Abstract
Background
The biochemical pathways underlying alcohol abuse and dependence are not well understood, although brain cell loss and neurotoxicity have been reported in subjects with alcohol dependence. Monoamine oxidase B (MAO B, which catabolizes neurotransmitters such as dopamine) is consistently increased in this psychiatric illness. MAO B has been implicated in the pathogenesis of alcohol dependence, neurodegenerative diseases and alcohol-induced brain neurotoxicity. Recently, the cell growth-inhibitor protein, Kruppel-like factor 11 (KLF11), has been reported to be an MAO-transcriptional activator. KLF11 is also known as TIEG2 (transforming growth factor-beta-inducible early gene 2) and mediates apoptotic cell death. This study investigates the protein expression of KLF11 and its relationship with MAO B using human postmortem prefrontal cortex from subjects with alcohol dependence.
Methods
Twelve subjects with alcohol dependence and the respective psychiatrically-normal control subjects were investigated. Expression of KLF11 and MAO B proteins in the prefrontal cortex were measured by Western blot analysis. A correlation study between KLF11 and MAO B protein expression was also performed.
Results
Levels of KLF11 protein were significantly increased by 44 percent (p<0.03) in the postmortem prefrontal cortex of subjects with alcohol dependence as compared to age- and gender-matched, psychiatrically-normal control subjects. In addition, KLF11 levels were significantly and positively correlated with the increased MAO B protein levels associated with alcohol dependence.
Conclusions
This novel study shows the important role of KLF11, an MAO-transcriptional activator, in human alcohol dependence. It further supports that the KLF11-MAO B cell death cascade may contribute to chronic alcohol-induced brain damage. This argues a case for KLF11-MAO B inhibition as a novel therapeutic strategy that may impact this highly prevalent, often treatment resistant, illness.
Keywords: alcohol dependence, Kruppel-like factor 11 (transforming growth factor-beta-inducible early gene 2), monoamine oxidase, human postmortem prefrontal cortex, brain tissue injury
INTRODUCTION
Alcohol use disorders including alcoholism are a major psychiatric condition because long term heavy ethanol consumption results in brain cell injury and neuropsychological difficulties. In general, alcohol use disorders are highly prevalent as 8.5% of American adults meet criteria for these disorders (Kogoj et al., 2010). According to the 2008 National Epidemiologic Survey on Alcohol and Related Conditions, approximately one-fourth of Americans partook in binge drinking within the 30 days prior to the survey and 6.9% Americans admitted to heavy drinking habits (Gold and Aronson, 2010). Because of the detrimental effects that alcohol dependence has on society, a further in-depth understanding of the pathogenesis of this illness is warranted (Gold and Aronson, 2010).
Alterations in brain monoamine oxidase (MAO) levels have been implicated in the pathogenesis of psychiatric disorders. As such, MAO B protein levels have been found to be significantly increased in human subjects with alcohol dependence (Carlsson et al., 1980; Ou et al., 2010b). Recently, a transcription factor, Kruppel-Like Factor 11 (KLF11), has been discovered to activate the gene expression of MAO B.
KLF11, also referred to as transforming growth factor-beta-inducible early gene 2 (TIEG2), is expressed ubiquitously in the body and up-regulates overall MAO B transcription via the overlapping Sp1-binding sites located at the MAO B promoter region. This promoter region has been identified as the upstream regulatory DNA segment of the MAO B coding region (Ou et al., 2004). Sequence analysis demonstrates that KLF11, along with the homologous KLF, belongs to an Sp1-like family of transcription factors containing the typical three zinc finger C-terminal array characteristic of several DNA-binding proteins (Fernandez-Zapico et al., 2003; Zhang et al., 2001). KLF11 is not only an activator of MAO B expression, but it also reduces exocrine cell growth and behaves as a tumor suppressor in pancreatic cancer (Buck et al., 2006; Cook et al., 1998; Fernandez-Zapico et al., 2003; Lomberk et al., 2012; Tachibana et al., 1997). KLF11 mediates the cellular apoptotic pathway in a caspase-3-dependent manner and decreases anti-apoptotic protein (Bcl-XL) levels in humans (Wang et al., 2007). KLF11 also impedes cell growth and stimulates apoptosis by binding to certain sequences in the promoter region of several genes, including MAO (Cook et al., 1998; Fernandez-Zapico et al., 2011; Ou et al., 2004; Tachibana et al., 1997).
The oxidation of neurotransmitters by MAO leads to the production of reactive oxygen species (ROS) such as hydrogen peroxide, the excess of which can cause oxidative stress resulting in a decline in cellular function (Maurel et al., 2003). MAO B, which degrades neurotransmitters such as phenylethylamine and dopamine (Bach et al., 1988), is involved in the pathogenesis of alcohol dependence (Carlsson et al., 1980; Ou et al., 2010b). Furthermore, KLF11 has already been identified as a transcriptional activator of MAO B (Lu et al., 2008; Ou et al., 2004), and also been reported to be increased upon chronic alcohol exposure in the rat brain prefrontal cortex (Ou et al., 2010a). Though other factors may be involved in the increased expression of MAO B, such as activation of retinoic acid response elements in the MAO B promoter region by retinoic acid receptor α and retinoid X receptor α (Wu et al., 2009), the KLF11-MAO B cell death pathway may play a crucial role in alcohol-induced brain malfunction and cytotoxicity.
This study was designed to determine 1) whether KLF11 protein levels are elevated in human subjects with alcohol dependence and 2) whether KLF11 expression correlates with levels of MAO B protein in the same human alcohol dependent subjects.
MATERIALS AND METHODS
Human Subjects and Tissue Collection
The study was conducted in accordance with the declaration of Helsinki and Institutional Review Board policies at University Hospitals of Cleveland and the University of Mississippi Medical Center. Samples of prefrontal cortex (Brodmann area 8/9; right hemisphere) were collected at autopsy at the Cuyahoga County Coroner’s Office (Cleveland, Ohio). Written consent was obtained from the next-of-kin for all subjects (Miguel-Hidalgo et al., 2006; Ou et al., 2010b; Stockmeier et al., 2009). A trained interviewer administered the Structured Clinical Interview for DSM-IV Psychiatric Disorders to knowledgeable informants for all subjects. Results of the interview, together with medical records and records from the coroner were used by a clinical psychologist and psychiatrist to determine current and lifetime Axis I psychopathology (First MB, 1996). The validity of diagnoses resulting from retrospective, informant-based interviews is in good agreement with diagnoses based on reviewing subject medical records or interviewing the subject (Deep-Soboslay et al., 2005; Dejong and Overholser, 2009; First MB, 1996; Johnson et al., 2011; Kelly and Mann, 1996).
Two groups of subjects were examined: Twelve subjects that met DSM-IV criteria for alcohol dependence (Table 1 and Supplemental Data Table 1B) (Ou et al., 2010b) and 12 psychiatrically-normal control subjects were matched to the alcohol dependent subjects (Table 1 and Supplemental Data Tables 1A) (Johnson et al., 2011). Criteria for matching are provided below. Control subjects did not ever meet criteria for an alcohol use disorder during their lifetimes.
Table 1.
Demographics, Sample Conditions, and Clinical Characteristics Data of Subjects with Alcohol Dependence and Corresponding Psychiatrically-Normal Control Subjects
| Control n=12 | Alcohol Dependent n=12 | |
|---|---|---|
| Age, y, mean (SEM) | 49.25 (3.0) | 48.92 (2.62) |
|
| ||
| Male, Number (%) | 8 (66.7) | 9 (75.0) |
|
| ||
| Female, Number (%) | 4 (33.3) | 3 (25.0) |
|
| ||
| Race, Number (%) | ||
| African American | 4 (33.3) | 3 (25.0) |
| Caucasian | 8 (66.7) | 9 (75.0) |
|
| ||
| PMI (h), mean (SEM) | 20.29 (2.2) | 23.36 (2.2) |
|
| ||
| Tissue pH, mean (SEM) | 6.64 (0.09) | 6.49 (0.1) |
|
| ||
| Storage time in freezer (y), mean (SEM) | 11.0 (0.76) | 10.83 (1.37) |
|
| ||
| Smoker | 8 (66.7) | 6 (50.0) |
|
| ||
| Age of onset (y), mean (SEM) | NA | 21.3 (1.77) |
|
| ||
| Duration of Dependence (y), mean (SEM) | NA | 24.9 (2.43) |
Abbreviations: n, number of subjects; y, years; SEM, Standard Error of the mean; PMI (h), post-mortem interval in hours; NA, not applicable
a. Psychiatrically-normal control subjects
Twelve psychiatrically-normal control subjects
The average age (years, mean±SEM) of this control group was 49.25±3.03, eight subjects were male and eight subjects were smokers (Table 1 and Supplemental Data Table 1A). Four subjects were African American and eight subjects were Caucasian. The average postmortem interval (PMI, time between death and freezing tissue) was 20.29±2.24 (hours, mean±SEM). The average pH of the brain tissue for this group was 6.64±0.09 (mean±SEM). The average freezer storage time during this investigation was 11±0.76 (years, mean±SEM). Assessment of postmortem blood and urine for the normal control subjects did not reveal the presence of psychoactive drugs. These control subjects were matched as closely as possible to the twelve subjects with alcohol dependence taking into account age, sex, race, PMI, pH, toxicology, smoking status and freezer storage time (Supplemental Data Table 1).
b. Alcohol dependent subjects
Twelve subjects with alcohol dependence
The average age (years, mean+SEM) for this group was 48.92±2.62 and nine subjects were male (Table 1 and Supplemental Data Table 1B). Each subject was diagnosed with alcohol dependence for at least seven years and the average duration of illness was 24.9 years. The average age of onset of alcohol dependence was 21.3 years of age for this group. One alcohol dependent subject was prescribed psychoactive medication within the last 30 days of life but was considered non-compliant as the medication was not detected in the postmortem toxicology screen. Based on the postmortem blood and urine analyses, none of the alcohol dependent subjects had taken psychoactive substances of abuse other than alcohol immediately prior to their death (Supplemental Data Table 1B).
The following subjects were excluded from the current study: 1) subjects with evidence of neurological disorders, including Korsakoff’s psychosis or Wernicke’s encephalopathy, based on neuropathological or clinical assessment; 2) subjects with other psychiatric disorders; 3) subjects with other psychoactive substance abuse (except nicotine); 4) subjects dying with prolonged hospitalization or life support measures prior to death; and 5) subjects with HIV, Hepatitis A, B or C infections.
Western Blot Analysis
Brain tissue from each subject was homogenized in a 0.5 ml solution containing 1 mM EDTA, 10 mM Tris-HCL and fresh protease inhibitor (Sigma), and centrifuged at 4°C (550 x g) for 10 min in a microcentrifuge. The supernatants were recovered and stored at −80°C. Forty micrograms (40 μg) of total protein were separated by 10.5 percent SDS-polyacrylamide gel electrophoresis. After transfer, the membranes were incubated with mouse anti-KLF11 antibody (1:500; BD Transduction Laboratory; 611402) or goat polyclonal anti-MAO B antibody (1:500; Santa Cruz, sc-18401) overnight at 4°C, followed by incubation with their respective secondary antibodies.
An equal number of matched samples from each group (control and alcohol-dependent subjects) were immunoblotted in duplicate on separate membranes. Protein bands were visualized by the ChemiDoc XRS+ Imaging System (Bio-Rad). Expression of β-actin was also measured by stripping each membrane immunoblotted for the determination of KLF11 and MAO B to establish loading controls. The band intensities for KLF11 were calculated and normalized to the band intensities for β-actin using Quantity One analysis software. Independently, a standard curve was established for KLF11 or MAO B using increasing concentrations of total protein from a human control subject; the optical densities for KLF11 or MAO B displayed a linear relationship relative to the total protein concentration as described previously (Johnson et al., 2011; Ou et al., 2010b).
Statistical Analysis
Statistical significance was evaluated using Student’s t test for two group comparisons. The data are reported as mean±SEM, and a value of p<0.05 is considered statistically significant. The potential influence of age, sex, race, smoking, PMI, tissue pH and tissue storage time, age of onset, and duration of illness on KLF11 was examined by linear regression and these variables had no significant effect on protein levels (Table 2).
Table 2. Statistical analysis of control subjects and subjects with alcohol dependence (Alcohol).
Summary of statistical analysis of KLF11 protein levels between human psychiatrically-normal control subjects and subjects with alcohol dependence (Alcohol)
| Control (n=12) | Alcohol (n=12) | Statistica | p-value | |
|---|---|---|---|---|
| Age | 49.25 ± 3.03 | 48.53 ± 2.37 | 0.083 | 0.934 |
| Gender (% Male) | 66.7 % | 75.0 % | 0.43 | 0.670 |
| Race (%AAb) | 33.3% | 25.0 % | 0.432 | 0.670 |
| PMI | 20.29 ± 2.2 | 23.36 ± 2.2 | −0.975 | 0.339 |
| Tissue pH | 6.64 ± 0.09 | 6.49 ± 0.1 | 1.12 | 0.276 |
| Storage Time | 11.0 ± 0.76 | 10.83 ± 1.37 | 0.12 | 0.916 |
| Smoker (%Yes) | 66.7 % | 50.0 % | −0.215 | 0.430 |
| KLF11/actin | 1.16 ± 0.25 | 2.09 ± 0.28 | −2.45 | 0.0227 |
Categorical data compared with chi-square test with 1 degree of freedom
Continuous data compared with t-statistic with 22 degrees of freedom.
AA, African American.
-
General outcomesThere were no significant differences between cohorts for demographic variables (Age, Gender, Race, PMI, pH, Storage Time and Smoking history).
-
The detailed analysis for KLF11In the univariate analysis using Student’s t-test and KLF11/actin ratio as the outcome, the mean value for subjects with alcohol dependence was significantly greater than control subjects (t=−2.45, df=10, p-value=0.0227).KLF11 was not significantly correlated with age, PHI, pH or storage time, nor was there a significant effect of gender, race or smoking status on KLF11 levels.There was no significant correlation between KLF11 and the age of onset or duration of alcohol dependence.
RESULTS
Increased Expression of KLF11 (TIEG2) Protein in the Prefrontal Cortex of Subjects with Alcohol Dependence
MAO B, a subtype of MAO, has been implicated in alcohol dependence (Ou et al., 2010b). Whether or not its transcriptional activator, KLF11, also plays a regulatory role in this psychiatric illness has not been investigated. To better characterize the function of this enzyme activator, KLF11 protein expression levels were analyzed in the postmortem prefrontal cortex of twelve alcohol-dependent subjects and twelve matched, psychiatrically-normal control subjects (Figure 1). Western blot analysis revealed that KLF11 levels were significantly increased by 44 percent (p<0.03) in alcohol-dependent subjects compared to controls (Figure 1A and B).
Figure 1.
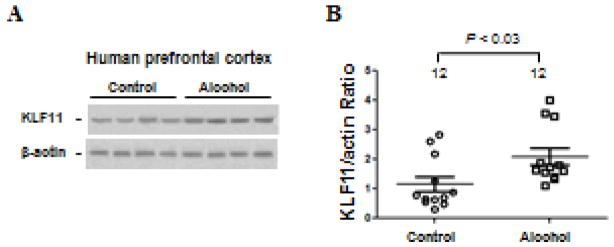
Quantitative analysis of KLF11 (TIEG2) protein expression in the postmortem prefrontal cortex of twelve subjects with alcohol dependence (Alcohol) and twelve matched, psychiatrically-normal control subjects was examined by Western blot analysis. (A) KLF11 protein levels. A representative immunoblot for 4 control subjects and 4 subjects with alcohol dependence is shown. β-actin was used as the loading control. (B) Quantitative analysis. Each KLF11 band was evaluated by its relative intensity and normalized to the density of β-actin. Graphs of the average optical density of KLF11/actin for the individual subjects (circles or squares) and mean values (horizontal lines) are shown.
The potential influence of age, sex, race, smoking habit, PMI, tissue pH, tissue storage time, age of onset and duration of illness on KLF11 were examined by linear regression. KLF11 levels were not significantly correlated with any of these variables (Tables 2).
Increased MAO B Protein Expression Correlates with KLF11 Expression in the Prefrontal Cortex of Subjects with Alcohol Dependence
To better establish KLF11-MAO B as a pathway related to alcohol use disorders, protein expression levels of MAO B were also determined in the prefrontal cortex of same alcohol-dependent subjects and twelve matched, psychiatrically-normal control subjects (Figure 2). Western blot analysis indicated that MAO B levels were significantly increased by 29 percent (p<0.05) in the prefrontal cortex of alcohol dependent subjects compared to controls (Figure 2A). This result is consistent with our previous findings (Ou et al., 2010b).
Figure 2.
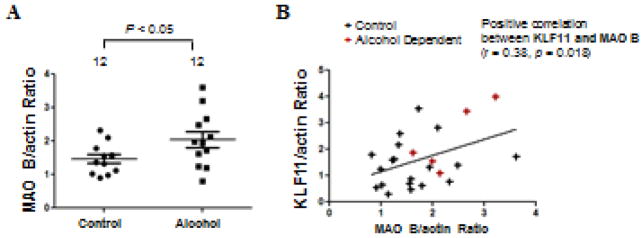
Western blot analysis of MAO B protein levels in the postmortem prefrontal cortex of twelve subjects with alcohol dependence (Alcohol) and twelve matched, psychiatrically-normal control subjects was examined by Western blot analysis. (A) Quantitative analysis. Each KLF11 band was evaluated by its relative intensity and normalized to the density of β-actin. Graphs of the average optical density of KLF11/actin for the individual subjects (solid circles or solid squares) and mean values (horizontal lines) are shown. (B) Graphic representation of the positive correlation between KLF11 and MAO B levels in control subjects (black crosses) and subjects with alcohol dependence (red crosses).
Because KLF11 is a transcriptional activator for MAO B, it was hypothesized that the increased KLF11 levels may positively correlate with the elevated MAO B levels. Indeed, there was a significant positive correlation between the protein expression levels of KLF11 and MAO B (p=0.018, Figure 2B).
DISCUSSION
Since altered regulation of monoamine oxidase levels have been associated with several psychiatric and neurodegenerative diseases including alcohol dependence (Du et al., 2004; Meyer et al., 2006; Ou et al., 2010b; Shih et al., 1999; Youdim et al., 2006), it is critical to determine the molecular mechanisms underlying the expression and enzymatic function of this enzyme. This study in postmortem human brain tissue reports new and robust insights into the role of a potentially important factor that may be crucial in the manifestation of human alcohol dependence and also provides additional insight into the underlying pathogenesis of alcohol dependence.
Recent in vitro and in vivo work demonstrates a role for KLF11 in alcohol use disorders. For example, exposure of neuronal cells to ethanol augments KLF11-mediated MAO B activation (Lu et al., 2008) and the KLF11-MAO B cell death cascade is increased in rat brains following exposure to ethanol (Ou et al., 2010a). These observations emphasize the important role of KLF11 as a transcriptional activator of MAO B (Ou et al., 2004). Likewise, the current study is the first to validate a role for KLF11 by examining the expression levels of this transcription protein in postmortem brain tissue from human alcohol-dependent subjects. Our study reveals that the expression of KLF11 protein is significantly increased in alcohol-dependent subjects as compared to normal control subjects. The noted increase of KLF11 protein in alcohol dependence is positively correlated with the increase in MAO B protein expression in the brains of these same subjects. These results in postmortem tissue are consistent with our previous observations in rats exposed to chronic ethanol (Ou et al., 2010a) and suggest that the elevated KLF11 levels in alcohol dependent subjects may be a pathobiological marker for alcohol-induced brain toxicity.
Examining the KLF11-MAO B pathway in alcohol dependence may provide more accurate insight into brain cytotoxicity underlying this disorder. Compounds that reduce KLF11-MAO B-mediated oxidative stress or apoptotic cell death by targeting the transcriptional activator at the gene, post-transcriptional, or protein level may promote neuroprotection, neuroplasticity and synaptic activities. By maximizing therapeutic effects upon these targets, more comprehensive treatments may be developed for ethanol-induced and frequently treatment-resistant alcohol-related disorders (Barr et al., 2004; Beasley et al., 2005; Dwivedi et al., 2006; Frazer, 1997; Mitchell et al., 2012; Sanacora, 2008; Sawada et al., 2005; Silberman et al., 2009; Wallace et al., 2007). Previous studies in human neuronal cell lines and rat brains have supported the existence of a KLF11-MAO B cell death cascade and its association with glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a metabolic enzyme. GAPDH, up-regulated by exposure to ethanol, is translocated into the nucleus and binds to KLF11, thereby forming a complex that mediates transcription of the MAO B gene (Ou et al., 2010b).
In summary, expression of KLF11 (TIEG2) protein was significantly elevated in postmortem prefrontal cortex associated with alcohol dependence in human subjects. Furthermore, the levels of KLF11 protein expression were positively correlated with increased MAO B expression in alcohol dependence. This relationship suggests a novel role for KLF11 as an MAO B transcriptional activator in human alcohol related disorders. Therefore, understanding the interactions between the transcriptional activator KLF11 and its target MAO B may reveal a new direction for treatment strategies to normalize levels of MAO B. Inhibiting expression of KLF11-MAO B cell death cascade could enhance neuroprotection and reduce alcohol-induced brain tissue injury.
Supplementary Material
Supplemental Data Table 1. Characteristics of subjects with or without alcohol dependence.
Table 1A. Characteristics of psychiatrically-normal control subjects.
Table 1B. Characteristics of alcohol dependent subjects.
Acknowledgments
This research was supported by Public Health Service Grants P20 RR 017701, MH67996, AA020103, The Brain & Behavior Research Foundation (NARSAD) and an Intramural Research Support grant from the University of Mississippi Medical Center.
We gratefully acknowledge the invaluable contributions made by the families consenting to the donation of brain tissue and to being interviewed. The kind assistance of the Cuyahoga County Coroner’s office, Cleveland, Ohio, is also noted. We thank Drs. James Overholser, George Jurjus and Herbert Y. Meltzer and Lesa Dieter for the psychiatric assessment of subjects, as well as Nicole Herbst, Timothy De Jong, Shawnnette Nelson and Nicole Peak for assisting with human tissue and obtaining written consent and Dr. Gouri Mahajan for assisting with tissue preparation.
References
- Bach AW, Lan NC, Johnson DL, Abell CW, Bembenek ME, Kwan SW, Seeburg PH, Shih JC. cDNA cloning of human liver monoamine oxidase A and B: molecular basis of differences in enzymatic properties. Proc Natl Acad Sci U S A. 1988;85:4934–8. doi: 10.1073/pnas.85.13.4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr AM, Young CE, Sawada K, Trimble WS, Phillips AG, Honer WG. Abnormalities of presynaptic protein CDCrel-1 in striatum of rats reared in social isolation: relevance to neural connectivity in schizophrenia. Eur J Neurosci. 2004;20:303–7. doi: 10.1111/j.0953-816X.2004.03457.x. [DOI] [PubMed] [Google Scholar]
- Beasley CL, Honer WG, Bergmann K, Falkai P, Lutjohann D, Bayer TA. Reductions in cholesterol and synaptic markers in association cortex in mood disorders. Bipolar Disord. 2005;7:449–55. doi: 10.1111/j.1399-5618.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- Buck A, Buchholz M, Wagner M, Adler G, Gress T, Ellenrieder V. The tumor suppressor KLF11 mediates a novel mechanism in transforming growth factor beta-induced growth inhibition that is inactivated in pancreatic cancer. Mol Cancer Res. 2006;4:861–72. doi: 10.1158/1541-7786.MCR-06-0081. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Adolfsson R, Aquilonius SM, Gottfries CG, Oreland L, Svennerholm L, Winblad B. Biogenic amines in human brain in normal aging, senile dementia, and chronic alcoholism. Adv Biochem Psychopharmacol. 1980;23:295–304. [PubMed] [Google Scholar]
- Cook T, Gebelein B, Mesa K, Mladek A, Urrutia R. Molecular cloning and characterization of TIEG2 reveals a new subfamily of transforming growth factor-beta-inducible Sp1-like zinc finger-encoding genes involved in the regulation of cell growth. J Biol Chem. 1998;273:25929–36. doi: 10.1074/jbc.273.40.25929. [DOI] [PubMed] [Google Scholar]
- Deep-Soboslay A, Akil M, Martin CE, Bigelow LB, Herman MM, Hyde TM, Kleinman JE. Reliability of psychiatric diagnosis in postmortem research. Biol Psychiatry. 2005;57:96–101. doi: 10.1016/j.biopsych.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Dejong TM, Overholser JC. Assessment of depression and suicidal actions: agreement between suicide attempters and informant reports. Suicide Life Threat Behav. 2009;39:38–46. doi: 10.1521/suli.2009.39.1.38. [DOI] [PubMed] [Google Scholar]
- Du L, Bakish D, Ravindran A, Hrdina PD. MAO-A gene polymorphisms are associated with major depression and sleep disturbance in males. Neuroreport. 2004;15:2097–101. doi: 10.1097/00001756-200409150-00020. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Mondal AC, Rizavi HS, Faludi G, Palkovits M, Sarosi A, Conley RR, Pandey GN. Differential and brain region-specific regulation of Rap-1 and Epac in depressed suicide victims. Arch Gen Psychiatry. 2006;63:639–48. doi: 10.1001/archpsyc.63.6.639. [DOI] [PubMed] [Google Scholar]
- Fernandez-Zapico ME, Lomberk GA, Tsuji S, DeMars CJ, Bardsley MR, Lin YH, Almada LL, Han JJ, Mukhopadhyay D, Ordog T, Buttar NS, Urrutia R. A functional family-wide screening of SP/KLF proteins identifies a subset of suppressors of KRAS-mediated cell growth. Biochem J. 2011;435:529–37. doi: 10.1042/BJ20100773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Zapico ME, Mladek A, Ellenrieder V, Folch-Puy E, Miller L, Urrutia R. An mSin3A interaction domain links the transcriptional activity of KLF11 with its role in growth regulation. EMBO J. 2003;22:4748–58. doi: 10.1093/emboj/cdg470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MBSR, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders—Patient Edition. Biometrics Research Department, New York State Psychiatric Institute; New York: 1996. Version 2.0 ed. [Google Scholar]
- Frazer A. Pharmacology of antidepressants. J Clin Psychopharmacol. 1997;17(Suppl 1):2S–18S. doi: 10.1097/00004714-199704001-00002. [DOI] [PubMed] [Google Scholar]
- Gold MS, Aronson MD. In: Alcohol abuse and dependence: Epidemiology, clinical manifestations, and diagnosis. Basow DS, editor. UpToDate; Waltham, MA: 2010. [Google Scholar]
- Johnson S, Stockmeier CA, Meyer JH, Austin MC, Albert PR, Wang J, May WL, Rajkowska G, Overholser JC, Jurjus G, Dieter L, Johnson C, Sittman DB, Ou XM. The reduction of R1, a novel repressor protein for monoamine oxidase a, in major depressive disorder. Neuropsychopharmacology. 2011;36:2139–48. doi: 10.1038/npp.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly TM, Mann JJ. Validity of DSM-III-R diagnosis by psychological autopsy: a comparison with clinician ante-mortem diagnosis. Acta Psychiatr Scand. 1996;94:337–43. doi: 10.1111/j.1600-0447.1996.tb09869.x. [DOI] [PubMed] [Google Scholar]
- Kogoj D, Addolorato G, Ferrulli A, Mouzas I, Okruhlica L, Poldrugo FA, Schlaff G, Zima T, Lesch O, Walter H. Alpe adria report 2010 - conclusions and recommendations for the treatment of alcohol dependence. Front Psychiatry. 2010;2:58. doi: 10.3389/fpsyt.2011.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomberk G, Mathison AJ, Grzenda A, Seo S, DeMars CJ, Rizvi S, Bonilla-Velez J, Calvo E, Fernandez-Zapico ME, Iovanna J, Buttar NS, Urrutia R. Sequence-specific recruitment of heterochromatin protein 1 via interaction with Kruppel-like factor 11, a human transcription factor involved in tumor suppression and metabolic diseases. J Biol Chem. 2012;287:13026–39. doi: 10.1074/jbc.M112.342634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Johnson C, Johnson S, Tazik S, Ou XM. The Neuroprotective Effect of Antidepressant Drug via Inhibition of TIEG2-MAO B Mediated Cell Death. Drug Discov Ther. 2008;2:289–295. [PMC free article] [PubMed] [Google Scholar]
- Maurel A, Hernandez C, Kunduzova O, Bompart G, Cambon C, Parini A, Frances B. Age-dependent increase in hydrogen peroxide production by cardiac monoamine oxidase A in rats. Am J Physiol Heart Circ Physiol. 2003;284:H1460–7. doi: 10.1152/ajpheart.00700.2002. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Ginovart N, Boovariwala A, Sagrati S, Hussey D, Garcia A, Young T, Praschak-Rieder N, Wilson AA, Houle S. Elevated monoamine oxidase a levels in the brain: an explanation for the monoamine imbalance of major depression. Arch Gen Psychiatry. 2006;63:1209–16. doi: 10.1001/archpsyc.63.11.1209. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Overholser JC, Meltzer HY, Stockmeier CA, Rajkowska G. Reduced glial and neuronal packing density in the orbitofrontal cortex in alcohol dependence and its relationship with suicide and duration of alcohol dependence. Alcohol Clin Exp Res. 2006;30:1845–55. doi: 10.1111/j.1530-0277.2006.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, Grossman LE, Coker AR, Messing RO. The anticonvulsant levetiracetam potentiates alcohol consumption in non-treatment seeking alcohol abusers. J Clin Psychopharmacol. 2012;32:269–72. doi: 10.1097/JCP.0b013e318248ba69. [DOI] [PubMed] [Google Scholar]
- Ou XM, Chen K, Shih JC. Dual functions of transcription factors, transforming growth factor-beta-inducible early gene (TIEG)2 and Sp3, are mediated by CACCC element and Sp1 sites of human monoamine oxidase (MAO) B gene. J Biol Chem. 2004;279:21021–8. doi: 10.1074/jbc.M312638200. [DOI] [PubMed] [Google Scholar]
- Ou XM, Johnson C, Lu D, Johnson S, Paul IA, Austin MC, Iyo AH, Miguel-Hidalgo JJ, Luo J, Bell RL, Grunewald M, Wang J, Sittman DB. Ethanol Increases TIEG2-MAO B Cell Death Cascade in the Prefrontal Cortex of Ethanol-Preferring Rats. Neurotox Res. 2010a doi: 10.1007/s12640-010-9164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou XM, Stockmeier CA, Meltzer HY, Overholser JC, Jurjus GJ, Dieter L, Chen K, Lu D, Johnson C, Youdim MB, Austin MC, Luo J, Sawa A, May W, Shih JC. A novel role for glyceraldehyde-3-phosphate dehydrogenase and monoamine oxidase B cascade in ethanol-induced cellular damage. Biol Psychiatry. 2010b;67:855–63. doi: 10.1016/j.biopsych.2009.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G. New understanding of mechanisms of action of bipolar medications. J Clin Psychiatry. 2008;69(Suppl 5):22–7. [PubMed] [Google Scholar]
- Sawada K, Barr AM, Nakamura M, Arima K, Young CE, Dwork AJ, Falkai P, Phillips AG, Honer WG. Hippocampal complexin proteins and cognitive dysfunction in schizophrenia. Arch Gen Psychiatry. 2005;62:263–72. doi: 10.1001/archpsyc.62.3.263. [DOI] [PubMed] [Google Scholar]
- Shih JC, Chen K, Ridd MJ. Monoamine oxidase: from genes to behavior. Annu Rev Neurosci. 1999;22:197–217. doi: 10.1146/annurev.neuro.22.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y, Bajo M, Chappell AM, Christian DT, Cruz M, Diaz MR, Kash T, Lack AK, Messing RO, Siggins GR, Winder D, Roberto M, McCool BA, Weiner JL. Neurobiological mechanisms contributing to alcohol-stress-anxiety interactions. Alcohol. 2009;43:509–19. doi: 10.1016/j.alcohol.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockmeier CA, Howley E, Shi X, Sobanska A, Clarke G, Friedman L, Rajkowska G. Antagonist but not agonist labeling of serotonin-1A receptors is decreased in major depressive disorder. J Psychiatr Res. 2009;43:887–94. doi: 10.1016/j.jpsychires.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana I, Imoto M, Adjei PN, Gores GJ, Subramaniam M, Spelsberg TC, Urrutia R. Overexpression of the TGFbeta-regulated zinc finger encoding gene, TIEG, induces apoptosis in pancreatic epithelial cells. J Clin Invest. 1997;99:2365–74. doi: 10.1172/JCI119418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MJ, Newton PM, Oyasu M, McMahon T, Chou WH, Connolly J, Messing RO. Acute functional tolerance to ethanol mediated by protein kinase Cepsilon. Neuropsychopharmacology. 2007;32:127–36. doi: 10.1038/sj.npp.1301059. [DOI] [PubMed] [Google Scholar]
- Wang Z, Spittau B, Behrendt M, Peters B, Krieglstein K. Human TIEG2/KLF11 induces oligodendroglial cell death by downregulation of Bcl-XL expression. J Neural Transm. 2007;114:867–75. doi: 10.1007/s00702-007-0635-6. [DOI] [PubMed] [Google Scholar]
- Wu JB, Chen K, Ou XM, Shih JC. Retinoic acid activates monoamine oxidase B promoter in human neuronal cells. J Biol Chem. 2009;284:16723–35. doi: 10.1074/jbc.M901779200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youdim MB, Edmondson D, Tipton KF. The therapeutic potential of monoamine oxidase inhibitors. Nat Rev Neurosci. 2006;7:295–309. doi: 10.1038/nrn1883. [DOI] [PubMed] [Google Scholar]
- Zhang JS, Moncrieffe MC, Kaczynski J, Ellenrieder V, Prendergast FG, Urrutia R. A conserved alpha-helical motif mediates the interaction of Sp1-like transcriptional repressors with the corepressor mSin3A. Mol Cell Biol. 2001;21:5041–9. doi: 10.1128/MCB.21.15.5041-5049.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data Table 1. Characteristics of subjects with or without alcohol dependence.
Table 1A. Characteristics of psychiatrically-normal control subjects.
Table 1B. Characteristics of alcohol dependent subjects.


