Abstract
Metabolic acidosis is a common complication of chronic kidney disease and believed to contribute to a number of sequelae, including bone disease, altered protein metabolism, skeletal muscle wasting, and progressive GFR loss. Small trials in animal models and humans suggest a role for alkali therapy to lessen these complications. Recent studies support this notion, although more definitive evidence is needed on the long-term benefits of alkali therapy and the optimal serum bicarbonate level. The role of dietary modification should also be given greater consideration. In addition, potential adverse effects of alkali treatment must be taken into consideration, including sodium retention and the theoretical concern of promoting vascular calcification. This teaching case summarizes the rationale for and the benefits and complications of base therapy in patients with chronic kidney disease.
Index Words: metabolic acidosis, chronic kidney disease, bicarbonate, alkali therapy
INTRODUCTION
Metabolic acidosis is associated with many of the complications of chronic kidney disease (CKD), including bone disease, muscle protein catabolism, and progressive glomerular filtration rate (GFR) loss. The Kidney Dialysis Outcomes Quality Initiative (KDOQI) guidelines, based on “evidence and opinion,” call for maintenance of serum bicarbonate ≥22 mEq/L to lessen these complications.1 A 2007 Cochrane review of alkali therapy in CKD found insufficient evidence for benefit.2 The evidence base has expanded since then and lends further support to the benefits of alkali therapy. However, a definitive randomized clinical trial has not yet been performed.
CASE REPORT
Clinical History and Initial Laboratory Data
A 58-year-old man with a history of sickle cell disease, hepatitis B, hypertension, and CKD stage 4 was seen in renal clinic. He had a progressive decline in kidney function over 13 years with a urine protein-to-creatinine ratio of 1.7 g/g. His kidney function had been stable for the past 2 years. Medications included nifedipine 60 mg daily, calcitriol 0.5mg daily, and folic acid. Physical examination revealed a blood pressure of 130/72 mmHg and no peripheral edema or signs of heart failure. Relevant laboratory data from one month prior and the day of the visit appear in Table 1.
Table 1.
Laboratory Data
| 1 month prior | Day of the visit | 3 months later | 12 months later | 16 months later | |
|---|---|---|---|---|---|
| Serum chemistry | |||||
| Sodium (mEq/L) | 140 | 139 | 141 | 140 | 141 |
| Potassium (mEq/L) | 5.8 | 5.4 | 4.6 | 4.4 | 4.9 |
| Chloride (mEq/L) | 111 | 110 | 108 | 101 | 106 |
| Bicarbonate (mEq/L) | 19 | 18 | 23 | 25 | 25 |
| Urea nitrogen (mg/dL) | 47 | 64 | 35 | 43 | 46 |
| Creatinine (mg/dL) | 3.4 | 3.3 | 3.8 | 4.9 | 4.2 |
| eGFR (ml/min/1.73m2) | 23 | 23 | 20 | 15 | 18 |
| Calcium (mg/dL) | 10.1 | 9.4 | 10.0 | 11.0* | 10.0 |
| Phosphorus (mg/dL) | 3.5 | 3.7 | - | 3.7 | 3.0 |
| Anion gap (mEq/L) | 10 | 11 | 10 | 14 | 10 |
| Albumin (g/dL) | - | 3.4 | 3.5 | 4.3 | 3.8 |
| PTH (pg/mL) | 309# | 229 | 273 | 140 | 241 |
| Urinalysis | |||||
| Specific gravity | 1.013 | - | - | 1.015 | - |
| pH | 6.0 | - | - | 7.0 | - |
Note: Conversion factors for units: serum creatinine in mg/dL to μmol/L, ×84.4; serum urea nitrogen in mg/dL to mmol/L, ×0.357; serum calcium in mg/dL to mmol/L, ×0.2495; serum phosphorous in mg/dL to mmol/L, ×0.3229.
Abbreviations: eGFR, estimated glomerular filtration rate calculated using the 4-variable Modification of Diet in Renal Disease Study equation; PTH, parathyroid hormone.
Hypercalcemia was secondary to exogenous activated vitamin D intake. Calcitriol was stopped.
PTH measured two months prior to the day of the visit.
Additional Investigations
Prior laboratory showed a metabolic acidosis 7 years earlier with a serum bicarbonate of 20 mEq/L and an estimated GFR of 38 ml/min/1.73 m2. Earlier urinalyses revealed a urine pH consistently above 6.0.
Diagnosis
Chronic metabolic acidosis (presumed given the lack of confirmatory pH and pCO2) and hyperkalemia in the setting of CKD, sickle cell disease, and distal nephron dysfunction.
Clinical Follow-up
The patient was started on oral sodium bicarbonate 1300 mg three times daily and returned to the clinic one month later. The blood pressure was 156/82 mmHg with trace peripheral edema. Nifedipine was increased to 90 mg daily. Three months later, the blood pressure was 122/67 mmHg with no peripheral edema. A basic metabolic panel revealed improvement in the serum bicarbonate (Table 1). Calcitriol was increased to 1 mg daily due to the persistently elevated parathyroid hormone (PTH) level. A year later the patient was found to have decreasing kidney function thought to be secondary to progression of CKD and hypercalcemia. After cessation of calcitriol and normalization of the serum calcium, kidney function improved. Sixteen months later, serum bicarbonate was maintained between 23 and 25 mEq/L and the serum potassium remained in the normal range. There was a slight increase in serum albumin, but no change in body weight (77 to 79 kg).
DISCUSSION
Metabolic acidosis is a common complication of advanced CKD, present in 30-50% of individuals with eGFR <30 ml/min/1.73 m2.3-5 It develops mainly secondary to reduced kidney mass and an inability to excrete the daily acid load via ammoniagenesis. Patients with CKD and acidosis have a positive acid balance of approximately 10-20 mEq/day.6-8 In a minority of patients, there is bicarbonate loss in the urine due to failure of renal bicarbonate conservation.9 The degree of acidosis in CKD is usually not severe. In uncomplicated acidosis, the serum bicarbonate is typically greater than 12 mEq/L, the blood pH >7.2, and the anion gap variable.9-11 The acidosis remains relatively stable, but can worsen as GFR declines.3,6 Some patients with CKD maintain close to normal serum bicarbonate levels even with severely compromised kidney function, including patients with diabetes who often develop less severe acidosis.12 Conversely, in patients with hypoaldosteronism or tubulointerstitial disease, metabolic acidosis often occurs with eGFR >30 ml/min/1.73 m2.13,14 The patient in this case developed metabolic acidosis before the eGFR fell below 30 ml/min/1.73 m2 with a persistently high urine pH. This suggests collecting duct damage with impaired acidification related to the patient’s sickle cell disease.
Low serum bicarbonate has been associated with increased mortality in patients with moderate and advanced CKD, as well as among patients receiving both peritoneal dialysis (PD) and hemodialysis.15-18 Chronic metabolic acidosis may have various adverse effects in patients with CKD (Table 2) on bone disease, insulin resistance, and muscle wasting, and it may contribute to the progression of kidney disease. Chronic acidosis results in negative calcium balance from bone buffering, which is ameliorated by treatment with alkali.19 There is so far no direct evidence that alkali therapy improves bone density in patients with pre-dialysis CKD. However, in children with renal tubular acidosis, sustained alkali therapy attained and maintained normal stature.20 In postmenopausal women with normal kidney function, administration of potassium bicarbonate to neutralize endogenous acid improved calcium and phosphorus balance, reduced bone resorption, and increased bone formation.21 Furthermore, in patients receiving hemodialysis, oral sodium bicarbonate supplementation to achieve pre-dialysis serum bicarbonate levels of 24 mEq/L decreased progression of secondary hyperparathyroidism in patients with high bone turnover and stimulated bone turnover in patients with low bone formation.22 Treatment of acidosis may also increase the sensitivity of the parathyroid glands to calcium.23 In our patient, PTH declined in the year after alkali therapy was initiated, but this may have been due to the concurrent increase in calcitriol dose.
Table 2.
Selected adverse effects of chronic metabolic acidosis in chronic kidney disease, and evidence for alkali therapy
| Adverse effect | Evidence for alkali therapy | |
|---|---|---|
| Bone disease | Enhances bone resorption and impairs bone formation48 | Reduces calcium losses in pre-dialysis CKD; has benefit in children with RTA20, postmenopausal women with normal kidney function21 and dialysis patients22 |
| Protein metabolism | Decreases albumin synthesis, increases muscle proteolysis, and causes negative nitrogen balance26 | Decreases protein degradation and may increase albumin concentration.27,31 The data is conflicting in dialysis patients.49,50 |
| Muscle mass | May contribute to muscle wasting24,25 | May increase muscle mass and improve function.31,34 |
| Kidney function | Could contribute to the progression of CKD | May slow the progression to kidney failure and development of ESRD31,37,38 |
Abbreviations: CKD, chronic kidney disease; RTA, renal tubular acidosis; ESRD, end stage renal disease
A large body of evidence suggests that metabolic acidosis is an important contributor to muscle wasting in patients with CKD. Acidosis impairs insulin and insulin-like growth factor (IGF)-1 signaling, leading to skeletal muscle protein breakdown via activation of caspase-3 and the ubiquitin-proteasome system.24,25 Chronic acidosis causes negative nitrogen balance and decreases albumin synthesis.26 Treating acidosis ameliorates the insulin signaling defect and decreases muscle breakdown.27,28 In a randomized, double-blind, placebo-controlled trial in 60 PD patients, oral sodium bicarbonate improved nutritional status and reduced hospitalizations.29 Correction of acidosis in another single-blinded trial of 200 PD patients resulted in increased lean body mass and fewer hospitalizations. 30 Interventional studies in hemodialysis patients have been inconsistent, and limited by small sample sizes, lack of control groups, and unmasked interventions. In patients with CKD stage 4, oral sodium bicarbonate increased albumin levels and mid-arm muscle circumference after 12 months.31 Currently, few studies have examined functional outcomes related to acidosis.32,33 In one single-blinded pilot study, increased lower-extremity muscle strength was seen after 6 weeks of oral sodium bicarbonate.34
Metabolic acidosis may also contribute to the progression of CKD by promoting tubulointerstitial injury via ammonia-induced complement activation and endothelin and aldosterone activation.35,36 In an open-label, randomized, prospective parallel-group study, de Brito-Ashurst et al. assigned 134 patients with CKD stage 4 and serum bicarbonate 16-20 mEq/L to oral sodium bicarbonate to maintain a bicarbonate level ≥23 mEq/L or to standard-of-care. Bicarbonate supplementation slowed the rate of GFR loss and reduced progression to end stage renal disease (ESRD) requiring dialysis.31 Phisitkul et al. noted similar findings in patients with hypertensive nephropathy with serum total carbon dioxide <22 mEq/L.37 Thirty patients prescribed sodium citrate were compared to 29 controls who were unable or unwilling to take the medication. After 24 months, urine endothelin-1 excretion was significantly lower in the treatment group, as was the rate of eGFR decline. Lastly, a 5-year randomized, placebo-controlled, blinded study compared sodium bicarbonate with placebo or equimolar sodium chloride (n=40 per group).38 The rate of eGFR decline was slower in patients treated with sodium bicarbonate (-1.47±0.19 ml/min per year) than in those given placebo (-2.13±0.19 ml/min per year) or sodium chloride (-2.05±0.19 ml/min per year).
The decision to initiate treatment of metabolic acidosis should be based on the severity of the acidosis as well as consideration of the patient’s clinical condition (Figure 1). Even large doses of alkali appear to be well-tolerated in individuals without edema who have preserved urine output. Given the variability of serum bicarbonate measurements, the presence of acidosis should be verified with a second measurement. In addition, in most cases the serum bicarbonate alone should not be the basis for starting treatment. A blood gas should be obtained to verify the acid-base disturbance, especially if there is suspicion of a respiratory disorder. A venous blood gas is sufficient for these purposes. In our patient with advanced CKD, treatment was appropriately begun after a second low serum bicarbonate measurement, although without a confirmatory blood gas. The initial prescribed dose was higher than we would generally recommend, but was tolerated in this patient with well-controlled hypertension and no edema. The need for a high dose might have been predicted based on the inappropriately high urine pH indicative of a defect in urine acidification from sickle cell disease. The serum potassium normalized after starting alkali, which may have been partly been due to the kaliuretic effect of sodium bicarbonate.34,39 Poor nutritional intake with advancing CKD also could have contributed, but this seems less likely given the increase in serum albumin and lack of weight loss. Fortunately, the patient did not experience gastrointestinal side effects and was able to continue treatment with long-term maintenance of a normal to low-normal serum bicarbonate.
Figure 1. Algorithm for treating chronic metabolic acidosis in patients with chronic kidney disease.
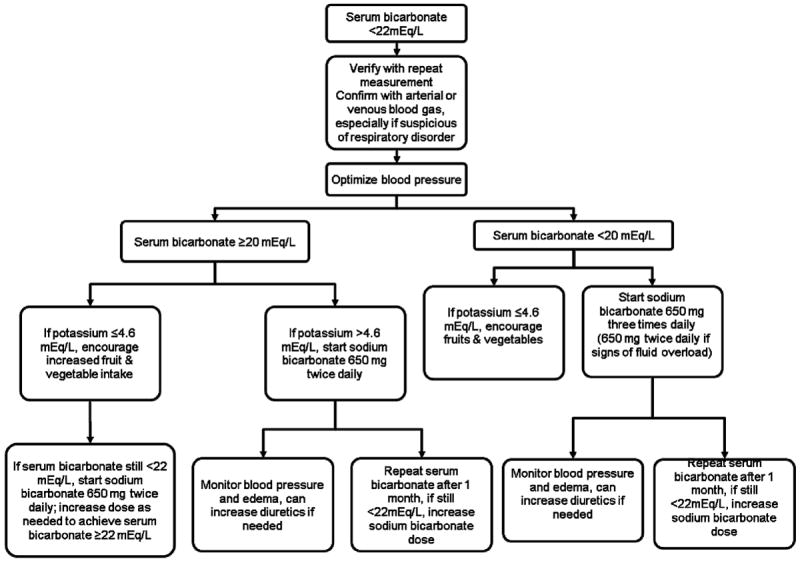
Baking soda (½ tsp = 26.8 mEq bicarbonate) can be substituted for sodium bicarbonate. Also consider sodium citrate if the patient does not tolerate sodium bicarbonate.
We suggest using sodium bicarbonate to treat the chronic acidosis of CKD, usually starting with 650 mg twice daily (15.5 mEq/day of bicarbonate) and titrating upward based on the response. In a short-term intervention, each 0.1 mEq/kg/day higher dose increased serum bicarbonate by 0.33 mEq/L (95% confidence interval, 0.23 -0.43 mEq/L),34 but the response may be greater over a period of months. If a poor response is observed and medication adherence is confirmed, renal bicarbonate wasting should be evaluated by measuring the urine pH. Baking soda is a cheaper alternative and especially useful for patients unable to tolerate the pill form (½ teaspoon dissolved in ½ cup water = 26.8 mEq bicarbonate). The most common side effect of sodium bicarbonate is bloating because of generation of carbon dioxide in the gastrointestinal tract. Sodium citrate is an alternative as citrate is rapidly metabolized to bicarbonate without produce bloating. However, it should be avoided in patients taking aluminum-containing antacids since citrate enhances intestinal aluminum absorption and increases the risk of aluminum toxicity.40 Enteric-coated sodium bicarbonate may prevent dose dumping in the stomach, but there are no data demonstrating improved gastrointestinal tolerability with this formulation.
Phosphorus binders, such as calcium acetate or carbonate and sevelamer carbonate, also contribute small quantities of exogenous base. In contrast, sevelamer hydrochloride may exacerbate acidosis; the base form is therefore most commonly used. Medications that impair net acid excretion, such as potassium-sparing diuretics and non-steroidal anti-inflammatory agents, should be avoided whenever possible.
The importance of diet should also be considered. The typical Western diet, high in animal protein, has a large dietary acid load. Conversely, a diet rich in fruits and vegetables contains greater quantities of base precursors. Increased fruit and vegetable consumption increased serum bicarbonate (19.9±1.7 versus 19.3±1.9 mEq/L) in 76 patients with CKD stage 4 and serum total carbon dioxide <22 mEq/L, although less effectively than sodium bicarbonate. 41 As the medication arm received a relatively high dose of alkali (1.0 mEq/kg/day), the difference with fruits and vegetables might have been smaller had a lower dose been used. After 1 year, eGFR was not different between the 2 groups. Furthermore, fruits and vegetables reduced systolic blood pressure and did not induce hyperkalemia in this cohort, all of whom had serum potassium ≤4.6 mEq/L upon study entry. Therefore, in patients at low-risk for hyperkalemia, increased fruit and vegetable intake should be routinely considered, with careful monitoring of the serum potassium early in the intervention.
The target serum bicarbonate with alkali therapy remains unclear. The goal set by KDOQI was opinion-based, and there are now intriguing data that a higher target may be preferable. The acid-inducing Western diet may increase tissue acidity without an observable change in plasma acid-base parameters.42 Such increased interstitial acidity could produce the same sequelae as overt acidosis,43 and beneficial effects of alkali might occur without a change in serum bicarbonate or pH. Thirty days of oral sodium bicarbonate in patients with mildly reduced eGFR reduced plasma endothelin-1 and aldosterone without a change in blood acid-base parameters. 44 In several positive studies of alkali in CKD patients, the mean serum bicarbonate at entry was 23-26 mEq/L, suggesting that even low-normal serum bicarbonate may be associated with deleterious effects.34,38,44 Despite this encouraging data, it seems premature to target this degree of acidosis for correction with medication. However, increased fruit and vegetable intake seems a reasonable intervention given its numerous potential health benefits. Clearly, further studies are needed to better define the long-term effects of alkali therapy and to determine the efficacy of treating mild metabolic acidosis.
Base administration in the form of sodium salts is not without potential complications, including worsened hypertension, volume overload, and congestive heart failure. However, less sodium retention occurs with sodium bicarbonate compared to equimolar sodium chloride.45 In a few studies, despite increased sodium intake, blood pressure remained similar between the sodium bicarbonate and control groups with no difference in antihypertensive medication requirements.31,38 Although these studies are reassuring, their populations were pre-selected and often excluded patients with congestive heart failure and uncontrolled hypertension. Thus, in certain patients the addition of a diuretic or the increase in diuretic dose may be needed to avoid volume overload.
There is also the possibility of increased vascular calcification from systemic alkalinization. This potentially serious complication has not been well studied. In animal and in vitro studies, metabolic acidosis inhibited extraskeletal calcification.46,47 Theoretically, alkali therapy could worsen vascular calcification, but no study has been performed in humans to test this effect. Finally, it is important to consider the pill burden and lesser side effects associated with alkali. Significant bloating or nausea may reduce appetite and food intake, which should be steadfastly avoided. The problems of high pill burden and polypharmacy have been well documented in the CKD population, and the impact of these additional pills should be considered on an individual basis.
In summary, chronic metabolic acidosis is associated with increased morbidity and mortality in patients with CKD. Existing evidence suggests alkali therapy might improve long-term outcomes in bone disease, muscle mass, and progression to ESRD. However, more data is needed to optimally guide treatment decisions. When initiating alkali therapy, one should consider the patient’s comorbidities, the tolerability of therapy, and the target serum bicarbonate. The key teaching points are listed in Box 1.
Box 1. Key Teaching Points.
Metabolic acidosis is a common complication of advanced CKD, especially with eGFR<30 ml/min/1.73 m2.
In patients with CKD, alkali therapy may decrease bone loss and protein degradation, increase muscle mass, and slow the progression of kidney disease. However, the optimal goal serum bicarbonate has not been defined.
It is important to verify metabolic acidosis by a second measurement of serum bicarbonate and a venous blood gas before initiation of alkali therapy.
Although alkali therapy is well-tolerated in most individuals, potential complications such as volume overload, congestive heart failure, and worsened hypertension need to be monitored.
It is important to consider a patient’s age, comorbidities, and pill burden when deciding to initiate alkali therapy.
Acknowledgments
Support: This research was supported by Clinical and Translational Science Award (CTSA) grants UL1RR025750, KL2RR025749 and TL1RR025748 from the National Center for Research Resources, a component of the National Institutes of Health (NIH), and by the National Center for Advancing Translational Sciences (NCATS), a component of the NIH, through CTSA grant numbers UL1TR000086, TL1RR000087, and KL2TR000088.
Footnotes
Disclosures: The authors declare that they have no relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2002 Feb;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 2.Roderick P, Willis NS, Blakeley S, Jones C, Tomson C. Correction of chronic metabolic acidosis for chronic kidney disease patients. Cochrane Database Syst Rev. 2007(1) doi: 10.1002/14651858.CD001890.pub3. CD001890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moranne O, Froissart M, Rossert J, et al. Timing of onset of CKD-related metabolic complications. Journal of the American Society of Nephrology : JASN. 2009 Jan;20(1):164–171. doi: 10.1681/ASN.2008020159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eustace JA, Astor B, Muntner PM, Ikizler TA, Coresh J. Prevalence of acidosis and inflammation and their association with low serum albumin in chronic kidney disease. Kidney international. 2004 Mar;65(3):1031–1040. doi: 10.1111/j.1523-1755.2004.00481.x. [DOI] [PubMed] [Google Scholar]
- 5.Shah SN, Abramowitz M, Hostetter TH, Melamed ML. Serum bicarbonate levels and the progression of kidney disease: a cohort study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2009 Aug;54(2):270–277. doi: 10.1053/j.ajkd.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodman AD, Lemann J, Jr, Lennon EJ, Relman AS. Production, Excretion, and Net Balance of Fixed Acid in Patients with Renal Acidosis. The Journal of clinical investigation. 1965 Apr;44:495–506. doi: 10.1172/JCI105163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Litzow JR, Lemann J, Jr, Lennon EJ. The effect of treatment of acidosis on calcium balance in patients with chronic azotemic renal disease. The Journal of clinical investigation. 1967 Feb;46(2):280–286. doi: 10.1172/JCI105530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uribarri J, Douyon H, Oh MS. A re-evaluation of the urinary parameters of acid production and excretion in patients with chronic renal acidosis. Kidney international. 1995 Feb;47(2):624–627. doi: 10.1038/ki.1995.79. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz WB, Hall PW, 3rd, Hays RM, Relman AS. On the mechanism of acidosis in chronic renal disease. The Journal of clinical investigation. 1959 Jan 1;38(1, Part 1):39–52. doi: 10.1172/JCI103794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Relman AS. Renal Acidosis and Renal Excretion of Acid in Health and Disease. Advances in internal medicine. 1964;12:295–347. [PubMed] [Google Scholar]
- 11.Kraut JA, Kurtz I. Metabolic acidosis of CKD: diagnosis, clinical characteristics, and treatment. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2005 Jun;45(6):978–993. doi: 10.1053/j.ajkd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Caravaca F, Arrobas M, Pizarro JL, Esparrago JF. Metabolic acidosis in advanced renal failure: differences between diabetic and nondiabetic patients. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1999 May;33(5):892–898. doi: 10.1016/s0272-6386(99)70422-1. [DOI] [PubMed] [Google Scholar]
- 13.Widmer B, Gerhardt RE, Harrington JT, Cohen JJ. Serum electrolyte and acid base composition. The influence of graded degrees of chronic renal failure. Archives of internal medicine. 1979 Oct;139(10):1099–1102. [PubMed] [Google Scholar]
- 14.Schambelan M, Sebastian A, Biglieri EG. Prevalence, pathogenesis, and functional significance of aldosterone deficiency in hyperkalemic patients with chronic renal insufficiency. Kidney international. 1980 Jan;17(1):89–101. doi: 10.1038/ki.1980.11. [DOI] [PubMed] [Google Scholar]
- 15.Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Association of serum bicarbonate levels with mortality in patients with non-dialysis-dependent CKD. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2009 Apr;24(4):1232–1237. doi: 10.1093/ndt/gfn633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menon V, Tighiouart H, Vaughn NS, et al. Serum bicarbonate and long-term outcomes in CKD. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2010 Nov;56(5):907–914. doi: 10.1053/j.ajkd.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 17.Raphael KL, Zhang Y, Wei G, et al. Serum bicarbonate and mortality in adults in NHANES III. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2013 May;28(5):1207–1213. doi: 10.1093/ndt/gfs609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vashistha T, Kalantar-Zadeh K, Molnar MZ, Torlen K, Mehrotra R. Dialysis modality and correction of uremic metabolic acidosis: relationship with all-cause and cause-specific mortality. Clinical journal of the American Society of Nephrology : CJASN. 2013 Feb;8(2):254–264. doi: 10.2215/CJN.05780612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemann J, Jr, Bushinsky DA, Hamm LL. Bone buffering of acid and base in humans. American journal of physiology Renal physiology. 2003 Nov;285(5):F811–832. doi: 10.1152/ajprenal.00115.2003. [DOI] [PubMed] [Google Scholar]
- 20.McSherry E, Morris RC., Jr Attainment and maintenance of normal stature with alkali therapy in infants and children with classic renal tubular acidosis. The Journal of clinical investigation. 1978 Feb;61(2):509–527. doi: 10.1172/JCI108962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sebastian A, Harris ST, Ottaway JH, Todd KM, Morris RC., Jr Improved mineral balance and skeletal metabolism in postmenopausal women treated with potassium bicarbonate. The New England journal of medicine. 1994 Jun 23;330(25):1776–1781. doi: 10.1056/NEJM199406233302502. [DOI] [PubMed] [Google Scholar]
- 22.Lefebvre A, de Vernejoul MC, Gueris J, Goldfarb B, Graulet AM, Morieux C. Optimal correction of acidosis changes progression of dialysis osteodystrophy. Kidney international. 1989 Dec;36(6):1112–1118. doi: 10.1038/ki.1989.309. [DOI] [PubMed] [Google Scholar]
- 23.Graham KA, Hoenich NA, Tarbit M, Ward MK, Goodship TH. Correction of acidosis in hemodialysis patients increases the sensitivity of the parathyroid glands to calcium. Journal of the American Society of Nephrology : JASN. 1997 Apr;8(4):627–631. doi: 10.1681/ASN.V84627. [DOI] [PubMed] [Google Scholar]
- 24.Bailey JL, Wang X, England BK, Price SR, Ding X, Mitch WE. The acidosis of chronic renal failure activates muscle proteolysis in rats by augmenting transcription of genes encoding proteins of the ATP-dependent ubiquitin-proteasome pathway. The Journal of clinical investigation. 1996 Mar 15;97(6):1447–1453. doi: 10.1172/JCI118566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding H, Gao XL, Hirschberg R, Vadgama JV, Kopple JD. Impaired actions of insulin-like growth factor 1 on protein Synthesis and degradation in skeletal muscle of rats with chronic renal failure. Evidence for a postreceptor defect. The Journal of clinical investigation. 1996 Feb 15;97(4):1064–1075. doi: 10.1172/JCI118499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ballmer PE, McNurlan MA, Hulter HN, Anderson SE, Garlick PJ, Krapf R. Chronic metabolic acidosis decreases albumin synthesis and induces negative nitrogen balance in humans. The Journal of clinical investigation. 1995 Jan;95(1):39–45. doi: 10.1172/JCI117668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reaich D, Channon SM, Scrimgeour CM, Daley SE, Wilkinson R, Goodship TH. Correction of acidosis in humans with CRF decreases protein degradation and amino acid oxidation. The American journal of physiology. 1993 Aug;265(2 Pt 1):E230–235. doi: 10.1152/ajpendo.1993.265.2.E230. [DOI] [PubMed] [Google Scholar]
- 28.Mak RH. Effect of metabolic acidosis on insulin action and secretion in uremia. Kidney international. 1998 Aug;54(2):603–607. doi: 10.1046/j.1523-1755.1998.00023.x. [DOI] [PubMed] [Google Scholar]
- 29.Szeto CC, Wong TY, Chow KM, Leung CB, Li PK. Oral sodium bicarbonate for the treatment of metabolic acidosis in peritoneal dialysis patients: a randomized placebo-control trial. Journal of the American Society of Nephrology : JASN. 2003 Aug;14(8):2119–2126. doi: 10.1097/01.asn.0000080316.37254.7a. [DOI] [PubMed] [Google Scholar]
- 30.Stein A, Moorhouse J, Iles-Smith H, et al. Role of an improvement in acid-base status and nutrition in CAPD patients. Kidney international. 1997 Oct;52(4):1089–1095. doi: 10.1038/ki.1997.433. [DOI] [PubMed] [Google Scholar]
- 31.de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate supplementation slows progression of CKD and improves nutritional status. Journal of the American Society of Nephrology : JASN. 2009 Sep;20(9):2075–2084. doi: 10.1681/ASN.2008111205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abramowitz MK, Hostetter TH, Melamed ML. Association of serum bicarbonate levels with gait speed and quadriceps strength in older adults. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2011 Jul;58(1):29–38. doi: 10.1053/j.ajkd.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abramowitz MK, Hostetter TH, Melamed ML. Lower serum bicarbonate and a higher anion gap are associated with lower cardiorespiratory fitness in young adults. Kidney international. 2012 May;81(10):1033–1042. doi: 10.1038/ki.2011.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abramowitz MK, Melamed ML, Bauer C, Raff AC, Hostetter TH. Effects of Oral Sodium Bicarbonate in Patients with CKD. Clinical journal of the American Society of Nephrology : CJASN. 2013 May;8(5):714–720. doi: 10.2215/CJN.08340812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phisitkul S, Hacker C, Simoni J, Tran RM, Wesson DE. Dietary protein causes a decline in the glomerular filtration rate of the remnant kidney mediated by metabolic acidosis and endothelin receptors. Kidney international. 2008 Jan;73(2):192–199. doi: 10.1038/sj.ki.5002647. [DOI] [PubMed] [Google Scholar]
- 36.Nath KA, Hostetter MK, Hostetter TH. Pathophysiology of chronic tubulo-interstitial disease in rats. Interactions of dietary acid load, ammonia, and complement component C3. The Journal of clinical investigation. 1985 Aug;76(2):667–675. doi: 10.1172/JCI112020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phisitkul S, Khanna A, Simoni J, et al. Amelioration of metabolic acidosis in patients with low GFR reduced kidney endothelin production and kidney injury, and better preserved GFR. Kidney international. 2010 Apr;77(7):617–623. doi: 10.1038/ki.2009.519. [DOI] [PubMed] [Google Scholar]
- 38.Mahajan A, Simoni J, Sheather SJ, Broglio KR, Rajab MH, Wesson DE. Daily oral sodium bicarbonate preserves glomerular filtration rate by slowing its decline in early hypertensive nephropathy. Kidney international. 2010 Aug;78(3):303–309. doi: 10.1038/ki.2010.129. [DOI] [PubMed] [Google Scholar]
- 39.Susantitaphong P, Sewaralthahab K, Balk EM, Jaber BL, Madias NE. Short- and long-term effects of alkali therapy in chronic kidney disease: a systematic review. American journal of nephrology. 2012;35(6):540–547. doi: 10.1159/000339329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nolan CR, Califano JR, Butzin CA. Influence of calcium acetate or calcium citrate on intestinal aluminum absorption. Kidney international. 1990 Nov;38(5):937–941. doi: 10.1038/ki.1990.294. [DOI] [PubMed] [Google Scholar]
- 41.Goraya N, Simoni J, Jo CH, Wesson DE. A Comparison of Treating Metabolic Acidosis in CKD Stage 4 Hypertensive Kidney Disease with Fruits and Vegetables or Sodium Bicarbonate. Clinical journal of the American Society of Nephrology : CJASN. 2013 Mar;8(3):371–381. doi: 10.2215/CJN.02430312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wesson DE. Dietary acid increases blood and renal cortical acid content in rats. The American journal of physiology. 1998 Jan;274(1 Pt 2):F97–103. doi: 10.1152/ajprenal.1998.274.1.F97. [DOI] [PubMed] [Google Scholar]
- 43.Wesson DE, Simoni J. Acid retention during kidney failure induces endothelin and aldosterone production which lead to progressive GFR decline, a situation ameliorated by alkali diet. Kidney international. 2010 Dec;78(11):1128–1135. doi: 10.1038/ki.2010.348. [DOI] [PubMed] [Google Scholar]
- 44.Wesson DE, Simoni J, Broglio K, Sheather S. Acid retention accompanies reduced GFR in humans and increases plasma levels of endothelin and aldosterone. American journal of physiology Renal physiology. 2011 Apr;300(4):F830–837. doi: 10.1152/ajprenal.00587.2010. [DOI] [PubMed] [Google Scholar]
- 45.Husted FC, Nolph KD. NaHCO3 and NaCl tolerance in chronic renal failure II. Clinical nephrology. 1977 Jan;7(1):21–25. [PubMed] [Google Scholar]
- 46.Mendoza FJ, Lopez I, Montes de Oca A, Perez J, Rodriguez M, Aguilera-Tejero E. Metabolic acidosis inhibits soft tissue calcification in uremic rats. Kidney international. 2008 Feb;73(4):407–414. doi: 10.1038/sj.ki.5002646. [DOI] [PubMed] [Google Scholar]
- 47.Lomashvili K, Garg P, O’Neill WC. Chemical and hormonal determinants of vascular calcification in vitro. Kidney international. 2006 Apr;69(8):1464–1470. doi: 10.1038/sj.ki.5000297. [DOI] [PubMed] [Google Scholar]
- 48.Kraut JA, Mishler DR, Singer FR, Goodman WG. The effects of metabolic acidosis on bone formation and bone resorption in the rat. Kidney international. 1986 Nov;30(5):694–700. doi: 10.1038/ki.1986.242. [DOI] [PubMed] [Google Scholar]
- 49.Movilli E, Zani R, Carli O, et al. Correction of metabolic acidosis increases serum albumin concentrations and decreases kinetically evaluated protein intake in haemodialysis patients: a prospective study. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 1998 Jul;13(7):1719–1722. doi: 10.1093/ndt/13.7.1719. [DOI] [PubMed] [Google Scholar]
- 50.Brady JP, Hasbargen JA. Correction of metabolic acidosis and its effect on albumin in chronic hemodialysis patients. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1998 Jan;31(1):35–40. doi: 10.1053/ajkd.1998.v31.pm9428449. [DOI] [PubMed] [Google Scholar]


