Abstract
Attention Deficit Hyperactivity Disorder (ADHD) is the most commonly diagnosed and studied cognitive and behavioral disorder in school-age children. The etiology of ADHD and ADHD-related behavior is unclear, but genetic and environmental factors, such as pesticides, have been hypothesized. The objective of this study was to investigate the relationship between in utero exposure to chlorpyrifos, chlorpyrifos-methyl, and/or 3, 5, 6-trichloro-2-pyridinol (TCPY) and ADHD in school-age Mexican children using TCPY as a biomarker of exposure. The temporal reliability of repeated maternal urinary TCPY concentrations across trimesters was also explored (N=21). To explore associations with ADHD-related outcomes in children, third trimester urinary TCPY concentrations in were measured in 187 mother-child pairs from a prospective birth cohort. Child neurodevelopment in children 6–11 years of age was assessed using Conners’ Parental Rating Scales-Revised (CRS-R), Conners’ Continuous Performance Test (CPT), and Behavior Assessment System for Children-2 (BASC-2). Multivariable linear regression models were used to test relationships for all children combined and also stratified by sex. Intraclass correlation coefficients (ICC) calculations were based on a random effects model. The ICC was 0.41 for uncorrected TCPY, and ranged from 0.29 to 0.32 for specific gravity-corrected TCPY. We did not observe any statistically significant associations between tertiles of maternal TCPY concentrations and ADHD-related outcomes in children. However, compared to the lowest tertile we found suggestive evidence for increased ADHD index in the highest TCPY tertile in boys (β= 5.55 points; 95% CI(−0.19, 11.3); p=0.06) and increased attention problems for the middle tertile in girls (β=5.81 points; 95% CI(−0.75, 12.4); p=0.08). Considering the continued widespread agricultural and possible residential use of chlorpyrifos and chlorpyrifos-methyl in Mexico and the educational implications of cognitive and behavior deficits, these relationships deserve further study.
Keywords: Biomarker, Organophosphates, Pesticides, Exposure, ADHD, Children
Introduction
Attention Deficit Hyperactivity Disorder (ADHD) is the most commonly diagnosed and studied cognitive and behavioral disorder in school-age children with parents reporting 9.5% or 5.4 million children 4–17 years of age ever being diagnosed in the U.S. (CDC, 2011; Banerjee et al 2007; Rowland et al 2002). ADHD is characterized by a persistent pattern of inattention and/or hyperactivity-impulsivity that is more severe than is typically observed in individuals at comparable levels of development (CDC, 2011). Boys are about three times more likely than girls to be diagnosed with ADHD (CDC, 2011). The etiology of ADHD and ADHD-related behavior, specifically inattention and hyperactivity, remains unclear, but genetic and environmental factors, including pesticides, have been hypothesized (Braun et al., 2006; Ernst et al., 2001; Garcia et al., 2003). Studies have shown that organophosphate (OP) exposures at low levels are associated with ADHD and ADHD-related symptoms (Rauh et al., 2006; Bouchard et al 2010; Marks et al., 2010).
Globally, over $30 billion is spent annually on pesticides with one third spent in the developing world (Handal et al., 2008; Karlsson et al., 2004). Chlorpyrifos (CPF), in particular, is the most widely used OP pesticide worldwide (Bradman et al., 2005; Timchalk et al., 2007; Ye et al., 2008). According to the most recent data available, approximately 10 million pounds of CPF are used annually in the U.S. (U.S. EPA, 2011) on crops and sprayed aerially to kill mosquitoes, mites, and termites (CDC, 2012). Human exposure is widespread and occurs through inhalation of vapors and aerosols from spray drift (Pang et al., 2002; Whyatt et al., 2009), ingestion of residuals on food and house dust/soil (Pang et al., 2002; Salas et al., 2003), and dermal absorption following skin contact (Panuwet et al., 2008) and is usually excreted within hours or days in urine (Bradman et al., 2005; Timchalk et al., 2007).
Indoor residential use of CPF was banned in the U.S. in 2001, and pre- and post-construction applications for termite control was phased out by 2005 (U.S. EPA, 2002) due to suspected effects on neurodevelopment in humans. However, it is still registered for agricultural use in the U.S. and allpurpose use in over 100 countries, including Mexico (Dow, 2012). In fact, CPF was one of the more highly used OPs in Mexico in 2000 (Salas et al., 2003). In Mexico, residuals of CPF have been found in raw and commercial pasteurized milk due to its use on crops destined for animal feed such as alfalfa, sorghum, soy, and maize (Salas et al., 2003). However, data on non-occupational human exposure to CPF in Mexico is lacking.
Human and experimental studies have found that the fetus and infant are more sensitive than adults to many environmental toxicants, including CPF (Timchalk et al., 2007) and that a mother’s exposure is a potential source of fetal exposure (Berkowitz et al., 2004). There is evidence that CPF readily passes through the blood-brain barrier, placenta, and amniotic fluid to potentially cause adverse health effects to developing fetuses, specifically, adverse neurodevelopment (Bradman et al., 2003; Whyatt et al., 2005; Perera et al., 2005). Studies have shown children prenatally exposed to OPs display adverse neurodevelopment, including: IQ, mental development, psychomotor development, and attention problems (Engel et al., 2011; Eskenazi et al., 2007; Bouchard et al., 2010; Marks et al., 2010). A study of 3-year old children in New York City found children with high prenatal exposure to CPF, assessed using cord blood, also showed problems with attention, attention-deficit/hyperactivity disorder, and pervasive developmental disorder (Rauh et al., 2006; Rauh et al., 2011). Also in the New York City study, significant morphological abnormalities in the cerebral surface of the brain that affects cognitive and behavioral processes, notably, attention and inhibitory control, was found in children prenatally exposed to higher levels of CPF compared to children with lower exposures (Rauh et al., 2012).
Several animal studies have demonstrated that gestational and/or postnatal exposures to CPF induce neurobehavioral alterations, even at doses that do not elicit measurable cholinergic responses (Dam et al., 2000; De Angelis et al., 2009; Garcia et al., 2005; Levin et al., 2001; Venerosi et al., 2009). As a result, behavioral changes, such as hyperactivity and memory errors, occur (Eaton et al., 2008) and impaired cognitive and behavioral functions may continue into adolescence (Icenogle et al., 2004).
The present study was conducted to explore the presence of urinary concentrations of 3, 5, 6-trichloro-2-pyridinol (TCPY; a metabolite of CPF and chlorpyrifos-methyl) in a study of pregnant women in Mexico City, and to explore relationships between urinary TCPY and measures of child neurodevelopment, specifically Attention Deficit Hyperactivity Disorder (ADHD)-related characteristics. We also explored the temporal reliability of repeated urinary TCPY concentrations across trimesters among a subset of the women. The degree of temporal variability and reliability in an exposure measure has important implications for epidemiology study design and interpretation, especially for chemicals such as CPF and chlorpyrifos-methyl, which are rapidly metabolized.
Methods
Study Population
Participants in the present study were from three sequentially-enrolled, prospective birth cohorts called the Early Life Exposures in Mexico to Environmental Toxicants (ELEMENT) study, which were conducted in Mexico City, Mexico during: 1994–1997 (cohort 1), 1997–2000 (cohort 2), and 2001–2005 (cohort 3). All three ELEMENT cohorts enrolled homogenous, low-to-moderate income, pregnant women from the National Institute of Perinatology, Hospital General Dr. Manuel Gea Gonzalez, or clinics affiliated with the Mexican Social Security Institute (Braun et al., 2012). Mother-child pairs from the three cohorts (N=827) were re-invited between 2007–2011 to examine childhood and adolescent neurodevelopmental characteristics. Exclusion characteristics for all ELEMENT cohorts included: plans to leave the area within the next 5 years; daily consumption of alcoholic beverages; addiction to illegal drugs; continuous use of prescription drugs; diagnosis of multiple pregnancy, pre-eclampsia, renal or heart disease, gestational diabetes; a history of infertility, diabetes, or psychosis; diagnosis of high risk pregnancy; or suffering from seizures requiring medical treatment (Hu et al., 2006). The major objectives of ELEMENT to date have been to examine fetal exposure and risk to heavy metals, particularly lead; however, specific objectives of each ELEMENT cohort have been previously published (Hernandez-Avila et al., 2003; Tellez-Rojo et al., 2004; Ettinger et al., 2009). Exposure, outcome, and demographic characteristics were collected from all eligible participants by the same group of investigators and field staff. The Institutional Review Boards of the National Institute of Public Health (Mexico), Harvard School of Public Health, University of Michigan, and participating hospitals approved all study materials and procedures. Participants were informed of the study, associated aims, and uses of biological samples/data and written consent was obtained before enrollment.
In the overall ELEMENT study, participants provided second morning void urine samples at each trimester of pregnancy. However, in this study, we utilized third trimester urine samples from mother-child pairs that had completed psychometric assessments for children (N=187) from cohorts 2 and 3. In a subset of women randomly selected from the 187 mother-child pairs (N=21), we measured urinary TCPY in samples collected during all three trimesters of pregnancy.
Psychometric Instruments
We used three psychometric assessments to evaluate behavioral characteristics of children 6 – 11 years of age: Conners' Parental Rating Scales-Revised (CPRS-R), Behavior Assessment System for Children – Parental Rating Scales (BASC-PRS), and Conners’ Continuous Performance Test (CPT). Scores from these psychometric instruments assess ADHD-related symptoms and are not designed as diagnostic tools, but rather for screening. The instructions and prompts were translated into Spanish by a researcher (L.S.) in our group who also trained and supervised the personnel who administered the assessments. Standardization and quality control checks were conducted by reviews of videotaped evaluations.
Conners' Parental Rating Scales-Revised (CPRS-R)
CPRS-R is a 27-question assessment tool for parents used to determine a child’s behavior for children and adolescents 3–17 years (Conner, et al. 1998). Most questions are based on behavioral characteristics that are described in the Diagnostic and Statistical Manual of Mental Disorders-IV (DSMIV) diagnostic guidelines for ADHD (American Psychiatric Association, 2000; Deb et al., 2008). In this study, we used the following scales: ADHD Index, DSM-IV Hyperactivity/Impulsivity, DSM-IV Inattention, DSM-IV Total (combined type of ADHD), and Global Restless/Impulsivity Index. For the ADHD Index and the Global Restlessness/Impulsivity scales, the higher the score typically indicates an elevated level of concern with a score of 40–59 considered average and <40 displaying even fewer concerns. The ADHD index identifies children and adolescents who are at risk for ADHD while the Global Restless/Impulsivity Index indicates tendencies toward hyperactivity as well as inattention. The DSM-IV scales yield scores between 0–9; scores of 6 and over suggest possible DSM-IV diagnosis (Conner Profile, 2012). DSM-IV Hyperactivity/Impulsivity and DSM-IV Inattention correspond to a diagnostic type of ADHD. DSM-IV Total represents the diagnostic criteria for the combined type of ADHD.
Behavior Assessment System for Children (BASC) – Parental Rating Scales (PRS)
BASC-PRS is used to measure adaptive and problem child behaviors in the community and home setting (Pearson Assessment, 2012). PRS for 6–11 year olds are used to assess attention problems and hyperactivity. For these scales, the higher the score typically indicates an elevated level of concern. Scores above 59 indicate increased levels of attention/hyperactivity problems for a child at that age.
Continuous Performance Test (CPT)
The CPT is a 14-minute computer test that measures sustained attention and impulsivity (Wilmshurst et al., 2009) and compares the participant’s answers to a reference group and results in a confidence index of a clinical profile. The clinical index measures the likelihood of an ADHD diagnosis. CPT has a high sensitivity (83–90%), but has a poorer specificity (59–61%) when measured against clinical ADHD diagnosis (Linnet et al, 2003). The CPT also provides a series of error and variability measures s such as omissions, commissions, hit reaction time (hit rt), hit rt standard error (hit rt se), and hit rt block change that provide information on specific characteristics of ADHD. Particularly, hit rt block change is the variability of reaction time for correct responses across blocks or sections of the test and has been indicated as a measure of vigilance or sustained attention (Conners’, 2000). Within the CPT, vigilance is a measure of consistency across the test (Conners’, 2000).
TCPY concentrations in urine
Maternal urine samples (2 mL) were transported on dry ice to Emory University for analysis of TCPY in urine. Samples were spiked with stable isotopically-labeled TCPY and then subjected to an enzyme hydrolysis. Hydrolysates were extracted using mixed-polarity solid-phase extraction cartridges (CDC, 2006; Olsson et al., 2004). Elutes were concentrated and analyzed using HPLC/tandem MS (Olsson et al., 2004) with both quantification and confirmation ions monitored. Metabolites were quantified using isotope dilution calibration. The LOD was 0.10 ng/mL for TCPY. Values below the LOD were assigned a value of LOD divided by the square root of two. Urinary specific gravity (SG) was determined using a handheld digital refractometer (ATAGO Company Ltd., Tokyo, Japan). SG-corrected urinary TCPY concentrations were calculated for use in certain statistical analyses.
Data Analysis
Statistical Analysis Software (SAS) (version 9.2; SAS Institute Inc., Cary, NC, USA) was used for most analyses. Descriptive statistics were calculated for demographic information along with the distribution of TCPY and the psychometric scales. ELEMENT third trimester geometric mean TCPY concentration was compared to the geometric mean among pregnant females aged 18–40 years from the U.S. National Health and Nutrition Examination Survey (NHANES), years 1999–2002, using a twosample t-test.
Bivariate analyses between the dependent variables (psychometric assessments- ADHD Index, DSM IV Hyperactivity/Impulsivity, DSM IV Inattention, DSM IV Total, and Global Restless/Impulsivity Index, BASC attention, BASC hyperactivity, CPT clinical index, and CPT Hit Reaction Time Block Change), the primary independent variable of interest (urinary TCPY), as well as other covariates (maternal IQ, maternal education, socioeconomic status, specific gravity, season, breastfeeding, maternal blood lead, child age at testing, child sex, birth length, and head circumference at birth) were conducted. Spearman correlations were used for continuous variables and Wilcoxon tests for categorical variables.
The intraclass correlation coefficient (ICC) was used to evaluate temporal variability and reliability of TCPY levels within individuals across all three trimesters of pregnancy among a subset of women. Calculations were based on a random effects model using PROC Mixed in SAS. ICC represents the reliability of repeated measures over time and is defined as the ratio of between-subject variance to total variance (between-subject plus within-subject) (Meeker et al. 2005). If a measure is highly reliable between time points, the ICC would be near 1.0, whereas a measure with low reliability would have an ICC closer to zero.
Multivariable regression models were created using variables found to be associated with ADHD-related behaviors and/or TCPY in bivariate analyses (p<0.05). Variables were also considered from a priori suspicion of being potential confounders of the association between prenatal CPF/chlorpyrifos-methyl exposure and psychometric outcome. Covariates included: maternal IQ, maternal education, income, maternal urine specific gravity, breastfeeding, maternal blood lead one month after delivery, season of sample collection, child’s age at testing, child’s sex, birth length, and head circumference at birth. Birth length and head circumference at birth were determined by a nurse at the time of delivery and were used as continuous variables. Maternal IQ was calculated on the basis of a mother’s scores on the Information, Comprehension, Similarities, and Block Designs scales of the Spanish Wechsler Adult Intelligence Scale (Téllez-Rojo et al., 2006; Weschler, 1968). A continuous variable was created to capture socioeconomic status and income based on material possessions. Maternal education was the cumulative number of years that the mother attended school. Breastfeeding (yes/no) from a questionnaire administered to the mother during the child’s infancy was included in the model and used categorically. Maternal blood lead was measured one month postpartum and was used continuously. Season was categorized as either rainy (June – October) or dry (November – May) based on the month of sample collection. Using Generalized Additive Models (GAM) adjusted for previously mentioned variables, we evaluated the shape of the TCPY-response relationship by fitting splines.
TCPY was categorized into tertiles for the multivariable models. This decision was based on the ICC analysis and the observation in our previous work that classifying TCPY concentrations into broad categories may be more robust to temporal within-person variability and measurement error (Meeker et al. 2005). The trend estimate for TCPY was calculated by creating a 3-level ordinal TCPY variable and including that variable in the regression model. Models were explored for all children combined and also stratified by sex.
Results
A total of 230 second morning void urine samples were analyzed for TCPY. These samples consisted of 187 third trimester samples from women who had children with data on at least one of the neurodevelopmental outcome measures of interest. The remaining samples were from the subset of women for whom we also measured urinary TCPY variability across all three trimesters. Characteristics of the women are presented in Table 1. The median maternal age at delivery was 26 years, with 11 years of schooling and a median IQ of 96. Most mothers were married (74%), did not smoke during pregnancy (99%), and breastfed their baby (90%). Median blood lead measures (5.4 ug/dl) in moms were slightly above the recommended CDC threshold of acceptable levels in pregnant and lactating women of 5.0 ug/dL (CDC, 2012).The distributions of TCPY concentrations (uncorrected for SG) in the present study and among pregnant women from NHANES 1999–2002 are presented in Table 2. TCPY was detected in over 90% of urine samples in the present study. Geometric mean TCPY concentration among ELEMENT women was significantly higher than pregnant women in NHANES (p-value =0.03).
Table 1.
Descriptive maternal characteristics (N=187) and for the subset with all three urine samples available (N=20)
| Full (N=187) | Sub-set (N=20) | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | N (%) | Median (25th, 75th) | N (%) | Median (25th, 75th) | p* | |||
| Maternal Age | 187 | 26 (22, 30) | 20 | 28(23, 31) | 0.59 | |||
| Maternal Education | 187 | 11 (9, 11) | 20 | 12(9,12) | 0.71 | |||
| Maternal IQ | 187 | 96 (88, 103) | 20 | 96(89, 107) | 0.72 | |||
| Socioeconomic Status | 178 | 8.0 (6.0, 11) | 18 | 7.5(6.0, 10.0) | 0.98 | |||
| Blood Lead (ug/dl) | 187 | 5.4(3.3, 7.8) | 20 | 5.9(2.6, 7.7) | 0.51 | |||
| Marital Status | 0.71 | |||||||
| Married | 139(74) | 16(80) | ||||||
| Divorced | 1(0.5) | 0 | ||||||
| Separated | 1(0.5) | 1(5) | ||||||
| Never married | 15(8) | 2(15) | ||||||
| Living with partner | 31(17) | 0 | ||||||
| Smoking during Pregnancy | ||||||||
| Some days | 2(1) | 1(10) | 0.8 | |||||
| Not at all | 185(99) | 9(90) | ||||||
| Season of Sample Collection | ||||||||
| rainy- June to October | 89 (48) | 5 (25) | 0.04 | |||||
| dry - November to May | 98 (52) | 15(75) | ||||||
| Breastfed | ||||||||
| Yes | 167(90) | 18(90) | 0.98 | |||||
| No | 19(10) | 2(10) | ||||||
| Trimester of Pregnancy | ||||||||
| 1st Trimester | 10(5) | 10(5) | ||||||
| 2nd Trimester | 11(6) | 11(6) | ||||||
| 3rd Trimester | 187(100) | 187(100) | ||||||
Two sample t-test
Table 2.
Distribution of uncorrected urinary TCPY concentrations among women in ELEMENT and NHANES
| N | Geomean (95% CI) | 10th | 25th | 50th | 75th | 90th | 95th | Max | |
|---|---|---|---|---|---|---|---|---|---|
| ELEMENT TCPYa,c | 187 | 1.76 (1.55, 2.02) | 0.45 | 0.91 | 1.78 | 3.57 | 6.40 | 11.6 | 44.8 |
| NHANES TCPY (Pregnant Women) | 177 | 1.41(1.23, 1.61) | <LOD | 0.61 | 1.60 | 3.05 | 5.00 | 6.85 | 15.2 |
TCPY=3,5,6-trichloro-2-pyridinol
Females are of reproductive age, 18–40 years
LOD-limit of detection; ELEMENT TCPY
LOD=0.10 ng/ml; NHANES TCPY LOD=0.40 ug/L
Two sample t-test: p=0.03
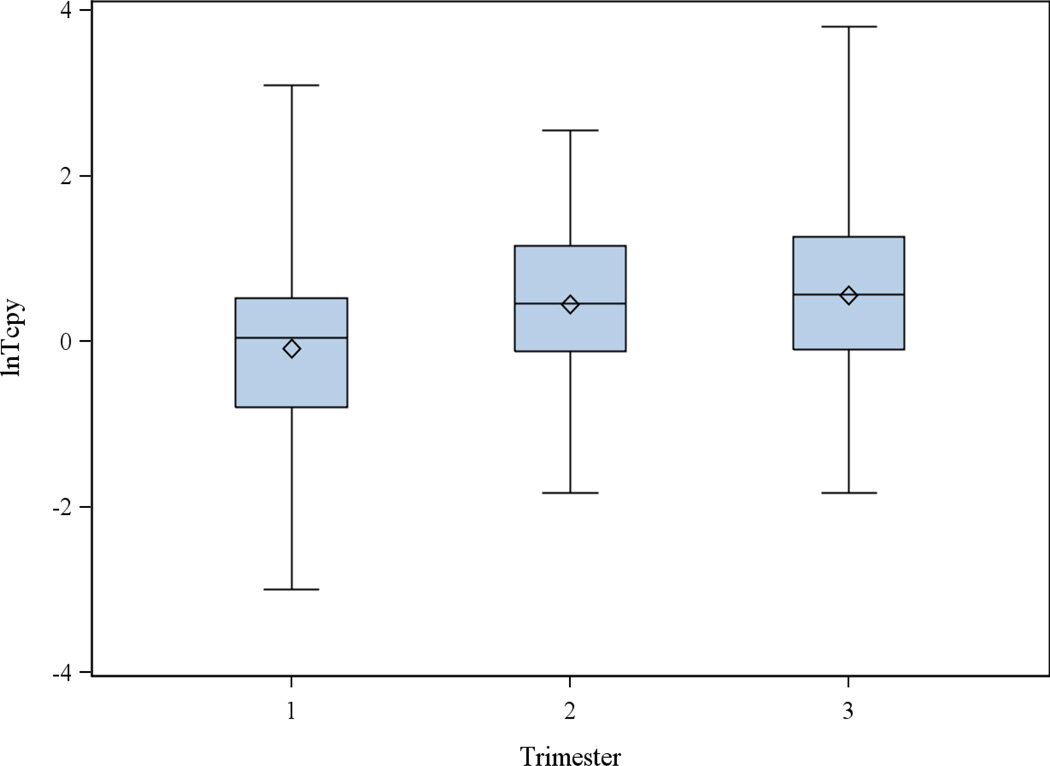
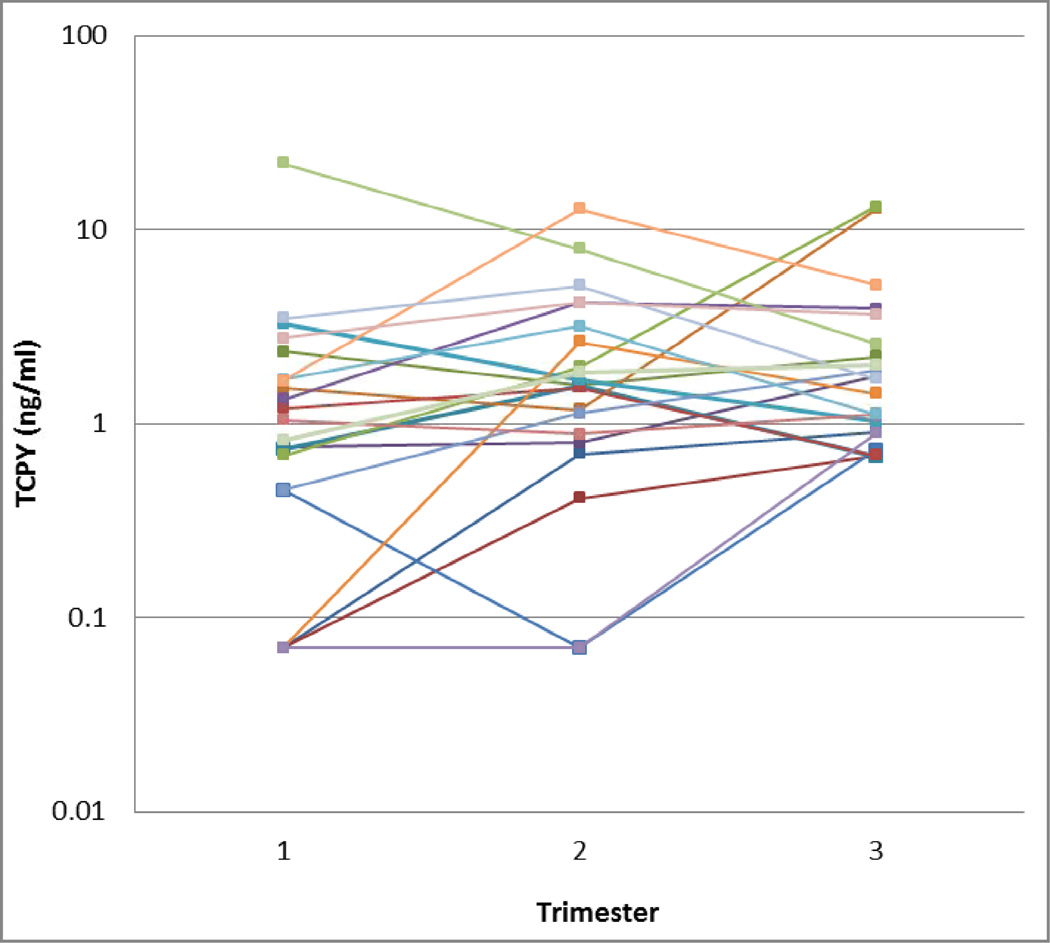
Among the subset of women with repeated measures, there were no significant differences in geometric mean TCPY concentrations between trimesters (Figure 1). However, there was significant within-woman variability across trimesters (Figure 2). ICCs for uncorrected and SG-corrected TCPY concentrations are shown in Table 3, calculated when considering only women in the variability subset as well as when including all women with TCPY measures. The ICC was 0.41 for uncorrected TCPY, and ranged from 0.29 to 0.32 for SG-corrected TCPY.
Figure 1.
Natural log-transformed TCPY concentrations in maternal spot urine samples by trimester of pregnancy
Figure 2.
TCPY concentrations in maternal spot urine samples collected in each trimester of pregnancy (N=21). Each color represents repeated samples collected from the same woman.
Table 3.
Variance estimates and intraclass correlation coefficients (ICC) for uncorrected and SG-corrected ln-transformed TCPY across trimesters of pregnancy
| Uncorrected (ng/ml) (N*=63) |
Uncorrected (ng/ml) (N*=230) |
||||
| Variance Estimate | ICC | Variance Estimate | ICC | ||
| Between-subject | 0.54 | 0.41 | Between-subject | 0.54 | 0.41 |
| Within-subject | 0.78 | Within-subject | 0.75 | ||
| Specific Gravity- Corrected (ng/ml) |
Specific Gravity- Corrected (ng/ml) |
||||
| Between-subject | 0.31 | 0.29 | Between-subject | 0.30 | 0.32 |
| Within-subject | 0.74 | Within-subject | 0.63 | ||
N represents the number of TCPY measurements used.
Table 4 describes characteristics of the children at birth and at the time of the psychometric assessments. In this population, 52% of children were female and the median age of the child at testing was 7.5 years. Results from the multivariable regression analysis are presented in Table 5. No statistically significant associations were observed in models that considered all children combined and models stratified by sex. However, we found a suggestive trend for increasing Hit RT Block Change in relation to increasing TCPY tertiles (p-value=0.09). In models stratified by sex, this relationship appeared to be stronger among boys than girls. In the sex-stratified analysis, we also found a suggestive trend for increased ADHD Index in relation to TCPY tertiles (p-value=0.06). In this model, the highest TCPY tertile was associated with an ADHD index score that was 5.55 points higher than children in the lowest tertile (p-value=0.06). Among girls, there was a suggestive increase in attention problems score in relation to exposure, but only for the middle TCPY tertile (p=0.08).
Table 4.
Child characteristics at birth and psychometric assessments in childhood
| N (%) | 10th | 25th | 50th | 75th | 90th | 95th | Max | ||
|---|---|---|---|---|---|---|---|---|---|
| Birth Characteristics | |||||||||
| Sex | 187 | ||||||||
| Male | 89 (48) | ||||||||
| Female | 97 (52) | ||||||||
| Head Circumference (cm) | 154 | 33 | 34 | 34 | 35 | 36 | 37 | 39 | |
| Weight (kg) | 186 | 2.6 | 2.9 | 3.2 | 3.5 | 3.8 | 3.9 | 4.2 | |
| Height (cm) | 181 | 48 | 49 | 50 | 51 | 52 | 54 | 57 | |
| Gestational Age (wks) | 185 | 38 | 38 | 39 | 40 | 40 | 41 | 42 | |
| Childhood Psychometric Assessments | |||||||||
| Age at Testing (yrs) | 178 | 7 | 7 | 7.5 | 9 | 10 | 10 | 11 | |
| Attention Deficit and Hyperactivity Index | 181 | 42 | 46 | 52 | 59 | 67 | 73 | 90 | |
| DSM IV Hyperactivity/Impulsivity | 181 | 45 | 49 | 55 | 63 | 71 | 77 | 89 | |
| DSM IV Inattention | 181 | 42 | 45 | 51 | 59 | 67 | 72 | 90 | |
| DSM IV Total | 181 | 44 | 48 | 53 | 62 | 69 | 75 | 88 | |
| Global Restlessness/Impulsivity Index | 181 | 43 | 46 | 52 | 60 | 68 | 74 | 90 | |
| Attention problems | 181 | 37 | 45 | 56 | 62 | 67 | 69 | 76 | |
| Hyperactivity | 181 | 39 | 43 | 47 | 55 | 64 | 68 | 83 | |
| CPT Conners II (Clinical) | 185 | 30 | 42 | 50 | 69 | 81 | 93 | 100 | |
| Hit Reaction Time Block Change | 185 | 38 | 44 | 50 | 58 | 67 | 73 | 100 | |
Table 5.
Adjusted*Multivariable linear regression models for change in psychometric assessment scores associated with medium and high tertiles of maternal third trimester urinary TCPY concentrations compared to the lowest tertile
| All** | Males | Females | |||||
|---|---|---|---|---|---|---|---|
| Psychometric Assessment | B(95%CI) | p | B(95%CI) | p | B(95%CI) | p | |
| ADHD Index | |||||||
| Middle TCPY | 2.61(−1.54, 6.75) | 0.22 | 2.32(−2.55, 7.20) | 0.34 | 1.63(−5.55, 8.82) | 0.65 | |
| High TCPY | 4.00(−0.91, 8.90) | 0.11 | 5.55(−0.19, 11.3) | 0.06 | 0.17(−8.28, 8.63) | 0.97 | |
| p for trend | 0.11 | 0.06 | 0.96 | ||||
| DSM IV Hyperactivity/Impulsivity | |||||||
| Middle TCPY | −0.56(−5.03, 3.91) | 0.81 | −0.17(−6.63, 6.29) | 0.96 | 0.33(−6.44, 7.10) | 0.92 | |
| High TCPY | −0.51(−5.80, 4.78) | 0.85 | 1.25(−6.36, 8.87) | 0.74 | −3.81(−11.8, 4.16) | 0.34 | |
| p for trend | 0.84 | 0.76 | 0.35 | ||||
| DSM IV Inattention | |||||||
| Middle TCPY | 2.37(−1.79, 6.53) | 0.26 | 2.33(−2.36, 7.02) | 0.32 | 1.19(−6.09, 8.47) | 0.74 | |
| High TCPY | 2.45(−2.47, 7.37) | 0.33 | 2.63(−2.89, 8.16) | 0.34 | −0.07(−8.64, 8.50) | 0.99 | |
| p for trend | 0.31 | 0.32 | 0.99 | ||||
| DSM IV Total | |||||||
| Middle TCPY | 1.23(−2.89, 5.35) | 0.56 | 0.80(−4.48, 6.09) | 0.76 | 1.64(−5.17, 8.45) | 0.63 | |
| High TCPY | 1.10(−3.77, 5.98) | 0.65 | 2.06(−4.17, 8.29) | 0.51 | −1.83(−9.84, 6.19) | 0.65 | |
| p for trend | 0.64 | 0.51 | 0.66 | ||||
| Restlessness/Impulsivity Index | |||||||
| Middle TCPY | −0.15(−4.57, 4.27) | 0.95 | 0.49(−5.71, 6.68) | 0.88 | −0.48(−7.10, 6.14) | 0.89 | |
| High TCPY | 0.38(−4.85, 5.61) | 0.89 | 3.78(−3.52, 11.1) | 0.31 | −4.90(−12.7, 2.89) | 0.21 | |
| p for trend | 0.89 | 0.32 | 0.22 | ||||
| Attention problems | |||||||
| Middle TCPY | 1.79(−2.66, 6.24) | 0.43 | −0.37(−7.02, 6.27) | 0.91 | 5.81(−0.75, 12.4) | 0.08 | |
| High TCPY | 3.46(−1.81, 8.73) | 0.20 | 5.59(−2.24, 13.4) | 0.16 | 1.82(−5.91, 9.55) | 0.64 | |
| p for trend | 0.19 | 0.18 | 0.62 | ||||
| Hyperactivity | |||||||
| Middle TCPY | −3.69(−7.88, 0.50) | 0.08 | −5.00(−12.0, 2.00) | 0.16 | −0.005(−5.17, 5.16) | 1.00 | |
| High TCPY | −3.35(−8.31, 1.60) | 0.18 | −3.49(−11.7, 4.73) | 0.40 | −2.77(−8.84, 3.31) | 0.37 | |
| p for trend | 0.17 | 0.36 | 0.37 | ||||
| CPT Conners II (Clinical) | |||||||
| Middle TCPY | −3.97(−12.5, 4.51) | 0.36 | −4.29(−15.8, 7.18) | 0.46 | 0.42(−13.2, 14.0) | 0.95 | |
| High TCPY | 2.19(−8.11, 12.5) | 0.68 | 0.84(−12.8, 14.5) | 0.90 | 8.55(−7.83, 24.9) | 0.30 | |
| p for trend | 0.73 | 0.95 | 0.31 | ||||
| Hit RT Block Change | |||||||
| Middle TCPY | −4.59(−9.55, 0.36) | 0.07 | −5.10(−13.1, 2.92) | 0.21 | −3.79(−10.6, 2.98) | 0.27 | |
| High TCPY | −5.10(−11.1, 0.91) | 0.10 | −6.86(−16.4, 2.68) | 0.16 | −2.33(−10.5, 5.82) | 0.57 | |
| p for trend | 0.09 | 0.14 | 0.55 | ||||
Adjusted by child sex, maternal IQ, Maternal education, income, child age at testing, Specific Gravity, Season, Breastfeeding, lead, delivery height, delivery head circumference
N=142
In parsimonious models that excluded delivery head circumference, delivery height, and breastfeeding, effect estimates were somewhat attenuated. For boys in the highest TCPY tertile, ADHD index was 4.13 points higher than boys in the lowest tertile (p-value=0.15). Among girls, the score for attention problem was 3.86 points higher for the middle TCPY tertile in comparison to the lowest TCPY tertile (p=0.19).
Discussion
The objectives of this study were to define the distribution of TCPY concentrations among a population of pregnant women in Mexico, to assess between- and within-individual variability of urinary TCPY levels over the course of pregnancy, and to explore the relationship between third trimester maternal urinary TCPY concentrations and child neurodevelopment, particularly attention and hyperactivity, using subscales from psychometric assessments. This is the first study to assess urinary TCPY concentrations in a Mexican population, as well as the first to explore associations with attention and hyperactivity, using urinary TCPY as a biomarker of exposure to CPF, chlorpyrifos-methyl or TCPY.
We found that TCPY levels were somewhat higher in our study population compared to U.S. pregnant women from NHANES investigations in overlapping years. While the ICC we report here indicates a fair level of reliability between TCPY measures across pregnancy (Landis et al. 1977; Portney et al. 2000), prior studies have also documented larger within-person variability relative to between-person variability in TCPY concentrations measured in repeat spot urine samples from the same individual. In a study in New York City, the ICC was 0.43 (corrected for creatinine) for TCPY in repeated urine samples from the third trimester of pregnancy (with one sample post-delivery) in participants enrolled 2001–2002 (Whyatt et al. 2009). This is quite similar to the uncorrected ICC reported in our study (0.41). Studies in non-pregnant women such as a study in Maryland found an ICC of 0.40 (uncorrected) in urinary TCPY after collecting six repeat first morning void urine samples from 80 participants over the course of a year (Egeghy et al., 2005). In an additional study, that collected nine repeat urine samples from 10 men over three months, ICCs ranged from 0.15 to 0.21 for uncorrected and corrected (for creatinine and specific gravity) TCPY (Meeker et al. 2005). However, the authors did report that a single urine measure of TCPY was able to adequately predict tertiles of exposure based on the average TCPY levels in repeated samples collected over three months (Meeker et al. 2005).
In the present study, we did not observe any statistically significant associations between tertiles of maternal third trimester urinary TCPY and measures of attention and hyperactivity in children. However, we found suggestive evidence for increases in the ADHD index in relation to TCPY tertiles among boys. This suggests that fetal exposure to increased quantities of chlorpyrifos, chlorpyrifosmethyl, or TCPY during the last trimester of pregnancy may influence the display of ADHD characteristics in childhood. Similarly, an animal study examining behavioral alterations after CPF exposure during neurolation found that Sprague-Dawley rats injected with 5 mg/kg of CPF during gestational days 9–12 compared to the control group (0 mg/kg) were more affected by adverse neurobehavior, particularly working and reference memory which involves attentional processes (Icenogle et al., 2004). There are limited human studies examining urinary TCPY and neurodevelopment and even fewer examining ADHD and ADHD-related behavior. One study in California did, however, utilize prenatal urinary TCPY, but did not find any association with attention or ADHD-related problems in 2-year old children (Eskenazi et al., 2007), however, such behaviors may not be apparent at that age. In another California study, where the association between prenatal urinary dialkyl phosphates (DAPs), a non-specific measure of OPs, and ADHD assessments at 3.5 and 5 years of age was examined, a stronger association with ADHD was found in boys than in girls and generally stronger associations in 5 year olds compared to the younger age children (Mark et al., 2010). In another study in New York City (N=228), it was found that 3 year olds more highly exposed to chlorpyrifos in utero, as measured in cord blood, had lower scores on the Bayley Scales of Infant Development Psychomotor Development (PDI) and Mental Development (MDI) Indices (Bayley, 1993) when compared to lower levels of exposure (Rauh et al., 2006). At the 7 year follow-up of the New York City study, significant associations were also found with working memory skills, thus consistent with previous findings (Rauh et al., 2011).
In this study, we found a suggestive association for increased attention problems when middle TCPY tertile was compared to the lowest tertile in girls. This is in contrast to the previously mentioned study in California of 348 mother-child pairs where urinary DAPs were associated with attention problems and ADHD symptoms that were stronger in 5 year-old boys than girls (A. R. Marks et al., 2010). We did, however, observe a 5.6-point increase in attention problems among boys in the highest TCPY tertile compared to the lowest (p=0.16). Animal studies have found that sex differences in the impacts on neurodevelopment occur when CPF exposure occurs in late pregnancy (17–20 gestational days in Sprague-Dawley rats) or soon after birth; however, CPF exposures throughout pregnancy may cause non-sex modified adverse neurodevelopment (Icenogle et al., 2004; Levin et al., 2001; Levin et al., 2002). It has been found that prenatal CPF exposure in rats, as early as neurulation or gestational days 9–12, resulted in increased locomotor activities, a characteristic of ADHD and impaired cognitive function in adolescence and adulthood (Icenogle et al., 2004). Other animal studies suggested that CPF may interfere with the development of sex differences in behavioral patterns that are dependent on the timing of exposure (Levin et al., 2001; Levin et al., 2002). However, there is not a clear mechanism to explain this occurrence. Studies have found that in the clinical presentation of attention deficit disorder, girls display more inattentive-type problems while boys display more hyperactive and impulsive behaviors (Biederman et al., 2002; Marks et al., 2010; Staller et al., 2006). In general, more boys are more likely to be diagnosed with ADHD than girls (CDC, 2011).
Our study had a number of strengths and limitations. Some imitations include a relatively modest sample size for exploring relationships between urinary TCPY and childhood neurodevelopment and the use of a single urinary measure to estimate exposure in those analyses. Despite our modest sample size, our study provides a foundation for a larger study and tentatively supports the limited previously published literature regarding urinary TCPY and childhood neurodevelopment. Strengths of our study included the use of urinary TCPY as a biomarker of exposure and the use of validated ADHD psychometric assessments by trained and experienced research team. Using urinary TCPY as a biomarker of chlorpyrifos exposure allowed the specific measure of chlorprifos and chlorprifos-methyl, whereas similar studies have measured DAPs, which examined OPs as a class. Also, urinary measures of chlorpyrifos are likely to be more reliable over time in comparison to blood given the rapid metabolism and low detection rates of non-persistent pesticides in blood. However, the presence of TCPY in urine may also reflect environmental or dietary exposure to TCPY following environmental degradation of CPF of CPF-methyl. To our knowledge, this is the first study to assess the association between urinary TCPY and a direct assessment of ADHD and ADHD-related behaviors in school-aged children. Previous studies have not directly assessed ADHD and ADHD-related behaviors in school-age children based on early life CPF, chlorpyrifos-methyl, and/or TCPY exposure. While previous studies have assessed OP exposure in relation to attention and ADHD in pre-school children, prenatal urinary TCPY exposure has not been previously examined with these outcomes in school age children. Also, this is the first study to assess in utero CPF, chlorpyrifos-methyl, and TCPY exposure in relation to neurodevelopment during childhood in a Mexico City, Mexico population.
In summary, these results are important considering the continued widespread agricultural and possibly residential use of chlorpyrifos and chlorpyrifos-methyl in Mexico and the educational implications of cognitive and behavioral deficits. Exactly how prenatal CPF and chlorpyrifos-methyl exposure might affect the onset of clinically diagnosed ADHD is unclear, but this study provides interesting information on CPF, CPF-methyl, and TCPY exposures in pregnancy and deserves further study in a larger population
Acknowledgements
This work was supported by the National Institute of Environmental Health Sciences, grant numbers: R01ES021446, R01ES007821, R01ES021465, P42ES017198, P20ES018171, and P30ES017885; by the U.S. Environmental Protection Agency, grant number: RD83480001; by: Consejo Nacional de Ciencia y Tecnología (CONACyT) grant number: 4150M9405; and by the Consejo de Estudios para la Restauración y Valoración Ambiental (CONSERVA), Department of Federal District, México. Authorship positions on this manuscript adhere to the long-standing policy of the ELEMENT partnership that requires the first and last author positions to be split between authors from either the US and Mexico or Mexico and the US.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aguiar A, Eubig PA, Schantz SL. Attention deficit/hyperactivity disorder: A focused overview for children's environmental health researchers. Environmental Health Perspectives. 2010;118(12):1646–1653. doi: 10.1289/ehp.1002326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayley N. Bayley Scales of Infant Development® - Second Edition (BSID®-II) 1993 [Google Scholar]
- 3.Berkowitz G, Wetmur J, Birman-Deych E, Obel J, Lapinski R, Godbold J, Wolff M. In utero pesticide exposure, maternal paraoxonase activity, and head circumference. Environmental Health Perspectives. 2004;112(3):388–391. doi: 10.1289/ehp.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biederman J, Mick E, Faraone SV, Braaten E, Doyle A, Spencer T, Johnson MA. Influence of gender on attention deficit hyperactivity disorder in children referred to a psychiatric clinic. The American Journal of Psychiatry. 2002;159(1):36–42. doi: 10.1176/appi.ajp.159.1.36. [DOI] [PubMed] [Google Scholar]
- 5.Bradman A, Whyatt R. Characterizing exposures to nonpersistent pesticides during pregnancy and early childhood in the national children's study: A review of monitoring and measurement methodologies. Environmental Health Perspectives. 2005;113(8):1092–1099. doi: 10.1289/ehp.7769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braun JM, Kahn RS, Froehlich T, Auinger P, Lanphear BP. Exposures to environmental toxicants and attention deficit hyperactivity disorder in U. S. children. Environmental Health Perspectives. 2006;114(12):1904–1909. doi: 10.1289/ehp.9478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braun JM, Hoffman E, Schwartz J, Sanchez B, Schnaas L, Mercado-Garcia A, Hernandez-Avila M. Assessing windows of susceptibility to lead-induced cognitive deficits in mexican children. Neurotoxicology. 2012;33(5):1040–1047. doi: 10.1016/j.neuro.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CDC. [Accessed October 10, 2012];2012 http://www.cdc.gov/biomonitoring/Chlorpyrifos_BiomonitoringSummary.html.
- 9.CDC. [Accessed November 24, 2012];2011 http://www.cdc.gov/ncbddd/adhd/data.html.
- 10.Conner Profile. [Accessed October 20, 2012]; http://www.damianbariexca.net/wp-content/docs/Conners%20ADHD%20Report.pdf. [Google Scholar]
- 11.Dam K, Seidler FJ, Slotkin TA. Chlorpyrifos exposure during a critical neonatal period elicits gender-selective deficits in the development of coordination skills and locomotor activity. Developmental Brain Research. 2000;121(2):179–187. doi: 10.1016/s0165-3806(00)00044-4. [DOI] [PubMed] [Google Scholar]
- 12.De Angelis S, Tassinari R, Maranghi F, Eusepi A, Di Virgilio A, Chiarotti F, Mantovani A. Developmental exposure to chlorpyrifos induces alterations in thyroid and thyroid hormone levels without other toxicity signs in CD-1 mice. Toxicological Sciences. 2009;108(2):311–319. doi: 10.1093/toxsci/kfp017. [DOI] [PubMed] [Google Scholar]
- 13.Deb S, Dhaliwal AJ, Roy M. The usefulness of conners' rating scales-revised in screening for attention deficit hyperactivity disorder in children with intellectual disabilities and borderline intelligence. Journal of Intellectual Disability Research : JIDR. 2008;52(11):950–965. doi: 10.1111/j.1365-2788.2007.01035.x. [DOI] [PubMed] [Google Scholar]
- 14.Dellu-Hagedorn F. Relationship between impulsivity, hyperactivity and working memory: a differential analysis in the rat. Behavioral and Brain Function. 2006;2(10) doi: 10.1186/1744-9081-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dow. [Accessed October 20, 2012];2012 http://www.chlorpyrifos.com/worldwide-use.htm. [Google Scholar]
- 16.Eaton D, Daroff R, Autrup H, Bridges J, Buffler P, Costa L, Spencer P. Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Critical Reviews in Toxicology. 2008;38(Suppl 2):1–125. doi: 10.1080/10408440802272158. [DOI] [PubMed] [Google Scholar]
- 17.Egeghy PP, Quackenboss JJ, Catlin S, Ryan PB. Determinants of temporal variability in NHEXAS-maryland environmental concentrations, exposures, and biomarkers. Journal of Exposure Analysis and Environmental Epidemiology. 2005;15(5):388–397. doi: 10.1038/sj.jea.7500415. [DOI] [PubMed] [Google Scholar]
- 18.Engel SM, Wetmur J, Chen J, Zhu C, Barr DB, Canfield RL, Wolff MS. Prenatal exposure to organophosphates, paraoxonase 1, and cognitive development in childhood. Environ Health Perspect. 2011;119(8) doi: 10.1289/ehp.1003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ernst M, Moolchan ET, Robinson ML. Behavioral and neural consequences of prenatal exposure to nicotine. Journal of the American Academy of Child & Adolescent Psychiatry. 2001;40(6):630–641. doi: 10.1097/00004583-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Ettinger AS, Lamadrid-Figueroa H, Tellez-Rojo MM, Mercado-Garcia A, Peterson KE, Schwartz J, Hernandez-Avila M. Effect of calcium supplementation on blood lead levels in pregnancy: A randomized placebo-controlled trial. Environmental Health Perspectives. 2009;117(1):26–31. doi: 10.1289/ehp.11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia S, Seidler F, Slotkin T. Developmental neurotoxicity elicited by prenatal or postnatal chlorpyrifos exposure: Effects on neurospecific proteins indicate changing vulnerabilities. Environmental Health Perspectives. 2003;111(3):297–303. doi: 10.1289/ehp.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia SJ, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: Targeting glial cells. Environmental Toxicology and Pharmacology. 2005;19(3):455–461. doi: 10.1016/j.etap.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Handal A, Harlow S, Breilh J, Lozoff B. Occupational exposure to pesticides during pregnancy and neurobehavioral development of infants and toddlers. Epidemiology. 2008;19(6):851–859. doi: 10.1097/EDE.0b013e318187cc5d. [DOI] [PubMed] [Google Scholar]
- 24.Hernandez-Avila M, Gonzalez-Cossio T, Hernandez-Avila JE, Romieu I, Peterson KE, Aro A, Hu H. Dietary calcium supplements to lower blood lead levels in lactating women: A randomized placebo-controlled trial. Epidemiology (Cambridge, Mass.) 2003;14(2):206–212. doi: 10.1097/01.EDE.0000038520.66094.34. [DOI] [PubMed] [Google Scholar]
- 25.Hu H, Tellez-Rojo MM, Bellinger D, Smith D, Ettinger AS, Lamadrid-Figueroa H, Hernandez-Avila M. Fetal lead exposure at each stage of pregnancy as a predictor of infant mental development. Environmental Health Perspectives. 2006;114(11):1730–1735. doi: 10.1289/ehp.9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Icenogle LM, Christopher NC, Blackwelder WP, Caldwell DP, Qiao D, Seidler FJ, Levin ED. Behavioral alterations in adolescent and adult rats caused by a brief subtoxic exposure to chlorpyrifos during neurulation. Neurotoxicology and Teratology. 2004;26(1):95–101. doi: 10.1016/j.ntt.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Inchem. [Accessed October 20, 2012];Pesticide residues in food. 1999 http://www.inchem.org/documents/jmpr/jmpmono/v99pr03.htm. [Google Scholar]
- 28.Karlsson SI. Agricultural pesticides in developing countries. Environment. 2004;46(4):22–41. [Google Scholar]
- 29.Linnet KM, Dalsgaard S, Obel C, Wisborg K. Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: Review of the current evidence. The American Journal of Psychiatry. 2003;160(6):1028–1040. doi: 10.1176/appi.ajp.160.6.1028. [DOI] [PubMed] [Google Scholar]
- 30.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 31.Levin ED, Addy N, Nakajima A, Christopher NC, Seidler FJ, Slotkin TA. Persistent behavioral consequences of neonatal chlorpyrifos exposure in rats. Developmental Brain Research. 2001;130(1):83–89. doi: 10.1016/s0165-3806(01)00215-2. [DOI] [PubMed] [Google Scholar]
- 32.Levin ED, Addy N, Baruah A, Elias A, Christopher NC, Seidler FJ, Slotkin TA. Prenatal chlorpyrifos exposure in rats causes persistent behavioral alterations. Neurotoxicology and Teratology. 2002;24(6):733–741. doi: 10.1016/s0892-0362(02)00272-6. [DOI] [PubMed] [Google Scholar]
- 33.MacIntosh DL, Needham LL, Hammerstrom KA, Ryan PB. A longitudinal investigation of selected pesticide metabolites in urine. Journal of Exposure Analysis and Environmental Epidemiology. 1999;9(5):494–501. doi: 10.1038/sj.jea.7500045. [DOI] [PubMed] [Google Scholar]
- 34.Marks AR, Harley K, Bradman A, Kogut K, Barr DB, Johnson C, Eskenazi B. Organophosphate pesticide exposure and attention in young mexican-american children: The CHAMACOS study. Environmental Health Perspectives. 2010;118(12):1768–1774. doi: 10.1289/ehp.1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meeker JD, Barr DB, Ryan L, Herrick RF, Bennett DH, Bravo R. Temporal variability of urinary levels of non-persistent insecticides in adult men. J Expo Anal Environ Epidemiology. 2005;15(3):271–281. doi: 10.1038/sj.jea.7500402. [DOI] [PubMed] [Google Scholar]
- 36.Middlemore-Risher ML, Buccafusco JJ, Terry J, A V. Repeated exposures to low-level chlorpyrifos results in impairments in sustained attention and increased impulsivity in rats. Neurotoxicology and Teratology. 2010;32(4):415–424. doi: 10.1016/j.ntt.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olsson A, Baker S, Nguyen J, Romanoff L, Udunka S, Walker R, Barr D. A liquid chromatography--tandem mass spectrometry multiresidue method for quantification of specific metabolites of organophosphorus pesticides, synthetic pyrethroids, selected herbicides, and deet in human urine. Analytical Chemistry. 2004;76(9):2453. doi: 10.1021/ac0355404. [DOI] [PubMed] [Google Scholar]
- 38.Pang Y, MacIntosh D, Camann D, Ryan PB. Analysis of aggregate exposure to chlorpyrifos in the NHEXAS-maryland investigation. Environmental Health Perspectives. 2002;110(3):235–240. doi: 10.1289/ehp.02110235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Panuwet P, Prapamontol T, Chantara S, Thavornyuthikarn P, Montesano MA, Whitehead R, Barr D. Concentrations of urinary pesticide metabolites in small-scale farmers in chiang mai province, thailand. The Science of the Total Environment. 2008;407(1):655–668. doi: 10.1016/j.scitotenv.2008.08.044. [DOI] [PubMed] [Google Scholar]
- 40.Pearson Assessments. [Accessed October 2012]; http://www.pearsonassessments.com/HAIWEB/Cultures/en-us/Productdetail.htm?Pid=PAa30000. [Google Scholar]
- 41.Perera FP, Rauh V, Whyatt RM, Tang D, Tsai WY, Bernert JT, Kinney PL. A summary of recent findings on birth outcomes and developmental effects of prenatal ETS, PAH, and pesticide exposures. Neurotoxicology. 2005;26(4):573–587. doi: 10.1016/j.neuro.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Portney LG, Watkins MP. Foundations of clinical research Applications to practice. New Jersey: Prentice Hall Inc.; 2000. pp. 560–567. ISBN 0-8385-2695-0. [Google Scholar]
- 43.Posner MI. Gazzaniga MS, editor. Attention in cognitive neuroscience: an overview. The Cognitive Neurosciences. 1995:615–624. [Google Scholar]
- 44.Rauh VA, Perera FP, Horton MK, Whyatt RM, Bansal R, Hao X, Peterson BS. Brain anomalies in children exposed prenatally to a common organophosphate pesticide. Proceedings of the National Academy of Sciences. 2012;109(20):7871–7876. doi: 10.1073/pnas.1203396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rauh V, Arunajadai S, Horton M, Perera F, Hoepner L, Barr DB, Whyatt R. Seven-year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environ Health Perspect. 2011;119(8) doi: 10.1289/ehp.1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rauh V, Garfinkel R, Perera F, Andrews H, Hoepner L, Barr D, Whyatt R. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118(6):e1845–e1859. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ricceri L, Venerosi A, Capone F, Cometa M, Lorenzini P, Fortuna S, Calamandrei G. Developmental neurotoxicity of organophosphorous pesticides: Fetal and neonatal exposure to chlorpyrifos alters sex-specific behaviors at adulthood in mice. Toxicological Sciences. 2006;93(1):105–113. doi: 10.1093/toxsci/kfl032. [DOI] [PubMed] [Google Scholar]
- 48.Salas J, Gonzlez M, Noa M, Prez N, Daz G, Gutirrez R, Osuna I. Organophosphorus pesticide residues in mexican commercial pasteurized milk. Journal of Agricultural and Food Chemistry. 2003;51(15):4468–4471. doi: 10.1021/jf020942i. [DOI] [PubMed] [Google Scholar]
- 49.Staller J, Faraone SV. Attention-deficit hyperactivity disorder in girls: Epidemiology and management. CNS Drugs. 2006;20(2):107–123. doi: 10.2165/00023210-200620020-00003. [DOI] [PubMed] [Google Scholar]
- 50.Téllez-Rojo MM, Bellinger DC, Arroyo-Quiroz C, Lamadrid-Figueroa H, Mercado-García A, Schnaas-Arrieta L, Hu H. Longitudinal associations between blood lead concentrations lower than 10 µg/dL and neurobehavioral development in environmentally exposed children in mexico city. Pediatrics. 2006;118(2):e323–e330. doi: 10.1542/peds.2005-3123. [DOI] [PubMed] [Google Scholar]
- 51.Téllez-Rojo MM, Hernández-Avila M, Lamadrid-Figueroa H, Smith D, Hernández-Cadena L, Mercado A, Hu H. Impact of bone lead and bone resorption on plasma and whole blood lead levels during pregnancy. American Journal of Epidemiology. 2004;160(7):668–678. doi: 10.1093/aje/kwh271. [DOI] [PubMed] [Google Scholar]
- 52.Timchalk C, Busby A, Campbell J, Needham L, Barr D. Comparative pharmacokinetics of the organophosphorus insecticide chlorpyrifos and its major metabolites diethylphosphate, diethylthiophosphate and 3,5,6-trichloro-2-pyridinol in the rat. Toxicology. 2007;237(1–3):145. doi: 10.1016/j.tox.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 53.U.S. Environmental Protection Agency (U.S. EPA) Interim registration eligibility decision for chlorpyrifos. [Accessed October 2012];U.S. EPA 738-R-01-007. 2002 February 2002. Available at URL: http://www.epa.gov/oppsrrd1/REDs/chlorpyrifos_ired.pdf.
- 54.U. S. Environmental Protection Agency (U.S. EPA) [Accessed October 2012];2011 http://www.epa.gov/oppsrrd1/REDs/factsheets/chlorpyrifos_fs.htm.
- 55.Venerosi A, Ricceri L, Scattoni M, Calamandrei G. Prenatal chlorpyrifos exposure alters motor behavior and ultrasonic vocalization in CD-1 mouse pups. Environmental Health. 2009;8:12. doi: 10.1186/1476-069X-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wechsler H. Wechsler Adult Intelligence Scale (WAIS), Spanish Version. San Antonio, TX: Psychological Corporation; 1968. [Google Scholar]
- 57.Whyatt R, Garfinkel R, Hoepner L, Andrews H, Holmes D, Williams M, Barr D. A biomarker validation study of prenatal chlorpyrifos exposure within an inner-city cohort during pregnancy. Environmental Health Perspectives. 2009;117(4):559–567. doi: 10.1289/ehp.0800041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilmshurst L, Peele M, Wilmshurst L. Resilience and well-being in college students with and without a diagnosis of ADHD. Journal of Attention Disorders. 2009 doi: 10.1177/1087054709347261. [DOI] [PubMed] [Google Scholar]
- 59.Ye X, Pierik F, Hauser R, Duty S, Angerer J, Park M, Longnecker M. Urinary metabolite concentrations of organophosphorous pesticides, bisphenol A, phthalates among pregnant women in rotterdam, the netherlands: The generation R study. Environmental Research. 2008;108(2):260–267. doi: 10.1016/j.envres.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]