Abstract
Puberty and adolescence are major life transitions during which an individual’s physiology and behavior changes from that of a juvenile to that of an adult. Here we review studies documenting the effects of stressors during pubertal and adolescent development on the adult brain and behavior. The experience of complex or compound stressors during puberty/adolescence generally increases stress reactivity, increases anxiety and depression, and decreases cognitive performance in adulthood. These behavioral changes correlate with decreased hippocampal volumes and alterations in neural plasticity. Moreover, stressful experiences during puberty disrupt behavioral responses to gonadal hormones both in sexual performance and on cognition and emotionality. These behavioral changes correlate with altered estrogen receptor densities in some estrogen-concentrating brain areas, suggesting a remodeling of the brain’s response to hormones. A hypothesis is presented that activation of the immune system results in chronic neuroinflammation that may mediate the alterations of hormone-modulated behaviors in adulthood.
Keywords: Stress, Immune Challenge, Depression, Anxiety, Cognitive function, Sexual Behavior, Estradiol, Progesterone, Stress Reactivity
1. Introduction
Puberty is a major life transition from that of a non-reproductive juvenile into a reproductively competent adult. Puberty and adolescence, the developmental period associated with puberty, are times of great physiological, psychosocial and cultural changes. As such, puberty and adolescence are also times of great developmental plasticity (Dahl and Gunnar, 2009; Patton and Viner, 2007). The neuroplasticity of puberty may also contribute to vulnerability for the development of mental diseases (Andersen, 2003). The National Comorbidity Survey Replication study, which assessed over 9000 people representative of the US population, indicate that affective disorders such as anxiety, bipolar disorder, and major depression emerge during adolescence, with the peak age of onset for these diseases of 14 years (Kessler et al., 2005; Paus et al., 2008). A major predictor of an individual’s susceptibility to mental disease is his or her sex: women show higher rates of anxiety and depression, and men have higher rates of autism spectrum disorders, schizophrenia, and attention deficit hyperactivity disorder (Astbury, 2001; Eaton et al., 2012). However, the 2:1 female-male prevalence in depression and anxiety disorders emerges only after puberty (Angold and Costello, 2006). Moreover, the pubertal status (Tanner stage III) predicts this sex difference better than age alone (Hayward and Sanborn, 2002; Patton et al., 1996), suggesting that ovarian hormones, particularly in puberty/adolescence, play a role in the etiology of affective disorders.
Recently, studies have demonstrated that peri-pubertal or adolescent exposure to stressful life experiences contributes greatly to an individual’s vulnerability to mental disease (Ge et al., 2001; Grant et al., 2003; Grant et al., 2004; Silverman et al., 1996; Turner and Lloyd, 2004). Yet little is known about how stress could disrupt normal neural development during the pubertal or adolescent period to produce a brain that is particularly vulnerable to mental diseases. Therefore, it is important to understand the neural mechanisms by which a pubertal stressor may produce a brain that is susceptible to specific mental diseases. Using animal models, we are beginning to learn the unique ways in which a pubertal organism responds to a stressor, and how the experience of stressors during pubertal development alters adult physiology and behavior.
The focus of this review is to present results of recent studies that demonstrate i) that stressors experienced in puberty alter steroid hormone-influenced behaviors in adulthood and ii) that these behavioral changes may be mediated through alterations in the ongoing processes of brain development. First, we provide a short background on some of the major developmental processes that occur during puberty and adolescence. Next, we discuss the ability of stressors during pubertal development to alter the display of sexual behaviors, anxiety- and depression-like behaviors, and learning, memory and cognitive behaviors, giving special attention to the possible neural correlates and mechanisms of these behavioral responses. Finally, we postulate a novel mechanism by which stressors may act to achieve enduring effects in the adult brain and behavior.
2. Puberty and Adolescence
2.1. Definitions of puberty and adolescence
Puberty and adolescence are commonly conflated; however, in order to understand the ways that puberty and adolescence can influence the brain’s response to hormones, it is critical to disambiguate and use precise definitions of both puberty and adolescence, as they are distinct processes. Puberty refers to the developmental transition from a non-reproductive state into a reproductive state, culminating in reproductive competence (Sisk and Foster, 2004; Waylen and Wolke, 2004). Even though it is sometimes referred to as a singular event, puberty is a prolonged, developmental process. The term is not only used to indicate the attainment of reproductive competence, but also it is used to describe the reproductive changes culminating in reproductive competence. Throughout this review, we will refer to the changes that occur in reproductive status as pubertal developments and the time during which these pubertal developments occur as the pubertal period. In contrast, adolescence refers to the social and cognitive maturation associated with and resulting from the hormonal changes of puberty (Sisk and Foster, 2004). While the biological hallmarks of pubertal development have been extensively categorized as the Tanner Stages, the biological hallmarks of adolescence are more nebulous and include the neuroplasticity and development of the cortical and limbic areas of the brain.
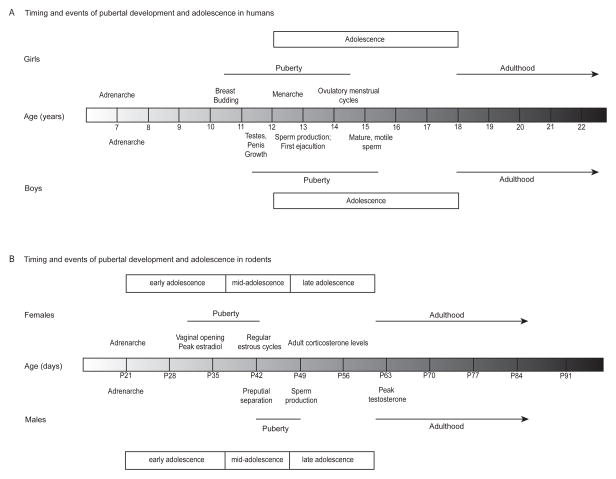
Pubertal development in humans comprises several physiological and physical changes (Figure 1), including the development of secondary sexual characteristics (e.g., breast budding in girls and testicular enlargement in boys and the growth of pubic hair) and culminating in the onset of reproductive competence (e.g., menarche and ovulatory menstrual cycles in girls and semenarche and spermarche in boys) (Marshall and Tanner, 1969; Marshall and Tanner, 1970; Tanner, 1962). The pubertal processes are dependent on the presence of estrogens and progestins in girls and androgens in boys (Tanner, 1962). Typically girls begin pubertal development around ages 10–11 and have completed the process by ages 15–16 (Marshall and Tanner, 1969; Tanner, 1962), whereas boys begin puberty slightly later at ages 11–12 and have completed it by 16–17 (Marshall and Tanner, 1969; Tanner, 1962).
Figure 1.
A generalization of the approximate timing and events of pubertal development and adolescence in (A) humans and (B) rats. (A) The average ages of the pubertal developments for girls (top) and boys (bottom) (Marshall and Tanner, 1969; Marshall and Tanner, 1970; Sizonenko, 1989; Tanner, 1962) and for (B) females (top) and males (bottom) (Korenbrot et al., 1977; Lohmiller and Sonya, 2006; Ojeda and Urbanski, 1994; Ojeda et al., 1976; Parker and Mahesh, 1976; Vandenbergh, 1967; Vandenbergh, 1969). Puberty onset is defined by the appearance of the secondary sex characteristics, and the offset is defined by the emergence of reproductive competence as indicated by ovulatory menstrual cycles and mature, motile sperm. In humans, the ages ranging from 12 to 18 are commonly used as the age range of adolescence (Spear, 2000); whereas, in rats adolescence is conceptualized by early, mid, and late adolescent periods (Tirelli et al., 2003). It should be noted that the times indicated for particular stages are quite variable, and can be influenced by nutrition and environmental conditions.
In female rodents, the first stage of pubertal development is typically the first external sign of ovarian activity (i.e., vaginal opening), and puberty is considered complete with the onset of the first reproductive (i.e., estrous) cycle. Although variable and dependent on housing conditions (e.g., (Vandenbergh, 1967; Vandenbergh, 1969)), the day of vaginal opening is approximately postnatal day 35 (P35) in rats (Ojeda and Urbanski, 1994; Ojeda et al., 1976) and P25 in C57Bl/6 mice and P30 in CD1 mice housed in single sex rooms (Ismail & Blaustein, unpublished observations). Female rats can have quite a short latency between vaginal opening and estrous cycles. This latency can be as prolonged as a week (Lohmiller and Sonya, 2006), but can be as brief as the same day (Ojeda et al., 1976; Parker and Mahesh, 1976). In female mice, however, the pubertal period may last several weeks when females are housed in the absence of male mice. While the presence of a male or male odor can both advance vaginal opening and shorten the interval between vaginal opening and first estrus (i.e., 7–10 days instead of 20 days), this pubertal period is still considered extended, compared to rats (Vandenbergh, 1967; Vandenbergh, 1969). It is important to note that girls also have an extended pubertal period of 2 – 3 years, if one considers the timing of analogous events beginning with the development of pubic hair and breast budding (Tanner Stage II) and ending with menarche and regular ovulatory menstrual cycles (Sizonenko, 1989).
The external markers of pubertal development in male rodents are the separation of the prepuce from the glans penis, or preputial separation, and the presence of mature motile sperm in the caput epididymis (Korenbrot et al., 1977). In rats, these events can be separated by a few days to a week (Korenbrot et al., 1977), but the average day of preputial separation is P42. Although preputial separation is also used as a marker of pubertal development in male mice, the timing of separation and the presence of mature sperm remain to be determined.
While animal work may inform the mechanism by which the pubertal brain is particularly vulnerable to insult, it can be difficult to reconcile animal work and the human condition. In particular, work in humans often does not precisely define pubertal stage but rather focuses on age or events that occur during adolescence. Additionally, studies using rodents typically use chronological age, rather than pubertal status, to categorize the animals using a classification system of early adolescence (P21–P34), mid-adolescence (P35–46), and late adolescence (P47–59) (Tirelli et al., 2003). Another widely used convention for classifications within the rat literature defines the adolescent period as P28–42 (Spear, 2000). Therefore, the adolescent and pubertal periods of males are not orthogonal; rather they are overlapping, resulting in ambiguity in the results of many experiments (Figure 1). That is, it can be difficult to determine whether the particular effects are due to manipulation at a particular chronological age or to a particular reproductive stage. Therefore, in this review, we will focus on the pubertal stage of development when possible.
2.2. Puberty as time of neuroplasticity and neural development
The pubertal and adolescent periods are times of great neuroplasticity and neural development in both humans and in rodent animal model species. The pubertal period shares many developmental processes with the prenatal and early postnatal periods: I) neuronal growth (Phoenix et al., 1959; Pinos et al., 2001) or death (Nunez et al., 2001); II) growth of axonal projections or axonal sprouting (Benes et al., 2000; Dahl, 2004; Lewis et al., 2004); III) reshaping of the dendritic tree by dendritic elaboration or retraction (Goldstein et al., 1990; Meyer et al., 1978); IV) synapse formation (Bourgeois et al., 1994; Bourgeois and Rakic, 1993) or elimination (Andersen et al., 2002; Gerocs et al., 1986; Huttenlocher and Dabholkar, 1997; Meyer et al., 1978); and V) myelination (Benes et al., 1994; Nunez et al., 2000; Woo et al., 1997).
Some brain regions, such as the prefrontal cortex, continue to develop into adulthood in humans. In particular, gross morphological changes result from both increases in white matter by continued axonal myelination and changes in grey matter (Barnea-Goraly et al., 2005; Giedd et al., 1999; Paus et al., 1999; Reiss et al., 1996; Sowell et al., 2001). The volume of grey matter in the frontal and parietal lobes first increases in early adolescence and then decreases in later adolescence (Shaw et al., 2008). The age-related decrease in grey matter volume has been proposed to be due to the increase in myelination and axonal growth, resulting in a net increase in white matter and subsequent decrease in grey matter (Paus et al., 2008). Alternatively, this decrease in grey matter may reflect the synaptic reorganization in the form of proliferation of neurons and/or synapses, followed by subsequent cell death and synapse elimination (Giedd et al., 1999; Huttenlocher, 1979; Petanjek et al., 2011; Sowell et al., 2001). Interestingly, sex differences in region volume or neural density emerge as a consequence of the brain remodeling. The hippocampal volume increases in girls, whereas the volume of the amygdala (Giedd et al., 1997) increases in boys. Additionally, the bed nucleus of the stria terminalis is larger and with a greater number of neurons in boys (Chung et al., 2002).
Rodents also experience neural development and brain remodeling during the pubertal period. These changes take the form of gross morphological alterations, such as volumetric changes in grey matter and neuronal cell numbers. The remodeling of the pubertal brain in rodents also includes reorganization at the pre-and post-synaptic levels (reviewed in Sisk and Zehr, 2005). Important for this review is the fact that many, but not all, of the processes that result in the remodeling of the pubertal and adolescent brain are driven by the pubertal hormones (Andersen et al., 2002; Davis et al., 1996; Goldstein et al., 1990; Guillamon et al., 1988; Nunez et al., 2000). Therefore, pubertal status, rather than chronological age, is likely to be the critical factor. Unfortunately, few studies focus on the precise, clearly defined, stage of reproductive development, so it can be difficult to determine the effects of the stressors during this time frame on subsequent behavioral and neurobiological processes in adulthood.
2.3. Puberty as a second period of organizational actions of steroid hormones
Multiple sensitive periods exist for the organization of the brain and nervous system, primarily occurring during periods of rapid developmental changes (Scott et al., 1974). One of the most well-studied and best-characterized periods is that of the perinatal period in rodents. During the perinatal period, the steroid hormones, testosterone and estradiol, have the ability to organize the neural circuits and permanently alter the brain and behavior. That is, even a brief exposure to testosterone or estradiol can sexually differentiate the brain (Lenz et al., 2012; McCarthy, 2010; Phoenix et al., 1959; Wallen, 2005). Organizational effects are contrasted with the activational effects of hormones, which are transient modifications or modulation of activity that can alter physiology and behavior (Phoenix et al., 1959). Additionally, the organizational actions of hormones are also limited to critical or vulnerable windows. Prior to and after these windows of plasticity, hormones may no longer affect the brain’s development (Phoenix et al., 1959). The pubertal period is now understood to be one such period of organization by steroid hormones (Sisk and Zehr, 2005).
Implicit in the discussion of the remodeling of the pubertal and adolescent brain is the conceptualization of the pubertal period as a second phase of steroid-dependent organization of behaviors and their underlying neural circuitry. Therefore, an individual’s life experience during pubertal development and adolescence may have enduring consequences, which profoundly affect adult life. Furthermore, as many of the neurodevelopmental processes occurring during the rather nebulous period of adolescence are hormonally-regulated or modulated, it may be that pubertal status and the underlying hormonal milieu are the critical factors in an individual’s susceptibility to the enduring consequences of experience. Indeed, stress during pubertal development and/or adolescence results in profound alterations in physiology and behavior as indicated by I) increases in anxiety and depression (Ge et al., 2001; Patton and Viner, 2007); II) risk-taking and novelty-seeking behaviors (Arnett, 1996; Roberti, 2004; Wagner, 2001); III) alterations in learning and cognition (Im-Bolter et al., 2013); and IV) drug and alcohol use and abuse (Bekman et al., 2010; Crawford et al., 2003; MacPherson et al., 2010). In this review, we will confine ourselves to the ability of stressors in the pubertal and adolescent periods to alter the brain’s response to gonadal (Section 3) and adrenal (Section 4) hormones.
3. Gonadal Steroid Hormones
3.1. Hypothalamic-pituitary-gonadal (HPG) Axis
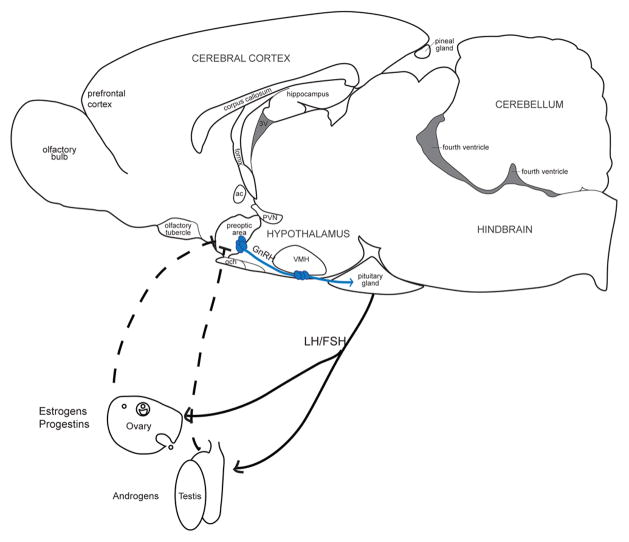
The hypothalamus and preoptic areas of the brain control much of the neuroendocrine activity of vertebrates via regulation of hormone secretion. The secretion of the gonadal hormones, primarily estrogens and progestins in females and androgens in males, is controlled by the HPG axis (Figure 2). The primary sources of gonadal hormones are the ovaries in females and the testes in males. The ovary primarily produces two classes of steroid hormones from cholesterol: estrogens and progestins. Of the estrogens, the most behaviorly potent is estradiol (Feder and Silver, 1974) and will be discussed in this review. Progestins, or progestogens, are the second class of steroid hormones synthesized in the ovary. Progesterone is the most biologically potent of the progestins. The testes produce androgens, of which testosterone is the primary androgen discussed in this review.
Figure 2.
Representation of the Hypothalamus-Pituitary-Gonadal Axis. Neurons in the preoptic area and mediobasal hypothalamus secrete gonadotropin-releasing hormone (GnRH), a neurohormone, into the hypophyseal portal system of the mediam eminence. The portal blood carries GnRH into the pituitary gland and activates its receptor, gonadotropin-releasing hormone receptor (GnRHR) located on gonadotrope cells. Once activated, the GnRHR triggers synthesis and secretion of pituitary gonadotropins: follicle stimulating hormone (FSH) and luteinizing hormone (LH). LH and FSH then act in concert to stimulate the production of gonadal steroid hormones (e.g., androgens from the testes and estrogens and progestins from the ovary) and maturation of gametes (e.g, the spermatozoa in the testes and an ovum in the ovary). The gonadal hormones act in the hypothalamus and pituitary to regulate gonadotropin secretion via feedback loops. solid lines, positive feedback; dashed lines, negative feedback.
3.2. Receptors of the HPG axis
In addition to acting other mechanisms of action to be discussed later, estradiol acts on two main so-called “nuclear” receptors in the brain: estrogen receptor (ER) α and β, also referred to as ESR1 and ESR2, respectively. High levels of ERα are present in hypothalamic and limbic nuclei in the rat brain, including but not limited to the anteroventral periventricular nucleus (AVPV), medial preoptic area (mPOA), bed nucleus of the stria terminalis (BNST), arcuate nucleus, paraventricular nucleus of the hypothalamus (PVN), ventromedial nucleus of the hypothalamus (VMN), and medial amygdala (MeA) (Shughrue et al., 1996; Shughrue et al., 1997). The distribution of ERβ is similar to ERα, but it is more highly expressed in the mPOA, hippocampus, and cerebellum (Shughrue et al., 1996; Shughrue et al., 1997). While no qualitative sex differences in the distribution of ERs in particular brain regions have been reported, there are sex differences in the density of ERs (Rainbow et al., 1982). Compared to male rats, female rats have higher concentrations of ERs in the BNST peri-pubertally (Kuhnemann et al., 1994) and in the mPOA, AVPV, and VMN pubertally and into adulthood (Brown et al., 1988; Kuhnemann et al., 1994). Specifically, female rats have a higher density of ERα in the VMN during pubertal development (Yokosuka et al., 1997) and in adulthood (Yamada et al., 2009) and more ERβ in the lateral amygdaloid nucleus and the dentate gyrus and CA3 region of the hippocampus in adulthood (Zhang et al., 2002).
Progesterone acts on two main receptors in the brain: PRA and PRB. The PRs are expressed throughout the female and male rat brains, especially in the BNST, mPOA, AVPV, PVN, VMN and arcuate nucleus (Parsons et al., 1982; Rainbow et al., 1982). Treatment with estradiol also induces the transcription of the genes for PRs within these brain regions (Intlekofer and Petersen, 2010; Parsons et al., 1982). Adult female rats have a higher density of PRs within the VMN and AVPV, compared to adult male rats (Rainbow et al., 1982). Prepubertal male rats have lower levels of PRs in the PVN compared to adult males (Romeo et al., 2005). It is unknown whether PR levels differ in pre-, peri-, and post-pubertal the brains of female rodents.
Androgen receptors (ARs) have a high degree of overlap with the distribution of ERs, particularly in the MeA, BNST, mPOA, PVN, VMN and arcuate nucleus (Commins and Yahr, 1985; Sheridan, 1978; Simerly et al., 1990). Adult male rats have a greater number and intensity of AR-staining in the hippocampus (Xiao and Jordan, 2002), BNST and PVN (Herbison, 1995) compared to females. Adult male mice also express increased levels of AR in the mPOA and BNST (Lu et al., 1998). It is unknown whether AR levels change following pubertal development.
Steroid hormone receptors can act via two mechanisms. The classic, or genomic, pathway involves the ligand-dependent dimerization and binding of the receptors to specific hormone response elements on target genes, leading to changes in gene transcription (Mangelsdorf et al., 1995; Novac and Heinzel, 2004). This classic activity of steroid receptors results in long lasting changes in cellular function. The genomic actions of the receptors also involve the recruitment of co-activators and co-repressors, but discussion of this is beyond the scope of this review (Tetel et al., 2009). More recent evidence suggests that steroid hormone receptors can also activate cytoplasmic signal transduction pathways (Filardo and Thomas, 2012; McEwen et al., 2012; Nilsson et al., 2001; Vasudevan and Pfaff, 2008).
3.3. Pubertal maturation of the HPG axis
The pubertal maturation of the HPG axis begins with the reactivation of GnRH neurons. While GnRH neurons maintain or increase expression of GnRH mRNA and proteins (Araki et al., 1975; Jakubowski et al., 1991; Wiemann et al., 1989), their secretory activity is quiescent during the prepubertal period (Sisk and Foster, 2004). At the onset of the pubertal period, the frequency and amplitude of GnRH secretion increases gradually until the GnRH neurons fire in synchrony to produce intermittent pulses of GnRH release (Plant and Barker-Gibb, 2004). Much work in recent years has helped to elucidate the triggers for the reinstatement of the GnRH pulsatility. A variety of permissive signals may converge on GnRH neurons, including photoperiod (i.e. melatonin), energy balance or metabolic (i.e., body fat, leptin), and psychosocial signals (Ellison et al., 2012; Sisk and Foster, 2004). These signals are mediated by the general excitatory and inhibitory neurotransmitters, glutamate and GABA, respectively (Parent et al., 2005).
Most of the work examining the re-emergence of GnRH pulsatility has centered on kisspeptin and its receptor, GPR54 (for further review see Ojeda et al., 2006; Sisk and Foster, 2004). Mutations in the genes for either kisspeptin or GPR54 are associated with low levels of gonadotropin and gonadal steroid levels and a failure to undergo spontaneous puberty in both human and rodent models (Oakley et al., 2009). Kisspeptin transcription increases dramatically prior to puberty, and GPR54 expression becomes localized to the GnRH secretory neurons (Gottsch et al., 2004; Ramaswamy et al., 2008). Additionally, kisspeptin signaling is both necessary and sufficient for pulsatile GnRH secretion (Kauffman et al., 2007). Finally, kisspeptin neurons in the arcuate nucleus express ERs, which may in part modulate steroid feedback of the HPG axis (Navarro et al., 2011).
3.4. Enduring effects of stressors on responses to gonadal steroids
The pubertal period is important for the maturation of the stress axis (see Section 4). Further, stressors experienced during puberty/adolescence alter stress reactivity. However, prior to a rather serendipitous discovery (reviewed in detail elsewhere: (Blaustein and Ismail, 2013)), it was unknown that pubertal stressors could alter the brain’s response to gonadal hormones. Here, we discuss the ability of a pubertal stressor to alter the response to gonadal, primarily ovarian, steroid hormones in regulation of sexual behavior (3.4.1), depression- and anxiety-like behaviors (3.4.2), and cognitive behavior (3.4.3). Throughout this review, we discuss alterations in the brain that may mediate the enduring effects of stressors on the response to ovarian hormones.
3.4.1. Sexual behavior in adulthood
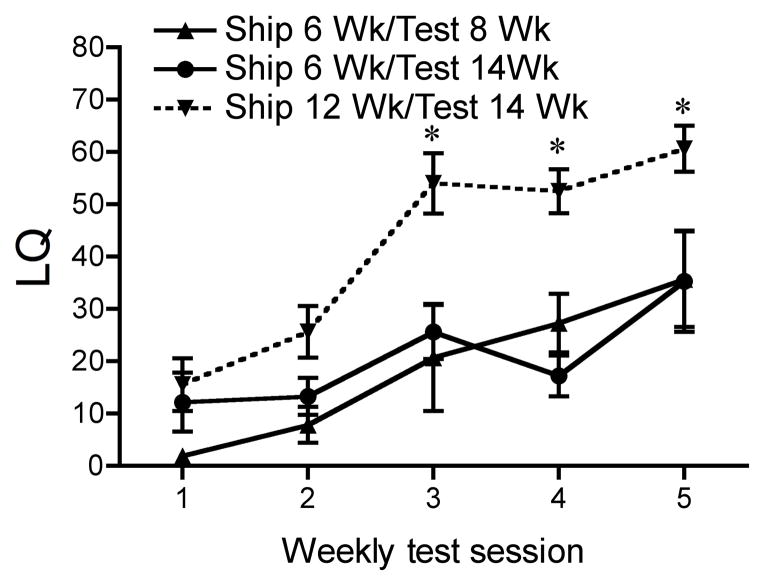
Ovariectomized (OVX) mice, unlike rats, guinea pigs, and hamsters, typically require several weekly injections of estradiol and progesterone followed by mating tests with sexually active males before they begin to express high levels of sexual receptivity (Blaustein, 2008). However, in the course of an extensive experiment to determine the most appropriate doses of estradiol and progesterone to induce female sexual behavior in C57Bl/6 mice, we discovered that mice shipped at six weeks old failed to respond to estradiol and progesterone injections in adulthood with typical increases in the level of sexual receptivity in response to mount attempts by a male mouse (Laroche et al., 2009b). However, mice shipped in adulthood (12 weeks old) expressed the expected behavioral response, indicating that the stress of shipping during pubertal development perturbs the behavioral response to ovarian hormones later in life (Figure 3). This vulnerability is limited to the pubertal period: C57Bl/6 mice shipped at four, five or six weeks old do not respond to estradiol and progesterone in adulthood with the usual high levels of receptivity. In fact, mice shipped at six weeks display the most profound perturbations in behavioral responses (Laroche et al., 2009b). Mice shipped at three weeks old or those shipped at seven weeks old or later responded to estradiol and progesterone with typical levels of sexual receptivity in adulthood. Therefore, in C57Bl/6 mice, this period between four to six weeks of age represents a sensitive or vulnerable period in which stressors have an enduring effect on adult behavioral response to ovarian hormones.
Figure 3.
The effect of pubertal shipping on sexual receptivity of C57Bl/6 mice. LQs of female mice shipped during the peripubertal period. Mice shipped at six weeks and tested at fourteen weeks display significantly lower LQs as compared to mice shipped at twelve weeks and tested at fourteen weeks during weekly test session 3, 4, and 5. Data represents mean ± SEM; *p<0.05. Reprinted with permission from The Endocrine Society.
This sensitivity is not limited to female mice. Male mice shipped at six weeks old also have a slightly decreased response to testosterone in adulthood compared to mice shipped in adulthood. That is, peripubertally-shipped male mice display fewer mounts and a trend towards longer intromission latencies (Laroche et al., 2009b). Recently, McCormick and colleagues (2013) demonstrated that social instability experienced during pubertal development (P30–45) leads to reduced sexual performance in male rats in adulthood. Specifically, pubertally-stressed males have fewer ejaculations, a longer latency to ejaculate, and a reduced copulatory efficiency, as indicated by more mounts and intromissions prior to ejaculation. This effect is confined to sexual performance, as sexual motivation in the pubertally-stressed male rats is unaffected. The stressed males also have lower plasma testosterone levels, but there is no difference in the levels of androgen or estrogen receptors in the mPOA of the stressed and control male rats (McCormick et al., 2013).
Several controlled stressors were used to duplicate the effect of shipping in the laboratory. However, standard restraint stress, food restriction for 36 hr, or a multiple stressor regimen (combination of heat, light and restraint) did not influence the behavioral response to estradiol and progesterone in adulthood (Laroche et al., 2009a). Shipping is a complex, multifactor stressor: animals experience noise and vibration, temperature fluctuations, possible predator odors, disruption in social hierarchy, etc. In situations such as our laboratory, they also transition from a light:dark cycle with lights on early in the morning at the breeders to a shifted and reversed light:dark cycle typical for behavioral testing (light off in the late morning; lights on in the early evening). It is possible that the shipping stressor may also have elements of circadian disruption beyond the random and intermittent exposure to light and dark during the process of shipping itself. It is therefore understandable that relatively simple stressors are unable to elicit similar perturbations in response to gonadal hormones.
Due to the similarities in duration of effect and in behavior of recently shipped animals and sick animals, injection of the bacterial endotoxin lipopolysaccharide (LPS) (Laugero and Moberg, 2000) was used as a stressor. LPS causes a robust inflammatory and immune response and induces the display of sickness behavior that lasts for less than forty-eight hours (Anisman, 2009; Dantzer, 2001). Female C57Bl/6 mice injected with LPS at four, five or six weeks, but not three, seven, eight, or ten weeks old expressed a decrease in sexual receptivity in adulthood, compared to saline-injected controls (Laroche et al., 2009a), duplicating the same developmental sensitive period demonstrated by mice shipped at these same ages (Laroche et al., 2009b). Subsequent work has demonstrated that pubertal and adult animals display similar increases sickness behavior (e.g., lethargy, huddling, piloerection, and ptosis) and decreases in body weight following LPS administration (Ismail and Blaustein, 2013; Ismail et al., 2011; Ismail et al., 2012; Olesen et al., 2011).
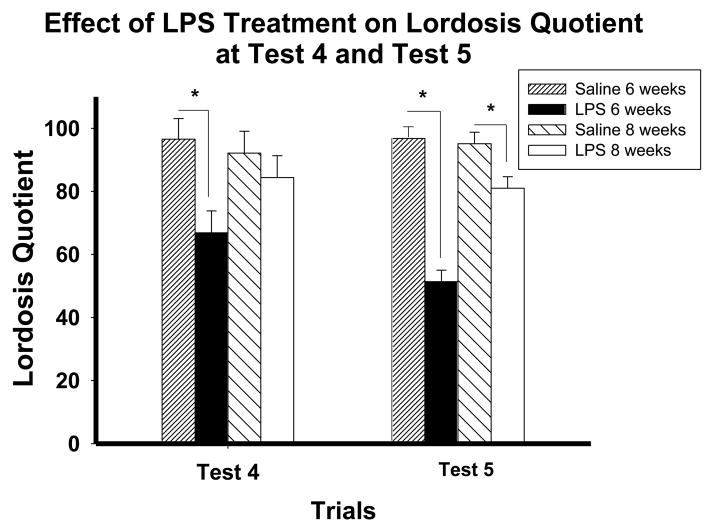
The effects of shipping and LPS exposure are not idiosyncratic to the inbred C57Bl/6 mice strain, as similar results have been obtained using the outbred CD1 mice strain (Figure 4) (Ismail et al., 2011). However, the sensitive period is shifted; in female C57Bl/6 mice, the onset of the sensitive period is four weeks old, but in CD1 mice, this vulnerability does not occur until after four weeks. The sensitive period may last longer in CD1 mice as indicated by the effects of injection of LPS and shipping at eight weeks, but not ten weeks old (Ismail et al., 2011).
Figure 4.
The effect of pubertal immune challenge on sexual receptivity in CD1 mice. Lordosis quotient on test weeks 4 and 5 of CD1 mice treated with either saline or LPS at 6 or 8 weeks old. Mice treated with LPS at six weeks display significantly lower LQs as compared to mice treated with saline at six weeks during the weekly test session 4 and 5. Mice treated with LPS at eight weeks display significantly lower LQs as compared to mice treated with saline at eight weeks only during weekly test session 5. Data represents mean ± SEM; *p<0.05. Reprinted with permission from Elsevier, Inc.
It should be noted that the effects of the controlled stressors, such as restraint or food deprivation, and LPS in pubertal male mice have not been examined. Although shipping is effective in reducing behavioral response to testosterone, the efficacy of these other stressors is unknown. Additionally, the effects of shipping or LPS have not been extended to male CD1 mice.
The decreased response to estradiol and progesterone in adulthood following the pubertal stressors may result from a decrease in ERα in some brain areas. That is, CD1 mice shipped at six weeks, but not at four weeks, express a decrease in ERα-immunopositive cells in the mPOA, VMN and arcuate nucleus, but not in the AVPV (Ismail et al., 2011). It is unknown whether this arises from potential volumetric changes in the brain areas, through cell death, or phenotypic changes in which cells express lower levels of ERα. Additionally, the neuronal (e.g., glutamatergic or GABAergic) or even the neural cell type (e.g., neuron or glia) in which this decrease in ERα occurs is unknown. Nonetheless, these data suggest a cellular basis for the decreased behavioral response after the pubertal stressor.
3.4.2. Ovarian hormone-modulated depression- and anxiety-like behavior in adulthood
While more commonly studied in premenstrual dysphoric disorder (Schmidt et al., 1998) or post-partum depression (Bloch et al., 2003), changes in hormone levels may contribute to the development of mental disease, such as depression and/or anxiety disorders in women. Studies of women with premenstrual dysphoric disorder or post-partum depression demonstrate that ovarian hormones trigger anxiety and/or depression during the luteal phase, or at times when ovarian hormone concentrations are relatively low (Bloch et al., 2003; Schmidt et al., 1998).
Animal models of depression-like behavior include the Porsolt forced swim test and the tail suspension test. In these tests, depressive symptoms of behavioral despair are inferred from decreases in times spent swimming and struggling to escape and an increase in time spent immobile (O’Neil and Moore, 2003; Palanza et al., 2001). Another widely used test measures anhedonia, defined by a decrease in sucrose preference (Grippo et al., 2003; Konkle et al., 2003). This test typically involves the presentation of two bottles, one with water and another with a solution of 1–10% sucrose. The consumption of the sucrose relative to the water is measured. Mice and rats typically prefer the sucrose solution, and failure to show this preference is taken as a sign of anhedonia.
Estradiol also modulates depression-like behaviors in rodents (Bekku and Yoshimura, 2005; Dalla et al., 2004; Okada et al., 1997; Rachman et al., 1998; Suda et al., 2008). OVX increases, and estradiol-replacement decreases, duration of immobility in the tail suspension (Bernardi et al., 1989) and forced-swim test (Koss et al., 2012; Okada et al., 1997; Rocha et al., 2005). Strain differences in the anti-depressive properties of estradiol have been reported. For example, Long-Evans rats have an anti-depressive response to estradiol. In contrast, Wistar rats show an increase in depression-like behavior following the withdrawal of estradiol (Koss et al., 2012). Additionally, female mice with a knockout of the aromatase gene, which are deficient in estradiol (as aromatase converts testosterone to estradiol), display greater depression-like behaviors compared to wild-type mice (Dalla et al., 2004). Overall, the evidence supports the hypothesis that estradiol is anti-depressive.
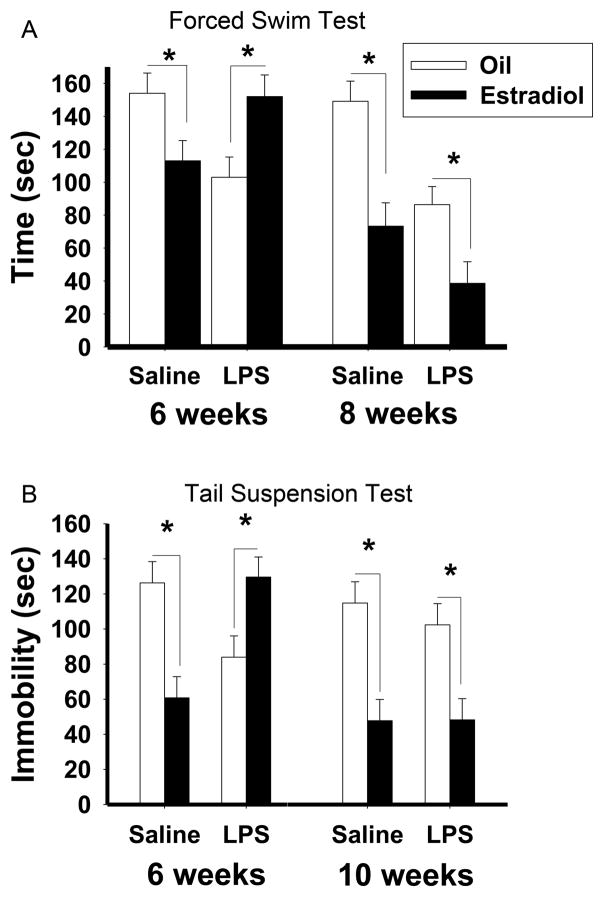
LPS exposure during the sensitive period perturbs the anti-depressive properties of estradiol in both C57Bl/6 and CD1 mice (Figure 5). Instead of decreasing the behavioral responses to estradiol, as previously demonstrated in sexual behavior, estradiol increased depression-like behavior in the tail suspension and forced swim tests (Ismail et al., 2012). Specifically, in mice treated with saline during pubertal development or adulthood or LPS in adulthood, estradiol induced the expected decrease in depression-like behaviors, as measured by a decrease in immobility. However, in mice treated with LPS during pubertal development, estradiol treatment increases the duration of immobility (Ismail et al., 2012). That is, the pubertal immune challenge reversed the effects of estradiol. Interestingly, neither estradiol treatment, nor pubertal LPS exposure, affected the sucrose preference test, which measures anhedonia. While it is possible that a higher concentration of sucrose would have resulted in a decrease in sucrose preference, these data may suggest that different mechanisms may mediate behavioral despair and anhedonia aspects of depression-like behaviors.
Figure 5.
The effects of pubertal immune challenge on depression-like behavior in adult female mice (A) Duration of immobility during the forced swim test in C57Bl/6 mice treated with either saline or LPS at six and eight weeks old and implanted with estradiol or oil vehicle capsules in adulthood. (B) Duration of immobility during the tail suspension test in CD1 mice treated with saline or LPS at six and ten weeks old and implanted with estradiol or oil vehicle capsules in adulthood. Data represents mean ± SEM; *p<0.05. Reprinted with permission from Elsevier, Inc.
Typical animal models of anxiety-like behaviors are based upon the innate aversion of rodents to open spaces. Two such tests are the elevated plus maze and the open field arena. The elevated plus maze is based upon the animal’s fear of open and unprotected spaces, with decreased time exploring the open arms interpreted as an increase in anxiety-like behavior (Roy and Chapillon, 2004; Wall and Messier, 2001). The open field test measures the exploratory behavior of the rat or mouse, as well as its willingness to move away from the arena wall and into the center. Reductions in both exploratory behavior and entries into the center are taken as evidence for an increase in anxiety (Roy and Chapillon, 2004). The light-dark box also takes advantage of the rodent’s natural preference for dark spaces, so increases in time spent in the light compartment are interpreted as decreases in anxiety-like behavior. Other measures of anxiety include enhanced startle reflexes, decreases in the approach to novel food or social interactions (File and Seth, 2003; Johnston and File, 1991), and more rapid marble burying in the marble burying test (which also may model compulsive behavior (Li et al., 2006)).
Ovarian hormones also influence anxiety-like behaviors in female rodents. Both rats (Diaz-Veliz et al., 1997; Diaz-Veliz et al., 1989; Diaz-Veliz et al., 1991; Mora et al., 1996; Olivera-Lopez et al., 2008; Pandaranandaka et al., 2006; Pandaranandaka et al., 2009) and mice (Galeeva and Tuohimaa, 2001) display cycles in anxiety-like behaviors that correlate with the estrous cycle. Specifically, anxiety-like behaviors are increased during diestrus, when estradiol levels are low, and females display a reduction in anxiety during proestrus and estrus (when estradiol levels are elevated). Moreover, exogenous estradiol treatment during diestrus reduces anxiety-like behaviors to proestrus-like levels (Marcondes et al., 2001). OVX also increases anxiety levels in rats (Diaz-Veliz et al., 1997; Pandaranandaka et al., 2009; Picazo et al., 2006) and mice (Ogawa et al.). Replacement of estradiol and progesterone decreases levels of anxiety-like behaviors in female rats (Diaz-Veliz et al., 1997; Diaz-Veliz et al., 1989; Diaz-Veliz et al., 1991; Mora et al., 1996; Olivera-Lopez et al., 2008; Pandaranandaka et al., 2006; Pandaranandaka et al., 2009). OVX mice, however, display a biphasic response to estradiol; while chronic treatment with low doses of estradiol reduces anxiety-like behaviors (Tomihara et al., 2009), higher levels of estradiol may increase anxiety (Morgan and Pfaff, 2001; Morgan and Pfaff, 2002; Tomihara et al., 2009).
As with the anti-depressive properties of estradiol, LPS exposure during the pubertal period perturbs the anxiolytic properties of estradiol and progesterone in both C57Bl/6 and CD1 mice. In control OVX mice, an injection of estradiol benzoate followed 44 h later by an injection of progesterone, similar to treatment used in tests of sexual receptivity, decreases anxiety-like behavior as assessed in the elevated plus maze, the light-dark box, and the marble burying task (Figure 6) (Olesen et al., 2011). In OVX mice that received the LPS during the pubertal period, hormone treatment either blocked the anxiolytic effect of estradiol and progesterone or had an anxiogenic effect (Olesen et al., 2011). That is, an immune challenge in puberty either eliminates or reverses the behavioral responses to ovarian hormones in adulthood.
Figure 6.

The effects of pubertal immune challenge on anxiety-like behavior in CD1 mice. Estradiol followed by progesterone administration 48 hour later increased the latency to bury marbles in the saline- but not the LPS- treated mice compared to respective oil controls. Data represents mean ± SEM; *p<0.05. Reprinted with permission from Elsevier, Inc.
It should be noted that there was a tendency for LPS exposure during pubertal development to affect the display of depression- and anxiety-like behaviors even in the absence of hormones. OVX mice exposed to LPS in the pubertal peroid and treated with oil in adulthood showed a reduction in time spent immobile, indicating a decrease in depression-like behavior (Ismail et al., 2012). There was also a tendency for pubertal LPS injection to reduce anxiety-like behaviors in adulthood in both the light-dark box and the elevated plus maze. This is consistent with previous research demonstrating that adolescent stressors (Conrad and Winder, 2011) or prenatal LPS treatment (Wang et al., 2010) reduce anxiety-like behaviors. Nevertheless, in all cases, hormone treatment was either without an effect or caused an increase in depression- and anxiety-like behaviors in mice that were injected with LPS during the pubertal period.
The alteration of the behavioral properties of the ovarian hormones, particularly estradiol, may be mediated in part by alteration of the ovarian hormone receptors in the brain. While the antidepressant effects of estradiol are mediated predominantly by ERβ (Osterlund, 2010; Rocha et al., 2005), both subtypes of ER have been implicated in the mediation of the anxiolytic properties of estradiol (Choleris et al., 2003; Choleris et al., 2006; Imwalle et al., 2005; Krezel et al., 2001; Ogawa et al., 1998). Therefore, the inability of estradiol, or estradiol and progesterone, to decrease depression- and/or anxiety-like behaviors in mice treated during pubertal development with LPS suggests an alteration in ERα and/or ERβ in relevant brain regions, such as the BNST, amygdala or hippocampus. Although the effects of shipping stress on ERα levels in some brain areas described above are suggestive, this hypothesis remains to be directly tested in future experiments.
3.4.3. Estradiol-modulated cognitive behaviors in adulthood
Cognition, learning, and memory are complex processes that involve the learning or acquisition of information, the consolidation and storing of information, and then the retrieval and utilization of the information. Some of the most commonly used learning and memory tasks involve spatial memory, or learning where an object or place is in relation to the environment. Spatial memory is typically measured using the radial arm maze, in which animals must learn the orientation of arms using spatial cues in order to retrieve food, and the Morris water maze, in which animals learn the location of a submerged escape platform. Other memory tasks involve the ability to recognize a familiar object or conspecific animal or a familiar object in a new place. The recognition tasks take advantage of the innate exploratory behavior of rodents. One major assumption of the recognition tasks is that rodents more readily investigate a new or novel object or conspecific than one that is familiar (Luine, 2008; Luine and Frankfurt, 2012). Both spatial memory tasks and recognition tasks depend upon an intact hippocampus (Broadbent et al., 2004).
In adulthood, estradiol generally has a positive effect on a variety of learning tasks, including spatial and recognition memory (reviewed in Luine, 2008), but the specific effects of estradiol depend upon the cognitive task and the brain regions(s) involved. Estradiol impairs performance in striatal or amygdalar tasks, in which some learned, associative response is required in female rats (Davis et al., 2005; Korol, 2004). In hippocampal, or spatial or place tasks, estradiol enhances performance in female rats (Daniel et al., 1997; Davis et al., 2005; Luine et al., 1998; Sandstrom and Williams, 2004) and mice (Li et al., 2004; Xu and Zhang, 2006).
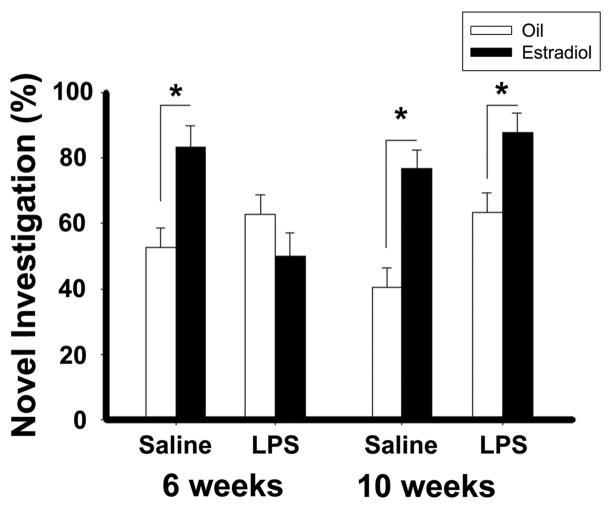
Pubertal exposure to LPS disrupts the positive cognitive effects of estradiol on social memory, as measured by social discrimination and recognition tasks, and object memory, as measured by novel object recognition and novel object placement tests (Ismail and Blaustein, 2013). More specifically, in mice treated with saline during pubertal development or with LPS or saline in adulthood, estradiol improves social memory, as measured by increases in the duration of investigation of a novel stimulus mouse. In contrast, in mice treated with LPS during pubertal development, estradiol treatment failed to increase the time spent investigating the novel mouse (Ismail and Blaustein, 2013). Estradiol also increased performance in the object recognition and object placement recognition tasks in mice treated with saline during the pubertal period or treated in adulthood (Figure 7). Additionally, estradiol treatment failed to increase the time spent investigating the novel object or the novel placement of a familiar object in mice treated with LPS during the vulnerable period, (Ismail and Blaustein, 2013). As with many other studies, the cognitive tasks used in this study depend upon the innate exploratory behavior of the mice, decreases of which are indicators of anxiety. Therefore, the possibility cannot be excluded that the apparent decreases in cognition are a result of the increased anxiety- and depression-like behaviors.
Figure 7.
The effect of pubertal immune challenge on cognitive behavior in CD1 mice. Estradiol increase the percentage of time spent investigating the novel object during the object recognition test in mice in treated pubertally with saline, but not LPS compared to respective oil controls. Data represents mean ± SEM; *p<0.05. Reprinted with permission from Elsevier, Inc.
Estradiol affects cognition in both a long lasting (from hours to days) manner and a short-term or rapid manner (occurring within minutes). The long-lasting effects are consistent with the classical, gene-transcription actions of ERs, while membrane receptors acting through signal transduction pathways mediate the short-term effects (McEwen et al., 2012). The preponderance of data demonstrate that the pro-cognitive effects of estradiol are mediated primarily through ERβ, rather than ERα (reviewed in Luine and Frankfurt, 2012). The numbers and types of dendritic spines, which are associated with increases in innervation and serve as sites of synaptic input, are critical mediators of neural plasticity in the adult brain (reviewed in Urbanska et al., 2012). Moreover, the mechanisms of hippocampal learning, memory, and cognition involve dendritic remodeling. In the CA1 region of the hippocampus of adult male (Jedlicka et al., 2008; Leuner et al., 2003) and female rats (Beltran-Campos et al., 2011; Woolley, 2000), learning is correlated with increases in dendritic spine density. Estradiol increases synaptic activity in the CA1 region of the hippocampus via alteration of both excitatory (Smejkalova and Woolley, 2010) and inhibitory transmission (Huang and Woolley, 2012), which can lead to new synaptic connections as indicated by dendritic spines (Yankova et al., 2001). Estradiol may enhance cognition through the generation of new spines or an alteration of existing spines (reviewed in Luine and Frankfurt, 2012). Therefore, it is possible that the experience of a pubertal stressor may interfere with these aspects of synaptic plasticity and learning and memory. This is an attractive hypothesis to be tested in subsequent experiments.
4. Adrenal Steroids
4.1. Hypothalamic-pituitary-adrenal (HPA) axis
The physiological response to a stressor occurs in two distinct components: activation of the sympathetic nervous system and activation of the hypothalamic-pituitary-adrenal (HPA) axis. The sympathetic nerves and adrenal medulla immediately release catecholamines following the experience of a stressor (Kvetnansky et al., 2009). This release of catecholamine in the periphery results in increased glucose utilization, heart rate and pupil dilation, bronchodilation, decreased gut motility, and vasoconstriction, all of which would promote survival in the case of an immediate physical stressor (Kvetnansky et al., 2009). The HPA axis, however, responds to a stressor by releasing glucocorticoids (primarily cortisol in humans, primates, and some other species and primarily corticosterone in rats and mice) in a slower, and potentially, a more long-lasting way (Figure 8). Blood levels of glucocorticoids peak around 10–20 min following a stressor; the glucocorticoids then initiate gene transcription, beginning approximately one hour following the stressor (Sapolsky et al., 2000; Schommer et al., 2003). The HPA axis serves to maintain “allostasis,” in which adaptive changes occur to both achieve physiological stability (McEwen, 2000) and influence a host of other processes, such as energy metabolism (Brandi et al., 1993; Rizza et al., 1982; Vitaliano et al., 2002), immune function (Straub et al., 2011), cardiovascular health (Sapolsky and Share, 1994; Whitworth et al., 1995), and as recent evidence suggests, even mental health (Pariante and Miller, 2001; Tafet and Bernardini, 2003).
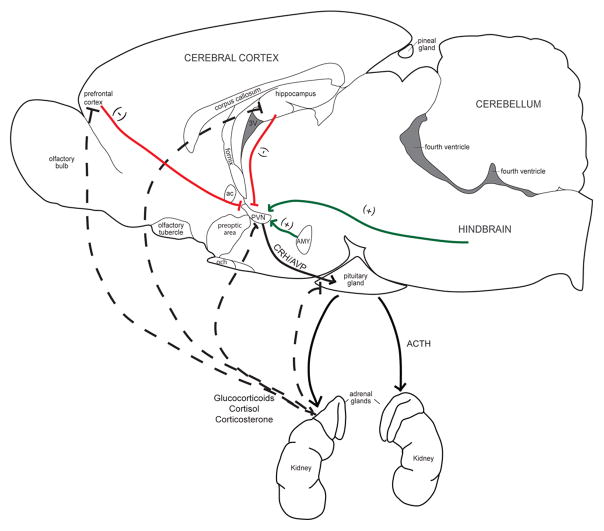
Figure 8.
Representation of the Hypothalamus-Pituitary-Adrenal Axis. The experience of a stressor cause sensory, hindbrain, limbic, and cortical regions to signal to neurons in the paraventricular nucleus of the hypothalamus (PVN). The PVN then secretes corticotrophin releasing hormone (CRH) and arginine vasopressin (AVP) into the hypophyseal portal system of the median eminence. The portal blood carries CRH and AVP into the pituitary gland and activates their respective receptors to triggers synthesis and secretion of adrenocorticotropic hormone (ACTH) from the anterior pituitary. ACTH then stimulates the release of glucocorticoids (cortisol in humans and primates and corticosterone in rats and mice) from the adrenal cortex. The elevated levels of glucocorticoids suppress further release of CRH, AVP and ACTH by binding to receptors in the PVN, hippocampus, and prefrontal cortex and pituitary. (+), solid lines, positive feedback; (−), dashed lines negative feedback.
4.2. Receptors of the HPA axis
Two types of receptors mediate the genomic actions of the glucocorticoids: the mineralocorticoid (MR) and the glucocorticoid (GR) receptor. While both MR and GR bind corticosterone, GRs are more directly involved in mediating a negative feedback loop for corticosterone secretion. MRs have a higher affinity for corticosterone than GRs; therefore, the majority of MRs being occupied even under low basal levels of glucocorticoids (reviewed in De Kloet et al., 2008). As only approximately 20% of GRs are occupied in basal conditions, GRs are more sensitive to changes in the concentrations of glucocorticoids (reviewed in Datson et al., 2008). Moreover, GRs are more widely distributed in the brain than MRs. The highest densities of GRs are in the hippocampus, medial prefrontal cortex, and the amygdala, which provides input, via the BNST, to the PVN (Ulrich-Lai and Herman, 2009). The hippocampus and infralimbic medial prefrontal cortex (mPFC) are particularly involved in the negative feedback loop, whereas the amygdala and prelimbic mPFC activate the HPA axis (Ulrich-Lai and Herman, 2009).
4.3. Pubertal Maturation of the HPA axis and Stress Response
Maturation of the HPA axis precedes the maturation of the HPG axis (Figure 1). This maturation results in increased secretion of hormones by the adrenal glands, termed adrenarche, which in humans takes places around 6–8 years of age (Parker, 1991) and is the result of increased 17-hydroxylase activity. At this stage of development, cortisol increases in humans (Apter et al., 1979; Walker et al., 2001), and corticosterone increases in rats and mice (Pignatelli et al., 2006; Spinedi et al., 1997). Basal corticosterone concentrations increase in both male and female rats during the peri-pubertal periods and peak around P45–60. Moreover, females have a steeper increase than males, resulting in a higher concentration of plasma corticosterone levels in female rats even in adulthood (Pignatelli et al., 2006). Other steroids not typically associated with the adult rat adrenal gland are present in the pre-pubertal period. Specifically, cortisol, androstenedione, and 17-hydroxyprogesterone are detected with peak concentrations at P20 in both female and male rats; these peaks correlate with an increase in 17-hydroxylase expression in the adrenal glands (Pignatelli et al., 2006).
Unlike gonadal hormones, basal ACTH and corticosterone levels are relatively stable during pubertal development and into adulthood (Pignatelli et al., 2006; Romeo et al., 2004a; Romeo et al., 2004b). Additionally, pre-pubertal (P28) and adult male and female rats respond to an acute stressor (e.g. restraint stress) with comparable increases in ACTH and corticosterone; however, pre-pubertal male and female rats have a more prolonged, stress-induced corticosterone response compared to adult rats (Romeo et al., 2004a; Romeo et al., 2004b). In adult rats, repetitive exposure to the same stressor results in a habituation of the corticosterone response (Girotti et al., 2006; Harris et al., 2004; Helmreich et al., 1997; Magarinos and McEwen, 1995; Marti and Armario, 1997), but male rats that experience repeated stressors in the pre-pubertal period do not show this same blunting of corticosterone response. In fact, they have higher peaks of ACTH, corticosterone and adrenal progesterone, but a more rapid return to baseline levels (Doremus-Fitzwater et al., 2009; Romeo et al., 2006). Pubertal female rats do not show this increase in corticosterone response (Doremus-Fitzwater et al., 2009).
It is well-established that gonadal hormones influence HPA reactivity. In adult male rats, testosterone decreases the peak of corticosterone levels and recovery time from a stressor (Handa et al., 1994; McCormick et al., 1998; McCormick et al., 2002; Viau, 2002; Viau and Meaney, 1996), whereas the ovarian hormones increase these measures in females (Carey et al., 1995; McCormick et al., 2002; Redei et al., 1994; Viau and Meaney, 1991; Young et al., 2001). While it seems plausible that the differential levels of gonadal hormones would contribute to the altered stress reactivity in pre-pubertal and adult animals, the protracted stress response in pre-pubertal male and female rats is not affected by the presence or absence of gonadal hormones (Romeo et al., 2004a; Romeo et al., 2004b). In mid-pubertal (P40) male rats, androgens modulate HPA reactivity, indicating that pubertal development need not be complete before adult-like patterns of stress reactivity emerge (Gomez et al., 2004). It is intriguing that this is also around the same time during which the adult-like sex differences in corticosterone levels emerge (Pignatelli et al., 2006).
One of the key brain regions of the HPA axis, the PVN, exhibits maturation in functionality during the pubertal period. The PVN volume, cellular size, and morphology are similar in pubertal and adult rats (Romeo et al., 2007). Moreover, the numbers of CRH and AVP cells are also similar (Romeo et al., 2006). There is, however, a greater activation (i.e., Fos expression) of cells within the PVN following a single stressor in pre-pubertal compared to adult male rats (Romeo et al., 2006). This greater activation is specific to the CRH cells, suggesting a correlation between this increased activation of CRH cells and the protracted corticosterone response following a stressor in pre-pubertal males.
4.4. Enduring effects of commonly-used stressors
In humans, the experience of extreme stress and trauma during adolescence is associated with a greater likelihood of mood disorders, such as anxiety, major depression, and post-traumatic stress disorder (PTSD) (Ge et al., 2001; Grant et al., 2003; Grant et al., 2004; Silverman et al., 1996; Turner and Lloyd, 2004). In recent years, several studies with animal models have addressed the enduring effects of stressors experienced during the juvenile and pubertal periods on stress reactivity in adulthood (4.4.1). These studies support the notion that stress experienced in pubertal development leads to an increase in anxiety- and depression-like behaviors in both male and female rats (4.4.2). There is also the suggestion that alterations in cognition occur in adulthood following pubertal stress (4.4.3). It is important to note that the maturation of the HPA axis occurs prior to maturation of the HPG axis. Therefore, many of the studies focus on stressors in the pre- or early pubertal periods. Additionally, once animals have progressed through gonadarche, they seem to be less susceptible to stress-induced changes, again demonstrating a vulnerable period to the enduring effects of stressors.
4.4.1. Stress response in adulthood
Examinations of the enduring effects of stressors in the pubertal period on adult HPA function and stress reactivity in adulthood have yielded somewhat mixed results. It has generally been reported that stressors experienced in puberty and adolescence increase or augment basal HPA function (Bazak et al., 2009; Jacobson-Pick and Richter-Levin, 2010; Pohl et al., 2007; Schmidt et al., 2010b; Schmidt et al., 2007; Sterlemann et al., 2008; Uys et al., 2006a; Uys et al., 2006b) or responses to stressors (Isgor et al., 2004; Jacobson-Pick and Richter-Levin, 2010; Mathews et al., 2008; Weathington et al., 2012) as indicated by corticosterone levels in adulthood (Table 1). While the basal increases of corticosterone occur in both male and female rats and male CD1 mice, there also may be interactions with gonadal hormones, as Mathews (2008) reported stressor-induced increases in corticosterone only in female rats in the diestrous stage of the estrous cycle. There may also be interactions with circadian rhythms, as some reports indicate that basal corticosterone levels are increased in the morning (Schmidt et al., 2010b; Schmidt et al., 2007; Sterlemann et al., 2008; Uys et al., 2006b) and decreased in the afternoon (Sterlemann et al., 2008; Toth et al., 2008a). Pubertal stressors lead to enhanced basal corticosterone and decreases in the expression of GR and MR in the hippocampus in adulthood (Isgor et al., 2004; Schmidt et al., 2007; Sterlemann et al., 2008; Uys et al., 2006b), suggesting enduring alterations in the negative feedback of the HPA axis.
Table 1.
Experiments investigating the effects of stressors in puberty and adolescence on the HPA axis reactivity in adulthood.
| Sex/strain/species | Age at Stress | Stressor | Age at Test | Test | Result: HPA Reactivity | Source |
|---|---|---|---|---|---|---|
| ♂ W rats | 21–32 | Chronic Variable Stress | 120 | Cort |

|
Maslova et al. (2002) |
| ♂ SD rats | 28–56 | Chronic Variable Stress | 77 | Cort |

|
Isgor et al (2004) |
| GR mRNA |
 in CA1, DG in CA1, DG |
|||||
| ♂ SD rats | 23, 35, 60 | Chronic Variable Stress | 68 | Basal ACTH | – | Uys et al (2006a) |
| Basal Cort |

|
|||||
| ♂ SD rats | 23, 35, 60 | Chronic Variable Stress | 61 | Basal Cort (am) |

|
Uys et al (2006b) |
| GR |
 in hilus, DG in hilus, DG |
|||||
| ♂ SD rats | 30–58 | Chronic Variable Stress | 60 | Basal Cort (am) | – | Toth et al (2008b) |
| Basal Cort (pm) |

|
|||||
| ♂ SD rats | 27–29 | Chronic Variable Stress | 60 | Basal Cort |

|
Ilin and Richter-Levin (2009) |
| ♂ SD rats | 27–29 | Chronic Variable Stress | 60 | Cort |

|
Jacobson-Pick and Richter-Levin (2010) |
| ♀ LE rats | 23–51 | Chronic Variable Stress | 72 | Basal Cort | mild/severe | Pohl et al (2007) |
–/

| ||||||
| ♂ LE rats | 22–33 | Chronic Variable Stress | 61 | Cort |

|
Wilkin et al (2012) |
| ♀ LE rats | 22–33 | Chronic Variable Stress | 61 | Cort |

|
|
| ♂ LE rats | 35–46 | Chronic Variable Stress | 73 | Cort | – | |
| ♀ LE rats | 35–46 | Chronic Variable Stress | 73 | Cort | – | |
| ♂ W rats | 26–28 | Repeated Elevated Platform | 56 | DHEA | – in hypothalamus, EC | Avital et al (2006) |
| DHEAS |
 in hypothalamus, EC in hypothalamus, EC |
|||||
| ♂ LE rats | 33–48 | Social Instability | 69 | Cort | – | McCormick et al (2005) |
| ♀ LE rats | 33–48 | Social Instability | 69 | Cort | – | |
| ♂ LE rats | 33–45 | Social Instability | 70 | Cort | – | Mathews et al (2008) |
| ♀ LE rats | 33–45 | Social Instability | 70 | Cort | – | |
| ♂ LE rats | 33–45 | Social Instability | 70 | Cort | – | McCormick et al (2008) |
| ♀ LE rats | 33–45 | Social Instability | 70 (estrus) |

|
||
| ♀ LE rats | 33–45 | Social Instability | 70 (disestrus) | Cort | – | |
| ♂ CD-1 mice | 28–84 | Social Instability | 92 | Basal Cort | – | Schmidt et al (2008) |
| Basal ACTH (am) |

|
|||||
| Basal ACTH (pm) | – | |||||
| MR mRNA |
 in CA2 in CA2 |
|||||
| GR mRNA |
 in CA1 in CA1 |
|||||
| ♂ CD-1 mice | 27–73 | Social Instability | 450 | Basal ACTH | – | Sterleman et al (2008) |
| Basal Cort (am) |

|
|||||
| Basal Cort (pm) |

|
|||||
| CRH mRNA | – | |||||
| AVP mRNA |
 in PVN in PVN |
|||||
| MR mRNA |
 in CA1, – in DG in CA1, – in DG |
|||||
| GR mRNA |
 in CA1-CA3, DG in CA1-CA3, DG |
|||||
| ♂ CD-1 mice | 30–80 | Social Instability | 120 | Basal Cort (am) | resilient/vulnerable | Schmidt et al (2010) |
–/

| ||||||
| ♂ W rats | 37–49 | Social Defeat | 96 | Cort | – | Bourke and Neigh (2011) |
| ♂ SD rats | 35–40 | Social Defeat | 63 | Basal Cort | – | Watt et al (2009) |
| ♂ LE rats | 28–41 | Social Defeat | 87 | Basal Cort | – | Weathington et al (2012) |
| Cort | – | |||||
| ♀ LE rats | 28–41 | Social Defeat | 87 | Basal Cort | – | |
| Cort |

|
|||||
| ♂ W rats | 28–30 | Predator Odor | 120 | Cort | – | Toledo-Rodriguez and Sandi (2007) |
| ♀ W rats | 28–30 | 120 | – | |||
| ♂ SD rats | 28 | Predator Odor | 60 | Basal Cort |

|
Bazak et al (2009) |
| ♂ LE rats | 40–48 | Predator Odor | 62 | Cort | – | Wright et al (2008) |
| ♀ LE rats | Cort | – |
LE, Long Evans; SD, Sprague-Dawley; W, Wistar; AVP, arginine vasopressin; CA1, Cornu Ammonis area 1 of the hippocampus; CA2, Cornu Ammonis area 2 of the hippocampus; CA3, Cornu Ammonis area 3 of the hippocampus; CRH, corticotropin-releasing hormone; DG; dentate gyrus of the hippocampus; EC, entorhinal cortex; GR, glucocorticoid receptor; MR, mineralocorticoid receptor; PVN, paraventricular nucleus of the hypothalamus
In contrast, several studies report that there are no enduring effects of a stressor during puberty in male or female rats on either basal (Watt et al., 2009; Weathington et al., 2012) or stress-induced plasma ACTH or corticosterone level following a second stressor in adulthood (Bourke and Neigh, 2011; Maslova et al., 2002; Mathews et al., 2008; McCormick et al., 2005; McCormick et al., 2008; Toledo-Rodriguez and Sandi, 2007; Wilkin et al., 2012; Wright et al., 2008) (Table 1). The adolescent stressors used in these studies include physical (e.g. restraint, foot shock, sleep deprivation), social (e.g., social instability or isolation), or predator odor; in adulthood these animals experienced well-established and commonly-used stressor procedures (e.g., restraint, foot-shock, elevated platform, and the forced swim). However, differences in procedures may explain the apparent discrepancies. In several of the studies in which no effect of pubertal stressor on adult stress reactivity was reported, the animals experienced the stressor beginning at 30 (McCormick et al., 2005; McCormick et al., 2008) or 40 days old (Wright et al., 2008), whereas the animals demonstrating alterations in stress response in adulthood experienced the stressors starting pre-pubertally (at or before P28) (Ilin and Richter-Levin, 2009; Isgor et al., 2004; Jacobson-Pick and Richter-Levin, 2010; Pohl et al., 2007; Schmidt et al., 2007; Sterlemann et al., 2008; Weathington et al., 2012). It will be important to determine if the difference in the findings between the different studies is due to the presence of a vulnerable period ending prior to P30. That is, perhaps pre-pubertal, not pubertal, rats and mice are sensitive to this enduring alteration of HPA axis function.
4.4.2. Anxiety- and depression-like behavior in adulthood
In general, the experience of repeated stressors such as chronic variable stress (Ilin and Richter-Levin, 2009; Jacobson-Pick and Richter-Levin, 2010; Jacobson-Pick and Richter-Levin, 2012; Wilkin et al., 2012), elevated platform stress (Avital et al., 2006; Avital and Richter-Levin, 2005), social instability (Green et al., 2012; Mathews et al., 2008; McCormick et al., 2008; Schmidt et al., 2010a; Schmidt et al., 2010b; Schmidt et al., 2007; Sterlemann et al., 2008), social defeat procedures (Bourke and Neigh, 2011; Vidal et al., 2007; Vidal et al., 2011a; Vidal et al., 2011b; Weathington et al., 2012), and predator odors (Bazak et al., 2009; Cohen et al., 2007; Tsoory et al., 2007; Wright et al., 2008; Wright et al., 2012a; Wright et al., 2012b) increases anxiety-like behaviors in male and female rats and mice in adulthood (Table 2; for further review see McCormick and Green, 2012). The increases in anxiety-like behaviors are typically indicated by decreases in the time spent in the open arm of the elevated plus maze (McCormick and Green, 2012; McCormick et al., 2008; Schmidt et al., 2007; Sterlemann et al., 2008; Tsoory et al., 2007; Uys et al., 2006a). In one study, both female and male rats that experienced severe, variable physical stress exhibited exaggerated anxiety-like behavior in the elevated plus maze, as indicated by jumping to escape from the maze (Pohl et al., 2007). An increase in anxiety-like behavior in adulthood is also displayed by decreases in locomotor and exploratory behavior in the open field following pre-pubertal (~P28) stress (Avital and Richter-Levin, 2005; Schmidt et al., 2007; Sterlemann et al., 2008; Tsoory et al., 2007). Social instability stress in the early pubertal period also increases open field anxiety and decreases social interaction in adult male rats, while maintaining exploratory behavior (Green et al., 2012). Social instability and social defeat also lead to a decrease in social interactions in adulthood (Green et al., 2012; Vidal et al., 2007; Vidal et al., 2011a; Vidal et al., 2011b). While a few studies report lack of effect of stressors during pubertal development on locomotor or exploratory behavior, these stressors began on P30 and continued until well into adulthood (Peleg-Raibstein and Feldon, 2011; Toth et al., 2008a; Toth et al., 2008b), perhaps missing an earlier vulnerable period.
Table 2.
Experiments investigating the effects of stressors in puberty and adolescence on anxiety-and depression-like behaviors in adulthood.
| Sex/strain/species | Age at Stress | Stressor | Age at Test | Test | Result: Anxiety and Depression | Source |
|---|---|---|---|---|---|---|
| ♂ W rats | 21–32 | Chronic Variable Stress | 120 | EPM | – | Maslova et al. (2002) |
| ♂ SD rats | 30–58 | Chronic Variable Stress | 67 | EPM | – | Toth et al (2008b) |
| FST | – | |||||
| SC | – | |||||
| ♂ SD rats | 27–29 | Chronic Variable Stress | 60 | EPM |

|
Ilin and Richter-Levin (2009) |
| OFT |

|
|||||
| ♂ SD rats | 27–29 | Chronic Variable Stress | 60 | EPM |

|
Jacobson-Pick and Richter-Levin (2010) |
| OFT |

|
|||||
| ♀ SD rats | 27–29 | Chronic Variable Stress | 60 | EPM |

|
|
| OFT |

|
|||||
| ♂ SD rats | 27–29 | Chronic Variable Stress | 60 | EPM |

|
Jacobson-Pick and Richter-Levin (2012) |
| ♂ SD rats | 30–90 | Chronic Variable Stress | 90 | OFT | – | Toth et al (2008a) |
| SI |

|
|||||
| mild/severe | Pohl et al (2007) | |||||
| ♂ LE rats | 23–51 | Chronic Variable Stress | 72 | EPM | –/

|
|
| SC | –/– | |||||
| ♀ LE rats | 23–51 | Chronic Variable Stress | 72 | EPM | –/

|
|
| SC |
 / /

|
|||||
| ♂ LE rats | 22–33 | Chronic Variable Stress | 61 | EPM |

|
Wilkin et al (2012) |
| FST |

|
|||||
| ♀ LE rats | 22–33 | Chronic Variable Stress | 61 | EPM |

|
|
| FST | – | |||||
| ♂ LE rats | 35–46 | Chronic Variable Stress | 73 | EPM |

|
|
| FST | – | |||||
| ♀ LE rats | 35–46 | Chronic Variable Stress | 73 | EPM |

|
|
| FST | – | |||||
| ♂ C57Bl6 mice | 25–29 | Chronic Variable Stress | 81–138 | EPM | – | Peleg-Rabistein and Feldon (2011) |
| OFT | – | |||||
| ♂ W rats | 28 | Repeated Elevated Platform | 90 | OFT | – | Avital and Richter-Levin (2005) |
| SR | – | |||||
| 26–28 | Repeated Elevated Platform | 60 | OFT |

|
||
| SR |

|
|||||
| ♂ W rats | 26–28 | Repeated Elevated Platform | 56 | OFT |

|
Avital et al (2006) |
| ♂ LE rats | 30–45 | Social Instability | 70 | FST | – | Mathews et al (2008) |
| ♀ LE rats | 30–45 | Social Instability | 70 | FST | – | |
| ♂ LE rats | 30–45 | Social Instability | 70 | EPM |

|
McCormick et al (2008) |
| ♀ LE rats | 30–45 | Social Instability | 70 (estrus) | EPM |

|
|
| ♀ LE rats | 70 (diestrus) |

|
||||
| ♂ LE rats | 30–45 | Social Instability | 70 | OFT |

|
Green et al (2012) |
| SI |

|
|||||
| SA | – | |||||
| ♂ CD-1 mice | 28–84 | Social Instability | 92 | EPM |

|
Schmidt et al. (2007) |
| NISF |

|
|||||
| ♂ CD-1 mice | 27–73 | Social Instability | 77 | EPM |

|
Sterleman et al (2008) |
| NISF |

|
|||||
| 450 | EPM |

|
||||
| NISF | – | |||||
| resilient/vulnerable | Schmidt et al (2010b) | |||||
| ♂ CD-1 mice | 30–80 | Social Instability | 120 | EPM | –/

|
|
| TST | –/

|
|||||
| ♀ CD-1 mice | 30–80 | Social Instability | 120 | EPM | – | Schmidt et al (2010a) |
| NISF |

|
|||||
| ♂ W rats | 45–57 | Social Defeat | 78 | SA |

|
Videl et al (2007) |
| ♂ W rats | 45–45 | Social Defeat | 67 | WCT |

|
Videl et at (2011a) |
| ♂ WT rats | – | |||||
| ♂ W rats | 45–58 | Social Defeat | 78, 108 | SA |

|
Videl et al (2011b) |
| ♂ WT rats | – | |||||
| ♀ W rats |

|
|||||
| ♀ WT rats | – | |||||
| ♂ W rats | 37–49 | Social Defeat | 96 | EPM | – | Bourke and Neigh (2011) |
| SC | – | |||||
| FST | – | |||||
| ♀ W rats | 37–49 | Social Defeat | EPM | – | ||
| SC |

|
|||||
| FST |

|
|||||
| ♂ SD rats | 35–40 | Social Defeat | 58 | EPM |

|
Watt et al (2009) |
| ♂ LE rats | 28–41 | Social Defeat | 63 | EPM |

|
Weathington et al (2012) |
| OFT | – | |||||
| SI | – | |||||
| FST | – | |||||
| ♀ LE rats | 28–41 | Social Defeat | 63 | EPM |

|
|
| OFT | – | |||||
| SI |

|
|||||
| FST |

|
|||||
| ♂ SD rats | 28 | Predator Odor | 63 | EPM |

|
Tsoory et al. (2007) |
| ♂ SD rats | 28 | Predator Odor | 60 | EPM |

|
Bazak et al (2009) |
| ♂ W rats | 28 | Predator Odor | 60 | EPM |

|
Cohen et al (2007) |
| ♂ W rats | 33–56 | Predator Odor | 58 | SI |

|
Kendig et al (2011) |
| EPM | – | |||||
| FST |

|
|||||
| ♂ LE rats | 40–48 | Predator Odor | 60 | OFT |

|
Wright et al (2008) |
| ♀ LE rats | OFT |

|
||||
| ♂ LE rats | 38–46 | Predator Odor | 68, 70 | OFT |

|
Wright et al (2012a, b) |
| SI |

|
|||||
| ♀ LE rats | 38–46 | Predator Odor | 68, 70 | OFT |

|
|
| SI |

|
LE, Long Evans; SD, Sprague-Dawley; W, Wistar; EPM, elevated plus maze; FST, forced swim test; NISF, novelty-induced suppression of feeding; OFT, open field test; SA, social approach; SC, sucrose consumption; SI, social interaction; SR, startle response; WCT, water conflict test
There may be sex differences in the effects of stressors during the pubertal period on subsequent anxiety-like behavior in adulthood. Following 10 days of foot-shock stress during the peri-pubertal period, female, but not male mice, display an increase in startle reflexes in adulthood (Table 2), suggestive of increased anxiety. If the mice are stressed with another foot-shock prior to anxiety testing, both male and female mice exhibit increases in anxiety-like behavior (Chester et al., 2008). The reverse effects have also been reported: under similar conditions, male rats displayed increases in anxiety in the shock probe burying task, while female rats expressed a decrease in anxiety-like behaviors (Pohl et al., 2007). It should be noted that the female rats in this study were intact and no mention was made regarding stage of estrous cycle. Therefore, the possibility of hormonal status affecting behavioral results remains. McCormick and colleagues (2008) have shown that female rats increase anxiety in adulthood following peri-pubertal stress (P30–45) only during the diestrous phase of the estrous cycle (i.e., a period characterized by low levels of estradiol and progesterone) (see 3.4.2).
The evidence for effects of pubertal stress on depression-like behavior in adulthood is not as strong. Female rats, exposed to chronic variable stressors during puberty (P23–51) exhibit an increase in anhedonia, as measured by a decrease in sucrose preference in adulthood (Pohl et al., 2007). Social defeat stress during pubertal development (P28–49) also increased depression-like behavioral despair in adult female, but not male rats (Bourke and Neigh, 2011; Weathington et al., 2012), reflecting a fundamental sex difference in the vulnerability to insults during the pubertal period on adult behavior. However, other studies report no effects of chronic variable stress or social instability stress on either sucrose preference (Toth et al., 2008b) or the forced swim test (Mathews et al., 2008; Toth et al., 2008b) in adulthood. Furthermore, in one report the experience of predator odor during the peri-pubertal period decreased depression-like behaviors in adult male rats (Kendig et al., 2011). There also may be inherent sensitivities to the pubertal stress on adult depression-like behaviors. For example, Schmidt and colleagues demonstrated that some outbred, CD1 male mice express increases in CRH and alterations in anxiety- or depression-like behavior in adulthood, whereas other strains of mice do not (Schmidt et al., 2010b). The vulnerable mice also express decreases in MR expression in the hippocampus, relative to the resilient mice, indicating a possible dysregulation in the tonic inhibitory tone on the HPA.
4.4.3. Cognitive behavior in adulthood
Areas such as the hippocampus are implicated in cognition, learning and memory and continue to develop over the course of puberty and adolescence (Andersen and Teicher, 2004; Yildirim et al., 2008). In addition to natural variations in performance of cognitive tasks due to age or developmental status (Altemus and Almli, 1997; Esmoris-Arranz et al., 2008; Schenk, 1985), acute stressors experienced during puberty also contribute to disruptions and impairments in cognitive performances (Table 3) (Avital and Richter-Levin, 2005; Isgor et al., 2004; Tsoory et al., 2007; Tsoory and Richter-Levin, 2006). Variable physical, but not social, stress experienced from prior to the onset of puberty (P28) into the late pubertal period (P56) in male rats results in impaired performance in the Morris water maze (a test of hippocampal-dependent, spatial learning in which an animal must find a hidden platform) in adulthood (Isgor et al., 2004). Moreover, the impaired cognition is not present during the pubertal period, but it emerges during adulthood. Learning in the Morris water maze is also impaired following repeated exposures to the stress of an elevated platform during the pre-pubertal period (P26–28) in adult male rats, but if the pre-pubertally-stressed rats are re-exposed to a stressor in adulthood, learning is improved (Avital and Richter-Levin, 2005). Taken together, these data suggest that physical stressors experienced in puberty contribute to enduring deficits in cognition in adulthood.
Table 3.
Experiments investigating the effects of stressors in puberty and adolescence on learning, memory, and cognition in adulthood.
| Sex/strain/species | Age at Stress | Stressor | Age at Test | Test | Result: Cognition | Source |
|---|---|---|---|---|---|---|
| ♂ SD rats | 28–56 | Chronic Variable Stress | 77 | WM |

|
Isgor et al (2004) |
| ♂ SD rats | 27–29 | Chronic Variable Stress | 60 | SA |

|
Tsoory and Richter-Levin (2006) |
| 33–35 | Chronic Variable Stress | 60 | SA |

|
||
| ♂ SD rats | 27–29 | Chronic Variable Stress | 63–65 | SA |

|
Tsoory et al (2007) |
| ♂ SD rats | 30–90 | Chronic Variable Stress | 90 | SA | – | Toth et al (2008a) |
| ♂ LE rats | 23–51 | Chronic Variable Stress | 72 | SFCT | –/– | Pohl et al (2007) |
| ♀ LE rats | 23–51 | Chronic Variable Stress | 72 | SFCT | –/– | |
| ♂ W rats | 26–28 | Repeated Elevated Platform | 60 | WM |

|
Avital and Richter-Levin (2005) |
| 28 | Repeated Elevated Platform | 90 | WM |

|
||
| 28, 90 | Repeated Elevated Platform | 90 |

|
|||
| 26–28, 60 | Repeated Elevated Platform | 90 | WM |

|
||
| ♂ SD rats | 28–56 | Social Instability | 77 | WM | – | Isgor et al (2004) |
| ♂ W rats | 28–30 | Predator Odor | 83, 85, 87 | Fear Conditioning | – | Toledo-Rodriguez and Sandi (2007) |
| Extinction |

|
|||||
| ♀ W rats | 28–30 | Predator Odor | 83.85.87 | Fear Conditioning | – | |
| Extinction | – |
LE, Long Evans; SD, Sprague-Dawley; W, Wistar; SA, shuttle avoidance; SFCT, social food choice transmission; WM, water maze
The impaired performance on the Morris water maze in animals exposed to variable physical stress in pubertal development is correlated with an inhibition of growth in the pyramidal cell layer of the CA1 and in the granular cell layer of the dentate gyrus of the hippocampus. Furthermore, the pre-pubertal stress also prevents further growth of the pyramidal cell layer of the CA3 of the hippocampus. Decreases in the volume of CA1 and CA3 of the hippocampus are also associated with down-regulation of hippocampal GR expression (Isgor et al., 2004). There is contradiction as to the effects of peri-pubertal stress on brain-derived growth factor (BDNF) in the hippocampus with either no effect or increases in BDNF reported (Toth et al., 2008b; Uys et al., 2006a). Intriguingly, in the work by Toth et al. (2008b), increases in BDNF were correlated with increased neurogenesis, which may represent an attempt to recover from the enduring effects of adolescent stressors on the hippocampus.
Three days of stress (forced swim, elevated platform, and foot-shock) during the pre-pubertal (P26–29) or early pubertal (P33–35) periods of male rats are sufficient to induce poor performance in the shuttle avoidance task (in which a rat learns to move to a different compartment to avoid an electric shock) in adulthood (Avital et al., 2006; Avital and Richter-Levin, 2005). In another study of shuttle avoidance learning, the experience of a multiple stressor paradigm (food restriction, acute swim test, and elevated platforms from P30 until P90) was without effect (Toth et al., 2008a). It is possible that the prolonged exposure to a stressor from early puberty until well into adulthood resulted in a desensitization or habituation to the stressors. This is illustrated by the habituation of adult rats do to repeated stressors (Girotti et al., 2006; Harris et al., 2004; Helmreich et al., 1997; Magarinos and McEwen, 1995; Marti and Armario, 1997).
The effect of pre-pubertal or pubertal stressors on learning and memory depends upon the specific neural development occurring during the exposure of the stressors and on the particular type of learning task. The use of predator odors are commonly used in fear learning, which is mediated by the central and basolateral amygdala and PFC (Farrell et al., 2013; Johansen et al., 2011), areas that continue to develop during the pubertal and adolescent periods (Coulter et al., 1996; Cunningham et al., 2002; Farber et al., 1995; Insel et al., 1990; Kalsbeek et al., 1988; Leslie et al., 1991). Exposure to a predator odor during the pre-pubertal period, which does not affect corticosterone levels in adulthood (Toledo-Rodriguez and Sandi, 2007; Wright et al., 2008), also does not affect fear conditioning in either male or female adult rats (Toledo-Rodriguez and Sandi, 2007). However, pre-pubertally-stressed male rats do not extinguish this fear conditioning (Toledo-Rodriguez and Sandi, 2007), suggesting abnormalities in PFC function. The PFC, which continues to develop during the pubertal period (Coulter et al., 1996; Farber et al., 1995; Insel et al., 1990; Kalsbeek et al., 1988; Leslie et al., 1991), also plays a critical role in extinction of fear memories (Milad and Quirk, 2002; Santini et al., 2004). Therefore, the experience of predator odor stress during puberty may disrupt the ability of the PFC to suppress or extinguish fear learning.
5. Immunity and Inflammation
During the last few decades, the importance of the intricate interactions of the nervous and immune systems has been realized, especially with regard to the regulation of several physical and psychological pathologies, as will be discussed.
5.1. Immune system
The immune system is highly complex and has several specialized components that protect an organism against disease. In order to function properly, the immune system must recognize various types of pathogens as ‘non-self’ and distinguish them from ‘self’ (for review Smith, 2012a; Smith, 2012b). Vertebrates have both an innate and an adaptive immune system. The innate immune system produces rapid response to evolutionarily conserved pathogens, such as bacterial, viral, fungal, or foreign proteins. Innate immune responses are not specific to the type of pathogen and consist of inflammatory, phagocytic, and lytic responses to destroy the pathogens (for further review see Janeway and Medzhitov, 2002; Medzhitov and Janeway, 2000). The complement system is part of the innate immune system; activation of complement proteases triggers a cascade of further cleavages, resulting in lysis of the target cell (for further information see Murphy, 2011). The other branch of the immune system, the adaptive immune system, responds with highly specialized cellular and highly targeted defenses to attack and destroy a pathogen. The adaptive immune responses are based upon the ability of B and T-lymphocytes to recognize and remember specific antigens found on the pathogens. Through the use of antibodies, the adaptive immune system provides stronger and more rapid responses upon each exposure to a pathogen (Janeway, 2001). Important for this review are the macrophages that phagocytose the pathogens, produce cytokines, and recruit additional immune cells.
5.2. Immune Signaling in the Brain
5.2.1. The Role of Microglia in the Healthy Brain
The brain had been thought to be ‘immune privileged’ and isolated from the immune system because of a lack of key immune proteins in neurons. Recently, immune molecules have been demonstrated to be present in the healthy brain and to play a critical role in brain development (Boulanger, 2009; Carpentier and Palmer, 2009; Deverman and Patterson, 2009; Garay and McAllister, 2010).
Microglia are the primary immune cells in the brain. Microglia do not communicate by gap junctions, and they are not connected like astrocytes (Eugenin et al., 2001). Rather, microglia maintain fairly uniform distances in which their cell processes constantly scan the microenvironment in the healthy adult brain, surveying for the presence of pathogens and monitoring the status of local synapses (Hanisch and Kettenmann, 2007; Nimmerjahn et al., 2005; Raivich, 2005; Tremblay et al., 2011; Wake et al., 2009). In cases of either damage to the brain or presence of infectious agents, the microglia migrate to the site of damage, up-regulate immune molecules such as major histocompatibility complex class II (MHC II) receptor and complement receptors of cluster of differentiation 11b (CD11b), and generate and maintain inflammatory responses (Graeber, 2010; Graeber and Streit, 2010; Streit et al., 1988; Streit et al., 2004; Streit et al., 1999).
Microglia undergo substantial morphological restructuring in response to free radicals or pro-inflammatory cytokines (Graeber and Streit, 2010; Kettenmann et al., 2011; Ransohoff and Perry, 2009; Streit et al., 1999). In the healthy brain, the microglia display a highly ramified structure with a round or cell soma, from which emerge four to six primary branches (Hinwood et al., 2012). Following chronic stress, ramified microglia transition into hyper-ramified microglia, with an increase in secondary but not primary branching (Hinwood et al., 2012). Hyper-ramified microglia also reorient their processes in response to changes in neural activity (Tremblay et al., 2011). That is, in response to injury or inflammatory signals, microglia first extend processes and reorient to the site of injury (Nimmerjahn et al., 2005), suggesting that the hyper-ramification may the first step in the activation of microglia. Next, the microglia transition into a reactive state, which is characterized by shorter and thicker processes (Graeber and Streit, 2010; Kettenmann et al., 2011; Streit et al., 1999). Finally, the reactive microglia become phagocytic, amoeboid cells that have no processes and act like macrophages (Graeber and Streit, 2010; Streit et al., 1999).
Microglia are also involved in synapse maturation or elimination via axonal guidance (Verney et al., 2010) and engulfing synaptic material (Graeber, 2010; Schafer et al., 2012; Stevens et al., 2007; Tremblay et al., 2011). These processes are particularly important during synaptic maturation, when synaptic turnover is high, or when synaptic pruning occurs (Paolicelli and Gross, 2011). This synaptic pruning by microglia is necessary for normal brain development in the neonatal period. As pubertally-born neurons and glia are functionally incorporated in neural circuits and contribute to the regulation of adult behaviors (Mohr and Sisk, 2013), we speculate that microglia activity may play a critical role in pubertal development as well.
5.2.2. The Immune Signals in Development
Cytokines are part of a large and diverse family of signaling molecules in the brain. They are typically described as either pro-inflammatory, serving to promote inflammation, and anti-inflammatory, limiting the effects of sustained or excessive inflammatory reactions. The major pro-inflammatory cytokines responsible for the early responses to injury are also the most widely studied and include interleukin 1α (IL-1α), IL-1β, IL-6, and tumor necrosis factor α (TNFα). The major anti-inflammatory cytokines include IL-1 receptor antagonist, IL-4, IL-10, 1L-11, IL-13, tumor growth factor β (TGFβ) (Vilcek, 1998). Caution, however, should be used in making generalizations about the pro- or anti-inflammatory nature of the cytokines, as a given cytokine can have both pro- and anti-inflammatory actions (for review see Cavaillon, 2001). The action of a particular cytokine depends upon the cytokine amount, timing and sequences of action, in addition to the nature of the target cell and of the activation signals. Further, the individual cytokines often act in concert with other cytokines, resulting in synergistic or antagonistic effects (reviewed in Hanisch, 2002; John et al., 2003).
The major cytokines are expressed in the healthy brain (Bauer et al., 2007) and the expression patterns of cytokines and their receptors vary throughout development (Gadient and Otten, 1994; Pousset, 1994; Takao et al., 1992). During development cytokines play a role in neurogenesis, migration, differentiation, synapse formation, neural plasticity, and response to injury (Boulanger, 2009; Carpentier and Palmer, 2009; Deverman and Patterson, 2009; Garay and McAllister, 2010). Cytokines, such as 1L-1β and TNFα, modulate synaptic transmission. Whereas 1L-1β inhibits excitatory synaptic transmission as measured by the frequency and/or amplitude of spontaneous excitatory postsynaptic currents (Yang et al., 2005), TNFα enhances inhibitory synaptic transmission (Beattie et al., 2002; Stellwagen et al., 2005; Stellwagen and Malenka, 2006). Cytokines also are involved in synapse refinement and plasticity in the adult cortex, hippocampus and cerebellum (Boulanger, 2009). Studies examining the role of cytokines in two models of activity-dependent plasticity, long-term plasticity (LTP) or long-term depression (LTD), indicate that exogenous cytokines typically inhibit LTP and LTD (for further review see Boulanger, 2009; Garay and McAllister, 2010).
5.3. Pubertal Maturation of Microglia
Microglia derive from primitive macrophages that originate in the extra-embryonic yolk sac (Ginhoux et al., 2010) and enter the brain in around embryonic day 14 (Bilbo et al., 2011; Ling and Wong, 1993). In rats, areas associated with cognition and emotionality, such as the hippocampus, amygdala and cortex, are among the first regions populated by microglia (approximately embryonic day 17), whereas the PVN is not colonized until the early postnatal period (P4) (Schwarz et al., 2012). Developing microglia are largely in an activated or amoeboid state during the early postnatal period, which correlates with increases in cytokine levels. Moreover, environmental factors, such as stressors, can increase the rate of colonization and the density of the microglia (Gomez-Gonzalez and Escobar, 2010). As microglia, particularly the amoeboid microglia, play an important role in synaptic pruning through the clearing of dead and dying cells and processes, alterations in the colonization or maturation rate could profoundly alter neural development and subsequently adult brain and behavior. It has been postulated that microglia may underlie the mechanisms by which insult in the early postnatal period results in deficits in cognition and disease states (Bilbo and Schwarz, 2009; Bilbo et al., 2011). Microglia are also excellent candidates for mediating the enduring effects of early insults due to their long life span and their ability to become and remain activated (Town et al., 2005).
There is a sex difference in the time course of brain colonization by the microglia (Schwarz et al., 2012). Male rat pups in the early postnatal period (P4) have several-fold more microglia (especially activated microglia) than female in the hippocampus, parietal cortex and amygdala (Schwarz et al., 2012). Male rats are also particularly sensitive to early infection-induced ‘glial priming’ by which early life insults, challenges or injury lead to exaggerated responses to challenges in later life (Bilbo and Schwarz, 2009). That is, the morphology of the primed microglial cells resembles that of the activated glial cells; however, primed microglia do not chronically produce cytokines or other pro-inflammatory mediators (Perry et al., 2003). The primed microglia overreact to a subsequent systemic challenge by overproduction of cytokines (Bilbo et al., 2011; Perry et al., 2003). This overproduction of cytokines in turn may lead to the development of psychopathologies (Bilbo and Schwarz, 2009). Peri-pubertal female (P30) and adult (P60) rats have more activated microglia than males of the same age in the hippocampus, parietal cortex and amygdala, suggesting that females are more sensitive to immune dysregulation during puberty/adolescence and early adulthood. We have recently observed that female CD1 mice display more activated microglia in the pubertal period (P28 and P42) than in adulthood (P70; Holder and Blaustein, unpublished observations). Although pubertal and adult mice show no apparent differences in sickness behavior in response to a bacterial endotoxin, the more activated microglia in puberty may lead to enhanced cytokine and/or stress responses in the brain following LPS, but this has yet to be empirically determined.
5.4. Working Hypothesis of Enduring Effects of Stress during Puberty
Neuroinflammation refers to the chronic activation of microglia and astrocytes (Aloisi, 2001; Kettenmann et al., 2011) and exaggerated expression of pro-inflammatory mediators (e.g., cytokines, reactive oxygen species that can damage cellular structures) within the brain (Streit, 2010). A growing body of experimental and clinical research reveals a pivotal role of neuroinflammation in the etiology of mental disease, such as major depression (Capuron and Miller, 2011; Miller et al., 2009), anxiety (Hou and Baldwin, 2012; Jones and Thomsen, 2012), post-traumatic stress disorders (Jones and Thomsen, 2012), and neurodegenerative diseases associated with cognitive decline such as Alzheimer’s disease and Parkinson’s disease (Streit et al., 2004).
While boys are at higher risk for early-onset neurological disorders (e.g., autism spectrum disorders, attention deficit hyperactivity disorder (ADHD)) and psychopathologies of brain organization (e.g., schizophrenia, Tourette’s disorder), girls and women are more vulnerable to conditions that emerge during puberty and adolescence (e.g., anxiety, depression, eating disorders) (Taylor and Rutter, 1985). Additionally, adolescent girls who experience a high rate of both sexual and physical abuse also show an increased rate of major depression (Silverman et al., 1996). Paralleling the ability of stressors to chronically activate microglia in adulthood leading to increased levels of cytokines (Bilbo and Schwarz, 2009), stressors experienced in childhood or adolescence induce short and long-term alterations in immune function (Danese et al., 2009; Danese et al., 2007; Lemieux et al., 2008) and higher levels of the inflammatory biomarker, C-reactive protein (CRP), in adulthood (Danese et al., 2007). It is, therefore, tempting to speculate that the vulnerability of pubertal and/or adolescent rats, mice, and humans may be due, in part, to the development of the neural immune systems and induction of enduring neuroinflammation. While these ideas have yet to be empirically tested, evidence to date is suggestive of the possibility of neuroinflammation as a common pathway by which pubertal stressors may alter the adult brain and behavior, especially as it relates to mental illness, such as anxiety and depression, as well as learning, memory and cognition (Bilbo et al., 2011; Cribbs et al., 2012; Miller et al., 2009; Salim et al., 2012).
6. Discussion and Conclusions
The pubertal period is a vulnerable period for the emergence of mental diseases, due in part to the neural developments occurring during this time (Andersen, 2003; Kessler et al., 2005; Paus et al., 2008). Moreover, exposure to extreme stressors during puberty and adolescence increases the risk of developing a mental disease (Ge et al., 2001; Grant et al., 2003; Grant et al., 2004; Silverman et al., 1996; Turner and Lloyd, 2004). Overall, the work discussed in this review demonstrates that particular stressors, specifically complex stressors or immune challenges, experienced during puberty and/or early-to-mid adolescence influence behaviors relevant to mental health and mental processes. Stressors during pubertal and/or adolescent development lead to increases in hormonally influenced anxiety-like and depression-like behaviors, a decrease sexual performance, and a decrease in learning, memory, and cognitive performance in adulthood (Ismail and Blaustein, 2013; Ismail et al., 2011; Ismail et al., 2012; Laroche et al., 2009a; Laroche et al., 2009b; Olesen et al., 2011). Ovarian hormones, particularly estradiol, modulate these behaviors in female mice, and the experience of a stressor can also lead a blockade or reversal of the effects of estradiol (Ismail and Blaustein, 2013; Ismail et al., 2011; Ismail et al., 2012; Laroche et al., 2009a; Laroche et al., 2009b; Olesen et al., 2011). Furthermore, stressors during pubertal and adolescent development cause alterations in stress reactivity, steroid hormone receptor densities, and synaptic plasticity of key brain regions such as the hippocampus (See Tables 1–3). What remains to be demonstrated are the mechanisms by which acute or short-term stressors experienced during puberty remodel the developing brain, behavior, and physiology.
One of the biggest challenges to precisely determining the neurobiological mechanisms and behavioral consequences of stressors is the lack of precision in the timing of stressors in many previous experiments. Hormones modulate many of the ongoing neurodevelopmental processes of puberty and adolescence. Recently, we have demonstrated that the ovary and, presumably, ovarian hormones are required for an immune challenge in the pubertal period to decrease sexual behavior in adulthood. (B. Rappleyea, N. Ismail, H. King, and J. Blaustein, unpublished observations). Therefore, we suggest that it is more appropriate to consider the pubertal or reproductive stage of the animal, rather than chronological age. This consideration is especially important in studies examining the effects of extreme stress and trauma in girls (Ge et al., 2001; Grant et al., 2003; Grant et al., 2004; Silverman et al., 1996; Turner and Lloyd, 2004), which often use the rather nebulous terms of ‘childhood’ and ‘adolescence’. As the pubertal status and hormonal milieu are critical for the etiology of affective disorders (Hayward and Sanborn, 2002; Patton et al., 1996), there may be sensitive periods in girls during which stressors contribute to the development of psychopathologies. However, these sensitive periods remain to be precisely defined in girls.
Another important matter to consider is that, rather than one vulnerable period, there may be several sensitive windows during which a particular stressor is more or less effective in altering the adult brain and behavior. In support of this notion, shipping or immune challenge in six, but not four or eight week old mice disrupts ovarian hormone signaling adulthood (Section 3.4). Chronic variable stressors during the early pubertal period (P28–30) are effective in altering the brain and behavior, but they are not effective at a later time point (Section 4.4). We suggest that the reasons for the maximally effective periods for each stressor might depend upon the nature of the stressor and on the particular developmental changes occurring in the brain regions that mediate the response to the stressor and the hormonal milieu. That is, the experience of the stressor may fundamentally alter the ongoing neural developmental processes occurring in key regions, such as the hippocampus and prefrontal cortex, leading to altered trajectories of the development of these regions. Hormones influence many developmental processes; therefore, it is even more important to carefully consider the hormonal status and stage of pubertal development at which the stressor is experienced, as the stressors may be disrupting these dynamic processes.
Finally, the enduring effects of stressors on mental illness-relevant behaviors in rodent models are accompanied by perturbations of the hormones and hormonal axes that modulate behaviors. Together, this review provides a novel focus on pubertal hormones as being responsible for the vulnerability to stressors, and that the enduring effects of stressors during puberty may be, in large part, due to aberrant responses to hormones. Aberrant responses to hormones have been recognized for specific mental diseases (Bloch et al., 2003; Schmidt et al., 1998); however, it is possible that disordered responses contribute to the etiology of other mental illnesses, such as depression. These possibilities need to be more fully studied in clinical populations in order to further elucidate the neurobiological mechanisms of mental illness and provide more targeted therapeutic treatments.
Highlights.
Pubertal stressors alter sexual, anxiety-and depression-like, and cognitive behaviors in adulthood
Pubertal stressors alter behavioral response to gonadal hormones
The authors propose that immune molecules or neuroinflammation may mediate these changes.
Acknowledgments
The work from the author’s laboratory was supported by grants NS 19327, MH093854 from the National Institutes of Health, IOS 1050179 from the National Science Foundation, and an Isis grant from the Society for Women’s Health Research. We thank the late Dr. Steven Zalcman for his suggestion that we consider the use of an immune challenge as a pubertal stressor, and the members of the Stress, Gender, Drugs and the Brain Network for Women’s Research for many thoughtful discussions in the early stages of the work. We also thank Bethany Rappleyea, Amarylis Velez-Perez, John Hernandez, and Christina Gagliardi on their comments on a previous version of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aloisi F. Immune function of microglia. Glia. 2001;36:165–79. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- Altemus KL, Almli CR. Neonatal hippocampal damage in rats: long-term spatial memory deficits and associations with magnitude of hippocampal damage. Hippocampus. 1997;7:403–15. doi: 10.1002/(SICI)1098-1063(1997)7:4<403::AID-HIPO6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology. 2004;29:1988–93. doi: 10.1038/sj.npp.1300528. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AP, Krenzel E, Teicher MH. Pubertal changes in gonadal hormones do not underlie adolescent dopamine receptor overproduction. Psychoneuroendocrinology. 2002;27:683–91. doi: 10.1016/s0306-4530(01)00069-5. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ. Puberty and depression. Child Adolesc Psychiatr Clin N Am. 2006;15:919–37. ix. doi: 10.1016/j.chc.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Anisman H. Cascading effects of stressors and inflammatory immune system activation: implications for major depressive disorder. J Psychiatry Neurosci. 2009;34:4–20. [PMC free article] [PubMed] [Google Scholar]
- Apter D, Pakarinen A, Hammond GL, Vihko R. Adrenocortical function in puberty. serum ACTH, cortisol and dehydroepiandrosterone in girls and boys. Acta Paediatr Scand. 1979;68:599–604. doi: 10.1111/j.1651-2227.1979.tb05062.x. [DOI] [PubMed] [Google Scholar]
- Araki S, Toran-Allerand CD, Ferin M, Vande Wiele RL. Immunoreactive gonadotropin-releasing hormone (Gn-RH) during maturation in the rat: Ontogeny of regional hypothalamic differences. Endocrinology. 1975;97:693–7. doi: 10.1210/endo-97-3-693. [DOI] [PubMed] [Google Scholar]
- Arnett JJ. Sensation seeking, aggressiveness, and adolescent reckless behavior. Personality and Individual Differences. 1996;20:693–702. [Google Scholar]
- Astbury J. Ministerial Round Tables 2001, 54th World Health Assemble. WHO; Geneva, Switzerland: 2001. Gender disparities in mental health. Mental Health; pp. 73–92. [Google Scholar]
- Avital A, Ram E, Maayan R, Weizman A, Richter-Levin G. Effects of early-life stress on behavior and neurosteroid levels in the rat hypothalamus and entorhinal cortex. Brain Res Bull. 2006;68:419–24. doi: 10.1016/j.brainresbull.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Avital A, Richter-Levin G. Exposure to juvenile stress exacerbates the behavioural consequences of exposure to stress in the adult rat. Int J Neuropsychopharmacol. 2005;8:163–73. doi: 10.1017/S1461145704004808. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Dant CC, Reiss AL. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005;15:1848–54. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Bauer S, Kerr BJ, Patterson PH. The neuropoietic cytokine family in development, plasticity, disease and injury. Nat Rev Neurosci. 2007;8:221–32. doi: 10.1038/nrn2054. [DOI] [PubMed] [Google Scholar]
- Bazak N, Kozlovsky N, Kaplan Z, Matar M, Golan H, Zohar J, Richter-Levin G, Cohen H. Pre-pubertal stress exposure affects adult behavioral response in association with changes in circulating corticosterone and brain-derived neurotrophic factor. Psychoneuroendocrinology. 2009;34:844–58. doi: 10.1016/j.psyneuen.2008.12.018. [DOI] [PubMed] [Google Scholar]
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–5. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- Bekku N, Yoshimura H. Animal model of menopausal depressive-like state in female mice: prolongation of immobility time in the forced swimming test following ovariectomy. Psychopharmacology (Berl) 2005;183:300–7. doi: 10.1007/s00213-005-0179-0. [DOI] [PubMed] [Google Scholar]
- Bekman NM, Cummins K, Brown SA. Affective and personality risk and cognitive mediators of initial adolescent alcohol use. J Stud Alcohol Drugs. 2010;71:570–80. doi: 10.15288/jsad.2010.71.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran-Campos V, Prado-Alcala RA, Leon-Jacinto U, Aguilar-Vazquez A, Quirarte GL, Ramirez-Amaya V, Diaz-Cintra S. Increase of mushroom spine density in CA1 apical dendrites produced by water maze training is prevented by ovariectomy. Brain Res. 2011;1369:119–30. doi: 10.1016/j.brainres.2010.10.105. [DOI] [PubMed] [Google Scholar]
- Benes FM, Taylor JB, Cunningham MC. Convergence and plasticity of monoaminergic systems in the medial prefrontal cortex during the postnatal period: implications for the development of psychopathology. Cereb Cortex. 2000;10:1014–27. doi: 10.1093/cercor/10.10.1014. [DOI] [PubMed] [Google Scholar]
- Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch Gen Psychiatry. 1994;51:477–84. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- Bernardi M, Vergoni AV, Sandrini M, Tagliavini S, Bertolini A. Influence of ovariectomy, estradiol and progesterone on the behavior of mice in an experimental model of depression. Physiol Behav. 1989;45:1067–8. doi: 10.1016/0031-9384(89)90238-2. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav Neurosci. 2009;3:14. doi: 10.3389/neuro.08.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Smith SH, Schwarz JM. A Lifespan Approach to Neuroinflammatory and Cognitive Disorders: A Critical Role for Glia. J Neuroimmune Pharmacol. 2011 doi: 10.1007/s11481-011-9299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein JD. Neuroendocrine regulation of feminine sexual behavior: lessons from rodent models and thoughts about humans. Ann Rev Psych. 2008;59:93–118. doi: 10.1146/annurev.psych.59.103006.093556. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Ismail N. Enduring influence of pubertal stressors on behavioral response to hormones in female mice. Horm Behav. 2013;64:390–8. doi: 10.1016/j.yhbeh.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch M, Daly RC, Rubinow DR. Endocrine factors in the etiology of postpartum depression. Compr Psychiatry. 2003;44:234–46. doi: 10.1016/S0010-440X(03)00034-8. [DOI] [PubMed] [Google Scholar]
- Boulanger LM. Immune proteins in brain development and synaptic plasticity. Neuron. 2009;64:93–109. doi: 10.1016/j.neuron.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- Bourgeois JP, Rakic P. Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. J Neurosci. 1993;13:2801–20. doi: 10.1523/JNEUROSCI.13-07-02801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke CH, Neigh GN. Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Horm Behav. 2011;60:112–20. doi: 10.1016/j.yhbeh.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandi LS, Santoro D, Natali A, Altomonte F, Baldi S, Frascerra S, Ferrannini E. Insulin resistance of stress: sites and mechanisms. Clin Sci (Lond) 1993;85:525–35. doi: 10.1042/cs0850525. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci U S A. 2004;101:14515–20. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TJ, Hochberg RB, Zielinski JE, MacLusky NJ. Regional sex differences in cell nuclear estrogen-binding capacity in the rat hypothalamus and preoptic area. Endocrinology. 1988;123:1761–70. doi: 10.1210/endo-123-4-1761. [DOI] [PubMed] [Google Scholar]
- Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2011;130:226–38. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey MP, Deterd CH, de Koning J, Helmerhorst F, de Kloet ER. The influence of ovarian steroids on hypothalamic-pituitary-adrenal regulation in the female rat. J Endocrinol. 1995;144:311–21. doi: 10.1677/joe.0.1440311. [DOI] [PubMed] [Google Scholar]
- Carpentier PA, Palmer TD. Immune influence on adult neural stem cell regulation and function. Neuron. 2009;64:79–92. doi: 10.1016/j.neuron.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaillon JM. Pro- versus anti-inflammatory cytokines: myth or reality. Cell Mol Biol (Noisy-le-grand) 2001;47:695–702. [PubMed] [Google Scholar]
- Chester JA, Barrenha GD, Hughes ML, Keuneke KJ. Age- and sex-dependent effects of footshock stress on subsequent alcohol drinking and acoustic startle behavior in mice selectively bred for high-alcohol preference. Alcohol Clin Exp Res. 2008;32:1782–94. doi: 10.1111/j.1530-0277.2008.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW, Ogawa S. An estrogen-dependent four-gene micronet regulating social recognition: a study with oxytocin and estrogen receptor-alpha and -beta knockout mice. Proc Natl Acad Sci U S A. 2003;100:6192–7. doi: 10.1073/pnas.0631699100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E, Ogawa S, Kavaliers M, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW. Involvement of estrogen receptor alpha, beta and oxytocin in social discrimination: A detailed behavioral analysis with knockout female mice. Genes Brain Behav. 2006;5:528–39. doi: 10.1111/j.1601-183X.2006.00203.x. [DOI] [PubMed] [Google Scholar]
- Chung WC, De Vries GJ, Swaab DF. Sexual differentiation of the bed nucleus of the stria terminalis in humans may extend into adulthood. J Neurosci. 2002;22:1027–33. doi: 10.1523/JNEUROSCI.22-03-01027.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H, Kaplan Z, Matar MA, Loewenthal U, Zohar J, Richter-Levin G. Long-lasting behavioral effects of juvenile trauma in an animal model of PTSD associated with a failure of the autonomic nervous system to recover. Eur Neuropsychopharmacol. 2007;17:464–77. doi: 10.1016/j.euroneuro.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Commins D, Yahr P. Autoradiographic localization of estrogen and androgen receptors in the sexually dimorphic area and other regions of the gerbil brain. J Comp Neurol. 1985;231:473–89. doi: 10.1002/cne.902310406. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Winder DG. Altered anxiety-like behavior and long-term potentiation in the bed nucleus of the stria terminalis in adult mice exposed to chronic social isolation, unpredictable stress, and ethanol beginning in adolescence. Alcohol. 2011;45:585–93. doi: 10.1016/j.alcohol.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter CL, Happe HK, Murrin LC. Postnatal development of the dopamine transporter: a quantitative autoradiographic study. Brain Res Dev Brain Res. 1996;92:172–81. doi: 10.1016/0165-3806(96)00004-1. [DOI] [PubMed] [Google Scholar]
- Crawford AM, Pentz MA, Chou CP, Li C, Dwyer JH. Parallel developmental trajectories of sensation seeking and regular substance use in adolescents. Psychol Addict Behav. 2003;17:179–92. doi: 10.1037/0893-164X.17.3.179. [DOI] [PubMed] [Google Scholar]
- Cribbs DH, Berchtold NC, Perreau V, Coleman PD, Rogers J, Tenner AJ, Cotman CW. Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: a microarray study. J Neuroinflammation. 2012;9:179. doi: 10.1186/1742-2094-9-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453:116–30. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Ann N Y Acad Sci. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Gunnar MR. Heightened stress responsiveness and emotional reactivity during pubertal maturation: implications for psychopathology. Dev Psychopathol. 2009;21:1–6. doi: 10.1017/S0954579409000017. [DOI] [PubMed] [Google Scholar]
- Dalla C, Antoniou K, Papadopoulou-Daifoti Z, Balthazart J, Bakker J. Oestrogen-deficient female aromatase knockout (ArKO) mice exhibit depressive-like symptomatology. Eur J Neurosci. 2004;20:217–28. doi: 10.1111/j.1460-9568.2004.03443.x. [DOI] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, Poulton R, Caspi A. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch Pediatr Adolesc Med. 2009;163:1135–43. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci U S A. 2007;104:1319–24. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Fader AJ, Spencer AL, Dohanich GP. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Horm Behav. 1997;32:217–25. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann N Y Acad Sci. 2001;933:222–34. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- Datson NA, Morsink MC, Meijer OC, de Kloet ER. Central corticosteroid actions: Search for gene targets. Eur J Pharmacol. 2008;583:272–89. doi: 10.1016/j.ejphar.2007.11.070. [DOI] [PubMed] [Google Scholar]
- Davis DM, Jacobson TK, Aliakbari S, Mizumori SJ. Differential effects of estrogen on hippocampal- and striatal-dependent learning. Neurobiol Learn Mem. 2005;84:132–7. doi: 10.1016/j.nlm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Davis EC, Shryne JE, Gorski RA. Structural sexual dimorphisms in the anteroventral periventricular nucleus of the rat hypothalamus are sensitive to gonadal steroids perinatally, but develop peripubertally. Neuroendocrinology. 1996;63:142–8. doi: 10.1159/000126950. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, de Jong IE, Oitzi MS. Neuropharmacology of glucocorticoids: focus on emotion, cognition and cocaine. Eur J Pharmacol. 2008;585:473–82. doi: 10.1016/j.ejphar.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64:61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Diaz-Veliz G, Alarcon T, Espinoza C, Dussaubat N, Mora S. Ketanserin and anxiety levels: influence of gender, estrous cycle, ovariectomy and ovarian hormones in female rats. Pharmacol Biochem Behav. 1997;58:637–42. doi: 10.1016/s0091-3057(97)90004-6. [DOI] [PubMed] [Google Scholar]
- Diaz-Veliz G, Soto V, Dussaubat N, Mora S. Influence of the estrous cycle, ovariectomy and estradiol replacement upon the acquisition of conditioned avoidance responses in rats. Physiol Behav. 1989;46:397–401. doi: 10.1016/0031-9384(89)90010-3. [DOI] [PubMed] [Google Scholar]
- Diaz-Veliz G, Urresta F, Dussaubat N, Mora S. Effects of estradiol replacement in ovariectomized rats on conditioned avoidance responses and other behaviors. Physiol Behav. 1991;50:61–5. doi: 10.1016/0031-9384(91)90498-d. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Social and non-social anxiety in adolescent and adult rats after repeated restraint. Physiol Behav. 2009;97:484–94. doi: 10.1016/j.physbeh.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton NR, Keyes KM, Krueger RF, Balsis S, Skodol AE, Markon KE, Grant BF, Hasin DS. An invariant dimensional liability model of gender differences in mental disorder prevalence: evidence from a national sample. J Abnorm Psychol. 2012;121:282–8. doi: 10.1037/a0024780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison PT, Reiches MW, Shattuck-Faegre H, Breakey A, Konecna M, Urlacher S, Wobber V. Puberty as a life history transition. Ann Hum Biol. 2012;39:352–60. doi: 10.3109/03014460.2012.693199. [DOI] [PubMed] [Google Scholar]
- Esmoris-Arranz FJ, Mendez C, Spear NE. Contextual fear conditioning differs for infant, adolescent, and adult rats. Behav Processes. 2008;78:340–50. doi: 10.1016/j.beproc.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, Eckardt D, Theis M, Willecke K, Bennett MV, Saez JC. Microglia at brain stab wounds express connexin 43 and in vitro form functional gap junctions after treatment with interferon-gamma and tumor necrosis factor-alpha. Proc Natl Acad Sci U S A. 2001;98:4190–5. doi: 10.1073/pnas.051634298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber NB, Wozniak DF, Price MT, Labruyere J, Huss J, St Peter H, Olney JW. Age-specific neurotoxicity in the rat associated with NMDA receptor blockade: potential relevance to schizophrenia? Biol Psychiatry. 1995;38:788–96. doi: 10.1016/0006-3223(95)00046-1. [DOI] [PubMed] [Google Scholar]
- Farrell MR, Sengelaub DR, Wellman CL. Sex differences and chronic stress effects on the neural circuitry underlying fear conditioning and extinction. Physiol Behav. 2013 doi: 10.1016/j.physbeh.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder HH, Silver R. Activation of lordosis in ovariectomized guinea pigs by free and esterified forms of estrone, estradiol-17 beta and estriol. Physiol Behav. 1974;13:251–5. doi: 10.1016/0031-9384(74)90042-0. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Thomas P. Minireview: G protein-coupled estrogen receptor-1, GPER-1: its mechanism of action and role in female reproductive cancer, renal and vascular physiology. Endocrinology. 2012;153:2953–62. doi: 10.1210/en.2012-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- Gadient RA, Otten U. Expression of interleukin-6 (IL-6) and interleukin-6 receptor (IL-6R) mRNAs in rat brain during postnatal development. Brain Res. 1994;637:10–4. doi: 10.1016/0006-8993(94)91211-4. [DOI] [PubMed] [Google Scholar]
- Galeeva A, Tuohimaa P. Analysis of mouse plus-maze behavior modulated by ovarian steroids. Behav Brain Res. 2001;119:41–7. doi: 10.1016/s0166-4328(00)00341-7. [DOI] [PubMed] [Google Scholar]
- Garay PA, McAllister AK. Novel roles for immune molecules in neural development: implications for neurodevelopmental disorders. Front Synaptic Neurosci. 2010;2:136. doi: 10.3389/fnsyn.2010.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Conger RD, Elder GH., Jr Pubertal transition, stressful life events, and the emergence of gender differences in adolescent depressive symptoms. Dev Psychol. 2001;37:404–17. doi: 10.1037//0012-1649.37.3.404. [DOI] [PubMed] [Google Scholar]
- Gerocs K, Rethelyi M, Halasz B. Quantitative analysis of dendritic protrusions in the medial preoptic area during postnatal development. Brain Res. 1986;391:49–57. doi: 10.1016/0165-3806(86)90006-4. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Castellanos FX, Rajapakse JC, Vaituzis AC, Rapoport JL. Sexual dimorphism of the developing human brain. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1185–201. doi: 10.1016/s0278-5846(97)00158-9. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–5. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girotti M, Pace TW, Gaylord RI, Rubin BA, Herman JP, Spencer RL. Habituation to repeated restraint stress is associated with lack of stress-induced c-fos expression in primary sensory processing areas of the rat brain. Neuroscience. 2006;138:1067–81. doi: 10.1016/j.neuroscience.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Goldstein LA, Kurz EM, Sengelaub DR. Androgen regulation of dendritic growth and retraction in the development of a sexually dimorphic spinal nucleus. J Neurosci. 1990;10:935–46. doi: 10.1523/JNEUROSCI.10-03-00935.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez F, Manalo S, Dallman MF. Androgen-sensitive changes in regulation of restraint-induced adrenocorticotropin secretion between early and late puberty in male rats. Endocrinology. 2004;145:59–70. doi: 10.1210/en.2003-0565. [DOI] [PubMed] [Google Scholar]
- Gomez-Gonzalez B, Escobar A. Prenatal stress alters microglial development and distribution in postnatal rat brain. Acta Neuropathol. 2010;119:303–15. doi: 10.1007/s00401-009-0590-4. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–7. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- Graeber MB. Changing face of microglia. Science. 2010;330:783–8. doi: 10.1126/science.1190929. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Streit WJ. Microglia: biology and pathology. Acta Neuropathol. 2010;119:89–105. doi: 10.1007/s00401-009-0622-0. [DOI] [PubMed] [Google Scholar]
- Grant KE, Compas BE, Stuhlmacher AF, Thurm AE, McMahon SD, Halpert JA. Stressors and child and adolescent psychopathology: moving from markers to mechanisms of risk. Psychol Bull. 2003;129:447–66. doi: 10.1037/0033-2909.129.3.447. [DOI] [PubMed] [Google Scholar]
- Grant KE, Compas BE, Thurm AE, McMahon SD, Gipson PY. Stressors and child and adolescent psychopathology: measurement issues and prospective effects. J Clin Child Adolesc Psychol. 2004;33:412–25. doi: 10.1207/s15374424jccp3302_23. [DOI] [PubMed] [Google Scholar]
- Green MR, Barnes B, McCormick CM. Social instability stress in adolescence increases anxiety and reduces social interactions in adulthood in male Long-Evans rats. Dev Psychobiol. 2012 doi: 10.1002/dev.21077. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Beltz TG, Johnson AK. Behavioral and cardiovascular changes in the chronic mild stress model of depression. Physiol Behav. 2003;78:703–10. doi: 10.1016/s0031-9384(03)00050-7. [DOI] [PubMed] [Google Scholar]
- Guillamon A, de Blas MR, Segovia S. Effects of sex steroids on the development of the locus coeruleus in the rat. Brain Res. 1988;468:306–10. doi: 10.1016/0165-3806(88)90143-5. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Nunley KM, Lorens SA, Louie JP, McGivern RF, Bollnow MR. Androgen regulation of adrenocorticotropin and corticosterone secretion in the male rat following novelty and foot shock stressors. Physiol Behav. 1994;55:117–24. doi: 10.1016/0031-9384(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40:140–55. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–94. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Harris RB, Gu H, Mitchell TD, Endale L, Russo M, Ryan DH. Increased glucocorticoid response to a novel stress in rats that have been restrained. Physiol Behav. 2004;81:557–68. doi: 10.1016/j.physbeh.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Hayward C, Sanborn K. Puberty and the emergence of gender differences in psychopathology. J Adolesc Health. 2002;30:49–58. doi: 10.1016/s1054-139x(02)00336-1. [DOI] [PubMed] [Google Scholar]
- Helmreich DL, Morano MI, Akil H, Watson SJ. Correlation between Changes in Stress-Induced Corticosterone Secretion and GR mRNA Levels. Stress. 1997;2:101–112. doi: 10.3109/10253899709014741. [DOI] [PubMed] [Google Scholar]
- Herbison AE. Sexually dimorphic expression of androgen receptor immunoreactivity by somatostatin neurones in rat hypothalamic periventricular nucleus and bed nucleus of the stria terminalis. J Neuroendocrinol. 1995;7:543–53. doi: 10.1111/j.1365-2826.1995.tb00791.x. [DOI] [PubMed] [Google Scholar]
- Hinwood M, Tynan RJ, Charnley JL, Beynon SB, Day TA, Walker FR. Chronic Stress Induced Remodeling of the Prefrontal Cortex: Structural Re-Organization of Microglia and the Inhibitory Effect of Minocycline. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs151. [DOI] [PubMed] [Google Scholar]
- Hou R, Baldwin DS. A neuroimmunological perspective on anxiety disorders. Hum Psychopharmacol. 2012;27:6–14. doi: 10.1002/hup.1259. [DOI] [PubMed] [Google Scholar]
- Huang GZ, Woolley CS. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron. 2012;74:801–8. doi: 10.1016/j.neuron.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–78. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Ilin Y, Richter-Levin G. Enriched environment experience overcomes learning deficits and depressive-like behavior induced by juvenile stress. PLoS One. 2009;4:e4329. doi: 10.1371/journal.pone.0004329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im-Bolter N, Cohen NJ, Farnia F. I thought we were good: social cognition, figurative language, and adolescent psychopathology. J Child Psychol Psychiatry. 2013 doi: 10.1111/jcpp.12067. [DOI] [PubMed] [Google Scholar]
- Imwalle DB, Gustafsson JA, Rissman EF. Lack of functional estrogen receptor beta influences anxiety behavior and serotonin content in female mice. Physiol Behav. 2005;84:157–63. doi: 10.1016/j.physbeh.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Insel TR, Miller LP, Gelhard RE. The ontogeny of excitatory amino acid receptors in rat forebrain--I. N-methyl-D-aspartate and quisqualate receptors. Neuroscience. 1990;35:31–43. doi: 10.1016/0306-4522(90)90117-m. [DOI] [PubMed] [Google Scholar]
- Intlekofer KA, Petersen SL. Distribution of mRNAs encoding classical progestin receptor, progesterone membrane components 1 and 2, serpine mRNA binding protein 1, and progestin and ADIPOQ receptor family members 7 and 8 in rat forebrain. Neuroscience. 2010;172:55–65. doi: 10.1016/j.neuroscience.2010.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isgor C, Kabbaj M, Akil H, Watson SJ. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 2004;14:636–48. doi: 10.1002/hipo.10207. [DOI] [PubMed] [Google Scholar]
- Ismail N, Blaustein JD. Pubertal immune challenge blocks the ability of estradiol to enhance performance on cognitive tasks in adult female mice. Psychoneuroendocrinology. 2013;38:1170–1177. doi: 10.1016/j.psyneuen.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail N, Garas P, Blaustein JD. Long-term effects of pubertal stressors on female sexual receptivity and estrogen receptor-alpha expression in CD-1 female mice. Horm Behav. 2011;59:565–71. doi: 10.1016/j.yhbeh.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail N, Kumlin AM, Blaustein JD. A pubertal immune challenge alters the antidepressant-like effects of chronic estradiol treatment in inbred and outbred adult female mice. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson-Pick S, Richter-Levin G. Differential impact of juvenile stress and corticosterone in juvenility and in adulthood, in male and female rats. Behav Brain Res. 2010;214:268–76. doi: 10.1016/j.bbr.2010.05.036. [DOI] [PubMed] [Google Scholar]
- Jacobson-Pick S, Richter-Levin G. Short- and long-term effects of juvenile stressor exposure on the expression of GABAA receptor subunits in rats. Stress. 2012;15:416–24. doi: 10.3109/10253890.2011.634036. [DOI] [PubMed] [Google Scholar]
- Jakubowski M, Blum M, Roberts JL. Postnatal development of gonadotropin-releasing hormone and cyclophilin gene expression in the female and male rat brain. Endocrinology. 1991;128:2702–8. doi: 10.1210/endo-128-6-2702. [DOI] [PubMed] [Google Scholar]
- Janeway CA., Jr How the immune system works to protect the host from infection: a personal view. Proc Natl Acad Sci U S A. 2001;98:7461–8. doi: 10.1073/pnas.131202998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- Jedlicka P, Vlachos A, Schwarzacher SW, Deller T. A role for the spine apparatus in LTP and spatial learning. Behav Brain Res. 2008;192:12–9. doi: 10.1016/j.bbr.2008.02.033. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–24. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John GR, Lee SC, Brosnan CF. Cytokines: powerful regulators of glial cell activation. Neuroscientist. 2003;9:10–22. doi: 10.1177/1073858402239587. [DOI] [PubMed] [Google Scholar]
- Johnston AL, File SE. Sex differences in animal tests of anxiety. Physiol Behav. 1991;49:245–50. doi: 10.1016/0031-9384(91)90039-q. [DOI] [PubMed] [Google Scholar]
- Jones KA, Thomsen C. The role of the innate immune system in psychiatric disorders. Mol Cell Neurosci. 2012 doi: 10.1016/j.mcn.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HB. Development of the dopaminergic innervation in the prefrontal cortex of the rat. J Comp Neurol. 1988;269:58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Clifton DK, Steiner RA. Emerging ideas about kisspeptin-GPR54 signaling in the neuroendocrine regulation of reproduction. Trends Neurosci. 2007;30:504–11. doi: 10.1016/j.tins.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Kendig MD, Bowen MT, Kemp AH, McGregor IS. Predatory threat induces huddling in adolescent rats and residual changes in early adulthood suggestive of increased resilience. Behav Brain Res. 2011;225:405–14. doi: 10.1016/j.bbr.2011.07.058. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Konkle AT, Baker SL, Kentner AC, Barbagallo LS, Merali Z, Bielajew C. Evaluation of the effects of chronic mild stressors on hedonic and physiological responses: sex and strain compared. Brain Res. 2003;992:227–38. doi: 10.1016/j.brainres.2003.08.047. [DOI] [PubMed] [Google Scholar]
- Korenbrot CC, Huhtaniemi IT, Weiner RI. Preputial separation as an external sign of pubertal development in the male rat. Biol Reprod. 1977;17:298–303. doi: 10.1095/biolreprod17.2.298. [DOI] [PubMed] [Google Scholar]
- Korol DL. Role of estrogen in balancing contributions from multiple memory systems. Neurobiol Learn Mem. 2004;82:309–23. doi: 10.1016/j.nlm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Koss WA, Einat H, Schloesser RJ, Manji HK, Rubinow DR. Estrogen effects on the forced swim test differ in two outbred rat strains. Physiol Behav. 2012;106:81–6. doi: 10.1016/j.physbeh.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krezel W, Dupont S, Krust A, Chambon P, Chapman PF. Increased anxiety and synaptic plasticity in estrogen receptor beta -deficient mice. Proc Natl Acad Sci U S A. 2001;98:12278–82. doi: 10.1073/pnas.221451898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnemann S, Brown TJ, Hochberg RB, MacLusky NJ. Sex differences in the development of estrogen receptors in the rat brain. Horm Behav. 1994;28:483–91. doi: 10.1006/hbeh.1994.1046. [DOI] [PubMed] [Google Scholar]
- Kvetnansky R, Sabban EL, Palkovits M. Catecholaminergic systems in stress: structural and molecular genetic approaches. Physiol Rev. 2009;89:535–606. doi: 10.1152/physrev.00042.2006. [DOI] [PubMed] [Google Scholar]
- Laroche J, Gasbarro L, Herman JP, Blaustein JD. Enduring influences of peripubertal/adolescent stressors on behavioral response to estradiol and progesterone in adult female mice. Endocrinology. 2009a;150:3717–25. doi: 10.1210/en.2009-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroche J, Gasbarro L, Herman JP, Blaustein JD. Reduced behavioral response to gonadal hormones in mice shipped during the peripubertal/adolescent period. Endocrinology. 2009b;150:2351–8. doi: 10.1210/en.2008-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugero KD, Moberg GP. Summation of behavioral and immunological stress: metabolic consequences to the growing mouse. Am J Physiol Endocrinol Metab. 2000;279:E44–9. doi: 10.1152/ajpendo.2000.279.1.E44. [DOI] [PubMed] [Google Scholar]
- Lemieux A, Coe CL, Carnes M. Symptom severity predicts degree of T cell activation in adult women following childhood maltreatment. Brain Behav Immun. 2008;22:994–1003. doi: 10.1016/j.bbi.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz KM, Nugent BM, McCarthy MM. Sexual differentiation of the rodent brain: dogma and beyond. Front Neurosci. 2012;6:26. doi: 10.3389/fnins.2012.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie CA, Robertson MW, Cutler AJ, Bennett JP., Jr Postnatal development of D1 dopamine receptors in the medial prefrontal cortex, striatum and nucleus accumbens of normal and neonatal 6-hydroxydopamine treated rats: a quantitative autoradiographic analysis. Brain Res Dev Brain Res. 1991;62:109–14. doi: 10.1016/0165-3806(91)90195-o. [DOI] [PubMed] [Google Scholar]
- Leuner B, Falduto J, Shors TJ. Associative memory formation increases the observation of dendritic spines in the hippocampus. J Neurosci. 2003;23:659–65. doi: 10.1523/JNEUROSCI.23-02-00659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Cruz D, Eggan S, Erickson S. Postnatal development of prefrontal inhibitory circuits and the pathophysiology of cognitive dysfunction in schizophrenia. Ann N Y Acad Sci. 2004;1021:64–76. doi: 10.1196/annals.1308.008. [DOI] [PubMed] [Google Scholar]
- Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, Magarinos AM, Allen PB, Greengard P, Luine V, McEwen BS. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci U S A. 2004;101:2185–90. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Morrow D, Witkin JM. Decreases in nestlet shredding of mice by serotonin uptake inhibitors: comparison with marble burying. Life Sci. 2006;78:1933–9. doi: 10.1016/j.lfs.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Ling EA, Wong WC. The origin and nature of ramified and amoeboid microglia: a historical review and current concepts. Glia. 1993;7:9–18. doi: 10.1002/glia.440070105. [DOI] [PubMed] [Google Scholar]
- Lohmiller J, Sonya P. Reproduction and Breeding. Elsevier; San Diego: 2006. [Google Scholar]
- Lu SF, McKenna SE, Cologer-Clifford A, Nau EA, Simon NG. Androgen receptor in mouse brain: sex differences and similarities in autoregulation. Endocrinology. 1998;139:1594–601. doi: 10.1210/endo.139.4.5863. [DOI] [PubMed] [Google Scholar]
- Luine VN. Sex steroids and cognitive function. J Neuroendocrinol. 2008;20:866–72. doi: 10.1111/j.1365-2826.2008.01710.x. [DOI] [PubMed] [Google Scholar]
- Luine VN, Frankfurt M. Estrogens facilitate memory processing through membrane mediated mechanisms and alterations in spine density. Front Neuroendocrinol. 2012;33:388–402. doi: 10.1016/j.yfrne.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Richards ST, Wu VY, Beck KD. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm Behav. 1998;34:149–62. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- MacPherson L, Magidson JF, Reynolds EK, Kahler CW, Lejuez CW. Changes in sensation seeking and risk-taking propensity predict increases in alcohol use among early adolescents. Alcohol Clin Exp Res. 2010;34:1400–8. doi: 10.1111/j.1530-0277.2010.01223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–9. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiol Behav. 2001;74:435–40. doi: 10.1016/s0031-9384(01)00593-5. [DOI] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti O, Armario A. Influence of Regularity of Exposure to Chronic Stress on the Pattern of Habituation of Pituitary-Adrenal Hormones, Prolactin and Glucose. Stress. 1997;1:179–189. doi: 10.3109/10253899709001107. [DOI] [PubMed] [Google Scholar]
- Maslova LN, Bulygina VV, Markel AL. Chronic stress during prepubertal development: immediate and long-lasting effects on arterial blood pressure and anxiety-related behavior. Psychoneuroendocrinology. 2002;27:549–61. doi: 10.1016/s0306-4530(01)00092-0. [DOI] [PubMed] [Google Scholar]
- Mathews IZ, Wilton A, Styles A, McCormick CM. Increased depressive behaviour in females and heightened corticosterone release in males to swim stress after adolescent social stress in rats. Behav Brain Res. 2008;190:33–40. doi: 10.1016/j.bbr.2008.02.004. [DOI] [PubMed] [Google Scholar]
- McCarthy MM. How it’s made: organisational effects of hormones on the developing brain. J Neuroendocrinol. 2010;22:736–42. doi: 10.1111/j.1365-2826.2010.02021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CM, Furey BF, Child M, Sawyer MJ, Donohue SM. Neonatal sex hormones have ‘organizational’ effects on the hypothalamic-pituitary-adrenal axis of male rats. Brain Res Dev Brain Res. 1998;105:295–307. doi: 10.1016/s0165-3806(97)00155-7. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Green MR. From the stressed adolescent to the anxious and depressed adult: Investigations in rodent models. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.08.063. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Green MR, Cameron NM, Nixon F, Levy MJ, Clark RA. Deficits in male sexual behavior in adulthood after social instability stress in adolescence in rats. Horm Behav. 2013;63:5–12. doi: 10.1016/j.yhbeh.2012.11.009. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Linkroum W, Sallinen BJ, Miller NW. Peripheral and central sex steroids have differential effects on the HPA axis of male and female rats. Stress. 2002;5:235–47. doi: 10.1080/1025389021000061165. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Robarts D, Kopeikina K, Kelsey JE. Long-lasting, sex- and age-specific effects of social stressors on corticosterone responses to restraint and on locomotor responses to psychostimulants in rats. Horm Behav. 2005;48:64–74. doi: 10.1016/j.yhbeh.2005.01.008. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Smith C, Mathews IZ. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav Brain Res. 2008;187:228–38. doi: 10.1016/j.bbr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Allostasis, allostatic load, and the aging nervous system: role of excitatory amino acids and excitotoxicity. Neurochem Res. 2000;25:1219–31. doi: 10.1023/a:1007687911139. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Akama KT, Spencer-Segal JL, Milner TA, Waters EM. Estrogen effects on the brain: actions beyond the hypothalamus via novel mechanisms. Behav Neurosci. 2012;126:4–16. doi: 10.1037/a0026708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, Janeway C., Jr Innate immune recognition: mechanisms and pathways. Immunol Rev. 2000;173:89–97. doi: 10.1034/j.1600-065x.2000.917309.x. [DOI] [PubMed] [Google Scholar]
- Meyer G, Ferres-Torres R, Mas M. The effects of puberty and castration on hippocampal dendritic spines of mice. A Golgi study. Brain Res. 1978;155:108–12. doi: 10.1016/0006-8993(78)90309-8. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–4. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–41. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr MA, Sisk CL. Pubertally born neurons and glia are functionally integrated into limbic and hypothalamic circuits of the male Syrian hamster. Proc Natl Acad Sci U S A. 2013;110:4792–7. doi: 10.1073/pnas.1219443110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora S, Dussaubat N, Diaz-Veliz G. Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology. 1996;21:609–20. doi: 10.1016/s0306-4530(96)00015-7. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Pfaff DW. Effects of estrogen on activity and fear-related behaviors in mice. Horm Behav. 2001;40:472–82. doi: 10.1006/hbeh.2001.1716. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Pfaff DW. Estrogen’s effects on activity, anxiety, and fear in two mouse strains. Behav Brain Res. 2002;132:85–93. doi: 10.1016/s0166-4328(01)00398-9. [DOI] [PubMed] [Google Scholar]
- Murphy K. Janeway’s Immunobiology. Garland Science; New York and London: 2011. [Google Scholar]
- Navarro VM, Castellano JM, McConkey SM, Pineda R, Ruiz-Pino F, Pinilla L, Clifton DK, Tena-Sempere M, Steiner RA. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab. 2011;300:E202–10. doi: 10.1152/ajpendo.00517.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA. Mechanisms of estrogen action. Physiol Rev. 2001;81:1535–65. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–8. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Novac N, Heinzel T. Nuclear receptors: overview and classification. Curr Drug Targets Inflamm Allergy. 2004;3:335–46. doi: 10.2174/1568010042634541. [DOI] [PubMed] [Google Scholar]
- Nunez JL, Jurgens HA, Juraska JM. Androgens reduce cell death in the developing rat visual cortex. Brain Res Dev Brain Res. 2000;125:83–8. doi: 10.1016/s0165-3806(00)00126-7. [DOI] [PubMed] [Google Scholar]
- Nunez JL, Lauschke DM, Juraska JM. Cell death in the development of the posterior cortex in male and female rats. J Comp Neurol. 2001;436:32–41. [PubMed] [Google Scholar]
- O’Neil MF, Moore NA. Animal models of depression: are there any? Hum Psychopharmacol. 2003;18:239–54. doi: 10.1002/hup.496. [DOI] [PubMed] [Google Scholar]
- Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30:713–43. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139:5070–81. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Lomniczi A, Mastronardi C, Heger S, Roth C, Parent AS, Matagne V, Mungenast AE. Minireview: the neuroendocrine regulation of puberty: is the time ripe for a systems biology approach? Endocrinology. 2006;147:1166–74. doi: 10.1210/en.2005-1136. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Urbanski HF. Puberty in the Rat. In: Knobil E, Neill J, editors. The Physiology of Reproduction. Raven Press; New York: 1994. pp. 363–409. [Google Scholar]
- Ojeda SR, Wheaton JE, Jameson HE, McCann SM. The onset of puberty in the female rat: changes in plasma prolactin, gonadotropins, luteinizing hormone-releasing hormone (LHRH), and hypothalamic LHRH content. Endocrinology. 1976;98:630–8. doi: 10.1210/endo-98-3-630. [DOI] [PubMed] [Google Scholar]
- Okada M, Hayashi N, Kometani M, Nakao K, Inukai T. Influences of ovariectomy and continuous replacement of 17beta-estradiol on the tail skin temperature and behavior in the forced swimming test in rats. Jpn J Pharmacol. 1997;73:93–6. doi: 10.1254/jjp.73.93. [DOI] [PubMed] [Google Scholar]
- Olesen KM, Ismail N, Merchasin ED, Blaustein JD. Long-term alteration of anxiolytic effects of ovarian hormones in female mice by a peripubertal immune challenge. Horm Behav. 2011;60:318–26. doi: 10.1016/j.yhbeh.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera-Lopez JI, Molina-Hernandez M, Tellez-Alcantara NP, Jaramillo MT. Estradiol and neuropeptide Y (intra-lateral septal) reduce anxiety-like behavior in two animal models of anxiety. Peptides. 2008;29:1396–403. doi: 10.1016/j.peptides.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Osterlund MK. Underlying mechanisms mediating the antidepressant effects of estrogens. Biochim Biophys Acta. 2010;1800:1136–44. doi: 10.1016/j.bbagen.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Palanza P, Gioiosa L, Parmigiani S. Social stress in mice: gender differences and effects of estrous cycle and social dominance. Physiol Behav. 2001;73:411–20. doi: 10.1016/s0031-9384(01)00494-2. [DOI] [PubMed] [Google Scholar]
- Pandaranandaka J, Poonyachoti S, Kalandakanond-Thongsong S. Anxiolytic property of estrogen related to the changes of the monoamine levels in various brain regions of ovariectomized rats. Physiol Behav. 2006;87:828–35. doi: 10.1016/j.physbeh.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Pandaranandaka J, Poonyachoti S, Kalandakanond-Thongsong S. Differential effects of exogenous and endogenous estrogen on anxiety as measured by elevated T-maze in relation to the serotonergic system. Behav Brain Res. 2009;198:142–8. doi: 10.1016/j.bbr.2008.10.043. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Gross CT. Microglia in development: linking brain wiring to brain environment. Neuron Glia Biol. 2011;7:77–83. doi: 10.1017/S1740925X12000105. [DOI] [PubMed] [Google Scholar]
- Parent AS, Matagne V, Bourguignon JP. Control of puberty by excitatory amino acid neurotransmitters and its clinical implications. Endocrine. 2005;28:281–6. doi: 10.1385/ENDO:28:3:281. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- Parker CR, Jr, Mahesh VB. Hormonal events surrounding the natural onset of puberty in female rats. Biol Reprod. 1976;14:347–53. doi: 10.1095/biolreprod14.3.347. [DOI] [PubMed] [Google Scholar]
- Parker LN. Adrenarche. Endocrinol Metab Clin North Am. 1991;20:71–83. [PubMed] [Google Scholar]
- Parsons B, Rainbow TC, MacLusky NJ, McEwen BS. Progestin receptor levels in rat hypothalamic and limbic nuclei. J Neurosci. 1982;2:1446–52. doi: 10.1523/JNEUROSCI.02-10-01446.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton GC, Hibbert ME, Carlin J, Shao Q, Rosier M, Caust J, Bowes G. Menarche and the onset of depression and anxiety in Victoria, Australia. J Epidemiol Community Health. 1996;50:661–6. doi: 10.1136/jech.50.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton GC, Viner R. Pubertal transitions in health. Lancet. 2007;369:1130–9. doi: 10.1016/S0140-6736(07)60366-3. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–57. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–11. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Peleg-Raibstein D, Feldon J. Differential effects of post-weaning juvenile stress on the behaviour of C57BL/6 mice in adolescence and adulthood. Psychopharmacology (Berl) 2011;214:339–51. doi: 10.1007/s00213-010-1991-8. [DOI] [PubMed] [Google Scholar]
- Perry VH, Newman TA, Cunningham C. The impact of systemic infection on the progression of neurodegenerative disease. Nat Rev Neurosci. 2003;4:103–12. doi: 10.1038/nrn1032. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HB, Rakic P, Kostovic I. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci U S A. 2011;108:13281–6. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–82. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Picazo O, Estrada-Camarena E, Hernandez-Aragon A. Influence of the post-ovariectomy time frame on the experimental anxiety and the behavioural actions of some anxiolytic agents. Eur J Pharmacol. 2006;530:88–94. doi: 10.1016/j.ejphar.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Pignatelli D, Xiao F, Gouveia AM, Ferreira JG, Vinson GP. Adrenarche in the rat. J Endocrinol. 2006;191:301–8. doi: 10.1677/joe.1.06972. [DOI] [PubMed] [Google Scholar]
- Pinos H, Collado P, Rodriguez-Zafra M, Rodriguez C, Segovia S, Guillamon A. The development of sex differences in the locus coeruleus of the rat. Brain Res Bull. 2001;56:73–8. doi: 10.1016/s0361-9230(01)00540-8. [DOI] [PubMed] [Google Scholar]
- Plant TM, Barker-Gibb ML. Neurobiological mechanisms of puberty in higher primates. Hum Reprod Update. 2004;10:67–77. doi: 10.1093/humupd/dmh001. [DOI] [PubMed] [Google Scholar]
- Pohl J, Olmstead MC, Wynne-Edwards KE, Harkness K, Menard JL. Repeated exposure to stress across the childhood-adolescent period alters rats’ anxiety- and depression-like behaviors in adulthood: The importance of stressor type and gender. Behav Neurosci. 2007;121:462–74. doi: 10.1037/0735-7044.121.3.462. [DOI] [PubMed] [Google Scholar]
- Pousset F. Developmental expression of cytokine genes in the cortex and hippocampus of the rat central nervous system. Brain Res Dev Brain Res. 1994;81:143–6. doi: 10.1016/0165-3806(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Rachman IM, Unnerstall JR, Pfaff DW, Cohen RS. Estrogen alters behavior and forebrain c-fos expression in ovariectomized rats subjected to the forced swim test. Proc Natl Acad Sci U S A. 1998;95:13941–6. doi: 10.1073/pnas.95.23.13941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainbow TC, Parsons B, McEwen BS. Sex differences in rat brain oestrogen and progestin receptors. Nature. 1982;300:648–9. doi: 10.1038/300648a0. [DOI] [PubMed] [Google Scholar]
- Raivich G. Like cops on the beat: the active role of resting microglia. Trends Neurosci. 2005;28:571–3. doi: 10.1016/j.tins.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM. Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology. 2008;149:4387–95. doi: 10.1210/en.2008-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–45. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Redei E, Li L, Halasz I, McGivern RF, Aird F. Fast glucocorticoid feedback inhibition of ACTH secretion in the ovariectomized rat: effect of chronic estrogen and progesterone. Neuroendocrinology. 1994;60:113–23. doi: 10.1159/000126741. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children. A volumetric imaging study. Brain. 1996;119(Pt 5):1763–74. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- Rizza RA, Mandarino LJ, Gerich JE. Cortisol-induced insulin resistance in man: impaired suppression of glucose production and stimulation of glucose utilization due to a postreceptor detect of insulin action. J Clin Endocrinol Metab. 1982;54:131–8. doi: 10.1210/jcem-54-1-131. [DOI] [PubMed] [Google Scholar]
- Roberti JW. A review of behavioral and biological correlates of sensation seeking. Journal of Research in Personality. 2004;38:256–279. [Google Scholar]
- Rocha BA, Fleischer R, Schaeffer JM, Rohrer SP, Hickey GJ. 17 Beta-estradiol-induced antidepressant-like effect in the forced swim test is absent in estrogen receptor-beta knockout (BERKO) mice. Psychopharmacology (Berl) 2005;179:637–43. doi: 10.1007/s00213-004-2078-1. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD, McEwen BS. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology. 2006;147:1664–74. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Bellani R, McEwen BS. Stress-induced progesterone secretion and progesterone receptor immunoreactivity in the paraventricular nucleus are modulated by pubertal development in male rats. Stress. 2005;8:265–71. doi: 10.1080/10253890500489320. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Karatsoreos IN, Jasnow AM, McEwen BS. Age- and stress-induced changes in corticotropin-releasing hormone mRNA expression in the paraventricular nucleus of the hypothalamus. Neuroendocrinology. 2007;85:199–206. doi: 10.1159/000102950. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Lee SJ, Chhua N, McPherson CR, McEwen BS. Testosterone cannot activate an adult-like stress response in prepubertal male rats. Neuroendocrinology. 2004a;79:125–32. doi: 10.1159/000077270. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Lee SJ, McEwen BS. Differential stress reactivity in intact and ovariectomized prepubertal and adult female rats. Neuroendocrinology. 2004b;80:387–93. doi: 10.1159/000084203. [DOI] [PubMed] [Google Scholar]
- Roy V, Chapillon P. Further evidences that risk assessment and object exploration behaviours are useful to evaluate emotional reactivity in rodents. Behav Brain Res. 2004;154:439–48. doi: 10.1016/j.bbr.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Salim S, Chugh G, Asghar M. Inflammation in anxiety. Adv Protein Chem Struct Biol. 2012;88:1–25. doi: 10.1016/B978-0-12-398314-5.00001-5. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Spatial memory retention is enhanced by acute and continuous estradiol replacement. Horm Behav. 2004;45:128–35. doi: 10.1016/j.yhbeh.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Santini E, Ge H, Ren K, Pena de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci. 2004;24:5704–10. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Share LJ. Rank-Related Differences in Cardiovascular Function among Wild Baboons - Role of Sensitivity to Glucocorticoids. Am J Primatol. 1994;32:261–275. doi: 10.1002/ajp.1350320404. [DOI] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Stevens B. The “quad-partite” synapse: Microglia-synapse interactions in the developing and mature CNS. Glia. 2012 doi: 10.1002/glia.22389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk F. Development of place navigation in rats from weaning to puberty. Behav Neural Biol. 1985;43:69–85. doi: 10.1016/s0163-1047(85)91510-9. [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Scharf SH, Liebl C, Harbich D, Mayer B, Holsboer F, Muller MB. A novel chronic social stress paradigm in female mice. Horm Behav. 2010a;57:415–20. doi: 10.1016/j.yhbeh.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Scharf SH, Sterlemann V, Ganea K, Liebl C, Holsboer F, Muller MB. High susceptibility to chronic social stress is associated with a depression-like phenotype. Psychoneuroendocrinology. 2010b;35:635–43. doi: 10.1016/j.psyneuen.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Sterlemann V, Ganea K, Liebl C, Alam S, Harbich D, Greetfeld M, Uhr M, Holsboer F, Muller MB. Persistent neuroendocrine and behavioral effects of a novel, etiologically relevant mouse paradigm for chronic social stress during adolescence. Psychoneuroendocrinology. 2007;32:417–29. doi: 10.1016/j.psyneuen.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. The New England journal of medicine. 1998;338:209–16. doi: 10.1056/NEJM199801223380401. [DOI] [PubMed] [Google Scholar]
- Schommer NC, Hellhammer DH, Kirschbaum C. Dissociation between reactivity of the hypothalamus-pituitary-adrenal axis and the sympathetic-adrenal-medullary system to repeated psychosocial stress. Psychosom Med. 2003;65:450–60. doi: 10.1097/01.psy.0000035721.12441.17. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Sholar PW, Bilbo SD. Sex differences in microglial colonization of the developing rat brain. J Neurochem. 2012;120:948–63. doi: 10.1111/j.1471-4159.2011.07630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JP, Stewart JM, De Ghett VJ. Critical periods in the organization of systems. Dev Psychobiol. 1974;7:489–513. doi: 10.1002/dev.420070602. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–94. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan PJ. Localization of androgen- and estrogen-concentrating neurons in the diencephalon and telencephalon of the mouse. Endocrinology. 1978;103:1328–34. doi: 10.1210/endo-103-4-1328. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Komm B, Merchenthaler I. The distribution of estrogen receptor-beta mRNA in the rat hypothalamus. Steroids. 1996;61:678–81. doi: 10.1016/s0039-128x(96)00222-x. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–25. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Silverman AB, Reinherz HZ, Giaconia RM. The long-term sequelae of child and adolescent abuse: a longitudinal community study. Child Abuse Negl. 1996;20:709–23. doi: 10.1016/0145-2134(96)00059-2. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7:1040–7. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26:163–74. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Sizonenko PC. Physiology of puberty. J Endocrinol Invest. 1989;12:59–63. [PubMed] [Google Scholar]
- Smejkalova T, Woolley CS. Estradiol acutely potentiates hippocampal excitatory synaptic transmission through a presynaptic mechanism. J Neurosci. 2010;30:16137–48. doi: 10.1523/JNEUROSCI.4161-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KA. Toward a molecular understanding of adaptive immunity: a chronology - part II. Front Immunol. 2012a;3:364. doi: 10.3389/fimmu.2012.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KA. Toward a molecular understanding of adaptive immunity: a chronology, part I. Front Immunol. 2012b;3:369. doi: 10.3389/fimmu.2012.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21:8819–29. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spinedi E, Chisari A, Pralong F, Gaillard RC. Sexual dimorphism in the mouse hypothalamic-pituitary-adrenal axis function after endotoxin and insulin stresses during development. Neuroimmunomodulation. 1997;4:77–83. doi: 10.1159/000097324. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J Neurosci. 2005;25:3219–28. doi: 10.1523/JNEUROSCI.4486-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–9. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Sterlemann V, Ganea K, Liebl C, Harbich D, Alam S, Holsboer F, Muller MB, Schmidt MV. Long-term behavioral and neuroendocrine alterations following chronic social stress in mice: implications for stress-related disorders. Horm Behav. 2008;53:386–94. doi: 10.1016/j.yhbeh.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW, Barres BA. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–78. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Straub RH, Buttgereit F, Cutolo M. Alterations of the hypothalamic-pituitary-adrenal axis in systemic immune diseases - a role for misguided energy regulation. Clin Exp Rheumatol. 2011;29:S23–31. [PubMed] [Google Scholar]
- Streit WJ. Microglial activation and neuroinflammation in Alzheimer’s disease: a critical examination of recent history. Front Aging Neurosci. 2010;2:22. doi: 10.3389/fnagi.2010.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Graeber MB, Kreutzberg GW. Functional plasticity of microglia: a review. Glia. 1988;1:301–7. doi: 10.1002/glia.440010502. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Mrak RE, Griffin WS. Microglia and neuroinflammation: a pathological perspective. J Neuroinflammation. 2004;1:14. doi: 10.1186/1742-2094-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Walter SA, Pennell NA. Reactive microgliosis. Prog Neurobiol. 1999;57:563–81. doi: 10.1016/s0301-0082(98)00069-0. [DOI] [PubMed] [Google Scholar]
- Suda S, Segi-Nishida E, Newton SS, Duman RS. A postpartum model in rat: behavioral and gene expression changes induced by ovarian steroid deprivation. Biol Psychiatry. 2008;64:311–9. doi: 10.1016/j.biopsych.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafet GE, Bernardini R. Psychoneuroendocrinological links between chronic stress and depression. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:893–903. doi: 10.1016/S0278-5846(03)00162-3. [DOI] [PubMed] [Google Scholar]
- Takao T, Culp SG, Newton RC, De Souza EB. Type I interleukin-1 receptors in the mouse brain-endocrine-immune axis labelled with [125I]recombinant human interleukin-1 receptor antagonist. J Neuroimmunol. 1992;41:51–60. doi: 10.1016/0165-5728(92)90195-q. [DOI] [PubMed] [Google Scholar]
- Tanner JM. Growth at Adolescence. Blackwell Scientific Publications; Oxford: 1962. [Google Scholar]
- Taylor E, Rutter M. Sex differences in neurodevelopmental and psychiatric disorders: One explanation or many? Behavioral and Brain Sciences. 1985;8:460–460. [Google Scholar]
- Tetel MJ, Auger AP, Charlier TD. Who’s in charge? Nuclear receptor coactivator and corepressor function in brain and behavior. Front Neuroendocrinol. 2009;30:328–42. doi: 10.1016/j.yfrne.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirelli E, Laviola G, Adriani W. Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neurosci Biobehav Rev. 2003;27:163–78. doi: 10.1016/s0149-7634(03)00018-6. [DOI] [PubMed] [Google Scholar]
- Toledo-Rodriguez M, Sandi C. Stress before puberty exerts a sex- and age-related impact on auditory and contextual fear conditioning in the rat. Neural Plasticity. 2007;2007:1–12. doi: 10.1155/2007/71203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomihara K, Soga T, Nomura M, Korach KS, Gustafsson JA, Pfaff DW, Ogawa S. Effect of ER-beta gene disruption on estrogenic regulation of anxiety in female mice. Physiol Behav. 2009;96:300–6. doi: 10.1016/j.physbeh.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth E, Avital A, Leshem M, Richter-Levin G, Braun K. Neonatal and juvenile stress induces changes in adult social behavior without affecting cognitive function. Behav Brain Res. 2008a;190:135–9. doi: 10.1016/j.bbr.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Toth E, Gersner R, Wilf-Yarkoni A, Raizel H, Dar DE, Richter-Levin G, Levit O, Zangen A. Age-dependent effects of chronic stress on brain plasticity and depressive behavior. J Neurochem. 2008b;107:522–32. doi: 10.1111/j.1471-4159.2008.05642.x. [DOI] [PubMed] [Google Scholar]
- Town T, Nikolic V, Tan J. The microglial “activation” continuum: from innate to adaptive responses. J Neuroinflammation. 2005;2:24. doi: 10.1186/1742-2094-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay ME, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A. The role of microglia in the healthy brain. J Neurosci. 2011;31:16064–9. doi: 10.1523/JNEUROSCI.4158-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoory M, Cohen H, Richter-Levin G. Juvenile stress induces a predisposition to either anxiety or depressive-like symptoms following stress in adulthood. Eur Neuropsychopharmacol. 2007;17:245–56. doi: 10.1016/j.euroneuro.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Tsoory M, Richter-Levin G. Learning under stress in the adult rat is differentially affected by ‘juvenile’ or ‘adolescent’ stress. Int J Neuropsychopharmacol. 2006;9:713–28. doi: 10.1017/S1461145705006255. [DOI] [PubMed] [Google Scholar]
- Turner RJ, Lloyd DA. Stress burden and the lifetime incidence of psychiatric disorder in young adults: racial and ethnic contrasts. Arch Gen Psychiatry. 2004;61:481–8. doi: 10.1001/archpsyc.61.5.481. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanska M, Swiech L, Jaworski J. Developmental plasticity of the dendritic compartment: focus on the cytoskeleton. Adv Exp Med Biol. 2012;970:265–84. doi: 10.1007/978-3-7091-0932-8_12. [DOI] [PubMed] [Google Scholar]
- Uys JD, Marais L, Faure J, Prevoo D, Swart P, Mohammed AH, Stein DJ, Daniels WM. Developmental trauma is associated with behavioral hyperarousal, altered HPA axis activity, and decreased hippocampal neurotrophin expression in the adult rat. Ann N Y Acad Sci. 2006a;1071:542–6. doi: 10.1196/annals.1364.060. [DOI] [PubMed] [Google Scholar]
- Uys JD, Muller CJ, Marais L, Harvey BH, Stein DJ, Daniels WM. Early life trauma decreases glucocorticoid receptors in rat dentate gyrus upon adult re-stress: reversal by escitalopram. Neuroscience. 2006b;137:619–25. doi: 10.1016/j.neuroscience.2005.08.089. [DOI] [PubMed] [Google Scholar]
- Vandenbergh JG. Effect of the presence of a male on the sexual maturation of female mice. Endocrinology. 1967;81:345–9. doi: 10.1210/endo-81-2-345. [DOI] [PubMed] [Google Scholar]
- Vandenbergh JG. Male odor accelerates female sexual maturation in mice. Endocrinology. 1969;84:658–60. doi: 10.1210/endo-84-3-658. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Pfaff DW. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocrinol. 2008;29:238–57. doi: 10.1016/j.yfrne.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Verney C, Monier A, Fallet-Bianco C, Gressens P. Early microglial colonization of the human forebrain and possible involvement in periventricular white-matter injury of preterm infants. J Anat. 2010;217:436–48. doi: 10.1111/j.1469-7580.2010.01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau V. Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. J Neuroendocrinol. 2002;14:506–13. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991;129:2503–11. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. The inhibitory effect of testosterone on hypothalamic-pituitary-adrenal responses to stress is mediated by the medial preoptic area. J Neurosci. 1996;16:1866–76. doi: 10.1523/JNEUROSCI.16-05-01866.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal J, Bie J, Granneman RA, Wallinga AE, Koolhaas JM, Buwalda B. Social stress during adolescence in Wistar rats induces social anxiety in adulthood without affecting brain monoaminergic content and activity. Physiol Behav. 2007;92:824–30. doi: 10.1016/j.physbeh.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Vidal J, Buwalda B, Koolhaas JM. Differential long-term effects of social stress during adolescence on anxiety in Wistar and wild-type rats. Behav Processes. 2011a;87:176–82. doi: 10.1016/j.beproc.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Vidal J, Buwalda B, Koolhaas JM. Male Wistar rats are more susceptible to lasting social anxiety than Wild-type Groningen rats following social defeat stress during adolescence. Behav Processes. 2011b;88:76–80. doi: 10.1016/j.beproc.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Vilcek J. The cytokines: an overview. In: Thomson A, editor. The Cytokine Handbook. Academic Press; San Diego: 1998. pp. 1–20. [Google Scholar]
- Vitaliano PP, Scanlan JM, Zhang J, Savage MV, Hirsch IB, Siegler IC. A path model of chronic stress, the metabolic syndrome, and coronary heart disease. Psychosom Med. 2002;64:418–35. doi: 10.1097/00006842-200205000-00006. [DOI] [PubMed] [Google Scholar]
- Wagner MK. Behavioral characteristics related to substance abuse and risk-taking, sensation-seeking, anxiety sensitivity, and self-reinforcement. Addict Behav. 2001;26:115–20. doi: 10.1016/s0306-4603(00)00071-x. [DOI] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–80. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EF, Walder DJ, Reynolds F. Developmental changes in cortisol secretion in normal and at-risk youth. Dev Psychopathol. 2001;13:721–32. doi: 10.1017/s0954579401003169. [DOI] [PubMed] [Google Scholar]
- Wall PM, Messier C. Methodological and conceptual issues in the use of the elevated plus-maze as a psychological measurement instrument of animal anxiety-like behavior. Neurosci Biobehav Rev. 2001;25:275–86. doi: 10.1016/s0149-7634(01)00013-6. [DOI] [PubMed] [Google Scholar]
- Wallen K. Hormonal influences on sexually differentiated behavior in nonhuman primates. Front Neuroendocrinol. 2005;26:7–26. doi: 10.1016/j.yfrne.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Wang H, Meng XH, Ning H, Zhao XF, Wang Q, Liu P, Zhang H, Zhang C, Chen GH, Xu DX. Age- and gender-dependent impairments of neurobehaviors in mice whose mothers were exposed to lipopolysaccharide during pregnancy. Toxicol Lett. 2010;192:245–51. doi: 10.1016/j.toxlet.2009.10.030. [DOI] [PubMed] [Google Scholar]
- Watt MJ, Burke AR, Renner KJ, Forster GL. Adolescent male rats exposed to social defeat exhibit altered anxiety behavior and limbic monoamines as adults. Behav Neurosci. 2009;123:564–76. doi: 10.1037/a0015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waylen A, Wolke D. Sex ‘n’ drugs ‘n’ rock ‘n’ roll: the meaning and social consequences of pubertal timing. Eur J Endocrinol. 2004;151(Suppl 3):U151–9. doi: 10.1530/eje.0.151u151. [DOI] [PubMed] [Google Scholar]
- Weathington JM, Arnold AR, Cooke BM. Juvenile social subjugation induces a sex-specific pattern of anxiety and depression-like behaviors in adult rats. Horm Behav. 2012;61:91–9. doi: 10.1016/j.yhbeh.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Whitworth JA, Brown MA, Kelly JJ, Williamson PM. Mechanisms of cortisol-induced hypertension in humans. Steroids. 1995;60:76–80. doi: 10.1016/0039-128x(94)00033-9. [DOI] [PubMed] [Google Scholar]
- Wiemann JN, Clifton DK, Steiner RA. Pubertal changes in gonadotropin-releasing hormone and proopiomelanocortin gene expression in the brain of the male rat. Endocrinology. 1989;124:1760–7. doi: 10.1210/endo-124-4-1760. [DOI] [PubMed] [Google Scholar]
- Wilkin MM, Waters P, McCormick CM, Menard JL. Intermittent physical stress during early- and mid-adolescence differentially alters rats’ anxiety- and depression-like behaviors in adulthood. Behav Neurosci. 2012;126:344–60. doi: 10.1037/a0027258. [DOI] [PubMed] [Google Scholar]
- Woo TU, Pucak ML, Kye CH, Matus CV, Lewis DA. Peripubertal refinement of the intrinsic and associational circuitry in monkey prefrontal cortex. Neuroscience. 1997;80:1149–58. doi: 10.1016/s0306-4522(97)00059-6. [DOI] [PubMed] [Google Scholar]
- Woolley CS. Effects of oestradiol on hippocampal circuitry. Novartis Found Symp. 2000;230:173–80. doi: 10.1002/0470870818.ch13. discussion 181–7. [DOI] [PubMed] [Google Scholar]
- Wright LD, Hebert KE, Perrot-Sinal TS. Periadolescent stress exposure exerts long-term effects on adult stress responding and expression of prefrontal dopamine receptors in male and female rats. Psychoneuroendocrinology. 2008;33:130–42. doi: 10.1016/j.psyneuen.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Wright LD, Muir KE, Perrot TS. Enhanced stress responses in adolescent versus adult rats exposed to cues of predation threat, and peer interaction as a predictor of adult defensiveness. Dev Psychobiol. 2012a;54:47–69. doi: 10.1002/dev.20575. [DOI] [PubMed] [Google Scholar]
- Wright LD, Muir KE, Perrot TS. Stress responses of adolescent male and female rats exposed repeatedly to cat odor stimuli, and long-term enhancement of adult defensive behaviors. Dev Psychobiol. 2012b doi: 10.1002/dev.21060. [DOI] [PubMed] [Google Scholar]
- Xiao L, Jordan CL. Sex differences, laterality, and hormonal regulation of androgen receptor immunoreactivity in rat hippocampus. Horm Behav. 2002;42:327–36. doi: 10.1006/hbeh.2002.1822. [DOI] [PubMed] [Google Scholar]
- Xu X, Zhang Z. Effects of estradiol benzoate on learning-memory behavior and synaptic structure in ovariectomized mice. Life Sci. 2006;79:1553–60. doi: 10.1016/j.lfs.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Yamada S, Noguchi D, Ito H, Yamanouchi K. Sex and regional differences in decrease of estrogen receptor alpha-immunoreactive cells by estrogen in rat hypothalamus and midbrain. Neurosci Lett. 2009;463:135–9. doi: 10.1016/j.neulet.2009.07.074. [DOI] [PubMed] [Google Scholar]
- Yang S, Liu ZW, Wen L, Qiao HF, Zhou WX, Zhang YX. Interleukin-1beta enhances NMDA receptor-mediated current but inhibits excitatory synaptic transmission. Brain Res. 2005;1034:172–9. doi: 10.1016/j.brainres.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Yankova M, Hart SA, Woolley CS. Estrogen increases synaptic connectivity between single presynaptic inputs and multiple postsynaptic CA1 pyramidal cells: a serial electron-microscopic study. Proc Natl Acad Sci U S A. 2001;98:3525–30. doi: 10.1073/pnas.051624598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim M, Mapp OM, Janssen WG, Yin W, Morrison JH, Gore AC. Postpubertal decrease in hippocampal dendritic spines of female rats. Exp Neurol. 2008;210:339–48. doi: 10.1016/j.expneurol.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokosuka M, Okamura H, Hayashi S. Postnatal development and sex difference in neurons containing estrogen receptor-alpha immunoreactivity in the preoptic brain, the diencephalon, and the amygdala in the rat. J Comp Neurol. 1997;389:81–93. doi: 10.1002/(sici)1096-9861(19971208)389:1<81::aid-cne6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Young EA, Altemus M, Parkison V, Shastry S. Effects of estrogen antagonists and agonists on the ACTH response to restraint stress in female rats. Neuropsychopharmacology. 2001;25:881–91. doi: 10.1016/S0893-133X(01)00301-3. [DOI] [PubMed] [Google Scholar]
- Zhang JQ, Cai WQ, Zhou DS, Su BY. Distribution and differences of estrogen receptor beta immunoreactivity in the brain of adult male and female rats. Brain Res. 2002;935:73–80. doi: 10.1016/s0006-8993(02)02460-5. [DOI] [PubMed] [Google Scholar]