Abstract
In birds, biological clock function pervades all aspects of biology, controlling daily changes in sleep: wake, visual function, song, migratory patterns and orientation, as well as seasonal patterns of reproduction, song and migration. The molecular bases for circadian clocks are highly conserved, and it is likely the avian molecular mechanisms are similar to those expressed in mammals, including humans. The central pacemakers in the avian pineal gland, retinae and SCN dynamically interact to maintain stable phase relationships and then influence downstream rhythms through entrainment of peripheral oscillators in the brain controlling behavior and peripheral tissues. Birds represent an excellent model for the role played by biological clocks in human neurobiology; unlike most rodent models, they are diurnal, they exhibit cognitively complex social interactions, and their circadian clocks are more sensitive to the hormone melatonin than are those of nocturnal rodents.
Each morning, and especially in the spring, we are greeted by a cacophony of small birds singing a dawn chorus. In eastern North America, spring mornings are sometimes defined by the merry roundelay of the American robin, Turdus migratorius, the varied staccato whistles of the Northern cardinal, Cardinalis cardinalis, the hey-hey of the white-breasted nuthatch, Sitta carolinensis, or even the cheery chirping of the introduced house sparrow, Passer domesticus. In the backdrop, we may hear the doleful ooh-wah-hoo-hoo of the aptly named mourning dove, Zenaida macroura, as the bass section above croons with the honking of migrating Canada geese, Branta canadensis. There is no particular order of who sings or who calls first, and the orchestration is peripatetic at best, seemingly random, although many of these garden songsters are reacting to each other’s songs. And yet, there is a coordination of the rhythm and timbre of this dawn chorus. These birds all possess an internal biological clock that is coincidentally entrained to the identical environmental signal, the rising of the morning sun, and, in turn, these internal clocks are tuned to the expression of clocks by their intraspecific and extra-specific neighbors.
While these appear to be the melodious embrace of the warming sun, they are, in fact, a cold war, defining territory for breeding and foraging in anticipation of reproductive success [98,151]. In no other group of animals are the seasonal changes in reproductive function so obvious to the casual observer. We hear them stake their claims. We see them build their nests, incubate the eggs, and raise and fledge their young.
At certain times of year, small songbirds fatten for their annual migrations and, at certain times of day, dusk usually, become increasingly agitated as they gather for their vernal and autumnal treks to breeding and wintering grounds [67,68]. These birds typically eschew their nightly drifts into slumber during this time, sleeping little or not at all, a phenomenon called Zugunruhe, as they migrate during the night, avoiding the gauntlet of diurnal predators as they cross vast areas of our continent.
Each of these processes and more are strictly timed to a time of day and to a time of year [31]. They are not restricted to eastern North America, either, as these processes are repeated time and time again throughout the world, albeit at different times of year, depending on the latitude and local environment [89,90,91]. The question that arises is, “Why do birds so strictly time so many of their behavioral and physiological functions, and how do they accomplish it?” In essence, the child-like question, “Why does the sparrow sing on spring mornings?”, is also a scientific question that is beginning to be answered, and these likely entail an understanding of the biological clock or clocks that underlie all rhythmic processes. Specifically, understanding of the molecular, physiological and behavioral mechanisms underlying the temporal coordination of these complex processes and behaviors in birds will tell us more about human chronobiology as well, because like humans and unlike the standard laboratory rodent models for biological clocks, birds exhibit a complex orchestration of circadian behavior that controls daily patterns of sleep: wake, visual sensitivity, cognition and social behavior. Further, study of the mechanisms underlying annual cycles of reproduction, migration and metabolism in birds will provide clues to anticipated ecological changes due to climatic disruption. In essence, birds are images in our own mirrors, and we should pay attention to them more than current biomedical science might prefer.
Biological Rhythms and the Clocks that Control Them
Biological rhythms and the endogenous clocks that control them are fundamental properties of nearly all living organisms, ranging from cyanobacteria to humans [12]. As diverse as the organisms that express biological rhythms, the formal properties of these rhythms are remarkably conserved [124]. These biological rhythms are functionally tied to environmental cycles they estimate; of these, we will concentrate in this review on two-circadian rhythms and circannual cycles.
The daily 24-hour cycle of day and night imposes a rhythmic cascade of positive and negative selective pressures on nearly all organisms on Earth [124]. Daily light cycles provide the energy for photosynthesis, to warm water to a consistently liquid state and to maintain ambient temperatures for life. It also provides daily cycles in deleterious teratogenic, carcinogenic and desiccating wavelengths. It is therefore no surprise that most free-living organisms, if not all, have adapted to these cycles through the expression of endogenously generated circadian (circa=approximately; dian = a day) oscillations that entrain to local time through the process of entrainment. Rhythmic processes cannot be identified as circadian unless they are experimentally observed to persist for at least 1 or 2 cycles, preferably more, when the organism in question is experimentally placed in constant environmental conditions of either constant darkness (DD) or constant dim light (dimLL) (Constant high light, LL, may have other effects, frequently abolishing circadian rhythms altogether and/or damaging photoreceptive elements in the system [3]). In fact, many circadian rhythms persist for weeks, months or years in constant environmental conditions.
In this scenario, organisms will repeatedly express patterns of behavior, physiology or biochemical processes with a period, τ, of close to but rarely exactly 24 hrs (Figure 1). These endogenously driven rhythms must then be entrained to the relevant environmental cycle, typically the light: dark cycle (LD) of day and night, such that the internal phase, φi, of the organism’s clock corresponds appropriately to the external phase, φe, of the LD cycle. Thus, a diurnal bird’s locomotor activity pattern entrains to the LD cycle so that activity onset φi corresponds approximately to dawn φe, maintaining a stable phase relationship, ψie.
Figure 1.
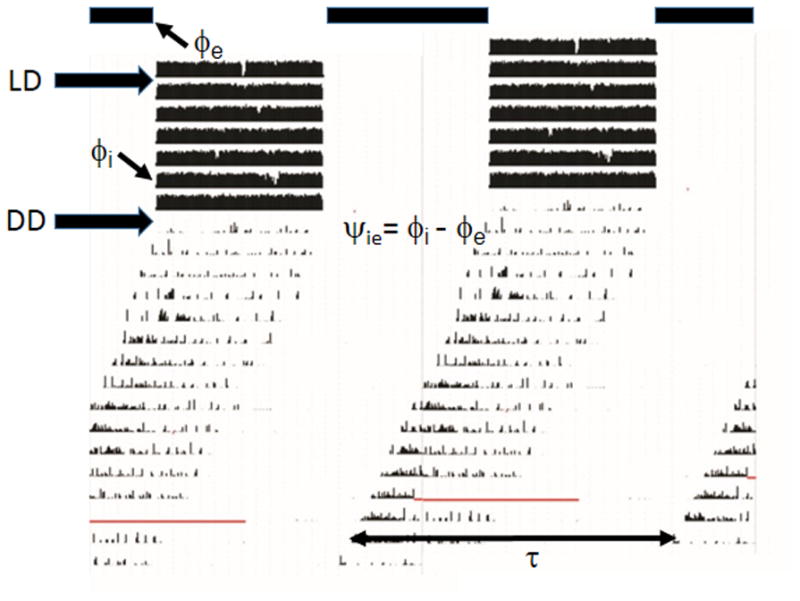
Actogram of locomotor activity from a single zebra finch, Taeniopygia guttata. The top bars indicate the times during which lights are off (black) vs on (white). These are plotted in a 48hr timespan in order to “double-plot” the data. The time off lights on, φe, indicated by the arrow, is a phase reference determined by the investigator. The internal phase, φi, is determined to be the activity onset. The relationship between φi and φe is called ψie. This relationship may change depending on the time of year and physiological condition of the bird. The internal period, τ, is indicated here as the average interval between activity onsets.
Similarly, annual environmental cycles correspond to circannual rhythms expressed by many organisms when placed experimentally in constant photoperiods of 12hrs of light and 12 hrs of dark (LD12:12) or another constant photoperiod [67,68]. Under these conditions many organisms, including birds, will express cycles of approximately 365 days. These in turn are believed to be entrained to the annual cycle by changes in photoperiod [67] and/or a physiological proxy, such as the duration of the hormone melatonin (see below). Circannual cycles are not as well understood as are circadian rhythms, but they are likely linked physiologically [69, 71].
The role of the circadian clock in annual cycles has been known for some time [24, 51,86,106, 130]. In many species of birds, exposure to photoperiods of longer than 11.5 hrs/day results in the rapid induction of the hypothalamo-hypophysial-gonad axis, causing development and growth of testes and ovarian follicles. Although there are differences among species of birds and between birds and other taxa, neither the absolute length of the photoperiod, length of the scotoperiod (the duration of the dark phase) or their ratio is the proximal causes of gonadal induction. Rather, it is the circadian φ at which light impinges on photoreceptive elements that causes reproductive changes. For example, male Japanese quail, Coturnix japonica, and white-crowned sparrows, Zonotrichia leucophys, which are maintained in LD 6:18 will exhibit regressed testes. However, if the last hr. of the 6 hr. photoperiod is extended each long night to a specific “photoinducible phase”, φpi, usually 11–12hrs following the onset of the short photoperiod, reproductive activity is commenced. Thus, birds exposed to LD 6:18 or L5: D1: L1: D17 (a single 1hr light pulse interrupting the night) will retain regressed gonads, but if the 1hr pulse occurs 5hrs later (L5:D6:L1:D12), gonads will recrudesce [48, 106, 131,132,161]. The same total amount of light (and dark) is present for each 24hrs, but the effect is dramatically different. It is the timing of light coinciding with an internal process that induces or prevents reproductive activity, suggesting a circadian clock underlies photoperiodic time measurement. Nanda and Hamner [116] had shown this to be the case in plants through an elegant series of experiments in which soy bean plants exposed to a 6hr photoperiod coupled with scotoperiods of varying lengths that were multiples of a 24hr cycle (e.g. LD6:18; LD6:42 or LD6:66) did not flower. However, when plants were exposed to 6hr photoperiods followed by scotoperiods of lengths that did not resonate with 24hrs (e.g. LD6:6; LD6:30 or LD6:54) flowering was observed. Similar studies in several species of birds showed that seasonal changes in both gonadal recrudescence and regression are regulated by a circadian clock [50,51,89].
These and other observations have led to two competing models for a role of circadian clocks in photoperiodic time measurement. In one, the “external coincidence model” proposed by Bünning [24] suggests that light has two complementary roles. First, light entrains the circadian clock with a stable ψie such that the photoinducible phase, φpi, is also maintained with a approximately 11.5 hrs following the beginning of the photoperiod (lights on). When stable ψ light coincides with the φpi either because the photoperiod is long under natural conditions or through exposure to an experimental light pulse, the reproductive axis is induced. The competing notion, the “internal coincidence model” proposed by Pittendrigh [cf. 124], stems from the observation that many circadian systems behave as if they are composed of at least two oscillators, one entrained to dawn and the other to dusk. In the internal coincidence model the phase relationship between the dawn oscillator to the dusk oscillator, ψdawndusk, induces seasonal changes in reproduction. As we will see below, each of these models has support from different experimental systems in birds. However, until specific structures and/or molecules become associated with these models, they are essentially untestable at the physiological level.
Molecular Genetics of Circadian Clock Function
Circadian rhythms are regulated by a highly conserved set of genes, collectively called “clock genes”, whose products are believed to dynamically interact to elicit rhythmic patterns of transcription, translation, biochemical and physiological processes, and behavior (Figure 2) [12,46]. In animals ranging from Drosophila to humans, the central core of this gene network can be broadly characterized as “positive elements” clock and bmal1 and “negative elements” Period 1 (Per1), period 2 (per2), period 3 (per3) and the cryptochromes cryptochrome 1 (cry1) and cryptochrome 2 (cry2). In contrast to mammals, birds do not express a per1 and have been shown to only express only per2 and per3 [5, 6, 156,157,163]. Clock and bmal1 are transcribed and then translated in the cytoplasm, where they dimerize and reenter the nucleus and activate transcription of the negative elements through the activation of E-box promoter elements. The pers and crys in turn are transcribed and translated in the cytoplasm, where the PER proteins are targeted for proteosomal proteolysis by a series of protein kinases, most notably casein kinase 1ε (CK1ε) and CK1δ. This process slows the accumulation of the cytoplasmic PER and thereby increases the period of the molecular cycle. In the cytoplasm, PER and CRY proteins form oligomers that reenter the nucleus and interfere with the CLOCK/BMAL1-mediated activation. A secondary cycle involving two genes containing E-box promoters, ReverbA and rorA, amplify the cycle by activating and inhibiting bmal1 transcription respectively. Disruption and/or knock-out of these genes’ action has profound effects on the expression of circadian rhythms in animals in which these technologies are possible (i.e. mice and Drosophila) ranging from changes in period to arrhythmicity.
Figure 2.
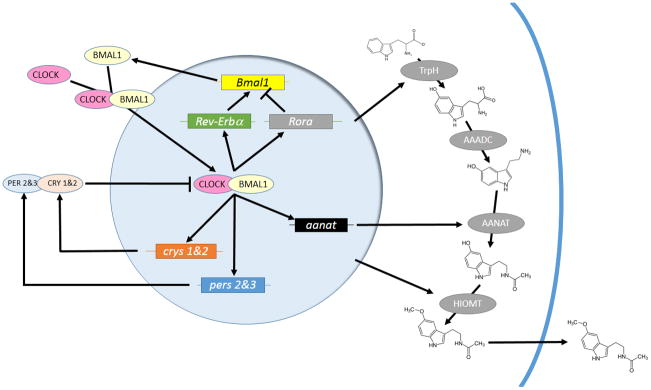
Schematic of the molecular clockworks regulating circadian patterns of melatonin biosynthesis in a pinealocytes or retinal photoreceptor. Positive elements CLOCK and BMAL1 enter the nucleus and activate expression of genes whose promoters contain an E-Box. Among these are the negative elements period 2 & 3 (pers 2&3) and cryptochromes 1& 2 (crys 1&2), Rev-Erbα and Rora, which form a secondary loop regulating Bmal1 transcription, and output, clock-controlled genes such as arylalkylamine-N-actyltransferase (aanat). The pers and crys are translated, form heterodimers with other components, such as the casein kinases, and reenter the nucleus to interfere with CLOCK/BMAL1 activation. Melatonin biosynthesis pathways are indicated on the right. Amino acid tryptophan is converted to 5-hydroxytryptophan by tryptophan hydroxylase (TrpH). Aromatic amino acid decarboxylase (AAADC) then converts 5-hydroxytryptophan to 5-hydroxytryptamine (5HT; serotonin), Then, during the night, AANAT converts 5HT to N-acetylserotonin, a substrate for hydroxyindole-O-methyltransferase (HIOMT), which produces melatonin itself. Presumably, melatonin diffuses out of the cell at this time, although a release mechanism may exist.
Unfortunately, these technologies are not routinely available in birds just yet. However, there are several observations that tentatively link clock gene expression with avian rhythmic behavior. Noting population studies in human populations of single nucleotide polymorphisms (SNP) in the clock gene’s 3′-UTR have revealed differences in times of sleep and sleep duration, Steinmeyer et al. [137] have shown weak (5.9%) to moderate (46.7%) associations between awakening time of blue tits, Cyanistes caeruleus, with single nucleotide polymorphisms (SNPs) in period2 and CK1ε. However, these studies were conducted in free-living tits using radio frequency transponders under semi-natural conditions, not under DD or dimLL, so that these effects cannot distinguish circadian clock effects from light-induced effects or effects on homeostatic regulation of sleep. Other studies have focused on allelic differences in C-terminal polyglutamine repeats in the CLOCK protein in breeding and migratory patterns of barn swallows, Hirundo rustica, suggesting negative selection on “deviant” genotypes [27]. However, other studies in several different swallow species in the genus Tachycineta failed to show similar associations [42].
Extraocular Photoreception
In addition to photoreceptors in the lateral eyes shared by all vertebrate classes, it has been known for some time that non-mammalian vertebrates express functional photopigments within the brain that are critical for entrainment of both circadian rhythms and circannual cycles. In birds, these reside in the pineal gland, the preoptic area, the lateral septum, and the tuberal hypothalamus. Early studies by Benoit in the 1930’s showed that domestic ducks, Anas platyrhynchos, that had been blinded (enucleated) continued to exhibit reproductive responses to changing photoperiod [13], but work by Menaker and colleagues in the 1960’s and 70’s in passerine birds clearly showed that the eyes are not necessary for circadian entrainment [99,102] or seasonal control of reproduction [105,107,108,142]. In a classic series of experiments, Menaker’s group demonstrated that enucleated house sparrows could entrain to a series of LD cycles of dimmer and dimmer illuminances. Once birds were no longer capable of entrainment, they showed that the responsible photoreceptor resided inside the head by simply plucking feathers, and entrainment was reinstated. They then blocked entrainment by injecting India ink beneath the scalp [105].
Subsequent research has now identified at least 4 distinct structures within the brain that are functionally photoreceptive, containing several opsin-based photopigments and photoisomerases [4,53,94,99]. These include the pineal gland, which expresses a pineal-specific opsin, pinopsin [5, 99,100,120], as well as melanopsin (OPN4) [4, 5, 36] and iodopsin (OPN1) [5, 99] and whose photoreceptive function will be discussed further below. In addition, neurons within the preoptic area express VA (vertebrate ancient) opsin [38,39,135], and project to the tuberal hypothalamus, while the tuberal hypothalamus expresses a plethora of photoreceptive cells that appear to be divergent among avian species. For example, in domestic turkeys, Meleagris gallopavo, melanopsin-expressing cells in the premammillary nucleus (PMM) in the dorsal tuberal hypothalamus project directly to the median eminence [79,80,87]. In Japanese quail, Coturnix coturnix, CSF-contacting neurons in the mediobasal hypothalamus (MBH), which may be homologous to the PMM, express both OPN4 and neuropsin (OPN5) [111,112]. In house sparrows, neurons within the arcuate nucleus express rhodopsin (OPN2) itself, in addition to OPN4 and OPN5 [52,149]. While it is not clear whether or which opsin-based photopigments are expressed in the lateral septal organ, illumination of this area of cerebrospinal fluid contacting neurons elicits a physiological response [93,94]. Further, it is not clear whether each of these photoreceptive organs and/or their photopigments subserve mutually exclusive physiological processes or whether these overlap in their functions. In addition to opsin-based photopigments, all animals express flavin-based cryptochromes [145]. While cryptochrome is the major photopigment responsible for photoentrainment in Drosophila [47], the multiple cryptochromes expressed by vertebrates have not been established as photoresponsive molecules. Even so, in birds, the role of cryptochromes in celestial orientation is light-dependent (see below).
The Pineal Gland is a Master Pacemaker for Avian Circadian Clocks
Searching for the location of the intracranial, extraretinal photoreceptors, Gaston and Menaker [60] surgically removed the pineal gland (PINX) from house sparrows. While the birds retained their ability to entrain to LD, they became arrhythmic when placed in DD, demonstrating that the pineal gland is necessary for self-sustained circadian rhythmicity. However, the data also showed that the pineal gland is part of a system of circadian clock components, since PINX sparrows could anticipate the time of lights on in an LD cycle and because birds only gradually became arrhythmic over 5–15 days following transfer from LD to DD. Further, the effect of PINX is not universal among avian species. PINX of European starlings, Sturnus vulgaris, results in a range of behavioral changes ranging from arrhythmicity akin to those seen in house sparrows to slight disruption of behavioral locomotor rhythmicity [69]. Circadian rhythms of locomotor behavior in columbiform and galliform birds are little or not affected at all by PINX [45,143].
Even so, the pineal gland represents both the capacity for rhythmicity and time of day. In an elegant experiment, Zimmerman and Menaker [167] transplanted pineal glands from two groups of house sparrows into the anterior chambers of the eye of PINX, arrhythmic sparrows maintained in DD. The first group of donor birds were entrained to an early LD cycle, with lights on at midnight, while the second set of donors were entrained to a late LD cycle, with lights on at 11 AM. Transplantation restored circadian rhythms to both groups of recipients within one day. Moreover, birds that received pineal glands from early donors, exhibited an early φi while the recipients of late donor pineal glands exhibited a late φi. Thus, the pineal gland is not only necessary for circadian rhythmicity in these birds, but it contains a correlate that confers time of day to recipient birds.
Importantly, the data also suggested that the pineal gland must affect behavior through the secretion of a hormone, because one day is not thought to be sufficient for re-innervation of target tissues, wherever they are. That hormone was known even then to be the indoleamine melatonin from earlier work of Lerner and later of Axelrod, Klein and their co-workers [cf. 85], who explored the biochemical basis for melatonin biosynthesis in the pineal gland of the chick, Gallus gallus domesticus. Research from a large number of investigators have shown that pinealocytes, the photoreceptive, secretory cells of the avian pineal gland, take up the amino acid tryptophan, which is converted to 5-hydroxytryptophan by tryptophan hydroxylase (TrH; EC 1.14.16.4) [37] and then decarboxylated to produce serotonin (5HT) by aromatic L-amino acid decarboxylase (AAADC; EC 4.1.1.28). During the night in LD and subjective night in DD, 5HT is converted to N-acetylserotonin (NAS) by arylalkylamine (or serotonin)-N-acetyltransferase (AANAT; EC 2.3.1.87) [19]. NAS is then converted to melatonin by hydroxyindole-O-methyltransferase (HIOMT; EC 2.1.1.4) [146]. The genes encoding each of these enzymes have been isolated, cloned and sequenced in several avian species. In chick, at least, TrH, AANAT and HIOMT are regulated by both the molecular clockworks within the pinealocytes and directly by light at the transcriptional, translational and post-translational levels, so that the enzymatic regulation of pineal melatonin is a dynamic, rhythmic process [85].
Avian pineal glands contain the circadian clockworks and photoreceptors to generate circadian patterns of melatonin biosynthesis in vitro as well as in vivo, which can be entrained to LD cycles directly [20]. Pineal tissue and pinealocyte cultures express circadian patterns of AANAT activity [20,83,148] and melatonin efflux [139] such that levels are high during the night and low during the day in LD. These rhythms persist for 4–10 days in DD before damping to arrhythmicity. Exposure to light has three effects on cultured pineal rhythms: 1) Light inhibits melatonin biosynthesis. 2) Light increases amplitude and decreases damping, and 3) light phase-shifts the clock within pineal cells [165]. In vivo, the avian pineal gland is innervated by post-ganglionic sympathetic nerves, and receives daily and circadian input through release of norepinephrine (NE) during the day and subjective day. Administration of NE to chick pineal glands in vivo and in vitro has two effects on pineal melatonin rhythms: 1) NE inhibits melatonin biosynthesis. 2) NE increases amplitude and decreases damping, but does not phase-shift the pineal circadian clock [28,32,166].
Using cDNA microarrays of chick pineal gland in vivo and in vitro and retinae in vivo, we have shown that many clock genes are expressed rhythmically in a fashion consistent with circadian patterns of clock gene expression in other model systems, such as Drosophila and mice [5,6,81]. In vivo, bmal1, bmal2 and clock are expressed predominantly during late subjective day/early subjective night, while putative negative elements per2 and per3 (per1 is not present avian genomes) are expressed during the subjective night to early subjective day. Interestingly, cry1 and cry2 are expressed during the early to mid-subjective day. In addition, transcripts associated with melatonin biosynthesis and photoreception are expressed rhythmically in patterns that are consistent with previous studies described above. Importantly, the 5′-flanking region of the chicken aanat gene contains an E-box [37], indicating the CLOCK-BMAL1 dimer may regulate aanat expression in the late subjective day/early subjective night. Transfection of COS cells with a reporter construct expressing luciferase and the chicken aanat promoter region in the presence of either chicken or human BMAL1 and CLOCK increases luciferase activity [37]. Mutation of the E-box region dramatically decreases luciferase activity, suggesting that binding of the aanat E-box by CLOCK/BMAL1 heterodimers is a critical component of the regulation of melatonin biosynthesis [37, 83]. Interestingly, in pineal glands, but not retinae, many transcripts associated with cytokine biosynthesis, immune function and lymphopoeisis are both highly and rhythmically expressed in chick pineal gland. These data are similar to those obtained by in situ hybridization in Japanese quail [156,157].
Although in situ hybridization for clock genes suggest that these genes are enriched in the pineal gland, retinae and other structures associated with clock function [5,6,152], quantitative real-time PCR for these transcripts indicate they are widely expressed in other parts of the brain and in the periphery. Daily and circadian patterns of cry1, per3 and bmal1 expression were observed in chick telencephalon, diencephalon and optic tectum in the brain, as well as in heart and liver. PINX and EX of chicks decreases the amplitude of these clock gene rhythms but does not completely abolish them [82].
Interestingly, the photoreceptors in the retinae of the lateral eyes also synthesize and release melatonin in many vertebrate species. In fact, in Japanese quail and domestic pigeon, Columba livia, the retinae release almost as much melatonin into the systemic circulation as does the pineal gland and removal of this source by enucleation (EX) or retinectomy in addition to PINX results in arrhythmic circadian locomotor behavior, similar to the effects of PINX alone in passerine birds [35,45,143]. Thus, the variability of the effects of PINX among birds may in part be due to this retinal component in some species and that it is not the pineal per se but rhythmic melatonin that is important for circadian locomotor behavior. To punctuate this view, rhythmic administration of melatonin to PINX house sparrows and European starlings or to EX/PINX pigeons [35, 71,74,96,150] restores a daily pattern of locomotor behavior. This synchronization of locomotor behavior by rhythmic melatonin administration represents entrainment of circadian clockworks in the PINX bird, since melatonin administration in a T-cycle different from 24hrs results in systematic changes in the phase relationship (ψ) of melatonin to the onset of locomotor activity [71,74].
Sites of Melatonin Action in Birds
In the 1980’s and 90’s, high affinity melatonin receptor binding using the radiolabeled agonist 2[125I]-iodomelatonin (IMEL) [144] revealed high densities of IMEL binding in retinal, retinorecipient structures and visual integrative structures in the avian brain as well as peripheral tissues [43,129] (Figure 3). Binding affinity studies indicated kD’s in the pM range with high specificity for melatonin itself. Brain structures that bind IMEL included retinorecipient structures in the visual suprachiasmatic nucleus (vSCN) of the circadian system, the ventrolateral and dorsal geniculate nuclei of the thalamofugal visual pathway, the optic tectum of the tectofugal pathway, and the nucleus of the basal optic root (nBOR) or the accessory optic pathway. In all species, integrative structures of the tectofugal pathway such as nucleus rotundus (Rt) and the ectopallium (Ep) also bind IMEL [33,129]. In some but not all species, hyperpallial structures, including the visual Wulst are sites of IMEL binding. In male passerine birds but not females, structures associated with bird song learning and control also revealed high affinity IMEL binding [58,153]. These will be discussed in more detail below.
Figure 3.
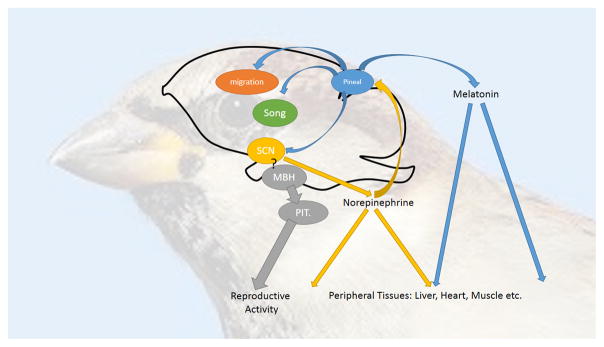
A chorus of clocks. The coordination of circadian and circannual patterns of behavior and physiology is the composite of multiple circadian oscillators and their interactions. At the core are circadian pacemakers in the pineal gland and suprachiasmatic nuclei (SCN), whose interactions maintain each other’s stability and self-sustainment through mutually inhibitory activity. Each of these pacemakers may influence downstream processes through entrainment of circadian oscillators controlling and residing in brain (e.g. song, vision and migration) and peripheral (e.g. liver, heart, muscle) function. In birds, regulation of primary gonadal activity has been separated from this circadian system with circadian oscillators residing in the mediobasal hypothalamus itself. There is little evidence that these oscillators are affected by pineal melatonin, but it is an open question whether SCM oscillators influence MBH function in the circadian and seasonal control of reproduction.
Reppert and colleagues were able to isolate and clone genes encoding two high affinity melatonin receptors; these were designated the Mel1A and Mel1C receptors [128]. Independent work isolated partial sequences encoding an ortholog of the Mel1B receptor in the same year [95]. Subsequent work has confirmed in birds that there are at least three melatonin receptors, the Mel1A, Mel1B and the Mel1C receptors [126]. Pharmacologists working with mammals have named the Mel1A and Mel1B receptors MT1 and MT2 receptors respectively [44]. However, mammals do not express the Mel1C receptor sub-type, and the Mel1A and Mel1B receptors have not been fully characterized pharmacologically in birds. In this review, we will employ the original nomenclature, the Mel1A, Mel1B and the Mel1C receptors. All three melatonin receptor sub-types represent 7-transmembrane domain, GTP-binding protein structures and all three are in the Gi GTP-binding protein category, although some cross-talk with Gq has been documented [126]. The distributions of these three receptor sub-types are not uniform in chicks, zebra finches and house sparrows. The Mel1A receptor predominates in central nervous neurons and peripheral tissues [82,117], while the Mel1B receptor is expressed in inner retinal neurons and photoreceptors as well as other central nervous neurons [117]. In passerines, the Mel1B receptor is the major receptor sub-type in song control nuclei, but the other 2 are expressed as well [17,78]. The Mel1C receptor, on the other hand, predominates in non-neuronal elements of the central nervous system [128]. Culture studies with chick astrocytes [2] show 95–100% diencephalic astrocytes express the Mel1C receptor, while an overlapping 5–10% expresses Mel1A. Astrocytes do not appear to express Mel1B. Intriguingly, neither IMEL binding nor strong melatonin receptor expression is present in the tuberal hypothalamus and/or hypophysis [33,128,129].
Avian circadian organization
Elimination of the pineal gland’s photic and neural inputs results in damped circadian patterns of melatonin release, which also can be restored by rhythmic administration of light and/or NE [28, 139, 165, 166]. Conversely, removal of the pineal pacemaker in passerine birds at least results in damped rhythmicity in behavioral and physiological output, suggesting whatever is left represents a damped circadian oscillator capable of entraining to light [60]. Whatever is left is likely at least in part the avian homologue for the mammalian hypothalamic nucleus (SCN) [25,26], which is a master pacemaker for mammalian circadian organization [cf. 110].
In birds, two sets of structures have been associated with SCN function: the medial suprachiasmatic nuclei (mSCN) and the visual suprachiasmatic nuclei (vSCN) [25,26,30, 162]. These structures are connected via neuronal projections and are contiguous in terms of their cellular populations, especially in the distribution of astrocytes. The vSCN, but not the mSCN, expresses metabolic and electrical rhythmicity and receives retinohypothalamic (RHT) input. Further, the vSCN, but not the mSCN, contains melatonin receptor binding [33,96,128,129], and exogenous melatonin inhibits metabolic activity in the vSCN [96] but not in the mSCN. Finally, light activates c-fos expression in the vSCN, but not in the mSCN [84]. In quail, only the mSCN expresses clock gene rhythmicity [157,158], while in the house sparrow, both structures rhythmically express per2 rhythms [1]. Lesions directed at the mSCN result in arrhythmicity similar to that observed following PINX [138]. The most likely scenario is that the functions subsumed by the mammalian SCN are regulated through the integration of both mSCN and vSCN.
Each component, the pineal gland, retinae and SCN, is integrated dynamically such that overt circadian organization is synchronized to environmental light cycles (LD) and such that internal processes are adaptively orchestrated [29,69]. Pineal (and retinal) melatonin, synchronized to LD cycles via endogenous photopigments, is secreted during the night and inhibits rhythmic metabolism and electrical activity of the vSCN. In turn, as the day approaches, oscillators within the pineal gland and retinae wane in their output, disinhibiting SCN activity. Oscillators within the mSCN and vSCN are active during the day, synchronized by LD via RHT input to the vSCN and possibly extraretinal input to the mSCN. One of the outputs of the vSCN at least is the rhythmic regulation of sympathetic activity, releasing norepinephrine (NE) within many peripheral targets. Among these is the pineal gland, where NE inhibits melatonin biosynthesis and release. It is not completely clear whether sympathetic NE synchronize circadian clockworks within the pineal gland, nor is it clear whether pineal melatonin affects clock gene expression in the SCN in vivo. However, in vitro, rhythmic melatonin administration synchronizes rhythms of both metabolic activity and the expression of both per2 and per3 [122].
Circadian Regulation of Visual System Function
The presence of dense, high affinity melatonin receptors within the retina, retinorecipient structures of all 4 visual pathways and visual integrative structures in the brains of multiple avian species [33], strongly suggests visual sensitivity, accommodation and more complex aspects of visual perception may be regulated on a circadian basis by melatonin. Circadian patterns in electroretinogram (ERG) and visually evoked potentials (VEP) recorded within the TeO show greater response amplitude during the day in LD and during subjective day in DD in both domestic chicks and pigeons [97,123,155]. In chicks and pigeons, the implicit time of the A-wave, which reflects photoreceptor activity, and B-wave, which reflects inner retinal activation, are greater during the night and subjective night than during the subjective day, while light sensitivity is greatest during the subjective night, reflecting the activity of dark-sensitive rods [123,155]. PINX of chicks reduces the amplitude of the circadian rhythm in ERG amplitude [101], and exogenous melatonin injections decreases ERG b-wave during the subjective day to levels similar to those described at night [101,123]. At this stage, it is not clear whether the effects of melatonin on avian visual system function directly influence physiological processes within visual system structures and/or regulate circadian clocks within the brain. The avian TeO expresses rhythms in clock gene expression in areas [156] superimposed with high affinity melatonin receptors [33], but the physiological link has yet to be made. Further, it is not clear how higher order visual function is affected by the circadian clock and melatonin.
Interestingly, this pattern of circadian regulation of visual system function, at least at the ERG level is nearly identical in humans with high implicit times during the night and high b-wave amplitudes during the day [72,119]. Further, administration of exogenous melatonin to human volunteers decreases b-wave amplitude during the day [59], similar to the situation in birds [101,123]. The role of melatonin in retinal physiology and pathophysiology is an emerging area of research [141], and birds provide an excellent model for human visual physiology in this regard.
Seasonal Cycles and Photoperiodism in Birds
Birds living in temperate zone latitudes generally restrict breeding to the spring and summer, maximizing the likelihood that young will be hatched during times at which food is plentiful [67,71,124]. As such, many primary and secondary sexual characteristics in birds undergo dramatic changes in both form and function. In the short days of winter, gonadal activity and gonad size regress and become inactive, while gonads recrudesce, becoming more active, in response to increased photoperiod. If birds are maintained in long photoperiod, their reproductive systems become insensitive to the photostimulatory effects of long photoperiod and spontaneous regress. This process is called photorefractoriness, and birds remain photorefractory until they are placed in short days for some time to make them photosensitive again [9,64,89,124].
In seasonally reproducing mammals such as hamsters and deer-mice, the nocturnal secretion of pineal melatonin is a critical signal that transduces time of year to the hypothalamo-hypophysial-gonadal axis to control reproductive function [11,63]. The duration of pineal melatonin biosynthesis faithfully reflects the duration of the scotoperiod; the duration is long during the long nights of winter and short during summer. These animals also exhibit a seasonal cycle of reproductive activity in which testes in males and estrus cyclicity in females increase as photoperiod increases in the spring. When photoperiod decreases, testes regress and females become anestrus. PINX in several species of hamsters prevents regression of gonads when the hamsters are transferred from long photoperiods to short photoperiods; their reproductive systems become blind to photoperiodic changes. When melatonin is infused into PINX Djungarian hamsters, Phodopus sungorus, long durations of melatonin, simulating winter, induce gonadal regression, while short durations, simulating summer, enable recrudescence. The sites for melatonin’s activity in this process are a combination of melatonin receptors in the SCN and in the pars tuberalis of the hypophysis. Therefore, the circadian control of pineal melatonin regulates the annual control of reproduction. This brief summary of mammalian seasonality is necessarily short in a review about birds; extensive reviews of these mechanisms can be found elsewhere [cf. 63,73].
Intriguingly, in spite of the fact that the rhythmic production of melatonin is critical for the expression of circadian locomotor rhythms in birds, melatonin does not affect seasonal changes in primary reproductive function in these species. As in mammals, pineal melatonin levels faithfully reflect the length of the scotoperiod both in vivo and in vitro [21, 23]. However, PINX and/or EX of several species of birds has little effect on seasonal changes in gonad size or activity [14, 90,131,132,133]. Moreover, administration of exogenous melatonin of different durations has little effect on primary reproductive function [34,104]. This corresponds to the relative absence of melatonin receptor activity in the tuberal hypothalamus and hypophysis [33], in stark contrast to the situation in seasonally reproducing mammals, where IMEL binding and melatonin receptor expression in pars tuberalis is a major site of melatonin action [63,73].
Yoshimura and colleagues have instead pointed to circadian clock function within the mediobasal hypothalamus (MBH) itself of Japanese quail controlling photoperiodic time measurement for reproductive function [161]. Earlier studies had shown that lesion of the MBH blocked testicular recrudescence in response to lengthening photoperiods and illumination of this area had resulted in in excitation of the tuberal hypothalamus and testicular growth [52, 91,113]. As stated above, the MBH of quail and the PMM of turkeys have been shown to express both OPN4 and OPN5 in cerebrospinal fluid (CSF) contacting neurons [80,112,113]. Noting that PINX or EX or even SCN lesion had little effect on photoperiodic regulation of gonadal function, Yoshimura’s group identified rhythmic expression of the clock genes in the MBH and hypothesized that this structure contained the circadian pacemaker associated with photoperiodic time measurement [114,121]. Using differential subtractive hybridization, they found type 2 iodothyronine deiodinase (Dio2) to be induced in the MBH by a light pulse associated with long-day induction. Dio2 encodes an enzyme that catalyzes the conversion of inactive thyroxine (T4) to active triiodothyroine (T3) [114,115,157,160]. Later, they showed that type 3 iodothyronine deiodinase (Dio3), which inactivates T3, was induced in the MBH by exposure to short days. The scenario they envision is that photoperiod is perceived by photopigments in the MBH that entrain a circadian oscillator within the MBH. In long days the circadian clock induces Dio2, while short days induce Dio3. Indeed, thyroidectomy induces gonadal recrudescence in starlings and Japanese quail [40, 49], and injection of T3 into the MBH induces quail gonadal growth [49,164]. These authors envision an external coincidence model in which circadian oscillators within the MBH are entrained by photoperiod co-localized in that structure. When the length of the photoperiod coincides with a φpi, Dio2 is induced, enabling a metabolic cascade in response to T3 hormone, and gonadal induction occurs. It is not clear, at this stage, what molecular components link the circadian clock to Dio2 or Dio3. The MBH of quail and the PMM of turkeys rhythmically express clock genes [76,77,92]. In humans, Dio2 is regulated by CCAAT/enhancer-binding proteins, and per2 is known to be a target of these transcription factors as well [61,140]. Perhaps, this link may provide clues to an analogous mechanism in birds.
Avian song production and perception
In parallel to primary reproductive processes, both the probability of a male bird to sing in response to a given stimulus as well as the size and complexity of the song control nuclei within its brain vary depending upon the time of year [40, 98,154]. The song control system of oscine passeriform birds is a specialized network of brain nuclei involved in singing and song learning. This system receives auditory input from ascending, primary auditory pathways beginning in the cochlear nuclei (Co), which project to the lateral dorsal mesencephalic nuclei (MLd). MLd in turn projects to the thalamic nucleus ovoidalis (Ov), which in turn projects to Field L in the forebrain. Song processing begins in secondary auditory areas in the caudal mesopallium (cM) and caudomedial nidopallium (NCM), which interact with the anterior forebrain pathway for song plasticity and learning. This pathway includes the HVC in the dorsal forebrain, which projects to Area X, whose projections form a loop between the dorsolateral thalamus (DLM) and the lateral magnocellular nucleus of the anterior nidopallium (LMAN). Then, both HVC and LMAN project to the robust nucleus of the archipallium (RA), which forms the song motor output pathway [7,8,118].
This system enables birds to process a complex species-specific identification of both self and con-specifics as well as other dynamics in birds’ acoustic environments (competitors, prey, predators etc.). These acoustic environments as well as their reproductive and survival relevance are not constant. Territories change hands and the available range of mates fluctuates depending on the time of day or time of year. Auditory sensitivities to these signals must be tuned to appropriate con-specific and perhaps inter-specific signals. At the same time, the structure and behavior of song itself must be tuned to reproductively appropriate situations.
Birds from temperate zones in the short days of winter possess small HVC, RA, and depending on the species other structures in the system. When birds are photostimulated as photoperiod increases, the song control nuclei grow in parallel with the growth of the testes, and, as they become photorefractory, these structures regress in both size and complexity. Seasonal fluctuations in androgens and estrogens appear to be critical for changes in song control [7–9]. Song control nuclei contain both androgen receptors (AR) and both estrogen receptor sub-types (ERα and ERβ), as well as aromatase (AA), capable of converting androgens into biologically active estrogens. Further, the rate of male song in several songbird species increases when testosterone levels are high, castration decreases this rate, and hormone replacement reestablishes vernal song patterns. Multiple studies have linked the activity of gonadal steroids to both song behavior and regulation of song control nuclei, which have been reviewed extensively [cf. 9].
Even so, a few studies have indicated gonad-independent regulation of song control nuclei under different photoperiods. In both American tree sparrows and house sparrows, HVC and RA increase in size in response to a change in photoperiod from a short day to long day [18, 66, 154]. Castrated birds in these studies also exhibit photostimulated song control nuclei, although the level of induction is not as great as in the sham-operated birds. In the house sparrow study, castrated birds also showed a blunted photorefractory phase. Together, the data indicate that, while the song system certainly responds to the seasonal changes in gonadal steroids, regulation of song control nuclei comprises a gonad-independent as well as a gonad-dependent aspect.
Role of pineal melatonin in song control
The observation that song control nuclei express melatonin receptors points to a role for melatonin in song behavior and in the growth and regression of song control nuclei [58, 153]. Melatonin binding and Mel1b receptor mRNA is affected by changing photoperiod, but not by castration [17, 154]. Bentley et al. [16] have shown that continuous administration exogenous melatonin attenuated the long-day-induced volumetric increase in HVC and also decreased the volume of another song-control nucleus, area X, in European starlings. This effect was independent of the birds’ reproductive state. However, seasonal changes in binding and expression of Mel1b receptor mRNA in Area X was affected by social conditions and laboratory captivity [17]. Further, Jansen et al. [78] have shown that neuronal firing in RA is decreased in vitro by administration of melatonin and that administration of the Mel1B antagonist luzindole decreases song behavior in male zebra finches the next day. However, neither of these studies addresses the rhythmic role of melatonin in the control of song control nuclei and/or song behavior.
We have asked whether PINX of house sparrows and subsequent rhythmic administration of summer-like (short duration) or winter-like (long duration) melatonin affected song control nuclei HVC and RA [34]. All birds were PINX and placed in constant darkness (DD), whereupon they all became arrhythmic in locomotor behavior for 30 days. Birds that received no melatonin administration in DD remained arrhythmic, exhibited small, regressed testes and small regressed HVC and RA volumes. Birds that received either the short duration or the long duration melatonin entrained to the melatonin cycle but still exhibited small testes and small song control nuclei. Therefore, we repeated the experiment in intact birds maintained in constant light (LL). In this case, all birds became arrhythmic as above, but all birds exhibited large testes. They also exhibited vocal behavior. Birds that received no melatonin or the short duration melatonin cycle (who entrained to the regime) exhibited large HVC and RA, but birds that received the long duration melatonin entrained to the melatonin regime but showed regressed HVC and RA. This showed that melatonin affects song control nuclei independently of gonadal state, but does melatonin affect song behavior?
As stated above, application of melatonin to brain slices from zebra finches containing RA decreases RA firing rate, and administration of the Mel1B antagonist lucindole affects song duration the next day [78]. Derégnaucourt et al. [41] recently have reported that PINX shortens the duration of song in an LD cycle. Recently, we have asked whether the pineal gland and melatonin cycles influence the daily and circadian pattern of song behavior in zebra finches [156]. In contrast to the Derégnaucourt et al. [41] study, we did not observe a shortening of the duration of song. However, we did observe effects of both PINX and melatonin under constant environmental conditions. Control birds expressed circadian rhythms of locomotion, song and call in constant dim light (dimLL) for up to 30 days. PINX birds’ locomotion, song and call in dimLL gradually became arrhythmic, but they became arrhythmic at different rates, such that song became arrhythmic more rapidly than did either locomotor or call rhythms. When PINX birds were placed in a melatonin cycle of 10 hrs melatonin/24 hrs, they entrained all three outputs. However, locomotion entrained in 4 days, song in 15 days and calls took 26 days to entrain. When PINX and control birds were placed in LL, locomotor, song and call rhythms all became arrhythmic, although PINX birds became arrhythmic more rapidly. In sham birds, song became arrhythmic most rapidly, but in PINX birds locomotor behavior damped most rapidly followed by song and then call behavior. Interestingly, all behaviors in both PINX and sham birds were entrained to melatonin cycles in LL almost immediately. The bottom line for these studies is that locomotor behavior, song production and call behavior are all under the regulation of the circadian clock and pineal melatonin. However, they are regulated differentially, perhaps via separable circadian clocks.
Biological clocks, migration and navigation
In migratory birds, circannual and circadian rhythms play integral roles in both the timing of migratory behavior and the capacity for orientation during migration [66,68,88]. Endogenously generated circannual rhythms regulate the initiation of both the vernal and autumnal migrations. Many species of migratory birds, including sparrows, finches and warblers, maintained in captivity under natural photoperiodic conditions spontaneously exhibit two bouts of migratory behavior in which normally diurnal birds express nocturnal activity called Zugunruhe or “migratory restlessness” at the same times of year that coincide with natural migration [57,136]. When birds are maintained for more than a year in a constantly equinoctial photoperiod (LD 12:12), they express two bouts of Zugunruhe approximately 6 months apart, strongly indicating an internally generated temporal program produces these migratory behaviors.
There is some evidence that a circadian clock and pineal melatonin are important for the expression of Zugunruhe [91]. First, when migratory white-throated sparrows, Zonotrichia albicollis, Sylvia warblers and other migratory birds are placed in DD or dimLL, Zugunruhe is expressed periodically with a stable ψ with circadian patterns of diurnal activity (ψzd) [10, 102,103]. Secondly, PINX of white-crowned sparrows abolishes both the free-running τ of circadian diurnal activity but also the expression of Zugunruhe [103]. Thirdly, the pattern of melatonin secretion is altered during Zugunruhe. During periods of nocturnal migratory restlessness in the lab, the amplitude of nocturnal melatonin titers is reduced [54,56,70]. If garden warblers, Sylvia borin, are disturbed during Zugunruhe by feeding, for example, the melatonin amplitude rebounds [55]. If melatonin is administered to bramblings, Fringilla montifringilla, during Zugunruhe, nocturnal activity is suppressed [54].
At this stage, the site for the circadian oscillators responsible for Zugunruhe is not known. However, there is evidence that Zugunruhe is regulated by circadian oscillators that are separate from, albeit coupled to, the circadian clock system controlling diurnal locomotor behavior. Bartell and Gwinner [10] have shown that garden warblers placed in a skeleton photoperiod of 11.5D: 1L: 10.55D: 1L entrain their diurnal locomotor behavior with a 24 hr period. However, in some birds, Zugunruhe free-ran with a long τ, indicating that a separate circadian oscillator must control Zugunruhe vs diurnal locomotor behavior. The authors suggest that the internal coincidence model best explains the data. In this scenario, the circadian clock(s) controlling normal diurnal behavior maintain a stable ψie with the photoperiod. Another clock or population of clocks associated with Zugunruhe, the hypothesis goes, is unexpressed unless and until their phase relationship with the circadian clocks controlling diurnal behavior coincide with the solstices. Then, Zugunruhe is induced. It is an intriguing idea that awaits the identification of specific structures or molecules associated with Zugunruhe.
Perhaps, one of the greatest mysteries associated with birds is their ability to navigate over great distances with remarkable accuracy during semiannual migrations or even in their day to day navigations within their home ranges [68]. There are many theories about avian orientation and navigation, including sensitivities to barometric pressures, olfactory cues and the Earth’s magnetic field [152]. These are neither mutually exclusive nor unrelated [22]. One of the most enduring ideas is the sun compass or time-compensated navigation. Early work by von Frisch in honeybees has shown that bees learn to visit a food source at a particular time of day and convey that information to the hive via its famous dance [147]. Using this information, bees navigate to the food source by interpolating the position of the sun’s azimuth with the bees’ internal sense of time. The fact that an internal sense of time (Zeitgedachnis) was required for this navigation was demonstrated by shifting the light: dark cycles of the hive by 6hrs (or 90° of the 24hrs day) and showing that the bees consistently make a 90° error in orientation. This capacity has also been demonstrated in several species of birds, including European starlings and domestic pigeons [75,88]. One question that has arisen from these studies was whether the internal sense of time was one and the same as the biological circadian clock. In an elegant series of studies, Hoffmann [75] showed that starlings maintained in constant dim light exhibited gradual shifting in the birds’ orientation with a period equivalent to the period of their free-running rhythms in locomotion. These data suggested that the orientation clock and the circadian clock shared clock properties, but the studies were conducted before any neuroscientific or molecular components of the clock had been identified.
An intriguing link between the circadian clock and avian magnetoreception has been made by several physicists in theoretical quantum coherence properties of cryptochromes. As noted above, cryptochromes are both photopigments in circadian entrainment in insects but a component of the negative element arm of the circadian molecular clockworks [47]. In birds, cry1 and cry2 are expressed rhythmically in the photoreceptor layer of retina of the lateral eyes [6]. Cryptochrome photosensitivity derives from light activated transfer of an electron from a pterin cofactor to the flavin moiety on the cryptochrome, but this process has also been shown to be modified by changes in magnetic inflection and amplitude. In insect species such as Drosophila and monarch butterflies, Danaus monarchus, the ability to perceive changes in magnetic field orientation are both dependent on ultraviolet-A/blue light and the expression of cry [47, 127]. Intriguingly, if human cry2 is expressed in cry-knockout Drosophila, magnetoreception is restored. Thus, it is possible that even humans have the capacity to detect magnetic fields [60, 127]. In European robins, Erithracus rubecula, and garden warblers, magnetic sensitivity is light-dependent with maximal sensitivity in the UVA/blue range, consistent with light sensitivity of cryptochromes [48, 122, 127]. At this stage, however, it is not clear that cryptochromes actually confer sensitivity to magnetic fields in birds nor is it clear whether the role of cryptochromes derive from their role as components of the molecular clock or whether the molecular clockworks are involved directly in time-compensated navigation [125]. It is, however, intriguing.
A chorus of clocks
This review began with an allegorical discussion of a dawn chorus of birds singing on a spring morning. In this allegory, some birds sing at their own pace, referring directly to their own biological clock entrained independently to the dawn. Others, the chickadees, perhaps, sing and call according to their own circadian clock but are also coupled to each other, responding to a conspecific just over the hedge. Still others, the jays, perhaps, respond to their clocks, their fellow jays and also to some of the other species of the garden, coupled to the community.
This is one way to envision the avian circadian system and perhaps vertebrate circadian clocks in general [65]. At the heart of it, all rhythmic processes are either entrained directly or indirectly to the photoperiod, the rising of the dawn sun. The central core of the avian circadian system, the pineal gland, the SCN and the retina, express robust molecular clockworks, but each component is not capable of self-sustained circadian rhythms. Instead, the pineal gland and retinae maintain SCN rhythmicity by inhibiting SCN output during subjective night through the actions of melatonin. These structures, being oscillators, wane in their outputs as dawn approaches, disinhibiting SCN output. We do not know if SCN activity affects retinal function, but it inhibits pineal melatonin biosynthesis during the day, and the system is maintained in a yin and yang mutually inhibitory relationship. Each of these core oscillators are also pacemakers and influence downstream processes in the brain and periphery via neuroendocrine (pineal and retinal melatonin) and neural (SCN modulation of sympathetic tone) output pathways. In turn, downstream processes possess their own circadian clockworks and entrain their tissue-specific rhythms to one, the other or both outputs of the circadian system. In this way, the system orchestrates coordinated physiological processes that maintain efficient circadian activities (Figure 3). This is the core of the neuroendocrine loop model for avian circadian organization [29].
Complementing these processes, other circadian oscillators in the tuberal hypothalamus, song control nuclei, visual system and un-described structures associated with migration and navigation maintain stable ψ with the circadian system, responding to its output in differential fashion. Reproductive function appears to be independent, but song and visual system structures are clearly regulated by melatonin, entraining to its nocturnal signal. And thus, it appears to be a cacophony, but it is not. It is a symphony or a chorus at least, coordinated in some processes but independent in others, each tuned to the rising sun and the dawn.
Highlights.
Biological clocks are fundamental to biological processes in birds, regulating many behaviors.
The formal properties and molecular components of clocks are highly conserved.
The core components of the clock interact to ensure stable phase relationships among them.
The outputs of this system entrain downstream oscillators and processes.
These processes include sleep, bird song, vision, migration and reproduction.
Acknowledgments
I’d like to thank Clifford Harpole, Jiffin Paulose, and Ye Li for helpful comments. My lab has been supported by NIH P01 NS39546 and the University of Kentucky.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abraham U, Albrecht U, Gwinner E, Brandstätter R. Spatial and temporal variation of passer Per2 gene expression in two distinct cell groups of the suprachiasmatic hypothalamus in the house sparrow (Passer domesticus) Eur J Neurosci. 2002 Aug;16(3):429–36. doi: 10.1046/j.1460-9568.2002.02102.x. [DOI] [PubMed] [Google Scholar]
- 2.Adachi A, Natesan AK, Whitfield-Rucker MG, Weigum SE, Cassone VM. Functional melatonin receptors and metabolic coupling in cultured chick astrocytes. Glia. 2002 Sep;39(3):268–78. doi: 10.1002/glia.10109. [DOI] [PubMed] [Google Scholar]
- 3.Aschoff J. Circadian rhythms: influences of internal and external factors on the period measured in constant conditions. Z Tierpsychol. 1979 Mar;49(3):225–49. doi: 10.1111/j.1439-0310.1979.tb00290.x. [DOI] [PubMed] [Google Scholar]
- 4.Bailey MJ, Cassone VM. Melanopsin expression in the chick retina and pineal gland. Brain Res Mol Brain Res. 2005 Apr 4;134(2):345–8. doi: 10.1016/j.molbrainres.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Bailey MJ, Beremand PD, Hammer R, Bell-Pedersen D, Thomas TL, Cassone VM. Transcriptional profiling of the chick pineal gland, a photoreceptive circadian oscillator and pacemaker. Mol Endocrinol. 2003 Oct;17(10):2084–95. doi: 10.1210/me.2003-0121. [DOI] [PubMed] [Google Scholar]
- 6.Bailey MJ, Beremand PD, Hammer R, Reidel E, Thomas TL, Cassone VM. Transcriptional profiling of circadian patterns of mRNA expression in the chick retina. J Biol Chem. 2004 Dec 10;279(50):52247–54. doi: 10.1074/jbc.M405679200. [DOI] [PubMed] [Google Scholar]
- 7.Ball GF, Balthazart J. Seasonal and hormonal modulation of neurotransmitter systems in the song control circuit. J Chem Neuroanat. 2010 Mar;39(2):82–95. doi: 10.1016/j.jchemneu.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Ball GF, Auger CJ, Bernard DJ, Charlier TD, Sartor JJ, Riters LV, Balthazart J. Seasonal plasticity in the song control system: multiple brain sites of steroid hormone action and the importance of variation in song behavior. Ann N Y Acad Sci. 2004 Jun;1016:586–610. doi: 10.1196/annals.1298.043. [DOI] [PubMed] [Google Scholar]
- 9.Balthazart J, Charlier TD, Barker JM, Yamamura T, Ball GF. Sex steroid-induced neuroplasticity and behavioral activation in birds. Eur J Neurosci. 2010 Dec;32(12):2116–32. doi: 10.1111/j.1460-9568.2010.07518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartell PA, Gwinner E. A separate circadian oscillator controls nocturnal migratory restlessness in the songbird Sylvia borin. J Biol Rhythms. 2005;20(6):538–49. doi: 10.1177/0748730405281826. [DOI] [PubMed] [Google Scholar]
- 11.Bartness TJ, Powers JB, Hastings MH, Bittman EL, Goldman BD. The timed infusion paradigm for melatonin delivery: what has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses? J Pineal Res. 1993;15(4):161–90. doi: 10.1111/j.1600-079x.1993.tb00903.x. [DOI] [PubMed] [Google Scholar]
- 12.Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, Zoran MJ. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet. 2005;6(7):544–56. doi: 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benoit J, Assenmacher I. Comparative sensitivity of superficial and deep receptors in photosexual reflex in duck. C R Hebd Seances Acad Sci. 1954;239(1):105–7. [PubMed] [Google Scholar]
- 14.Bentley GE. Unraveling the enigma: the role of melatonin in seasonal processes in birds. Microsc Res Tech. 2001;53(1):63–71. doi: 10.1002/jemt.1069. [DOI] [PubMed] [Google Scholar]
- 15.Bentley GE, Ball GF. Photoperiod-dependent and -independent regulation of melatonin receptors in the forebrain of songbirds. J Neuroendocrinol. 2000;12(8):745–52. doi: 10.1046/j.1365-2826.2000.00523.x. [DOI] [PubMed] [Google Scholar]
- 16.Bentley GE, Van’t Hof TJ, Ball GF. Seasonal neuroplasticity in the songbird telencephalon: a role for melatonin. Proc Natl Acad Sci U S A. 1999;96(8):4674–9. doi: 10.1073/pnas.96.8.4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bentley GE, Perfito N, Calisi RM. Season- and context-dependent sex differences in melatonin receptor activity in a forebrain song control nucleus. Horm Behav. 2012 doi: 10.1016/j.yhbeh.2012.11.015. S0018-506X(12)00287-5. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Bernard DJ, Wilson FE, Ball GF. Testis-dependent and -independent effects of photoperiod on volumes of song control nuclei in American tree sparrows (Spizella arborea) Brain Res. 1997;760(1–2):163–9. doi: 10.1016/s0006-8993(97)00277-1. [DOI] [PubMed] [Google Scholar]
- 19.Bernard M, Iuvone PM, Cassone VM, Roseboom PH, Coon SL, Klein DC. Avian melatonin synthesis: photic and circadian regulation of serotonin N-acetyltransferase mRNA in the chicken pineal gland and retina. J Neurochem. 1997;68(1):213–24. doi: 10.1046/j.1471-4159.1997.68010213.x. [DOI] [PubMed] [Google Scholar]
- 20.Binkley S, Riebman JB, Reilly KB. Timekeeping by the pineal gland. Science. 1977;197(4309):1181–3. doi: 10.1126/science.897660. [DOI] [PubMed] [Google Scholar]
- 21.Binkley S, Stephens JL, Riebman JB, Reilly KB. Regulation of pineal rhythms in chickens: photoperiod and dark-time sensitivity. Gen Comp Endocrinol. 1977;32(4):411–6. doi: 10.1016/0016-6480(77)90222-2. [DOI] [PubMed] [Google Scholar]
- 22.Bischof HJ, Nießner C, Peichl L, Wiltschko R, Wiltschko W. Avian ultraviolet/violet cones as magnetoreceptors: The problem of separating visual and magnetic information. Commun Integr Biol. 2011;4(6):713–6. doi: 10.4161/cib.17338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brandstätter R, Kumar V, Abraham U, Gwinner E. Photoperiodic information acquired and stored in vivo is retained in vitro by a circadian oscillator, the avian pineal gland. Proc Natl Acad Sci U S A. 2000;97(22):12324–8. doi: 10.1073/pnas.200354997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bünning E. Common features of photoperiodism in plants and animals. Photochem Photobiol. 1969;9(3):219–28. doi: 10.1111/j.1751-1097.1969.tb07286.x. [DOI] [PubMed] [Google Scholar]
- 25.Cantwell EL, Cassone VM. Chicken suprachiasmatic nuclei: I. Efferent and afferent connections. J Comp Neurol. 2006;496(1):97–120. doi: 10.1002/cne.20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cantwell EL, Cassone VM. Chicken suprachiasmatic nuclei: II. Autoradiographic and immunohistochemical analysis. J Comp Neurol. 2006;499(3):442–57. doi: 10.1002/cne.21124. [DOI] [PubMed] [Google Scholar]
- 27.Caprioli M, Ambrosini R, Boncoraglio G, Gatti E, Romano A, Romano M, Rubolini D, Gianfranceschi L, Saino N. Clock gene variation is associated with breeding phenology and maybe under directional selection in the migratory barn swallow. PLoS One. 2012;7(4):e35140. doi: 10.1371/journal.pone.0035140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cassone VM, Menaker M. Sympathetic regulation of chicken pineal rhythms. Brain Res. 1983;272(2):311–7. doi: 10.1016/0006-8993(83)90578-4. [DOI] [PubMed] [Google Scholar]
- 29.Cassone VM, Menaker M. Is the avian circadian system a neuroendocrine loop? J Exp Zool. 1984;232(3):539–49. doi: 10.1002/jez.1402320321. [DOI] [PubMed] [Google Scholar]
- 30.Cassone VM, Moore RY. Retinohypothalamic projection and suprachiasmatic nucleus of the house sparrow, Passer domesticus. J Comp Neurol. 1987;266(2):171–82. doi: 10.1002/cne.902660204. [DOI] [PubMed] [Google Scholar]
- 31.Cassone VM, Westneat DF. The bird of time: cognition and the avian biological clock. Front Mol Neurosci. 2012;5:32. doi: 10.3389/fnmol.2012.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cassone VM, Takahashi JS, Blaha CD, Lane RF, Menaker M. Dynamics of noradrenergic circadian input to the chicken pineal gland. Brain Res. 1986;384(2):334–41. doi: 10.1016/0006-8993(86)91169-8. [DOI] [PubMed] [Google Scholar]
- 33.Cassone VM, Brooks DS, Kelm TA. Comparative distribution of 2[125I]iodomelatonin binding in the brains of diurnal birds: out-group analysis with turtles. Brain Behav Evol. 1995;45(5):241–56. doi: 10.1159/000113553. [DOI] [PubMed] [Google Scholar]
- 34.Cassone VM, Bartell PA, Earnest BJ, Kumar V. Duration of melatonin regulates seasonal changes in song control nuclei of the house sparrow, Passer domesticus: independence from gonads and circadian entrainment. J Biol Rhythms. 2008;23(1):49–58. doi: 10.1177/0748730407311110. [DOI] [PubMed] [Google Scholar]
- 35.Chabot CC, Menaker M. Effects of physiological cycles of infused melatonin on circadian rhythmicity in pigeons. J Comp Physiol A. 1992;170(5):615–22. doi: 10.1007/BF00199337. [DOI] [PubMed] [Google Scholar]
- 36.Chaurasia SS, Rollag MD, Jiang G, Hayes WP, Haque R, Natesan A, Zatz M, Tosini G, Liu C, Korf HW, Iuvone PM, Provencio I. Molecular cloning, localization and circadian expression of chicken melanopsin (Opn4): differential regulation of expression in pineal and retinal cell types. J Neurochem. 2005;92(1):158–70. doi: 10.1111/j.1471-4159.2004.02874.x. [DOI] [PubMed] [Google Scholar]
- 37.Chong NW, Bernard M, Klein DC. Characterization of the chicken serotonin N-acetyltransferase gene. Activation via clock gene heterodimer/E box interaction. J Biol Chem. 2000;275(42):32991–8. doi: 10.1074/jbc.M005671200. [DOI] [PubMed] [Google Scholar]
- 38.Davies WI, Turton M, Peirson SN, Follett BK, Halford S, Garcia-Fernandez JM, Sharp PJ, Hankins MW, Foster RG. Vertebrate ancient opsin photopigment spectra and the avian photoperiodic response. Biol Lett. 2012;8(2):291–4. doi: 10.1098/rsbl.2011.0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davies WL, Hankins MW, Foster RG. Vertebrate ancient opsin and melanopsin: divergent irradiance detectors. Photochem Photobiol Sci. 2010;9(11):1444–57. doi: 10.1039/c0pp00203h. [DOI] [PubMed] [Google Scholar]
- 40.Dawson A, Goldsmith AR, Nicholls TJ, Follett BK. Endocrine changes associated with the termination of photorefractoriness by short daylengths and thyroidectomy in starlings (Sturnus vulgaris) J Endocrinol. 1986;110(1):73–9. doi: 10.1677/joe.0.1100073. [DOI] [PubMed] [Google Scholar]
- 41.Derégnaucourt S, Saar S, Gahr M. Melatonin affects the temporal pattern of vocal signatures in birds. J Pineal Res. 2012;53(3):245–58. doi: 10.1111/j.1600-079X.2012.00993.x. [DOI] [PubMed] [Google Scholar]
- 42.Dor R, Cooper CB, Lovette IJ, Massoni V, Bulit F, Liljesthrom M, Winkler DW. Clock gene variation in Tachycineta swallows. Ecol Evol. 2012;2(1):95–105. doi: 10.1002/ece3.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dubocovich ML, Takahashi JS. Use of 2-[125I]iodomelatonin to characterize melatonin binding sites in chicken retina. Proc Natl Acad Sci U S A. 1987;84(11):3916–20. doi: 10.1073/pnas.84.11.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dubocovich ML, Delagrange P, Krause DN, Sugden D, Cardinali DP, Olcese J. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev. 2010;62(3):343–80. doi: 10.1124/pr.110.002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ebihara S, Uchiyama K, Oshima I. Circadian organization in the pigeon, Columba livia. J Comp Physiol A. 1984;154:59–69. [Google Scholar]
- 46.Eckel-Mahan K, Sassone-Corsi P. Metabolism and the circadian clock converge. Physiol Rev. 2013;93(1):107–35. doi: 10.1152/physrev.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Emery P, So WV, Kaneko M, Hall JC, Rosbash M. CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell. 1998;95(5):669–79. doi: 10.1016/s0092-8674(00)81637-2. [DOI] [PubMed] [Google Scholar]
- 48.Follett BK, Mattocks PW, Jr, Farner DS. Circadian function in the photoperiodic induction of gonadotropin secretion in the white-crowned sparrow, Zonotrichia leucophrys gambelii. Proc Natl Acad Sci U S A. 1974 May;71(5):1666–9. doi: 10.1073/pnas.71.5.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Follett BK, Nicholls TJ. Influences of thyroidectomy and thyroxine replacement on photoperiodically controlled reproduction in quail. J Endocrinol. 1985;107(2):211–21. doi: 10.1677/joe.0.1070211. [DOI] [PubMed] [Google Scholar]
- 50.Follett BK, Pearce-Kelly AS. Photoperiodic induction in quail as a function of the period of the light-dark cycle: implications for models of time measurement. J Biol Rhythms. 1991;6(4):331–41. doi: 10.1177/074873049100600404. [DOI] [PubMed] [Google Scholar]
- 51.Follett BK, Kumar V, Juss TS. Circadian nature of the photoperiodic clock in Japanese quail. J Comp Physiol A. 1992;171(4):533–40. doi: 10.1007/BF00194586. [DOI] [PubMed] [Google Scholar]
- 52.Foster RG, Follett BK, Lythgoe JN. Rhodopsin-like sensitivity of extra-retinal photoreceptors mediating the photoperiodic response in quail. Nature. 1985;313(5997):50–2. doi: 10.1038/313050a0. [DOI] [PubMed] [Google Scholar]
- 53.Foster RG, Hankins MW. Non-rod, non-cone photoreception in the vertebrates. Prog Retin Eye Res. 2002;21(6):507–27. doi: 10.1016/s1350-9462(02)00036-8. [DOI] [PubMed] [Google Scholar]
- 54.Fusani L, Gwinner E. Reduced amplitude of melatonin secretion during migration in the blackcap (Sylvia atricapilla) In: Goos H, Rastogi RK, Vaudry H, Pierpantoni R, editors. Perspective in Comparative Endocrinology: Unity and Diversity. Naples IT; 2001. pp. 295–300. [Google Scholar]
- 55.Fusani L, Gwinner E. Simulation of migratory flight and sopover affects night levels of melatonin in a nocturnal migrant. Proc R Soc Lond. 2004;B271:205–211. doi: 10.1098/rspb.2003.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fusani L, Cardinale M, Schwabl I, Goymann W. Food availability but not melatonin affects nocturnal restlessness in a wild migrating passerine. Horm Behav. 2011;59(1):187–92. doi: 10.1016/j.yhbeh.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 57.Fusani L, Cardinale M, Carere C, Goymann W. Stopover decision during migration: physiological conditions predict nocturnal restlessness in wild passerines. Biol Lett. 2009;5(3):302–5. doi: 10.1098/rsbl.2008.0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gahr M, Kosar E. Identification, distribution, and developmental changes of a melatonin binding site in the song control system of the zebra finch. J Comp Neurol. 1996;367(2):308–18. doi: 10.1002/(SICI)1096-9861(19960401)367:2<308::AID-CNE11>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 59.Gagné AM, Danilenko KV, Rosolen SG, Hébert M. Impact of oral melatonin on the electroretinogram cone response. J Circadian Rhythms. 2009;7:14. doi: 10.1186/1740-3391-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gaston S, Menaker M. Pineal function: the biological clock in the sparrow? Science. 1968;160(3832):1125–7. doi: 10.1126/science.160.3832.1125. [DOI] [PubMed] [Google Scholar]
- 61.Gegear RJ, Foley LE, Casselman A, Reppert SM. Animal cryptochromes mediate magnetoreception by an unconventional photochemical mechanism. Nature. 2010;463(7282):804–7. doi: 10.1038/nature08719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gery S, Koeffler HP. Per2 is a C/EBP target gene implicated in myeloid leukemia. Integr Cancer Ther. 2009;8(4):317–20. doi: 10.1177/1534735409352084. [DOI] [PubMed] [Google Scholar]
- 63.Goldman BD. Mammalian photoperiodic system: formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J Biol Rhythms. 2001;16(4):283–301. doi: 10.1177/074873001129001980. [DOI] [PubMed] [Google Scholar]
- 64.Goldsmith AR, Ivings WE, Pearce-Kelly AS, Parry DM, Plowman G, Nicholls TJ, Follett BK. Photoperiodic control of the development of the LHRH neurosecretory system of European starlings (Sturnus vulgaris) during puberty and the onset of photorefractoriness. J Endocrinol. 1989;122(1):255–68. doi: 10.1677/joe.0.1220255. [DOI] [PubMed] [Google Scholar]
- 65.Granados-Fuentes D, Herzog ED. The clock shop: Coupled circadian oscillators. Exp Neurol. 2013;243:21–7. doi: 10.1016/j.expneurol.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gulledge CC, Deviche P. Photoperiod and testosterone independently affect vocal control region volumes in adolescent male songbirds. J Neurobiol. 1998;36(4):550–8. [PubMed] [Google Scholar]
- 67.Gwinner E. Photoperiod as a modifying and limiting factor in the expression of avian circannual rhythms. J Biol Rhythms. 1989;4(2):237–50. [PubMed] [Google Scholar]
- 68.Gwinner E. Circannual rhythms in birds. Curr Opin Neurobiol. 2003;13(6):770–8. doi: 10.1016/j.conb.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 69.Gwinner E, Brandstätter R. Complex bird clocks. Philos Trans R Soc Lond B Biol Sci. 2001;356(1415):1801–10. doi: 10.1098/rstb.2001.0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gwinner E, Subbaraj R, Bluhm CK, Gerkema M. Differential effects of pinealectomy on circadian rhythms of feeding and perch hopping in the European starling. J Biol Rhythms. 1987;2(2):109–20. doi: 10.1177/074873048700200203. [DOI] [PubMed] [Google Scholar]
- 71.Gwinner E, Hau M, Heigl S. Melatonin: generation and modulation of avian circadian rhythms. Brain Res Bull. 1997;44(4):439–44. doi: 10.1016/s0361-9230(97)00224-4. [DOI] [PubMed] [Google Scholar]
- 72.Hankins MW, Jones RJ, Ruddock KH. Diurnal variation in the b-wave implicit time of the human electroretinogram. Vis Neurosci. 1998;15(1):55–67. doi: 10.1017/s0952523898151118. [DOI] [PubMed] [Google Scholar]
- 73.Hastings MH, Follett BK. Toward a molecular biological calendar? J Biol Rhythms. 2001;16(4):424–30. doi: 10.1177/074873001129002015. [DOI] [PubMed] [Google Scholar]
- 74.Heigl S, Gwinner E. Synchronization of circadian rhythms of house sparrows by oral melatonin: effects of changing period. J Biol Rhythms. 1995;10(3):225–33. doi: 10.1177/074873049501000305. [DOI] [PubMed] [Google Scholar]
- 75.Hoffmann K. Experimental manipulation of the orientational clock in birds. Cold Spring Harb Symp Quant Biol. 1960;25: 379–387. doi: 10.1101/sqb.1960.025.01.040. [DOI] [PubMed] [Google Scholar]
- 76.Ikegami K, Yoshimura T. Circadian clocks and the measurement of daylength in seasonal reproduction. Mol Cell Endocrin. 2012;349:76–81. doi: 10.1016/j.mce.2011.06.040. [DOI] [PubMed] [Google Scholar]
- 77.Ikegami K, Katou Y, Higashi K, Yoshimura T. Localization of circadian clock protein BMAL1 in the photoperiodic signal transduction machinery in Japanese quail. J Comp Neurol. 2009;517(3):397–404. doi: 10.1002/cne.22165. [DOI] [PubMed] [Google Scholar]
- 78.Jansen R, Metzdorf R, van der Roest M, Fusani L, ter Maat A, Gahr M. Melatonin affects the temporal organization of the song of the zebra finch. FASEB J. 2005;19(7):848–50. doi: 10.1096/fj.04-2874fje. [DOI] [PubMed] [Google Scholar]
- 79.Kang SW, Thayananuphat A, Bakken T, El Halawani ME. Dopamine-melatonin neurons in the avian hypothalamus controlling seasonal reproduction. Neuroscience. 2007;150(1):223–33. doi: 10.1016/j.neuroscience.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 80.Kang SW, Leclerc B, Kosonsiriluk S, Mauro LJ, Iwasawa A, El Halawani ME. Melanopsin expression in dopamine-melatonin neurons of the premammillary nucleus of the hypothalamus and seasonal reproduction in birds. Neuroscience. 2010;170(1):200–13. doi: 10.1016/j.neuroscience.2010.06.082. [DOI] [PubMed] [Google Scholar]
- 81.Karaganis SP, Kumar V, Beremand PD, Bailey MJ, Thomas TL, Cassone VM. Circadian genomics of the chick pineal gland in vitro. BMC Genomics. 2008;9:206. doi: 10.1186/1471-2164-9-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Karaganis SP, Bartell PA, Shende VR, Moore AF, Cassone VM. Modulation of metabolic and clock gene mRNA rhythms by pineal and retinal circadian oscillators. Gen Comp Endocrinol. 2009;161(2):179–92. doi: 10.1016/j.ygcen.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kasal CA, Menaker M, Perez-Polo JR. Circadian clock in culture: N-acetyltransferase activity of chick pineal glands oscillates in vitro. Science. 1979;203(4381):656–8. doi: 10.1126/science.569904. [DOI] [PubMed] [Google Scholar]
- 84.King VM, Follett BK. c-fos expression in the putative avian suprachiasmatic nucleus. J Comp Physiol A. 1997;180(5):541–51. doi: 10.1007/s003590050071. [DOI] [PubMed] [Google Scholar]
- 85.Klein DC, Coon SL, Roseboom PH, Weller JL, Bernard M, Gastel JA, Zatz M, Iuvone PM, Rodriguez IR, Bégay V, Falcón J, Cahill GM, Cassone VM, Baler R. The melatonin rhythm-generating enzyme: molecular regulation of serotonin N-acetyltransferase in the pineal gland. Recent Prog Horm Res. 1997;52:307–57. [PubMed] [Google Scholar]
- 86.Konishi H, Foster RG, Follett BK. Evidence for a daily rhythmicity in the acute release of luteinizing hormone in response to electrical stimulation in the Japanese quail. J Comp Physiol A. 1987;161(2):315–9. doi: 10.1007/BF00615251. [DOI] [PubMed] [Google Scholar]
- 87.Kosonsiriluk S, Mauro LJ, Chaiworakul V, Chaiseha Y, El Halawani ME. Photoreceptive oscillators within neurons of the premammillary nucleus (PMM) and seasonal reproduction in temperate zone birds. Gen Comp Endocrinol. 2013 doi: 10.1016/j.ygcen.2013.02.015. S0016-6480(13)00081-6. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 88.Kramer G. Recent experiments of bird navigation. Ibis. 1959;101:399–416. [Google Scholar]
- 89.Kumar V, Jain N, Follett BK. The photoperiodic clock in blackheaded buntings (Emberiza melanocephala) is mediated by a self-sustaining circadian system. J Comp Physiol A. 1996;179(1):59–64. doi: 10.1007/BF00193434. [DOI] [PubMed] [Google Scholar]
- 90.Kumar V, Singh S, Misra M, Malik S, Rani S. Role of melatonin in photoperiodic time measurement in the migratory redheaded bunting (Emberiza bruniceps) and the nonmigratory Indian weaver bird (Ploceus philippinus) J Exp Zool. 2002;292(3):277–86. doi: 10.1002/jez.10079. [DOI] [PubMed] [Google Scholar]
- 91.Kumar V, Wingfield JC, Dawson A, Ramenofsky M, Rani S, Bartell P. Biological clocks and regulation of seasonal reproduction and migration in birds. Physiol Biochem Zool. 2010;83(5):827–35. doi: 10.1086/652243. [DOI] [PubMed] [Google Scholar]
- 92.Leclerc B, Kang SW, Mauro LJ, Kosonsiriluk S, Chaiseha Y, El Halawani ME. Photoperiodic modulation of clock gene expression in the avian premammillary nucleus. J Neuroendocrinol. 2010;22(2):119–28. doi: 10.1111/j.1365-2826.2009.01942.x. [DOI] [PubMed] [Google Scholar]
- 93.Li H, Ferrari MB, Kuenzel WJ. Light-induced reduction of cytoplasmic free calcium in neurons proposed to be encephalic photoreceptors in chick brain. Brain Res Dev Brain Res. 2004;153(2):153–61. doi: 10.1016/j.devbrainres.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 94.Li H, Kuenzel WJ. A possible neural cascade involving the photoneuroendocrine system (PNES) responsible for regulating gonadal development in an avian species, Gallus gallus. Brain Res Bull. 2008;76(6):586–96. doi: 10.1016/j.brainresbull.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 95.Liu F, Yuan H, Sugamori KS, Hamadanizadeh A, Lee FJ, Pang SF, Brown GM, Pristupa ZB, Niznik HB. Molecular and functional characterization of a partial cDNA encoding a novel chicken brain melatonin receptor. FEBS Lett. 1995;374(2):273–8. doi: 10.1016/0014-5793(95)01129-3. [DOI] [PubMed] [Google Scholar]
- 96.Lu J, Cassone VM. Daily melatonin administration synchronizes circadian patterns of brain metabolism and behavior in pinealectomized house sparrows, Passer domesticus. J Comp Physiol A. 1993;173:775–782. [Google Scholar]
- 97.Lu J, Zoran MJ, Cassone VM. Daily and circadian variation in the electroretinogram of the domestic fowl: effects of melatonin. J Comp Physiol A. 1995;177(3):299–306. doi: 10.1007/BF00192419. [DOI] [PubMed] [Google Scholar]
- 98.Marler P. Bird calls: their potential for behavioral neurobiology. Ann N Y Acad Sci. 2004;1016:31–44. doi: 10.1196/annals.1298.034. [DOI] [PubMed] [Google Scholar]
- 99.Masuda H, Oishi T, Ohtani M, Michinomae M, Fukada Y, Shichida Y, Yoshizawa T. Visual pigments in the pineal complex of the Japanese quail, Japanese grass lizard and bullfrog: immunocytochemistry and HPLC analysis. Tissue Cell. 1994;26(1):101–13. doi: 10.1016/0040-8166(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 100.Max M, McKinnon PJ, Seidenman KJ, Barrett RK, Applebury ML, Takahashi JS, Margolskee RF. Pineal opsin: a nonvisual opsin expressed in chick pineal. Science. 1995;267(5203):1502–6. doi: 10.1126/science.7878470. [DOI] [PubMed] [Google Scholar]
- 101.McGoogan JM, Cassone VM. Circadian regulation of chick electroretinogram: effects of pinealectomy and exogenous melatonin. Am J Physiol. 1999;277(5 Pt 2):R1418–27. doi: 10.1152/ajpregu.1999.277.5.R1418. [DOI] [PubMed] [Google Scholar]
- 102.McMillan JP. Pinealectomy abolishes circadian rhythm of migratory restlessness. J Comp Physiol. 1970;79:105–110. [Google Scholar]
- 103.McMillan JP. An endogenous rhythm of migratory restlessness in caged birds. Amer Zool. 1969;9: 1065–1072. [Google Scholar]
- 104.Meddle SL, Follett BK. Photoperiodically driven changes in Fos expression within the basal tuberal hypothalamus and median eminence of Japanese quail. J Neurosci. 1997;17(22):8909–18. doi: 10.1523/JNEUROSCI.17-22-08909.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Menaker M. Extraretinal light perception in the sparrow. I. Entrainment of the biological clock. Proc Natl Acad Sci U S A. 1968;59(2):414–21. doi: 10.1073/pnas.59.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Menaker M, Eskin A. Circadian clock in photoperiodic time measurement: a test of the Bünning hypothesis. Science. 1967;157(3793):1182–5. doi: 10.1126/science.157.3793.1182. [DOI] [PubMed] [Google Scholar]
- 107.Menaker M, Keatts H. Extraretinal light perception in the sparrow. II. Photoperiodic stimulation of testis growth. Proc Natl Acad Sci U S A. 1968;60(1):146–51. doi: 10.1073/pnas.60.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Menaker M, Underwood H. Extraretinal photoreception in birds. Photophysiology. 1976;23(4):299–306. doi: 10.1111/j.1751-1097.1976.tb07251.x. [DOI] [PubMed] [Google Scholar]
- 109.Menaker M, Roberts R, Elliott J, Underwood H. Extraretinal light perception in the sparrow. 3. The eyes do not participate in photoperiodic photoreception. Proc Natl Acad Sci U S A. 1970;67(1):320–5. doi: 10.1073/pnas.67.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Moore RY, Silver R. Suprachiasmatic nucleus organization. Chronobiol Int. 1998;15(5):475–87. doi: 10.3109/07420529808998703. [DOI] [PubMed] [Google Scholar]
- 111.Nakane Y, Yoshimura T. Deep brain photoreceptors and a seasonal signal transduction cascade in birds. Cell Tissue Res. 2010;342(3):341–4. doi: 10.1007/s00441-010-1073-6. [DOI] [PubMed] [Google Scholar]
- 112.Nakane Y, Ikegami K, Ono H, Yamamoto N, Yoshida S, Hirunagi K, Ebihara S, Kubo Y, Yoshimura T. A mammalian neural tissue opsin (Opsin 5) is a deep brain photoreceptor in birds. Proc Natl Acad Sci U S A. 2010;107(34):15264–8. doi: 10.1073/pnas.1006393107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nakao N, Yasuo S, Nishimura A, Yamamura T, Watanabe T, Anraku T, Okano T, Fukada Y, Sharp PJ, Ebihara S, Yoshimura T. Circadian clock gene regulation of steroidogenic acute regulatory protein gene expression in preovulatory ovarian follicles. Endocrinology. 2007;148(7):3031–8. doi: 10.1210/en.2007-0044. [DOI] [PubMed] [Google Scholar]
- 114.Nakao N, Ono H, Yoshimura T. Thyroid hormones and seasonal reproductive neuroendocrine interactions. Reproduction. 2008;136(1):1–8. doi: 10.1530/REP-08-0041. [DOI] [PubMed] [Google Scholar]
- 115.Nakao N, Ono H, Yamamura T, Anraku T, Takagi T, Higashi K, Yasuo S, Katou Y, Kageyama S, Uno Y, Kasukawa T, Iigo M, Sharp PJ, Iwasawa A, Suzuki Y, Sugano S, Niimi T, Mizutani M, Namikawa T, Ebihara S, Ueda HR, Yoshimura T. Thyrotrophin in the pars tuberalis triggers photoperiodic response. Nature. 2008;452(7185):317–22. doi: 10.1038/nature06738. [DOI] [PubMed] [Google Scholar]
- 116.Nanda KK, Hamner KC. Studies on the nature of the endogenous rhythm affecting photoperiodic response in the Biloxi soy bean. Bot Gaz. 1958;120: 14–25. [Google Scholar]
- 117.Natesan AK, Cassone VM. Melatonin receptor mRNA localization and rhythmicity in the retina of the domestic chick, Gallus domesticus. Vis Neurosci. 2002;19(3):265–74. doi: 10.1017/s0952523802192042. [DOI] [PubMed] [Google Scholar]
- 118.Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary. J Comp Neurol. 1976;165: 457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- 119.Nozaki S, Wakakura M, Ishikawa S. Circadian rhythm of human electroretinogram. Jpn J Ophthalmol. 1983;27(2):346–52. [PubMed] [Google Scholar]
- 120.Okano T, Yoshizawa T, Fukada Y. Pinopsin is a chicken pineal photoreceptive molecule. Nature. 1994;372(6501):94–7. doi: 10.1038/372094a0. [DOI] [PubMed] [Google Scholar]
- 121.Ono H, Nakao N, Yoshimura T. Identification of the photoperiodic signaling pathway regulating seasonal reproduction using the functional genomics approach. Gen Comp Endocrinol. 2009;163(1–2):2–6. doi: 10.1016/j.ygcen.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 122.Paulose JK, Peters JL, Karaganis SP, Cassone VM. Pineal melatonin acts as a circadian zeitgeber and growth factor in chick astrocytes. J Pineal Res. 2009;46(3):286–94. doi: 10.1111/j.1600-079X.2008.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Peters JL, Cassone VM. Melatonin regulates circadian electroretinogram rhythms in a dose- and time-dependent fashion. J Pineal Res. 2005;38(3):209–15. doi: 10.1111/j.1600-079X.2004.00195.x. [DOI] [PubMed] [Google Scholar]
- 124.Pittendrigh CS. Temporal organization: reflections of a Darwinian clockwatcher. Annu Rev Physiol. 1993;55:16–54. doi: 10.1146/annurev.ph.55.030193.000313. [DOI] [PubMed] [Google Scholar]
- 125.Pohl H. Circadian control of migratory restlessness and the effects of melatonin in the brambling (Fringilla montifringila) Chronobiol Int. 2000;17: 471–488. doi: 10.1081/cbi-100101058. [DOI] [PubMed] [Google Scholar]
- 126.Reppert SM. Melatonin receptors: molecular biology of a new family of G protein-coupled receptors. J Biol Rhythms. 1997;12(6):528–31. doi: 10.1177/074873049701200606. [DOI] [PubMed] [Google Scholar]
- 127.Reppert SM. The ancestral circadian clock of monarch butterflies: role in time-compensated sun compass orientation. Cold Spring Harb Symp Quant Biol. 2007;72:113–8. doi: 10.1101/sqb.2007.72.056. [DOI] [PubMed] [Google Scholar]
- 128.Reppert SM, Weaver DR, Cassone VM, Godson C, Kolakowski LF., Jr Melatonin receptors are for the birds: molecular analysis of two receptor subtypes differentially expressed in chick brain. Neuron. 1995;15(5):1003–15. doi: 10.1016/0896-6273(95)90090-x. [DOI] [PubMed] [Google Scholar]
- 129.Rivkees SA, Cassone VM, Weaver DR, Reppert SM. Melatonin receptors in chick brain: characterization and localization. Endocrinology. 1989;125(1):363–8. doi: 10.1210/endo-125-1-363. [DOI] [PubMed] [Google Scholar]
- 130.Rowan W. On photoperiodism, reproductive periodicity and the annual migration of birds and certain fishes. Proc Bost Nat History. 1926;38:147–189. [Google Scholar]
- 131.Schleussner G, Gwinner E. Photoperiodic time measurement during the termination of photorefractoriness in the starling (Sturnus vulgaris L.) Gen Comp Endocrinol. 1989;75(1):54–61. doi: 10.1016/0016-6480(89)90007-5. [DOI] [PubMed] [Google Scholar]
- 132.Sharp PJ. Photoperiodic regulation of seasonal breeding in birds. Ann N Y Acad Sci. 2005;1040:189–99. doi: 10.1196/annals.1327.024. [DOI] [PubMed] [Google Scholar]
- 133.Siopes TD. Effect of pinealectomy on broodiness of turkey hens. Poult Sci 1983. 1983 Nov;62(11):2245–8. doi: 10.3382/ps.0622245. [DOI] [PubMed] [Google Scholar]
- 134.Solov’yov IA, Mouritsen H, Schulten K. Acuity of a cryptochrome and vision-based magnetoreception system in birds. Biophys J. 2010;99(1):40–9. doi: 10.1016/j.bpj.2010.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Soni BG, Foster RG. A novel and ancient vertebrate opsin. FEBS Lett. 1997;406(3):279–83. doi: 10.1016/s0014-5793(97)00287-1. [DOI] [PubMed] [Google Scholar]
- 136.Stapput K, Güntürkün O, Hoffmann KP, Wiltschko R, Wiltschko W. Magnetoreception of directional information in birds requires nondegraded vision. Curr Biol. 2010;20(14):1259–62. doi: 10.1016/j.cub.2010.05.070. [DOI] [PubMed] [Google Scholar]
- 137.Steinmeyer C, Kempenaers B, Mueller JC. Testing for associations between candidate genes for circadian rhythms and individual variation in sleep behavior in blue tits. Genetica. 2012;140(4–6):219–28. doi: 10.1007/s10709-012-9673-6. [DOI] [PubMed] [Google Scholar]
- 138.Takahashi JS, Menaker M. Role of the suprachiasmatic nuclei in the circadian system of the house sparrow, Passer domesticus. J Neurosci. 1982;2(6):815–28. doi: 10.1523/JNEUROSCI.02-06-00815.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Takahashi JS, Hamm H, Menaker M. Circadian rhythms of melatonin release from individual superfused chicken pineal glands in vitro. Proc Natl Acad Sci U S A. 1980;77(4):2319–22. doi: 10.1073/pnas.77.4.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Thoennissen NH, Thoennissen GB, Abbassi S, Nabavi-Nouis S, Sauer T, Doan NB, Gery S, Müller-Tidow C, Said JW, Koeffler HP. Transcription factor CCAAT/enhancer-binding protein alpha and critical circadian clock downstream target gene PER2 are highly deregulated in diffuse large B-cell lymphoma. Leuk Lymphoma. 2012;53(8):1577–85. doi: 10.3109/10428194.2012.658792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tosini G, Baba K, Hwang CK, Iuvone PM. Melatonin: an underappreciated player in retinal physiology and pathophysiology. Exp Eye Res. 2012;103:82–9. doi: 10.1016/j.exer.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Underwood H, Menaker M. Photoreception in sparrows: response to photoperiodic stimuli. Science. 1970;169(3948):893. doi: 10.1126/science.169.3948.893. [DOI] [PubMed] [Google Scholar]
- 143.Underwood H, Siopes T. Circadian organization in Japanese quail. J Exp Zool. 1984;232: 557–566. doi: 10.1002/jez.1402320323. [DOI] [PubMed] [Google Scholar]
- 144.Vakkuri O, Lämsä E, Rahkamaa E, Ruotsalainen H, Leppäluoto J. Iodinated melatonin: preparation and characterization of the molecular structure by mass and 1H NMR spectroscopy. Anal Biochem. 1984;142(2):284–9. doi: 10.1016/0003-2697(84)90466-4. [DOI] [PubMed] [Google Scholar]
- 145.Van Gelder RN. Non-visual ocular photoreception. Ophthalmic Genet. 2001;22(4):195–205. doi: 10.1076/opge.22.4.195.2215. [DOI] [PubMed] [Google Scholar]
- 146.Voisin P, Guerlotté J, Bernard M, Collin JP, Cogné M. Molecular cloning and nucleotide sequence of a cDNA encoding hydroxyindole O-methyltransferase from chicken pineal gland. Biochem J. 1992;282 (Pt 2):571–6. doi: 10.1042/bj2820571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.von Frisch K. The “language” of bees and its utilization in agriculture 1946. Experientia. 1946/1994;50(4):406–13. [PubMed] [Google Scholar]
- 148.Wainwright SD, Wainwright LK. Chick pineal serotonin acetyltransferase: a diurnal cycle maintained in vitro and its regulation by light. Can J Biochem. 1979;57(6):700–9. doi: 10.1139/o79-088. [DOI] [PubMed] [Google Scholar]
- 149.Wang G, Wingfield JC. Immunocytochemical study of rhodopsin-containing putative encephalic photoreceptors in house sparrow, Passer domesticus. Gen Comp Endocrinol. 2011;170(3):589–96. doi: 10.1016/j.ygcen.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 150.Wang G, Harpole CE, Trivedi AK, Cassone VM. Circadian regulation of bird song call, and locomotor behavior by pineal melatonin in the zebra finch. J Biol Rhythms. 2012;27(2):145–55. doi: 10.1177/0748730411435965. [DOI] [PubMed] [Google Scholar]
- 151.Williams H. Birdsong and singing behavior. Ann N Y Acad Sci. 2004;1016:1–30. doi: 10.1196/annals.1298.029. [DOI] [PubMed] [Google Scholar]
- 152.Wiltschko R, Wiltschko W. The magnetite-based receptors in the beak of birds and their role in avian navigation. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2013;199(2):89–98. doi: 10.1007/s00359-012-0769-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Whitfield-Rucker MG, Cassone VM. Melatonin binding in the house sparrow song control system: sexual dimorphism and the effect of photoperiod. Horm Behav. 1996;30(4):528–37. doi: 10.1006/hbeh.1996.0056. [DOI] [PubMed] [Google Scholar]
- 154.Whitfield-Rucker M, Cassone VM. Photoperiodic regulation of the male house sparrow song control system: gonadal dependent and independent mechanisms. Gen Comp Endocrinol. 2000;118(1):173–83. doi: 10.1006/gcen.2000.7455. [DOI] [PubMed] [Google Scholar]
- 155.Wu WQ, McGoogan JM, Cassone VM. Circadian regulation of visually evoked potentials in the domestic pigeon, Columba livia. J Biol Rhythms. 2000;15(4):317–28. doi: 10.1177/074873000129001422. [DOI] [PubMed] [Google Scholar]
- 156.Yasuo S, Ebihara S, Yoshimura T. Circadian expression of clock gene in theoptic tectum of Japanese quail. Brain Res. 2004;1005(1–2):193–6. doi: 10.1016/j.brainres.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 157.Yasuo S, Yoshimura T. Comparative analysis of the molecular basis of photoperiodic signal transduction in vertebrates. Integr Comp Biol. 2009;49(5):507–18. doi: 10.1093/icb/icp011. [DOI] [PubMed] [Google Scholar]
- 158.Yasuo S, Yoshimura T, Bartell PA, Iigo M, Makino E, Okabayashi N, Ebihara S. Effect of melatonin administration on qPer2, qPer3, and qClock gene expression in the suprachiasmatic nucleus of Japanese quail. Eur J Neurosci. 2002;16(8):1541–6. doi: 10.1046/j.1460-9568.2002.02222.x. [DOI] [PubMed] [Google Scholar]
- 159.Yasuo S, Watanabe M, Tsukada A, Takagi T, Iigo M, Shimada K, Ebihara S, Yoshimura T. Photoinducible phase-specific light induction of Cry1 gene in the pars tuberalis of Japanese quail. Endocrinology. 2004;145(4):1612–6. doi: 10.1210/en.2003-1285. [DOI] [PubMed] [Google Scholar]
- 160.Yasuo S, Watanabe M, Iigo M, Yamamura T, Nakao N, Takagi T, Ebihara S, Yoshimura T. Molecular mechanism of photoperiodic time measurement in the brain of Japanese quail. Chronobiol Int. 2006;23(1–2):307–15. doi: 10.1080/07420520500521913. [DOI] [PubMed] [Google Scholar]
- 161.Yoshimura T. Neuroendocrine mechanism of seasonal reproduction in birds and mammals. Anim Sci J. 2010;81(4):403–10. doi: 10.1111/j.1740-0929.2010.00777.x. [DOI] [PubMed] [Google Scholar]
- 162.Yoshimura T, Yasuo S, Suzuki Y, Makino E, Yokota Y, Ebihara S. Identification of the suprachiasmatic nucleus in birds. Am J Physiol Regul Integr Comp Physiol. 2001;280(4):R1185–9. doi: 10.1152/ajpregu.2001.280.4.R1185. [DOI] [PubMed] [Google Scholar]
- 163.Yoshimura T, Suzuki Y, Makino E, Suzuki T, Kuroiwa A, Matsuda Y, Namikawa T, Ebihara S. Molecular analysis of avian circadian clock genes. Brain Res Mol Brain Res. 2000;78(1–2):207–15. doi: 10.1016/s0169-328x(00)00091-7. [DOI] [PubMed] [Google Scholar]
- 164.Yoshimura T, Yasuo S, Watanabe M, Iigo M, Yamamura T, Hirunagi K, Ebihara S. Light-induced hormone conversion of T4 to T3 regulates photoperiodic response of gonads in birds. Nature. 2003;426(6963):178–81. doi: 10.1038/nature02117. [DOI] [PubMed] [Google Scholar]
- 165.Zatz M, Mullen DA. Two mechanisms of photoendocrine transduction in cultured chick pineal cells: pertussis toxin blocks the acute but not the phase-shifting effects of light on the melatonin rhythm. Brain Res. 1988;453(1–2):63–71. doi: 10.1016/0006-8993(88)90143-6. [DOI] [PubMed] [Google Scholar]
- 166.Zatz M, Mullen DA. Norepinephrine, acting via adenylate cyclase, inhibits melatonin output but does not phase-shift the pacemaker in cultured chick pineal cells. Brain Res. 1988;450(1–2):137–43. doi: 10.1016/0006-8993(88)91553-3. [DOI] [PubMed] [Google Scholar]
- 167.Zimmerman NH, Menaker M. The pineal gland: a pacemaker within the circadian system of the house sparrow. Proc Natl Acad Sci U S A. 1979;76(2):999–1003. doi: 10.1073/pnas.76.2.999. [DOI] [PMC free article] [PubMed] [Google Scholar]


