Abstract
Objective
Prior studies have suggested treatment and outcome disparities between men and women for lower extremity peripheral arterial disease after surgical bypass. Given the recent shift toward endovascular therapy, which has increasingly been used to treat claudication, we sought to analyze sex disparities in presentation, revascularization, amputation, and inpatient mortality.
Methods
We identified individuals with intermittent claudication and critical limb ischemia (CLI) using International Classification of Diseases, Ninth Revision codes in the Nationwide Inpatient Sample from 1998 to 2009. We compared presentation at time of intervention (intermittent claudication vs CLI), procedure (open surgery vs percutaneous transluminal angioplasty or stenting vs major amputation), and in-hospital mortality for men and women. Regional and ambulatory trends were evaluated by performing a separate analysis of the State Inpatient and Ambulatory Surgery Databases from four geographically diverse states: California, Florida, Maryland, and New Jersey.
Results
From the Nationwide Inpatient Sample, we identified 1,797,885 patients (56% male) with intermittent claudication (26%) and CLI (74%), who underwent 1,865,999 procedures (41% open surgery, 20% percutaneous transluminal angioplasty or stenting, and 24% amputation). Women were older at the time of intervention by 3.5 years on average and more likely to present with CLI (75.9% vs 72.3%; odds ratio [OR], 1.21; 95% confidence interval [CI], 1.21–1.23; P < .01). Women were more likely to undergo endovascular procedures for both intermittent claudication (47% vs 41%; OR, 1.27; 95% CI, 1.25–1.28; P < .01) and CLI (21% vs 19%; OR, 1.14; 95% CI, 1.13–1.15; P < .01). From 1998 to 2009, major amputations declined from 18 to 11 per 100,000 in men and 16 to 7 per 100,000 in women, predating an increase in total CLI revascularization procedures that was seen starting in 2005 for both men and women. In-hospital mortality was higher in women regardless of disease severity or procedure performed even after adjusting for age and baseline comorbidities (.5% vs .2% after percutaneous transluminal angioplasty or stenting for intermittent claudication; 1.0% vs .7% after open surgery for intermittent claudication; 2.3% vs 1.6% after percutaneous transluminal angioplasty or stenting for CLI; 2.7% vs 2.2% after open surgery for CLI; P < .01 for all comparisons).
Conclusions
There appears to be a preference to perform endovascular over surgical revascularization among women, who are older and have more advanced disease at presentation. Percutaneous transluminal angioplasty or stenting continues to be popular and is increasingly being performed in the outpatient setting. Amputation and in-hospital mortality rates have been declining, and women now have lower amputation but higher mortality rates than men. Recent improvements in outcomes are likely the result of a combination of improved medical management and risk factor reduction.
Individuals age >65 years now constitute the fastest growing segment of the U.S. population.1 As the population has matured, the number of individuals with peripheral arterial disease (PAD) has proportionately increased. Estimates from the Centers for Disease Control indicate PAD affects approximately 8 million Americans.2 Women continue to outnumber men in the older age groups. In 2010, women ≥65 years of age outnumbered their male counterparts by 32%, and there were more than twice as many women age ≥85 years as men.1
Sex-related differences have been demonstrated in other vascular diseases. For instance, women may derive less benefit from carotid endarterectomy for symptomatic moderate carotid stenosis than men3 and have a higher risk of postoperative strokes, transient ischemic attacks, and death after carotid endarterectomy for asymptomatic disease.4 Women with abdominal aortic aneurysms (AAAs) have a higher rate of rupture at any given diameter.5 Endoleaks, access-related problems, and limb occlusion are more common after endovascular AAA repair,6–8 and perioperative mortality is higher among women following open AAA repair.9 Multiple hypotheses have been proposed to explain these differences, including fewer screening and preventative measures in women, the influence of sex hormones, and differences in body size and anatomy.
Studies evaluating sex differences after lower extremity bypass have been equivocal. Earlier reports suggested worse long-term patency, perioperative mortality, and long-term survival in women.10,11 More recent studies, including several systematic reviews, found no major differences in patency rates, amputation-free, and overall survival.12–14 The emergence of endovascular therapy as a safe and effective adjunct to the gold standard of surgical bypass15,16 has transformed the treatment of lower extremity PAD and angioplasty, and stenting has supplanted peripheral bypass as the most commonly performed treatment.17,18 We therefore sought to assess sex-related differences in treatment and outcomes of lower extremity PAD associated with this shift in treatment paradigm.
METHODS
This study examined revascularization and amputation procedures among patients with PAD using three databases from the Healthcare Cost and Utilization Project (HCUP): the Nationwide Inpatient Sample (NIS), the State Inpatient Databases (SIDs), and the State Ambulatory Surgery Databases (SASDs). The NIS is the largest all-payer inpatient care database in the United States. Capturing roughly 8 million discharges per year, it approximates a 20% sample of all U.S. hospital stays. Discharge weights are used to generate national estimates. Using data from 1998 to 2009, we performed a search using International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes to identify patients with any primary diagnosis code (reason for admission) or secondary diagnosis code indicating arterial occlusive disease (PAD) of the extremities (Supplementary Table, online only), categorizing them by presentation: intermittent claudication vs critical limb ischemia (CLI). CLI was defined by any primary or secondary diagnosis code for rest pain, ulcer, osteomyelitis, or gangrene. Patients without a primary or secondary code for PAD and those whose disease severity could not be identified (ie, no codes for either claudication or CLI) were excluded. Using ICD-9 procedure codes, we then identified patients who underwent open revascularization (endarterectomy, aortoiliacfemoral bypass, or infrainguinal bypass), endovascular revascularization (angioplasty, stenting, atherectomy, or mechanical thrombectomy) or major amputation (below or above knee). Admissions involving both endovascular and open procedures during the same hospital admission are labeled “open+endo.” These admissions represent either hybrid procedures, open surgery after failed endovascular intervention, or procedures performed on different limbs.
HCUP does not offer a national ambulatory surgery database. We therefore utilized the SASD, which contains information from hospital-affiliated and freestanding ambulatory surgery sites, to evaluate trends in outpatient procedures. The SASD captures all surgeries that are performed on the same day in which patients are admitted and released. Therefore, patients admitted under “observation” status who stay overnight would be captured in the NIS rather than the SASD. Additionally, we verified our findings from the NIS using the SID. The NIS is a 20% weighted sample drawn from individual SIDs. However, because not all states contribute data to the NIS and because different states began contributing at different times, it is advisable to verify results from the NIS against other sources. Both the SID and SASD contain 100% of discharge abstracts from each participating state, and thus, the SID serves as a useful comparator for the NIS. Forty-six states participate in the SID, and 32 in the SASD. We used the 2005 to 2009 SID and SASD from four geographically diverse states: California, Florida, Maryland, and New Jersey. Each state was initially analyzed separately. Because we found that the overall procedural trends were consistent from state to state, we aggregated the states into one composite dataset for simplicity. As with the NIS data, diagnoses and procedures were identified using ICD-9 codes. However, the California and Maryland SASDs report procedures using current procedural terminology (CPT) codes rather than ICD-9 codes. Thus, to classify the procedures performed, we chose CPT codes that most closely corresponded to the ICD-9 codes used to classify procedures within the other databases (Supplementary Table, online only).
Statistical analyses were performed using SAS (v. 9.3; SAS Institute, Cary, NC) and SAS Callable SUDAAN v. 10 (Research Triangle Institute, Research Triangle Park, NC). Data were indexed to the population using national-, state-, and sex-specific population data from the U.S. Census Bureau. Comparisons of categorical covariates (demographics, procedures, and outcomes) between men and women were performed using the χ2 test. Continuous variables were compared using the Student t-test and Wilcoxon rank-sum test. The Cochrane-Armitage trend test was used to detect significant changes in categorical outcomes over time. A multivariable logistic regression model, which included age, sex, race, disease severity, and baseline comorbidities, was used to determine predictors of in-hospital mortality. Covariates included in the model have been previously shown to influence in-hospital survival in patients with PAD and therefore were likely sufficient to control for most confounding. Statistical significance was defined as P < .005 using the Bonferroni adjustment.
RESULTS
NIS (inpatient procedures)
Using the discharge weights supplied by HCUP, we identified an estimated 12,248,188 admissions for extremity atherosclerosis (Fig 1). From this group, we identified 1,797,885 admissions (56% male) for intermittent claudication (26.2%) or CLI (73.8%) with 1,865,999 procedures.
Fig 1.
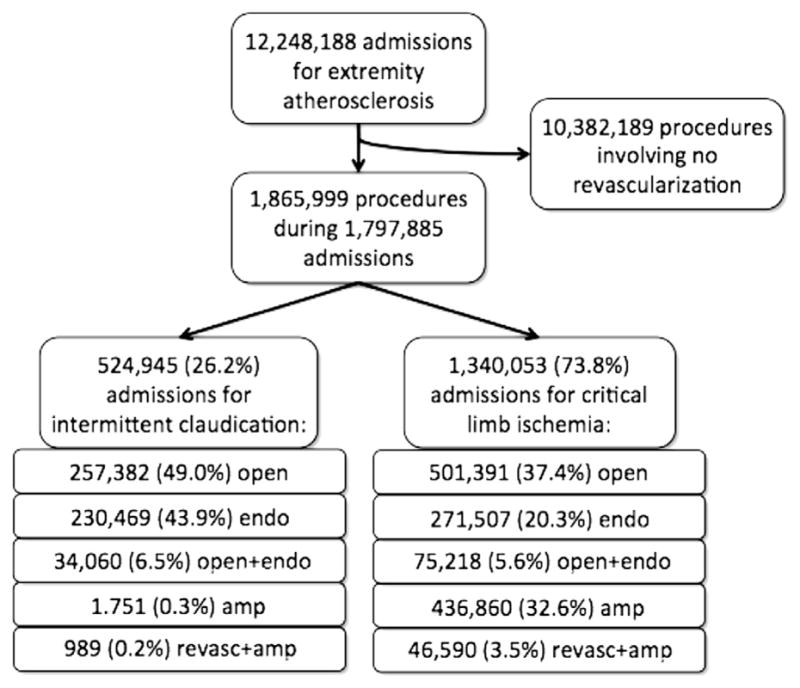
Flowchart diagramming identification of the study cohort.
Demographics and comorbidities
Overall, women were older than men for both intermittent claudication and CLI (Table I). Between 1998 and 2009, the age of individuals with intermittent claudication increased (men: 65.6 to 65.8 years; women: 67.8 to 68.5 years), whereas that for CLI decreased (men: 69.4 to 68.1 years; women: 73.0 to 71.4 years). The age of patients undergoing amputation fell by 2.4 years for men and 3.0 years for women. Compared with patients with intermittent claudication, those with CLI were older, less likely to be white, and had a higher prevalence of diabetes mellitus, congestive heart failure, and chronic renal failure (Table I). Men were more likely to be white, have concurrent diagnoses of diabetes mellitus, coronary artery disease, chronic obstructive pulmonary disease, and chronic renal failure. In contrast, women had higher rates of hypertension, congestive heart failure, and cerebrovascular disease.
Table I.
Demographics and comorbidities of patients undergoing revascularization or amputation by presentation (intermittent claudication vs critical limb ischemia [CLI])
| Intermittent claudication
|
CLI
|
|||||
|---|---|---|---|---|---|---|
| Men (n = 281,852), % | Women (n = 189,273), % | P value | Men (n = 734,059), % | Women (n = 593,731), % | P value | |
| Mean age, years | 65.9 | 68.3 | <.001 | 68.7 | 72.4 | <.001 |
| Race | ||||||
| White | 84.3 | 82.6 | 71.1 | 65.5 | ||
| Black | 7.7 | 10.1 | <.001 | 16.6 | 22.9 | <.001 |
| Other | 8.0 | 7.3 | 12.3 | 11.6 | ||
| DM | 30.7 | 29.3 | <.001 | 54.7 | 51.7 | <.001 |
| CAD | 50.9 | 41.6 | <.001 | 41.2 | 34.6 | <.001 |
| HTN | 61.6 | 66.5 | <.001 | 44.4 | 53.2 | <.001 |
| CHF | 6.9 | 7.8 | <.001 | 19.5 | 21.3 | <.001 |
| COPD | 21.0 | 20.3 | <.001 | 20.9 | 16.9 | <.001 |
| CVD | 6.3 | 7.3 | <.001 | 4.4 | 4.8 | <.001 |
| CRF | 4.6 | 4.1 | <.001 | 14.4 | 12.7 | <.001 |
CAD, Coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CRF, chronic renal failure; CVD, cerebral vascular disease; DM, diabetes mellitus; HTN, hypertension.
Presentation
Among all patients with PAD, severity of disease was classified as intermittent claudication in 26% of patients and CLI in 74%. When considering only those undergoing revascularization, 35% were classified as intermittent claudication and 65% as CLI. For all years of the study except 2009, women were more likely than men to present with CLI than intermittent claudication with an overall 1.2 higher odds (95% confidence interval [CI], 1.21–1.23; P < .01). Among only patients undergoing revascularization, women were again more likely to present with CLI (odds ratio [OR], 1.19; 95% CI, 1.18–1.20; P < .001). After adjusting for age and baseline comorbidities, women were still more likely to present with CLI (OR, 1.15; 95% CI, 1.13–1.16; P < .001).
Treatment modality
Intermittent claudication–inpatient (NIS)
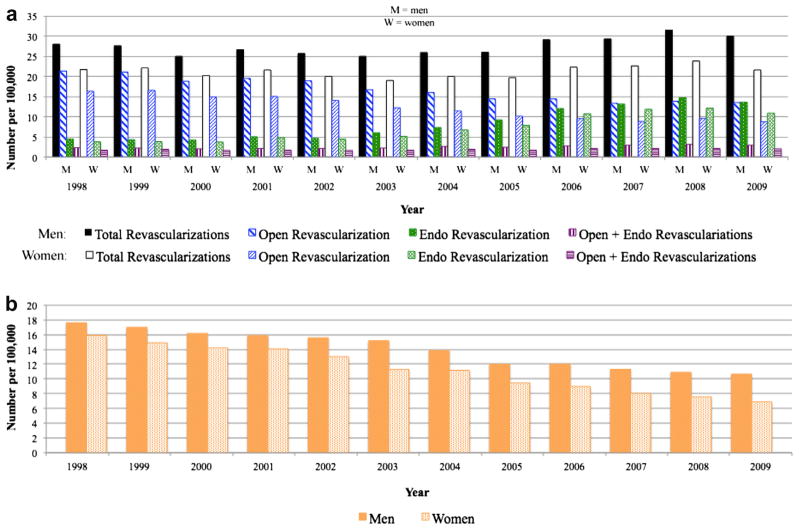
The number of endovascular procedures rose over the 12-year study period (Fig 2). Between 1998 and 2009, endovascular procedures increased by 126% for men and 167% for women. A peak in endovascular procedures was seen in 2007 when numbers reached 13 per 100,000 in men and nine per 100,000 in women. Concurrently, open procedures fell by 26% for men and 26% for women. The total number of revascularization procedures remained stable (around 16–17 per 100,000 in men and 10–11 per 100,000 in women) until 2005 when numbers began climbing. This increase in revascularization procedures was attributable to the sharp rise in endovascular procedures since the number of open procedures continued to fall until 2005 whereafter they remained stable, and the number of open+endo procedures remained stable throughout. Total inpatient revascularizations peaked in 2006 at 23 per 100,000 in men and 14 per 100,000 in women, subsequently declining to eight per 100,000 in men and seven per 100,000 in women.
Fig 2.
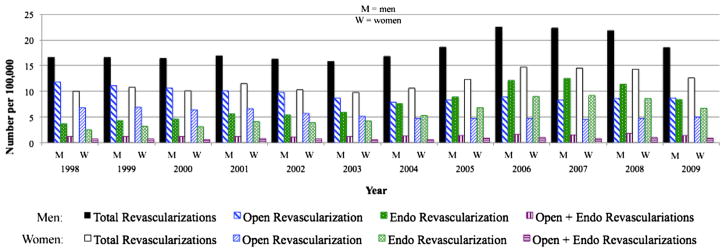
Open and endovascular procedures performed for intermittent claudication. Women were more likely than men to undergo an endovascular procedure.
As a proportion of all inpatient procedures performed, endovascular revascularizations increased steadily (from 22% in men and 24% in women in 1998), peaking in 2007 (at 56% in men and 63% in women), and declining thereafter (to 45% in men and 53% in women by 2009). This was mirrored by a decline and subsequent rebound in the proportion of inpatient open procedures. The proportion of open+endo procedures remained stable around 7% for both men and women. Women with intermittent claudication were more likely to undergo an endovascular procedure (OR, 1.27; 95% CI, 1.25–1.28; P < .001) compared with men. This remained the case after adjustment for demographics and comorbidities (OR, 1.20; 95% CI, 1.19–1.23; P < .001).
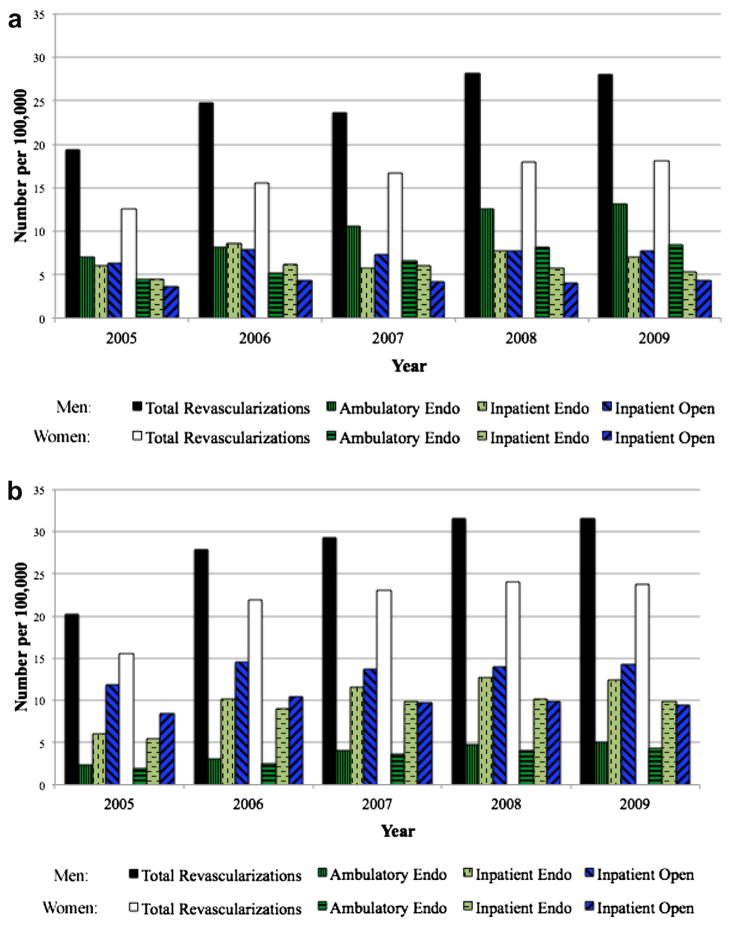
Intermittent claudication–inpatient and outpatient (SID and SASD)
The number of open revascularization procedures remained relatively stable for both men and women across the 5-year period at approximately seven per 100,000 for men and four per 100,000 for women (Fig 3, a). Rather than seeing a decline in endovascular procedural volume after 2007 as was observed with the NIS (inpatient) data, the volume of endovascular procedures documented in the state data continued to increase. Because the state data includes procedures performed in the ambulatory setting, this discrepancy represents a shift toward performing endovascular procedures in the outpatient setting.
Fig 3.
Volume of revascularization procedures in California, Florida, Maryland, and New Jersey during admissions for (a) intermittent claudication and (b) critical limb ischemia (CLI). Women were more likely to undergo endovascular procedures and less likely to undergo open surgery.
Inpatient endovascular procedures increased from 6.0 to 7.1 per 100,000 in men and 4.5 to 7.1 per 100,000 in women, representing a 17% increase in men and 19% increase in women. The growth in volume of endovascular procedures was even more evident in the ambulatory setting, increasing from 7.0 to 13.2 per 100,000 for men and 4.5 to 8.4 per 100,000 in women. This represented an 88% increase in men and 87% increase in women. As a proportion of all endovascular procedures, those being performed in the outpatient setting increased from 54% in 2005 to 65% in 2009 for men and from 50% in 2005 to 61% in 2009 for women. As seen in the NIS, women were more likely to undergo endovascular procedures (univariate: OR, 1.22; 95% CI, 1.18–1.26; P < .001; multivariable: OR, 1.32; 95% CI, 1.30–1.46; P = .002) and less likely to undergo open surgery.
CLI–inpatient (NIS)
The trend in procedural volumes for patients with CLI was similar to that observed for patients with intermittent claudication (Fig 4, a). Endovascular procedures increased 206% (from four to 14 per 100,000) in men and 191% (from four to 11 per 100,000) in women. Open procedures fell 37% in men (from 21 to 14 per 100,000) and 46% in women (from 16 to 9 per 100,000). The rate of expansion of endovascular procedures approximated the rate of decline in open procedures such that the number of total revascularizations performed for CLI remained relatively stable until 2006. From 2006 to 2008, the rise in endovascular procedures continued while open procedures stabilized, producing an overall increase in total revascularization procedures. Amputations performed for CLI also declined in both men and women (Fig 4, b): by 40% (from 18 to 11 per 100,000) in men and by 57% in women (from 16 to seven per 100,000). Notably, amputation numbers were decreasing prior to the rise of total revascularization procedures in 2006.
Fig 4.
a, Revascularization and (b) amputation procedures performed for critical limb ischemia (CLI). Women were more likely than men to undergo endovascular intervention whereas men were more likely to undergo open revascularization. Amputations were more frequent in women except after 2005.
The volume of open+endo procedures increased slightly in men and remained stable for women (Fig 4, a). Hospitalizations that involved both revascularization and amputation remained stable around one to two per 100,000 for both men and women.
Compared with other vascular procedures performed for CLI, the proportion of inpatient endovascular revascularizations increased dramatically from 9% in men and 10% in women in 1998, reaching a plateau in 2007 around 32% in men and 37% in women. The proportion of open revascularizations correspondingly fell from 45% in men and 42% in women in 1998, also plateauing from 2007 to 2008 at 31% for men and 28% for women. Amputations were performed less often relative to revascularization procedures, declining from 38% to 25% in men and from 41% to 23% in women. Women with CLI were more likely to undergo endovascular procedures (univariate: OR, 1.14; 95% CI, 1.13–1.15; P < .001; multivariable: OR, 1.11; 95% CI, 1.09–1.12; P = .004) and less likely to undergo an open procedure. Overall, women were more likely than men to undergo major amputation (univariate: OR, 1.04; 95% CI, 1.03–1.05; P < .001; multivariable: OR, 1.07; 95% CI, 1.01–1.12; P < .001) but were consistently less likely to after 2005.
CLI–inpatient and outpatient (SID and SASD)
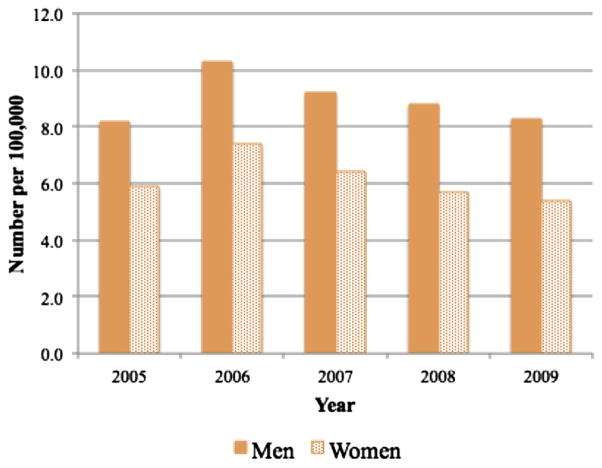
Open procedural volume remained stable (14 per 100,000 for men and nine per 100,000 for women) (Fig 3, b). Inpatient endovascular procedural volume increased 103% for men and 83% for women. Outpatient endovascular procedural volume made even greater gains, increasing 114% for men and 132% for women. However, there were still 2.7 times more inpatient than outpatient endovascular procedures for both men and women. Again, the decline in the volume of endovascular procedures after 2007 that was observed in the inpatient (NIS) data was not observed for the state data, indicating procedures have shifted to the outpatient setting. This trend was observed to be greater for CLI than intermittent claudication (Fig 3, b). Consistent with the NIS, amputations declined for both men and women (Fig 5). Women with CLI were more likely to have an endovascular intervention compared with men (univariate: OR, 1.20; 95% CI, 1.17–1.23; P < .001; multivariable: OR, 1.24; 95% CI, 1.20–1.30; P = .001) and were less likely to undergo major amputation (univariate: OR, .89, 95% CI, .87–.92; P < .001; multivariable: OR, 0.97, 95% CI, 0.93–0.98; P < .001).
Fig 5.
Volume of major amputations performed in California, Florida, Maryland, and New Jersey. Women were less likely to undergo amputation but the number of amputations declined for both men and women.
In-hospital mortality–inpatient (NIS)
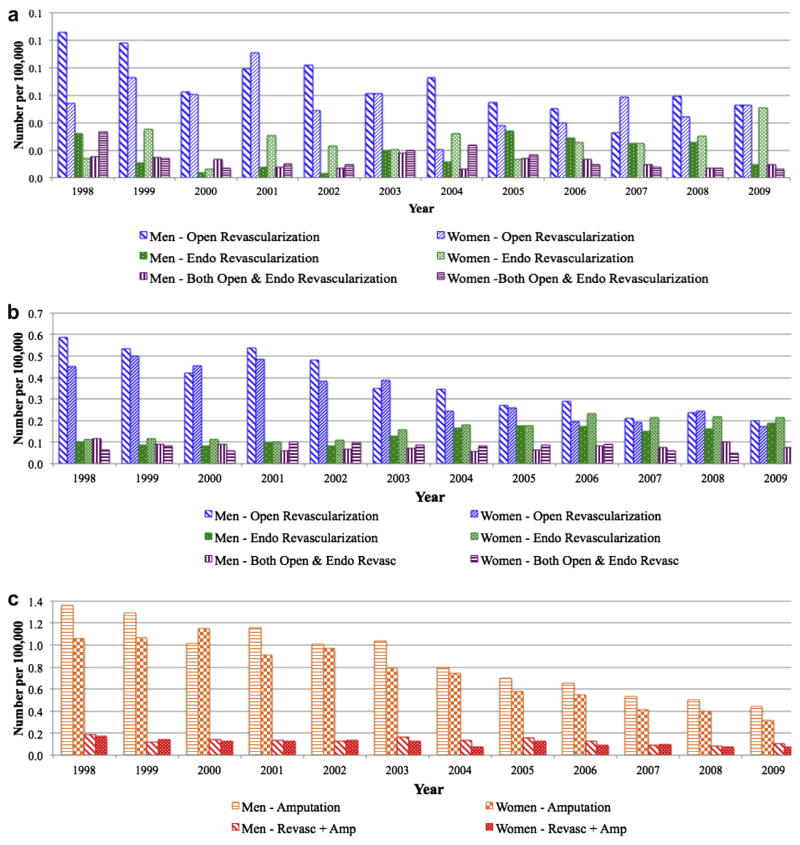
Between 1998 and 2009, the number of in-hospital deaths among patients with intermittent claudication remained low (less than .10 per 100,000) (Fig 6, a) and declined significantly for both men and women regardless of procedure (P < .01 for open, endovascular, and open-+endo procedures). Mortality was lowest among patients undergoing endovascular procedures and highest among those undergoing open+endo procedures. However, women had higher mortality rates than men for all procedures (open: 1.0% vs .7%; OR, 1.37; 95% CI, 1.25–1.49; P < .01; endovascular: .5% vs .2%; OR, 1.99; 95% CI, 1.72–2.30; P < .01; open+endo: 1.8% vs .8%, OR, 2.13; 95% CI, 1.76–2.58; P < .01).
Fig 6.
Annual in-hospital mortality by procedure and gender among (a) patients with intermittent claudication, (b) patients with critical limb ischemia (CLI) undergoing revascularization, and (c) patients with CLI undergoing amputation. Over the study period, in-hospitality mortality declined for both men and women, but was persistently higher in women.
For patients with CLI, the number of in-hospital deaths declined for all patients except for those undergoing endovascular procedures, who experienced an increase of 86% (from .10 to .19 per 100,000 for men and 88% from .11 to .21 per 100,000 for women) (Fig 6, b). Mortality rates were similar among men and women undergoing open and endovascular intervention. Both absolute number of deaths and mortality rate of men and women undergoing amputation declined (Fig 6, c). Inhospital mortality rates declined significantly (P < .01) for both men and women for all procedures evaluated. However, women again experienced higher mortality than men regardless of procedure (open: 2.7% vs 2.2%; OR, 1.23; 95% CI, 1.19–1.28; P < .01; endovascular: 2.3% vs 1.6%; OR, 1.41; 95% CI, 1.33–1.49; P < .01; open+endo: 4.0% vs 3.2%; OR, 1.28; 95% CI, 1.18–1.38; P < .01; major amputation: 6.6% vs 6.2%; OR, 1.06; 95% CI, 1.04–1.09; P < .01; revascularization with amputation: 9.7% vs 8.7%; OR, 1.13; 95% CI, 1.06–1.20; P ≤ .01).
Multivariable logistic regression revealed female sex to be a significant predictor of in-hospital mortality after adjustment for age, disease severity, race, and other baseline demographics and comorbidities (Table II). The negative impact of female sex was greatest for endovascular procedures and least for major amputation. Another interesting finding was that age had a substantially more negative impact on in-hospital mortality among patients undergoing bypass compared with those undergoing endovascular procedures or amputation whereas disease severity (CLI vs intermittent claudication) had a more negative impact on in-hospital mortality among patients undergoing endovascular procedures.
Table II.
Multivariable predictors of in-hospital mortality by procedure
| Open
|
Endovascular
|
Major amputation
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Female | 1.2 | 1.2–1.3 | <.001 | 1.4 | 1.3–1.5 | <.001 | 1.1 | 1.0–1.1 | <.001 |
| CLI | 2.5 | 2.4–2.6 | <.001 | 4.8 | 4.4–5.3 | <.001 | 1.0 | 0.8–1.2 | 0.650 |
| Age category, years | |||||||||
| <40 | - | - | - | - | - | - | - | - | - |
| 40–49 | 2.0 | 1.3–3.0 | .001 | 0.6 | 0.4–0.9 | .027 | 1.5 | 1.3–1.9 | <.001 |
| 50–59 | 3.0 | 2.0–4.5 | <.001 | 0.9 | 0.6–1.3 | .607 | 1.8 | 1.5–2.2 | <.001 |
| 60–69 | 4.7 | 3.2–7.0 | <.001 | 1.4 | 0.9–2.0 | .113 | 2.4 | 2.0–2.8 | <.001 |
| 70–79 | 6.6 | 4.5–9.8 | <.001 | 1.8 | 1.2–2.7 | .003 | 2.9 | 2.4–3.4 | <.001 |
| ≥80 | 8.5 | 5.7–12.6 | <.001 | 2.3 | 1.5–3.4 | <.001 | 2.7 | 2.3–3.3 | <.001 |
| CHF | 2.7 | 2.6–2.8 | <.001 | 2.6 | 2.5–2.8 | <.001 | 1.9 | 1.9–2.0 | <.001 |
| CRF | 1.5 | 1.5–1.6 | <.001 | 1.1 | 1.1–1.2 | .001 | 1.3 | 1.3–1.4 | <.001 |
| CVD | 1.7 | 1.6–1.8 | <.001 | 1.7 | 1.6–1.9 | <.001 | 1.8 | 1.7–1.9 | <.001 |
| COPD | 1.2 | 1.1–1.2 | <.001 | 1.4 | 1.3–1.5 | <.001 | 1.2 | 1.1–1.2 | <.001 |
| DM | 0.6 | 0.6–0.7 | <.001 | 0.7 | 0.6–0.7 | <.001 | 0.7 | 0.6–0.7 | <.001 |
| CAD | 0.7 | 0.65–0.69 | <.001 | 0.7 | 0.6–0.7 | <.001 | 0.69 | 0.68–0.71 | <.001 |
| HTN | 0.3 | 0.31–0.34 | <.001 | 0.3 | 0.3–0.4 | <.001 | 0.36 | 0.35–0.37 | <.001 |
| White race | 1.00 | 0.97–1.03 | .846 | 1.1 | 1.0–1.1 | .109 | 1.11 | 1.09–1.14 | <.001 |
CAD, Coronary artery disease; CHF, congestive heart failure; CI, confidence interval; CLI, critical limb ischemia; COPD, chronic obstructive pulmonary disease; CRF, chronic renal failure; CVD, cerebral vascular disease; DM, diabetes mellitus; HTN, hypertension; OR, odds ratio.
DISCUSSION
The goal of our study was to elucidate differences in presentation, treatment, and outcomes between men and women undergoing revascularization for lower extremity PAD. We found that women undergoing procedural hospitalizations presented at an older age, with more advanced disease, were preferentially treated with endovascular procedures, and experienced higher mortality for both intermittent claudication and CLI.
Reviewing inpatient discharge data from 3 state agencies from 1998 to 2007, Egorova et al similarly found that women were less likely to undergo surgical bypass.19 However, they found no differential utilization of endovascular procedures between sexes, possibly because they analyzed all hospitalizations (including those in which no procedure was performed), whereas we only considered procedural hospitalizations. There are several possible reasons women are less likely to undergo open revascularizations. The Bypass vs Angioplasty in Severe Ischaemia of the Leg (BASIL) study compared angioplasty and stenting to open bypass for infrainguinal CLI20 and suggested patients should be offered open bypass as first line therapy as long as they have suitable vein conduit, are fit enough to undergo surgery, and have a life expectancy greater than 2 years.21 Given that women with lower extremity PAD are generally older and present with more advanced disease, fewer may be good candidates for surgical bypass than men. Additionally, it is possible women are less likely to have adequate venous conduit due to smaller vessel size and in fact, vein grafts are reportedly used less frequently in women than in men.22
Though the trend was more dramatic in women, amputations declined substantially for both sexes throughout the study period. Of note, this decline was well under way prior to the steep rise in endovascular (and consequently total revascularization) procedures in the latter one-half of the 2000s. Indeed, between 1998 and 2003, the number of amputations performed per year had already fallen 17% in men and 30% in women. This observation challenges proposed theories that declines in amputations have been related to increased utilization of and/or advances in endovascular technology23 or the increase in total numbers of all revascularization procedures.24,25 Rather, it suggests that other factors, perhaps more aggressive control of cardiovascular risk factors (smoking cessation, cholesterol-lowering medications, antiplatelet therapy, aggressive diabetes management) and improved wound care may have had a greater impact on limb salvage than procedural interventions.
Over the study period, in-hospitality mortality declined for both men and women, but was persistently higher in women. Female sex remained a significant independent predictor of mortality regardless of procedure. Voyouka et al also found that women had higher procedural mortality than men for open and endovascular revascularization and major amputation, even after adjusting for age and other demographic factors and comorbidities.26 In fact, they found that advanced age (>70 years) actually cancelled the negative effect of female sex on mortality, suggesting we cannot attribute the discrepancy in mortality rates entirely to the age gap between men and women. One possibility is the relative underutilization of preventative care in women. For example, antiplatelet, lipid-lowering, and beta-blocker medications are underutilized to a greater degree in women compared with men among patients with cardiovascular disease and PAD.27–29 Even when treated, women are less likely to achieve goal blood pressure, target low-density lipoprotein, and hemoglobin A1c levels.30,31 Inferior optimization of medical management may put women at higher risk for postoperative cardiovascular events and death.
Using state ambulatory data, we found that endovascular (and thus total revascularization procedural) volume is not declining but rather being shifted into the outpatient setting, at least in these four states. Egorova et al found a similar increase in ambulatory endovascular therapy using 1998–2007 New York state data.19 Although outpatient endovascular interventions for CLI are growing at a faster rate than those for intermittent claudication, the latter still make up the majority of those undergoing same-day surgery. Patients with CLI are more likely to experience complications and require admission for wound care and parenteral antibiotics. Multiple studies have championed the safety and cost-effectiveness of outpatient endovascular revascularization but most have looked only at patients with claudication,32–34 and there is currently limited evidence for patients with CLI.35
Our study has several limitations resulting from the use of administrative databases. In addition to coding error, because HCUP databases lack information indicating laterality, it was impossible to know if patients who had procedure codes for both percutaneous transluminal angioplasty or stenting and open surgical bypass (approximately 6%–7% of patients), as well as patients who underwent both revascularization (either percutaneous transluminal angioplasty or stenting or open) and amputation (approximately 3% of patients) had those procedures performed on the same leg. Because the NIS is an administrative database with limited clinical information, aside from in-hospital mortality, outcomes of revascularization (eg, patency, wound healing, etc), including postdischarge events, cannot be assessed. Additionally, there is no way to track patients in the NIS after discharge, and therefore, long-term outcomes cannot be studied. For this reason, it is also unknown how many admissions actually represent readmission and reintervention. Furthermore, whereas the majority of the HCUP databases used ICD-9 procedure codes, the California and Maryland SASD used CPT codes. Using two separate classification systems may make state vs nationwide and inpatient vs outpatient comparisons more difficult to interpret. However, while CPT codes are more specific (they indicate site/anatomic level), the categories of procedures we chose (eg, endovascular vs open revascularization and major amputation) are broad enough such that they should be captured at similar rates regardless of classification system used. Indeed, results observed in the California and Maryland SASDs correlated well with those from the New Jersey and Florida SASDs. Finally, although trends were similar between the state databases when analyzed individually, results derived from the SIDs and SASDs of the four states we chose may be less generalizable since they include only four states.
CONCLUSIONS
Women undergoing vascular procedures for lower extremity PAD are older and have more advanced disease than men. Perhaps for these reasons, there appears to be a preference to perform endovascular procedures in women and open procedures in men. Endovascular procedures continue to increase, and many of these are now being performed in the outpatient setting. Amputation rates have declined steadily, and women now have lower rates than men. In-hospital mortality for both men and women also continues to decline. These improvements do not necessarily correlate with the increased utilization of endovascular (or total number of) procedures and likely resulted from both continued improvements in medical management and risk factor reduction as well as advances in surgical technology.
Supplementary Material
Acknowledgments
This work was supported by the NIH T32 Harvard-Longwood Research Training in Vascular Surgery grant HL007734.
Footnotes
Author conflict of interest: Dr Hamdan is a consultant for Endologix Data Safety and Monitoring Board. Dr Wyers is a consultant for Endologix Data Safety and Monitoring Board. Dr Schermerhorn is a consultant for Endologix Data Safety and Monitoring Board and Medtronic.
Presented at the Fortieth Annual Symposium of the Society for Clinical Vascular Surgery, Las Vegas, Nev, March 14, 2012.
Additional material for this article may be found online at www.jvascsurg.org.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
AUTHOR CONTRIBUTIONS
Conception and design: RL, RB, RM, MS
Analysis and interpretation: RL, RB, SD, AH, MW, EC, MS
Data collection: RL
Writing the article: RL, MS
Critical revision of the article: RL, RB, AH, MW, EC, MS
Final approval of the article: RL, RB, AH, MW, EC, MS
Statistical analysis: RL, RB, SD
Obtained funding: MS
Overall responsibility: MS
References
- 1.Werner CA. The Older Population: 2010, in 2010 Census Briefs. US Census Bureau; 2011. [Google Scholar]
- 2.Center for Disease Control and Prevention. [Accessed July 15, 2011];Peripheral Arterial Disease (PAD) Fact Sheet. 2011 Available at: http://www.cdc.gov/DHDSP/data_statistics/fact_sheets/fs_PAD.html.
- 3.Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998;339:1415–25. doi: 10.1056/NEJM199811123392002. [DOI] [PubMed] [Google Scholar]
- 4.Seiden SW. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;274:1506. doi: 10.1001/jama.1995.03530190019017. author reply 1506–7. [DOI] [PubMed] [Google Scholar]
- 5.Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. The UK Small Aneurysm Trial Participants. Lancet. 1998;352:1649–55. [PubMed] [Google Scholar]
- 6.Wolf YG, Arko FR, Hill BB, Olcott CT, Harris RJ, Jr, Fogarty TJ, et al. Gender differences in endovascular abdominal aortic aneurysm repair with the AneuRx stent graft. J Vasc Surg. 2002;35:882–6. doi: 10.1067/mva.2002.123754. [DOI] [PubMed] [Google Scholar]
- 7.Buth J, Laheij RJ. Early complications and endoleaks after endovascular abdominal aortic aneurysm repair: report of a multicenter study. J Vasc Surg. 2000;31:134–46. doi: 10.1016/s0741-5214(00)70075-9. [DOI] [PubMed] [Google Scholar]
- 8.Ouriel K, Greenberg RK, Clair DG, O’Hara JP, Srivastava SD, Lyden SP, et al. Endovascular aneurysm repair: gender-specific results. J Vasc Surg. 2003;38:93–8. doi: 10.1016/s0741-5214(03)00127-7. [DOI] [PubMed] [Google Scholar]
- 9.Dillavou ED, Muluk SC, Makaroun MS. A decade of change in abdominal aortic aneurysm repair in the United States: have we improved outcomes equally between men and women? J Vasc Surg. 2006;43:230–8. doi: 10.1016/j.jvs.2005.09.043. discussion: 238. [DOI] [PubMed] [Google Scholar]
- 10.Magnant JG, Cronenwett JL, Walsh DB, Schneider JR, Besso SR, Zwolak RM. Surgical treatment of infrainguinal arterial occlusive disease in women. J Vasc Surg. 1993;17:67–76. discussion: 76–8. [PubMed] [Google Scholar]
- 11.Mays BW, Towne JB, Fitzpatrick CM, Smart SC, Cambria RA, Seabrook GR, et al. Women have increased risk of perioperative myocardial infarction and higher long-term mortality rates after lower extremity arterial bypass grafting. J Vasc Surg. 1999;29:807–12. doi: 10.1016/s0741-5214(99)70207-7. discussion: 812–3. [DOI] [PubMed] [Google Scholar]
- 12.Eugster T, Gurke L, Obeid T, Stierli P. Infrainguinal arterial reconstruction: female gender as risk factor for outcome. Eur J Vasc Endovasc Surg. 2002;24:245–8. doi: 10.1053/ejvs.2002.1712. [DOI] [PubMed] [Google Scholar]
- 13.Harthun NL, Cheanvechai V, Graham LM, Freischlag JA, Gahtan V. Arterial occlusive disease of the lower extremities: do women differ from men in occurrence of risk factors and response to invasive treatment? J Thorac Cardiovasc Surg. 2004;127:318–21. doi: 10.1016/j.jtcvs.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 14.Ballotta E, Gruppo M, Lorenzetti R, Piatto G, Dagiau G, Toniato A. The impact of gender on outcome after infrainguinal arterial reconstructions for peripheral occlusive disease. J Vasc Surg. 2012;56:343–52. doi: 10.1016/j.jvs.2012.01.040. [DOI] [PubMed] [Google Scholar]
- 15.Bradbury AW. Bypass versus Angioplasty in Severe Ischemia of the Leg (BASIL) trial in perspective. J Vasc Surg. 2010;51(5 Suppl):1S–4S. doi: 10.1016/j.jvs.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Conrad MF, Crawford RS, Hackney LA, Paruchuri V, Abularrage CJ, Patel VI, et al. Endovascular management of patients with critical limb ischemia. J Vasc Surg. 2011;53:1020–5. doi: 10.1016/j.jvs.2010.10.088. [DOI] [PubMed] [Google Scholar]
- 17.Nowygrod R, Egorova N, Greco G, Anderson P, Gelijns A, Moskowitz A, et al. Trends, complications, and mortality in peripheral vascular surgery. J Vasc Surg. 2006;43:205–16. doi: 10.1016/j.jvs.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Sachs T, Pomposelli F, Hamdan A, Wyers M, Schermerhorn M. Trends in the national outcomes and costs for claudication and limb threatening ischemia: angioplasty vs bypass graft. J Vasc Surg. 2011;54:1021–31. e1021. doi: 10.1016/j.jvs.2011.03.281. [DOI] [PubMed] [Google Scholar]
- 19.Egorova N, Vouyouka AG, Quin J, Guillerme S, Moskowitz A, Marin M, et al. Analysis of gender-related differences in lower extremity peripheral arterial disease. J Vasc Surg. 2010;51:372–8. e371. doi: 10.1016/j.jvs.2009.09.006. discussion: 378–9. [DOI] [PubMed] [Google Scholar]
- 20.Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FG, Gillespie I, et al. Multicentre randomised controlled trial of the clinical and cost-effectiveness of a bypass-surgery-first versus a balloon-angioplasty-first revascularisation strategy for severe limb ischaemia due to infrainguinal disease. The Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial. Health Technol Assess. 2010;14:1–210. iii–iv. doi: 10.3310/hta14140. [DOI] [PubMed] [Google Scholar]
- 21.Bradbury AW. Bypass versus angioplasty in severe ischaemia of the leg (BASIL) trial: what are its implications? Semin Vasc Surg. 2009;22:267–74. doi: 10.1053/j.semvascsurg.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Jain AK, Velazquez-Ramirez G, Goodney PP, Edwards MS, Corriere MA. Gender-based analysis of perioperative outcomes associated with lower extremity bypass. Am Surg. 2011;77:844–9. [PMC free article] [PubMed] [Google Scholar]
- 23.Rowe VL, Lee W, Weaver FA, Etzioni D. Patterns of treatment for peripheral arterial disease in the United States: 1996–2005. J Vasc Surg. 2009;49:910–7. doi: 10.1016/j.jvs.2008.11.054. [DOI] [PubMed] [Google Scholar]
- 24.Hallett JW, Jr, Byrne J, Gayari MM, Ilstrup DM, Jacobsen SJ, Gray DT. Impact of arterial surgery and balloon angioplasty on amputation: a population-based study of 1155 procedures between 1973 and 1992. J Vasc Surg. 1997;25:29–38. doi: 10.1016/s0741-5214(97)70318-5. [DOI] [PubMed] [Google Scholar]
- 25.Goodney PP, Beck AW, Nagle J, Welch HG, Zwolak RM. National trends in lower extremity bypass surgery, endovascular interventions, and major amputations. J Vasc Surg. 2009;50:54–60. doi: 10.1016/j.jvs.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 26.Vouyouka AG, Egorova NN, Salloum A, Kleinman L, Marin M, Faries PL, et al. Lessons learned from the analysis of gender effect on risk factors and procedural outcomes of lower extremity arterial disease. J Vasc Surg. 2010;52:1196–202. doi: 10.1016/j.jvs.2010.05.106. [DOI] [PubMed] [Google Scholar]
- 27.Miller M, Byington R, Hunninghake D, Pitt B, Furberg CD. Sex bias and underutilization of lipid-lowering therapy in patients with coronary artery disease at academic medical centers in the United States and Canada. Prospective Randomized Evaluation of the Vascular Effects of Norvasc Trial (PREVENT) Investigators. Arch Intern Med. 2000;160:343–7. doi: 10.1001/archinte.160.3.343. [DOI] [PubMed] [Google Scholar]
- 28.McDermott MM, Greenland P, Reed G, Mazor KM, Merriam PA, Graff R, et al. Gender differences in cholesterol-lowering medication prescribing in peripheral artery disease. Vasc Med. 2011;16:428–35. doi: 10.1177/1358863X11425879. [DOI] [PubMed] [Google Scholar]
- 29.Chou AF, Scholle SH, Weisman CS, Bierman AS, Correa-de-Araujo R, Mosca L. Gender disparities in the quality of cardiovascular disease care in private managed care plans. Womens Health Issues. 2007;17:120–30. doi: 10.1016/j.whi.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Chou AF, Brown AF, Jensen RE, Shih S, Pawlson G, Scholle SH. Gender and racial disparities in the management of diabetes mellitus among Medicare patients. Womens Health Issues. 2007;17:150–61. doi: 10.1016/j.whi.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Wexler DJ, Grant RW, Meigs JB, Nathan DM, Cagliero E. Sex disparities in treatment of cardiac risk factors in patients with type 2 diabetes. Diabetes Care. 2005;28:514–20. doi: 10.2337/diacare.28.3.514. [DOI] [PubMed] [Google Scholar]
- 32.Akopian G, Katz SG. Peripheral angioplasty with same-day discharge in patients with intermittent claudication. J Vasc Surg. 2006;44:115–8. doi: 10.1016/j.jvs.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 33.Maurel B, Paumier A, Jacobi D, Bleuet F, Martinez R, Lermusiaux P. Ambulatory percutaneous angioplasty in patients with claudication. Ann Vasc Surg. 2011;25:191–6. doi: 10.1016/j.avsg.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Kruse JR, Cragg AH. Safety of short stay observation after peripheral vascular intervention. J Vasc Interv Radiol. 2000;11:45–9. doi: 10.1016/s1051-0443(07)61277-9. [DOI] [PubMed] [Google Scholar]
- 35.Zayed HA, Fassiadis N, Jones KG, Edmondson RD, Edmonds ME, Evans DR, et al. Day-case angioplasty in diabetic patients with critical ischemia. Int Angiol. 2008;27:232–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.