Abstract
In North America, tick-borne relapsing fever of humans is most frequently caused by infection with the spirochete Borrelia hermsii. Prior to our investigation, this spirochete was not known to infect dogs although another species, Borrelia turicatae, has been isolated from domestic canids in Florida and Texas. A clinically ill dog in Washington, USA, was spirochetemic upon examination. Spirochetes were isolated from the dog’s serum and examined by PCR and multi-locus sequence typing. DNA sequences for 7 loci all typed the spirochete as B. hermsii and a member of genomic group II of this species. Therefore, companion dogs that reside in rustic cabins in higher elevation forests are at risk of infection with B. hermsii.
Keywords: Canine borreliosis, Ornithodoros hermsi, Argasid ticks, Veterinary pathogens, Relapsing fever
Introduction
Spirochetes in the genus Borrelia are transmitted primarily by ticks and cause 2 diseases of humans in North America, Lyme borreliosis and tick-borne relapsing fever (Piesman and Schwan, 2010). The hard ticks Ixodes scapularis and Ixodes pacificus are the primary vectors of Borrelia burgdorferi (Lane et al., 1991), which is the etiological agent of Lyme borreliosis (Burgdorfer et al., 1982). In humans, this spirochete causes a pathognomonic lesion in the skin, erythema migrans, and more debilitating clinical outcomes may develop subsequently that include arthritis, carditis, and various neuropathies (Steere, 1989). Soft ticks in the genus Ornithodoros transmit 3 species of Borrelia that cause tick-borne relapsing fever (TBRF) in humans: Borrelia hermsii, Borrelia turicatae, and Borrelia parkeri (Davis, 1942; Dworkin et al., 2002). Patients with TBRF experience recurrent episodes of fever, myalgia, and a variety of other symptoms when spirochetemic (Dworkin et al., 2002).
While the clinical effects of Lyme borreliosis in dogs are well established (Littman et al., 2006; Little et al., 2010), few dogs infected with relapsing fever spirochetes have been reported. In 1939, Brumpt and Brumpt (1939) demonstrated that a 3-week-old dog was susceptible to infection with B. turicatae when fed upon by 10 infected Ornithodoros turicata ticks. The young dog became spirochetemic on days 5-15 after tick bite, relapsed on day 18, and died 2 days later when spirochetes were still detectable in the blood. Prior to our report, B. turicatae is the only species of relapsing fever spirochete that has been identified previously in clinically ill dogs in North America (Breitschwerdt et al., 1994; Rawlings, 1995; Schwan et al., 2005; Whitney et al., 2007). In 1979, a 6-year-old, spayed female bluetick hound with fever (104°F) and altered gait was admitted to a veterinary hospital in Bakersfield, California (Schalm, 1979). Spirochetes were found in a stained blood smear, but the organisms were not isolated or identified. In 1990, a 2-year-old Siberian husky in Texas was reported to be infected with B. burgdorferi (Moreland et al., 1990), however, the presence of spirochetes visible in the animal’s blood suggests that this dog was more likely infected with an unidentified relapsing fever spirochete. In the early 1990s, 2 clinically ill dogs in Florida were diagnosed with spirochete infections (Breitschwerdt et al., 1994) that were later identified as B. turicatae from one of the animals (Schwan et al., 2005). From 1999 to 2001, 3 dogs in Texas were infected with B. turicatae (Whitney et al., 2007). Herein, we document the isolation of the relapsing fever spirochete, B. hermsii, from a clinically ill domestic dog. We are unaware of any previous reports that demonstrate dogs having been infected with this species of spirochete.
Materials and methods
Bacterial strains and cultivation
Isolates of B. hermsii and B. turicatae in our collection that had been passaged only 1-3 times in vitro were included in the characterization of the spirochete isolated from the dog described here. Borrelia hermsii LAK-1, LAK-2, LAK-4, SIL, HAN, YOR, DAH, and WAR originated from human patients infected in Montana, Idaho, Washington, and California (Schwan et al., 2007), while B. turicatae TCB-1 and TCB-2 originated from dogs infected in Texas (Schwan et al., 2005; Whitney et al., 2007). Spirochetes from the dog described in this report were isolated by first inoculating a laboratory mouse (Mus musculus) with the dog’s infected serum. On day 2 post-inoculation, the mouse had a spirochetemia detected by microscopy. Infected blood from this mouse was passaged into a second mouse by intraperitoneal inoculation, and 2 days later the infected blood was collected. Thin smears of the blood from the first infected mouse were made and fixed with 100% methanol and stained with monoclonal antibody H9826 that is specific for B. hermsii (Schwan et al., 1992). From the second mouse, 500 μl of infected blood was inoculated into 100 ml of BSK-H medium (Sigma-Aldrich, St. Louis, MO) supplemented with 12% rabbit serum and incubated at 35°C. Spirochetes subsequently grew to a high cell density stationary-phase culture, were cryopreserved at −80°C and analyzed as described below. The Animal Care and Use Committee of the Rocky Mountain Laboratories, NIAID, NIH, approved our study protocol #2009-32 for mouse infection and isolation of relapsing fever spirochetes.
PCR and DNA sequence analysis
DNA was extracted from the spirochete culture using a phenol/chloroform extraction and ethanol precipitation method that we have used previously (Simpson et al., 1990). Genomic DNA samples were prepared from the spirochetes isolated from the dog and from a previously established, but uncharacterized isolate of B. hermsii WAR from a human patient infected in the same geographic location as the dog. These samples were subjected to PCR amplification and DNA sequence analysis for 7 loci: 16S rRNA, flagellin (flaB), gyrase subunit B (gyrB), glycerophosphodiester phosphodiesterase (glpQ), factor H binding protein (fhbA), variable tick protein (vtp), and the 16S-23S rRNA intergenic spacer (IGS) (Table 1). The 50-μL PCR reaction mixtures were initially heated to 96°C for 3 min, followed by 35 cycles at 94°C for 30 s, 55°C for 30 s and 72°C for 2.5 min. An additional 7-min extension was done at 72°C. PCR products were electrophoresed in a 1% agarose gel stained with gel red to visualize the amplicons. If a band of the expected size was visible, the PCR product was purified using the QIAquick® PCR Purification Kit (Qiagen®, Valencia, CA). If a band was not visible, another PCR reaction was performed with the annealing temperature lowered to 53°C. The purified PCR products were sequenced using the BigDye® Terminator v3.1 Cycle Sequencing Kit (Invitrogen Life Technologies, Grand Island, NY). The 10-μL sequencing reactions were initially heated to 95°C for 5 s and followed by 45 cycles at 95°C for 10 s, 55°C for 5 s, and 60°C for 4 min. Sequence reaction mixtures were cleaned using the BigDye® XTerminator® Purification Kit (Life Technologies, Grand Island, NY) and then sequenced on an Applied Biosystems 3730XL DNA Sequencer. Nucleotide sequences were first analyzed with Sequencher 5.0, transferred to Mega 5.05, and aligned using ClustalW with sequences from other Borrelia isolates determined by us previously and available in GenBank. A neighbor-joining tree was constructed with Mega 5.05 using a bootstrap value of 1000. Undigested genomic DNA samples from Borrelia isolates were electrophoresed in a reverse pulse-field agarose gel to resolve plasmids as previously described (Porcella et al., 2005) and stained with gel red (Phenix Research, Candler, NC).
Table 1.
Locus primers used for DNA amplification and sequencing of the isolate.
| Locus and primers | Sequence (5′–3′) |
|---|---|
|
| |
| 16S rRNA | |
| FD3 | AGAGTTTGATCCTGGCTTAG |
| T50 | GTTACGACTTCACCCTCCT |
| Rec4 | ATGCTAGAAACTGCATGA |
| Rec9 | TCGTCTGAGTCCCCATCT |
| 16S+ | TACAGGTGCTGCATGGTTGTCG |
| 16S− | TAGAAGTTCGCCTTCGCCTCTG |
|
| |
| flaB | |
| Bh fla 5′ | AATCTTTGAATTTACAGCGACAAAACAGG |
| Bh fla 3′ | AAACTCCAATGCGAAAACATTACAATCC |
| fla LL | ACATATTCAGATGCAGACAGAGGT |
| fla RL | GCAATCATAGCCATTGCAGATTGT |
| fla LS | AACAGCTGAAGAGCTTGGAATG |
| fla RS | CTTTGATCACTTATCATTCTAATAGC |
|
| |
| glpQ | |
| F+1 | GGGGTTCTGTTACTGCTAGTGCCATTAC |
| SPR2 | CAATACTAAGACCAGTTGCTCCTCCGCC |
| F-1 | CAATTTTAGATATGTCTTTACCTTGTTGTTTATGCC |
| SPR1 | GCACAGGTAGGAATGTTGGAATTTATCCTG |
|
| |
| gyrB | |
| New 5′-1 | TGTGGAAGGTTTATGAGTTATGTTG |
| 3D | GCCTTACAAGTGCTTCGTAAATCGACTG |
| 5′+1 | TTATCAAAGAGACTTAGGGAACTTGC |
| 5′+2 | GAAAGATGTTCCAAGTCTTACATTAGATG |
| 5′+3 | GCTGATGCTGATGTTGATGG |
| 3′+1 | TGCCCATTCTCAATTAACTCCC |
| 3′+2 | CATCATGCACAATAGTTTCAACG |
| 3′+3 | TTCCACCTTCAAAATAAAATTCC |
|
| |
| fhbA | |
| 5′ | CTTAAAACAACAGATAGACTCAATTTACAG |
| 3′ | TTGGAGTACTGCCTATACTCTCAGG |
| fhbA 20F | TAGCTGTGATTTATTCAATAAAAAC |
| fhbA 192R | CAACTTAAGTTTTTAAATATTCC |
|
| |
| vtp | |
| 5′-1 | GCACTATTAATGTATGTTAATAAGGAGGCAC |
| sp-12 | GCTTTCTATTTATTGACTTTATTTTTCCAG |
| V33-12 | CTTTATTTTTCCAGTTAC |
|
| |
| IGS | |
| IGS F | GTATGTTTAGTGAGGGGGGTG |
| IGS R | GGATCATAGCTCAGGTGGTTAG |
| IGS Fn | AGGGGGGTGAAGTCGTAACAAG |
| IGS Rn | GTCTGATAAACCTGAGGTCGGA |
Primers in bold were used for PCR amplification of the target DNA, and all primers were used for sequence reactions.
Nucleotide sequence accession numbers
The 14 DNA sequences determined for B. hermsii isolated from the dog and the previously uncharacterized isolate of B. hermsii WAR were deposited in the GenBank database with the accession numbers KC883459 to KC883472.
Results
Case report
On November 3, 2009, a 9-year-old dog (a spayed female Labrador-cross) was brought to Kenmore Veterinary Hospital in Kenmore, Washington, because she had been lethargic and anorexic for 2 days. The dog had recently spent time in the owner’s cabin located close to Nathan Creek near Stevens Pass (4062 ft elevation), Chelan County, Washington. During the initial examination the dog had a temperature of 102.7°F and normal heart and respiratory rates. The capillary refill time was greater than 2 s. No obvious masses were seen in chest and abdominal x-rays, although the x-rays demonstrated the dog was constipated and had spondylosis of the L1–L2 and lumbosacral joints. Awaiting complete blood count (CBC) and chemistry results, the dog received an enema, fluids, famotidine (Pepcid®) (2 mg q 24 h), and prednisone (40 mg). The blood chemistry profile showed mild increases in alkaline phosphatase (160 U/L), cholesterol (330 mg/dL), and lipase (688 U/L), and other routine chemistry values were within normal limits. The CBC showed a pancytopenia characterized by a moderate thrombocytopenia (platelet clumping precluded an accurate count), mild nonregenerative anemia (PCV 28.3% with 67,000/ul reticulocytes), and a leukopenia (3500/μL) due to a mild neutropenia (2310/μL) and lymphopenia (525/μL). Microscopic analysis of a Wright-stained, thin blood smear demonstrated the presence of numerous spirochetes. With this finding, the prednisone was discontinued, polyflex and maropitant citrate (Cerenia®) injections were given for nausea, and the dog began treatment with doxycycline (200 mg q 12 h) and amoxicillin (400 mg q 12 h) for 14 days. The dog responded well to the treatment and was lost to follow up. A blood sample taken from the dog prior to treatment was sent to Rocky Mountain Laboratories for further analysis.
Indirect immunofluorescence assay
A sample of the infected dog blood produced microscopically detectable spirochetes in the experimentally inoculated mice. Spirochetes in the thin smear from one infected mouse were reactive with the B. hermsii species-specific monoclonal antibody H9826 (Schwan et al., 1992) (data not shown).
DNA sequence analysis
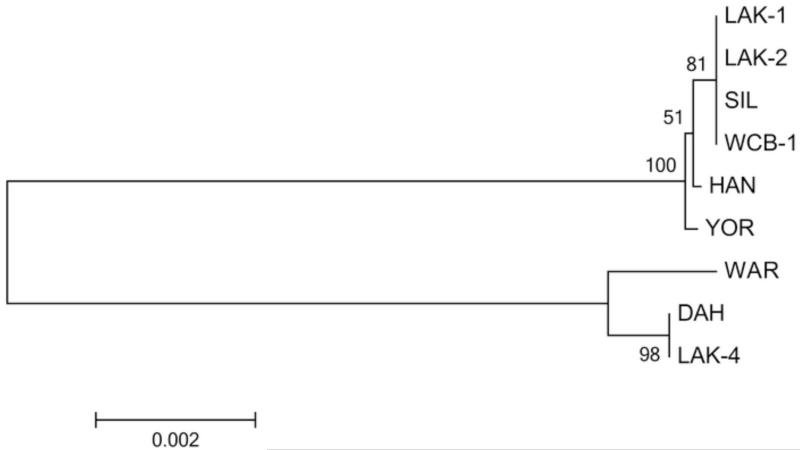
DNA sequences were determined for the spirochete isolated from the dog, here designated WCB-1 for Washington Canine Borrelia, and a previously uncharacterized isolate (WAR) from a human patient that originated from the same county where the dog likely became infected. The extent of the DNA sequences determined included the 16S rRNA (1272 bp), flaB (1002 bp), gyrB (1902 bp), glpQ (1011 bp), fhbA (546 bp), vtp (626 bp), and the IGS locus (649 bp). BLAST searches with all individual sequences identified the isolate from the dog as B. hermsii belonging to genomic group II (GGII). A ClustalW alignment with the IGS sequence clearly grouped WCB-1 with B. hermsii GGII spirochetes, while the WAR isolate grouped with the B. hermsii GGI isolates (Fig. 1). The phylogram based on the concatenated sequences comprised of $ loci (16S rRNA, flaB, gyrB, and glpQ) that totaled 5187 bp also placed WCB-1 in GGII with SIL, HAN, LAK-1 and LAK-2 isolates and WAR with GGI isolates (Fig. 2). Borrelia hermsii HAN and SIL originated from people infected in northern Idaho, and LAK-1 and LAK-2 were isolated from patients infected in western Montana (Schwan et al., 2007).
Fig. 1.
Phylogram of 16S-23S rRNA intergenic spacer (IGS) sequence from WCB-1 and other representative GGI and GGII isolates of B. hermsii. The tree was constructed using ClustalW and the Neighbor-joining method with 1000 bootstrap replicates (values shown on tree). Branch lengths represent the number of substitutions per site. Borrelia turicatae isolates collected from 2 dogs in Texas were used for the outgroup. The WCB-1 clearly groups with GGII B. hermsii.
Fig. 2.
Phylogram of the concatenated (16S rRNA-flaB-gyrB-glpQ) sequences of WCB-1 compared to other B. hermsii isolates. The tree was constructed using ClustalW and the neighbor-joining method with 1000 bootstrap replicates. The scale bar represents the number of substitutions per site.
The vtp gene from WCB-1 grouped with other Type 1 sequences with 100% identity to vtp sequences for B. hermsii LAK-1, LAK-2, and HAN (Porcella et al., 2005). The vtp sequence for WAR grouped most closely (78% identity) with other vtp Type 2 sequences in GGI isolates. However, this locus is highly polymorphic (Porcella et al., 2005), and due to possible horizontal transfer of this plasmid-encoded gene between B. hermsii spirochetes, vtp is a poor candidate for phylogenetic analysis and was not included in our phylograms.
Analysis of plasmids
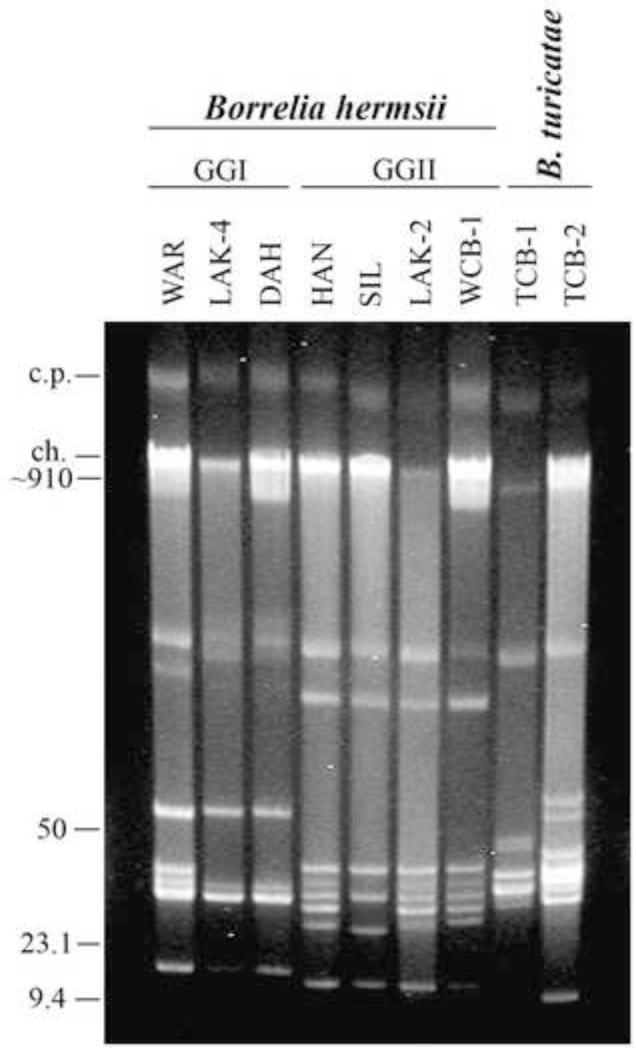
Reverse pulse-field agarose gel electrophoresis was used to further characterize the new isolate from the dog. The plasmid profile of WCB-1 was compared to 3 B. hermsii in GGII (HAN, SIL, LAK-2), 3 B. hermsii in GGI (WAR, LAK-4, DAH), and 2 B. turicatae isolates from Texas dogs (TCB-1, TCB-2) (Fig. 3). The Washington isolate WCB-1 from the dog contained at least 8 resolved linear plasmids, one circular plasmid, and the linear chromosome (~910 kbp). The plasmid profile of the isolate from the dog shared a similar plasmid pattern with other GGII B. hermsii, in particular the LAK-2 and HAN isolates from western Montana and northern Idaho, respectively.
Fig. 3.
Reverse pulse-field agarose gel of uncut genomic DNA from B. hermsii WCB-1 compared to other GGI and GGII isolates and B. turicatae isolated from domestic dogs in Texas. The circular plasmids (c.p.) and linear chromosome (ch.) are shown on the left with estimated DNA sizes shown in kilobases. The remaining bands are linear plasmids of varying sizes.
Discussion
Among the 3 recognized species of tick-borne relapsing fever spirochetes in North America, the vast majority of reported human infections are caused by B. hermsii (Dworkin et al., 2002). Endemic foci for this spirochete have been known in the United States since 1915 (Meader, 1915), prior to the identification of the spirochete and its tick vector (Wheeler, 1935; Davis, 1942). Yet in the nearly one century of known human disease caused by this species of spirochete, no prior evidence of this bacterial infection in dogs has been reported. Most human infections occur while sleeping in tick-infested cabins located in higher elevation coniferous forests (Dworkin et al., 1998). The vector, O. hermsi, is a nocturnal fast-feeding tick that parasitizes numerous species of diurnal small mammals such as chipmunks and squirrels (Wynns and Beck, 1935; Dworkin et al., 1998, 2002). Humans are accidental hosts for the ticks when the wild mammals are not available or accessible to the ticks when hungry. Companion dogs that sleep in tick-infested cabins would also be prone to being fed upon by O. hermsi. The dog discussed in this report had spent time in a forest cabin prior to becoming ill, and while a site investigation was not performed, this cabin exposure may have been where the dog became infected.
Dogs infected with another species of relapsing fever spirochete, B. turicatae, have been described from Florida and Texas (Breitschwerdt et al., 1994; Rawlings, 1995; Schwan et al., 2005; Whitney et al., 2007). The ecology of this spirochete and its tick vector, O. turicata, is strikingly different from that of B. hermsii. The few dogs that have been described with B. turicatae infection roamed freely during the day and likely encountered O. turicata ticks while the dogs foraged in, excavated, or explored underground burrows or caves. No dogs have yet been described with clinical illness and infection with B. parkeri.
Borrelia hermsii is found in the appropriate forested habitats from southern British Columbia, Canada, to the mountains of southern California, and scattered foci as far east as central Colorado (Wynns and Beck, 1935; Beck, 1937; Banerjee et al., 1998; Trevejo et al., 1998; Fritz et al., 2004; Schwan et al., 2007, 2009). Genetic characterization of the spirochete isolates has identified 2 genomic groups (GGI and GGII) in the species (Porcella et al., 2005; Schwan et al., 2007). The WCB-1 isolate from the dog belonged to GGII, which has been found throughout much of the geographic range for the species. Mammalian species may vary in their susceptibility to infection with B. hermsii (Burgdorfer and Mavros, 1970), and the basis for this difference is not known. Whether dogs and other canid species are more susceptible to infection with B. hermsii of one genomic group versus the other await further studies.
We included other GGI and GGII isolates of B. hermsii along with our description of WCB-1 from the dog. One of these isolates (B. hermsii WAR) originated from a human relapsing fever patient that was infected in the same general vicinity as was the dog in Chelan County, Washington. These 2 isolates again represent near sympatry of both genomic groups of B. hermsii (WCB-1 in GGII and WAR in GGI), as we have found in an enzootic focus in western Montana (Schwan et al., 2003, 2007). The ability of different genetic types of B. hermsii to superinfect single O. hermsi ticks and be co-transmitted during a single tick feeding (Policastro et al., 2013) helps explain how a diversity of these spirochetes can coexist in the same natural focus. The division of B. hermsii into 2 genomic groups began to emerge with the agarose gel analysis of plasmids contained in total genomic DNA preparations (Schwan et al., 1995). As we established more isolates from clinical samples and performed multi-locus sequence typing of the spirochetes, we observed a content of plasmids that was unique to each genomic group as defined by their DNA sequences (Porcella et al., 2005; Lopez et al., 2008). Thus the number and size of plasmids contained in an isolate of B. hermsii is sufficient to identify the genomic group to which the spirochete belongs. In our characterization of the isolate of B. hermsii WCB-1 that infected the dog, its plasmid content clearly grouped the spirochete with other GGII isolates (Fig. 3). Early reports demonstrated that dogs are susceptible to infection with Old World species of relapsing fever spirochetes (reviewed in Felsenfeld, 1965). Evidence has been slowly emerging during the past 20 years that at least 2 species of North American species of relapsing fever spirochetes, B. turicatae and now B. hermsii, can infect and cause a variety of clinical syndromes in dogs. All the infected dogs reported to date were observed to have spirochetes in stained thin blood smears. While microscopy is not the most sensitive method to detect spirochetes in the blood, this technique remains as the gold standard in most clinical laboratories for veterinary and human medicine. Our report demonstrates that dogs are susceptible in infection with B. hermsii, which is widespread in southern British Columbia, Canada, and the western United States. Clinically ill dogs that have recently resided in rustic forest cabins should be evaluated for possible spirochete infection.
Acknowledgements
This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banerjee SN, Banerjee M, Fernando K, Burgdorfer W, Schwan TG. Tick-borne relapsing fever in British Columbia, Canada: first isolation of Borrelia hermsii. J. Clin. Microbiol. 1998;36:3503–3508. doi: 10.1128/jcm.36.12.3505-3508.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck MD. California field and laboratory studies on relapsing fever. J. Infect. Dis. 1937;60:64–80. [Google Scholar]
- Breitschwerdt EB, Nicholson WL, Kiehl AR, Steers C, Meuten DJ, Levine JF. Natural infections with Borrelia spirochetes in two dogs from Florida. J. Clin. Microbiol. 1994;32:352–357. doi: 10.1128/jcm.32.2.352-357.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumpt E, Brumpt LC. Identité du spirochete des fièvres récurrentes a tiques des plateaux mexicains et du Spirochaeta turicatae agent de la fièvre recurrente sporadique des Etats-Unis. Ann. Parasitol. Hum. Compar. 1939;17:287–298. [Google Scholar]
- Burgdorfer W, Mavros AJ. Susceptibility of various species of rodents to the relapsing fever spirochete, Borrelia hermsii. Infect. Immun. 1970;2:256–259. doi: 10.1128/iai.2.3.256-259.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Lyme disease-a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Davis GE. Species unity or plurality of the relapsing fever spirochetes. In: Moulton FR, editor. A Symposium of Relapsing Fever in the Americas. Am. Assoc. Adv. Sci.; Washington, D.C.: 1942. pp. 41–47. [Google Scholar]
- Dworkin MS, Schwan TG, Anderson DE. Tick-borne relapsing fever in North America. Med. Clin. North Amer. 2002;86:417–433. doi: 10.1016/s0025-7125(03)00095-6. [DOI] [PubMed] [Google Scholar]
- Dworkin MS, Anderson DE, Jr., Schwan TG, Shoemaker PC, Banerjee SN, Kassen BO, Burgdorfer W. Tick-borne relapsing fever in the northwestern United States and southwestern Canada. Clin. Infect. Dis. 1998;26:122–131. doi: 10.1086/516273. [DOI] [PubMed] [Google Scholar]
- Felsenfeld O. Borreliae, human relapsing fever, and parasite-vector-host relationships. Bacteriol. Rev. 1965;29:46–74. doi: 10.1128/br.29.1.46-74.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz CL, Bronson LR, Smith CR, Schriefer ME, Tucker JR, Schwan TG. Isolation and characterization of Borrelia hermsii associated with two foci of tick-borne relapsing fever in California. J. Clin. Microbiol. 2004;42:1123–1128. doi: 10.1128/JCM.42.3.1123-1128.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RS, Piesman J, Burgdorfer W. Lyme borreliosis: relation of its causative agent to its vectors and hosts in North America and Europe. Annu. Rev. Entomol. 1991;36:587–609. doi: 10.1146/annurev.en.36.010191.003103. [DOI] [PubMed] [Google Scholar]
- Little SE, Heise SR, Blagburn BL, Callister SM, Mead PS. Lyme borreliosis in dogs and humans in the USA. Trends Parasitol. 2010;26:213–218. doi: 10.1016/j.pt.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Littman MP, Goldstein RE, Labato MA, Lappin MR, Moore GE. ACVIM small animal consensus statement on Lyme disease in dogs: diagnosis, treatment, and prevention. J. Vet. Intern. Med. 2006;20:422–434. doi: 10.1892/0891-6640(2006)20[422:asacso]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Lopez JE, Schrumpf ME, Raffel SJ, Policastro PF, Porcella SF, Schwan TG. Relapsing fever spirochetes retain infectivity after prolonged in vitro cultivation. Vector-Borne Zoonotic Dis. 2008;8:813–820. doi: 10.1089/vbz.2008.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meader CN. Five cases of relapsing fever originating in Colorado, with positive blood findings in two. Colorado Med. 1915;12:365–369. [Google Scholar]
- Moreland KJ, Wilson EA, Simpson RB. Concurrent Ehrlichia canis and Borrelia burgdorferi infections in a Texas dog. J. Am. Anim. Hosp. Assoc. 1990;26:635–639. [Google Scholar]
- Piesman J, Schwan TG. Ecology of borreliae and their arthropod vectors. In: Samuels DS, Radolf JD, editors. Borrelia: Molecular Biology, Host Interaction and Pathogenesis. Caister Academic Press; Norfolk, UK: 2010. pp. 251–278. [Google Scholar]
- Policastro PF, Raffel SJ, Schwan TG. Borrelia hermsii acquisition order in superinfected ticks determines transmission efficiency. Infect. Immun. 2013;81:2899–2908. doi: 10.1128/IAI.00542-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcella SF, Raffel SJ, Anderson DE, Jr., Gilk SD, Bono JL, Schrumpf ME, Schwan TG. Variable tick protein in two genomic groups of the relapsing fever spirochete Borrelia hermsii in western North America. Infect. Immun. 2005;73:6647–6658. doi: 10.1128/IAI.73.10.6647-6658.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings JA. An overview of tick-borne relapsing fever with emphasis on outbreaks in Texas. Tex. Med. 1995;91:56–59. [PubMed] [Google Scholar]
- Schalm OW. Uncommon hematologic disorders: spirochetosis, trypanosomiasis, leishmaniasis, Pelger-Huet anomaly. Canine Pract. 1979;6:46–49. [Google Scholar]
- Schwan TG, Raffel SJ, Schrumpf ME, Porcella SF. Diversity and distribution of Borrelia hermsii. Emerg. Infect. Dis. 2007;13:436–442. doi: 10.3201/eid1303.060958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan TG, Gage KL, Hinnebusch J. Analysis of relapsing fever spirochetes from the western United States. J. Spirochetal Tick-Borne Dis. 1995;2:3–8. [Google Scholar]
- Schwan TG, Gage KL, Karstens RH, Schrumpf ME, Hayes SF, Barbour AG. Identification of the tick-borne relapsing fever spirochete Borrelia hermsii by using a species-specific monoclonal antibody. J. Clin. Microbiol. 1992;30:790–795. doi: 10.1128/jcm.30.4.790-795.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan TG, Policastro PF, Miller Z, Thompson RL, Damrow T, Keirans JE. Tick-borne relapsing fever caused by Borrelia hermsii, Montana. Emerg. Infect. Dis. 2003;9:1151–1154. doi: 10.3201/eid0909.030280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan TG, Raffel SJ, Schrumpf ME, Policastro PF, Rawlings JA, Lane RS, Breitschwerdt EB, Porcella SF. Phylogenetic analysis of the spirochetes Borrelia parkeri and Borrelia turicatae and the potential for tick-borne relasping fever in Florida. J. Clin. Microbiol. 2005;43:3851–3859. doi: 10.1128/JCM.43.8.3851-3859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan TG, Raffel SJ, Schrumpf ME, Webster LS, Marques AR, Spano R, Rood M, Burns J, Hu R. Tick-borne relapsing fever and Borrelia hermsii, Los Angeles County, California, USA. Emerg. Infect. Dis. 2009;15:1026–1031. doi: 10.3201/eid1507.090223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson WJ, Garon CF, Schwan TG. Analysis of supercoiled circular plasmids in infectious and non-infectious Borrelia burgdorferi. Microb. Pathog. 1990;8:109–118. doi: 10.1016/0882-4010(90)90075-2. [DOI] [PubMed] [Google Scholar]
- Steere AC. Lyme disease. N. Engl. J. Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- Trevejo RT, Schriefer ME, Gage KL, Safranek TJ, Orloski KA, Pape WJ, Montenieri JA, Campbell GL. An interstate outbreak of tick-borne relapsing fever among vacationers at a Rocky Mountain cabin. Am. J. Trop. Med. Hyg. 1998;58:743–747. doi: 10.4269/ajtmh.1998.58.743. [DOI] [PubMed] [Google Scholar]
- Wheeler CM. A new species of tick which is a vector of relapsing fever in California. Am. J. Trop. Med. 1935;15:435–438. [Google Scholar]
- Whitney MS, Schwan TG, Sultemeier KB, McDonald PS, Brillhart MN. Spirochetemia caused by Borrelia turicatae infection in 3 dogs in Texas. Vet. Clin. Pathol. 2007;36:212–216. doi: 10.1111/j.1939-165x.2007.tb00213.x. [DOI] [PubMed] [Google Scholar]
- Wynns HL, Beck MD. Epidemiological studies on relapsing fever in California. Am. J. Publ. Health. 1935;7:270–276. doi: 10.2105/ajph.25.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]