Abstract
The purpose of this study was to assess the relationship between APOE, life events and engagement, and subjective well-being (as measured by positive and negative affect) among centenarians. Based on the life stress paradigm, we predicted that higher levels of stress would allow APOE to influence positive and negative affect. One hundred and ninety six centenarians and near centenarians (98 years and older) of the Georgia Centenarian Study participated in this research. APOE, positive and negative affect, number of recent (last two years) and life-long (more than 20 years prior to testing) events, as well as a number of life engagement tasks were assessed. Results suggested that centenarians carrying the APOE ε4 allele rated lower in positive affect, number of life-long events, and in engaged lifestyle when compared to centenarians without the APOE ε4 allele (t = 3.43, p < .01, t = 3.19, p < .01, and t = 2.33, p < .05, respectively). Blockwise multiple regressions indicated that APOE ε4 predicted positive but not negative affect after controlling for demographics. Gene-environment interactions were obtained for APOE ε4 and life-long events, suggesting that carriers of the APOE ε4 allele had higher scores of negative affect after having experienced more events, whereas non-carriers had reduced negative affect levels after having experienced more events.
The ε4 allele of apolipoprotein E (APOE) on chromosome 19 has been related to late age onset of Alzheimer's disease and cognitive decline (Corder, Saunders, & Risch, 1994; Saunders, Schmader, & Breitner, 1993). The ε4 allele has also been associated with a number of physical diseases, such as coronary artery disease (Lehtinin, Lehtimaki, & Sisto, 1995), vascular disease in diabetes (Vauhkonen, Niskanen, & Ryynanen, 1997), and stroke (Ferrucci, Gurlanik, & Pahor, 1997). It then comes as no surprise that APOE is also related to increased mortality (Corder, Lannfelt, & Vitanen, 1996; Tilvis, Strandberg, & Juva, 1998), even though some centenarian studies did not find significant differences in the frequency of the ε4 allele between Alzheimer's disease and nondemented centenarians (Asada et al., 1996), whereas others did (Choi et al., 2003).
To date, however, little research has considered the role of APOE ε4 for other psychological outcomes. During the past 15 years, considerable attention has been devoted to genetic associations with human longevity (e.g., Perls & Terry, 2003; Vijg & Campisi, 2008). One approach taken is to assess long-lived individuals, such as centenarians, and compare them to control individuals who did not or who are not likely to achieve such an advanced age (e.g., Perls, Kunkel, & Puca, 2002). Even though studying links between genes and longevity in itself is important, it may be even more important to study the relationship between genes and functioning in very late life. The purpose of this study was to assess the relationship between one particular gene, APOE, and affect among centenarians.
Why is it important to study APOE in a highly select group of survivors? Centenarians are obviously long-lived individuals and genetic disadvantages – if they existed – did not compromise the unusual life span of centenarians. The question of genetic and gene-environment interaction effects may inform us whether survivorship comes with a high price (e.g., cognitive, functional, or mental health problems). One of the consequences of extended lives may be to become more susceptible to impairments in very late life. Although a large number of centenarians are quite frail and functional impairments may be quite high (Andersen-Ranberg, Schroll, & Jeune, B., 2001; Martin, Rott, Hagberg, & Morgan, 2000), there are also individual differences distinguishing relatively well-functioning centenarians from centenarians who may be severely impaired. The question is whether genetic markers, alone or in interaction with stress, can account for these individual differences in very late life.
Explaining individual differences in functioning is our conceptual starting point for this study. Very few studies have examined an association of the ε4 allele with overall functioning. Blazer, Fillenbaum, and Burchett (2001) assessed whether functional decline was associated with the APOE ε4 allele and noted no direct association. However, a statistically significant interaction of the ε4 allele with gender and baseline functional status was obtained. Greater functional decline was observed among women with the ε4 allele who also had poorer baseline functioning. Another interaction effect was reported by Seeman, Huang, Bretsky, Crimmins, Launer, and Guralnik (2005) who noted that the presence of at least one ε4 allele reduced the protective effects of education resulting in steeper cognitive declines with age.
We propose to extend findings on the relationship between APOE ε4 and physical functioning to mental functioning. A number of studies have investigated the association between APOE ε4 and mental health. Borroni, Costanzi, and Padovani (2010) in reviewing recent literature linking the APOE gene with behavioral and psychological symptoms in dementia reported conflicting results about the effect of APOE ε4 on mental health. Delano-Wood et al. (2008) reported that the frequency of the APOE ε4 allele was significantly higher in depressed vs. non-depressed persons with Alzheimer's disease. In addition, women possessing the APOE ε4 allele were almost four times more likely to be depressed than those without the ε4 allele. Other studies did not find an overall association between APOE ε4 and depression (Garcia-Pena et al., 2010; Steffens, Norton, Hart, Skoog, Corgoran, & Breitner, 2003). A significant interaction effect of APOE ε4 and age was reported such that the relationship of late-onset depression with respect to presence of the ε4 allele was larger among those 80 years and older compared to those at younger ages. A third study (Gallagher-Thomson, O'Hara, Simmons, Kraemer, & Murphy, 2001) reported that increased stress levels were associated with increased depressive symptoms in caregivers who had the ε4 allele.
Although there is emerging literature indicating that the APOE e4 allele has a direct effect on some functional outcomes, several studies point to the importance of gene-environment interactions (e.g., Chou, 2010). For example, Dar-Nimrod, Chapman, Robbins, Porsteinsson, Mapstone, and Duberstein (2012) reported that APOE ε4 moderated the relationship between neuroticism and cognitive function. Neighborhood environmental factors also appear to attenuate the association of the APOE ε4 allele with depressive symptoms (Yen, Rebok, Yang, & Lung, 2008). Less is known about the interactive relationship between life experiences, the APOE ε4 allele and functioning. We therefore assessed to what extent life experiences can play such a moderating influence on the gene-environment relationship.
A “molar approach” to studying stress, health, and aging places a focus on major life events (Almeida, Piazza, Stawski, & Klein, 2011; Dohrenwend, 2006). Stressful life events can accumulate over time leading to poor health outcomes. The life stress (“stress-conditioning”) hypothesis indicates that the relationship between resources and distress is moderated by stressful experiences (Ensel & Lin, 1991). Stressful events can be distal (i.e., those experienced in the distant past) or proximal (i.e., experiences of more recent nature) (Ensel, Peek, Lin, & Lai, 1996).
Positive and negative affect are important parts of the adaptation process for older adults. Several studies have reported that measures of subjective well-being decline across adulthood (Gerstorf, Ram, Mayraz, Hidajat, Lindenberger, Wagern, & Schupp, 2010). Late life appears to be particularly associated with lower levels of positive affect but not with negative affect (Kunzmann, Little, & Smith, 2000; Poon, Martin, & Margrett, 2010). A number of studies have provided evidence for the relationship between stress and positive and negative affect (e.g., Folkman & Moskowitz, 2000; Mroczek & Almeida, 2004). Previous research indicates that stress has a negative effect on mood states (e.g., Bolger, DeLongis, Kessler, & Schilling, 1989), and most studies link proximal stress to affective states. However, it is also important to consider life-long cumulative stressors when assessing the link between stress and well-being. Life-long stress is particularly important to consider in a sample of very old individuals who may have accumulated many events over their life time. Our previous studies demonstrated that positive and negative events were associated with negative affect, and distal events were associated with positive affect (Martin, da Rosa, & Poon, 2011). This study evaluated both proximal and distal events in its relationship to affective outcomes.
Our research was guided by a life stress hypothesis (Ensel & Lin, 1991) suggesting that the relationship between APOE ε4 and mental health is moderated by stressful experiences. Life stressors can be taxing but can also serve as challenges that keep older adults active and involved. Consistent with the stress paradigm (Ensel & Lin, 1991), there are two pathways which we tested through which genes and life experiences may influence functional outcomes: (a) genes and experiences may be independent influences or (b) life experiences can serve as a moderator in the relationship between genes and functioning. It is unclear whether the hypothesized effect of APOE e4 is equally noticeable when predicting positive and negative affect. We hypothesized that APOE e4 and life stress had independent influences on positive and negative affect. The relationship between APOE e4 and affect was hypothesized to be moderated by distal and proximal stressors. The direct effect is consistent with the independence model proposed by Ensel and Lin suggesting a direct effect of stressors and biological markers on distress. The moderating hypothesis is consistent with the stress-conditioning model proposed by Ensel and Lin suggesting that stressful events moderate the relationship between biological markers and distress.
Method
Participants
The overall study included a total of 234 community-dwelling and institutionalized centenarians and near-centenarians from the second Georgia Centenarian Study (Phase III, Poon et al., 2007). Excluded from this particular analysis were 38 centenarians without genetic data. We therefore included 196 centenarians (mean age = 100.4 years) in this specific study.
Although we have genetic information on all 196 of these participants, a number of them were classified as “admixed.” Admixture occurs in populations because allele frequencies can vary widely between the ancestral populations of which they are composed (Cardon & Palmer, 2003). The net effect of this admixture depends on patterns of geographical migration, mating practices, reproductive expansions and stochastic variation that can result in differences in allele frequencies between individuals rather than the association with a particular phenotype of interest (Cardon & Palmer, 2003). Because study populations may be confounded by genetic admixture, we identified 33 participants characterized by admixture and we conducted our analyses separately for the total population and for the population without these admixed participants.
A large proportion of our participants (85.7 percent) were women and 75.5 percent were Caucasian. Most participants (89.2%) were widowed, only 2.6 percent were married. A sizeable group (37.4%) had no more than eight years of education, whereas 23.7 percent had a college degree. About a third of the centenarians resided in their private home or apartment (36.7 %), whereas 17.9 percent resided in assisted living facilities and 45.4 percent in skilled nursing facilities. Only 4.2 percent were rated by proxies as being in “poor” health, 20.0 percent were rated as being in “fair” health, whereas 48. 3 percent were rated as being in “good” and 27.5% in “excellent” health. The average Mini Mental State Exam (MMSE, Folstein, Folstein, & McHugh, 1975) score of the participants was M = 16.36. Therefore, a large number of participants had modest to substantial deficits in cognitive functioning. Demographic characteristics are summarized in Table 1. About 19 percent of our centenarians were classified with APOE e4, which is comparable with frequencies reported in other population-based studies (Gerdes, Klausen, Sihm, Faergeman, & Vogler, 1992).
Table 1. Demographic Characteristics.
| Variable | N | Percentage | M | SD |
|---|---|---|---|---|
| Age | 100.44 | 1.967 | ||
| Gender | ||||
| Female | 168 | 85.7 | ||
| Male | 28 | 14.3 | ||
| Total | 196 | 100.0 | ||
| Ethnicity | ||||
| White | 148 | 75.5 | ||
| African American | 48 | 24.5 | ||
| Total | 196 | 100.0 | ||
| Education | ||||
| 0-8 years | 71 | 37.4 | ||
| 9-12 years | 74 | 39.0 | ||
| 13-16 years | 37 | 19.5 | ||
| 17+ | 8 | 4.2 | ||
| Total | 190 | 100.1 | ||
| Residence | ||||
| Private Home | 72 | 36.7 | ||
| Assisted Living | 35 | 17.9 | ||
| Skilled Nursing | 89 | 45.4 | ||
| Total | 196 | 100.0 | ||
| ε4 allele | ||||
| No | 159 | 81.1 | ||
| Yes | 37 | 18.9 | ||
| Total | 196 | 100.0 | ||
| MMSE | 196 | 16.36 | 9.08 |
Information about affect, life events, and lifetime activities for all centenarians was obtained by proxy informants. Proxies are commonly used in centenarian research because many very old persons are not able to participate in a structured interview and asking proxy informants remains the only option to assess low functioning centenarians (Gu, 2008). Proxy respondents are particularly useful in research with oldest-old samples to avoid bias in favor of healthy older persons who are able to answer for themselves (Gu, 2008; Rodgers & Herzog, 1992). Several studies have indicated that proxies may in some cases overrate disabilities (Rothman, Hedrick, Bulcroft, Kickam, & Rubinstein, 1991), but they tend to be more knowledgeable about personal, familial, and economic situations (Gu, 2008). Our own work suggests that proxy responses and self-ratings in mental health are not significantly different on the mean level (MacDonald, Martin, Margrett, & Poon, 2009).
A proxy-selection decision tree was used to determine proxy participation. Close family, such as spouses or children were first considered as proxies. If more than one child was alive, the centenarian nominated a proxy, or in the case of cognitive impairment, a contacted child made the decision regarding who could provide the most accurate information. Other relatives served as proxies, if no children were alive or available or if nominated by the participant. If no other relatives were alive or available, friends, neighbors, nurses, clergy or other knowledgeable persons could also serve as proxies. Proxy selection continued until an agreeable participant was found.
Most proxies (61.1%) were adult children. Additional proxies included nieces and nephews (13.9%), granddaughters (9.9%), and additional informants, such as spouses, siblings, or friends (15.1%). Proxy informants received a questionnaire booklet and were asked to fill out all questions and return the information in a self-addressed, stamped envelope. All original scale items were reworded so that they were made in reference to the centenarian. Proxies were specifically instructed to evaluate the centenarians how they were at the time of the assessment.
Measures
Positive and negative affect
Proxies assessed affect with the Bradburn Affect Balance Scale (Bradburn, 1969). This scale was used because it had been successfully applied in our earlier centenarian studies. The scale consists of the two dimensions “positive affect” and “negative affect.” Each scale consists of five items with the categories “not at all,” “once,” “several times,” and “often.” Internal consistency was α = .79 for positive affect and α = .74 for negative affect. Higher scores for positive affect indicated better mental health, whereas higher scores for negative affect were indicative of poorer mental health. The scores could range from 5 to 20.
Although it may be difficult to assess a participant's affect levels by proxy, it is still important to take into account their observations. Proxy informants typically can observe whether centenarians are “depressed and very unhappy,” “restless,” “bored,” or “lonely.” Of course, these observations may not match those that would be obtained by self-ratings.
Proximal events and distal events
Proxy informants were also asked to select proximal and distal life events from a commonly used life events list that had been applied in our earlier centenarian studies (Dohrenwend, Askenasy, Krasnoff, & Dohrenwend, 1978). These events refer to occurrences such as separation or divorce of parents, marriage, divorce, death of close family members, birth and loss of children, job events (first job, change of jobs, and retirement), a major financial loss, a residential change including institutionalization, a major decrease in activities that one really enjoyed, or worsening relationship with a child. We assessed a total of 23 events and their time of occurrence; events were then classified as to whether they had happened two or fewer years ago (“proximal events, two years”) or whether these events had happened more than 20 years ago (“distal experiences”). Time frames were chosen to obtain enough variability in life event occurrence. Consistent with the notion of cumulative advantage and adversity (Ryff, Singer, Love, & Essex, 1998) we assessed the effect of cumulative events rather than discrete events or negative versus positive events.
Engaged lifestyle
Past engaged lifestyle activities were defined by a series of cognitive engagement tasks participants may have engaged in at any time of their lives (Hultsch, Hertzog, Small, & Dixon, 1999). These past engaged lifestyle activities included eight dichotomous questions such as learning a foreign language, volunteerism, traveling, preparing income taxes, and public speaking. Although it would have been advantageous to use a “current engaged life style” assessment, it is quite unlikely that centenarians would be still involved in these activities. Cronbach's alpha for this scale was .62 for proxies. Higher scores were indicative of greater engagement.
APOE measurement
APOE genotype was determined by resequencing exon 4 of the APOE gene. DNA was extracted from blood spotted on FTA cards (Whatman) using the Gentra Generation DNA Purification System. Exon 4 sequences were amplified by the polymerase chain reaction (PCR), using 5′-CTTGGGTCTCTCTGGCTCATC-3′ and 5′-GCAGCCTGCACCTTCTCC-3′ as the forward and reverse primers, respectively. The correct size was verified by agarose gel electrophoresis, and the PCR products were cleaned using the Edge Biosystems Performa Ultra 96-well plate cleaning kit. The cleaned DNA was subjected to cycle-sequencing using the Applied Biosystems (ABI) Big-Dye Terminator Reagent version 3.1 and the ABI 3130×l DNA sequencing system. Sequencing primers were the same as those used for PCR amplicaton. Trace files were analyzed with the ABI SeqScape version 2.5 software. Both DNA strands were sequenced twice by two independent investigators, and the sequence calls were validated by comparison between them. For statistical purposes, we classified participants as either APOE ε4 (ε2/ε4, ε3/ε4, ε4/ε4) or non-ε4(ε2/ε2, ε2/ε3, ε3/ε3).
Population stratification was carried out using the program Structure (Pritchard et al., 2000). DNA samples were genotyped at 100 Alu insertion polymorphisms by PCR amplification using locus specific primers followed by gel electrophoresis. These Alu sequences served as ethnic affiliation markers that were analyzed using Structure. For this purpose, the same Alu genotypes from 715 independent DNA samples from different geographic regions of the world were used. No departure from Hardy-Weinberg proportions was found for the APOE variants. The details of these analyses have been published elsewhere (Jazwinski et al., 2010).
Statistical Analyses
Two analysis steps were undertaken. First, mean differences between APOE ε4 carriers and non-carriers were computed with independent-group t-tests. Second, blocked hierarchical multiple regressions were computed separately for positive affect and negative affect. The first block contained demographic variables age, ethnicity, and gender, as well as the covariates residence (i.e., care facility vs. independent living). We did not control for cognitive status in the statistical analyses given that APOE ε4 is associated with cognitive impairment. Adding cognitive status to the equation would have removed some of the variability that should be attributed to genetic markers. The second block contained the ε4 variable, the third block contained the life experience variables (i.e., proximal events, distal events, and engaged lifestyle), and the fourth block contained the ε4 variable by life experience interactions. Analyses were computed with SPSS 19.
Results
Results are presented in three sections: first, we report differences on life events, engaged lifestyle and mental health for the two genetic groups: those carrying the APOE ε4 allele as compared to those not carrying the APOE ε4 allele. Next we report on the direct (“non-mediated”) effects of genes and events on mental health outcome variables. Finally, we will report results of the moderating (gene × environment) interaction effects.
The results comparing the different genetic groups are summarized in Table 2. Carriers of the ε4 allele had lower positive affect scores, t(155) = 3.43, p < .01, and centenarians who were carriers of the ε4 allele had experienced fewer distal events t(175) = 3.19, p < .001, and were lower in engaged lifestyle, t(137) = 2.33, p < .05. We repeated the analysis by excluding admixed participants and the same significant differences were obtained.
Table 2. Mean Differences by APOE ε4 Allele.
| n | ε4 | ε4 | t | |
|---|---|---|---|---|
| No | Yes | |||
| M | M | |||
| Negative Affect | 159 | 8.25 | 8.32 | 0.09 |
| Positive Affect | 157 | 12.28 | 9.43 | 3.43** |
| Proximal Events | 176 | 0.66 | 0.54 | 0.60 |
| Distal Events1 | 176 | 4.13 | 2.60 | 3.19** |
| Engaged Lifestyle | 139 | 4.14 | 3.13 | 2.33* |
Note.
Equal variance not assumed.
p< .05.
p< .01.
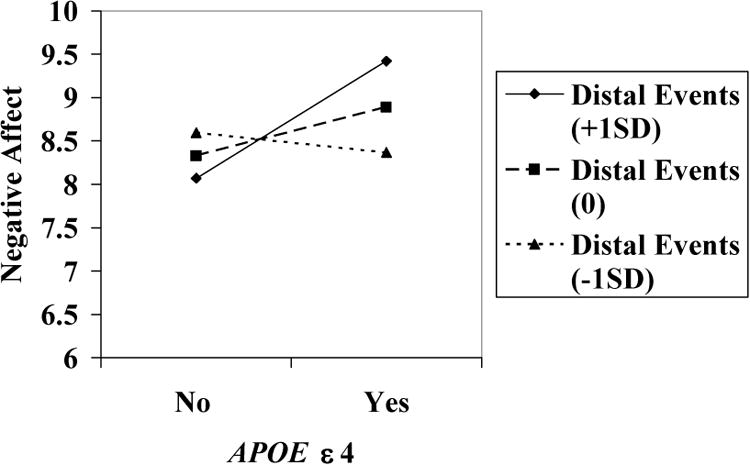
Tables 3 and 4 summarize the direct effects of the ε4 allele and events on positive and negative affect. For positive affect, carriers of ε4 had lower scores in positive affect, β =-.21, p < .05. Engaged lifestyle also significantly predicted positive affect, β =,18 p < .05, indicating that those who had higher scores in engaged lifestyle were also perceived as having greater positive affect (Table 3). For negative affect, only distal events were significant predictors, β =-.22, p < .05, indicating that centenarians who reportedly had experienced more life events had lower negative affect scores. Table 4 also highlights a significant gene × environment interaction for negative affect, β = .25, p < .05. The effect of the ε4 allele on negative affect was stronger when participants reportedly had more distal (lifetime) experiences (Figure 1). Additional sex × ε4 allele interactions on positive and negative affect were also computed but were not significant (data not shown).
Table 3. Effect of APOE ε4 and Life Events on Positive Affect (n = 126).
| Model 1 | Model 2 | Model 3 | Model 4 | FΔ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | β | B | SE | β | B | SE | β | B | SE | β | ||
| Age | -.28 | .20 | -.13 | -.30 | .19 | -.14 | -.22 | .19 | -.10 | -.22 | .19 | -.10 | |
| Ethnicity | -56 | .86 | -.06 | -.23 | .84 | -.02 | -.25 | .85 | .03 | .26 | .86 | .03 | |
| Gender | 1.12 | 1.04 | -.09 | -1.45 | 1.01 | -.12 | -1.58 | 1.00 | -.13 | -1.60 | 1.02 | -.13 | |
| Residence | -1.04 | .42 | -.22* | -.95 | .40 | -.20* | -.81 | .41 | -.17 | -.81 | .41 | -.17 | |
| ε4 | -2.84 | .90 | -.26** | -2.31 | .92 | -.21* | -2.27 | 1.00 | -.21* | 9.90** | |||
| Proximal Events | .12 | .35 | .03 | .13 | .35 | .03 | |||||||
| Distal Events | .15 | .12 | .11 | .14 | .13 | .11 | |||||||
| Engaged Lifestyle | .40 | .20 | .18* | .40 | .20 | .18* | |||||||
| ε4×Proximal Events | -.55 | .94 | .05 | 0.34 | |||||||||
| ε4×Distal Events | .04 | .39 | .01 | 0.01 | |||||||||
| ε4×Engaged Lifestyle | .78 | .58 | .13 | 1.83 | |||||||||
| R2 | .10 | .17 | .21 | ||||||||||
Note. Interaction terms were entered separately.
p< .05.
p< .01.
Table 4. Effect of APOE ε4 and Life Events on Negative Affect (n = 130).
| Model 1 | Model 2 | Model 3 | Model 4 | FΔ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | β | B | SE | β | B | SE | β | B | SE | β | ||
| Age | .26 | .18 | .14 | .26 | .18 | .14 | .24 | .19 | .12 | .26 | .16 | .13 | |
| Ethnicity | -.73 | .80 | -.08 | -.74 | .81 | -.08 | -.99 | .84 | -.11 | -.75 | .83 | -.08 | |
| Gender | -1.08 | .97 | -.10 | -1.07 | .98 | -.10 | -1.09 | .98 | -.10 | -1.53 | .98 | -.14 | |
| Residence | .21 | .39 | .05 | .21 | .39 | .05 | .13 | .40 | .03 | .16 | .40 | .04 | |
| ε4 | .05 | .88 | .01 | -.24 | .90 | -.02 | .63 | .96 | .06 | ||||
| Proximal Events | -.04 | .35 | -.01 | -.01 | .34 | .00 | |||||||
| Distal Events | -.18 | .12 | -.14 | -.27 | .12 | -.22* | |||||||
| Engaged Lifestyle | -.05 | .19 | -.02 | -.01 | .19 | -.01 | |||||||
| ε4×Proximal Events | .39 | .92 | .04 | .18 | |||||||||
| ε4×Distal Events | .88 | .37 | .25* | 5.60* | |||||||||
| ε4×Engaged Lifestyle | .69 | .57 | .13 | 1.46 | |||||||||
| R2 | .03 | .03 | .05 | ||||||||||
Note. Interaction terms were entered separately.
p< .05.
Figure 1. Interaction Effect of ε4 and Distal Experiences on Negative Affect (Including Admixed Participants).
Once again, we repeated the regression analyses for the sample that excluded admixed participants. With regard to positive affect, the ε4 allele and engaged lifestyle again were significant predictors and no significant interactions were obtained. For negative affect, distal events was again significant but there was only a statistical trend for the gene × distal event interaction, β = .21, p = .086.
Discussion
Although there is quite a bit of research highlighting the relationship between the ε4 allele and Alzheimer's disease, cardiovascular disease, and mortality, much less is known about the direct and moderating effect of ε4 on mental health. This research attempted to fill this gap by assessing the relationship between ε4 and affect by advancing stressful life events hypotheses of adaptation. We had hypothesized that APOE ε4 and life stress had independent influences on positive and negative affect. The relationship between APOE ε4 and affect was hypothesized to be moderated by distal and proximal stressors.
Two primary results emerged from our analyses. First, APOE ε4 allele carriers differed significantly in positive affect, distal events, and engaged lifetime activities. The effect of APOE ε4 was maintained even after adjusting for covariates. Second, ε4 interacted significantly with distal life events to predict negative affect but not positive affect. Specifically, the effects of distal life experiences more strongly predicted negative affect for carriers of an APOE ε4 allele than for non-carriers.
By following a life stress paradigm (Ensel & Lin, 1991), we evaluated whether APOE and stress had an independent effect on late life functioning or whether the effect of APOE on functioning was moderated by stressful life experiences. Carriers of the ε4 allele were more likely to have lower positive affect, had fewer distal experiences and lower engaged lifestyle scores. This result is supported by other research indicating that the APOE ε4 allele is directly related to mental health. For example, Delano-Wood et al. (2008) reported that the frequency of APOE e4 alleles was significantly higher in a depressed group of persons with Alzheimer's disease. Our results extend these findings by indicating that for very old adults the APOE ε4 appears to relate to reduced positive affect levels but does not appear to be associated with increased negative affect levels. Even after controlling for a number of covariates, our results also suggest that the APOE ε4 allele may be event-suppressing and may lessen the frequency with which individuals seek events or engage in activities.
To support the importance of combining the effects of genes and stress, we also found an important gene-environment interaction. The effect of ε4 on negative affect was enhanced by distal events. Apparently, the ε4 allele has a stronger effect on negative affect if very old adults have a larger number of distal experiences. It is important to note, however, that no significant interaction was obtained for ε4 and proximal events or engaged lifestyle. The time frame for proximal events in this study was somewhat arbitrary. We used a two-year time interval to increase variability in the measure, but perhaps a shorter time-frame would have been more effective in distinguishing centenarians who were still dealing with acute stressful events from those who had perhaps already overcome them.
APOE does not act in isolation to influence longevity and healthy aging. We have described an interaction between APOE, HRAS1, and LASS1 that plays a role in determining these phenotypes (Jazwinski et al., 2010). The interaction of these genes supports a model describing a network of molecular and cellular interactions that impact both physical and cognitive function ability. Favorable genotypes support healthy aging and delay frailty and morbidity. Thus, the role of APOE we describe here may emanate from this network and be moderated by interactions with other genes.
This study, like others, has a number of limitations. The results are only generalizable to a very old population in Georgia and would perhaps not be obtained for other age groups or in other regions. In addition, results may be limited to the assessed cohort of centenarians. Other cohorts may not necessarily show the same results. We also recognize that the main results are based on a sample that included admixed participants. Although our findings were essentially the same when excluding admixture participants, the only significant interaction reported barely missed the conventional significance cut-off. This may be because of the loss of power when excluding participants from a relatively small sample, or the results may be confounded because of unique genetic variation unrelated to the phenotype studied here, among admixed participants.
Variables of mental health were obtained from proxy informants, because centenarians who were low in cognitive functioning would not have been able to answer many of our questions. The environmental assessments, therefore, were biased by the views held by close family members of centenarians. Other variables, such as the measure of engaged lifestyle, could be modified to fit an oldest-old population. For example, active engagement in this group may take the form of visiting with others, eating meals in a group room, or taking short walks. Finally, many of our assessments were brief, because they had to be performed together with a long overall assessment battery. The response rate for proxies was very high because we inquired about proxies until we found a suitable person. From our experience, proxy respondents (typically family members) were quite willing to participate in our study.
Future research could consider including different measures of stress. This study only included major life events (i.e., molar stress, Almeida et al., 2011). “Microscopic stressors” (Almeida et al.), including daily stressors, chronic stressors, and daily hassles may also have a direct impact on negative and positive affect, and these stressors could moderate the relationship between biological markers and emotional affect. Likewise, different genetic markers involved in regulating the stress response system may play an important role in predicting positive and negative affect (Kremen & Lyons, 2011). Finally, different mental health measures, such as depression and depressive symptoms as well as overall life satisfaction may be relevant outcome variables that should be studied.
Our results provide first insight into the relationship between the ε4 allele of the APOE gene and its relationship to functioning and resources for a unique group of survivors. The APOE gene appears to be important for predicting positive emotional states directly and negative emotional states in interaction with distal experiences. Taken together, the results suggest that more attention should be paid to resources and emotional states of the oldest members in our society.
Acknowledgments
The Georgia Centenarian Study (Leonard W. Poon, PI) was funded by 1P01AG17553 from the National Institute on Aging, a collaboration among The University of Georgia, Tulane University Health Sciences Center, Boston University, University of Kentucky, Emory University, Duke University, Wayne State University, Iowa State University, Temple University, and University of Michigan. Authors acknowledge the valuable recruitment and data acquisition effort from M. Burgess, K. Grier, E. Jackson, E. McCarthy, K. Shaw, L. Strong and S. Reynolds, data acquisition team manager; S. Anderson, E. Cassidy, M. Janke, and J. Savla, data management; M. Poon for project fiscal management. We also appreciate the assistance of M. Allen, L. Cosenza, V. Greco, S. Hadie, B. Kimball, B. McEvoy-Hein, J. Owens, and J. Walker, who performed genotyping.
References
- Almeida DM, Piazza JR, Stawski RS, Klein LC. The speedometer of life: Stress, health, and aging. In: Schaie KW, Willis SL, editors. Handbook of the psychology of aging. 7th. Amsterdam: Elsevier; 2011. pp. 191–206. [DOI] [Google Scholar]
- Andersen-Ranberg K, Schroll M, Jeune B. Healthy centenarians do not exist, but autonomous do: A population-based study of morbidity among Danish centenarians. Journal of the American Geriatrics Society. 2001;49:900–908. doi: 10.1046/j.1532-5415.2001.49180.x. [DOI] [PubMed] [Google Scholar]
- Asada T, Yamagata Z, Kinoshita T, et al. Prevalence of dementia and distribution of ApoE alleles in Japanese centenarians: an almost-complete survey in Yamanashi prefecture, Japan. Journal of the American Geriatrics Society. 1996;44:151–155. doi: 10.1111/j.1532-5415.1996.tb02431.x. [DOI] [PubMed] [Google Scholar]
- Blazer DG, Fillenbaum G, Burchett B. The APOE-E4 allele and the risk of functional decline in a community sample of African American and White older adults. Journal of Gerontology: Biological and Medical Sciences. 2001;56:M785–M789. doi: 10.1093/gerona/56.12.m785. [DOI] [PubMed] [Google Scholar]
- Bolger N, DeLongis A, Kessler RC, Schilling EA. Effects of daily stress on negative mood. Journal of Personality and Social Psychology. 1989;57:808–818. doi: 10.1037//0022-3514.57.5.808. [DOI] [PubMed] [Google Scholar]
- Borroni B, Costanzi C, Padovani A. Genetic susceptibility to behavioral and psychological symptoms in Alzheimer disease. Current Alzheimer Research. 2010;7:158–164. doi: 10.2174/156720510790691173. [DOI] [PubMed] [Google Scholar]
- Bradburn NM. The structure of psychological well-being. Chicago: Aldine; 1969. [Google Scholar]
- Cardon LR, Palmer LJ. Population stratification and spurious allelic association. The Lancet. 2003;361:598–604. doi: 10.1016/S0140-6736(03)12520-2. [DOI] [PubMed] [Google Scholar]
- Choi YH, Kim JH, Kwan Kim D, Kim JW, Kim DK, Lee MS, Kim CH, Park SC. Distributions of ACE and APOE polymorphisms and their relations with dementia status in Korean centenarians. Journal of Gerontology: Medical Sciences. 2003;58A:227–231. doi: 10.1093/gerona/58.3.m227. [DOI] [PubMed] [Google Scholar]
- Chou KL. Moderating effect of Apolipoprotein genotype on loneliness leading to depressive symptoms in Chinese older adults. American Journal of Geriatric Psychiatry. 2010;18:313–322. doi: 10.1097/JGP.0b013e3181c37b2a. [DOI] [PubMed] [Google Scholar]
- Corder E, Lanfelt L, Vitanen M. Apolipoprotein E genotype determines survival in the oldst old (85 years or older) who have good cognition. Arch Neurol. 1996;53:418–422. doi: 10.1001/archneur.1996.00550050048022. [DOI] [PubMed] [Google Scholar]
- Corder E, Saunders A, Risch N. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer's disease. Nat Genet. 1994;7:180–184. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- Dar-Nimrod I, Chapman BP, Robbins JA, Porsteinsson A, Mapstone M, Duberstein PR. Gene by neuroticism interaction and cognitive function among older adults. International Journal of Geriatric Psychiatry. 2012;27:1147–1154. doi: 10.1002/gps.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delano-Wood L, Houston WS, Emond JA, Marchant NL, Salmon DP, Jeste DV, Thal LJ, Bondi MW. APOE genotype predicts depression in women with Alzheimer's disease: A retrospective study. International Journal of Geriatric Psychiatry. 2008;23:632–666. doi: 10.1002/gps.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohrenwend BP. Inventorying stressful life events as risk factors for psychopathology: Toward resolution of the problem of intracategory variability. Psychological Bulletin. 2006;132:477–495. doi: 10.1037/0033-2909.132.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohrenwend BS, Krasnoff L, Askenasy AR, Dohrenwend BP. Exemplification of a method for scaling life events: The PERI Life Events Scale. Journal of Health and Social Behavior. 1978;19:205–229. [PubMed] [Google Scholar]
- Ensel WM, Lin N. The life stress paradigm and psychological distress. Journal of Health and Social Behavior. 1991;32:321–341. [PubMed] [Google Scholar]
- Ensel WM, Peek MK, Lin N, Lai G. Stress and the life course: A life history approach. Journal of Aging and Health. 1996;8:389–416. doi: 10.1177/089826439600800305. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Gurlanik J, Pahor M. Apolipoprotein E epsilon 2 allele and risk of stroke in the older population. Stroke. 1997;28:2410–2416. doi: 10.1161/01.str.28.12.2410. [DOI] [PubMed] [Google Scholar]
- Folkman S, Moskowitz JT. Positive affect and the other side of coping. American Psychologist. 2000;55:647–654. doi: 10.1037/0003-066X.55.6.647. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gallagher-Thompson D, O'Hara R, Simmons A, Kraemer HC, Murphy GM., Jr Apolipoprotein E ε4 allele affects the relationship between stress and depression in caregivers of patients with Alzheimer's disease. Journal of Geriatric Psychiatry and Neurology. 2001;14:115–119. doi: 10.1177/089198870101400303. [DOI] [PubMed] [Google Scholar]
- Garcia-Pena C, Juarez-Cedillo T, Cruz-Robles D, Fragoso JM, Sanchez-Garcia S, Rodriguez-Perez J, M…Vargas-Alarcon G. Depressive symptoms and APOE polymorphisms in an elderly population-based sample. Psychiatric Genetics. 2010;20:215–220. doi: 10.1097/YPG.0b013e32833a211a. [DOI] [PubMed] [Google Scholar]
- Gerdes LU, Klausen C, Sihm I, Faergeman O, Vogler GP. Apolipoprotein E polymorphism in a Danish population compared to findings in 45 other study populations around the world. Genetic Epidemiology. 1992;9:155–167. doi: 10.1002/gepi.1370090302. [DOI] [PubMed] [Google Scholar]
- Gerstorf D, Ram N, Mayraz G, Hidajat M, Lindenberger U, Wagner GG, Schupp J. Late-life decline in well-being across adulthood in Germany, the United Kingdom, and the United States. Something is seriously wrong at the end of life. Psychology and Aging. 2010;25:477–485. doi: 10.1037/a0017543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu D. General data quality assessment of the CLHLS. In: Yi Z, Poston DL Jr, Vlosky DA, Gu D, editors. Healthy longevity in China: Demographic, socioeconomic, and psychological dimensions. New York: Springer; 2008. pp. 39–59. [Google Scholar]
- Hultsch DF, Hertzog C, Small BJ, Dixon RA. Use it or lose it: Engaged lifestyle as a buffer of cognitive decline in aging? Psychology and Aging. 1999;14:245–263. doi: 10.1037//0882-7974.14.2.245. [DOI] [PubMed] [Google Scholar]
- Jazwinski SM, Kim S, Dai J, Li L, Bi X, Jiang JC, Arnold J, Batzer MA, Walker JA, Welsh DA, Lefante CM, Volaufova J, Myers L, Su LJ, Hausman DB, Miceli MV, Ravussin E, Poon LW, Cherry KE, Welsch MA Georgia Centenarian Study & Louisiana Healthy Aging Study. HRAS1 and LASS1 with APOE are associated with human longevity and healthy aging. Aging Cell. 2010;9:698–708. doi: 10.1111/j.1474-9726.2010.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen WS, Lyons MJ. Behavior genetics of aging. In: Schaie KW, Willis SL, editors. Handbook of the psychology of aging. 7. Amsterdam: Elsevier; 2011. pp. 93–107. [DOI] [Google Scholar]
- Kunzmann U, Little TD, Smith J. Is age-related stability and subjective well-being a paradox? Cross-sectional and longitudinal evidence from the Berlin Aging Study. Psychology and Aging. 2000;15:511–526. doi: 10.1037//0882-7974.15.3.511. [DOI] [PubMed] [Google Scholar]
- Lehtinin S, Lehtimaki T, Sisto T. Apolipoprotein E polymorphism, serum lipids, myocardial infarction and severity of angiographically verified coronary artery disease in men and women. Artherosclerosis. 1995;114:83–91. doi: 10.1016/0021-9150(94)05469-y. [DOI] [PubMed] [Google Scholar]
- MacDonald M, Martin P, Margrett J, Poon LW. Correspondence of perceptions about centenarians' mental health. Aging and Mental Health. 2009;13:827–837. doi: 10.1080/13607860902918249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P, Rott C, Hagberg B, Morgan K. Centenarians - Autonomy vs dependence in the oldest old. New York: Springer; 2000. [Google Scholar]
- Martin P, daRosa G, Poon LW. The impact of life events on the oldest old. In: Poon LW, Cohen-Mansfield J, editors. Understanding the well-being of the oldest old. Cambridge: Cambridge University Press; 2011. pp. 96–110. [Google Scholar]
- Mroczek DK, Almeida DM. The effect of daily stress, personality, and age on daily negative affect. Journal of Personality. 2004;72:355–378. doi: 10.1111/j.0022-3506.2004.00265.x. [DOI] [PubMed] [Google Scholar]
- Perls T, Kunkel LM, Puca AA. The genetics of exceptional human longevity. Journal of the American Geriatrics Society. 2002;50:359–368. doi: 10.1046/j.1532-5415.2002.49283.x. [DOI] [PubMed] [Google Scholar]
- Perls T, Terry D. Genetics of exceptional longevity. Experimental Gerontology. 2003;38:725–730. doi: 10.1016/s0531-5565(03)00098-6. [DOI] [PubMed] [Google Scholar]
- Poon LW, Jazwinski SM, Green RC, Woodard JL, Martin P, Rodgers WL, Johnson MA, Hausman D, Arnold J, Davey A, Batzer MA, Markesbery WR, Gearing M, Siegler IC, Reynolds S, Dai J. Methodological considerations in studying centenarians: Lessons learned from the Georgia Centenarian Studies. In: Poon LW, Perls TT, editors. Annual Review of Gerontology and Geriatrics: Biopsychosocial approaches to longevity. Vol. 27. New York: Springer; 2007. pp. 231–264. [PMC free article] [PubMed] [Google Scholar]
- Poon LW, Martin P, Margrett J. Cognition and emotion in centenarians. In: Depp CA, Jeste DV, editors. Successful cognitive and emotional aging. Arlington, VA: American Psychiatric Publishing, Inc; 2010. pp. 115–133. [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers WL, Herzog AR. Collecting data about the oldest: Problems and procedures. In: Suzman RM, Willis DP, Manton KG, editors. The oldest old. New York: Oxford University Press; 1992. pp. 135–156. [Google Scholar]
- Rothman ML, Hedrick KA, Bulcroft KA, Kickam DH, Rubenstein LZ. The validity of proxy-generated scores as measures of patient health status. Medical Care. 1991;29:115–124. doi: 10.1097/00005650-199102000-00004. [DOI] [PubMed] [Google Scholar]
- Ryff CD, Singer B, Love GD, Essex MJ. Resilience in adulthood and later life: Defining features and dynamic processes. In: Lomranz J, editor. Handbook of aging and mental health. New York: Plenum; 1998. pp. 69–96. [Google Scholar]
- Saunders A, Schmader K, Breitner J. Apolipoprotein E epsilon 4 allele distributions in late onset Alzheimer's disease and in other amyloid forming diseases. Lancet. 1993;342:710–711. doi: 10.1016/0140-6736(93)91709-u. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Huang MH, Bretsky P, Crimmins E, Launer L, Guralnik JM. Education and APOE-e4 in longitudinal cognitive decline: MacArthur Studies of Successful Aging. Journal of Gerontology: Psychological Sciences. 2005;60B:74–83. doi: 10.1093/geronb/60.2.p74. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Norton MC, Hart AD, Skoog I, Corcoran C, Breitner JCS. Apolipoprotein E genotype and major depression in a community of older adults. The Cache County Study. Psychological Medicine. 2003;33:541–547. doi: 10.1017/s0033291702007201. [DOI] [PubMed] [Google Scholar]
- Tilvis RS, Strandberg TE, Juva K. Apolipoprotein E phenotypes, dementia and mortality in a prospective population sample. Journal of the American Geriatrics Society. 1998;46:712–715. doi: 10.1111/j.1532-5415.1998.tb03805.x. [DOI] [PubMed] [Google Scholar]
- Vauhkonen I, Niskanen L, Ryynanen M. Divergent association of apolipoprotein E polymorphism with vascular disease in patients with NIDDM and control subjects. Diabetes Med. 1997;14:748–756. doi: 10.1002/(SICI)1096-9136(199709)14:9<748::AID-DIA469>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Vijg J, Campisi J. Puzzles, promises, and a cure for ageing. Nature. 2008;454:1065–1071. doi: 10.1038/nature07216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen YC, Rebok GW, Yang MJ, Lung FW. A multilevel analysis of the influence of Apolipoprotein E genotypes on depressive symptoms in late-life moderated by the environment. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2008;32:479–486. doi: 10.1016/j.pnpbp.2007.09.023. [DOI] [PubMed] [Google Scholar]


