Abstract
Inbred mouse strains such as C57BL/6J (B6) and DBA/2J (D2) and related strains have been used extensively to help identify genetic controls for a number of ethanol-related behaviors, including acute intoxication and sensitivity to repeated exposures. The disparate ethanol drinking behaviors of B6 mice expressing high-drinking/preference and D2 mice expressing low-drinking/preference have yielded considerable insight into the heritable control of alcohol drinking. However, the B6-high and D2-low drinking phenotypes are contrasted with ethanol-conditioned reward-like behaviors, which are robustly expressed by D2 mice and considerably less expressed by B6 mice. This suggests that peripheral factors, chiefly ethanol taste, may help drive ethanol drinking by these and related strains, which complicates mouse genetic studies designed to understand the relationships between reward-related behaviors and ethanol drinking. Traditional approaches such as the sucrose/saccharin-substitution procedure that normally accentuate ethanol drinking in rodents have had limited success in low drinking/preferring mice such as the D2 line. This may be due to allelic variations of the sweet taste receptor subunit, expressed by many ethanol low-drinking/preferring strains, which would limit the utility of these types of substitution approaches. We have recently shown (McCool & Chappell, 2012) that monosodium glutamate (MSG), the primary component of umami taste, can be used in a substitution procedure to initiate ethanol drinking in both B6 and D2 mice that greatly surpasses that initiated by a more traditional sucrose-substitution procedure. In this study, we show that ethanol drinking initiated by MSG substitution in D2 mice, but not sucrose substitution, can persist for several weeks following removal of the flavor. These findings further illustrate the utility of MSG substitution to initiate ethanol drinking in distinct mouse strains.
Keywords: monosodium glutamate, sucrose, substitution procedure, inbred mice, ethanol drinking
Introduction
Voluntary ethanol consumption in rodents is a valuable model for understanding the neurobiological and genetic mechanisms that drive drinking behaviors such as ethanol preference and reward. Because many rodent species do not show an innate drive to consume ethanol, investigators sometimes employ a substitution procedure in which animals are allowed to initially consume a solution containing ethanol sweetened with sucrose or saccharin (Samson, 1986). The sweetener is then gradually removed until the animal is drinking only ethanol in water. This procedure has proved invaluable for delineation of the neurobiological control of ethanol drinking/self-administration (reviewed in (McBride and Li, 1998; Samson and Czachowski, 2003)). It has been routinely employed to initiate ethanol drinking in many rodent species.
Inbred mouse strains provide a valuable resource for defining genetic contributions to a number of pathologies, including drug and alcohol abuse. These diverse genotypes exhibit unique ethanol drinking characteristics, and extensive interbreeding programs using strains such as the C57BL/6J (B6) and DBA/2J (D2) strains have been used to define potential genetic contributions to numerous alcohol-related behaviors including ethanol preference (Fehr et al., 2005; Weng et al., 2009), acute intoxication/sedation (Browman and Crabbe, 2000; DuBose et al., 2013; Hood and Buck, 2000), tolerance (Kirstein et al., 2002), and withdrawal severity (Shirley et al., 2004). For drinking phenotypes, many strains have a strong aversion to ethanol taste, which may dramatically influence the interpretation of behaviors such as preference, home-cage intake, and reward learning. For example, it is well established that D2 mice exhibit very limited voluntary ethanol drinking in the home cage, but this strain exhibits robust intragastric self-administration (Fidler et al., 2011) as well as strong ethanol-conditioned place preference (Font and Cunningham, 2012). Taste aversion-conditioning studies show that D2 mice generalize the taste of bitter compounds with ethanol taste while B6 mice taste both a sweet and a bitter component of ethanol (Blizard, 2007). Importantly, D2 mice carry an allele of the sac locus which encodes a sweet ‘taste’ receptor that has reduced response to sweet compounds like saccharin and sucrose (Max et al., 2001; Montmayeur et al., 2001; Reed et al., 2004). These biological characteristics strongly suggest that the taste of ethanol is a major factor limiting ethanol drinking in D2 mice.
Both sweet and umami flavors elicit natural ‘seeking’ behaviors (Uematsu et al., 2011). More importantly, these tastants bind to unique receptors on the tongue (Zhao et al., 2003), suggesting that genetic determinants decreasing the efficacy of sweet tastes are unlikely to be the same as those influencing umami tastes. This suggests that umami (monosodium glutamate [MSG]) might be used as an alternative to sucrose in substitution procedures to initiate ethanol drinking in rodents. Indeed we have recently shown that MSG substitution can engender substantial ethanol drinking in the home cage in both B6 and D2 mice (McCool and Chappell, 2012). However, there are many variations of the tastant-substitution approach in the literature, and it was unclear if the details within the substitution process could ultimately influence ethanol drinking. It was also unclear if ethanol drinking induced by MSG substitution produced persistent ethanol drinking above that achieved with sucrose. Both questions were explored in the current study.
Materials and methods
Animals
Adult male C57BL6/J (B6) and DBA/2J (D2) mice (5 weeks old; n = 8 in each treatment group) were purchased from a commercial supplier (Jackson Laboratories, Bar Harbor, ME) and were individually housed on a reverse light–dark cycle (lights off at 7:00 AM) under standard conditions with food and water available ad libitum. Animals were handled every day throughout the study. All procedures were approved by the Wake Forest Animal Care and Use Committee and were consistent with the NIH Guide for the Care and Use of Animals.
Tastant-substitution procedure and limited-access drinking
Individuals were initially exposed to the different tastants (sucrose or MSG) using continuous access in the home cage for 2–3 days. During this period, mice had free access to water and 10% sucrose (in the sucrose-substitution group) or water and 100 mM MSG (in the MSG-substitution group). Ten percent sucrose engenders near maximal consumption in both B6 and D2 mice (Lewis et al., 2005; Pothion et al., 2004). Additionally, we have shown that 100 mM MSG is maximally ‘preferred’ in both B6 and D2 mice (McCool and Chappell, 2012).
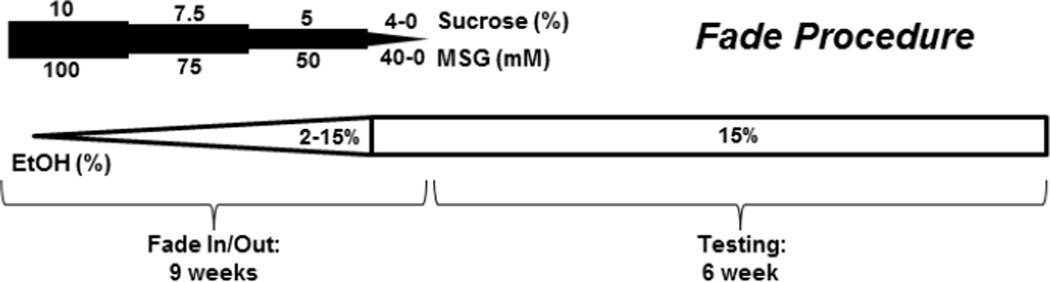
Following this continuous-access tastant exposure, ethanol drinking was initiated using a modified ‘drinking in the dark’ (DID) procedure (Rhodes et al., 2005) as previously described (McCool and Chappell, 2012). Briefly, 2-hr access to a drinking ‘tube’ (5 mL serological pipette with 0.05 mL accuracy) was initiated 30 min after the beginning of the dark cycle 5 days each week. During the 2-hr limited access, mice were given single tubes containing either 100 mM MSG or 10% sucrose on 2 consecutive days; food was available ad libitum during this period. Following this initial exposure to the tastants alone, ethanol concentrations were increased incrementally from 2% to 7% (Fig. 1). MSG and sucrose concentrations were then lowered to 75 mM and 7.5%, respectively, and ethanol concentrations were again incrementally increased from 8% to 12%. MSG and sucrose concentrations were again lowered to 50 mM and 5%, respectively, and mice were given access to ethanol concentrations increasing from 13% to 15%. Finally, MSG and sucrose were gradually diminished completely, using decreasing concentrations from 40, 30, 20, and 10 mM MSG or 4%, 3%, 2%, and 1% sucrose in the continued presence of 15% ethanol. Animals were always given access to each tastant/ethanol combination for at least 2 consecutive days. Novel tastant/ethanol combinations were never introduced on Mondays following weekends where no drinking took place, such that animals received some ethanol/tastant combinations for 3 days (Thursday, Friday, and Monday). Experiments with the sucrose or MSG substitution were conducted in parallel. The entire tastant fade-out/ethanol (15%) fade-in procedure took approximately 8 weeks. At the end of the tastant substitution, animals were then given DID access to 15% ethanol 5 days a week over 6 consecutive weeks.
Figure 1.
Tastant-substitution procedure used in the current study. The concentrations of sucrose and monosodium glutamate (MSG) used in the procedure are indicated above and below the dark bar, respectively. The precise concentration changes for the tastants occurred at specific ethanol concentrations as described in the Methods section. Ethanol concentrations (white bars) were gradually increased from 2% to 15% over an 8-week period with animals experiencing each ethanol concentration for 2–3 days. After 15% ethanol was introduced for 2 days, tastants were finally faded away over the span of about a week, and drinking of 15% ethanol alone was assessed for 6 consecutive weeks.
Statistics
Ethanol drinking, expressed as either g ethanol/kg/session or mL/session, was averaged across days for any given condition within each animal. Mean consumption was then averaged across animals within each treatment group. Drinking data were typically analyzed using a standard 2-way ANOVA, and main factors are described within each experiment. Significance levels were assumed at p < 0.05. Bonferroni’s multiple comparisons test was used post hoc to compare individual data points within the main factors when there were significant within-factor effects. Interactions between main factors were not analyzed post hoc. In some cases, standard t tests were used to compare groups when appropriate.
Results
B6 and D2 mice exhibit differential consumption of sucrose and MSG
The tastant substitution procedures began with an initial exposure to MSG or sucrose alone. During this 2-day period, B6 and D2 mice consumed different amounts of these tastants (illustrated in Fig. 2A1/B1 and Fig. 3A1/B1 at 0% ethanol). Two-way analysis of the volume of tastant consumed found a significant interaction (p < 0.001, F = 47.65) between strain (B6 versus D2) and tastant (MSG versus sucrose) as main factors. Within a strain, B6 mice drank significantly more of the 10% sucrose (2.4 ± 0.1 mL) than the 100 mM MSG (1.2 ± 0.2 mL; p < 0.001, Bonferroni multiple comparison post-test), while D2 mice drank significantly more 100 mM MSG (1.8 ± 0.1 mL) than 10% sucrose (0.9 ± 0.2 mL; p < 0.01, Bonferroni). Previous work has shown that, while 10% sucrose and 100 mM MSG engender unique magnitudes of absolute consumption within B6 and D2 mice, these concentrations are maximally preferred by both strains (Lewis et al., 2005; McCool and Chappell, 2012).
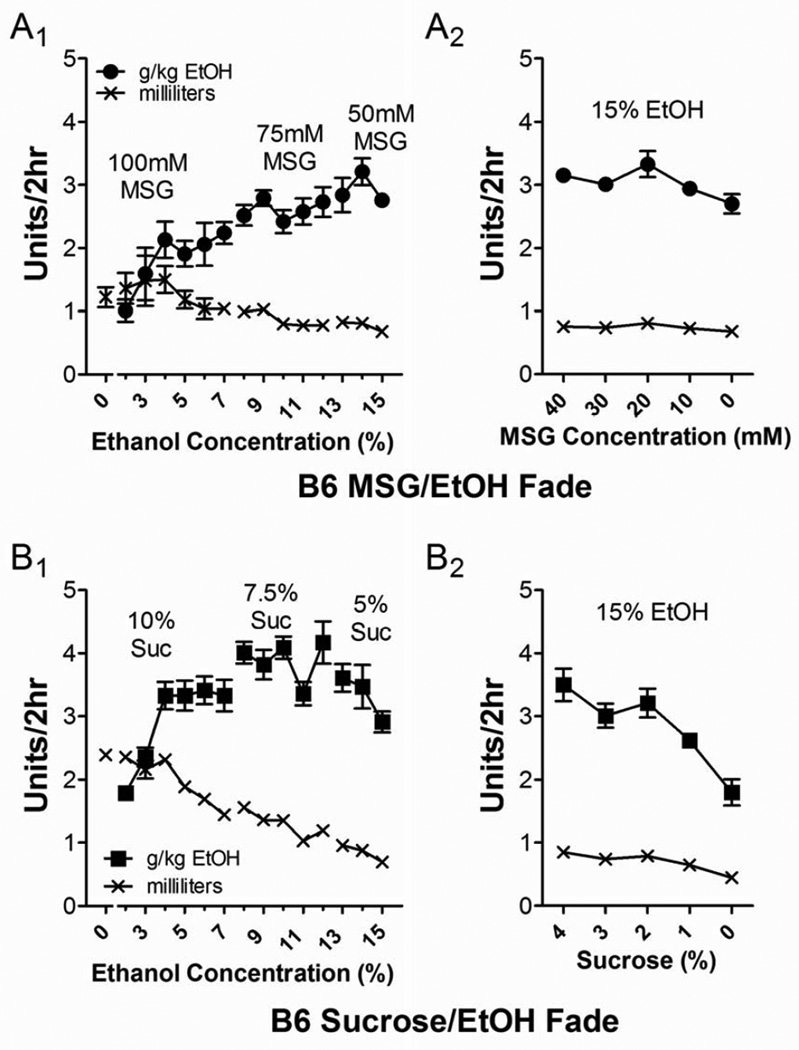
Figure 2.
MSG and sucrose substitution initiated robust ethanol drinking in B6 mice. (A) MSG substitution with 15% ethanol produced robust ethanol intakes (●, g/kg/2 hr; x, mL/2 hr) across a range of both ethanol and MSG concentrations (A1). During the MSG fade-out (A2), ethanol drinking was relatively stable despite declining MSG concentrations. (B) In the sucrose-substitution group with B6 mice, ethanol intake (■, g/kg/2 hr; x, mL/2 hr) tended to remain relatively stable across sucrose concentrations when ethanol concentrations were > 3% (B1). However, during the sucrose fade-out (B2), ethanol drinking declined in parallel with declining sucrose concentrations.
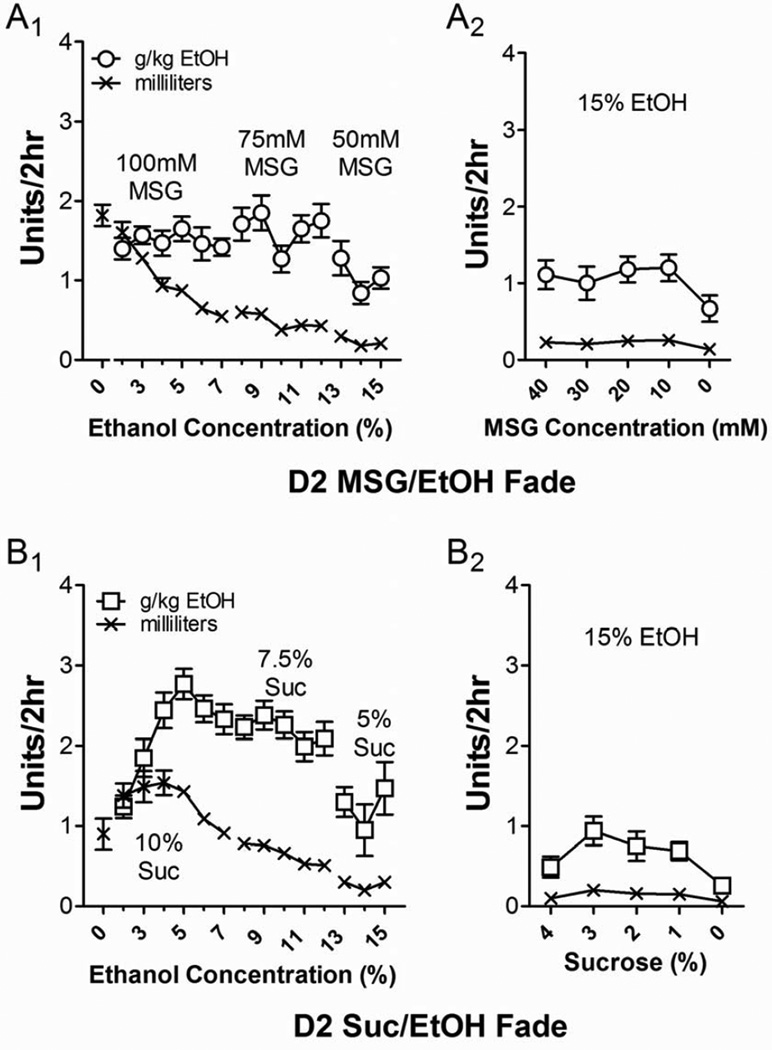
Figure 3.
MSG and sucrose substitution initiated ethanol drinking in D2 mice. (A) Unlike B6 mice, ethanol intake (○, g/kg/2 hr) in D2-MSG mice remained relatively constant across a range of both MSG and ethanol concentrations. This reflects a very precise titration of the volume of ethanol/MSG consumed by these mice (A1). During the MSG substitution (A2), D2 mice maintained ethanol drinking except at the point where MSG was entirely removed from the solution. (B) D2 mice undergoing the sucrose-substitution procedure (□, g/kg/2 hr, B1) rapidly increased intakes until ethanol concentrations reached 5%. Intakes were then titrated by apparently decreasing the volume of ethanol/sucrose consumed (x, mL). After sucrose concentrations declined to < 5% during the substitution, ethanol drinking decreased dramatically. During the sucrose substitution (B2), ethanol drinking continued to decline.
MSG and sucrose substitution initiate ethanol drinking in B6 mice
The MSG and sucrose substitution initiated considerable ethanol drinking in B6 mice (Fig. 2). Ethanol intake (g/kg/2 hr, ● and ■) during the ethanol ‘fade-in’ (Fig. 2A1 and 2B1) was characterized by a relatively stable increase across a range of ethanol and tastant concentrations. For the 100 mM MSG concentration, there was a significant increase in intake (g/kg/2 hr) as ethanol concentrations increased (p < 0.01, repeated-measures ANOVA, F = 3.8), but a Neuman-Keuls multiple comparison post-test showed that this effect was driven by low intakes when ethanol concentrations were < 3% (p < 0.05 to 0.01 comparing 2% and 3% ethanol to 4–7%), with animals drinking 1.9–2.1 g/kg across the 4–7% ethanol concentrations. The volume of the 100 mM MSG-containing solution did not significantly differ between any of the ethanol concentrations (p > 0.05, F = 1.4, repeated-measures ANOVA). During the 75 mM MSG period, ethanol intakes ranged from 2.4–2.7 g/kg/2 hr and were not significantly different across the 7–12% ethanol concentrations (p > 0.05, F = 1.2, repeated-measures ANOVA). This likely reflected precise titration of the volume of MSG-ethanol consumed because this declined significantly across these concentrations (p < 0.001, F = 6.5, repeated-measures ANOVA, Neuman-Keuls multiple comparison post-test). For the 50 mM-MSG period, we found intakes ranging from 2.8–3.2 g/kg, and these were not significantly different from one another across 13–15% ethanol concentrations (p > 0.05, F = 1.4, repeated-measures ANOVA). The volumes consumed during this period were also not different from each other (p > 0.05, F = 1.5, repeated-measures ANOVA).
In the B6 mice undergoing the sucrose substitution, we found similar results. During the 10% sucrose period, intakes (g/kg/2 hr) of 3–7% ethanol were significantly greater (p < 0.001, F = 34.2, repeated-measures ANOVA) than that of 2% and plateaued around 3.3–3.4 g/kg/2 hr for concentrations above 3% ethanol (p < 0.001, Neuman-Keuls multiple comparison post-test). The 7% and 5% sucrose periods could be similarly described with intakes across different concentrations not being significantly different from one another except for intake of 11% ethanol during the 7% sucrose period (p < 0.05, F = 3.2, repeated-measures ANOVA). The volume of ethanol/sucrose consumed declined significantly throughout the 10%, 7%, and 5% sucrose periods (p < 0.001 and F = 7.5 for 10% sucrose, p < 0.001 and F = 12.6 for 7% sucrose, and p < 0.05 and F = 5.0 for 5% sucrose, repeated-measures ANOVA).
During the tastant fade-out period in the B6 mice, both MSG and sucrose groups significantly declined their intakes across the range of tastant concentrations (Fig. 2A2 & 2B2). For the B6-MSG mice, intakes significantly declined from 3.2 ± 0.1 g/kg/2 hr to 2.7 ± 0.4 g/kg/2 hr (p < 0.05, F = 3.2, repeated-measures ANOVA). However, the total volume consumed by B6-MSG mice during the ‘fade-out’ of MSG decreased modestly but not significantly during this period (p ≈ 0.1, F = 2.2, repeated-measures ANOVA). Mice were still gaining weight during the fade-out period (28.6 ± 0.5 g to 29.9 ± 0.6 g, p < 0.001, F = 21.9, repeated-measures AVOVA), suggesting the modest decrease in g/kg/2 hr intakes were substantially influenced by increasing body weights. For the B6-sucrose mice, the same effects were evident although they appeared more pronounced with both intake and volume consumed decreasing significantly across the tastant ‘fade-out’ period (p < 0.0001, F = 23.3 for intake and F = 22.5 for volume consumed, repeated-measures ANOVA). Like the B6-MSG mice, B6-sucrose mice gained a significant amount of body weight throughout the ‘fade-out’ period (29.1 ± 0.5 g to 30.1 ± 0.6 g, p < 0.0001, F = 20.0, repeated-measures ANOVA). However, the body weights of B6-MSG and B6-sucrose mice did not significantly differ from one another at the end of this period (p > 0.05, t test).
MSG and sucrose substitution initiate ethanol drinking in D2 mice
Unlike B6-MSG mice, D2-MSG mice did not significantly alter ethanol intakes (○, g/kg/2 hr; Fig. 3A1) during the 100 mM and 75 mM periods of the MSG/ethanol substitution (p > 0.05, F = 0.5 for 100 mM and F = 1.8 for 75 mM, repeated-measures ANOVA). This precise titration of intakes may be reflected by the significant decrease in the volume of ethanol/MSG consumed (x, mL, Fig. 3A1) during these periods (p < 0.001, F = 23.1 for 100 mM MSG and p < 0.05, F = 3.9 for 75 mM MSG, repeated-measures ANOVA). During the 50 mM MSG period, D2-MSG intakes at 14% and 15% ethanol were significantly less than those for 13% ethanol (p < 0.05, F = 7.3, repeated-measures ANOVA), consistent with the significant decrease in the volume of ethanol/MSG consumed across these concentrations (p < 0.01, F = 8.1, repeated-measures ANOVA). During the MSG ‘fade-out’ period of the substitution procedure (Fig. 3A2), there was a trend for declining ethanol intakes (1.1 ± 0.2 g/kg/2 hr with 40 mM MSG to 0.7 ± 0.2 g/kg/2 hr at 0 mM MSG) and volumes (0.23 ± 0.03 mL to 0.15 ± 0.04 mL) as the MSG concentration decreased, but these did not reach statistical significance (p > 0.05, F = 2.3 for intake and F = 2.1 for volume, repeated-measures ANOVA).
For the D2-sucrose mice, intakes (□, g/kg/2 hr, Fig. 3B1) dramatically and significantly increased as ethanol concentrations increased from 2% to 4% (p < 0.001, F = 16.9, repeated-measures ANOVA) but then plateaued around 4% (p > 0.05, Neuman-Keuls multiple comparison post-test). This was mirrored by the volumes of ethanol/sucrose consumed (x, mL; p < 0.001, F = 6.5, repeated-measures ANOVA) which was not significantly different from 2% to 4% ethanol (p > 0.05, Neuman-Keuls multiple comparison post-test), then decreased significantly across ethanol concentrations greater than 4% (p < 0.05 to p < 0.001, Neuman-Keuls multiple comparison post-test). Ethanol intakes during both the 7.5% and 5% sucrose periods did not differ significantly across ethanol concentrations (p > 0.05, F = 0.9 for 7.5% and F = 3.1 for 5%, repeated-measures ANOVA), although the volume of ethanol consumed during the 7.5% sucrose period continued the trend from the end of the 10% sucrose period and significantly declined across the 8–12% ethanol concentrations (p < 0.01, F = 6.1, repeated-measures ANOVA). During the sucrose fade-out period (Fig. 3B2), D2 mice intakes of 15% ethanol varied significantly across the range of sucrose concentrations (p < 0.01, F = 5.0, repeated-measures ANOVA), with the significant decline in the volume of ethanol/sucrose being consumed (p < 0.01, F = 4.7, repeated-measures ANOVA) from 3% to 0% sucrose (p < 0.05 to p < 0.01, Neuman-Keuls multiple comparison post-test), possibly driving most of this change in intake. As with the B6 mice, body weights at the end of the substitution procedure were not significantly different between the D2-MSG (26.4 ± 0.7 g) and the D2-sucrose mice (26.1 ± 0.3 g; p > 0.05, t test).
Strain comparisons within the MSG- or sucrose-substitution procedures
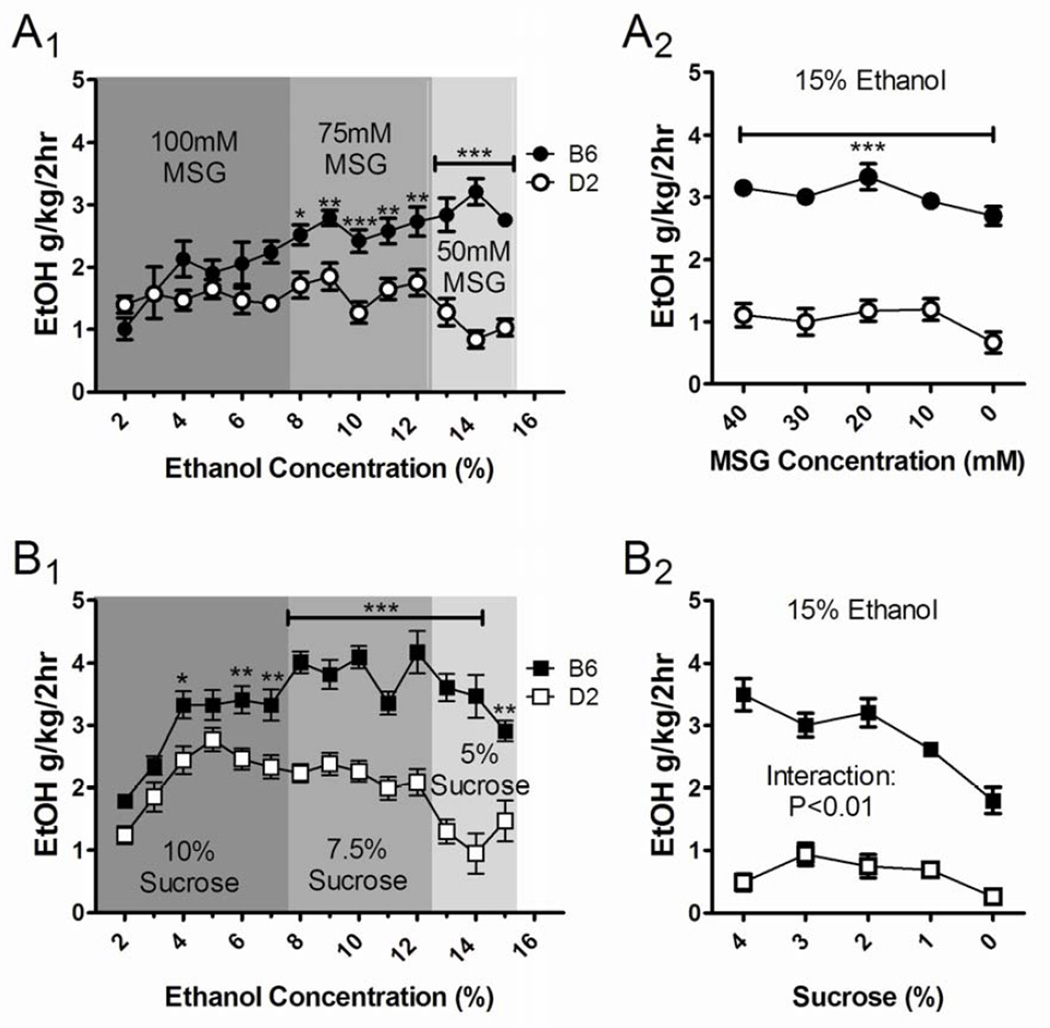
The data from Figures 2 and 3 were subsequently re-analyzed to compare across strain and ethanol concentration within a given substitution procedure (Fig. 4). Across all 3 MSG concentrations, the B6 mice had significantly higher intake levels than D2 mice (p < 0.05, F = 6.4 strain effect for the 100 mM period, p < 0.001 and F = 63.4 for the 75 mM period, and p < 0.001 and F = 150.7 for the 50 mM period, 2-way ANOVA; Fig. 4A1). There were no significant main effects of ethanol concentration or any interaction between ethanol concentration and strain. Post hoc analysis (Bonferroni multiple comparison post-test) revealed significant strain differences during both the 75 mM and 50 mM MSG periods. During the MSG fade-out period, both strain (p < 0.001, F = 379.9) and MSG concentration (p < 0.05, F = 3.5, 2-way ANOVA) significantly influenced ethanol intake (Fig. 4A2), but there was no interaction between these factors. For the sucrose groups, 2-way ANOVA analysis of intake data with strain and ethanol concentration as main factors found significant differences between strains at each sucrose concentration (Fig. 4B1). During the 10% sucrose period, there were significant main effects of both strain (p < 0.001, F = 42.4) and ethanol concentration (p < 0.001, F = 19.0) but no significant interaction between factors. Post hoc analysis with Bonferroni’s multiple comparison test defined significant strain differences at 4% ethanol (p < 0.05) and both 6% and 7% ethanol (p < 0.01). These strain differences were also evident for both the 7.5% sucrose and 5% sucrose periods (p < 0.001 for the strain factor, F = 172.3 for 7.5% and F = 89.4 for 5%), but ethanol concentration as a factor was no longer significant. With 15% ethanol, the sucrose fade-out (Fig. 4B2) revealed a significant interaction (p < 0.01, F = 5.1) between strain and sucrose concentration, possibly due to an ethanol concentration-dependent decrease in intake in the B6 mice. This was supported by significant main effects for both strain (p < 0.001, F = 396.1) and ethanol concentration (p < 0.001, F = 11.25), along with significant differences between all the concentrations defined by post hoc analysis (p < 0.001, Bonferroni’s multiple comparison test).
Figure 4.
Strain comparisons during the substitution procedure suggest that the MSG substitution did not alter the relative differences in ethanol drinking behavior between B6 and D2 mice. For mice undergoing the MSG-substitution procedure (A), B6 and D2 mice did not significantly diverge from one another until the procedure employed 75 mM MSG. Two-way ANOVA with strain and ethanol concentration as the main factors revealed a significant difference between strains but no effect of ethanol during both the 75 mM and 50 mM MSG periods (p < 0.001, A1). Significant differences between strains remained during the MSG substitution (A2, p < 0.001, 2-way ANOVA). During the sucrose-substitution procedure (B), ethanol intakes by B6 and D2 mice diverged significantly during the 10% sucrose period as ethanol concentrations were raised above 3% (B1; 2-way ANOVA, p < 0.001 for the strain factor and for the ethanol concentration factor). The significant strain differences, but not the ethanol concentration effect, were also present during the 7.5% and 5% sucrose periods (p < 0.001, 2-way ANOVA). There was a significant interaction between factors during the sucrose fade-out (B2, p < 0.01, strain and sucrose concentrations as main factors). Post hoc analysis of the strain factor used Bonferroni’s multiple comparison tests, *p < 0.05, **p < 0.01, ***p < 0.001.
MSG-enhanced ethanol drinking persists for several weeks
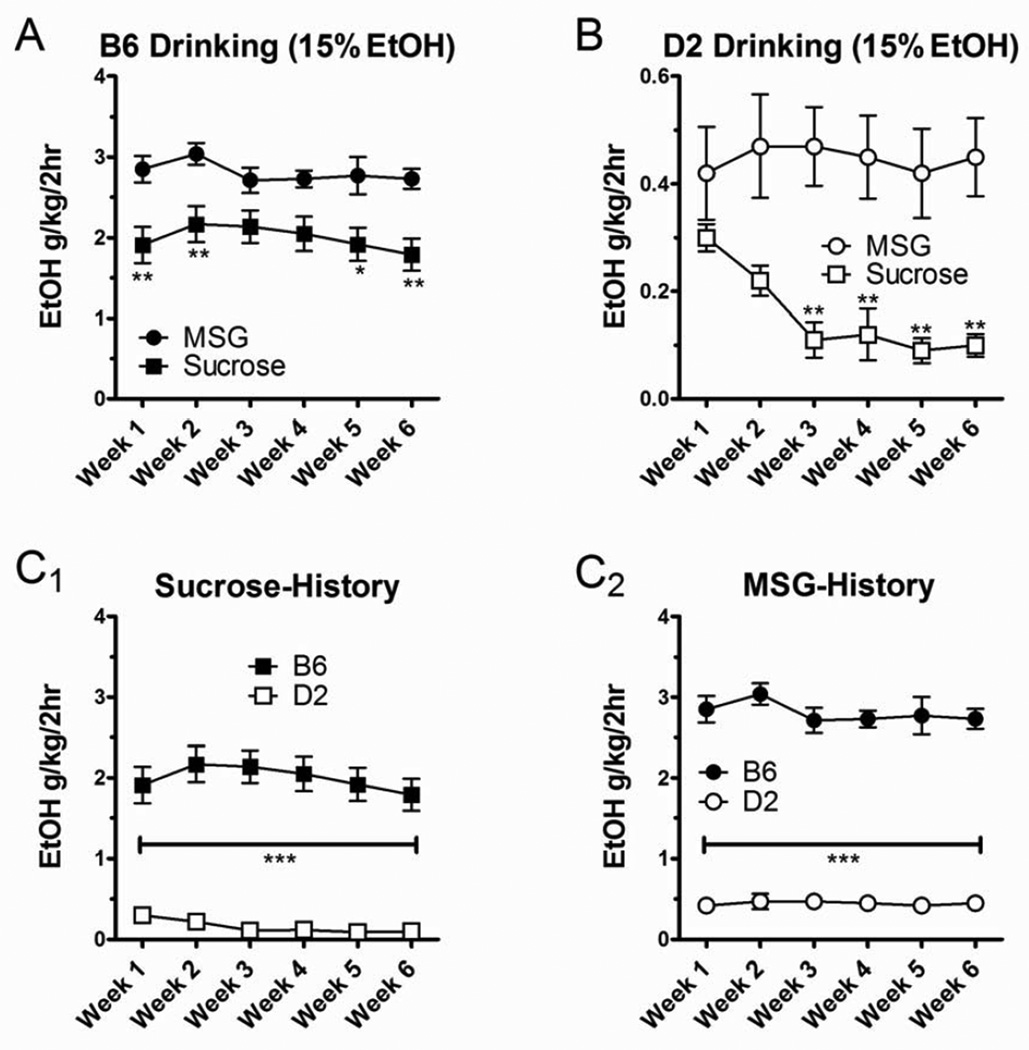
Following the sucrose and MSG substitution, B6 and D2 mice were allowed 2-hr daily access to 15% ethanol, 5 days a week, for 6 consecutive weeks. We found that intake levels were differentially stable during the prolonged period of 15% ethanol drinking and appeared to depend upon both strain and sucrose or MSG history. In B6 mice we found that ethanol intake was significantly larger in animals following MSG substitution compared to the sucrose substitution (Fig. 5A). Two-way ANOVA with tastant history and week of drinking as the main factors indicated a significantly larger ethanol intake in B6-MSG mice compared to B6-sucrose mice (p < 0.001, F = 56.1), but there was no significant main effect of the week of ethanol drinking and no significant interaction between tastant history and drinking week. Post hoc analysis with Bonferroni’s multiple comparison test found significant differences between B6 mice from the different tastant histories in weeks 1 (p < 0.01), 2 (p < 0.01), 5 (p < 0.05), and 6 (p < 0.01).
Figure 5.
Enhanced ethanol drinking initiated by the MSG substitution persisted for 6 weeks. (A) In B6 mice, the MSG-substitution procedure produced significantly higher ethanol intakes than the sucrose-substitution procedure (p < 0.001 for across the tastant factor, 2-way ANOVA). Post hoc analysis with Bonferroni’s multiple comparison tests showed that the differences between MSG substitution and sucrose substitution were evident in weeks 1, 2, 5, and 6 (*p < 0.05, **p < 0.01). (B) For D2 mice, ethanol drinking was also significantly greater following the MSG substitution relative to sucrose substitution (p < 0.001, 2-way ANOVA) and was particularly pronounced in weeks 3–6 (Bonferroni’s post-test). (C) Strain comparisons within a given substitution procedure showed that neither the sucrose substitution (C1) nor the MSG substitution (C2) altered the well-characterized differences between B6 and D2 mice (see text). ***p < 0.001, Bonferroni’s multiple comparison test between strains across weeks.
Ethanol drinking was also enhanced in D2 mice following MSG substitution compared to sucrose substitution. It should be noted that one of the D2-sucrose mice in this experiment consumed significantly more than the rest of the individuals in this experimental group. The average intake from this animal was 0.9 ± 0.1 g/kg/2 hr across all 6 weeks; and Grubb’s test of weekly averages indicate it was a significant outlier (at least p < 0.05) for 5 out of the 6 weeks. In fact, ethanol intake by this animal in the last 3 weeks of the experiment was almost 10 times greater than the rest of this experimental group and approached levels seen in some of the D2-MSG group (see below). Data from this animal were excluded from the following analysis. In the remaining D2 mice, ethanol intake following MSG substitution was significantly greater than following sucrose substitution (Fig. 5B, p < 0.001, F = 58.6, 2-way ANOVA with tastant history and week as main factors). There was no significant main effect of the week of drinking and no significant interaction between main factors. Bonferroni post hoc analysis revealed significantly more drinking in the D2-MSG mice in weeks 3–6 (p < 0.01 for each). This reflects a substantial decline in ethanol drinking in the D2-sucrose mice across the 6 weeks – from 0.30 ± 0.02 g/kg/2 hr in week 1 to 0.10 ± 0.02 g/kg/2 hr in week 6 (a ~67% decrease) – while ethanol drinking in the D2-MSG mice was stable during this same period. These data suggest that MSG substitution initiates stable ethanol drinking in D2 mice while sucrose substitution does not.
Importantly, the MSG substitution and sucrose substitution did not alter the relative differences in drinking phenotype between the 2 strains. B6 mice consumed significantly more 15% ethanol than D2 mice across all the drinking weeks regardless of the tastant used in the substitution procedure (Fig. 5C). Similarly, B6 mice weighed significantly more (20.6 ± 0.3 g) than D2 mice (16.4 ± 0.4 g; p < 0.001, t test) despite being similar ages (~5 weeks). These differences in body weight that were apparent from the beginning of the experiment were also evident at the end of the experiment 15 weeks later: B6-sucrose mice weighed 33.9 ± 0.7 g, B6-MSG mice weighed 33.6 ± 0.8 g, D2-sucrose mice weighed 30.3 ± 1.3 g, and D2-MSG mice weighed 31.3 ± 0.8 g. Two-way analysis using strain and tastant history as main factors showed a significant effect of strain (p < 0.01, F = 10.0) but not of tastant history. These findings suggest that the simultaneous MSG/ethanol-substitution procedure produces a greater ethanol intake than the sucrose/ethanol-substitution in both B6 and D2 mice but does not alter relative differences between the strains for either ethanol drinking or the physical characteristic of body weight.
Discussion
In the current study we found that a substitution procedure using monosodium glutamate produced ethanol drinking by inbred mouse strains more effectively than a more traditional sucrose substitution. Each strain exhibited unique drinking patterns directed at the different tastants alone: B6 mice drank more 10% sucrose than 100 mM MSG while D2 mice drank more 100 mM MSG than 10% sucrose. However, the volumes of MSG and sucrose consumed during this initial tastant-only exposure did not significantly correlate either with 15% ethanol intake (g/kg/2 hr) or with the volume of 15% ethanol consumed. Mice also exhibited unique drinking behaviors directed at the 15% ethanol once the substitution procedure had been completed. In B6 mice, the MSG substitution increased drinking of 15% ethanol by ~40% above that produced with sucrose substitution, and this drinking difference persisted for the entire 6-week experimental period. The relative difference between MSG- and sucrose-substitution procedures in B6 mice compares favorably with the ~30% increase we found in a previous study using a different substitution procedure (McCool and Chappell, 2012). In D2 mice, the effect of MSG substitution was even more dramatic and increased ethanol consumption by ~40% in week 1 and by ~450% in weeks 4–6 when ethanol drinking following sucrose substitution dramatically decreased (Fig. 5). Moreover, despite the dramatic MSG-dependent increase in ethanol drinking in both strains, the well-established drinking phenotypes of these inbred strains was not altered in this particular study, with B6 mice drinking 8–10 fold more ethanol than D2 mice. This is consistent with a large body of literature describing differences in voluntary ethanol consumption in these strains across different drinking paradigms (Belknap et al., 1993; Mittleman et al., 2003; Rhodes et al., 2007; Risinger et al., 1998; Yoneyama et al., 2008).
There are limited reports of novel substitution procedures being employed with inbred mouse strains making comparisons across studies problematic. However, our limited experience with MSG substitution suggests that the precise construct of the substitution procedure may ultimately influence the magnitude of the resulting ethanol consumption in some inbred mouse strains. For example, our recent study (McCool and Chappell, 2012) found that a variant of the MSG substitution used here produced higher ethanol drinking in the D2 mice than the current study was able to generate. In that work, D2 mice drinking 15% ethanol produced intakes of ~1.5 g/kg/2 hr compared to the 0.4–0.5 g/kg/2 hr intakes in the current work. However, ethanol drinking in B6 mice appears to be relatively insensitive to the exact construct of the substitution procedure. For example, B6-MSG ethanol intakes were almost identical between the current study (2.7–3.0 g/kg/2 hr) and our previous publication (~2.9 g/kg/2 hr). Additionally, these intake levels are comparable to DID drinking studies in which ethanol is placed on the home cage of B6 mice 1 hour after lights-out without using any substitution procedure (Rhodes et al., 2005). Regardless, differential response of D2 mice in the different studies is potentially interesting. Our previous study employed an ‘incremental substitution” – first ‘fading in’ ethanol to a low concentration (5%) then ‘fading out’ the tastant before ultimately raising ethanol concentrations to 15%. The current study altered both ethanol and tastant concentrations simultaneously. The ethanol ‘fade-in periods’ to 5% were identical between studies; the ethanol intakes of this concentration likewise did not differ between studies. It is likely then that the different approaches used for the MSG ‘fade-out’ produced marked differences in ethanol intake at least in the D2 mice. Importantly, the incremental substitution used by McCool and Chappell (2012) allowed mice to consume 5% ethanol for 18 days as MSG was slowly removed and offered an additional week of drinking 5% ethanol alone prior to increasing ethanol concentrations from 5% to 10%, and finally to 15% on separate weeks. This gave the D2 mice a full week to experience drinking each ethanol concentration. In the current study, mice had access to each ethanol concentration for only 2–3 days at a time and also drank ethanol across a constantly changing background of tastant concentrations. These differences in the precise scheduling of the substitution procedure together suggest that prolonged access to lower ethanol concentrations might engender greater ethanol drinking in D2 mice. This is consistent with the robust ethanol-conditioned taste aversion learning in this strain (Horowitz and Whitney, 1975), which has typically employed higher ethanol concentrations. Indeed, ‘pre-exposure’ to ethanol reduces subsequent conditioned taste aversion in D2 mice (Risinger and Cunningham, 1995). The longer access to lower, presumably less aversive, ethanol concentrations in the ‘incremental substitution’ might provide an opportunity for this strain to ‘learn’ the pharmacological salience of ethanol which is presumed to play a critical role in reducing/avoiding aversions to higher ethanol concentrations. But, we should note that direct, simultaneous comparisons between the schedules used in the current study and that employed in our previous work (McCool and Chappell, 2012) would be needed to directly test this hypothesis.
The mechanism responsible for increased ethanol drinking following MSG substitution is uncertain. It is possible that MSG acts directly on specific neurobiological mechanisms within the central nervous system to influence ethanol preference/consumption. However, glutamate is a non-essential amino acid, and this amino acid is rapidly integrated into numerous biosynthetic pathways and converted to other biochemical intermediary metabolites. Indeed, oral administration of 1 g/kg glutamate – similar to the largest amount any mouse would have consumed in our study (~1.2 g/kg by B6 mice consuming 100 mM MSG) – does not change brain levels of this amino acid (Caccia et al., 1982). And ‘glutamate-enriched’ diets engendering ~25 g/kg/day oral intake in mice do not cause any of the hypothalamic neurotoxicity that is apparent with subcutaneous injection of 4–5 g/kg MSG (Takasaki, 1978). Since our animals had a nutritionally complete diet available throughout the drinking period, it is likely that < 10% of the glutamate consumed during a limited access session would have even been found in the circulation (Bourdel et al., 1981). Since MSG activates a cadre of taste receptors that are unique from the ‘sweet’ receptors on the tongue, it is also possible that it is the distinct taste of MSG relative to ethanol that provides mice an opportunity to associate ethanol taste with any internal cues that drive consumption. Conditioned taste aversion experiments show nicely that B6 mice generalize the taste of ethanol with both bitter and sweet tastes while D2 mice generalize ethanol to only bitter tastes (Blizard, 2007). This finding offers some insight into the limited utility of sucrose or saccharin to induce ethanol drinking by D2 mice. Further, these data suggest that the unique character of MSG flavor might offer a better contrast between ethanol and the tastant during the substitution procedure.
In summary, we show here that monosodium glutamate can be used in a substitution procedure to induce more robust ethanol drinking than traditional tastants such as sucrose in both C57BL/6J and DBA/2J inbred mouse strains. Further, MSG-dependent increases in ethanol drinking persist for at least 6 weeks. These findings suggest that the MSG substitution may offer a useful avenue to study ethanol drinking within rodent species that typically do not consume ethanol.
Acknowledgments
This work is supported by NIH/NIAAA award U01 AA020942 (B.A.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology (Berl) 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Blizard DA. Sweet and bitter taste of ethanol in C57BL/6J and DBA2/J mouse strains. Behav Genet. 2007;37:146–159. doi: 10.1007/s10519-006-9121-4. [DOI] [PubMed] [Google Scholar]
- Bourdel G, Kande J, Robin D, Robin P. Quantitative and qualitative circadian variations of amino acid intestinal efflux in mixed-fed and in protein-meal-fed rats. J Nutr. 1981;111:1528–1535. doi: 10.1093/jn/111.9.1528. [DOI] [PubMed] [Google Scholar]
- Browman KE, Crabbe JC. Quantitative trait loci affecting ethanol sensitivity in BXD recombinant inbred mice. Alcohol Clin Exp Res. 2000;24:17–23. [PubMed] [Google Scholar]
- Caccia S, Garattini S, Ghezzi P, Zanini MG. Plasma and brain levels of glutamate and pyroglutamate after oral monosodium glutamate to rats. Toxicol Lett. 1982;10:169–175. doi: 10.1016/0378-4274(82)90070-4. [DOI] [PubMed] [Google Scholar]
- DuBose CS, Chesler EJ, Goldowitz D, Hamre KM. Use of the expanded panel of BXD mice narrow QTL regions in ethanol-induced locomotor activation and motor incoordination. Alcohol Clin Exp Res. 2013;37:170–183. doi: 10.1111/j.1530-0277.2012.01865.x. [DOI] [PubMed] [Google Scholar]
- Fehr C, Shirley RL, Crabbe JC, Belknap JK, Buck KJ, Phillips TJ. The syntaxin binding protein 1 gene (Stxbp1) is a candidate for an ethanol preference drinking locus on mouse chromosome 2. Alcohol Clin Exp Res. 2005;29:708–720. doi: 10.1097/01.alc.0000164366.18376.ef. [DOI] [PubMed] [Google Scholar]
- Fidler TL, Dion AM, Powers MS, Ramirez JJ, Mulgrew JA, Smitasin PJ, Crane AT, Cunningham CL. Intragastric self-infusion of ethanol in high- and low-drinking mouse genotypes after passive ethanol exposure. Genes Brain Behav. 2011;10:264–275. doi: 10.1111/j.1601-183X.2010.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Font L, Cunningham CL. Post-retrieval propranolol treatment does not modulate reconsolidation or extinction of ethanol-induced conditioned place preference. Pharmacol Biochem Behav. 2012;101:222–230. doi: 10.1016/j.pbb.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood HM, Buck KJ. Allelic variation in the GABA A receptor gamma2 subunit is associated with genetic susceptibility to ethanol-induced motor incoordination and hypothermia, conditioned taste aversion, and withdrawal in BXD/Ty recombinant inbred mice. Alcohol Clin Exp Res. 2000;24:1327–1334. [PubMed] [Google Scholar]
- Horowitz GP, Whitney G. Alcohol-induced conditioned aversion: genotypie specificity in mice (Mus musculus) J Comp Physiol Psychol. 1975;89:340–346. doi: 10.1037/h0076803. [DOI] [PubMed] [Google Scholar]
- Kirstein SL, Davidson KL, Ehringer MA, Sikela JM, Erwin VG, Tabakoff B. Quantitative trait loci affecting initial sensitivity and acute functional tolerance to ethanol-induced ataxia and brain cAMP signaling in BXD recombinant inbred mice. J Pharmacol Exp Ther. 2002;302:1238–1245. doi: 10.1124/jpet.302.3.1238. [DOI] [PubMed] [Google Scholar]
- Lewis SR, Ahmed S, Dym C, Khaimova E, Kest B, Bodnar RJ. Inbred mouse strain survey of sucrose intake. Physiol Behav. 2005;85:546–556. doi: 10.1016/j.physbeh.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Max M, Shanker YG, Huang L, Rong M, Liu Z, Campagne F, Weinstein H, Damak S, Margolskee RF. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat Genet. 2001;28:58–63. doi: 10.1038/ng0501-58. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Li TK. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- McCool BA, Chappell AM. Using monosodium glutamate to initiate ethanol self-administration in inbred mouse strains. Addiction Biology. 2012;17:121–131. doi: 10.1111/j.1369-1600.2010.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittleman G, Van Brunt CL, Matthews DB. Schedule-induced ethanol self-administration in DBA/2J and C57BL/6J mice. Alcohol Clin Exp Res. 2003;27:918–925. doi: 10.1097/01.ALC.0000071930.48632.AE. [DOI] [PubMed] [Google Scholar]
- Montmayeur JP, Liberles SD, Matsunami H, Buck LB. A candidate taste receptor gene near a sweet taste locus. Nat Neurosci. 2001;4:492–498. doi: 10.1038/87440. [DOI] [PubMed] [Google Scholar]
- Pothion S, Bizot JC, Trovero F, Belzung C. Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress. Behav Brain Res. 2004;155:135–146. doi: 10.1016/j.bbr.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Reed DR, Li S, Li X, Huang L, Tordoff MG, Starling-Roney R, Taniguchi K, West DB, Ohmen JD, Beauchamp GK, Bachmanov AA. Polymorphisms in the taste receptor gene (Tas1r3) region are associated with saccharin preference in 30 mouse strains. J Neurosci. 2004;24:938–946. doi: 10.1523/JNEUROSCI.1374-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, Jr, Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Brown MM, Doan AM, Oakes RA. Mouse strain differences in oral operant ethanol reinforcement under continuous access conditions. Alcohol Clin Exp Res. 1998;22:677–684. doi: 10.1111/j.1530-0277.1998.tb04311.x. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Cunningham CL. Genetic differences in ethanol-induced conditioned taste aversion after ethanol preexposure. Alcohol. 1995;12:535–539. doi: 10.1016/0741-8329(95)00040-2. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Samson HH, Czachowski CL. Behavioral measures of alcohol self-administration and intake control: rodent models. Int Rev Neurobiol. 2003;54:107–143. doi: 10.1016/s0074-7742(03)54004-1. [DOI] [PubMed] [Google Scholar]
- Shirley RL, Walter NA, Reilly MT, Fehr C, Buck KJ. Mpdz is a quantitative trait gene for drug withdrawal seizures. Nat Neurosci. 2004;7:699–700. doi: 10.1038/nn1271. [DOI] [PubMed] [Google Scholar]
- Takasaki Y. Studies on brain lesions after administration of monosodium L-glutamate to mice. II. Absence of brain damage following administration of monosodium L-glutamate in the diet. Toxicology. 1978;9:307–318. doi: 10.1016/0300-483x(78)90014-8. [DOI] [PubMed] [Google Scholar]
- Uematsu A, Tsurugizawa T, Kitamura A, Ichikawa R, Iwatsuki K, Uneyama H, Torii K. Evaluation of the 'liking' and 'wanting' properties of umami compound in rats. Physiol Behav. 2011;102:553–558. doi: 10.1016/j.physbeh.2011.01.005. Epub 2011 Jan 1012. [DOI] [PubMed] [Google Scholar]
- Weng J, Symons MN, Singh SM. Studies on Syntaxin 12 and alcohol preference involving C57BL/6J and DBA/2J strains of mice. Behav Genet. 2009;39:183–191. doi: 10.1007/s10519-008-9249-5. Epub 12008 Dec 10524. [DOI] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]