, Nederkoorn C, Stankiewicz K, Alberts H, Geschwind N, Martijn C, et al. The influence of trait and induced state impulsivity on food intake in normal-weight healthy women. Appetite. 2007;49:66–73. doi: 10.1016/j.appet.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Epstein LH, Leddy JJ, Temple JL, Faith MS. Food reinforcement and eating: a multilevel analysis. Psychological bulletin. 2007;133:884–906. doi: 10.1037/0033-2909.133.5.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis C, Levitan RD, Carter J, Kaplan AS, Reid C, Curtis C, et al. Personality and eating behaviors: a case-control study of binge eating disorder. The International journal of eating disorders. 2008;41:243–50. doi: 10.1002/eat.20499. [DOI] [PubMed] [Google Scholar]
- 23.Galanti K, Gluck ME, Geliebter A. Test meal intake in obese binge eaters in relation to impulsivity and compulsivity. The International journal of eating disorders. 2007;40:727–32. doi: 10.1002/eat.20441. [DOI] [PubMed] [Google Scholar]
- 24.Logue A. Self-control and health behavior. Reframing health behavior change with behavioral economics. 2000:167–92. [Google Scholar]
- 25.Bogg T, Roberts BW. Conscientiousness and health-related behaviors: a meta-analysis of the leading behavioral contributors to mortality. Psychological bulletin. 2004;130:887–919. doi: 10.1037/0033-2909.130.6.887. [DOI] [PubMed] [Google Scholar]
- 26.Saelens BE, Epstein LH. The rate of sedentary activities determines the reinforcing value of physical activity. Health Psychol. 1999;18:655–9. doi: 10.1037//0278-6133.18.6.655. [DOI] [PubMed] [Google Scholar]
- 27.Epstein LH, Saelens BE. Behavioral economics of obesity: food intake and energy expenditure. Reframing health behavior change with behavioral economics. 2000:293–311. [Google Scholar]
- 28.Tuson KM, Sinyor D. On the affective benefits of acute aerobic exercise: Taking stock after twenty years of research. In: Sereganian P, editor. On the affective benefits of acute aerobic exercise: Taking stock after twenty years of research. New York, NY: John Wiley & Sons; 1993. pp. 80–121. [Google Scholar]
- 29.Lind E, Joens-Matre RR, Ekkekakis P. What intensity of physical activity do previously sedentary middle-aged women select? Evidence of a coherent pattern from physiological, perceptual, and affective markers. Prev Med. 2005;40:407–19. doi: 10.1016/j.ypmed.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Ekkekakis P, Lind E. Exercise does not feel the same when you are overweight: the impact of self-selected and imposed intensity on affect and exertion. Int J Obes (Lond) 2006;30:652–60. doi: 10.1038/sj.ijo.0803052. [DOI] [PubMed] [Google Scholar]
- 31.Trost SG, Owen N, Bauman AE, Sallis JF, Brown W. Correlates of adults’ participation in physical activity: review and update. Medicine & Science in Sports & Exercise. 2002;34:1996–2001. doi: 10.1097/00005768-200212000-00020. [DOI] [PubMed] [Google Scholar]
- 32.McAuley E, Blissmer B. Self-efficacy determinants and consequences of physical activity. American College of Sports Medicine. 1999;28:85–8. [PubMed] [Google Scholar]
- 33.Bartholomew JB, Miller BM. Affective responses to an aerobic dance class: the impact of perceived performance. Res Q Exerc Sport. 2002;73:301–9. doi: 10.1080/02701367.2002.10609024. [DOI] [PubMed] [Google Scholar]
- 34.Donnelly JE, Washburn RA, Smith BK, Sullivan DK, Gibson C, Honas JJ, et al. A randomized, controlled, supervised, exercise trial in young overweight men and women: The Midwest Exercise Trial II (MET2) Contemp Clin Trials. 2012 doi: 10.1016/j.cct.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirk EP, Jacobsen DJ, Gibson C, Hill JO, Donnelly JE. Time-course for changes in aerobic capacity and body composition in overweight men and women in response to long-term exercise: The Midwest Exercise Trial (MET) International Journal of Obesity. 2003;27:912–9. doi: 10.1038/sj.ijo.0802317. [DOI] [PubMed] [Google Scholar]
- 36.Taylor H, Jacobs D, Schucker B, Knudsen J, Leon A, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31:741–55. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 37.Kansas Behavioral Risk Factor Surveillance System. Health Risk Behaviors of Kansans: Annual Report. Kansas Department of Health and Environment; 2011. http://www.kdheks.gov/brfss/publications.html. [Google Scholar]
- 38.Garner DM, Olmsted MP, Bohr Y, Garfinkel PE. The eating attitudes test: psychometric features and clinical correlates. Psychol Med. 1982;12:871–8. doi: 10.1017/s0033291700049163. [DOI] [PubMed] [Google Scholar]
- 39.Gormally J, Black S, Daston S, Rardin D. The assessment of binge eating severity among obese persons. Addictive behaviors. 1982;7:47–55. doi: 10.1016/0306-4603(82)90024-7. [DOI] [PubMed] [Google Scholar]
- 40.Radloff LS. The CED-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:395–401. [Google Scholar]
- 41.Wechsler D. Wechsler abbreviated intelligence scale. San Antonio: The Psychological Corporation; 1999. [Google Scholar]
- 42.Strauss A, Corbin J. Basics of qualitative research: Techniques and procedures for developing grounded theory. 1998 [Google Scholar]
- 43.Rogers RD, Ramnani N, Mackay C, Wilson JL, Jezzard P, Carter CS, et al. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biological psychiatry. 2004;55:594–602. doi: 10.1016/j.biopsych.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 44.Delgado MR, Nystrom LE, Fissell D, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. Journal of Neurophysiology. 2000;84:3072–7. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- 45.Delgado MR, Nystrom LE, Fissell C, Noll D, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. Journal of neurophysiology. 2000;84:3072–7. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- 46.Berns GS, McClure SM, Pagnoni G, Montague PR. Predictability modulates human brain response to reward. The Journal of Neuroscience. 2001;21:2793–8. doi: 10.1523/JNEUROSCI.21-08-02793.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abler B, Walter H, Erk S, Kammerer H, Spitzer M. Prediction error as a linear function of reward probability is coded in human nucleus accumbens. Neuro Image. 2006;31:790–5. doi: 10.1016/j.neuroimage.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 48.Ainslie G. Specious reward: a behavioral theory of impulsiveness and impulse control. Psychological bulletin. 1975;82:463. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- 49.McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–7. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- 50.Wittmann M, Leland DS, Paulus MP. Time and decision making: differential contribution of the posterior insular cortex and the striatum during a delay discounting task. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 2007;179:643–53. doi: 10.1007/s00221-006-0822-y. [DOI] [PubMed] [Google Scholar]
- 51.Ballard K, Knutson B. Dissociable neural representations of future reward magnitude and delay during temporal discounting. Neuro Image. 2009;45:143–50. doi: 10.1016/j.neuroimage.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin LE, Potts GF. Medial frontal event-related potentials and reward prediction: do responses matter? Brain and cognition. 2011;77:128–34. doi: 10.1016/j.bandc.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Potts GF, Martin LE, Kamp SM, Donchin E. Neural response to action and reward prediction errors: Comparing the error-related negativity to behavioral errors and the feedback-related negativity to reward prediction violations. Psychophysiology. 2010 doi: 10.1111/j.1469-8986.2010.01049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martin LE, Potts GF, Burton PC, Montague PR. Electrophysiological and hemodynamic responses to reward prediction violation. Neuroreport. 2009;20:1140–3. doi: 10.1097/WNR.0b013e32832f0dca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Potts GF, Martin LE, Burton P, Montague PR. When things are better or worse than expected: the medial frontal cortex and the allocation of processing resources. Journal of cognitive neuroscience. 2006;18:1112–9. doi: 10.1162/jocn.2006.18.7.1112. [DOI] [PubMed] [Google Scholar]
- 56.Martin LE, Potts GF. Reward sensitivity in impulsivity. Neuroreport. 2004;15:1519–22. doi: 10.1097/01.wnr.0000132920.12990.b9. [DOI] [PubMed] [Google Scholar]
- 57.Deichmann R, Gottfried JA, Hutton C, Turner R. Optimized EPI for fMRI studies of the orbitofrontal cortex. Neuroimage. 2003;19:430–41. doi: 10.1016/s1053-8119(03)00073-9. [DOI] [PubMed] [Google Scholar]
- 58.Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Champaign, Ill: Human Kinetics Books; 1988. [Google Scholar]
- 59.Treuth MS, Hunter GR, Kekes-Szabo T. Estimating intraabdominal adipose tissue in women by dual-energy X-ray absorptiometry. Am J Clin Nutr. 1995;62:527–32. doi: 10.1093/ajcn/62.3.527. [DOI] [PubMed] [Google Scholar]
- 60.Treuth MS, Hunter GR, Kekes-Szabo T. Estimating intraabdominal adipose tissue in women by dual- energy X-ray absorptiometry. Am J Clin Nutr. 1995;62:527–32. doi: 10.1093/ajcn/62.3.527. [DOI] [PubMed] [Google Scholar]
- 61.Kim J, Heshka S, Gallagher D, Kotler DP, Mayer L, Albu J, et al. Intermuscular adipose tissue-free skeletal muscle mass: estimation by dual-energy X-ray absorptiometry in adults. J Appl Physiol. 2004;97:655–60. doi: 10.1152/japplphysiol.00260.2004. [DOI] [PubMed] [Google Scholar]
- 62.Donnelly JE, Kirk EP, Jacobsen DJ, Hill JO, Sullivan DK, Johnson SL. Effects of 16-months of verified, supervised aerobic exercise on macronutrient intake in overweight men and women: The midwest exercise trial (MET) American Journal of Clinical Nutrition. 2003 doi: 10.1093/ajcn/78.5.950. [DOI] [PubMed] [Google Scholar]
- 63.Donnelly JE, Honas JJ, Smith BK, Mayo MS, Gibson CA, Sullivan DK, et al. Aerobic exercise alone results in clinically significant weight loss for men and women: midwest exercise trial 2. Obesity (Silver Spring) 2013;21:E219–28. doi: 10.1002/oby.20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Donnelly JE, Herrmann SD, Lambourne K, Szabo AN, Honas JJ, Washburn RA. Does increased exercise or physical activity alter ad-libitum daily energy intake or macronutrient composition in healthy adults? A systematic review. PLoS One. doi: 10.1371/journal.pone.0083498. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]