Abstract
Failed axon regeneration and retinal ganglion cell (RGC) death after trauma or disease, including glaucomatous and mitochondrial optic neuropathies, are increasingly linked to mitochondrial dysfunction. Mitochondria are highly dynamic organelles whose size, organization, and function are regulated by a balance between mitochondrial fission and fusion. Mitochondria are ubiquitous in axonal growth cones both in vitro and in vivo and during development and regeneration. However, the roles that mitochondrial fission and fusion dynamics play in the growth cone during axon regeneration are largely unstudied. Here we discuss recent data suggesting mitochondria in the distal axon and growth cone play a central role in axon growth by integrating intrinsic axon growth states with signaling from extrinsic cues. Mitochondrial fission and fusion are intrinsically regulated in the distal axon in the growth cones of acutely purified embryonic and postnatal RGCs with differing intrinsic axon growth potentials. These differences in fission and fusion correlate with differences in mitochondrial bioenergetics; embryonic RGCs with high intrinsic axon growth potential rely more on glycolysis whereas RGCs with low intrinsic axon growth potential rely more on oxidative phosphorylation. Mitochondrial fission and fusion are also differentially modulated by KLFs that either promote or suppress intrinsic axon growth, and altering the balance between mitochondrial fission and fusion can differentially regulate axon growth rate and growth cone guidance responses to both inhibitory and permissive guidance cues.
Keywords: Mitochondria, Growth cone, Regeneration, Retinal ganglion cell, KLF
Introduction
Vision loss after optic nerve injury remains a persistent clinical problem due in part to the failure of injured or diseased retinal ganglion cells (RGCs) to regenerate their axons, which often degenerate after injury, leading to RGC cell death and irreversible vision loss [1]. In RGCs, like other central nervous system (CNS) neurons, the failure to regenerate after injury or disease is multifactorial, including reduced intrinsic axon growth ability in adult neurons [2-5], tissue destructive inflammatory responses [6], extrinsic glial-associated inhibitory molecules [7-9], and insufficient neurotrophic factor support [10-12]. Most efforts to improve CNS axon survival and regeneration have focused on overcoming extrinsic glial-associated inhibitors in the injured CNS like Nogo, oligodendrocyte myelin glycoprotein (Omgp), semaphorin 3A, myelin associated glycoprotein, and chondroitin sulfate proteoglycans (CSPGs) [13-19], increasing deficient neurotrophic factor signaling [10,12,20-23], modulating inflammation [24], and manipulating intrinsic signaling molecules like phosphatase and tensin homolog (PTEN) [25,26], SOCS3 [27], and the Krüppel-like family (KLF) family of transcription factors [28,29] among others well reviewed [30]. Addressing extrinsic factors individually or in combination with other extrinsic or intrinsic factors can slow RGC death, promote partial axon regeneration, and in some cases, restore limited visual function in animal models [26]. However, the overall number of axons that regenerate successfully is low, many regenerating axons fail to pathfind correctly [25,31], and many of the molecular manipulations are not clinically translatable, arguing for new strategic and therapeutic approaches to overcoming barriers to successful axon regeneration.
Mitochondria
Mitochondria integrate and modulate numerous cellular signaling pathways [32] and are increasingly recognized as central players in modulating signaling by both intrinsic and extrinsic factors that regulate neuronal survival and axon growth (Figure 1). Generally, mitochondria regulate neuronal growth, maintenance, and function by regulating basic metabolic functions, including ATP production through oxidative phosphorylation, calcium homeostasis, cellular apoptosis, lipid metabolism, and reactive oxygen species (ROS), nitric oxide (NO) and cyclic AMP (cAMP) signaling [33]. Moreover, evidence suggests mitochondria play key modulatory roles in the signaling pathways of many intrinsic and extrinsic factors that regulate axon regeneration [34]. Specifically, mitochondrial fission, fusion, and transport dynamics, appear to regulate numerous signal transduction pathways that control axon growth, suggesting mitochondria in axonal growth cones (GC) are optimally positioned to integrate and modulate signaling from both intrinsic and extrinsic factors that regulate axon growth rate and guidance.
Figure 1.
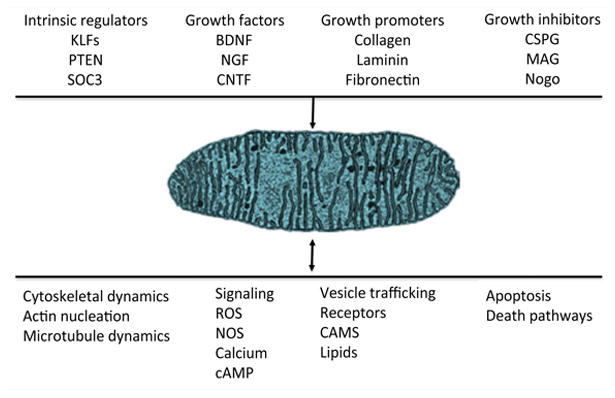
Mitochondria are positioned opportunistically to modulate numerous signaling pathways. Numerous intrinsic and extrinsic factors that modulate axon growth also modulate mitochondrial dynamics and function. In turn, mitochondria regulate and are regulated by signaling pathways that regulate axon growth.
Mitochondria, Intrinsic and Extrinsic Signaling, and Second Messengers
In previous studies, both intrinsic and extrinsic regulators of axon growth have been shown to regulate mitochondrial activities (Figure 1). For example, intrinsic regulators, like KLFs and PTEN, influence mitochondrial dynamics and function. PTEN is a negative regulator of the mammalian target of rapamycin (mTOR) pathway [35] and both PTEN and mTOR integrate signals regulating mitochondrial transport, oxygen consumption, and oxidative capacity [36-39]. PTEN may also mediate mitochondrial trafficking via PTEN induced Kinase 1 (PINK1). PINK1 forms a multi-protein complex with the calcium-dependent transport protein Miro and the adaptor protein Milton [40], a complex that links kinesin to mitochondria for anterograde transport along microtubules [41,42]. The KLF transcription factors can also differentially alter intrinsic axon growth ability in RGCs. The downstream KLF axon growth effectors in CNS neurons are not yet defined, but potential links to mitochondria exist. Overexpressing KLFs that differentially suppress, KLF4, or enhance, KLF6, axon growth potential [28] differentially regulates mitochondrial organization and mitochondrial DNA replication in the distal axons and growth cones of cultured RGCs [43]. In non-neuronal cells KLF2 is regulated by the mTOR pathway [44], KLF4 can up-regulate Mfn2 expression [45], and KLF6 can up-regulate p21 [46-48] which can enhance axon regeneration in the spinal cord [49]. Moreover, p21 can activate p21-activated kinase 5 (PAK5), which localizes to mitochondria [50,51].
Similarly, extrinsic axon growth regulators also act on mitochondrial dynamics and function. BDNF can increase mitochondrial respiration in neurons, via a MAP kinase dependent pathway linked to increased complex I activity [52], whereas decreased BDNF levels are linked to reduced mitochondrial respiration in neurons [53]. Interestingly, the full-length TrkB receptor is present in brain mitochondria [54] and BDNF protein has been localized to mitochondria in axon terminals in the visual system [55], suggesting that neurotrophins may act as signaling molecules directly on mitochondria. Nerve growth factor (NGF) can also regulate mitochondrial activities. In culture, applying NGF locally to either axons or to pre-synaptic terminals can locally direct mitochondrial transport and docking [56-58] by a mechanism that locally increases mitochondrial membrane potential [59] and is directed by the actin cytoskeleton [57,58]. Similarly, locally applying semaphorin 3A, an inhibitory guidance cue [60,61], also directs mitochondrial transport and increases mitochondrial membrane potential (Δψm) [59]. Both NGF and semaphorin 3A, altered Δψm via PI3 kinase and, like BDNF, MAP kinase dependent pathways [59], suggesting that these pathways can regulate Δψm and that mitochondrial functions requiring increased Δψm may regulate both positive and negative regulators of axon growth. Finally, Nogo, a potent inhibitor of axon growth, has been suggested to regulate electron transport chain (ETC) function by binding to a complex III protein or to a putative Nogo-interacting mitochondrial protein [62]. Surprisingly, despite the number of cues that regulate both signaling pathways that regulate axon growth, little is known about how these intrinsic and extrinsic signaling pathways modulate or are modulated by mitochondrial dynamics in the growth cone.
Mitochondria also share a reciprocal relationship with second messenger systems that regulate axon growth (Figure 1). Mitochondria are prominent in axonal growth cones in vitro [63] and in vivo [64] and regulate numerous second messenger pathways, including Ca2+, cAMP, and NOS, that can regulate both axon growth rate and growth cone motility and guidance [65-67]. Moreover, mitochondrial organization, in turn, is influenced by second messenger systems [68,69] that alter axon growth, including Ca2+ signaling [70-72], ROS signaling [73], NOS signaling [74], and mitophagy [75-77], suggesting mitochondrial dynamics and second messenger signaling share a reciprocal relationship. Though progress has been made in the temporal and spatial control of second messenger transients in the growth cone [78,79], studies on how various second messenger pathways alter mitochondrial dynamics in the growth cone and, in turn, how such changes in mitochondrial dynamics regulate second messenger signaling to regulate axonal growth and guidance are limited [43]. Moreover, little is known about how mitochondrial dynamics function in the axonal growth cone.
The Axonal Growth Cone
Growth cones, the motile tips of axons, determine the rate and the direction axons grow by altering three distinguishable processes: protrusion, engorgement, and consolidation (Figure 2) [80] in response to signaling from cellular [81] and extracellular matrix guidance cues [82] integrated with intrinsic axon growth states [83,84]. During protrusion, actin meshworks in the growth cone's peripheral domain protrude sheet-like, lamellipodia, whereas filamentous actin (F-actin) bundles protrude finger-like, filopodia. These protrusions advance the growth cone's membrane [85], distributing receptors, cell adhesion molecules, and lipids peripherally [86] to detect and modulate extrinsic cue signal transduction [87]. In the central domain, at the base of the growth cone, bundled microtubules extending from the neurite shaft fan out providing structural support and conduits for transporting vesicular cargos, including mitochondria, essential to growth cone function and remodeling. Mitochondria are ubiquitous residents of the axonal growth cone's central domain. In actively growing axons, mitochondria are highly dynamic often undergoing rapid fission and fusion events and bi-directional transport along dynamic microtubules extending from the central domain and into the peripheral domain. The persistent presence of mitochondria in the growth cone's central domain is hypothesized to provide the ATP required to support growth cone motility. However, recent observations suggest the role of mitochondria in the growth cone is not so straightforward and that mitochondrial dynamics themselves may play a critical signaling role.
Figure 2.
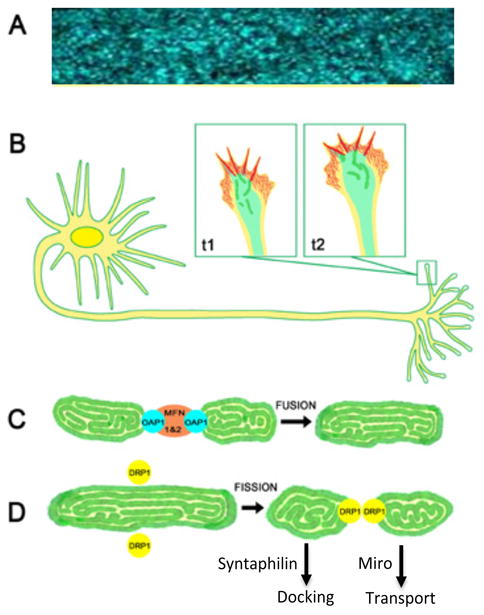
A. Mitochondria (blue) are highly dynamic organelles localized throughout retinal ganglion cells axons shown in this live whole mount image of the optic nerve [166]. B. Growth cones (boxed regions) at the tips of growing axons and dendrites have a protrusive, actin-rich, peripheral domain (red) that pushes the growth cone's membrane peripherally. A transition zone separates the peripheral domain from the microtubule rich central domain (light green). Within the central domain, mitochondria (dark green) are highly dynamic, often undergoing fission and fusion events that constantly change the organization of the mitochondrial network in active growth cones. C. Mitochondrial fission and fusion events are regulated by distinct classes of proteins. Mfn1 & 2 and Oap1 regulate mitochondrial outer and inner membrane fusion respectively, whereas D. DRP-1 is a major factor in mediating mitochondrial fission. Additional proteins, like Miro and syntaphilin, among others, regulate mitochondrial transport and docking respectively.
Mitochondrial Dynamics
Mitochondria are heterogeneous organelles, differing in subcellular distribution, membrane potential (Δψm), and metabolic activity that act individually or in dynamic networks regulated by fission, fusion, transport, docking, biogenesis, and mitophagy (Figure 3) [88-90]. These dynamics are critical to regulating mitochondrial bioenergetics, network organization, and distribution in different neuronal compartments [91-93]. Specifically, mitochondrial fission, fusion, and transport proteins play critical roles in maintaining mitochondrial fidelity and cellular health. Among other adaptor and regulatory proteins, the mitofusins, Mfn-1 and -2 [94], and optic atrophy 1 (OAP-1) [95] cooperate to regulate outer and inner mitochondrial membrane fusion respectively, dynamin related protein (DRP-1) mediates fission [72], and Miro [70] and syntaphilin [96] regulate mitochondrial transport and docking respectively (Figure 2C-2D). Mutations in these proteins can lead to mitochondrial network dysfunction and eventually poor cellular and organism health [97]. In the CNS, dysfunctional mitochondrial fission, fusion, and transport dynamics are broadly linked to impaired neuroplasticity and neuronal degeneration in Alzheimer's disease [98], Huntington's disease [99], Parkinson's disease [100], ALS [101,102], psychiatric disorders [103], and stroke [104] among others well reviewed [97].
Figure 3.
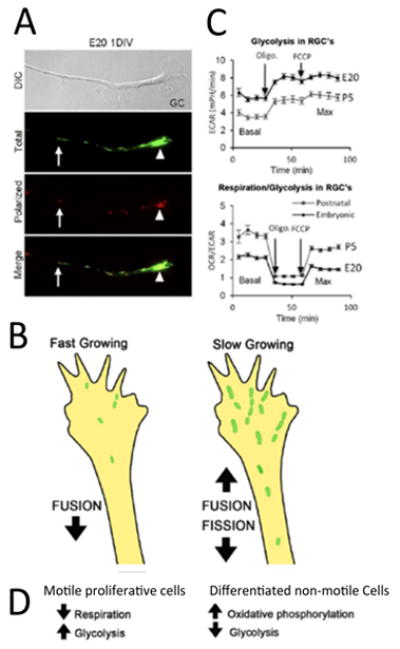
In nascent RGC axons, mitochondrial organization and bioenergetics correlate with axon growth rate. A. DIC image of a distal RGC axon and growth cone. JC-1 monomer emission at 530 nm (green) reveals total mitochondria and JC-1 J-aggregate emission at 590 nm (red) reveals the high potential, polarized, regions within mitochondria, demonstrating heterogeneity within the mitochondrial network. Mitochondria are detected as individuals (arrows) and as complexes (arrowheads) that differ in their degree of polarization. B. Glycolysis is higher in fast growing embryonic RGCs whereas the basal and maximal OCR (oxygen consumption rate)/ECAR (extracellular acidification rate) ratios are greater in P5 RGCs, indicating a greater reliance on oxidative phosphorylation in slow growing RGC axons [43]. C. Schematic showing that fast growing RGCs are associated with small active mitochondria in the growth cone compared to slower growing postnatal RGC axons, which harbor more, longer mitochondria likely due to differences in mitochondrial fission and fusion dynamics. D. Data from RGCs agree with data from motile, proliferative cells, which rely more on glycolysis, whereas differentiated, non-motile cells, rely primarily on oxidative phosphorylation.
In the visual system, mitochondrial dysfunction is also implicated in both traumatic and disease related disorders [105], including RGC axon degeneration after trauma [106,107], glaucoma [102], Charcot-Marie tooth disease (CMT) [108,109], and autosomal dominant optic atrophy (ADOA) [110,111]. Interestingly, these disorders are linked either primarily or secondarily to proteins regulating mitochondrial fission and fusion. For example, mutations in the fusion proteins Mfn-2 or OPA-1 are considered causal to axon loss in CMT type 2A [112] and progressive RGC axon loss in ADOA [111] respectively. Mitochondrial fission abnormalities are also implicated in eye disorders related to Parkinson's and Alzheimer's disease [97], and abnormal brain development [109,113-117]. Moreover, dominant negative mutations in Drp-1 cause lethality in addition to the rapid loss of RGC axons [118]. In glaucomatous eyes, ischemia due to increased ocular pressure can increase OPA-1 release from the inner mitochondrial membranes, leading to increased mitochondrial fission and eventual RGC axon degeneration [119]. Neurodegeneration in these diseases suggests RGC survival and function depend on mitochondrial fission/fusion dynamics, not only to meet the high energy demands in highly polarized cells like neurons [120], but also (as discussed below) to regulate mitochondrial fission/fusion-dependent signaling necessary to regulate RGC axon regeneration.
Mitochondrial Dynamics are Intrinsically Regulated in RGCs
In non-neuronal cells, mitochondrial morphology, biogenesis, distribution, and signaling change with the state of the cell [121-123]. However, little is known about how mitochondrial fission/fusion dynamics function in the neuronal growth cone. RGCs' intrinsic ability to rapidly regenerate axons declines progressively in postnatal RGCs shortly after birth compared to embryonic RGCs [2]. In culture, this decline in axon growth ability correlates with changes in mitochondrial fission/fusion dynamics; fewer, smaller mitochondria in the distal axon and growth cone support rapid axon regeneration [43], whereas longer more fused mitochondria do not (Figure 3B). For example, RGCs with enhanced axon growth potential, such as embryonic RGCs or RGCs overexpressing the axon growth enhancer KLF6, have smaller, more dynamic mitochondria. Moreover, chronically inhibiting the fission protein DRP-1 prior to axon initiation, which increases mitochondrial fusion (Figure 4), significantly reduces mitochondrial number and size in RGCs axons and growth cones without altering initial axon growth rates. In contrast, slow growing postnatal RGC axons harbor longer mitochondria in their distal axons and growth cones [43], which are less mobile, consistent with studies in cultured peripheral neurons [124] and the localization of immobile mitochondria to non-motile regions like branch points [125]. Whether similar differences in mitochondrial fission/fusion dynamics are specific to RGCs or applicable to regenerating axons in general remains to be determined.
Figure 4.
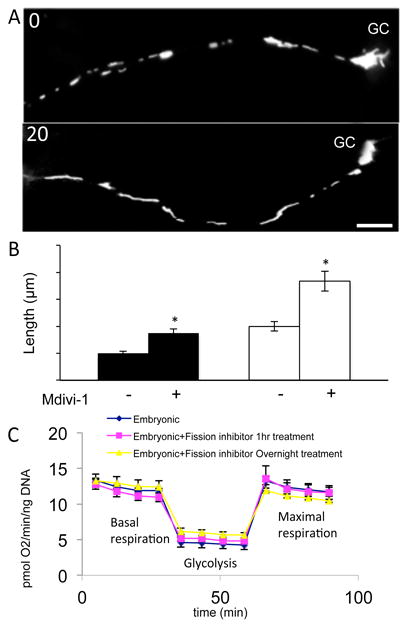
Inhibiting DRP-1 with Mdivi-1 induces long reticulated mitochondria within minutes without altering mitochondrial ATP production. A. Cultured RGCs labeled with mitotracker, before and 20 minutes after Mdivi-1 (20 μm). B. After Mdivi-1, mitochondrial length doubles in both the faster growing embryonic and the slow growing RGC axons. C. Neither acute nor chronic Mdivi-1 alters basal or maximal respiration or glycolysis [43]. Bar is 10 μm.
Mitochondrial Bioenergetics and Axon Growth
Mitochondrial fission/fusion dynamics regulate mitochondrial bioenergetics [126,127], which appears to play an important role in pro-growth signaling. Consistent with smaller, less dense mitochondria in fast growing axons, embryonic RGCs appear to rely less on mitochondrial oxidative phosphorylation and more on cytoplasmic glycolysis compared to postnatal RGCs, which rely primarily on oxidative phosphorylation (Figure 3C) [43]. This is consistent with the low oxygen environment during prenatal development and the onset of respiration in postnatal development [128]. Thus, in contrast to the conventional roles prescribed to growth cone mitochondria [63], these data support the hypothesis that increased oxidative phosphorylation may suppress axon regeneration and suggest a switch from primarily oxidative phosphorylation to glycolysis, known as the Warburg effect (Figure 3D) [129], may be necessary to enhance postnatal axon regeneration. A switch from oxidative phosphorylation to glycolysis, or at least an increase in glycolytic metabolites from neighboring cells [130], is necessary for cancer metastasis [131] and proper retinal progenitor [132] and stem cell [133] proliferation and biosynthesis, consistent with the hypothesis that pro-growth signaling pathways are supported by glycolysis [134]. Thus, postnatal RGCs and other mature CNS neurons may be unable to meet the unique metabolic demands required to support axon regeneration [100,135]. In addition, mitochondrial bioenergetics also regulate the molecular relationships between mitochondria and both cytoskeletal [136] and cytoplasmic proteins [137]. Thus, changes in mitochondrial fission/fusion dynamics and mitochondrial bioenergetics, and the immobilization of mature actively respiring mitochondria may help explain the decline in postnatal RGC axon growth ability and the distinct differences in embryonic and postnatal RGC axon growth ability. Unquestionably, the evolutionary development of highly polarized neurons that make up the nervous system depended upon mitochondrial activities. However, perhaps mature mitochondria are more suited to support neuronal maintenance than active axon growth.
The temporal and spatial regulation of growth cone motility is fundamental to the patterning of the nervous system [138,139], neuroplasticity in learning and memory [140], and regeneration [141]. Though many of the cytoskeletal mechanisms underlying growth cone motility [85,142] and growth cone protrusive initiations [143,144] have been described, the precise control over the level and the location of lamellar and filopodial protrusion in the growth cone is unclear. Interestingly, increasing mitochondrial fusion by acutely inhibiting DRP-1 pharmacologically (Figure 4A and 4B) reversibly suppresses both axon growth rate and lamellar protrusion but not filopodial protrusion (Figure 5A and 5B), independent of ATP production (Figure 4C), suggesting that mitochondrial fission/fusion dynamics play a central role in controlling growth cone motility. In addition to filopodial signaling [145], lamellae play important sensory roles, mediating neurotrophic factor [87] and second messenger signaling feedback loops [146] that can locally amplify lamellar protrusions to regulate the direction and the rate of growth. Thus, these results also suggest that multiple different cues could act on a single control point regulating mitochondrial fission/fusion dynamics to, in turn; regulate axon growth rate and growth cone motility.
Figure 5.
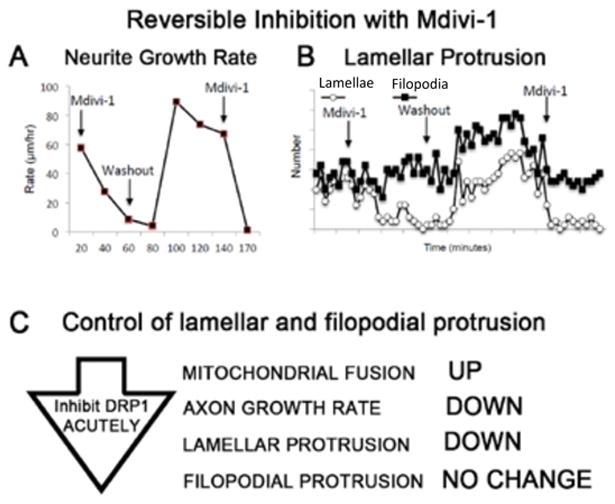
The DRP-1 fission protein inhibitor, Mdivi-1, reversibly reduces neurite growth rate and lamellar, but not filopodial protrusion. A. Neurite growth rate and B. lamellar protrusion (white circles) were reversibly and repeatedly inhibited by Mdivi-1. Filopodial protrusion frequency (black squares) was unchanged with the initial Mdivi-1 perfusion, but increased briefly after washout [43]. C. Summary of acute effects of inhibiting DRP-1 in already established axons.
Several lines of evidence suggest mitochondrial fission/fusion dynamics play an important role in neuronal architecture. Mitochondrial dynamics can regulate axon guidance responses; increasing mitochondrial fusion either genetically or pharmacologically alters growth cone turning responses both to inhibitory, CSPGs, and to permissive netrin and fibronectin guidance cues (Figure 6) [43]. Mitochondrial dynamics also appear to play a role in axogenesis [147], synapse formation [148], dendritic arbor morphology [149], and dendritic spine and filopodial protrusion [150]. Though the underlying molecular mechanisms of how mitochondrial fission/fusion dynamics regulate such diverse axonal and dendritic processes are unknown, some evidence points to the differential regulation of filamentous actin dynamics through the WASP-ARP2/3 complex. Inhibiting protrusion locally at the growth cone by extrinsic cues can redirect protrusion proximally to promote branching [151,152] at sites where mitochondria are localized [152,153]. At these sites, mitochondria appear to regulate the formation of F-actin patches [154] that can promote lamellar and filopodial nucleation by ARP2/3 [155,156], which also localizes to pre-branch sites [144] and can be regulated by mitochondrial associated WAVE1 [157]. Interestingly, inhibiting ARP2/3 in cultured cerebellar granule neurons can induce a similar phenotype as increasing mitochondrial fusion in cultured RGCs axons [43], reducing lamellar but not filopodial protrusion [158]. WAVE1 also regulates ARP2/3 in growth cones [157]. However, whether WAVE1 also associates with or is activated by mitochondria in growth cones is unknown. Finally, mitochondrial dynamics also appear to play a role in regulating microtubule dynamics [159] which can regulate neurite initiation [160] and extension in PC12 cells [161] and primary cultured neurons [162,163] possibly by locally effecting actin dynamics [164]. How mitochondrial dynamics differentially regulate lamellar and filopodial protrusions, which are regulated by distinct sets of proteins [165], within the neuronal soma, neurites, and growth cones remains an interesting unanswered question.
Figure 6.
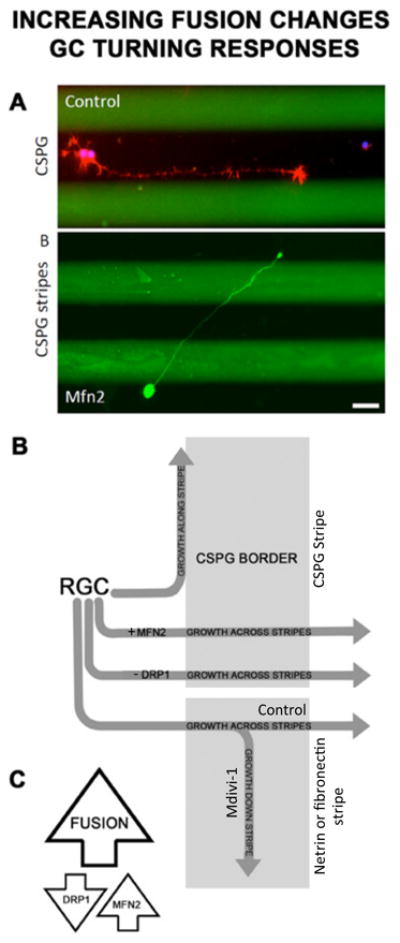
Increasing fusion chronically, either genetically or pharmacologically, alters RGC growth cone responses to both inhibitory and to permissive guidance cues. A. RGCs normally turn and fail to cross chondroitin sulfate proteoglycan (CSPG) borders (green). B. Overexpressing Mfn2 or treating RGCs with Mdivi-1 (not shown) prior to axon initiation permits RGC growth cones to cross CSPG stripes without altering neurite growth rate (not shown). B. Schematic of increasing fusion on RGC axon decision-making. Untreated RGCs, turn and grow along CSPG stripes whereas Mfn2 overexpressing or DRP-1 inhibited RGC axons cross CSPG stripes. Inhibiting DRP-1 with Mdivi-1 also alters RGC responses to permissive cues netrin-1 and fibronectin by decreasing crossing from netrin-1 and fibronectin onto laminin [43]. Bar is 10 μm. C. Summary: Increasing fusion by either inhibiting DRP-1 mediated fission or increasing fusion by overexpressing Mfn2 alters growth cone decision making.
Conclusion
The development of therapeutics aimed to enhance RGC axon regeneration as well as other CNS neuronal population is a persistent challenge due to the number of intrinsic and extrinsic factors known to suppress axon regeneration in the injured CNS. Numerous lines of evidence suggest many of these factors share a reciprocal relationship with mitochondrial dynamics and functions. Thus, understanding how mitochondrial dynamics integrate and modulate multiple intrinsic and extrinsic signaling pathways to regulate axon growth and guidance as well as how mitochondrial dynamics and bioenergetics function in the growth cone may reveal novel therapeutic strategies for treating neurodegenerative diseases and for enhancing regeneration in the injured CNS.
Acknowledgments
We gratefully acknowledge funding from the National Institutes of Health Core Grant for Vision Research P30 EY008098, the Eye and Ear Foundation of Pittsburgh, PA, the Department of Ophthalmology at the University of Pittsburgh (MBS, start-up funds) as well as an unrestricted Grant to the University of Pittsburgh from Research to Prevent Blindness, Inc. New York, NY. We thank Alex Kreymerman for constructive comments.
References
- 1.Goldberg JL, Barres BA. The relationship between neuronal survival and regeneration. Annu Rev Neurosci. 2000;23:579–612. doi: 10.1146/annurev.neuro.23.1.579. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg JL, Klassen MP, Hua Y, Barres BA. Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science. 2002;296:1860–1864. doi: 10.1126/science.1068428. [DOI] [PubMed] [Google Scholar]
- 3.Blackmore M, Letourneau PC. Changes within maturing neurons limit axonal regeneration in the developing spinal cord. J Neurobiol. 2006;66:348–360. doi: 10.1002/neu.20224. [DOI] [PubMed] [Google Scholar]
- 4.Chen DF, Jhaveri S, Schneider GE. Intrinsic changes in developing retinal neurons result in regenerative failure of their axons. Proc Natl Acad Sci U S A. 1995;92:7287–7291. doi: 10.1073/pnas.92.16.7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dusart I, Airaksinen MS, Sotelo C. Purkinje cell survival and axonal regeneration are age dependent: an in vitro study. J Neurosci. 1997;17:3710–3726. doi: 10.1523/JNEUROSCI.17-10-03710.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitch MT, Silver J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp Neurol. 2008;209:294–301. doi: 10.1016/j.expneurol.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nash M, Pribiag H, Fournier AE, Jacobson C. Central nervous system regeneration inhibitors and their intracellular substrates. Mol Neurobiol. 2009;40:224–235. doi: 10.1007/s12035-009-8083-y. [DOI] [PubMed] [Google Scholar]
- 8.Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cafferty WB, Duffy P, Huebner E, Strittmatter SM. MAG and OMgp synergize with Nogo-A to restrict axonal growth and neurological recovery after spinal cord trauma. J Neurosci. 2010;30:6825–6837. doi: 10.1523/JNEUROSCI.6239-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou LJ, Ren WJ, Zhong Y, Yang T, Wei XH, et al. Limited BDNF contributes to the failure of injury to skin afferents to produce a neuropathic pain condition. Pain. 2010;148:148–157. doi: 10.1016/j.pain.2009.10.032. [DOI] [PubMed] [Google Scholar]
- 11.Gordon T, Gordon K. Nerve regeneration in the peripheral nervous system versus the central nervous system and the relevance to speech and hearing after nerve injuries. J Commun Disord. 2010;43:274–285. doi: 10.1016/j.jcomdis.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Mansour-Robaey S, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc Natl Acad Sci U S A. 1994;91:1632–1636. doi: 10.1073/pnas.91.5.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JK, Geoffroy CG, Chan AF, Tolentino KE, Crawford MJ, et al. Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice. Neuron. 2010;66:663–670. doi: 10.1016/j.neuron.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giger RJ, Hollis ER, 2nd, Tuszynski MH. Guidance molecules in axon regeneration. Cold Spring Harb Perspect Biol. 2010;2:a001867. doi: 10.1101/cshperspect.a001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandvig A, Berry M, Barrett LB, Butt A, Logan A. Myelin-, reactive glia-, and scar-derived CNS axon growth inhibitors: expression, receptor signaling, and correlation with axon regeneration. Glia. 2004;46:225–251. doi: 10.1002/glia.10315. [DOI] [PubMed] [Google Scholar]
- 16.Buriani A, Savage MJ, Burmeister DW, Goldberg DJ. Early changes in nuclear proteins following axotomy. J Neurochem. 1990;55:1817–1820. doi: 10.1111/j.1471-4159.1990.tb04975.x. [DOI] [PubMed] [Google Scholar]
- 17.Caudy M, Bentley D. Pioneer growth cone steering along a series of neuronal and non-neuronal cues of different affinities. J Neurosci. 1986;6:1781–1795. doi: 10.1523/JNEUROSCI.06-06-01781.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Domeniconi M, Filbin MT. Overcoming inhibitors in myelin to promote axonal regeneration. J Neurol Sci. 2005;233:43–47. doi: 10.1016/j.jns.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 19.Ramer MS, Duraisingam I, Priestley JV, McMahon SB. Two-tiered inhibition of axon regeneration at the dorsal root entry zone. J Neurosci. 2001;21:2651–2660. doi: 10.1523/JNEUROSCI.21-08-02651.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldberg JL, Espinosa JS, Xu Y, Davidson N, Kovacs GT, et al. Retinal ganglion cells do not extend axons by default: promotion by neurotrophic signaling and electrical activity. Neuron. 2002;33:689–702. doi: 10.1016/s0896-6273(02)00602-5. [DOI] [PubMed] [Google Scholar]
- 21.Gordon T. The physiology of neural injury and regeneration: The role of neurotrophic factors. J Commun Disord. 2010;43:265–273. doi: 10.1016/j.jcomdis.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Lingor P, Tönges L, Pieper N, Bermel C, Barski E, et al. ROCK inhibition and CNTF interact on intrinsic signalling pathways and differentially regulate survival and regeneration in retinal ganglion cells. Brain. 2008;131:250–263. doi: 10.1093/brain/awm284. [DOI] [PubMed] [Google Scholar]
- 23.Muller A, Hauk TG, Leibinger M, Marienfeld R, Fischer D. Exogenous CNTF stimulates axon regeneration of retinal ganglion cells partially via endogenous CNTF. Mol Cell Neurosci. 2009;41:233–246. doi: 10.1016/j.mcn.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Leibinger M, Müller A, Gobrecht P, Diekmann H, Andreadaki A, et al. Interleukin-6 contributes to CNS axon regeneration upon inflammatory stimulation. Cell Death Dis. 2013;4:e609. doi: 10.1038/cddis.2013.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park KK, Liu K, Hu Y, Smith PD, Wang C, et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Lima S, Koriyama Y, Kurimoto T, Oliveira JT, Yin Y, et al. Full-length axon regeneration in the adult mouse optic nerve and partial recovery of simple visual behaviors. Proc Nat Acad Sci U S A. 2012;109:9149–9154. doi: 10.1073/pnas.1119449109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun F, Park KK, Belin S, Wang D, Lu T, et al. Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature. 2011;480:372–375. doi: 10.1038/nature10594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore DL, Blackmore MG, Hu Y, Kaestner KH, Bixby JL, et al. KLF family members regulate intrinsic axon regeneration ability. Science. 2009;326:298–301. doi: 10.1126/science.1175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blackmore MG, Moore DL, Smith RP, Goldberg JL, Bixby JL, et al. High content screening of cortical neurons identifies novel regulators of axon growth. Mol Cell Neurosci. 2010;44:43–54. doi: 10.1016/j.mcn.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore DL, Goldberg JL. Multiple transcription factor families regulate axon growth and regeneration. Dev Neurobiol. 2011;71:1186–1211. doi: 10.1002/dneu.20934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parisiadou L, Xie C, Cho HJ, Lin X, Gu XL, et al. Phosphorylation of ezrin/radixin/moesin proteins by LRRK2 promotes the rearrangement of actin cytoskeleton in neuronal morphogenesis. J Neurosci. 2009;29:13971–13980. doi: 10.1523/JNEUROSCI.3799-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldenthal MJ, Marin-Garcia J. Mitochondrial signaling pathways: a receiver/integrator organelle. Mol Cell Biochem. 2004;262:1–16. doi: 10.1023/b:mcbi.0000038228.85494.3b. [DOI] [PubMed] [Google Scholar]
- 33.Newmeyer DD, Ferguson-Miller S. Mitochondria: releasing power for life and unleashing the machineries of death. Cell. 2003;112:481–490. doi: 10.1016/s0092-8674(03)00116-8. [DOI] [PubMed] [Google Scholar]
- 34.Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007;8:870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- 35.Park KK, Liu K, Hu Y, Kanter JL, He Z. PTEN/mTOR and axon regeneration. Exp Neurol. 2010;223:45–50. doi: 10.1016/j.expneurol.2009.12.032. [DOI] [PubMed] [Google Scholar]
- 36.Valente EM, Salvi S, Ialongo T, Marongiu R, Elia AE, et al. PINK1 mutations are associated with sporadic early-onset parkinsonism. Ann Neurol. 2004;56:336–341. doi: 10.1002/ana.20256. [DOI] [PubMed] [Google Scholar]
- 37.Thomas KJ, Cookson MR. The role of PTEN-induced kinase 1 in mitochondrial dysfunction and dynamics. Int J Biochem Cell Biol. 2009;41:2025–2035. doi: 10.1016/j.biocel.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Desai BN, Myers BR, Schreiber SL. FKBP12-rapamycin-associated protein associates with mitochondria and senses osmotic stress via mitochondrial dysfunction. Proc Natl Acad Sci U S A. 2002;99:4319–4324. doi: 10.1073/pnas.261702698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rieker C, Engblom D, Kreiner G, Domanskyi A, Schober A, et al. Nucleolar disruption in dopaminergic neurons leads to oxidative damage and parkinsonism through repression of mammalian target of rapamycin signaling. J Neurosci. 2011;31:453–460. doi: 10.1523/JNEUROSCI.0590-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weihofen A, Thomas KJ, Ostaszewski BL, Cookson MR, Selkoe DJ. Pink1 forms a multiprotein complex with Miro and Milton, linking Pink1 function to mitochondrial trafficking. Biochemistry. 2009;48:2045–2052. doi: 10.1021/bi8019178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glater EE, Megeath LJ, Stowers RS, Schwarz TL. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J Cell Biol. 2006;173:545–557. doi: 10.1083/jcb.200601067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stowers RS, Megeath LJ, Gorska-Andrzejak J, Meinertzhagen IA, Schwarz TL. Axonal transport of mitochondria to synapses depends on milton, a novel Drosophila protein. Neuron. 2002;36:1063–1077. doi: 10.1016/s0896-6273(02)01094-2. [DOI] [PubMed] [Google Scholar]
- 43.Steketee MB, Moysidis SN, Weinstein JE, Kreymerman A, Silva JP, et al. Mitochondrial dynamics regulate growth cone motility, guidance, and neurite growth rate in perinatal retinal ganglion cells in vitro. Invest Ophthalmol Vis Sci. 2012;53:7402–7411. doi: 10.1167/iovs.12-10298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sinclair LV, Finlay D, Feijoo C, Cornish GH, Gray A, et al. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol. 2008;9:513–521. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang R, Han M, Zheng B, Li YJ, Shu YN, et al. Kruppel-like factor 4 interacts with p300 to activate mitofusin 2 gene expression induced by all-trans retinoic acid in VSMCs. Acta Pharmacol Sin. 2010;31:1293–1302. doi: 10.1038/aps.2010.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benzeno S, Narla G, Allina J, Cheng GZ, Reeves HL, et al. Cyclin-dependent kinase inhibition by the KLF6 tumor suppressor protein through interaction with cyclin D1. Cancer Res. 2004;64:3885–3891. doi: 10.1158/0008-5472.CAN-03-2818. [DOI] [PubMed] [Google Scholar]
- 47.Tahara E, Kadara H, Lacroix L, Lotan D, Lotan R. Activation of protein kinase C by phorbol 12-myristate 13-acetate suppresses the growth of lung cancer cells through KLF6 induction. Cancer Biol Ther. 2009;8:801–807. doi: 10.4161/cbt.8.9.8186. [DOI] [PubMed] [Google Scholar]
- 48.Narla G, Kremer-Tal S, Matsumoto N, Zhao X, Yao S, et al. In vivo regulation of p21 by the Kruppel-like factor 6 tumor-suppressor gene in mouse liver and human hepatocellular carcinoma. Oncogene. 2007;26:4428–4434. doi: 10.1038/sj.onc.1210223. [DOI] [PubMed] [Google Scholar]
- 49.Tanaka H, Yamashita T, Yachi K, Fujiwara T, Yoshikawa H, et al. Cytoplasmic p21(Cip1/WAF1) enhances axonal regeneration and functional recovery after spinal cord injury in rats. Neuroscience. 2004;127:155–164. doi: 10.1016/j.neuroscience.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 50.Wu X, Carr HS, Dan I, Ruvolo PP, Frost JA. p21 activated kinase 5 activates Raf-1 and targets it to mitochondria. J Cell Biochem. 2008;105:167–175. doi: 10.1002/jcb.21809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cotteret S, Jaffer ZM, Beeser A, Chernoff J. p21-Activated kinase 5 (Pak5) localizes to mitochondria and inhibits apoptosis by phosphorylating BAD. Mol Cell Biol. 2003;23:5526–5539. doi: 10.1128/MCB.23.16.5526-5539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Markham A, Cameron I, Franklin P, Spedding M. BDNF increases rat brain mitochondrial respiratory coupling at complex I, but not complex II. Eur J Neurosci. 2004;20:1189–1196. doi: 10.1111/j.1460-9568.2004.03578.x. [DOI] [PubMed] [Google Scholar]
- 53.Aguiar AS, Jr, Tuon T, Pinho CA, Silva LA, Andreazza AC, et al. Intense exercise induces mitochondrial dysfunction in mice brain. Neurochem Res. 2008;33:51–58. doi: 10.1007/s11064-007-9406-x. [DOI] [PubMed] [Google Scholar]
- 54.Wiedemann FR, Siemen D, Mawrin C, Horn TF, Dietzmann K. The neurotrophin receptor TrkB is colocalized to mitochondrial membranes. Int J Biochem Cell Biol. 2006;38:610–620. doi: 10.1016/j.biocel.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 55.Avwenagha O, Bird MM, Lieberman AR, Yan Q, Campbell G. Patterns of expression of brain-derived neurotrophic factor and tyrosine kinase B mRNAs and distribution and ultrastructural localization of their proteins in the visual pathway of the adult rat. Neuroscience. 2006;140:913–928. doi: 10.1016/j.neuroscience.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 56.Chada SR, Hollenbeck PJ. Mitochondrial movement and positioning in axons: the role of growth factor signaling. J Exp Biol. 2003;206:1985–1992. doi: 10.1242/jeb.00263. [DOI] [PubMed] [Google Scholar]
- 57.Lee CW, Peng HB. Mitochondrial clustering at the vertebrate neuromuscular junction during presynaptic differentiation. J Neurobiol. 2006;66:522–536. doi: 10.1002/neu.20245. [DOI] [PubMed] [Google Scholar]
- 58.Chada SR, Hollenbeck PJ. Nerve growth factor signaling regulates motility and docking of axonal mitochondria. Curr Biol. 2004;14:1272–1276. doi: 10.1016/j.cub.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 59.Verburg J, Hollenbeck PJ. Mitochondrial membrane potential in axons increases with local nerve growth factor or semaphorin signaling. J Neurosci. 2008;28:8306–8315. doi: 10.1523/JNEUROSCI.2614-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fan J, Mansfield SG, Redmond T, Gordon-Weeks PR, Raper JA. The organization of F-actin and microtubules in growth cones exposed to a brain-derived collapsing factor. J Cell Biol. 1993;121:867–878. doi: 10.1083/jcb.121.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mann F, Rougon G. Mechanisms of axon guidance: membrane dynamics and axonal transport in semaphorin signalling. J Neurochem. 2007;102:316–323. doi: 10.1111/j.1471-4159.2007.04578.x. [DOI] [PubMed] [Google Scholar]
- 62.Hu WH, Hausmann ON, Yan MS, Walters WM, Wong PK, et al. Identification and characterization of a novel Nogo-interacting mitochondrial protein (NIMP) J Neurochem. 2002;81:36–45. doi: 10.1046/j.1471-4159.2002.00788.x. [DOI] [PubMed] [Google Scholar]
- 63.Morris RL, Hollenbeck PJ. The regulation of bidirectional mitochondrial transport is coordinated with axonal outgrowth. J Cell Sci. 1993;104:917–927. doi: 10.1242/jcs.104.3.917. [DOI] [PubMed] [Google Scholar]
- 64.Gordon-Weeks PR. Neuronal growth cones. Cambridge University Press; Cambridge, UK; New York: 2000. pp. xii–260. [Google Scholar]
- 65.Zhang XF, Forscher P. Rac1 modulates stimulus-evoked Ca(2+) release in neuronal growth cones via parallel effects on microtubule/endoplasmic reticulum dynamics and reactive oxygen species production. Mol Biol Cell. 2009;20:3700–3712. doi: 10.1091/mbc.E08-07-0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tojima T, Hines JH, Henley JR, Kamiguchi H. Second messengers and membrane trafficking direct and organize growth cone steering. Nat Rev Neurosci. 2011;12:191–203. doi: 10.1038/nrn2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ernst AF, Gallo G, Letourneau PC, McLoon SC. Stabilization of growing retinal axons by the combined signaling of nitric oxide and brain-derived neurotrophic factor. J Neurosci. 2000;20:1458–1469. doi: 10.1523/JNEUROSCI.20-04-01458.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.MacAskill AF, Kittler JT. Control of mitochondrial transport and localization in neurons. Trends Cell Biol. 2010;20:102–112. doi: 10.1016/j.tcb.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 69.Rui Y, Tiwari P, Xie Z, Zheng JQ. Acute impairment of mitochondrial trafficking by beta-amyloid peptides in hippocampal neurons. J Neurosci. 2006;26:10480–10487. doi: 10.1523/JNEUROSCI.3231-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Macaskill AF, Rinholm JE, Twelvetrees AE, Arancibia-Carcamo IL, Muir J, et al. Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron. 2009;61:541–555. doi: 10.1016/j.neuron.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Szabadkai G, Simoni AM, Chami M, Wieckowski MR, Youle RJ, et al. Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Mol Cell. 2004;16:59–68. doi: 10.1016/j.molcel.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 72.Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, et al. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol. 2009;11:958–966. doi: 10.1038/ncb1907. [DOI] [PubMed] [Google Scholar]
- 73.Miriyala S, Holley AK, St Clair DK. Mitochondrial superoxide dismutase--signals of distinction. Anticancer Agents Med Chem. 2011;11:181–190. doi: 10.2174/187152011795255920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marks JD, Boriboun C, Wang J. Mitochondrial nitric oxide mediates decreased vulnerability of hippocampal neurons from immature animals to NMDA. J Neurosci. 2005;25:6561–6575. doi: 10.1523/JNEUROSCI.1450-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Twig G, Hyde B, Shirihai OS. Mitochondrial fusion, fission and autophagy as a quality control axis: the bioenergetic view. Biochim Biophys Acta. 2008;1777:1092–1097. doi: 10.1016/j.bbabio.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Parone PA, Da Cruz S, Tondera D, Mattenberger Y, James DI, et al. Preventing mitochondrial fission impairs mitochondrial function and leads to loss of mitochondrial DNA. PLoS One. 2008;3:e3257. doi: 10.1371/journal.pone.0003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nicol X, Hong KP, Spitzer NC. Spatial and temporal second messenger codes for growth cone turning. Proc Natl Acad Sci U S A. 2011;108:13776–13781. doi: 10.1073/pnas.1100247108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mai J, Fok L, Gao H, Zhang X, Poo MM. Axon initiation and growth cone turning on bound protein gradients. J Neurosci. 2009;29:7450–7458. doi: 10.1523/JNEUROSCI.1121-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goldberg DJ, Burmeister DW. Stages in axon formation: observations of growth of Aplysia axons in culture using video-enhanced contrast-differential interference contrast microscopy. J Cell Biol. 1986;103:1921–1931. doi: 10.1083/jcb.103.5.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oakley RA, Tosney KW. Contact-mediated mechanisms of motor axon segmentation. J Neurosci. 1993;13:3773–3792. doi: 10.1523/JNEUROSCI.13-09-03773.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Steketee MB, Tosney KW. Three functionally distinct adhesions in filopodia: shaft adhesions control lamellar extension. J Neurosci. 2002;22:8071–8083. doi: 10.1523/JNEUROSCI.22-18-08071.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kolodkin AL, Tessier-Lavigne M. Mechanisms and molecules of neuronal wiring: a primer. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yu TW, Bargmann CI. Dynamic regulation of axon guidance. Nat Neurosci. 2001;4:1169–1176. doi: 10.1038/nn748. [DOI] [PubMed] [Google Scholar]
- 85.Dent EW, Gertler FB. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron. 2003;40:209–227. doi: 10.1016/s0896-6273(03)00633-0. [DOI] [PubMed] [Google Scholar]
- 86.Steketee MB, Goldberg JL. Signaling endosomes and growth cone motility in axon regeneration. Int Rev Neurobiol. 2012;106:35–73. doi: 10.1016/B978-0-12-407178-0.00003-X. [DOI] [PubMed] [Google Scholar]
- 87.Zhou P, Porcionatto M, Pilapil M, Chen Y, Choi Y, et al. Polarized signaling endosomes coordinate BDNF-induced chemotaxis of cerebellar precursors. Neuron. 2007;55:53–68. doi: 10.1016/j.neuron.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Youle RJ, Karbowski M. Mitochondrial fission in apoptosis. Nat Rev Mol Cell Biol. 2005;6:657–663. doi: 10.1038/nrm1697. [DOI] [PubMed] [Google Scholar]
- 89.Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1alpha. Cardiovasc Res. 2008;79:208–217. doi: 10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]
- 90.Chan DC. Dissecting mitochondrial fusion. Dev Cell. 2006;11:592–594. doi: 10.1016/j.devcel.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 91.Wakabayashi J, Zhang Z, Wakabayashi N, Tamura Y, Fukaya M, et al. The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. J Cell Biol. 2009;186:805–816. doi: 10.1083/jcb.200903065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Detmer SA, Chan DC. Complementation between mouse Mfn1 and Mfn2 protects mitochondrial fusion defects caused by CMT2A disease mutations. J Cell Biol. 2007;176:405–414. doi: 10.1083/jcb.200611080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alavi MV, Bette S, Schimpf S, Schuettauf F, Schraermeyer U, et al. A splice site mutation in the murine Opa1 gene features pathology of autosomal dominant optic atrophy. Brain. 2007;130:1029–1042. doi: 10.1093/brain/awm005. [DOI] [PubMed] [Google Scholar]
- 94.Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, et al. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cipolat S, Martins de Brito O, Dal Zilio B, Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci U S A. 2004;101:15927–15932. doi: 10.1073/pnas.0407043101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kang JS, Tian JH, Pan PY, Zald P, Li C, et al. Docking of axonal mitochondria by syntaphilin controls their mobility and affects short-term facilitation. Cell. 2008;132:137–148. doi: 10.1016/j.cell.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen H, Chan DC. Mitochondrial dynamics--fusion, fission, movement, and mitophagy--in neurodegenerative diseases. Hum Mol Genet. 2009;18:R169–R176. doi: 10.1093/hmg/ddp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moreira PI, Cardoso SM, Santos MS, Oliveira CR. The key role of mitochondria in Alzheimer's disease. J Alzheimers Dis. 2006;9:101–110. doi: 10.3233/jad-2006-9202. [DOI] [PubMed] [Google Scholar]
- 99.Bossy-Wetzel E, Petrilli A, Knott AB. Mutant huntingtin and mitochondrial dysfunction. Trends Neurosci. 2008;31:609–616. doi: 10.1016/j.tins.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Henchcliffe C, Beal MF. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol. 2008;4:600–609. doi: 10.1038/ncpneuro0924. [DOI] [PubMed] [Google Scholar]
- 101.Magrane J, Hervias I, Henning MS, Damiano M, Kawamata H, et al. Mutant SOD1 in neuronal mitochondria causes toxicity and mitochondrial dynamics abnormalities. Hum Mol Genet. 2009;18:4552–4564. doi: 10.1093/hmg/ddp421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Osborne NN. Mitochondria: Their role in ganglion cell death and survival in primary open angle glaucoma. Exp Eye Res. 2010;90:750–757. doi: 10.1016/j.exer.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 103.Mattson MP, Gleichmann M, Cheng A. Mitochondria in neuroplasticity and neurological disorders. Neuron. 2008;60:748–766. doi: 10.1016/j.neuron.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jung JE, Kim GS, Chen H, Maier CM, Narasimhan P, et al. Reperfusion and neurovascular dysfunction in stroke: from basic mechanisms to potential strategies for neuroprotection. Mol Neurobiol. 2010;41:172–179. doi: 10.1007/s12035-010-8102-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yu-Wai-Man P, Griffiths PG, Chinnery PF. Mitochondrial optic neuropathies - disease mechanisms and therapeutic strategies. Prog Retin Eye Res. 2011;30:81–114. doi: 10.1016/j.preteyeres.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Carelli V, Ross-Cisneros FN, Sadun AA. Mitochondrial dysfunction as a cause of optic neuropathies. Prog Retin Eye Res. 2004;23:53–89. doi: 10.1016/j.preteyeres.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 107.Yu-Wai-Man P, Bailie M, Atawan A, Chinnery PF, Griffiths PG. Pattern of retinal ganglion cell loss in dominant optic atrophy due to OPA1 mutations. Eye (Lond) 2011;25:596–602. doi: 10.1038/eye.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zuchner S, Mersiyanova IV, Muglia M, Bissar-Tadmouri N, Rochelle J, et al. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat Genet. 2004;36:449–451. doi: 10.1038/ng1341. [DOI] [PubMed] [Google Scholar]
- 109.Cuesta A, Pedrola L, Sevilla T, García-Planells J, Chumillas MJ, et al. The gene encoding ganglioside-induced differentiation-associated protein 1 is mutated in axonal Charcot-Marie-Tooth type 4A disease. Nat Genet. 2002;30:22–25. doi: 10.1038/ng798. [DOI] [PubMed] [Google Scholar]
- 110.Alexander C, Votruba M, Pesch UE, Thiselton DL, Mayer S, et al. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet. 2000;26:211–215. doi: 10.1038/79944. [DOI] [PubMed] [Google Scholar]
- 111.Delettre C, Lenaers G, Griffoin JM, Gigarel N, Lorenzo C, et al. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet. 2000;26:207–210. doi: 10.1038/79936. [DOI] [PubMed] [Google Scholar]
- 112.Zuchner S, De Jonghe P, Jordanova A, Claeys KG, Guergueltcheva V, et al. Axonal neuropathy with optic atrophy is caused by mutations in mitofusin 2. Ann Neurol. 2006;59:276–281. doi: 10.1002/ana.20797. [DOI] [PubMed] [Google Scholar]
- 113.Waterham HR, Koster J, van Roermund CW, Mooyer PA, Wanders RJ, et al. A lethal defect of mitochondrial and peroxisomal fission. N Engl J Med. 2007;356:1736–1741. doi: 10.1056/NEJMoa064436. [DOI] [PubMed] [Google Scholar]
- 114.Deng H, Dodson MW, Huang H, Guo M. The Parkinson's disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc Natl Acad Sci U S A. 2008;105:14503–14508. doi: 10.1073/pnas.0803998105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, et al. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci U S A. 2008;105:1638–1643. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang Y, Ouyang Y, Yang L, Beal MF, McQuibban A, et al. Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc Natl Acad Sci U S A. 2008;105:7070–7075. doi: 10.1073/pnas.0711845105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cho DH, Nakamura T, Lipton SA. Mitochondrial dynamics in cell death and neurodegeneration. Cell Mol Life Sci. 2010;67:3435–3447. doi: 10.1007/s00018-010-0435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Buoni S, Zannolli R, Waterham H, Wanders R, Fois A. D-bifunctional protein deficiency associated with drug resistant infantile spasms. Brain Dev. 2007;29:51–54. doi: 10.1016/j.braindev.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 119.Ju WK, Kim KY, Lindsey JD, Angert M, Duong-Polk KX, et al. Intraocular pressure elevation induces mitochondrial fission and triggers OPA1 release in glaucomatous optic nerve. Invest Ophthalmol Vis Sci. 2008;49:4903–4911. doi: 10.1167/iovs.07-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Knott AB, Bossy-Wetzel E. Impairing the mitochondrial fission and fusion balance: a new mechanism of neurodegeneration. Ann N Y Acad Sci. 2008;1147:283–292. doi: 10.1196/annals.1427.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kondoh H, Lleonart ME, Nakashima Y, Yokode M, Tanaka M, et al. A high glycolytic flux supports the proliferative potential of murine embryonic stem cells. Antioxid Redox Signal. 2007;9:293–299. doi: 10.1089/ars.2006.1467. [DOI] [PubMed] [Google Scholar]
- 122.Prigione A, Adjaye J. Modulation of mitochondrial biogenesis and bioenergetic metabolism upon in vitro and in vivo differentiation of human ES and iPS cells. Int J Dev Biol. 2010;54:1729–1741. doi: 10.1387/ijdb.103198ap. [DOI] [PubMed] [Google Scholar]
- 123.Benard G, Bellance N, James D, Parrone P, Fernandez H, et al. Mitochondrial bioenergetics and structural network organization. J Cell Sci. 2007;120:838–848. doi: 10.1242/jcs.03381. [DOI] [PubMed] [Google Scholar]
- 124.Saxton WM, Hollenbeck PJ. The axonal transport of mitochondria. J Cell Sci. 2012;125:2095–2104. doi: 10.1242/jcs.053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Courchet J, Lewis TL, Jr, Lee S, Courchet V, Liou DY, et al. Terminal axon branching is regulated by the LKB1-NUAK1 kinase pathway via presynaptic mitochondrial capture. Cell. 2013;153:1510–1525. doi: 10.1016/j.cell.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Westermann B. Bioenergetic role of mitochondrial fusion and fission. Biochim Biophys Acta. 2012;1817:1833–1838. doi: 10.1016/j.bbabio.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 127.Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337:1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rust RS. Energy metabolism of developing brain. Curr Opin Neurol. 1994;7:160–165. doi: 10.1097/00019052-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 129.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- 130.Sotgia F, Whitaker-Menezes D, Martinez-Outschoorn UE, Flomenberg N, Birbe RC, et al. Mitochondrial metabolism in cancer metastasis: visualizing tumor cell mitochondria and the “reverse Warburg effect” in positive lymph node tissue. Cell Cycle. 2012;11:1445–1454. doi: 10.4161/cc.19841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Agathocleous M, Love NK, Randlett O, Harris JJ, Liu J, et al. Metabolic differentiation in the embryonic retina. Nat Cell Biol. 2012;14:859–864. doi: 10.1038/ncb2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Varum S, Rodrigues AS, Moura MB, Momcilovic O, Easley CA, 4th, et al. Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS One. 2011;6:e20914. doi: 10.1371/journal.pone.0020914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lopez-Lazaro M. The warburg effect: why and how do cancer cells activate glycolysis in the presence of oxygen? Anticancer Agents Med Chem. 2008;8:305–312. doi: 10.2174/187152008783961932. [DOI] [PubMed] [Google Scholar]
- 135.Diaz G, Setzu MD, Zucca A, Isola R, Diana A, et al. Subcellular heterogeneity of mitochondrial membrane potential: relationship with organelle distribution and intercellular contacts in normal, hypoxic and apoptotic cells. J Cell Sci. 1999;112:1077–1084. doi: 10.1242/jcs.112.7.1077. [DOI] [PubMed] [Google Scholar]
- 136.Wagner OI, Lifshitz J, Janmey PA, Linden M, McIntosh TK, et al. Mechanisms of mitochondria-neurofilament interactions. J Neurosci. 2003;23:9046–9058. doi: 10.1523/JNEUROSCI.23-27-09046.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jin SM, Lazarou M, Wang C, Kane LA, Narendra DP, et al. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J Cell Biol. 2010;191:933–942. doi: 10.1083/jcb.201008084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bentley D, Toroian-Raymond A. Disoriented pathfinding by pioneer neurone growth cones deprived of filopodia by cytochalasin treatment. Nature. 1986;323:712–715. doi: 10.1038/323712a0. [DOI] [PubMed] [Google Scholar]
- 139.Butler SJ, Tear G. Getting axons onto the right path: the role of transcription factors in axon guidance. Development. 2007;134:439–448. doi: 10.1242/dev.02762. [DOI] [PubMed] [Google Scholar]
- 140.Gutierrez H, Davies AM. Regulation of neural process growth, elaboration and structural plasticity by NF-κB. Trends Neurosci. 2011;34:316–325. doi: 10.1016/j.tins.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hur EM, Yang IH, Kim DH, Byun J, Saijilafu, et al. Engineering neuronal growth cones to promote axon regeneration over inhibitory molecules. Proc Natl Acad Sci U S A. 2011;108:5057–5062. doi: 10.1073/pnas.1011258108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jung H, O'Hare CM, Holt CE. Translational regulation in growth cones. Curr Opin Genet Dev. 2011;21:458–464. doi: 10.1016/j.gde.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Steketee M, Balazovich K, Tosney KW. Filopodial initiation and a novel filament-organizing center, the focal ring. Mol Biol Cell. 2001;12:2378–2395. doi: 10.1091/mbc.12.8.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Spillane M, Ketschek A, Jones SL, Korobova F, Marsick B, et al. The actin nucleating Arp2/3 complex contributes to the formation of axonal filopodia and branches through the regulation of actin patch precursors to filopodia. Dev Neurobiol. 2011;71:747–758. doi: 10.1002/dneu.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mattila PK, Lappalainen P. Filopodia: molecular architecture and cellular functions. Nat Rev Mol Cell Biol. 2008;9:446–454. doi: 10.1038/nrm2406. [DOI] [PubMed] [Google Scholar]
- 146.Cheng PL, Song AH, Wong YH, Wang S, Zhang X, et al. Self-amplifying autocrine actions of BDNF in axon development. Proc Natl Acad Sci U S A. 2011;108:18430–18435. doi: 10.1073/pnas.1115907108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mattson MP, Partin J. Evidence for mitochondrial control of neuronal polarity. J Neurosci Res. 1999;56:8–20. doi: 10.1002/(SICI)1097-4547(19990401)56:1<8::AID-JNR2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 148.Liu QA, Shio H. Mitochondrial morphogenesis, dendrite development, and synapse formation in cerebellum require both Bcl-w and the glutamate receptor delta2. PLoS Genet. 2008;4:e1000097. doi: 10.1371/journal.pgen.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chihara T, Luginbuhl D, Luo L. Cytoplasmic and mitochondrial protein translation in axonal and dendritic terminal arborization. Nat Neurosci. 2007;10:828–837. doi: 10.1038/nn1910. [DOI] [PubMed] [Google Scholar]
- 150.Sung JY, Engmann O, Teylan MA, Nairn AC, Greengard P, et al. WAVE1 controls neuronal activity-induced mitochondrial distribution in dendritic spines. Proc Natl Acad Sci U S A. 2008;105:3112–3116. doi: 10.1073/pnas.0712180105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Steketee MB, Tosney KW. Contact with isolated sclerotome cells steers sensory growth cones by altering distinct elements of extension. J Neurosci. 1999;19:3495–3506. doi: 10.1523/JNEUROSCI.19-09-03495.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Davenport RW, Thies E, Cohen ML. Neuronal growth cone collapse triggers lateral extensions along trailing axons. Nat Neurosci. 1999;2:254–259. doi: 10.1038/6360. [DOI] [PubMed] [Google Scholar]
- 153.Dedov VN, Armati PJ, Roufogalis BD. Three-dimensional organisation of mitochondrial clusters in regenerating dorsal root ganglion (DRG) neurons from neonatal rats: evidence for mobile mitochondrial pools. J Peripher Nerv Syst. 2000;5:3–10. doi: 10.1046/j.1529-8027.2000.00153.x. [DOI] [PubMed] [Google Scholar]
- 154.Ketschek A, Spillane M, Gallo G. Mechanism of NGF-induced formation of axonal filopodia: NGF turns up the volume, but the song remains the same? Commun Integr Biol. 2011;4:55–58. doi: 10.4161/cib.4.1.13689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rogers SL, Wiedemann U, Stuurman N, Vale RD. Molecular requirements for actin-based lamella formation in Drosophila S2 cells. J Cell Biol. 2003;162:1079–1088. doi: 10.1083/jcb.200303023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Korobova F, Svitkina T. Arp2/3 complex is important for filopodia formation, growth cone motility, and neuritogenesis in neuronal cells. Mol Biol Cell. 2008;19:1561–1574. doi: 10.1091/mbc.E07-09-0964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kim Y, Sung JY, Ceglia I, Lee KW, Ahn JH, et al. Phosphorylation of WAVE1 regulates actin polymerization and dendritic spine morphology. Nature. 2006;442:814–817. doi: 10.1038/nature04976. [DOI] [PubMed] [Google Scholar]
- 158.Tahirovic S, Hellal F, Neukirchen D, Hindges R, Garvalov BK, et al. Rac1 regulates neuronal polarization through the WAVE complex. J Neurosci. 2010;30:6930–6943. doi: 10.1523/JNEUROSCI.5395-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Barrientos A, Moraes CT. Titrating the effects of mitochondrial complex I impairment in the cell physiology. J Biol Chem. 1999;274:16188–16197. doi: 10.1074/jbc.274.23.16188. [DOI] [PubMed] [Google Scholar]
- 160.Mattson MP. Mitochondrial regulation of neuronal plasticity. Neurochem Res. 2007;32:707–715. doi: 10.1007/s11064-006-9170-3. [DOI] [PubMed] [Google Scholar]
- 161.Tomaselli B, Podhraski V, Heftberger V, Bock G, Baier-Bitterlich G. Purine nucleoside-mediated protection of chemical hypoxia-induced neuronal injuries involves p42/44 MAPK activation. Neurochem Int. 2005;46:513–521. doi: 10.1016/j.neuint.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 162.Bocklinger K, Tomaselli B, Heftberger V, Podhraski V, Bandtlow C, et al. Purine nucleosides support the neurite outgrowth of primary rat cerebellar granule cells after hypoxia. Eur J Cell Biol. 2004;83:51–54. doi: 10.1078/0171-9335-00362. [DOI] [PubMed] [Google Scholar]
- 163.Ren Y, Feng J. Rotenone selectively kills serotonergic neurons through a microtubule-dependent mechanism. J Neurochem. 2007;103:303–311. doi: 10.1111/j.1471-4159.2007.04741.x. [DOI] [PubMed] [Google Scholar]
- 164.Sanchez M, Gastaldi L, Remedi M, Caceres A, Landa C. Rotenone-induced toxicity is mediated by Rho-GTPases in hippocampal neurons. Toxicol Sci. 2008;104:352–361. doi: 10.1093/toxsci/kfn092. [DOI] [PubMed] [Google Scholar]
- 165.Le Clainche C, Carlier MF. Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol Rev. 2008;88:489–513. doi: 10.1152/physrev.00021.2007. [DOI] [PubMed] [Google Scholar]
- 166.Misgeld T, Kerschensteiner M, Bareyre FM, Burgess RW, Lichtman JW. Imaging axonal transport of mitochondria in vivo. Nat Methods. 2007;4:559–561. doi: 10.1038/nmeth1055. [DOI] [PubMed] [Google Scholar]


