Abstract
Objectives
We aimed to investigate the characteristics and outcomes of patients with heart failure with preserved ejection fraction (HFpEF) and angina pectoris (AP).
Background
AP is a predictor of adverse events in patients with heart failure with reduced EF. The implications of AP in HFpEF are unknown.
Methods
We analyzed HFpEF patients (EF≥50%) who underwent coronary angiography at Duke University Medical Center from 2000–2010 with and without AP in the previous 6 weeks. Time to first event was examined using Kaplan-Meier methods for the primary endpoint of death/myocardial infarction (MI)/revascularization/stroke (i.e., MACE) and secondary endpoints of death/MI/revascularization, death/MI/stroke, death/MI, death and cardiovascular death/cardiovascular hospitalization.
Results
In the Duke Databank, 3517 patients met criteria for inclusion and 1402 (40%) had AP. Those with AP were older with more comorbidities, and prior revascularization vs. non-AP patients. AP patients more often received beta-blockers, ACE-inhibitors, nitrates, and statins (all P<0.05). In unadjusted analysis, AP patients had increased MACE and death/MI/revascularization (both P <0.001), lower rates of death and death/MI (both P<0.05), and similar rates of death/MI/stroke and cardiovascular death/cardiovascular hospitalization (both P>0.1). After multivariable adjustment, those with AP remained at increased risk for MACE (Hazard Ratio [HR] 1.30; 95% Confidence Interval [CI], 1.17–1.45) and death/MI/revascularization (HR 1.29; 95% CI, 1.15–1.43), but were at similar risk for other endpoints (P>0.06).
Conclusions
AP in HFpEF patients with a history of coronary artery disease is common despite medical therapy and is independently associated with increased MACE due to revascularization with similar risk of death, MI, and hospitalization.
Keywords: heart failure with preserved ejection fraction, angina pectoris, outcomes
Angina pectoris (AP) is the symptomatic condition related to ischemia and has different prognostic implications in various patient populations(1). We have previously shown that the presence of AP in patients with heart failure (HF) with reduced ejection fraction (EF) is common despite medical therapy and previous revascularization and is associated with increased cardiovascular death or rehospitalization(2). Heart failure with preserved ejection fraction (HFpEF) accounts for upwards of 50% of all patients with HF(3) and the evidence for therapies to reduce adverse events in this population is limited(4). The implications of AP in HFpEF are not well defined, since these patients have generally been excluded from AP studies(5). We compared the clinical characteristics and the outcomes of patients with and without AP in a cohort of HFpEF patients.
Methods
Patient data was obtained from the Duke Databank for Cardiovascular Disease (DDCD), an ongoing databank of all patients undergoing diagnostic cardiac catheterization at Duke University Medical Center. Patients were included in the study population if they underwent coronary angiography from January 2000 through December 2010, had HFpEF and a history of ≥50% stenosis in at least one epicardial coronary vessel (only those patients with a history of significant coronary artery disease [CAD] receive DDCD follow-up). Coronary stenoses were graded by visual consensus of at least two experienced observers. HFpEF was defined as patients with New York Heart Association (NYHA) class II to IV symptoms in the 2 weeks prior to index catheterization and EF≥50%(6). Patients were excluded from analysis if they had EF<50%, unknown EF, unknown NYHA class, primary valvular heart disease (defined as moderate or severe aortic or mitral insufficiency or severe stenosis of any heart valve), congenital heart disease, acquired immunodeficiency syndrome or metastatic cancer.
Data from the index catheterization was prospectively collected as part of routine patient care. Baseline clinical variables for each patient were stored in the DDCD using methods previously described(7). Follow-up was obtained through self-administered questionnaires, with telephone follow-up to nonresponders. Patients not contacted through this mechanism had vital status determined through a search of the National Death Index(8).
AP classification was based on physician-obtained patient history just prior to cardiac catheterization and was defined as chest pain within the previous 6 weeks. Since many groups (e.g. women, elderly) present with atypical angina(9,10), we did not want to bias our results by using a classic angina definition alone. Given the prognostic value of angina characteristics, the severity, frequency, and pattern of occurrence were recorded at baseline. Revascularization was defined as treatment with percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG). Death, myocardial infarction (MI), stroke and cardiovascular rehospitalization were determined using methods previously described(7).
Statistical Analysis
Baseline characteristics are described with medians and interquartile ranges for continuous variables and percentages for discrete variables in HFpEF patients with vs. without AP. These characteristics were compared using the Wilcoxon rank-sum test for continuous variables, and chi-square tests for categorical variables unless otherwise noted. The primary endpoint was death/MI/revascularization/stroke [i.e., major adverse cardiac events (MACE)] and secondary endpoints were death/MI/revascularization, death/MI/stroke, death/MI, death and cardiovascular death/cardiovascular hospitalization. Multivariable Cox proportional hazards regression analysis was used to adjust for baseline differences between groups. A comprehensive set of covariates was used for the adjustment analysis (see Table 3 footnote) based on clinical relevance and data from previous investigation(2). With the large number of events in each analysis, there was no overfitting problem with adjustment variables. Adjusted time-to-event results were generated for the endpoints, and comparisons were made using the log-rank test. A p-value <0.05 was used to indicate statistical significance for all comparisons. Statistical analyses were performed by Duke Clinical Research Institute (Durham, NC, USA) using SAS system version 9.2 (Cary, NC, USA). The protocol was approved by the institutional review board at Duke University and all patients voluntarily provided written informed consent.
Table 3.
Angina pectoris as a predictor of outcome on adjusted analysis.
| End point | Adjusted* Hazard Ratio (95% Confidence Interval) |
P-Value |
|---|---|---|
| Death/myocardial infarction/revascularization/stroke | 1.30 (1.17–1.45) | <0.0001 |
| Death/myocardial infarction/revascularization | 1.29 (1.15–1.43) | <0.0001 |
| Death/myocardial infarction/stroke | 0.99 (0.87–1.11) | 0.81 |
| Death/myocardial infarction | 0.93 (0.82–1.06) | 0.27 |
| Death | 0.94 (0.82–1.06) | 0.30 |
| Cardiovascular death/cardiovascular hospitalization | 0.95 (0.85–1.05) | 0.32 |
Adjusted for age, ejection fraction, sex, race, hypertension, diabetes, prior myocardial infarction, hyperlipidemia, New York Heart Association class, cerebrovascular disease, peripheral vascular disease, previous smoking history, previous percutaneous coronary intervention, previous coronary artery bypass grafting, ventricular gallop, Charlson Index, body mass index, heart rate, systolic blood pressure, use of beta-blocker, ACE-inhibitor or angiotensin receptor blocker, hydralazine, nitrates, calcium channel blocker, aspirin, clopidogrel, statin, diuretic and serum creatinine, sodium, blood urea nitrogen and hemoglobin.
Results
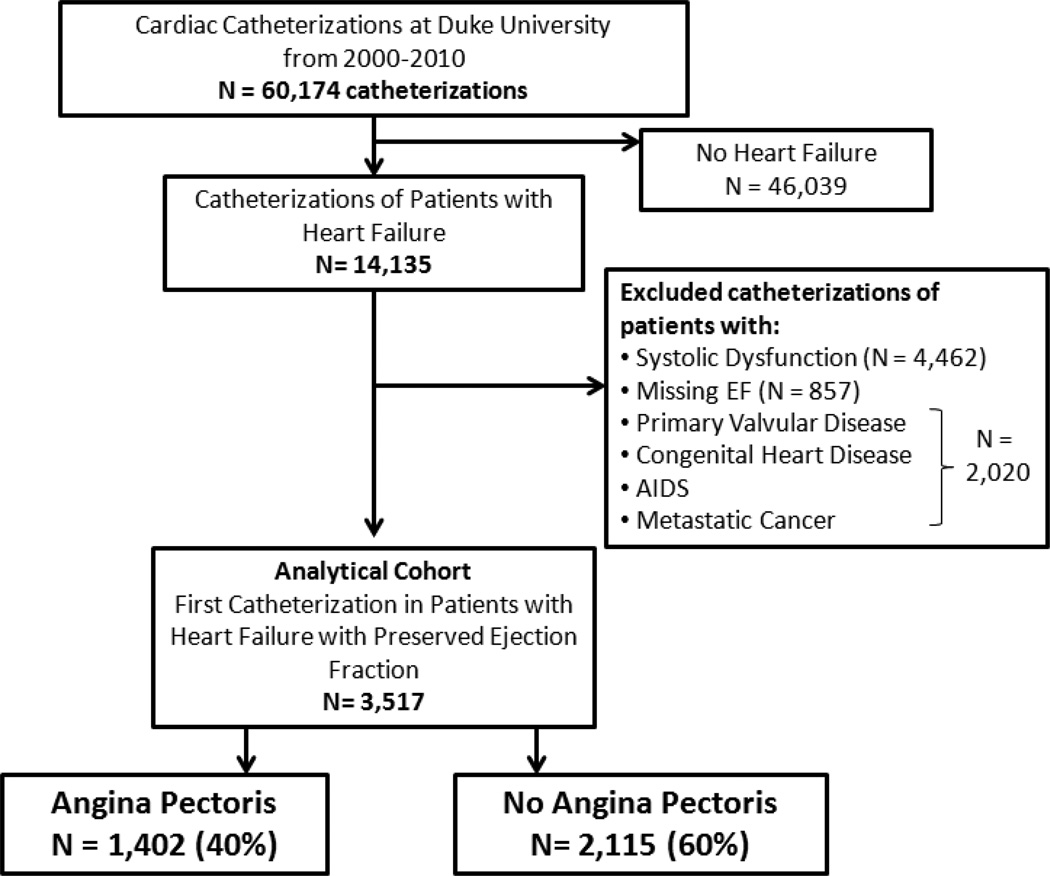
A total of 3517 patients met the criteria for the study (Figure 1) and 1402 (40%) had AP. In the AP cohort, 48% had typical angina and 49% had atypical angina in the previous 6 weeks. AP was described as stable, progressing or unstable in 24%, 47% and 27% of patients in the preceding 6 weeks, respectively. Using a modification of the Canadian Cardiovascular Society (CCS) angina grade(11), the percentage of AP patients with CCS class I (no symptoms with ordinary activity), II (symptoms with moderate exertion), III (symptoms with ordinary exertion), IV (symptoms with any exertion or at rest) and symptoms unrelated to exertion were 0.2%, 13.3%, 15.0%, 41.5% and 30.1%, respectively. The median frequency per week of chest pain episodes was 4 (interquartile range: 3–7).
Figure 1.
Patients included in this analysis.
Abbreviation: AIDS=acquired immunodeficiency syndrome.
Baseline characteristics for the AP and no AP groups are provided in Table 1. As expected, a number of baseline characteristics differed significantly between the cohorts, with AP patients tending to be older and more likely to have a prior history of hypertension, diabetes, hyperlipidemia, vascular disease, smoking and coronary revascularization. Notably, those with AP tended to have less severe NYHA class symptoms and were less likely to have rales or an S3 gallop. Systolic blood pressure was significantly higher in the AP group. The basic laboratory parameters were fairly similar between the two groups even though there were statistically significant differences in several of the laboratory parameters due to the large sample size. AP patients more often received beta-blockers, ACE-inhibitors, nitrates, and statins but were less likely to receive diuretics as compared to non-AP patients. In this HFpEF population, both groups had fairly high baseline use of beta-blockers and ACE-inhibitors, but modest use of calcium channel blockers, nitrates and hydralazine. In the AP group, 77% of patients received a beta-blocker, calcium channel blocker or nitrate at baseline compared to 68% in the non-AP group.
Table 1.
Baseline patient characteristics.
| Variable | Angina Pectoris (Past 6 Weeks) |
P-value | |
|---|---|---|---|
| NO (N=2115) |
YES (N=1402) |
||
| Age (years) | 62 (51, 71) | 65 (55, 73) | <0.001 |
| Men | 44% | 48% | 0.062 |
| Race | |||
| White | 69% | 69% | <0.001 |
| Black | 28% | 26% | |
| Hypertension | 58% | 78% | <0.001 |
| Diabetes mellitus | 29% | 41% | <0.001 |
| Prior myocardial infarction | 13% | 26% | <0.001 |
| Hyperlipidemia* | 37% | 66% | <0.001 |
| 3 vessel coronary disease | 13% | 24% | <0.001 |
| Ejection fraction (%) | 60 (55–60) | 60 (55–67) | <0.001 |
| New York Heart Association III, IV | 75% | 60% | <0.001 |
| Cerebrovascular disease | 9% | 15% | <0.001 |
| Peripheral vascular disease | 5% | 13% | <0.001 |
| Previous smoking | 36% | 48% | <0.001 |
| Previous percutaneous coronary intervention | 6% | 23% | <0.001 |
| Previous coronary bypass | 12% | 25% | <0.001 |
| Indications for index catheterization | |||
| Shortness of breath | 53% | 35% | <0.001 |
| Heart failure | 39% | 30% | <0.001 |
| Charlson Index | |||
| 0 | 49% | 38% | |
| 1 | 31% | 33% | <0.001 |
| ≥ 2 | 20% | 29% | |
| Body mass index (kg/m2) | 28 (24, 35) | 31 (26, 36) | <0.001 |
| Heart rate (beats/minute) | 78 (68, 90) | 71 (62, 83) | <0.001 |
| Systolic blood pressure (mmHg) | 135 (119, 153) | 147 (130, 166) | <0.001† |
| Rales | 20% | 14% | <0.001 |
| S3 gallop | 9% | 4% | <0.001 |
| LVEDP, mmHg‡ | 16 (11–22) | 16 (12–22) | 0.37 |
| Serum sodium (mmol/L) | 139 (137, 141) | 140 (138, 141) | 0.003 |
| Blood urea nitrogen (mg/dL) | 20 (14, 30) | 18 (13, 25) | <0.001 |
| Serum creatinine (mg/dL) | 1.1 (0.9, 1.5) | 1.1 (0.9, 1.4) | 0.016 |
| Hemoglobin (g/dL) | 12.6 (11.0, 14.0) | 12.9 (11.5, 14.1) | <0.001 |
| Beta-blocker use | 60% | 72% | <0.001 |
| ACE-inhibitor/Angiotensin receptor blocker | 54% | 64% | <0.001 |
| Hydralazine use | 11% | 7% | <0.001 |
| Nitrates use | 12% | 15% | 0.006 |
| Calcium channel blocker use | 42% | 45% | 0.12 |
| Ranolazine use§ | 0.6% | 0.4% | 0.29 |
| Aspirin use | 58% | 77% | <0.001 |
| Clopidogrel use | 18% | 37% | <0.001 |
| Statin use | 39% | 60% | <0.001 |
| Diuretic use | 71% | 66% | <0.001 |
Expressed as %, or median (Q1, Q3).
Hyperlipidemia = Cholesterol >200 mg/dl, low-density lipoprotein >130 mg/dl, high-density lipoprotein <30 mg/dl, or triglycerides >150 mg/dl.
P-value here is calculated using a T-test.
LVEDP=left ventricular end-diastolic pressure. This information was available for 2280 patients (65%) of the overall cohort; 1104 patients (52%) in the no AP group and 1176 patients (84%) in the AP group.
Ranolazine was FDA approved in 2006.
The median follow-up time for all patients was 4.0 years (interquartile range: 1.6–7.6). Five-year unadjusted KM survival for the study population was 66.3%. AP patients were observed to have a significantly increased event rate for the primary endpoint of MACE as well as death/MI/revascularization (Table 2). Of note, many of the events for the MACE composite occurred early following the index catheterization (30-day and 6-month unadjusted KM event rates of 32.4% and 37.4% for the AP group, respectively). In contrast, the event rates were lower in the AP patients for the endpoints of death/MI and death compared to those without AP (both P<0.05). There were no significant differences between the event rates in those with and without AP for the endpoints of death/MI/stroke, and cardiovascular death/cardiovascular hospitalization (Table 2).
Table 2.
Five- and ten-year unadjusted event rates for those with and without angina pectoris.
| End point | 5 Year | 10 Year | P- Value* |
||
|---|---|---|---|---|---|
| Angina Pectoris | Angina Pectoris | ||||
| NO | YES | NO | YES | ||
| Death/myocardial infarction/revascularization/stroke | |||||
| Events for composite (first events) | 848 | 797 | 948 | 910 | |
| Death | 528 | 299 | 608 | 382 | |
| Myocardial infarction | 19 | 15 | 22 | 16 | |
| Revascularization | 222 | 425 | 230 | 436 | |
| Stroke | 79 | 58 | 88 | 76 | |
| KM rate for composite (95% CI) | 47.0 (44.6, 49.5) | 59.2 (56.6, 61.9) | 61.7 (58.5, 64.9) | 73.6 (70.7, 76.4) | <0.001 |
| Death/myocardial infarction/revascularization | |||||
| Events for composite (first events) | 803 | 768 | 908 | 876 | |
| Death | 525 | 243 | 614 | 336 | |
| Myocardial infarction | 30 | 23 | 37 | 27 | |
| Revascularization | 248 | 502 | 257 | 513 | |
| KM rate for composite (95% CI) | 44.5 (42.1, 47.0) | 57.1 (54.4, 59.8) | 59.8 (56.5, 63.1) | 71.2 (68.2, 74.1) | <0.001 |
| Death/myocardial infarction/stroke | |||||
| Events for composite (first events) | 716 | 502 | 844 | 693 | |
| Death | 573 | 325 | 680 | 468 | |
| Myocardial infarction | 42 | 53 | 49 | 73 | |
| Stroke | 101 | 124 | 115 | 152 | |
| KM rate for composite (95% CI) | 40.9 (38.5, 43.3) | 38.4 (35.7, 41.1) | 58.2 (54.9, 61.4) | 60.1 (57.0, 63.2) | 0.33 |
| Death/myocardial infarction | |||||
| Events for composite (first events) | 657 | 428 | 790 | 621 | |
| Death | 612 | 369 | 735 | 536 | |
| Myocardial infarction | 45 | 59 | 55 | 85 | |
| KM rate for composite (95% CI) | 37.7 (35.3, 40.1) | 32.9 (30.4, 35.5) | 55.4 (52.2, 58.8) | 55.3 (52.1, 58.6) | 0.019 |
| Death | |||||
| Events | 632 | 391 | 767 | 583 | |
| KM rate (95% CI) | 36.2 (33.9, 38.5) | 30.1 (27.6, 32.7) | 54.2 (50.9, 57.5) | 52.7 (49.5, 56.0) | 0.002 |
| Cardiovascular death/cardiovascular hospitalization | |||||
| Events for composite (first events) | 1115 | 787 | 1179 | 881 | |
| Cardiovascular death | 199 | 82 | 212 | 105 | |
| Cardiovascular hospitalization | 916 | 705 | 967 | 776 | |
| KM rate for composite (95% CI) | 63.7 (61.2, 66.2) | 61.1 (58.4, 63.9) | 75.0 (71.8, 78.1) | 75.9 (72.7, 78.9) | 0.12 |
P-value represents the result of a Log-Rank Test between those with angina pectoris and those without over the entire follow up for the composite endpoints.
Abbreviation: KM=Kaplan-Meier, CI=Confidence Interval.
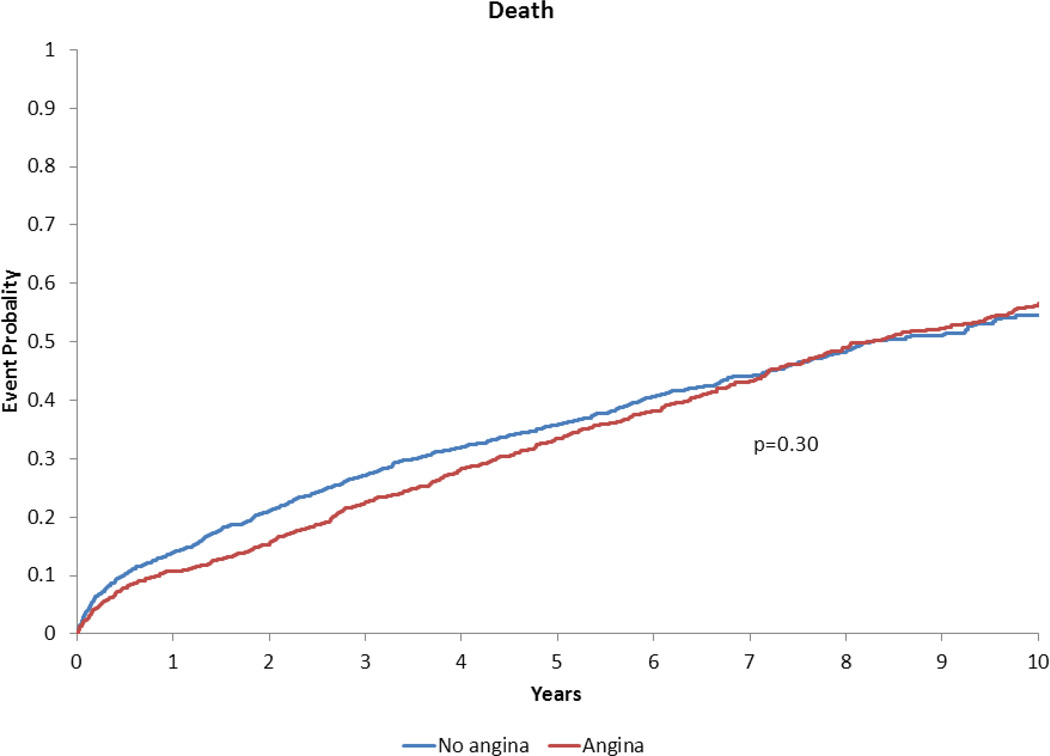
Following risk adjustment, AP was associated with a significantly higher risk of MACE and death/MI/revascularization compared to no AP (both P<0.0001) (Table 3). AP was an independent predictor of MACE (Hazard Ratio 1.30; 95% Confidence Interval, 1.17–1.45) (Table 3). Patients with and without AP had similar risk for death/MI/stroke, death/MI, death, and cardiovascular death/cardiovascular hospitalization (all P>0.06) (Table 3). Results for the composite endpoint of all-cause death/hospitalization in those with AP (adjusted HR 0.91; 95% CI, 0.83–1.00; P=0.056) were similar to the results for cardiovascular death/cardiovascular hospitalization. The adjusted time-to-event plots in patients with vs. without AP are presented in Figures 2–5.
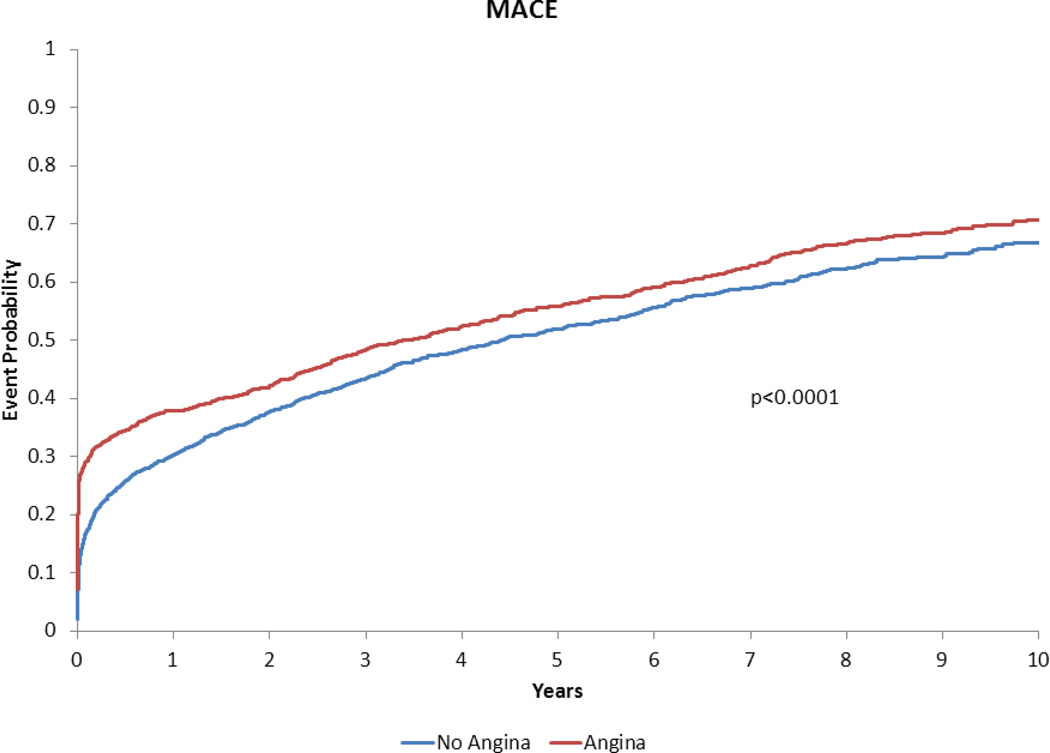
Figure 2.
Adjusted time-to-event plot for death, myocardial infarction, revascularization or stroke in heart failure with preserved ejection fraction patients with vs. without angina pectoris*.
*Adjusted for variables listed in Table 3 footnote.
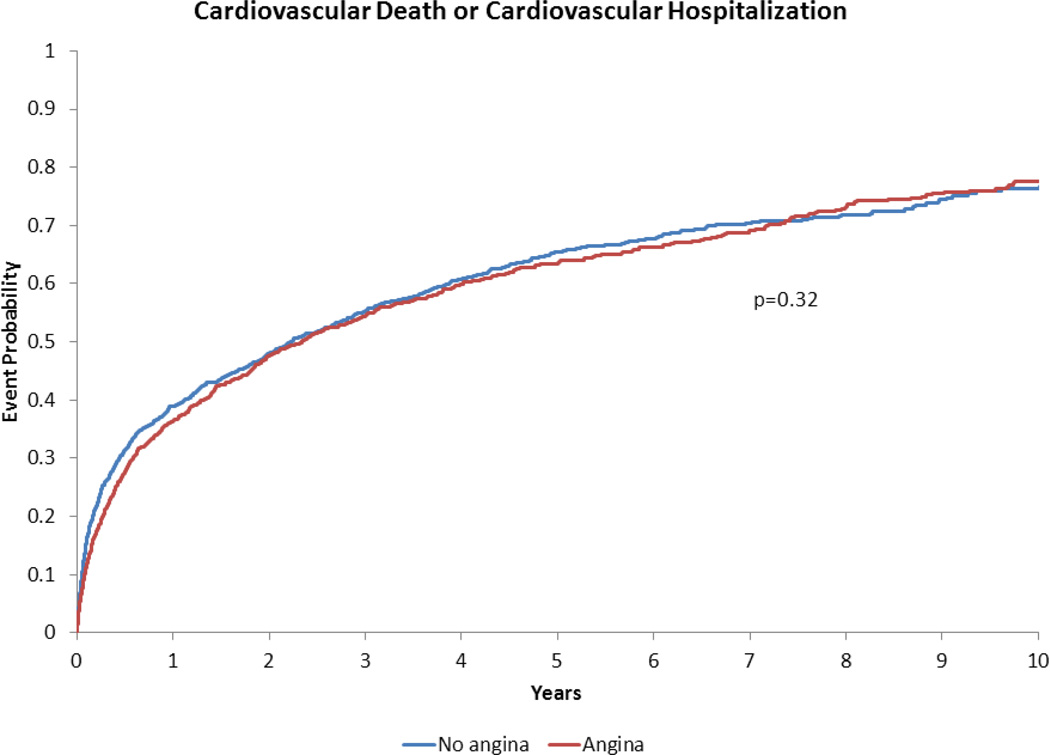
Figure 5.
Adjusted time-to-event plot for cardiovascular death, or cardiovascular hospitalization in heart failure with preserved ejection fraction patients with vs. without angina pectoris*.
*Adjusted for variables listed in Table 3 footnote.
Discussion
There were several important findings from this study. First, AP was common in this HFpEF cohort despite medical therapy and previous revascularization. Most of these patients had angina that was progressive or unstable in the preceding weeks, with more than half experiencing Canadian Cardiovascular Society class III or IV symptoms. Second, HFpEF patients with AP had more comorbidities and more prior revascularization procedures than non-AP patients. After multivariable risk adjustment, those with recent AP were at significantly increased risk for MACE. However, AP was not associated with increased risk for death/MI/stroke, death/MI, death or cardiovascular death/hospitalization following adjustment for baseline characteristics. Thus, AP was an independent predictor of major adverse cardiac events driven by increased revascularization, but was not associated with increased risk of death, MI, stroke or rehospitalization.
While the prevalence of AP in HFpEF patients is lower than in patients with HFrEF(2,6), a significant percentage of HFpEF patients suffer from AP. We found that 40% of HFpEF patients had AP despite previous revascularization (25% with prior CABG and 23% with prior PCI) and high usage of beta-blockers. The modest usage of calcium channel blockers, nitrates and ranolazine in this cohort suggests that there is room for significant improvement in the use of medical therapies to reduce AP in these patients(1). These findings are particularly relevant in the context of the paucity of treatments for HFpEF patients. Potentially, by targeting angina symptoms with presently available medical therapies, the morbidity related to repeat revascularizations in HFpEF patients could be reduced. Despite the relative contraindication to calcium channel blockers in HFrEF patients(12), further investigation is needed to define their use as anti-anginals in HFpEF patients.
The AP patients in this cohort had a distinct phenotype from those without AP. Specifically, the AP patients were less likely to have rales, an S3 or baseline diuretic use and tended to have a lower NYHA class. Thus, these patients may have had more prominent anginal symptoms rather than volume overload with fatigue and dyspnea, which are used to characterize NYHA class.
The death/MI/revascularization/stroke event curves for the cohorts began to diverge early (i.e. within the first 6 months) with a persistent effect up to 10 years after the index catheterization. After adjusting for baseline comorbidities and medication use, AP remained a strong independent predictor of MACE. It was found that AP was associated with a 30% increased risk of long-term death/MI/revascularization/stroke. These findings, along with the lack of association between AP and other endpoints on adjusted analysis, suggest that the implications for AP are most strongly correlated with increased revascularization. These results support previous data that revascularization may be performed to relieve anginal symptoms, but may not improve prognosis unless the patient demonstrates other high-risk features(1).
These results have important clinical applications given the procedural costs and quality of life implications for revascularization procedures. Previous studies have also suggested that HFpEF patients with coronary disease who present with pulmonary edema tend to have recurrence of pulmonary edema despite revascularization(13). Thus, a reappraisal of the utility of revascularization in HFpEF patients may be warranted given potential limitations in preventing HF decompensation. Future studies will need to explore whether improved management of AP may reduce revascularization rates.
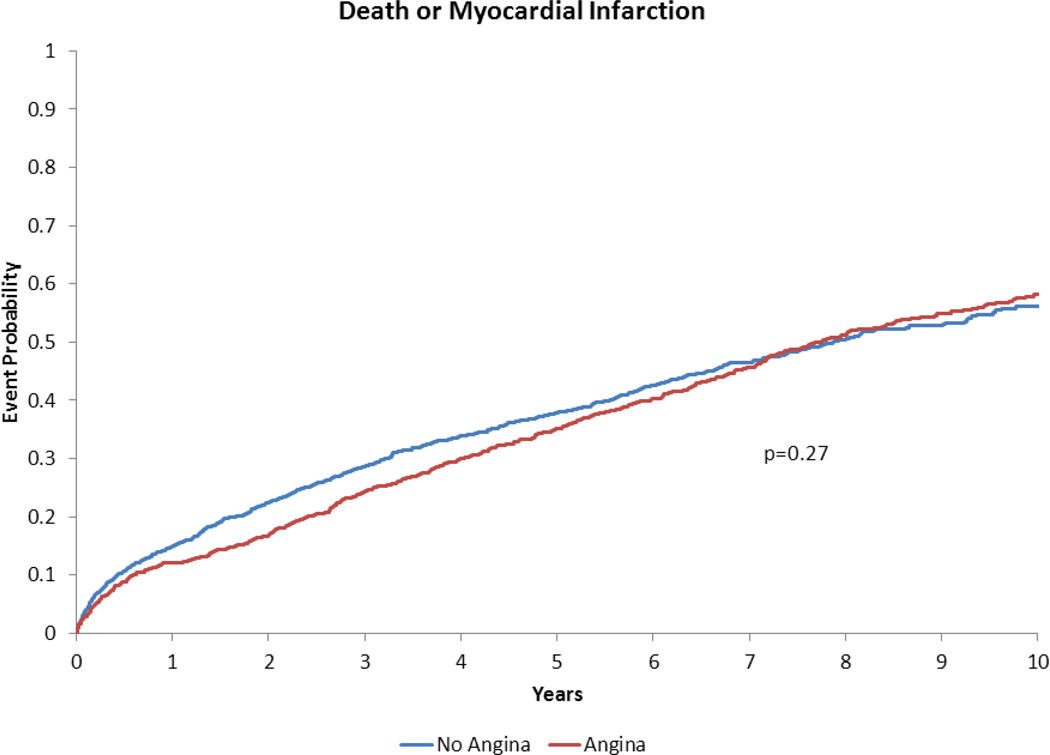
Our findings that AP did not portend increased death or hospitalization following risk adjustment is concordant with previous studies of stable AP in the general (i.e., non-HF) population. Follow-up of the Angina Prognosis Study in Stockholm (APSIS) demonstrated that patients with stable AP had similar all-cause mortality compared to patients without AP over a median follow-up of 9 years(14). We have previously shown that AP in the ischemic cardiomyopathy population with reduced EF was not associated with increased long-term death, death/MI or death/all-cause hospitalization(2). The present study extends these results into the HFpEF population.
The observation that AP was associated with reduced death and death/MI on unadjusted analysis was unexpected. Potential reasons for reduced mortality associated with angina include increased use of prevention therapies (e.g., aspirin, statins), heightened physician follow-up, “ischemic preconditioning” protecting against subsequent adverse outcomes(15,16) or statistical chance. Interestingly, the between-group difference in outcomes narrowed over time. Our previous study in the reduced EF population demonstrated a trend toward reduced mortality associated with AP on unadjusted analysis(2). Similar to the present results, the association between AP and death in reduced EF patients was further attenuated with risk adjustment. Thus, these data present consistent evidence that AP is not associated with mortality across the spectrum of HF patients when baseline characteristics are accounted for.
Our findings should be considered in the context of several limitations. The DDCD captures a subset of cardiac patients undergoing cardiac catheterization, which limits the population studied and may not reflect event rates in a broader population. For instance, the requirement to undergo cardiac catheterization likely reduced the age of the patients in the study cohort compared to other HFpEF datasets. On the other hand, the robust representation of both women and minorities in the DDCD provides important insight into patient characteristics and outcomes in frequently underrepresented patient groups. A limitation related to this dataset is that only those patients with a history of significant coronary artery disease receive DDCD follow-up. Further empiric testing is required to explore outcomes in HFpEF patients without epicardial coronary disease, since underlying significant CAD likely influenced subsequent revascularization considerations. Given this study’s long accrual time, the subjective AP classification was recorded by many investigators such that there was inherent variability in the databank. This is a recognized limitation of the databank but also represents the reality of clinical practice where clinicians may categorize subjective symptoms differently. It is also possible that patients in both the AP and non-AP groups would be weighted toward those with a higher index of suspicion for intervenable CAD. Future studies should also explore whether the degree of ischemia confounds the association between AP and outcomes, since chest pain in HFpEF patients does not always represent underlying myocardial ischemia. Our use of AP classification at a single time point (index catheterization) is another potential limitation, since we did not investigate persistent AP or the relation of a subsequent revascularization to AP. Given the multiple analyses conducted in the present study, these results should be viewed as exploratory given the increased likelihood of a Type I error. Our study provides the foundation for future studies of AP in HFpEF in an attempt to improve patients’ symptoms and reduce revascularization rates.
Conclusion
AP in HFpEF patients with a history of coronary artery disease is common despite medical therapy and prior revascularization and is independently associated with increased MACE due to revascularization with similar risk of death, MI, stroke and hospitalization. Given the paucity of treatments for HFpEF patients, these data provide the foundation for pharmacologic studies targeting anginal symptoms to reduce the morbidity associated with repeat revascularizations. Future prospective studies of angina in HFpEF patients are warranted.
Figure 3.
Adjusted time-to-event plot for death or myocardial infarction in heart failure with preserved ejection fraction patients with vs. without angina pectoris*.
*Adjusted for variables listed in Table 3 footnote.
Figure 4.
Adjusted time-to-event plot for death in heart failure with preserved ejection fraction patients with vs. without angina pectoris*.
*Adjusted for variables listed in Table 3 footnote.
Acknowledgments
Disclosures: R.J.M, M.F., and C.M.O have received research funding from Gilead Sciences, Inc.
Abbreviations
- AP
angina pectoris
- HFpEF
heart failure with preserved ejection fraction
- EF
ejection fraction
- NYHA
New York Heart Association
- MI
myocardial infarction
- CCS
Canadian Cardiovascular Society
- MACE
major adverse cardiac events
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fraker TD, Jr, Fihn SD, Gibbons RJ, et al. 2007 chronic angina focused update of the ACC/AHA 2002 guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines Writing Group to develop the focused update of the 2002 guidelines for the management of patients with chronic stable angina. J Am Coll Cardiol. 2007;50:2264–2274. doi: 10.1016/j.jacc.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Mentz RJ, Fiuzat M, Shaw LK, et al. Comparison of Clinical characteristics and long-term outcomes of patients with ischemic cardiomyopathy with versus without angina pectoris (from the Duke Databank for Cardiovascular Disease) Am J Cardiol. 2012;109:1272–1277. doi: 10.1016/j.amjcard.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 3.Udelson JE. Heart failure with preserved ejection fraction. Circulation. 2011;124:e540–e543. doi: 10.1161/CIRCULATIONAHA.111.071696. [DOI] [PubMed] [Google Scholar]
- 4.Steinberg BA, Zhao X, Heidenreich PA, et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 2012;126:65–75. doi: 10.1161/CIRCULATIONAHA.111.080770. [DOI] [PubMed] [Google Scholar]
- 5.Rehnqvist N, Hjemdahl P, Billing E, et al. Effects of metoprolol vs verapamil in patients with stable angina pectoris. The Angina Prognosis Study in Stockholm (APSIS) Eur Heart J. 1996;17:76–81. doi: 10.1093/oxfordjournals.eurheartj.a014695. [DOI] [PubMed] [Google Scholar]
- 6.Felker GM, Shaw LK, O'Connor CM. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol. 2002;39:210–218. doi: 10.1016/s0735-1097(01)01738-7. [DOI] [PubMed] [Google Scholar]
- 7.Harris PJ, Lee KL, Harrell FE, Jr, Behar VS, Rosati RA. Outcome in medically treated coronary artery disease. Ischemic events: nonfatal infarction and death. Circulation. 1980;62:718–726. doi: 10.1161/01.cir.62.4.718. [DOI] [PubMed] [Google Scholar]
- 8.Boyle CA, Decoufle P. National sources of vital status information: extent of coverage and possible selectivity in reporting. Am J Epidemiol. 1990;131:160–168. doi: 10.1093/oxfordjournals.aje.a115470. [DOI] [PubMed] [Google Scholar]
- 9.Douglas PS, Ginsburg GS. The evaluation of chest pain in women. N Engl J Med. 1996;334:1311–1315. doi: 10.1056/NEJM199605163342007. [DOI] [PubMed] [Google Scholar]
- 10.Canto JG, Fincher C, Kiefe CI, et al. Atypical presentations among Medicare beneficiaries with unstable angina pectoris. Am J Cardiol. 2002;90:248–253. doi: 10.1016/s0002-9149(02)02463-3. [DOI] [PubMed] [Google Scholar]
- 11.Campeau L. The Canadian Cardiovascular Society grading of angina pectoris revisited 30 years later. Can J Cardiol. 2002;18:371–379. [PubMed] [Google Scholar]
- 12.Mahe I, Chassany O, Grenard AS, Caulin C, Bergmann JF. Defining the role of calcium channel antagonists in heart failure due to systolic dysfunction. Am J Cardiovasc Drugs. 2003;3:33–41. doi: 10.2165/00129784-200303010-00004. [DOI] [PubMed] [Google Scholar]
- 13.Kramer K, Kirkman P, Kitzman D, Little WC. Flash pulmonary edema: association with hypertension and reoccurrence despite coronary revascularization. Am Heart J. 2000;140:451–455. doi: 10.1067/mhj.2000.108828. [DOI] [PubMed] [Google Scholar]
- 14.Hjemdahl P, Eriksson SV, Held C, Forslund L, Nasman P, Rehnqvist N. Favourable long term prognosis in stable angina pectoris: an extended follow up of the angina prognosis study in Stockholm (APSIS) Heart. 2006;92:177–182. doi: 10.1136/hrt.2004.057703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kloner RA, Shook T, Przyklenk K, et al. Previous angina alters in-hospital outcome in TIMI 4. A clinical correlate to preconditioning? Circulation. 1995;91:37–45. doi: 10.1161/01.cir.91.1.37. [DOI] [PubMed] [Google Scholar]
- 16.Abete P, Ferrara N, Cacciatore F, et al. Angina-induced protection against myocardial infarction in adult and elderly patients: a loss of preconditioning mechanism in the aging heart? J Am Coll Cardiol. 1997;30:947–954. doi: 10.1016/s0735-1097(97)00256-8. [DOI] [PubMed] [Google Scholar]