Abstract
Purpose
To explore the activity of dasatinib in combination with docetaxel, gemcitabine, topotecan and doxorubicin in ovarian cancer cells.
Experimental Design
Cells with previously determined SRC pathway and protein expression (SRC pathway/SRC protein: IGROV1, both high; SKOV3, both low) were treated with dasatinib in combination with the cytotoxic agents. SRC and paxillin protein expression were determined pre- and post-treatment. Dose-response curves were constructed and the combination index (CI) for drug interaction was calculated.
Results
In the IGROV1 cells, dasatinib alone reduced pSRC/tSRC 71% and p-paxillin/t-paxillin ratios 77%. pSRC (3-33%, p=0.002-0.04) and p-paxicillin (6-19%; p=0.01-0.05) levels were significantly reduced with dasatinib in combination with each cytotoxic agent. The combination of dasatinib and docetaxel, gemcitabine or topotecan had a synergistic anti-proliferative effect (CI 0.49-0.68), while dasatinib combined with doxorubicin had an additive effect (CI 1.08).
In SKOV3 cells, dasatinib resulted in less pronounced reductions of pSRC/tSRC (49%) and p-paxillin/t-paxillin (62%). pSRC (18%; p<0.001) and p-paxillin levels (18%; p=0.001; 9%; p=0.007) were significantly decreased when dasatinib was combined with docetaxel and topotecan (p-paxillin only). Furthermore, dasatinib combined with the cytotoxics in the SKOV3 cells produced an antagonistic interaction on proliferation of these cells (CI 1.49-2.27).
Conclusion
Dasatinib in combination with relapse chemotherapeutic agents appears to interact in a synergistic or additive manner in cells with high SRC pathway activation and protein expression. Further evaluation of dasatinib in combination with chemotherapy in ovarian cancer animal models and exploration of the use of biomarkers to direct therapy is warranted.
Keywords: SRC pathway, dasatinib, ovarian cancer
INTRODUCTION
Dasatinib is an oral inhibitor of SRC family kinases1, and also inhibits at least four other protein tyrosine kinases and kinase families including BCR-ABL, c-KIT, EPHA2 and PDGFRβ.2 SRC is a nonreceptor tyrosine kinase that mediates multiple cell signaling pathways, including cell proliferation, growth, and survival 1. SRC and its activated form, phospho-SRC (pSRC), are aberrantly activated in a number of solid tumors, including ovarian cancers.1,3,4 In previous studies, treatment of ovarian cancer cells in vitro or in vivo with various SRC-inhibitors resulted in decreased activation of survival pathways and cell growth, and synergistically enhanced the activity of standard chemotherapeutics.5-8
We previously demonstrated synergistic activity of dasatinib in combination with paclitaxel and carboplatin in select ovarian cancer cell lines.5 Based on this data we conducted a phase I trial of combination paclitaxel and carboplatin in women with advanced and recurrent epithelial ovarian, peritoneal, or tubal cancer.9 We observed a response rate of 40% including 3 complete responses (15%) and 5 partial responses (25%) with stable disease in 10 patients (50%). The combination demonstrated clinical activity based on the response rates and survival outcomes in this patient population that included women with platinum-resistant disease. While first-line therapy is important to evaluate, there is a critical need to develop novel agents for women with recurrent or persistent ovarian cancer. Commonly used second-line chemotherapeutics have low response rates in women with taxane and platin-resistant ovarian cancer. Therefore, it is imperative to find ways to enhance the anti-tumor activity of commonly used relapse chemotherapy regimens including liposomal doxorubicin, docetaxel, gemcitabine, and topotecan.
There have been reports of enhanced anti-proliferative activity using anti-SRC treatments in combination with docetaxel, gemcitabine, and doxorubin in ovarian, pancreatic, and breast cancer cells, respectively.10-13 Docetaxel in combination with SRC inhibitors, AP23846 and AP23846, increased growth inhibition in ovarian cancer cells and reduced tumor burden by 95–98% in an orthotopic murine ovarian cancer model compared to docetaxel alone.10 In pancreatic cell lines, gemcitabine resistance was associated with higher SRC phosphorylation and could be reversed with SRC inhibitors.13 Furthermore, the combination of dasatinib, erlotinib, and gemcitabine inhibited pancreatic cancer cell migration and invasion at drug concentrations that were ineffective as single agents or as doublets.11 Dasatinib combined with doxorubicin also demonstrated synergistic anti-proliferative activity, and significantly inhibited cellular migration and invasion in breast cancer cell lines.12 The promising anti-tumor activity of dasatinib in combination with chemotherapy in a variety of solid tumors warrants further evaluation.
Therefore, the aim of this study was to evaluate the anti-proliferative activity of dasatinib in combination with typical relapse chemotherapy agents in ovarian cancer cell lines. In addition, we sought to determine if SRC substrates are potential biomarkers to predict the anti-proliferative activity of dasatinib in combination with these cytotoxic agents.
MATERIALS AND METHODS
Drugs
Dasatinib (BMS-354825) was provided by Bristol-Myers-Squibb (Princeton, NJ). Docetaxel, gemcitabine, topotecan and doxorubicin were purchased from Sigma-Aldrich (St. Louis, MO). Docetaxel, gemcitabine, doxorubicin and dasatinib were dissolved in dimethylsulfoxide (DMSO), and topotecan was dissolved in distilled water. Concentrated stock solutions were stored at −20°C.
Ovarian Cancer Cell Lines
The SKOV3 human ovarian cancer cell line was obtained from the American Type Culture Collection (Manassas, VA). The A2780 and IGROV1 human ovarian cancer cell lines were gifts from Drs. Robert Brown and Johnathan Lancaster, respectively. Cell line authentication was performed by DNA genotyping at the University of Colorado DNA Sequencing and Analysis Core (Denver, CO). Prior immunohistochemical assessment of SRC protein expression in the untreated cell lines was lowest in the SKOV3 cells (1+), while the A2780 cellshad heterogeneous 2+ immunostaining, and IGROV1 exhibited diffuse 4+ membrane and cytoplasmic staining. Total SRC (tSRC) protein expression by Western blot analysis demonstrated low relative SRC protein expression in SKOV3 (1.713) and A2780 (0.404), and high relative SRC expression in IGROV1 (2.27).5 The relative expression of the SRC pathway signature on a scale from 0% to 100% was: SKOV3 (38.3%), A2780 (55.1%), and IGROV1 (60.4%).5 Evaluation of the SRC pathway was based on a previously defined SRC pathway gene signature.14
Meso Scale Discovery (MSD) Analysis
MSD analysis was performed for SRC and paxillin. Paxillin is a SRC pathway substrate that is phosphorylated by SRC and FAK upon integrin binding or growth factor stimulation. Anti-tSrc antibody (Cell Signaling Technology, Inc., Danvers, MA, Cat#2108), anti-pSrc pY418 antibody (Invitrogen, Carlsbad, CA, Cat# 44660G), anti-total Paxillin antibody (Cell Signaling Technology, Inc., Danvers, MA, Cat#2542), or anti-phospho Paxillin (Tyr118) antibody (Cell Signaling Technology, Inc., Danvers, MA, Cat#2541) were added at 1ug/ml to goat anti-mouse plates (MSD, Gaithersburg MD), and incubated at room temperature (R.T.) for 1 hour. The plates were washed with TBS/ 0.05% Tween-20 three times and protein lysate from IGROV1 (10 ug total protein) or SKOV3 (20 ug total protein) cells were added and incubated for 2 hours at R.T. Sulfo-TAG (MSD, Cat#R91AN-1) labeled anti-Src antibody (R&D, Cat# AF3389) or a mixture of Sulfo-TAG Streptavidin at 0.5ug/ml (MSD, Cat#R32AD-1) and anti-Paxillin antibody (R&D, Minneapolis, MN, Cat# BAF4259) were then added to the plates and incubated for 1 hour at R.T. after plates were washed. The plates were imaged and analyzed using a MSD Sector Imager 2400 and associated software. The electroluminescence value was normalized to each control and plotted as a percent of control. Statistical analysis was performed using two-tailed unpaired t test.
Cell proliferation assay
Tumor cells were seeded at 2,000 cells/well in a 96-well plate, and allowed to reach 40% confluence over 24 hours. Cells were incubated with each drug at 37°C for 72 hours: docetaxel (range 0.05-25nM); gemcitabine (0.025-25nM); topotecan (2.5-25nM); doxorubicin (25-1000nM); dasatinib (25 -2000nM). Control wells contained RPMI media only. Cell proliferation was measured using the CellTiter 96 AQueous One Solution Cell Proliferation Assay according to manufacturer's recommendations (Promega, Madison, WI). All experiments were performed in duplicate or triplicate. Single-agent dose response curves were constructed and the IC50 computed from the best fitting transition functions (determined by F-statistic) using GraphPad Prism software, version 4.03 (San Diego, CA). The cells were subsequently treated with dasatinib in doublet combination with docetaxel, gemcitabine, topotecan and doxorubicin at fixed-ratio molar concentrations ranging from 0.125 to 4 multiples of the single-drug IC50 doses. The cell proliferation assay and IC50 computations were performed as above. The degree of dasatinib-inhibited cell growth had previously been determined. The dasatinib IC50 values were 74.7 nM in the IGROV1 cell line, 273 nM in SKOV3, and 4100 nM in A2780.5
In vitro drug interaction studies and statistical methodologies
The median effect method, which takes into account the potency of each drug combination and the shape of the dose-response curve, was used to evaluate growth inhibition 5. Composite dose response curves were obtained from three independent experiments and the median effective dose, Dm (equivalent to the IC50) was computed using CalcuSyn software (Biosoft, Cambridge, UK). Drug interaction was assessed by the combination index (CI) method of Chou and Talalay as previously described 5. A synergistic, additive, or antagonistic drug interaction is defined as CI < 1, CI = 1, CI >1, respectively.
RESULTS
SRC protein expression
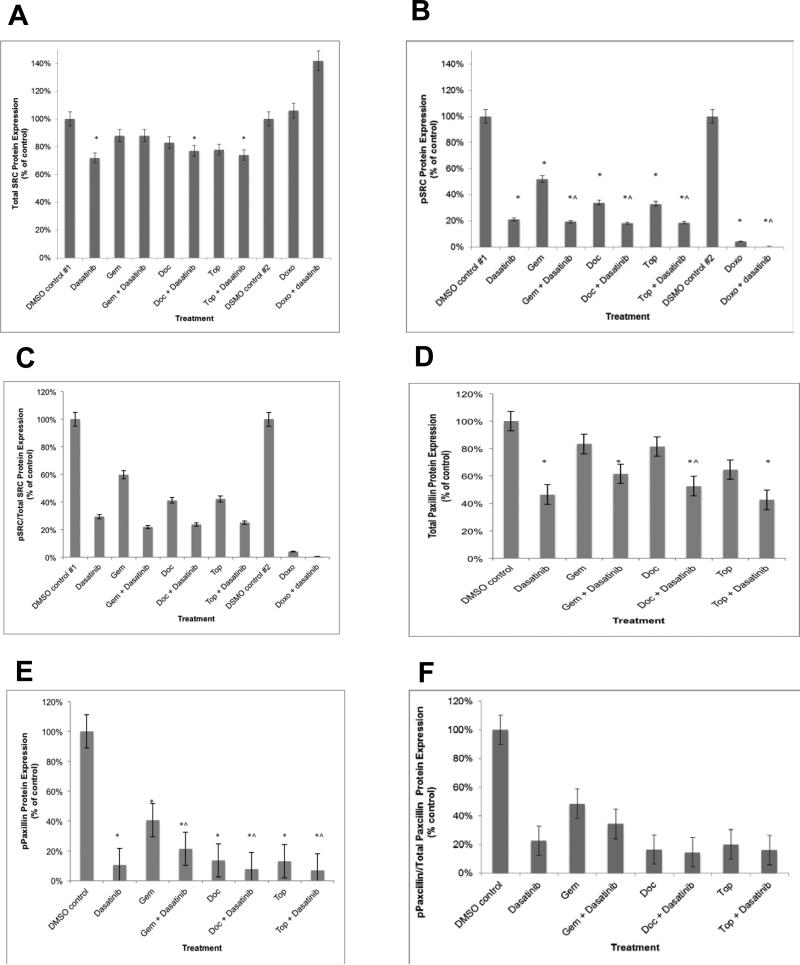
In IGROV1 (high SRC activation) cells, there was a slight but statistically significant loss of tSRC protein expression after dasatinib alone (28%; p=0.03) and in combination with either docetaxel (23%; p=0.05) or topotecan (26%; p=0.04) (Figure 1A). There was a marginal decrease in tSRC after treatment with docetaxel (17%; p=0.07) and topotecan (22%; 0.07). There was no significant change in tSRC levels after treatment with dasatinib combined with any of the agents compared to the respective chemotherapy alone.
Figure 1.
SRC and paxillin protein expression in the IGROV1 cells after treatment with dasatinib and/or chemotherapeutic agents. (A) tSRC protein expression decreased significantly after treatment with single-agent dasatinib, and dasatinib in combination with docetaxel and topotecan. (B). pSRC levels dramatically decreased in the presence of any of the single-agent treatments. (C) The ratio of pSRC/tSRC was reduced after treatment with dasatinib alone (71%), gemcitabine (40%), doxorubicin (96%), docetaxel (59%), and topotecan (58%). (D) t-paxillin protein expression decreased after treatment with dasatinib alone as well as in combination with gemcitabine, docetaxel, or topotecan. (E) p-paxillin levels were dramatically decreased after treatment with any agent. (F) The ratio of p-paxillin/t-paxillin was reduced by with dasatinib alone (77%), gemcitabine (52%), docetaxel (83%), and topotecan (80%). All experiments were performed in duplicate. * p < 0.05 compared to controls. ^ p < 0.05 compared to respective chemotherapy.
In contrast, pSRC levels were dramatically decreased with dasatinib and any of the single-agent chemotherapy agents (48-99%; p=0.002-0.01) (Figure 1B). The ratio of pSRC/tSRC was reduced by 71% with dasatinib alone. Treatment with any of the chemotherapy agents alone resulted in a loss of SRC activation (pSRC/tSRC); gemcitabine (40% inhibited), doxorubicin (66% [data not shown]-96%), docetaxel (59%), and topotecan (58%) (Figure 1C). The addition of dasatinib to chemotherapy resulted in significantly decreased pSRC levels (3-33%; p=0.002-0.04) and pSRC/tSRC (4-38% further inhibition) compared to chemotherapy alone.
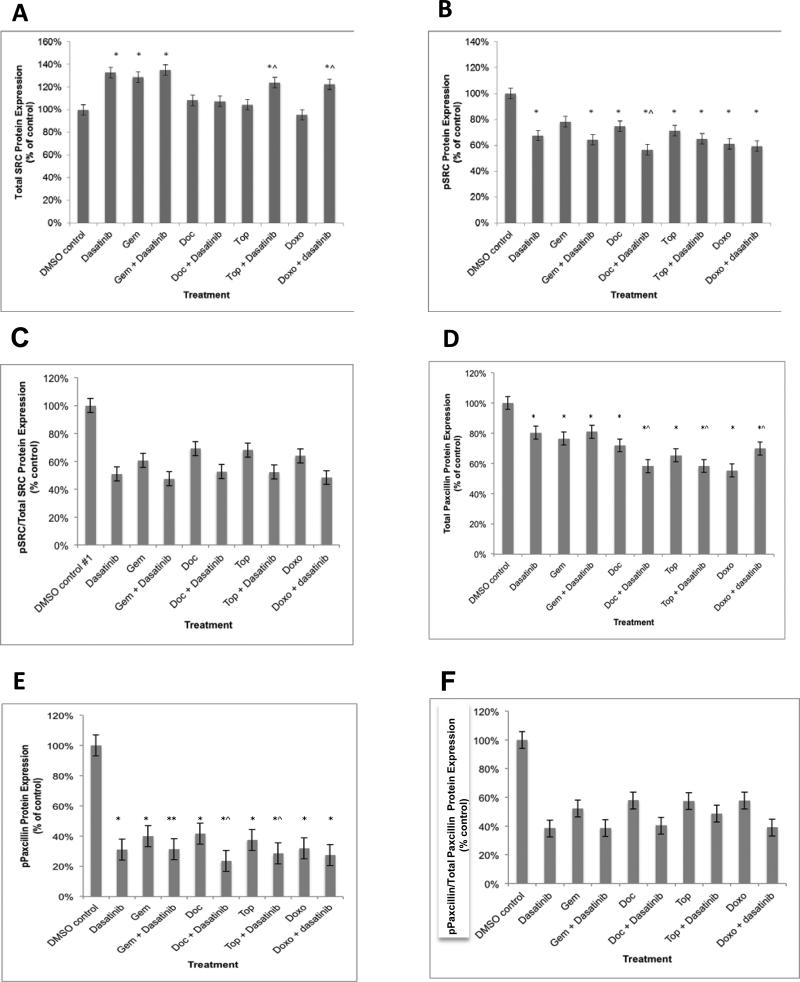
The levels of tSRC protein in SKOV3 cells (low SRC activation) were approximately 40% of what was observed in IGROV1 cells (normalized by total protein). In contrast to IGROV cells, tSRC protein expression in SKOV3 cells did not significantly decrease after treatment with any of the cytotoxics alone or dasatinib doublets (Figure 2A). In fact, the tSRC levels significantly increased after treatment with dasatinib (33%; p=0.01); gemcitabine (29%; p=0.01); and combinations of dasatinib with gemcitabine (35%; p=0.01), topotecan (24%; p=0.01), and doxorubicin (22% p=0.01). There was no significant alteration in tSRC levels after exposure to docetaxel, topotecan, doxorubicin or dasatinib combined with docetaxel. There was a significant decrease in pSRC in cells treated with dasatinib (32%; 0.02); docetaxel (25%; p=0.01); doxorubicin (39%; p=0.001); topotecan (29%; p=0.001); any cytotoxic in combination with dasatinib (35-43%; p <0.001 - 0.02) (Figure 2B). There was a significant reduction in pSRC in cells treated with dasatinib and docetaxel compared to those treated with docetaxel alone (11%; p=0.009). Treatment with single-agent dasatinib, gemcitabine, doxorubicin, docetaxel, and topotecan resulted in inhibition of pSRC/tSRC by 49%, 39%, 36%, 31%, and 32%, respectively (Figure 2C). The addition of dasatinib to chemotherapy reduced the pSRC/tSRC ratio (14-16% further inhibition) compared to chemotherapy alone (Figure 2C).
Figure 2.
SRC and paxillin protein expression in the SKOV3 cells after treatment with dasatinib and/or chemotherapeutic agents. (A) tSRC levels significantly increased after treatment with dasatinib; gemcitabine; and combination dasatinib with gemcitabine, topotecan, and doxorubicin compared to controls. (B) pSRC level was minimally reduced after treatment with dasatinib; docetaxel; doxorubicin; topotecan; and any cytotoxic in combination with dasatinib compared to controls. (C) Treatment with single-agent dasatinib, gemcitabine, doxorubicin, docetaxel, and topotecan resulted in inhibition of pSRC/tSRC by 49%, 39%, 36%, 31%, and 32%, respectively. (D) There was a significant decrease in t-paxillin with all agents. (E) There was a significant reduction in p-paxillin in cells treated with dasatinib as well as docetaxel and topotecan compared to the respective chemotherapy alone. (F) Treatment with single-agent dasatinib, gemcitabine, doxorubicin, docetaxel, and topotecan resulted in inhibition of p-paxillin/t-paxillin by 62%, 48%, 42%, 43%, and 42%, respectively. All experiments were performed in triplicate. * p < 0.05 compared to controls. ^ p < 0.05 compared to respective chemotherapy.
Paxillin protein expression
In IGROV1 cells, a significant loss of t-paxillin protein expression was noted after treatment with dasatinib alone (54%; p=0.03) as well as in combination with gemcitabine (38%; p=0.05), docetaxel (47%; p=0.05), or topotecan (57%; p=0.04) (Figure 1D). There was a significant 29% reduction in t-paxillin levels after treatment of dasatinib combined with docetaxel compared to docetaxel alone.
In contrast, p-paxillin levels were dramatically decreased in the presence of dasatinib and any of the single-agent chemotherapeutics (60-89%; p=0.01) (Figure 1E). The ratio of p-paxillin/t-paxillin was reduced by with dasatinib alone (77%), gemcitabine (52%), docetaxel (83%), and topotecan (80%) (Figure 1F). The addition of dasatinib to chemotherapy resulted in significantly decreased p-paxillin levels (6-7%; p=0.005-0.02) and there was a 2-14% further inhibition of p-paxillin/t-paxillin compared to chemotherapy alone.
The levels of t-paxillin protein in SKOV3 cells were similar to IGROV1, however, p-paxillin levels were 25% lower (normalized by total protein). Total paxillin levels significantly decreased after treatment with any of the cytotoxics in singlet or with any of the dasatinib doublets (20-45%; p=0.02-<0.00001; Figure 2D). There was a significant decrease in p-paxillin with dasatinib (69%; p<0.001); gemcitabine (69%; p<0.001); (docetaxel (58%; p<0.001); doxorubicin (68%; p<0.001); topotecan (63%; p<0.001); any cytotoxic in combination with dasatinib (69-76%; p <0.001) (Figure 2E). There was a significant reduction in p-paxillin in cells treated with dasatinib and docetaxel (18%; p=0.001) and topotecan (9%; p=0.007) compared to the respective chemotherapy alone. Treatment with single-agent dasatinib, gemcitabine, doxorubicin, docetaxel, and topotecan resulted in inhibition of p-paxillin/t-paxillin by 62%, 48%, 42%, 43%, and 42%, respectively (Figure 2F). The addition of dasatinib to chemotherapy reduced the p-paxillin/t-paxillin ratio (8-19% further inhibition) compared to chemotherapy alone (Figure 2F).
Drug interaction assessment for combinations of dasatinib and cytotoxic chemotherapy
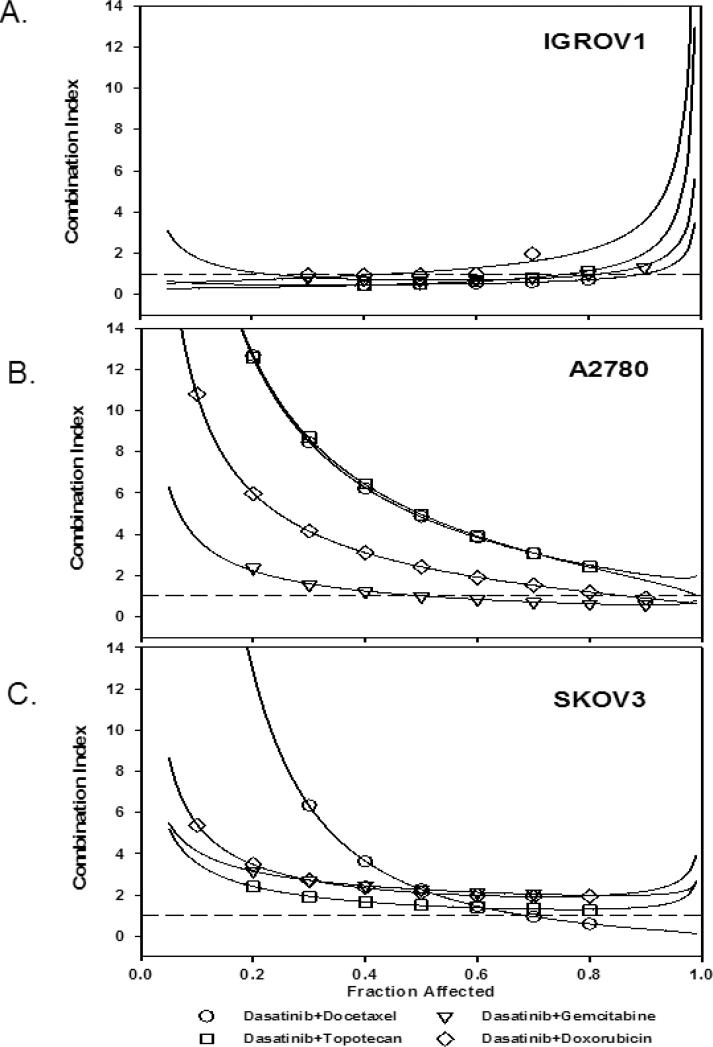
The IC50 results for single agent dasatinib, docetaxel, gemcitabine, topotecan and doxorubicin are detailed in Table 1. There was enhanced cytotoxic activity in the IGROV1 cell line and no enhancement of the cytotoxic effect in the SKOV3 cell line (Table 2) (Figure 3). In the IGROV1 cell line, the addition of dasatinib to docetaxel, gemcitabine or topotecan had a synergistic effect (CI 0.49-0.68), while the addition of dasatinib to doxorubicin had an additive effect (CI 1.08) (Figure 3A). In the A2780 cell line, the addition of dasatinib to docetaxel, topotecan and doxorubicin had an antagonistic interaction (CI 2.42-4.96), while the addition of dasatinib to gemcitabine was additive (CI 1.01) (Figure 3B). Conversely, the addition of dasatinib to all cytotoxics in the SKOV3 cell line produced an antagonistic interaction (CI 1.49-2.27) (Figure 3C).
Table 1.
Potency of single agent therapies based on the concentration required for 50% inhibition of cell proliferation.
| SKOV3 | A2780 | IGROV1 | |
|---|---|---|---|
| Dasatinib IC50 | 273 nM | 4100 nM | 74.7 nM |
| Docetaxel IC50 | 0.194 nM | 0.592 nM | 9.63 nM |
| Gemcitabine IC50 | 4.05 nM | 0.998 nM | 13.6 nM |
| Topotecan IC50 | 12.11 nM | 5.76 nM | 38.8 nM |
| Doxorubicin IC50 | 223.1 nM | 42.9 nM | 324 nM |
Table 2.
The combination index (CI) of dasatinib combined with docetaxel, gemcitabine, topotecan, and doxorubicin. CI <1 is synergistic, CI=1 is additive, CI >1 is antagonistic.
| SKOV3 | A2780 | IGROV1 | |
|---|---|---|---|
| Dasatinib + Docetaxel | 2.26 | 4.83 | 0.486 |
| Dasatinib + Gemcitabine | 2.27 | 1.01 | 0.682 |
| Dasatinib + Topotecan | 1.49 | 4.96 | 0.525 |
| Dasatinib + Doxorubicin | 2.10 | 2.42 | 1.08 |
Figure 3.
Combination index plots (CI) of Chou-Talalay in the (A) IGROV1, (B) A2780, and (C) SKOV3 cell lines. In the IGROV1 cell lines with high SRC pathway deregulation and high SRC protein expression (Panel A), dasatinib combinations are synergistic or additive. In the A2780 cell line (B), which has a dysregulated SRC pathway and low SRC protein expression, the addition of dasatinib to gemcitabine yielded an additive interaction, while other combinations were antagonistic. In the SKOV3 cell line (C), which has a regulated SRC pathway and low SRC protein expression, the addition of dasatinib to chemotherapy agents does not yield a synergistic interaction.
DISCUSSION
Our data demonstrated that dasatinib inhibited the SRC pathway based on reduction of pSRC/tSRC and p-paxillin/t-paxillin ratios in ovarian cancer cell lines regardless of pathway deregulation status. However, the decrease in pSRC/tSRC and p-paxillin/t-paxillin ratios was greatest in the ovarian cancer cells with both high SRC pathway deregulation and high total and pSRC protein expression. Moreover, the enhanced anti-proliferative effects with dasatinib when combined with standard relapse chemotherapy agents was seen only in these ovarian cancer cells with both high SRC pathway deregulation and high total and pSRC protein expression. Conversely, in the A2780 and SKOV3 cell lines the addition of dasatinib to chemotherapy demonstrated an antagonistic effect. This finding is very interesting and indicates a potentially unintended and detrimental effect of adding dasatinib to a cytotoxic agent in certain ovarian cancers. Our data, as well as the lack of activity of SRC inhibitors in clinical trials as a single agent or in combination with chemotherapy in unselected ovarian cancer patients indicate the need to identify biomarkers to direct dasatinib therapy.
pSRC has been identified as potential pharmacodynamic biomarkers of dasatinib activity in human prostate and colorectal cancer cells.1,15 Dasatinib had a dose-dependent anti-proliferative effect, which correlated with the inhibition of tumoral pSRC and SRC activity. The SRC pathway inhibition observed in our study indicated a statistically significant, albeit small, additive effect when chemotherapy was combined with dasatinib. These findings imply that important signaling pathways, other than SRC, drive cell growth in ovarian cancer. These other important signaling pathways are also inhibited by dasatinib. Dasatinib has been shown to inhibit the SRC family kinases (SRC, YES1, FYN, FGR, LCK16, HCK, BLK, LYN17, and FRK) as well as at least four other protein tyrosine kinases including BCR/ABL, KIT, PDGFRB and EPHA218,19. Dasatinib also mediates its action via beclin 1, AKT1, and BCL2 signaling and interacts with several important receptor tyrosine kinases including EGFR, PDGFR, FGFR, MET, IGF1R, ERBB2, and KIT.7,20,21
Our preclinical findings regarding a synergistic anti-proliferative effect of dasatinib combined with chemotherapy is consistent with prior reports.10-12 Preclinical data has demonstrated that dasatinib has additive or synergistic activity in combination with several cytotoxic and biologic agents, providing rationale for combination therapy.22 Several authors have reported preliminary clinical activity and recommended doses of dasatinib in combination with paclitaxel23, gemcitabine24, dacarbazine25, and ixabepilone26. Our phase I trial of dasatinib in combination with paclitaxel and carboplatin demonstrated clinical activity in women with platin-resistant and –sensitive recurrent ovarian cancer.9 However, a randomized placebo-controlled phase II clinical trial (OVERT-1) of another SRC inhibitor, saracatinib, in combination with paclitaxel and carboplatin in patients with platinum-sensitive EOC demonstrated no statistically significant difference in response rates or clinical outcome.27 In addition the combination saracatinib with weekly paclitaxel did not improve 6-month progression-free survival in women with platinum-resistant ovarian cancer.28 While saracatinib and dasatinib target different pathways other than SRC, it is clear from the clinical experience with these agents that biomarkers are needed to predict response and direct therapy. There are currently several phase I and II trials that are actively recruiting and include a wide array of biomarkers.21,29 Some studies are evaluating only tumor protein expression while others are evaluating protein expression in the stromal compartments, plasma, and PBMCs. There are even trials that are being conducting in enriched patient populations. A phase II study of dasatinib in chronic lymphocytic leukemia only includes subjects whose cells exhibit in vitro dasatinib cytotoxicity based on anti-proliferative assays. Another trial only includes patients whose tumors harbor a DDR2 or inactivating BRAF mutation.
Chemotherapy alone also reduced pSRC/tSRC and p-paxillin/t-paxillin ratios in cell lines regardless of SRC pathway status and protein expression. One potential explanation is that the reduction in SRC and paxillin phosphorylation may be secondary to cytotoxicity. In order to confirm this hypothesis, the relationship of SRC and paxillin dephosphorylation to cell viability would need to be assessed. Alternatively, the decreased SRC and paxillin phosphorylation we observed may be secondary to chemotherapy induced global inhibition of cellular signaling.
While we acknowledge the limitations of our study, which include the small number of cell lines evaluated, the limited range of SRC protein expression and the use of in vitro models, the synergistic activity of dasatinib in combination with second-line chemotherapeutic agents in the IGROV1 cells is intriguing. In addition, preliminary phase 1 and 2 data have demonstrated activity of dasatinib combination therapy in participants with breast, prostate, and lung cancers. Further pre-clinical and clinical evaluation of dasatinib in combination with cytotoxics should be considered in recurrent platinum-resistant ovarian cancer given the low response rate to chemotherapy and enhanced anti-tumor activity of combination chemotherapy and dasatinib in select cell lines. However, studies will need to be rationally developed based on biomarker-directed therapy in order to successfully identify women with ovarian cancer who will benefit from dasatinib.
Acknowledgments
This study was supported by the National Cancer Institute grant supporting the Duke Clinical Oncology Research Career Development Award (K12 # CA100639), a research grant from Bristol-Myers-Squibb, and an anonymous philanthropic research fund.
Footnotes
CONFLICT OF INTEREST STATEMENT
One author has received funding from Bristol-Myers-Squibb, GlaxoSmithKline, Sanofi-aventis and Genetech for clinical trials. She has also served on an Advisory Board for (<$5000 annually) Genentech. Another author received funding from Bristol-Myers-Squibb. All other authors declare that there are no conflicts of interest.
REFERENCES
- 1.Luo FR, Barrett YC, Yang Z, et al. Identification and validation of phospho-SRC, a novel and potential pharmacodynamic biomarker for dasatinib (SPRYCEL), a multi-targeted kinase inhibitor. Cancer Chemother Pharmacol. 2008;62(6):1065–1074. doi: 10.1007/s00280-008-0699-5. [DOI] [PubMed] [Google Scholar]
- 2.Lombardo LJ, Lee FY, Chen P, et al. Discovery of N-(2-chloro-6-methyl phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4- ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47(27):6658–6661. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- 3.Budde RJ, Ke S, Levin VA. Activity of pp60c-src in 60 different cell lines derived from human tumors. Cancer Biochem Biophys. 1994;14(3):171–175. [PubMed] [Google Scholar]
- 4.Wiener JR, Windham TC, Estrella VC, et al. Activated Src protein tyrosine kinase is overexpressed in late-stage human ovarian cancers. Gynecol Oncol. 2003;88(1):73–79. doi: 10.1006/gyno.2002.6851. [DOI] [PubMed] [Google Scholar]
- 5.Teoh D, Ayeni TA, Rubatt JM, et al. Dasatinib (BMS-35482) has synergistic activity with paclitaxel and carboplatin in ovarian cancer cells. Gynecol Oncol. 2011;121(1):187–192. doi: 10.1016/j.ygyno.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konecny GE, Glas R, Dering J, et al. Activity of the multikinase inhibitor dasatinib against ovarian cancer cells. Br J Cancer. 2009;101(10):1699–1708. doi: 10.1038/sj.bjc.6605381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le XF, Mao W, Lu Z, Carter BZ, Bast RC., Jr. Dasatinib induces autophagic cell death in human ovarian cancer. Cancer. 2010;116(21):4980–4990. doi: 10.1002/cncr.25426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le XF, Mao W, He G, et al. The role of p27(Kip1) in dasatinib-enhanced paclitaxel cytotoxicity in human ovarian cancer cells. J Natl Cancer Inst. 2011;103(18):1403–1422. doi: 10.1093/jnci/djr280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Secord AA, Teoh DK, Barry WT, et al. A phase I trial of dasatinib, an SRC- family kinase inhibitor, in combination with paclitaxel and carboplatin in patients with advanced or recurrent ovarian cancer. Clin Cancer Res. 2012;18(19):5489–5498. doi: 10.1158/1078-0432.CCR-12-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han LY, Landen CN, Trevino JG, et al. Antiangiogenic and antitumor effects of SRC inhibition in ovarian carcinoma. Cancer Res. 2006;66(17):8633–8639. doi: 10.1158/0008-5472.CAN-06-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagaraj NS, Washington MK, Merchant NB. Combined blockade of Src kinase and epidermal growth factor receptor with gemcitabine overcomes STAT3- mediated resistance of inhibition of pancreatic tumor growth. Clin Cancer Res. 2011;17(3):483–493. doi: 10.1158/1078-0432.CCR-10-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pichot CS, Hartig SM, Xia L, et al. Dasatinib synergizes with doxorubicin to block growth, migration, and invasion of breast cancer cells. Br J Cancer. 2009;101(1):38–47. doi: 10.1038/sj.bjc.6605101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. Inhibition of SRC tyrosine kinase impairs inherent and acquired gemcitabine resistance in human pancreatic adenocarcinoma cells. Clin Cancer Res. 2004;10(7):2307–2318. doi: 10.1158/1078-0432.ccr-1183-3. [DOI] [PubMed] [Google Scholar]
- 14.Bild AH, Yao G, Chang JT, et al. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature. 2006;439(7074):353–357. doi: 10.1038/nature04296. [DOI] [PubMed] [Google Scholar]
- 15.Serrels A, Macpherson IR, Evans TR, et al. Identification of potential biomarkers for measuring inhibition of Src kinase activity in colon cancer cells following treatment with dasatinib. Mol Cancer Ther. 2006;5(12):3014–3022. doi: 10.1158/1535-7163.MCT-06-0382. [DOI] [PubMed] [Google Scholar]
- 16.Lee KC, Ouwehand I, Giannini AL, Thomas NS, Dibb NJ, Bijlmakers MJ. Lck is a key target of imatinib and dasatinib in T-cell activation. Leukemia. 2010;24(4):896–900. doi: 10.1038/leu.2010.11. [DOI] [PubMed] [Google Scholar]
- 17.Choi YL, Bocanegra M, Kwon MJ, et al. LYN is a mediator of epithelial mesenchymal transition and a target of dasatinib in breast cancer. Cancer Res. 2010;70(6):2296–2306. doi: 10.1158/0008-5472.CAN-09-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang Q, Jorgensen C, Pawson T, Hedley DW. Effects of dasatinib on EphA2 receptor tyrosine kinase activity and downstream signalling in pancreatic cancer. Br J Cancer. 2008;99(7):1074–1082. doi: 10.1038/sj.bjc.6604676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montero JC, Seoane S, Ocana A, Pandiella A. Inhibition of SRC family kinases and receptor tyrosine kinases by dasatinib: possible combinations in solid tumors. Clin Cancer Res. 2011;17(17):5546–5552. doi: 10.1158/1078-0432.CCR-10-2616. [DOI] [PubMed] [Google Scholar]
- 20.Nautiyal J, Majumder P, Patel BB, Lee FY, Majumdar AP. Src inhibitor dasatinib inhibits growth of breast cancer cells by modulating EGFR signaling. Cancer Lett. 2009;283(2):143–151. doi: 10.1016/j.canlet.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 21.Puls LN, Eadens M, Messersmith W. Current status of SRC inhibitors in solid tumor malignancies. Oncologist. 2011;16(5):566–578. doi: 10.1634/theoncologist.2010-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Araujo J, Logothetis C. Dasatinib: a potent SRC inhibitor in clinical development for the treatment of solid tumors. Cancer Treat Rev. 2010;36(6):492–500. doi: 10.1016/j.ctrv.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fornier MN, Morris PG, Abbruzzi A, et al. A phase I study of dasatinib and weekly paclitaxel for metastatic breast cancer. Ann Oncol. 2011;22(12):2575–2581. doi: 10.1093/annonc/mdr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong DS, Choe JH, Naing A, et al. A phase 1 study of gemcitabine combined with dasatinib in patients with advanced solid tumors. Invest New Drugs. 2013;31(4):918–926. doi: 10.1007/s10637-012-9898-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Algazi AP, Weber JS, Andrews SC, et al. Phase I clinical trial of the Src inhibitor dasatinib with dacarbazine in metastatic melanoma. Br J Cancer. 2012;106(1):85–91. doi: 10.1038/bjc.2011.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herbolsheimer P, Kapoor R, Smith KL, et al. Phase I trial of dasatinib and ixabepilone in patients with solid tumors. Invest New Drugs. 2013;31(1):92–98. doi: 10.1007/s10637-012-9805-y. [DOI] [PubMed] [Google Scholar]
- 27.Poole C, Lisyanskaya A, Rodenhuis S, et al. A randomized phase II clinical trial of the SRC inhibitor saracatinib (AZD0530) and carboplatin 1 paclitaxel (C1P) versus C1P in patients with advanced platinum-sensitive epithelial ovarian cancer. Ann Oncol. 2010;21(8):313. abstract 9720. [Google Scholar]
- 28.McNeish I, Ledermann JA, Webber LC, et al. A randomized placebo-controlled trial of saracatinib (AZD0530) plus weekly paclitaxel in platinum-resistant ovarian, fallopian-tube, or primary peritoneal cancer. J Clin Oncol. 2013:31. doi: 10.1093/annonc/mdu363. abstract 5514. [DOI] [PubMed] [Google Scholar]
- 29.Clinical Trials.gov. 2013. Accessed January 22, 2013.