Abstract
Background
In the Western world, bladder cancer is the fourth most common cancer in men and the eighth most common in women. Recurrences frequently occur and continued surveillance is necessary to identify and treat recurrent tumors. Efforts to identify risk factors that are potentially modifiable to reduce the rate of recurrence are needed.
Methods
We investigated cigarette smoking behavior and body mass index (BMI) at diagnosis for associations with bladder cancer recurrence in a population-based study of 726 bladder cancer patients in New Hampshire, US. Patients diagnosed with non-muscle invasive urothelial-cell carcinoma were followed to ascertain long-term prognosis. Analysis of time to recurrence was performed using multivariate Cox regression models.
Results
Smokers experienced shorter time to recurrence (continuing smoker HR 1.51 95%CI 1.08-2.13). Although being overweight (BMI>24.9 kg/m2) at diagnosis was not a strong independent factor (HR 1.33 95%CI 0.94-1.89), among continuing smokers, being overweight more than doubled the risk of recurrence compared to smokers of normal weight (HR 2.67 95%CI 1.14-6.28).
Conclusions
These observational results suggest that adiposity is a risk factor for bladder cancer recurrence, particularly among tobacco users. Future intervention studies are warranted to evaluate whether both smoking cessation and weight reduction strategies reduce bladder tumor recurrences.
Keywords: bladder cancer, urothelial-cell carcinoma, obesity, smoking, body mass index, BMI, recurrence
Introduction
Bladder cancer is among the top ten most prevalent cancers worldwide [1]. In the Western world, bladder cancer is the fourth most common cancer in men, and eighth most common in women [2]. An estimated 54,610 new cases of bladder cancer and 10,820 deaths are expected for U.S. males in 2013 [3]. Bladder cancer risk is up to four-fold higher among smokers compared with non-smokers [4]. The median age at diagnosis is 65 [5], and greater than 90% of cases are urothelial cell carcinoma (UCC). Of these, 75% are non-muscle invasive [6]. Current treatments are often effective at eliminating primary disease but rates of recurrent disease are high, exceeding 40% [7]. Bladder cancer is among the most expensive malignancies to treat due to the need for frequent, painful procedures to identify and surgically resect recurrent tumors [8]. Non-muscle invasive tumors are prevalent in the population, with an estimated 500,000 patients with a history of urothelial cell carcinoma currently residing in the U.S. [2]. Predictors of recurrent bladder cancer include a history of recurrences, primary tumor characteristics, including multiplicity, tumor size, T category (depth of invasion), presence of carcinoma in situ, tumor grade, and patient gender [9]. To date, analyses of smoking and recurrence are suggestive, but have yielded somewhat inconsistent results [10-13]. Several studies report an increased risk of incident bladder cancer associated with obesity [14, 15], however, little data are available with regard to body mass index (BMI) and bladder cancer recurrence. Numerous studies focus on rare subtypes of bladder cancer and, to our knowledge, no studies on recurrence of non-muscle invasive tumors and BMI have been published. A recent study found no significant difference in recurrence or survival associated with BMI in a Japanese population diagnosed with upper tract urothelial carcinoma which is a rare, aggressive malignancy representing just 5% of urothelial neoplasms [16, 17]. A study of muscle invasive urothelial carcinoma patients treated by radical cystectomy found a decreased risk of osseous recurrence, however this study examined only tumor recurrences at secondary sites following radical cystectomy [18] and is not generalizable to the majority of bladder cancer patients that present with non-invasive UCC.
Our study provides the unique opportunity to examine recurrence of non-muscle invasive tumors in the context of a large U.S. population-based patient cohort identified from the New Hampshire State Cancer Registry. We have collected detailed data on patient characteristics, behaviors, exposures and clinical information and performed long-term patient follow-up (up to 15 years) to test the hypothesis that smoking and BMI (as a measure of adiposity) are associated with tumor recurrence.
Materials and Methods
Study population
We identified all incident bladder cancers among New Hampshire residents, ages 25 to 74 years, diagnosed from July 1, 1994 to December 31, 2001 from the New Hampshire State Cancer Registry. Detailed methods have been described previously [19, 20]. Briefly, we interviewed a total of 857 individuals with bladder cancer, which was 85% of the cases confirmed to be eligible for the study. After re-review, 21 were non-cancer and 7 undetermined, leaving n=829 tumors which were either deemed cancerous by histopathology re-review (~90%), or the original diagnosis if the pathology materials were unavailable. The histologic composition of these tumors was 817 urothelial-cell (transitional-cell) carcinoma (660 papillary urothelial cell carcinoma, 133 urothelial cell carcinoma, 4 papillary carcinoma, 20 carcinoma NOS), as well as 2 spindle cell carcinoma, 3 small cell carcinoma, 5 squamous cell carcinoma, 2 adenocarcinoma. Omitting n=9 participants with primary diagnosis dates >2 years prior to the diagnosis date assumed in the interview, a total of n=808 participants were diagnosed with urothelial-cell (transitional-cell) carcinoma of the bladder. We restricted our analyses to the participants with non-muscle invasive disease (n=726) as muscle invasive cases likely have a different clinical experience and prognosis [1].
Personal interviews
Informed consent was obtained from each participant. All procedures and study materials were approved by the Committee for the Protection of Human Subjects at Dartmouth College. Consenting participants underwent a detailed in-person interview, usually at their home. The main objectives of the study were not disclosed to the interviewers. To ensure consistent quality of the study interviewer, interviews were tape recorded with the consent of the participants and routinely monitored by the interviewer supervisor. Questionnaires covered detailed socio-demographic as well as medical, personal, and family history information.
Follow-up
Information on bladder cancer recurrences was obtained from medical records provided by the treating hospital(s) (both in and outpatient records, including any pathology reports) covering the follow-up period. Records were reviewed by an experienced, certified tumor registrar to abstract the data on bladder tumors occurring subsequent to the incident tumor. Hospital registry data were used if the medical record could not be obtained. The first recurrent tumor was defined as any tumor identified after a disease-free remission period, more than 90 days after the date of initial primary bladder tumor diagnosis. Persistent primary tumors that did not have a remission period were excluded from the analysis of recurrence (n=1). Time to first event was calculated as the time between the date of initial diagnosis and the first recurrence. The diagnosis of a tumor with a greater stage or grade than the initial primary bladder tumor was defined as progression. If no events were reported, the date the patient was last seen documented in the medical record was used for censoring. Life status (alive or deceased) was determined as of January 2011 using the Social Security and the National Death Indices (NDI). Survival time was calculated from the date of initial diagnosis to date of death. Patients were re-contacted in 2007 and we obtained data from a brief questionnaire to collect information on continuing smoking after diagnosis.
Statistical analysis
For the analysis of prognosis, smoking status was available on n=716 non-muscle invasive urothelial cell carcinoma patients coded as: never smokers, former smokers (quit at or before diagnosis date), and continuing smokers (smokers who continued to smoke after diagnosis). Individuals without smoking data available after diagnosis were assumed to have maintained the smoking status collected at the diagnostic interview, as our data demonstrated that nearly all cases (93%) who reported smoking at diagnosis continued to smoke. Body mass index (BMI) was calculated from weight and height at diagnosis, as reported in this questionnaire on n=350 non-muscle invasive urothelial cell carcinoma patients according to common standards (kg/m2) and was used as a measure of adiposity. Using NIH guidelines, normal BMI was defined as ≤24.9 kg/m2; overweight (high BMI) was defined as BMI 25 kg/m2 - 29.9 kg/m2; obesity was defined as ≥30 kg/m2.
The main goals of the statistical analysis were to assess the relationship between cigarette smoking status, being overweight, and bladder cancer recurrence among non-muscle invasive urothelial-cell carcinoma cases (n=726). Time to first recurrence analysis was performed using Kaplan-Meier plots and differences were assessed using the log-rank test. To adjust for additional factors related to patient survival, Cox-proportional hazards regression analysis was performed in non-muscle invasive tumors (stages 0 and I) with adjustment for age at diagnosis, gender, smoking status (never, former, continuing), as well as grade (low, high), presence of carcinoma in situ (TIS), first course treatment (transurethral resection (TURB), immunotherapy, chemotherapy, radiotherapy, cystectomy), tumor size (<3cm, ≥3cm), and multiplicity (single, >1) in the model. P values represent two-sided statistical tests with statistical significance at P<0.05. Statistical significances of the interactions were assessed using likelihood ratio tests comparing the models with and without interaction terms.
Results
Medical data were reviewed on 94% of the cases (679 out of 726) and 75% of the cases (545 out of 726) were followed >2 years. Follow-up times ranged from 3 months to 15 years, with a median of 6 years. Of the non-muscle invasive urothelial-cell carcinoma patients with follow-up data available, 55% experienced recurrences during a median of 5.6 years of post-diagnosis follow-up time (Table 1). The median recurrence-free survival time was 4.2 years. Only 5% of the patients had tumors that progressed during the follow-up period and the median time to progression for these patients was 1 year. Recurrences were more common among older patients with primary tumor characteristics including high-grade or Tis, large size, or multiple primaries. Most (>95%) of the participants in this study are of Caucasian origin, reflecting the racial mix of the New Hampshire population; restricting to Caucasians does not appreciably alter our analyses (data not shown). At diagnosis, the majority of the bladder cancer cases had a BMI >=24.9 (74%), and 83% were current or former smokers. In addition to the medical record review, we also re-contacted the patients who were surviving in 2007 (n=448). The response rate for the patient follow-up questionnaire to collect updated smoking status information was 85% (n=382). Of the smokers, 64% quit smoking at or before diagnosis, while 36% continued smoking after diagnosis.
Table 1.
Characteristics of non-muscle invasive urothelial cell carcinoma cases.
| Overall N (%) | Non-recurrent N (%) | Recurrent N (%) | Univariate P-value | ||
|---|---|---|---|---|---|
| Gender | Female | 171 (24) | 77 (25) | 80 (21) | |
| Male | 555 (76) | 229 (75) | 293 (79) | 0.18 | |
| total | 726 | 306 | 373 | ||
| Reference agea | <40 | 21 (3) | 12 (4) | 7(2) | |
| 40-49 | 58 (8) | 19 (6) | 30 (8) | ||
| 50-59 | 172 (24) | 81 (26) | 82 (22) | ||
| 60-69 | 295 (41) | 119 (39) | 160 (43) | ||
| >=70 | 180 (25) | 75 (10) | 94 (13) | 0.038 | |
| Race | Caucasian | 693 (97) | 292 (97) | 355 (97) | |
| Other | 22 (3) | 10 (3) | 12 (3) | 0.91 | |
| Tumor Type | TaT1 Low-grade | 538 (74) | 254 (83) | 246 (66) | |
| TaT1 High-grade | 142 (20) | 37 (12) | 98 (26) | 0.0025 | |
| Tis | 46 (6) | 15 (5) | 29 (8) | <0.0001 | |
| Treatment | TURB | 539 (75) | 229 (75) | 273 (73) | |
| TURB + other | 112 (16) | 41 (13) | 69 (18) | 0.0154 | |
| other | 68 (9) | 34 (11) | 31 (8) | 0.038 | |
| Tumor Size | <3cm | 435 (64) | 201 (70) | 136 (83) | |
| >3cm | 241 (33) | 86 (30) | 27 (17) | 0.0028 | |
| Multiplicity | Single | 749 (72) | 226 (80) | 223 (66) | |
| >1 | 185 (28) | 58 (20) | 116 (34) | 0.0001 | |
| Follow-up time | 25th | 1.7 years | 1.56 years | 2.1 years | |
| 50th | 5.6 years | 4.54 years | 6.6 years | ||
| 75th | 9.2 years | 8.91 years | 9.6 years | ||
| 95th | 12.4 years | 12.6 years | 12.2 years |
Data were missing on race n=11, smoking n=10, treatment n=7, size n=50, multiplicity n=62, follow-up n=47.
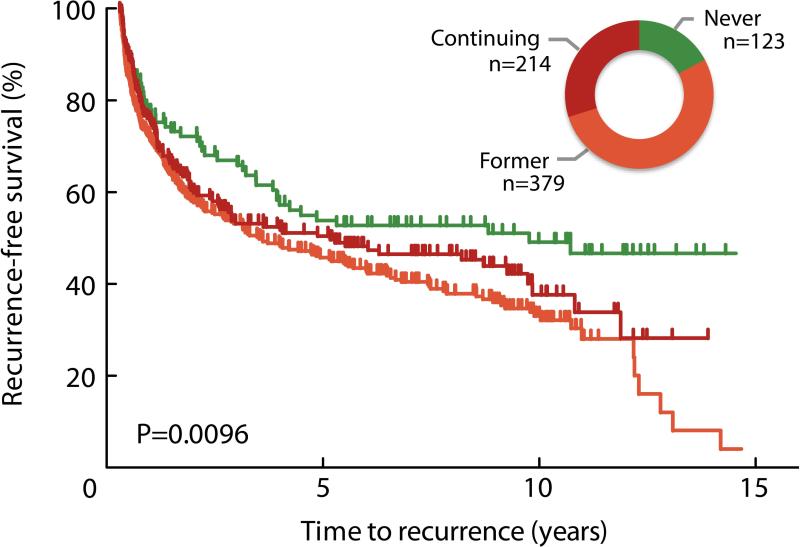
Cigarette smoking was associated with shorter time to first recurrence (log-rank P=0.0096, Figure 1). After adjustment for age, gender, stage / grade, tumor size, multiplicity and treatment, patients were at a higher risk of recurrence if they had a history of smoking when compared to non-smokers, whether they continued smoking after diagnosis (HR 1.51 95%CI 1.08-2.13) or quit at or before diagnosis (HR 1.61 95%CI 1.17-2.20; Table 2). We did not observe a difference in the rate of tumor progression associated with smoking, however the statistical power for this analysis was limited. Continued smoking after diagnosis, but not former smoking, was associated with shorter overall survival (continued smoking survival HR 3.42 95%CI 1.29-9.07; former smoking survival HR 1.69 95%CI 0.70-4.10).
Figure 1.
Bladder cancer recurrence by cigarette smoking status after diagnosis. The Kaplan-Meier plot indicates shorter time to recurrence among patients who reported being either former or continued smokers when compared to never smokers (Log-rank p=0.0096). Inset graphic shows relative group size of never (green; 17.2%), former (orange; 52.9%), and current (red; 29.9%) smokers.
Table 2.
Bladder cancer recurrence and smoking after diagnosis.
| Time to recurrence | ||||
|---|---|---|---|---|
| Smoking status at follow-up | N (%) | Median years | HR* (95%CI) | P-value |
| Never | 123 (17) | 9.76 | 1.00 (ref) | |
| Former | 379 (53) | 3.48 | 1.61 (1.17 - 2.20) | 0.0031 |
| Continuing | 214 (30) | 5.14 | 1.51 (1.08 - 2.13) | 0.018 |
adjusted for age, gender, stage/grade, treatment, smoking status, tumor size, and multiplicity.
We also evaluated the relationship between tumor recurrence and the time since smoking cessation among patients who reported that they had quit smoking. We observed a modest decrease in median years to recurrence associated with smoking more recently (Table 3). Patients who quit smoking within the past 18 years from diagnosis experienced a shorter time to recurrence (median 2.4 years), compared with a median of 4.7 years for those who quit greater than 29 years ago, and 9.8 years for never smokers (quitting within 1-18 years vs. never smokers HR 1.83 95%CI 1.30-2.59; Table 3).
Table 3.
Bladder cancer recurrence and time since smoking cessation.
| Time to recurrence | |||
|---|---|---|---|
| Smoking status | N (%) | Median years | HR* (95%CI) |
| Never smoker | 123 (27) | 9.76 | 1.00 (ref) |
| Quit ≥29 yrs prior | 83 (18) | 4.68 | 1.37 (0.89 - 2.10) |
| Quit 19-28 yrs prior | 73 (16) | 3.57 | 1.44 (0.94 - 2.21) |
| Quit 1-18 yrs prior | 184 (40) | 2.39 | 1.83 (1.30 - 2.59) |
adjusted for age, gender, stage/grade, treatment, smoking status, tumor size, and multiplicity.
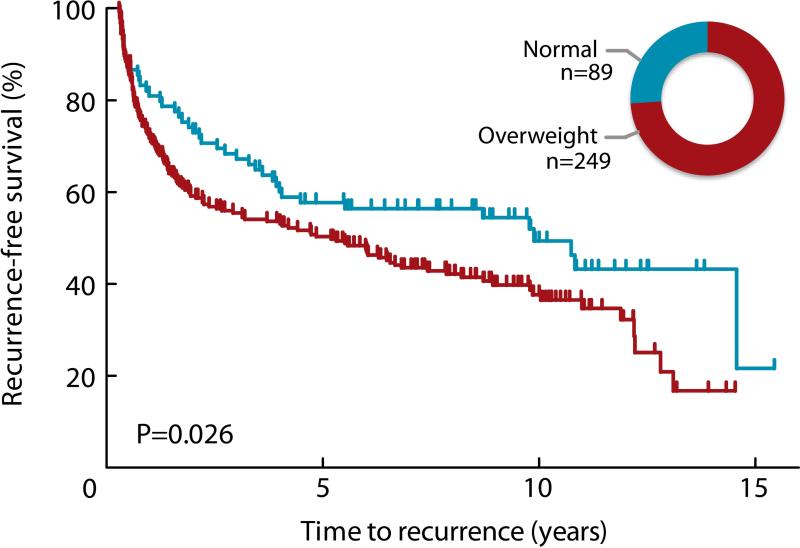
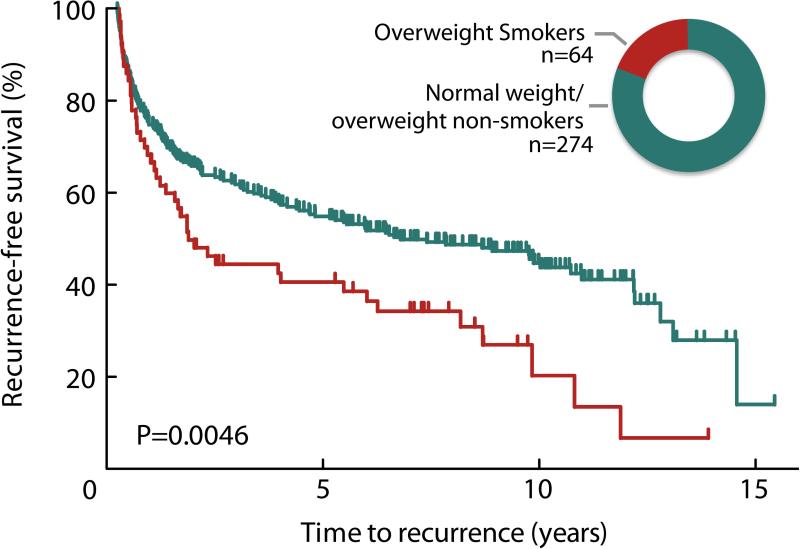
Being overweight at diagnosis (BMI ≥ 24.9) was associated with a shorter time to bladder tumor recurrence (log-rank P=0.026, Figure 2). In a multivariate Cox regression analysis, high BMI at diagnosis remained modestly associated with an increased risk of recurrence (HR 1.33 95%CI 0.94-1.89) after adjustment for age, gender, smoking status, stage, grade, tumor size, multiplicity, and treatment (Table 4); however, among individuals who continued smoking in the follow-up period, those who were overweight were at greater risk for recurrence (HR 2.67 95%CI 1.14-6.28) (P for multiplicative interaction=0.239) (Table 4). These overweight smokers experienced a median recurrence-free survival time of 1.9 years, compared to 6.8 years for all other patients combined (log-rank P=0.0046, Figure 3). We did not observe a clear difference in time to progression (HR 1.56 95%CI 0.41-5.89), although the statistical power for this analysis was limited by few events. The effects of smoking and BMI did not differ by gender, or by immunotherapy treatment status (data not shown). Pack-years, as a dose measure of exposure to cigarette smoke, was not associated with increased risk of recurrence (P=0.98) and inclusion of pack-years in our model did not alter results appreciably (overweight current smoker HR 2.57 95%CI 1.08-6.12). We further considered whether the effects we observed for BMI might be due to diabetes, which is in itself associated with being overweight. We performed the analysis excluding the 20% of the patients who reported being diagnosed as diabetic; high BMI remained associated with a greater than two-fold hazard ratio in the continuing smokers (HR 2.43 95%CI 1.01-5.89).
Figure 2.
Bladder cancer recurrence by BMI at diagnosis. The Kaplan-Meier plot indicates shorter time to recurrence for individuals who were overweight (BMI >24.9) at diagnosis compared with normal weight individuals (Log-rank p=0.026). Inset graphic shows relative group size of normal (blue; 26.6%) and overweight (red; 73.4%) individuals.
Table 4.
Bladder cancer recurrence and adiposity by smoking status.
| Overall time to recurrence | Time to recurrence by smoking status | ||||||
|---|---|---|---|---|---|---|---|
| BMI at diagnosis | N % | Median years | HR ( 95%CI ) | Never HR ( 95%CI ) | Former HR ( 95%CI ) ) | Current HR ( 95%CI ) | |
| Normal | ≤24.9 | 89 ( 26 ) | 9.87 | 1.00 ( ref ) | 1.00 ( ref ) | 1.00 ( ref ) | 1.00 ( ref ) |
| Overweight | 24.9-29.9 | 158 ( 47 ) | 4.39 | 1.39 ( 0.96 - 2.01 ) | 1.76 ( 0.69 - 4.49 ) | 1.19 ( 0.71 - 1.98 ) | 2.67 ( 1.13 - 6.32 ) |
| Obese | 30+ | 91 ( 27 ) | 5.97 | 1.22 ( 0.80 - 1.87 ) | 0.76 ( 0.20 - 2.98 ) | 1.22 ( 0.69 - 2.16 ) | 2.68 ( 0.88 - 8.23 ) |
| Normal | ≤24.9 | 89 ( 26 ) | 9.87 | 1.00 ( ref ) | 1.00 ( ref ) | 1.40 ( 0.69 - 2.84 ) | 1.13 ( 0.47 - 2.70 ) |
| Over./obese | 24.9+ | 249 ( 74 ) | 5.16 | 1.33 ( 0.94 - 1.89 ) | 1.05 ( 0.49 - 2.23 ) | 1.68 ( 0.92 - 3.08 ) | 2.24 ( 1.15 - 4.34 ) |
*adjusted for age, gender, stage/grade, treatment, smoking status, tumor size, and multiplicity.
Figure 3.
Bladder cancer recurrence by BMI at diagnosis among continued cigarette smokers. The Kaplan-Meier plot indicates shorter time to recurrence for overweight (BMI >24.9) continuing smokers compared with all normal weight and non-smoking overweight patients (log-rank p=0.0046). Inset graphic shows relative group size of overweight smokers (red; 18.9%) and all normal weight and non-smoking overweight patients combined (green; 81.1%).
Discussion
Bladder cancer is among the top ten most prevalent cancers worldwide [1]. Although the mean 10-year survival rate is nearly 70%, more than half of the patients experience recurrent tumors which require extensive screening and treatment [8]. The association between adiposity and bladder cancer incidence has been inconsistent [15, 21]. The relationship between bladder cancer recurrence and BMI is also poorly understood and no studies of BMI on recurrence of non-muscle invasive urothelial tumors have been published to date to our knowledge. Our unique study with detailed long-term prognostic data on patients from the general population of New Hampshire, permitted us to evaluate BMI and smoking in relation to recurrent disease following the primary diagnosis of early stage bladder cancer. We identified BMI as a modifier of time to recurrence in patients who continued to smoke after diagnosis. Among continuing smokers, those who were overweight had a greater than two-fold increased risk of recurrence, compared to those of normal weight. Obesity and body mass are associated with recurrence of several cancers [22], including the recurrence of prostate cancer after radical prostatectomy [23].
The biological mechanism for obesity-related tumorigenesis is not yet well characterized, but many possibilities have been suggested. High levels of adipose tissue correlate with high levels of cholesterol, a precursor for the androgen testosterone, which stimulates epithelial cell proliferation [24, 25]. High adipose levels have also been correlated with high plasma levels of VEGF and FGF2 [26], which both stimulate proliferation of epithelial cells [24, 27, 28]. Adipose tissue also secretes leptin, which has been implicated in enhancing angiogenesis [29] and, consequently, may also enhance tumor development. Adiposity has also been correlated with reduced mitochondrial function and in turn, increased circulating reactive oxygen species, which can cause DNA damage [22]. Obesity related psychological depression [30], increased circulating lipids [22], and cigarette smoke [31, 32] depress immune surveillance for tumor cells.
It is unclear whether the biologic mechanisms relating obesity to recurrences differ from those driving incident tumors. There are several mechanisms that seem to play a unique role in tumor recurrence. BMI has been studied extensively in the context of breast cancer prognosis, with high BMI significantly increasing risk of distant recurrences [33]. In breast, as well as in colon cancer patients, an insulin resistant phenotype characterized by over-expression of insulin receptors in the tumor cells of obese patients is associated with poor prognosis [34]. Adipocytes have also been shown to create localized microenvironmental conditions that promote growth of circulating tumor cells deposited nearby [35]. Mechanistic studies to understand whether these obesity-related biologic factors are similar in the context of bladder cancer are necessary.
Our study is limited by its observational nature. While our study is representative of the New Hampshire population, the racial homogeneity of the state limits the generalizability of our findings. Nevertheless, the strengths of this population-based study lie in the recruitment of patients across diverse types of healthcare facilities throughout the state, the collection of detailed prognostic data, and the long duration of follow-up. Self-reported weight and height were used to compute body mass index. Previous studies show that inaccuracies in self-reported BMI are skewed towards overweight individuals reporting a lower BMI than actual [36]. This underreporting would be expected to dampen the observed association with prognosis, rather than strengthen it. Additionally, we used a threshold to dichotomize BMI, thus misclassification only occurs if a reporting error spans the normal – overweight threshold. Thus, the association between BMI and recurrence is unlikely to be due to BMI misclassification, and may be stronger than observed.
BMI and smoking are factors that are modifiable post-diagnosis. A large majority of patients in our study population were overweight or obese smokers. During our review, we did not observe widespread behavioral intervention efforts documented in the medical record, and few patients achieved weight loss or smoking cessation following the primary diagnosis. The effectiveness and clinical utility of various approaches to modifying these factors must be empirically determined in additional studies. Despite the high prevalence of recurrences, the reasonably high survival rates for non-muscle invasive bladder cancer patients may allow for a ‘teachable moment’ at the time of the primary diagnosis that should be utilized to improve the overall health and prognosis for patients [34]. Smoking cessation is an underutilized cost-effective intervention [37] that reduces risk of numerous diseases, including recurrent bladder cancer [10, 11]. Furthermore, with the advent of advanced bariatric weight loss interventions, as well as advances in health awareness programs, both active and passive weight loss interventions are increasingly obtainable. Physical activity after cancer diagnosis has been associated with longer cancer-specific and overall survival among early-stage breast, prostate and colorectal cancer patients, with current trials underway, including a 3-year structured physical activity intervention in colon cancer survivors. A low-fat diet intervention for early-stage breast cancer patients also resulted in weight loss and a lower rate of recurrence [34]. The results of this study contribute to the growing body of evidence implicating adiposity in addition to smoking as potentially modifiable predictors of bladder cancer recurrence [14]. Obesity and smoking are risk factors for multiple other types of cancer, as well as cardiovascular disease. Thus, interventions that promote weight reduction and smoking cessation in bladder cancer survivors have a significant sociological impact and improve overall health and longevity. Our findings highlight the importance of evaluating these individual and lifestyle factors as predictors of clinical outcomes in bladder cancer and present an opportunity for testing interventions in survivors to reduce risk of subsequent malignancies.
Precis.
BMI and smoking are associated with bladder cancer recurrence. Consideration of these lifestyle factors in diagnosis and intervention therapies should be examined in future studies.
Acknowledgments
The authors wish to thank the staff and participants of the New Hampshire Health Study and the New Hampshire State Cancer Registry for making this project possible.
Funding:
This publication was funded in part by the National Cancer Institute, NIH (K07CA102327, CA121382, CA82354, RR018787), and by the National Institute of Environmental Health Sciences, NIH (ES07373, ES00002, ES05947). The New Hampshire State Cancer Registry is supported by the Centers for Disease Control and Prevention's National Program of Cancer Registries (NPCR) through cooperative agreement (U58/DP000798) awarded to the New Hampshire Department of Health and Human Services, Division of Public Health Services, Bureau of Public Health Statistics & Informatics, Health Statistics and Data Management Section.
Footnotes
Financial Disclosures: None.
Conflicts of Interest Disclosures: There are no conflicts of interest between any author and the work presented. Dr. Wyszynski is an advisor for Genextropy Inc., Bar Harbor Biotechnology Inc., and Borealis Ventures on unrelated topics.
References
- 1.Murta-Nascimento C, Schmitz-Drager BJ, Zeegers MP, et al. Epidemiology of urinary bladder cancer: from tumor development to patient's death. World J Urol. 2007;25(3):285–95. doi: 10.1007/s00345-007-0168-5. [DOI] [PubMed] [Google Scholar]
- 2.Burger M, Catto JW, Dalbagni G, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63(2):234–41. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 4.Kirkali Z, Chan T, Manoharan M, et al. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology. 2005;66(6 Suppl 1):4–34. doi: 10.1016/j.urology.2005.07.062. [DOI] [PubMed] [Google Scholar]
- 5.Montie J, Shipley W. Bladder Cancer Including Upper Tract Tumors and Transitional Cell Carcinoma of the Prostate. National Comprehensive Cancer Network Practice Guidelines in Oncology: National Comprehensive Cancer Network. 2004 [Google Scholar]
- 6.Silverman DT, Morrison AS, Devesa SS. Bladder Cancer. In: Schottenfeld DFJ, editor. Cancer Epidemiology and Prevention. Oxford University Press; New York: 1996. pp. 1156–79. [Google Scholar]
- 7.Honma I, Masumori N, Sato E, et al. Local recurrence after radical cystectomy for invasive bladder cancer: an analysis of predictive factors. Urology. 2004;64(4):744–48. doi: 10.1016/j.urology.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Botteman MF, Pashos CL, Redaelli A, Laskin B, Hauser R. The health economics of bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics. 2003;21(18):1315–30. doi: 10.1007/BF03262330. [DOI] [PubMed] [Google Scholar]
- 9.Schmitz-Drager BJ. Identifying risk factors in patients with non-muscle-invasive bladder cancer: clinical implications. Eur Urol. 2011;60(4):721–3. doi: 10.1016/j.eururo.2011.07.065. [DOI] [PubMed] [Google Scholar]
- 10.Strope SA, Montie JE. The causal role of cigarette smoking in bladder cancer initiation and progression, and the role of urologists in smoking cessation. J Urol. 2008;180(1):31–7. doi: 10.1016/j.juro.2008.03.045. discussion 37. [DOI] [PubMed] [Google Scholar]
- 11.Fleshner N, Garland J, Moadel A, et al. Influence of smoking status on the disease-related outcomes of patients with tobacco-associated superficial transitional cell carcinoma of the bladder. Cancer. 1999;86(11):2192–45. [PubMed] [Google Scholar]
- 12.Lammers RJ, Witjes WP, Hendricksen K, Caris CT, Janzing-Pastors MH, Witjes JA. Smoking status is a risk factor for recurrence after transurethral resection of non-muscleinvasive bladder cancer. Eur Urol. 2011;60(4):713–20. doi: 10.1016/j.eururo.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Aveyard P, Adab P, Cheng KK, Wallace DM, Hey K, Murphy MF. Does smoking status influence the prognosis of bladder cancer? A systematic review. BJU Int. 2002;90(3):228–39. doi: 10.1046/j.1464-410x.2002.02880.x. [DOI] [PubMed] [Google Scholar]
- 14.Koebnick C, Michaud D, Moore SC, et al. Body mass index, physical activity, and bladder cancer in a large prospective study. Cancer Epidemiol Biomarkers Prev. 2008;17(5):1214–21. doi: 10.1158/1055-9965.EPI-08-0026. [DOI] [PubMed] [Google Scholar]
- 15.Holick CN, Giovannucci EL, Stampfer MJ, Michaud DS. Prospective study of body mass index, height, physical activity and incidence of bladder cancer in US men and women. Int J Cancer. 2007;120(1):140–6. doi: 10.1002/ijc.22142. [DOI] [PubMed] [Google Scholar]
- 16.Inamoto T, Komura K, Watsuji T, Azuma H. Specific body mass index cut-off value in relation to survival of patients with upper urinary tract urothelial carcinomas. Int J Clin Oncol. 2011 doi: 10.1007/s10147-011-0284-5. [DOI] [PubMed] [Google Scholar]
- 17.Latchamsetty KC, Porter CR. Treatment of upper tract urothelial carcinoma: a review of surgical and adjuvant therapy. Rev Urol. 2006;8(2):61–70. [PMC free article] [PubMed] [Google Scholar]
- 18.Umbreit EC, Crispen PL, Shimko MS, Farmer SA, Blute ML, Frank I. Multifactorial, sitespecific recurrence model after radical cystectomy for urothelial carcinoma. Cancer. 2010;116(14):3399–407. doi: 10.1002/cncr.25202. [DOI] [PubMed] [Google Scholar]
- 19.Karagas MR, Tosteson TD, Blum J, Morris JS, Baron JA, Klaue B. Design of an epidemiologic study of drinking water arsenic exposure and skin and bladder cancer risk in a U.S. population. Environ Health Perspect. 1998;106(Suppl 4):1047–50. doi: 10.1289/ehp.98106s41047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrew AS, Gui J, Sanderson AC, et al. Bladder cancer SNP panel predicts susceptibility and survival. Hum Genet. 2009;125(5-6):527–39. doi: 10.1007/s00439-009-0645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larsson SC, Andersson SO, Johansson JE, Wolk A. Diabetes mellitus, body size and bladder cancer risk in a prospective study of Swedish men. Eur J Cancer. 2008;44(17):2655–60. doi: 10.1016/j.ejca.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Mydlo JH. The impact of obesity in urology. Urol Clin North Am. 2004;31(2):275–87. doi: 10.1016/j.ucl.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Jayachandran J, Banez LL, Aronson WJ, et al. Obesity as a predictor of adverse outcome across black and white race: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) Database. Cancer. 2009;115(22):5263–71. doi: 10.1002/cncr.24571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mydlo JH, Tieng NL, Volpe MA, Chaiken R, Kral JG. A pilot study analyzing PSA, serum testosterone, lipid profile, body mass index and race in a small sample of patients with and without carcinoma of the prostate. Prostate Cancer Prostatic Dis. 2001;4(2):101–05. doi: 10.1038/sj.pcan.4500514. [DOI] [PubMed] [Google Scholar]
- 25.Berry PA, Maitland NJ, Collins AT. Androgen receptor signalling in prostate: effects of stromal factors on normal and cancer stem cells. Mol Cell Endocrinol. 2008;288(1-2):30–7. doi: 10.1016/j.mce.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 26.Mydlo JH, Gerstein MI, Harris CF, Braverman AS. Immune function, mitogenicity, and angiogenic growth factor concentrations in lean and obese rodent sera: implications in obesity-related prostate tumor biology. Prostate Cancer Prostatic Dis. 2003;6(4):286–9. doi: 10.1038/sj.pcan.4500693. [DOI] [PubMed] [Google Scholar]
- 27.Kassouf W, Brown GA, Black PC, et al. Is vascular endothelial growth factor modulation a predictor of the therapeutic efficacy of gefitinib for bladder cancer? J Urol. 2008;180(3):1146–53. doi: 10.1016/j.juro.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szarvas T, Jager T, Droste F, et al. Serum levels of angiogenic factors and their prognostic relevance in bladder cancer. Pathol Oncol Res. 2009;15(2):193–201. doi: 10.1007/s12253-008-9107-z. [DOI] [PubMed] [Google Scholar]
- 29.Cirillo D, Rachiglio AM, la Montagna R, Giordano A, Normanno N. Leptin signaling in breast cancer: an overview. J Cell Biochem. 2008;105(4):956–64. doi: 10.1002/jcb.21911. [DOI] [PubMed] [Google Scholar]
- 30.Godbout JP, Glaser R. Stress-induced immune dysregulation: implications for wound healing, infectious disease and cancer. J Neuroimmune Pharmacol. 2006;1(4):421–7. doi: 10.1007/s11481-006-9036-0. [DOI] [PubMed] [Google Scholar]
- 31.Mian MF, Lauzon NM, Stampfli MR, Mossman KL, Ashkar AA. Impairment of human NK cell cytotoxic activity and cytokine release by cigarette smoke. J Leukoc Biol. 2008;3(3):774–84. doi: 10.1189/jlb.0707481. [DOI] [PubMed] [Google Scholar]
- 32.Choi JM, Cho YC, Cho WJ, Kim TS, Kang BY. Hydroquinone, a major component in cigarette smoke, reduces IFN-gamma production in antigen-primed lymphocytes. Arch Pharm Res. 2008;31(3):337–41. doi: 10.1007/s12272-001-1161-1. [DOI] [PubMed] [Google Scholar]
- 33.Goodwin PJ, Ennis M, Pritchard KI, et al. Insulin- and obesity-related variables in early-stage breast cancer: correlations and time course of prognostic associations. J Clin Oncol. 2012;30(2):164–71. doi: 10.1200/JCO.2011.36.2723. [DOI] [PubMed] [Google Scholar]
- 34.Demark-Wahnefried W, Platz EA, Ligibel JA, et al. The role of obesity in cancer survival and recurrence. Cancer Epidemiol Biomarkers Prev. 2012;21(8):1244–59. doi: 10.1158/1055-9965.EPI-12-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dirat B, Bochet L, Escourrou G, Valet P, Muller C. Unraveling the obesity and breast cancer links: a role for cancer-associated adipocytes? Endocr Dev. 2010;19:45–52. doi: 10.1159/000316896. [DOI] [PubMed] [Google Scholar]
- 36.Gillum RF, Sempos CT. Ethnic variation in validity of classification of overweight and obesity using self-reported weight and height in American women and men: the Third National Health and Nutrition Examination Survey. Nutr J. 2005;4:27. doi: 10.1186/1475-2891-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noyes K, Singer EA, Messing EM. Healthcare economics of bladder cancer: costenhancing and cost-reducing factors. Curr Opin Urol. 2008;18(5):533–9. doi: 10.1097/MOU.0b013e32830b8910. [DOI] [PMC free article] [PubMed] [Google Scholar]