Abstract
Objectives
While randomized trials demonstrated efficacy of infliximab for both pediatric Crohn’s disease (CD) and ulcerative colitis (UC), few patients in these studies exhibited colitis requiring hospitalization. The aims of this study were to determine the rate of subsequent infliximab failure and dose escalation in pediatric patients started on infliximab during hospitalization for colitis-predominant IBD, and to identify potential predictors of these endpoints.
Methods
Single center retrospective cohort study of patients admitted from 2005 to 2010 with Crohn’s colitis, UC, or IBD-unspecified (IBD-U) and initiated on infliximab.
Results
We identified 29 patients (12 Crohn’s colitis, 15 UC, and 2 IBD-U; median age 14 years) with a median follow-up of 923 days. 18 patients (62%) required infliximab dose escalation (increased dose or decreased infusion interval). Infliximab failure occurred in 18 patients (62%), due to ineffectiveness in 12 (67%) and adverse reactions in 6 (33%). 12 patients (41%) underwent colectomy. Subsequent need for infliximab dose escalation was associated with lower body mass index (BMI) z-score (P=0.01), lower serum albumin (P=0.03), and higher ESR (P=0.002) at baseline. ESR predicted subsequent infliximab dose escalation with an area under the curve of 0.89 (95% CI 0.72–1.00) and a sensitivity and specificity at a cutoff of 38 mm/hr of 0.79 (95% CI 0.49–0.95) and 0.88 (95% CI 0.47–0.99), respectively.
Conclusions
Most hospitalized pediatric patients with colitis treated with infliximab require early dose escalation and fail the drug long term. Low BMI and albumin, and high ESR may identify patients who would benefit from a higher infliximab starting dose.
Keywords: infliximab, inpatient, pediatrics, inflammatory bowel disease, colitis
Despite the proven efficacy of infliximab for the treatment of both Crohn’s disease (CD) and ulcerative colitis (UC) in children, there are many patients who do not respond or lose response to therapy.1–3 Randomized studies indicate that 44% and 61% of children with moderate or severe CD and UC, respectively, fail to achieve clinical remission on every 8 week infusions of infliximab 5 mg/kg at 52 weeks.1, 3 Protocols in these studies did not permit further dose escalation, and in an observational pediatric UC cohort, approximately 50% of patients received infliximab dose escalation.4 It is not known if children with increased disease severity or specific disease distributions are more likely to require higher infliximab doses.
Children with inflammatory bowel disease (IBD), whether it is CD or UC, often present with a colitis-predominant phenotype.5, 6 Those admitted to the hospital with severe steroid-refractory colitis represent a particularly difficult therapeutic challenge.7 Outcomes after infliximab in these patients are not reflected in pediatric randomized controlled studies, which enrolled very few hospitalized patients.1, 3 In a prospective cohort study of pediatric patients admitted with severe UC, of those refractory to intravenous steroids and treated with infliximab, 52% required a colectomy and an additional 12% remained steroid dependent at 1 year.7
Our clinical experience suggested that hospitalized pediatric patients treated with infliximab for refractory colitis, regardless of IBD type, have poor outcomes and frequently require early dose escalation. However, outcomes after infliximab and the subsequent incidence of dose escalation in this combined inpatient colitis population have not been described. Furthermore, it is not known if any baseline characteristics are associated with infliximab failure or dose escalation in this population. In adults with UC, early detectable serum infliximab trough levels are associated with clinical remission and lower risk of colectomy.8 If clinicians could identify patients who are at high likelihood for needing future dose escalation and treat them with higher dose upfront, we might be able to speed recovery and improve outcomes in those patients. Therefore, the aims of this study were to 1) determine the incidence of infliximab failure and dose escalation in hospitalized pediatric patients initiated on infliximab for refractory UC or Crohn’s colitis, and 2) to identify baseline characteristics predictive of these outcomes. We hypothesized that subsequent infliximab failure and dose escalation would occur frequently in this population, and be associated with elevated baseline erythrocyte sedimentation rate (ESR) and low serum albumin.
METHODS
Subject Identification
We conducted a single center retrospective cohort at the Monroe Carell Jr. Children’s Hospital at Vanderbilt (MCJCHV). The cohort consisted of anti-TNF naïve children and adolescents 2–20 years old hospitalized between January 2005 and December 2010 with refractory colitis-predominant IBD (UC, Crohn’s colitis, or IBD-unspecified (IBD-U)) and initiated on infliximab. Potential subjects were identified by 2 separate searches. In the first search, all patients admitted to MCJCHV between January 2005 and December 2010 with International Classification of Diseases, Ninth Revision (ICD-9) discharge diagnosis codes for UC (556.9) or Crohn’s disease (555.x) were identified from a hospital administrative billing database. In the second search, inpatient pharmacy records were queried for all patients who received infliximab during their hospital admission between January 2005 and December 2010. We cross-referenced the results of both searches to create a list of potential subjects, the records of whom were reviewed for eligibility for abstraction. Patients were excluded if they had no significant colitis, were started on an anti-TNF as an outpatient prior to admission, did not start on Infliximab during admission, were started on an anti-TNF other than infliximab (e.g. adalimumab or certolizumab pegol) during admission, or if infliximab was given for another disease.
Chart abstraction
Patient information was abstracted from the electronic medical record using a structured chart abstraction tool. Baseline information collected included demographic information, age at diagnosis and hospitalization, IBD type and anatomic distribution, admission laboratory values, medications, and infliximab dose. Follow-up data collected included physician global assessment (PGA) at approximately 6 months and 1 year, corticosteroid and immunomodulator use, serum infliximab levels or antibodies to infliximab (ATIs) if obtained, and time to infliximab dose escalation, interval reduction, cessation of infliximab, and/or colectomy.
Outcomes
The primary outcomes were infliximab failure and infliximab dose escalation. Infliximab failure was defined as cessation of infliximab for inefficacy or adverse reaction, or total colectomy. Infliximab dose escalation was defined as an increase in the infliximab dose or reduction in the infusion interval from the standard 5 mg/kg at 0, 2, and 6 weeks induction followed by every 8-week maintenance regimen. Secondary outcomes included total colectomy and steroid-free clinical remission at 6 months and 1 year, defined as off systemic corticosteroids and physician global assessment of inactive disease.
Statistical Methods
Time to event analyses were performed using Kaplan-Meier survival curves. Fisher’s exact test was used to compare binary outcomes between IBD diagnosis groups and treatment groups, and odds rations (ORs) were reported. Since baseline variables followed skewed distributions, median values were compared between outcome groups using the non-parametric Mann-Whitney U test. To limit the problem of multiple comparisons, we only explored six baseline variables based on hypothesis and prior literature. Univariate logistic regression was applied for baseline variables that were different between outcome groups. A receiver operating characteristic (ROC) curve was plotted to determine the predictive value of ESR across various discrimination thresholds. Data analysis was performed using SPSS 20.0 (IBM, Armonk, NY) and R version 2.15.0. Figures were prepared using GraphPad Prism (Graphpad Software, La Jolla, CA).
Ethical considerations
The Vanderbilt University Institutional Review Board approved the study. All data was recorded in a de-identified fashion, and no protected health information was recorded during chart abstraction. Data was transferred to secure web-based Research Electronic Data Capture (REDCap) software.9
RESULTS
Patient Characteristics
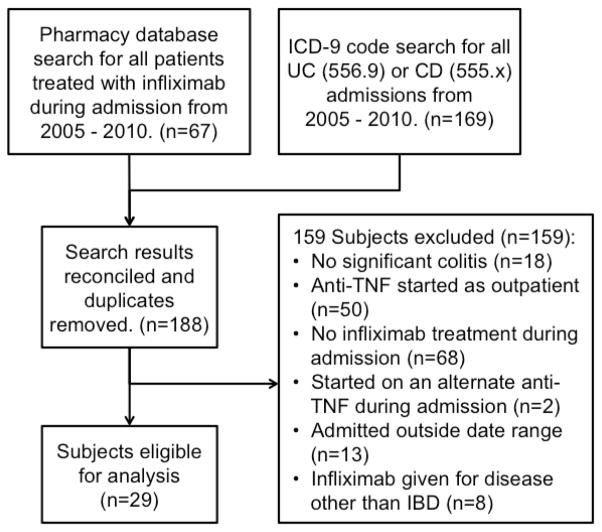
Hospital administrative and pharmacy record search identified 188 subjects for screening. After chart abstraction, 29 patients met inclusion criteria and were included in the analysis (Figure 1). Baseline characteristics, diagnosis, disease location, medications, starting infliximab dose, laboratory values and follow up for our population are shown in Table 1. 52% of the patients in our cohort had ulcerative colitis, 41% Crohn’s disease, and 7% IBD-U.
Figure 1.
Flow diagram of data base searches and subject selection.
TABLE 1.
Patient Baseline Characteristics
| Patient Characteristics | n = 29 |
|---|---|
| Age, years | 13.6 ± 3 |
| Male gender, n (%) | 15 (52) |
| IBD Diagnosis, n (%) | |
| Ulcerative colitis | 15 (52) |
| Crohn’s disease | 12 (41) |
| IBD-Unspecified | 2 (7) |
| UC classification (UC and IBD-U patients), n (%) | |
| E1 (limited proctitis) | 0 (0) |
| E2 (distal to the splenic flexure) | 1 (6) |
| E3 (proximal to the splenic flexure) | 16 (94) |
| Crohn’s disease classification, n (%) | |
| L1 (ileal only) | 0 (0) |
| L2 (colonic only) | 4 (33) |
| L3 (ileocolonic) | 8 (67) |
| L4 (upper) | 8 (67) |
| Medications at admission, n (%) | |
| Oral 5-ASA | 18 (62) |
| Rectal 5-ASA | 1 (3) |
| Oral steroids | 17 (58) |
| Rectal steroids | 1 (3) |
| 6-MP or Azathioprine | 13 (45) |
| Methotrexate | 2 (17) |
| Antibiotics | 3 (25) |
| Laboratory values, median (interquartile range) | |
| Erythrocyte sedimentation rate (mm/hr) | 40.5 (25.3,67) |
| Albumin (g/dL) | 3.1 (2.6,3.2) |
| Hemoglobin (g/dL) | 9.5 (7.5,11.2) |
| C-reactive protein (mg/L) | 46.6 (28.5,74.4) |
| Median follow up, days (range) | 923 (133,1952) |
| Duration of disease (years) | 0.9 (0,2.8) |
| Initial infliximab dose, mg/kg | 5 ± 0 |
Outcome Measures
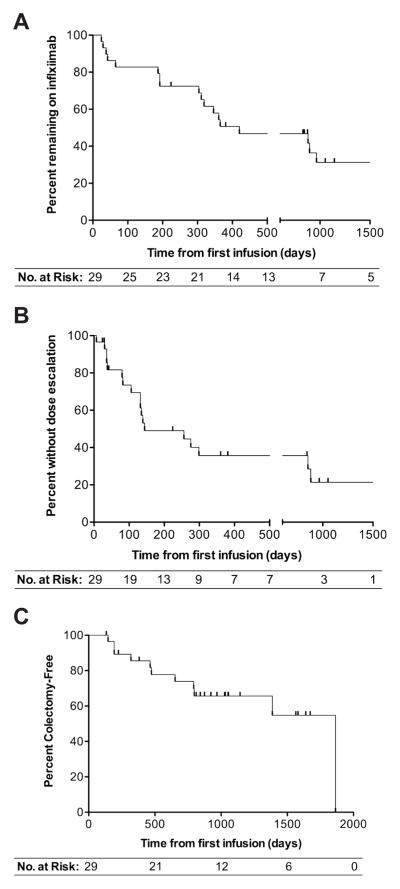
Infliximab failure occurred in 18 patients (62%, 95% CI [42%,79%]) with a median time to infliximab failure of 420 days (range 24–965 days) (Figure 2A). Infliximab dose escalation occurred in 18 patients (62%, 95% CI [42%,79%]) with a median time to dose escalation of 144 days (range 7–881 days) (Figure 2B). Infliximab failure was due to ineffectiveness in 12 patients (67%, 95% CI [41%,87%]) and adverse reaction in 6 patients (33%, 95% CI [13%,59%]). 12 patients (41%, 95% CI [24%,61%]) were in steroid-free clinical remission at both 6 months and 1 year after initiating Infliximab. 11 patients (38%, 95% CI [21%,58%]) underwent colectomy within three years of initiating infliximab (Figure 2C). Since we included patients diagnosed with UC, Crohn’s colitis, and IBD-U in this study, we compared outcome measures between CD and UC/IBD-U combined (there were 2 patients diagnosed with IBD-U). We did not observe a statistically significant difference in the rates of steroid-free clinical remission at 6 months or 1 year, infliximab failure, dose escalation, or colectomy between these IBD phenotypes (Table 2).
Figure 2.
Kaplan-Meier analysis of time from first infliximab infusion to infliximab failure (A), infliximab dose escalation (B), and colectomy (C).
Table 2.
Comparison of outcomes between IBD subtypes
| CD n=12 | UC/IBD-U n=17 | Difference of Proportions [95% CI] | P-value | |
|---|---|---|---|---|
| 26 week steroid-free remission | 5 (42%) | 7 (41%) | 1% [−36%, 37%] | 0.98 |
| 52 week steroid-free remission | 5 (42%) | 7 (41%) | 1% [−36%, 37%] | 0.98 |
| Infliximab dose escalation | 8 (67%) | 10 (59%) | 8% [−28%, 43%] | 0.67 |
| Infliximab failure | 6 (50%) | 12 (71%) | −21% [−56%, 15%] | 0.26 |
| Colectomy | 5 (42%) | 7 (41%) | 1% [−36%, 37%] | 0.98 |
CI, confidence interval
Predictors of Infliximab Dose Escalation
Baseline body mass index (BMI) z-score and serum albumin were lower, and baseline ESR was higher in patients subsequently requiring infliximab dose escalation compared to those remaining on a stable dose (Table 3). There was no statistically significant difference in baseline factors between patients with and without infliximab failure (Table 3). Median BMI z-score was −1.4 (interquartile range [IQR] [−1.9,−0.1]) in those requiring dose escalation compared to 0.0 (IQR [−1.0, 0.7]) in those not requiring dose escalation (P = 0.01) (Figure 3A). Median serum albumin was 3.0 g/dL (IQR [2.6,3.1]) in those requiring dose escalation compared to 3.3 g/dl (IQR [3.0,3.6]) in those not requiring dose escalation (P = 0.03) (Figure 3B). Median ESR was 53 mm/hr (IQR [37,74]) in those requiring dose escalation versus 23 mm/hr (IQR [21,34]) in those not requiring dose escalation (P = 0.002) (Figure 3C).
Table 3.
Comparison of baseline factors between infliximab outcome groups
| Variable n (%) or median (IQR) | Infliximab Failure
|
P-value | Infliximab Dose Escalation
|
P-value | ||
|---|---|---|---|---|---|---|
| Yes | No | Yes | No | |||
| Male gender | 10 (56%) | 5 (46%) | 0.71 | 10 (56%) | 5 (46%) | 0.71 |
| BMI z-score | 0.0 (−1.8,−0.4) | −1.3 (−1.6,−1.0) | 0.16 | −1.4 (−1.9,−0.1) | 0.0 (−1.0,0.7) | 0.01 |
| Albumin (g/dL) | 3.1 (2.6,3.2) | 3.2 (2.4,3.4) | 0.57 | 3.0 (2.6,3.1) | 3.3 (3.0,3.6) | 0.03 |
| ESR (mm/hr) | 50 (27,75) | 38 (23,52) | 0.42 | 53 (37,74) | 23 (21,33) | 0.002 |
| Hemoglobin (g/dL) | 9.5 (7.4,11.1) | 9.5 (8.9,11.2) | 0.58 | 9.3 (7.9,10.9) | 10.2 (7.0,11.2) | 0.72 |
| Disease Duration (yrs) | 1 (0,2.5) | 0.7 (0,4.25) | 0.81 | 1 (0,2.5) | 1 (0,3) | 0.73 |
IQR, interquartile range; BMI, body mass index
Figure 3.
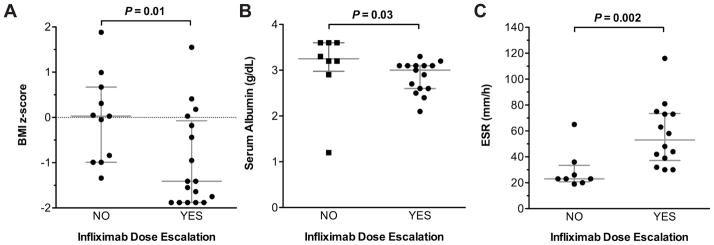
Baseline BMI z-score (A), serum albumin (B) and ESR (C) in patients requiring versus those not requiring infliximab dose escalation. Lines and error bars represent median and interquartile range, respectively.
To determine whether variation in baseline BMI, albumin, or ESR is associated with the binary outcomes of infliximab failure and infliximab dose escalation, logistic regression analysis was applied (Table 4). Each unit increase in baseline BMI z-score was associated with a relative decrease in the odds of infliximab dose escalation of 0.41 (95% CI [0.18,0.95]; P = 0.04). However, there was a trend towards BMI influencing infliximab failure in the opposite direction, where for each unit increase in BMI z-score, there was a relative increase in the odds of infliximab failure of 2.10 (95% CI [0.90,4.90], P = 0.09. Each unit increase in baseline ESR was associated with a relative increase in the odds of infliximab dose escalation of 1.1 (95% CI [1.01,1.19]; P = 0.03). We did not detect any statistically significant associations between variation in baseline serum albumin or ESR and the outcome of infliximab failure by logistic regression.
Table 4.
Univariate logistic regression to assess predictors of infliximab outcomes
| Variable | Infliximab Failure | Infliximab Dose Escalation | ||
|---|---|---|---|---|
|
| ||||
| OR1 [95% CI] | P-value | OR [95% CI] | P-value | |
| BMI z-score | 2.10 [0.90,4.90] | 0.09 | 0.41 [0.18,0.95] | 0.04 |
| Albumin (g/dL) | 1.25 [0.23,6.86] | 0.80 | 0.38 [0.05,2.91] | 0.35 |
| ESR (mm/hr) | 1.02 [0.98,1.07] | 0.23 | 1.10 [1.01,1.19] | 0.03 |
Relative change in odds of event occurring for each unit increase in variable
CI, confidence interval; OR, odds ratio
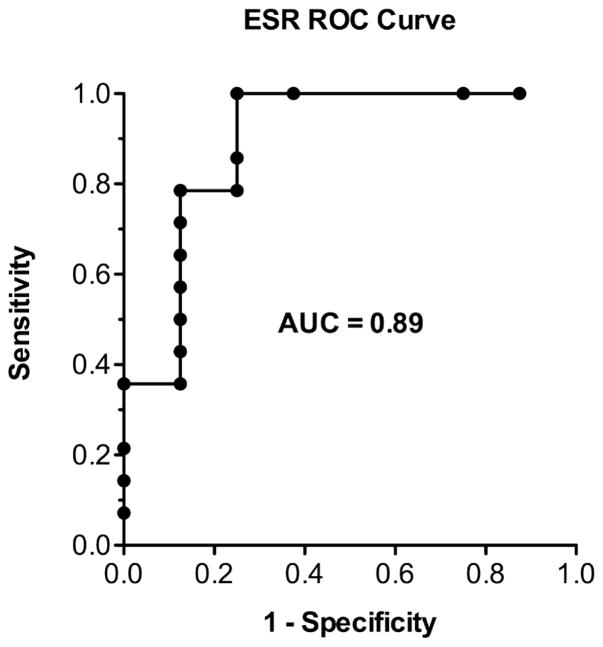
The predictive value of ESR at infliximab initiation at various discriminatory thresholds was explored ROC curve anlaysis (Figure 4). ESR predicted subsequent infliximab dose escalation with an area under the curve of 0.89 (95% CI [0.72–1.00]). At an ESR cutoff of 38 mm/hr, the sensitivity and specificity were 0.79 (95% CI [0.49–0.95]) and 0.88 (95% CI [0.47–0.99]), respectively.
Figure 4.
Receiving operating characteristic (ROC) curve of ESR at first infliximab infusion as a predictor of subsequent infliximab dose escalation.
Use of concomitant immunodulators has been shown to reduce the risk of developing ATIs and result in high serum infliximab concentrations.10. Of 9 patients (31%) on concomitant immunomodulators in this cohort, only 2 (22%) required dose escalation, compared to 16 of 20 (80%) started on infliximab monotherapy (OR 0.71 [95% CI 0.01,0.49], P = 0.01) (Figure 5A). There was no statistically significant difference in infliximab failure rates between patients treated with concomitant immunomodulators or infliximab monotherapy (78% vs. 55%; OR 2.86 [95% CI 0.47,17.35], P = 0.41 (Figure 5B).
Figure 5.
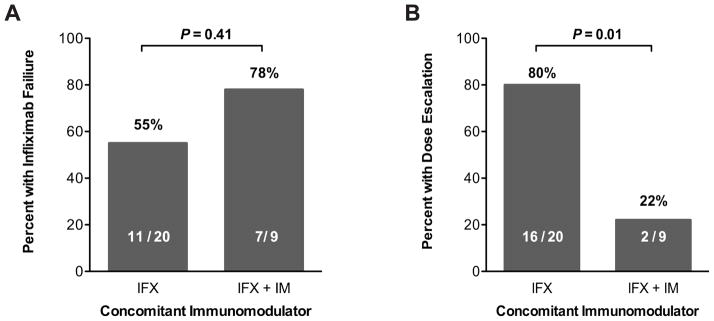
Incidence of infliximab failure (A) and infliximab dose escalation (B) in patients started on infliximab monotherapy versus those started on infliximab with a concomitant immunomodulator (thiopurine or methotrexate).
Outcomes after Infliximab Dose Escalation
To determine whether patients may benefit from infliximab dose escalation, we performed Kaplan-Meier analysis of time to infliximab failure and time to colectomy from the time of infliximab dose escalation. Of the 18 patients with infliximab dose escalation during follow-up, 39% remained on infliximab 1 and 2 years after dose escalation, and 29% remained on infliximab 3 years after dose escalation. For the outcome of colectomy, 76% remained colectomy-free 1 year after dose escalation, and 52% remained colectomy-free 2 and 3 years after dose escalation.
Serum Infliximab Levels and Antibodies to Infliximab
Serum infliximab levels and antibodies to infliximab (ATI) were obtained in 15 of 29 (52%) patients. A low serum infliximab level was defined as less than 12 μg/ml between 0 and 4 weeks after the last infusion, or undetectable between 0 and 8 weeks after the last infusion.8, 10 Serum infliximab levels from 2 patients were considered uninterpretable either because they were obtained greater than 8 weeks after the last infusion, or less than 4 weeks after the last infusion and were greater than 12 μg/ml. Of 13 patients with interpretable serum infliximab levels, 12 patients (92%) had low levels including 7 (58%) patients with undetectable levels. Since ATI were assessed by enzyme-linked immunosorbent assay, they could only be detected when serum infliximab levels were undetectable. Of the 7 patients with undetectable serum infliximab levels, 3 (43%) had ATI. Amongst patients in whom ATI were measured, 3 of 12 (25%) who did not receive concomitant immunomodulators at the start of infliximab therapy developed ATI, whereas 0 of 3 (0%) patients who did receive concomitant immunomodulators developed ATI.
DISCUSSION
In this high-risk population of hospitalized pediatric patients initiated on infliximab for colitis-predominant IBD, we found a majority require early dose escalation and fail to continue infliximab in the long-term. Furthermore, we demonstrated that subsequent need for infliximab dose escalation in this population is associated with baseline laboratory values at the start of treatment including high ESR and low albumin. Need for dose escalation was also associated with lack of use of a concomitant immunomodulator (i.e. infliximab monotherapy).
At inception of this study, we chose to do a combined analysis of children admitted with UC, Crohn’s colitis, or IBD-unspecified for several reasons. First, children with CD often present with colitis, which can be difficult to distinguish from UC.5 Second, the latest genome-wide association meta-analysis clearly demonstrate broad genetic overlap between both types of IBD.11 Lastly, our clinical experience suggested that outcomes in hospitalized children with both Crohn’s colitis and UC treated with infliximab were similar. In fact, we did not detect a statistically significant difference in incidence infliximab failure, infliximab dose escalation, or colectomy between IBD types; however we cannot rule out differences given the wide confidence intervals.
We observed that 50% of patients with hospitalized colitis-predominant IBD subsequently discontinued infliximab by 1 year, which was higher than the 18% incidence observed in the every 8 week infusion arm of the major randomized trial of infliximab for pediatric UC, and more similar to incidences of infliximab discontinuation observed in observational studies of both general (41%) and severe inpatient pediatric UC (48%).1, 4, 7 This difference is likely explained by two major factors. First, we focused on hospitalized patients who likely had more severe disease, compared to the randomized trial, which included few hospitalized patients.1 Second, patients are less likely to discontinue a drug when participating in a tightly protocol-driven clinical trial. In pediatric CD, the likelihood of infliximab discontinuation at 1 year was also lower in both a major randomized trial (6%) and an observational cohort (7%). In the context of these prior studies, our results suggest that children admitted for colitis-predominant IBD, whether it be CD or UC, respond to infliximab more similarly to a general pediatric UC population than CD.3, 12
In our study, 65% of patients received infliximab dose escalation by 1 year. This incidence of dose escalation was higher than that observed in the every 8 week infusion maintenance arm of the randomized trials for both pediatric UC (40%) and CD (19%), and in a large observational pediatric UC cohort (33%).1, 4, 13 We suspect this difference is due to our specific focus on hospitalized children with colitis, who are more likely to have severe disease. Providers typically dose escalate when patients are primary non-responders or lose response to infliximab. There are two suggested explanations for primary non-response or loss of response: 1) chronic intestinal inflammation driven by factors other than TNF, or 2) inadequate serum infliximab levels due to the development of antibodies to infliximab, high TNF burden, or rapid infliximab clearance.14 Detectable trough serum infliximab levels are associated with improved clinical outcomes in both UC and CD.8, 15 Some have proposed that patients with UC may have higher infliximab clearance than those with CD.14 A limitation of our study is that serum infliximab levels were only obtained in approximately half the patients at varying time points. Nonetheless, the high rate of early infliximab dose escalation and the very high frequency of low serum infliximab levels observed in our population, supports the notion that patients with colitis, and disease severe enough to warrant hospitalization, likely clear infliximab rapidly.
We questioned whether there were baseline characteristics that would identify patients likely to fail infliximab or require dose escalation. Based on studies linking serum infliximab levels to clinical outcomes, it is plausible that many of these patients exhibit rapid infliximab clearance and that high initial infliximab dosing might result in improved and earlier disease control. We observed need for dose escalation was associated with lower serum albumin levels and high ESR. Low serum albumin, as an indicator of gut protein loss, may identify individuals likely to leak infliximab through a highly permeable intestine. Alternatively, low serum albumin may also be a marker for decreased protein and antibody salvage and recirculation in the setting of high systemic inflammation.14 Accordingly, an inverse correlation between serum albumin and infliximab levels has been observed in both UC and CD.16, 17 In an observational cohort of children with UC, baseline albumin less than 3.5 g/dL was associated with increased risk for earlier colectomy.18 We did not observe a similar association in our cohort, likely because our cohort was composed of more ill hospitalized patients; all but 3 patients in our cohort had a serum albumin less than 3.5 g/dL.
We also observed that low baseline BMI z-score was associated with need for dose escalation. In contrast to the inverse association of BMI with infliximab dose escalation, we observed a trend toward higher BMI z-score predicting infliximab failure. This finding is similar to that reported in an abstract of high BMI associated with decreased likelihood of 6-month remission in children with UC, but not CD.19 We can speculate that very low BMI may represent severe malnutrition associated worse disease severity, higher TNF and/or inflammatory burden, and increased gut protein loss, which may lead to a higher infliximab requirement. However, long-term, amongst more normally weighted and overweight individuals, high BMI may adversely effects infliximab response, perhaps due to the additional TNF burden of excess adipose tissue20, 21
Infliximab is cleared through the reticuloendothelial system which is activated in the presence of high systemic inflammation.14 Indeed, serum infliximab levels are inversely associated with fecal calprotectin and serum CRP.17, 22, 23 We chose to study ESR as an inflammatory marker over CRP because CRP was not consistently obtained, especially during the early portion of the study period. Additionally, there are some IBD patients who do not exhibit increases in CRP levels.24–26 We found that ESR cutoff of 38 mm/hr provided high sensitivity and specificity for subsequent need for infliximab dose escalation. Future studies will be needed to validate the predictive accuracy of high ESR. Similar to our finding, in adult CD, high pretreatment CRP is associated with subsequent clinical relapse and need for intervention.27
Approximately one third of our patients were maintained on a concomitant immunomodulator at infliximab initiation, and these patients were less likely to require subsequent infliximab dose escalation. This finding is consistent with substantial evidence from post-hoc analyses of infliximab CD and UC clinical trials, and from the SONIC trial, demonstrating that use of concomitant immunomodulators increases serum infliximab concentrations.28, 29 This effect, in part, is due to decreased formation of ATI, and likely also through reductions in infliximab clearance by other mechanisms.
We did not detect significant associations between baseline factors and the outcome of infliximab failure. One explanation for this result is that one third of the patients with infliximab failure discontinued infliximab due to adverse reactions. We do not suspect that the baseline factors studied would predict risk for adverse reactions to infliximab. Our study lacked the precision to rule out meaningful differences in baseline factors in those who discontinued infliximab exclusively for non-response or loss of response.
Our study has several limitations. While the retrospective observational design provides results that reflect real world clinical outcomes at a tertiary care children’s hospital, it presents several challenges such as variations in laboratory studies obtained, individual physician practice styles, and in physician practices over time. Furthermore, the retrospective design is susceptible to confounding. Our relatively small study population yielded results with wide confidence intervals and precluded the use of a multivariable analysis due to risk for over fitting a model.
In conclusion, children treated with infliximab for colitis-predominant IBD of sufficient severity to warrant hospitalization exhibit a high incidence of infliximab dose escalation and infliximab failure. Low baseline BMI z-score and serum albumin, and high ESR are associated with need for early infliximab dose escalation. Future prospective studies are needed to determine whether BMI, serum albumin, and ESR can identify children hospitalized with colitis-predominant IBD who would benefit from higher initial infliximab dosing regimens.
Acknowledgments
Support: Supported in part by NIH Training Grant T32DK00767320-S1 (TOF), NIH Award K23DK094832 (MJR), a NASPGHAN George Ferry Young Investigator Award (MJR), Vanderbilt Physician Scientist Development Award (MJR), NIH Award R01 AT004821 (KTW) and NIH CTSA Award UL1TR000445.
Footnotes
Financial Disclosure: The authors have no financial affiliations to disclose.
References
- 1.Hyams J, Damaraju L, Blank M, et al. Induction and maintenance therapy with infliximab for children with moderate to severe ulcerative colitis. Clin Gastroenterol Hepatol. 2012;10:391–9. e1. doi: 10.1016/j.cgh.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 2.Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–76. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 3.Hyams J, Crandall W, Kugathasan S, et al. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn’s disease in children. Gastroenterology. 2007;132:863–73. doi: 10.1053/j.gastro.2006.12.003. quiz 1165–6. [DOI] [PubMed] [Google Scholar]
- 4.Hyams JS, Lerer T, Griffiths A, et al. Outcome following infliximab therapy in children with ulcerative colitis. Am J Gastroenterol. 2010;105:1430–6. doi: 10.1038/ajg.2009.759. [DOI] [PubMed] [Google Scholar]
- 5.Heyman MB, Kirschner BS, Gold BD, et al. Children with early-onset inflammatory bowel disease (IBD): analysis of a pediatric IBD consortium registry. J Pediatr. 2005;146:35–40. doi: 10.1016/j.jpeds.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 6.Gupta N, Bostrom AG, Kirschner BS, et al. Presentation and disease course in early- compared to later-onset pediatric Crohn’s disease. Am J Gastroenterol. 2008;103:2092–8. doi: 10.1111/j.1572-0241.2008.02000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner D, Mack D, Leleiko N, et al. Severe pediatric ulcerative colitis: a prospective multicenter study of outcomes and predictors of response. Gastroenterology. 2010;138:2282–91. doi: 10.1053/j.gastro.2010.02.047. [DOI] [PubMed] [Google Scholar]
- 8.Seow CH, Newman A, Irwin SP, et al. Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut. 2010;59:49–54. doi: 10.1136/gut.2009.183095. [DOI] [PubMed] [Google Scholar]
- 9.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med. 2003;348:601–8. doi: 10.1056/NEJMoa020888. [DOI] [PubMed] [Google Scholar]
- 11.Jostins L, Ripke S, Weersma RK, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–24. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hyams JS, Lerer T, Griffiths A, et al. Long-term outcome of maintenance infliximab therapy in children with Crohn’s disease. Inflamm Bowel Dis. 2009;15:816–22. doi: 10.1002/ibd.20845. [DOI] [PubMed] [Google Scholar]
- 13.Wultanska D, Banaszkiewicz A, Radzikowski A, et al. Clostridium difficile infection in Polish pediatric outpatients with inflammatory bowel disease. Eur J Clin Microbiol Infect Dis. 2010;29:1265–70. doi: 10.1007/s10096-010-0997-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ordas I, Feagan BG, Sandborn WJ. Therapeutic drug monitoring of tumor necrosis factor antagonists in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2012;10:1079–87. doi: 10.1016/j.cgh.2012.06.032. quiz e85–6. [DOI] [PubMed] [Google Scholar]
- 15.Maser EA, Villela R, Silverberg MS, et al. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn’s disease. Clin Gastroenterol Hepatol. 2006;4:1248–54. doi: 10.1016/j.cgh.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 16.Fasanmade AA, Adedokun OJ, Olson A, et al. Serum albumin concentration: a predictive factor of infliximab pharmacokinetics and clinical response in patients with ulcerative colitis. Int J Clin Pharmacol Ther. 2010;48:297–308. doi: 10.5414/cpp48297. [DOI] [PubMed] [Google Scholar]
- 17.Fasanmade AA, Adedokun OJ, Ford J, et al. Population pharmacokinetic analysis of infliximab in patients with ulcerative colitis. Eur J Clin Pharmacol. 2009;65:1211–28. doi: 10.1007/s00228-009-0718-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelley-Quon LI, Jen HC, Ziring DA, et al. Predictors of proctocolectomy in children with ulcerative colitis. J Pediatr Gastroenterol Nutr. 2012;55:534–40. doi: 10.1097/MPG.0b013e3182619d26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grossi V, Lerer T, Markowitz J, et al. Impact of Body Mass Index on Efficacy of Infliximab in Children With Inflammatory Bowel Disease. Gastroenterol. 2013;144(5):S128. [Google Scholar]
- 20.Hotamisligil GS, Arner P, Caro JF, et al. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–15. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kern PA, Saghizadeh M, Ong JM, et al. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J Clin Invest. 1995;95:2111–9. doi: 10.1172/JCI117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamalainen A, Sipponen T, Kolho KL. Serum infliximab concentrations in pediatric inflammatory bowel disease. Scand J Gastroenterol. 2013;48:35–41. doi: 10.3109/00365521.2012.741619. [DOI] [PubMed] [Google Scholar]
- 23.Wolbink GJ, Voskuyl AE, Lems WF, et al. Relationship between serum trough infliximab levels, pretreatment C reactive protein levels, and clinical response to infliximab treatment in patients with rheumatoid arthritis. Ann Rheum Dis. 2005;64:704–7. doi: 10.1136/ard.2004.030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandborn WJ, Hanauer SB. Infliximab in the treatment of Crohn’s disease: a user’s guide for clinicians. Am J Gastroenterol. 2002;97:2962–72. doi: 10.1111/j.1572-0241.2002.07093.x. [DOI] [PubMed] [Google Scholar]
- 25.Sono K, Yamada A, Yoshimatsu Y, et al. Factors associated with the loss of response to infliximab in patients with Crohn’s disease. Cytokine. 2012;59:410–6. doi: 10.1016/j.cyto.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 26.Imaeda H, Andoh A, Fujiyama Y. Development of a new immunoassay for the accurate determination of anti-infliximab antibodies in inflammatory bowel disease. J Gastroenterol. 2012;47:136–43. doi: 10.1007/s00535-011-0474-y. [DOI] [PubMed] [Google Scholar]
- 27.Jurgens M, Mahachie J, Cleynen I, et al. Levels of C-reactive protein are associated with response to infliximab therapy in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2011;9:421–7. e1. doi: 10.1016/j.cgh.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–95. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 29.Lichtenstein GR, Diamond RH, Wagner CL, et al. Clinical trial: benefits and risks of immunomodulators and maintenance infliximab for IBD-subgroup analyses across four randomized trials. Aliment Pharmacol Ther. 2009;30:210–26. doi: 10.1111/j.1365-2036.2009.04027.x. [DOI] [PubMed] [Google Scholar]