Abstract
It is believed that primary tumor resection modulates host-tumor immune interaction, but this has not been characterized in a stringent breast cancer tumor model. This report, using the 4T1 murine mammary tumor model, characterizes for the first time the dynamic longitudinal changes in immunosuppressive and effector components of the immune system after resection of an established orthotopic primary tumor with a defined natural history of developing lung metastases. More specifically, we analyzed changes of absolute numbers and frequencies of MDSC, regulatory T cells (Treg), as well as activated CD4 and CD8 positive T cells in spleens and, in some studies, lungs of 4T1 tumor-bearing mice and mice after primary tumor resection. Importantly, using mathematical analyses we established that primary resection of an orthotopic tumor had created a “window of opportunity” with decreased tumor-associated immune suppression that existed for approximately 10 days. Although tumor resection did slightly prolong survival, it did not affect the ultimate development of metastatic disease since animals with resected tumors or intact primary tumors eventually died by day 47 and 43, respectively. This window of opportunity likely occurs in humans providing a rationale and parameters for integration and testing of immunotherapeutic strategies in this critical “window of opportunity” to combat the development of metastatic disease.
Keywords: mammary carcinoma, animal model of cancer, immunosuppression, clonogenic tumor cells, window of opportunity for immunotherapy
Introduction
Breast cancer is the most commonly diagnosed cancer worldwide. Almost 234,580 new cases of breast cancer will be diagnosed in the USA, and nearly 40,030 deaths are expected in 2013, the second leading cause of death in women [1]. Although standard treatments (surgery, radiation and chemotherapy) are beneficial and lead to increased disease-free and overall survival, a substantial proportion of patients experiences disease recurrence and eventually succumbs to metastatic disease. One of the most promising strategies for fighting the development of metastatic disease is the stimulation of patient's immune system to “seek out and destroy” disseminated tumor cells. Unfortunately, numerous immunotherapeutic strategies that showed promise in pre-clinical animal models have poor or mediocre clinical results [2–4].
Several factors are responsible for the high failure rate, but tumor-associated immune suppression is a major factor, as exemplified by the recent clinical efficacy of anti-CTLA-4 [5] and anti-PD-1 [6] treatments. Although these treatments abrogate tumor-associated immune suppression, they do not directly induce or enhance tumor associated antigen (TAA)-specific immune responses. Two subpopulations of cells, myeloid-derived suppressor cells (MDSC) and regulatory T cells (Treg), are recognized to play major roles in establishing this tumor-associated immune suppression and inhibiting the efficacy of anti-tumor immunotherapeutic strategies [7–13], arguing for application of therapeutics when this tumor-associated immune suppression is at a minimum.
One major factor leading to inability to successfully translate preclinical animal model work into the clinical arena is the limited number of tumor cell lines recapitulating human disease, including the spontaneous development of metastases, some of which are poorly characterized, or subjected to extensive genetic drifts from serial passage [14]. Various animal tumor models currently used for immunotherapeutic strategies are based on (i) genetically modified or non-modified tumor cells implanted into normal or transgenic congeneic mice; (ii) human tumors injected into immunodeficient animals (i.e, SCID mice); (iii) injection of tumor-inducing chemicals or viruses; (iv) transgenic animals with genetic knock-in or knock-out genes. Although, these animal models have been instructional in understanding the basic principles of human cancer biology, only few of them closely resemble human cancers and spontaneously develop metastatic disease. In recent years, several groups including us have used the 4T1 mouse model of breast cancer for evaluation of anti-tumor immunotherapeutic strategies. The 4T1 mouse tumor cell line was isolated and characterized by Dr. F. Miller and coworkers from a spontaneously arising mammary tumor in a BALB/cfC3H mice [15,16], and subsequently was extensively studied by the Ostrand-Rosenberg's group who advocated that this model is the closest in representing of the human clinical situation [17]. As in human mammary carcinoma, the major cause of morbidity and mortality for this model is a development of spontaneous metastatic disease during primary tumor growth [18]. This group has established that there is a positive correlation between the size of the primary orthotopic 4T1 tumor and the number of clonogenic tumor cells [19]. More recently, other reports have demonstrated a positive correlation between the tumor size and the frequency of MDSC at the tumor inoculation site, blood, spleen, lung, and liver [20,21]. Younos et al. also reported that the numbers of MDSC inversely correlated with the CD3+ T-cell frequency and levels of pro-inflammatory Th1 cytokines, and correlated with increasing anti-inflammatory Th2 cytokines in 4T1 tumor-bearing mice [21], potentially enhancing a development or progression of metastatic tumors. Additional mechanisms can have a role in inhibition of anti-tumor immune responses such as: L-arginine depletion through arginase, apoptosis of T cells through high levels of reactive oxygen species, and induction of Treg activation [11]. Increased Treg cells at tumor sites [22] and in draining lymph nodes of 4T1 tumor-bearing mice [23] have been associated with primary tumor growth. Although the resection of primary 4T1 tumors restored the immune responses to allogeneic tumors and protein antigens, and partially decreased the levels of splenic MDSC at 9–10 days post surgery, it did not significantly affect metastatic disease in this mouse model of breast cancer [24].
Even though the studies described above characterized the 4T1 mouse model in terms of an association of tumor size with a development of metastatic disease and MDSC-mediated immunosuppression in tumor-bearing mice, along with demonstrating immunologic changes in post-surgery mice at day 9–10 post-resection, they did not address important questions related to the longitudinal dynamics of these changes, nor did they broadly examine cellular immune elements (e.g., Tregs, MDSC, and immunocompetent effector T cells) in mice following post-surgical resection of primary tumor and during the growth of occult metastatic disease. Notably, surgery is considered the primary treatment modality for breast cancer. Although retrospective studies indicate that patients with metastatic breast cancer may benefit from the surgical removal of tumor, the mechanisms of this effect and the impact of tumor resection on the tumor-associated immune suppression are unclear [25]. Additionally, it has been established that the general anesthesia and a surgery itself lead to a transient immune suppression [26]. We sought to characterize, in a stringent animal model, the longitudinal impact of primary 4T1 tumor resection on the host immune system, on the tumor-associated immune suppression, and on the development of metastatic disease. Therefore, we analyzed the numbers of clonogenic tumor cells in lungs, as well as the frequency and the absolute numbers of suppressor cells (MDSC, Tregs) and activated effector T cells (CD8+ and CD4+) in the spleens at different time points in post-surgery mice and, in some studies, in lungs of the post-surgery mice. Using mathematical analyses we defined a short, but critical “window" for optimal immunotherapeutic intervention. Understanding changes in the immunosuppressive environment after tumor resection provides a basis for identifying factors or biomarkers that define/characterize an optimal time for integration of post-surgery anti-cancer immunomodulatory or immunotherapeutic treatment in humans.
Materials and Methods
Mice
Female 8- to 10-wk-old BALB/c mice were purchased from The Jackson Laboratory (Bar Harbor, ME). All animals were housed in the temperature- and light cycle-controlled facility, and their care was under the guidelines of the National Institutes of Health and the approved Institutional Animal Care and Use Committee protocol at the University of California, Irvine.
Reagents and Abs
Monoclonal antibodies (mAbs) CD3-PerCP-eFluor710, CD4-eFluor450, CD8-APC, NKp46-PE, FoxP3-FITC, CD69-FITC, rat IgG2a, k-FITC isotype control, and armenian hamster IgG-FITC isotype control, rat IgG2a k-PE isotype control and FoxP3 staining buffer set were purchased from eBioscience, San Diego, CA. Gr1-FITC, CD11b-APC were from Miltenyi Biotec, Auburn, CA.
Tumor inoculations and metastasis Assays
Unmodified 4T1 mammary carcinoma cells were obtained from Dr. F.R. Miller (WSU, School of Medicine, Detroit, MI) and were cultured as previously described [27]. Freshly prepared, 15×103 tumor cells in mid-log growth phase were injected into the mammary fat pads as described in [28,29]. Tumor growth was monitored daily starting at day when tumors became palpable (usually at days 5–7 after injection of 4T1 cells). Mean tumor diameter (TD) was calculated as the square root of the product of the two measurements (√ab) of the tumor that are perpendicular to each other and span the largest portion of the tumor in each direction as described [17]. Mice were sacrificed when TD reached 2–3mm, 3.5–5mm and 10–15mm, spleens and lungs were taken for further analysis. In another group of mice, tumors were surgically removed when they reached a diameter of 3.5–5mm. Mice were sacrificed at 2, 4, 6, 13, 17–20, 30–33 days after surgery. The number of clonogenic tumor cells in the lungs was analyzed as described [17,30]. Briefly, the lungs were harvested in aseptic conditions, digested with collagenase type IV (1mg/ml in 1× HBSS) (Sigma-Aldrich, St. Louis, MO) at 4°C for 75min. Digested samples were filtrated through the 70-µm nylon cell strainer, washed and grown in medium containing 6-thioguanine for 10–14 days. The 4T1 colonies were fixed by methanol and stained with 0.03% (w/v) methylene blue solution. Two independent observers have evaluated the numbers of blue colonies in the culture dishes.
Surgical removal of tumor
All surgical supplies and surgical equipment were purchased from Roboz (Rockville, MD). Mice were anesthetized with isoflurane and were injected with the analgesic ketoprofen in the back (6 mg/kg body weight) subcutaneously (s.c.). Once the animals were unconscious, the surgical area was shaved to enhance visualization of the surgical site and sterilized with Betadine surgical scrub (Purdue products L.P., Stamford, CT) and 85% isopropanol. Tumors were removed with sterilized surgical instruments using blunt dissection to isolate the tumor. Wounds were closed with stainless steel wound clips (CellPoint Scientific, Gaithersburg, MD). Mice were moved to a warm dry area and monitored during recovery. Skin closures were removed 10–14 days post-operatively with 100% of the mice surviving surgery. 5% of post-surgery mice had recurrence of tumor likely due to incomplete removal of primary tumors. These mice were excluded from the experiments to which they were initially assigned.
Mononuclear cell isolation from mouse lungs
Mice were anesthetized and euthanized with nembutal (i.p., 40mg/kg), spleens were immediately harvested, then mice underwent transcardial perfusion of the lungs with ice-cold PBS. Lungs were harvested for isolation of pulmonary mononuclear cells (PMC) and digested with type IV collagenase (1mg/ml in 1× HBSS) (Sigma-Aldrich, St. Louis, MO) for 75 minutes at 4°C. Digested organs were dissociated mechanically by passing through 70µm mesh. The collected cell suspension was washed with 1× HBSS. PMC were isolated via Ficoll-paque density gradient centrifugation (density=1.077 g/L), washed with HBSS × 1, and counted for further flow cytometric analyses.
Flow cytometry staining
Spleens of mice were processed for isolation of mononuclear cells by a commonly used method: preparing single cell suspensions from mouse spleens followed by the removal of red blood cells.
Splenocytes or mononuclear cells from lungs were re-suspended in PBS containing 0.1% BSA (Sigma-Aldrich, St Luis, MO) and 0.1% sodium azide. For surface staining of CD4, CD8, CD3, CD69, GR1a and CD11b, cells were incubated with fluorochrome-conjugated antibodies to the indicated cell surface markers at the recommended dilutions for 20 minutes at 4°C. The frequency of activated CD4+ and CD8+ cells was calculated as the percent of CD69+ cells in CD4+ and CD8+ cell populations, respectively. For intracellular staining of Foxp3, cells were fixed and permeabilized with Foxp3 staining buffer before incubation with the conjugated antibody or isotype control. Non-specific "background" was distinguished by staining with isotype control primary Abs. The frequency of Treg cells was calculated as the percent of Foxp3+ cells in CD4+ cell population. Stained cells were analyzed with MacsQuant cytometer (Miltenyi Biotec, Auburn, CA).
Mathematical analyses
The region for the lowest level of MDSC and the highest percent of activated T cells in the spleen was defined by mathematical analyses of data using OriginPro software. The experimental data in the region of interest (from day 0 to day 33) were linearly fitted by polynomial functions in the form where coefficients were defined by Ordinary Least Squares method with the following weights: weights = 1/SD2, SD = experimental standard deviation. Basis up to the third order was used (N=3). Local maximum and minimum values of observed functions were calculated using basic Newton's method for finding a minimum or maximum of a function. Then the variance of the function value at the extreme point was defined according to the fitting of experimental data, under condition that the argument (x) does not have errors. The interval of the function which corresponds to the error of the extreme value was defined as follows: [ϰleft, ϰright]: F(ϰ) = Extr ± VarExtr where Extr - the function value at the extreme point, VarExtr - an error of the function value at the extreme point.
Statistical Analysis
All statistical parameters were calculated using GraphPad Prism 5.0 Software. Statistical significance between groups was determined by two-tailed unpaired t test (P values less than 0.05 were considered significant) and one-way Anova post Tukey comparison test. Survival curves were generated in GraphPad Prism 5.0 Software and compared using Log-rank (Mantel-Cox) test. An association between the groups was evaluated using Pearson correlation coefficient.
Results
Metastatic disease and immunosuppression are associated with tumor size in 4T1 tumor-bearing mice
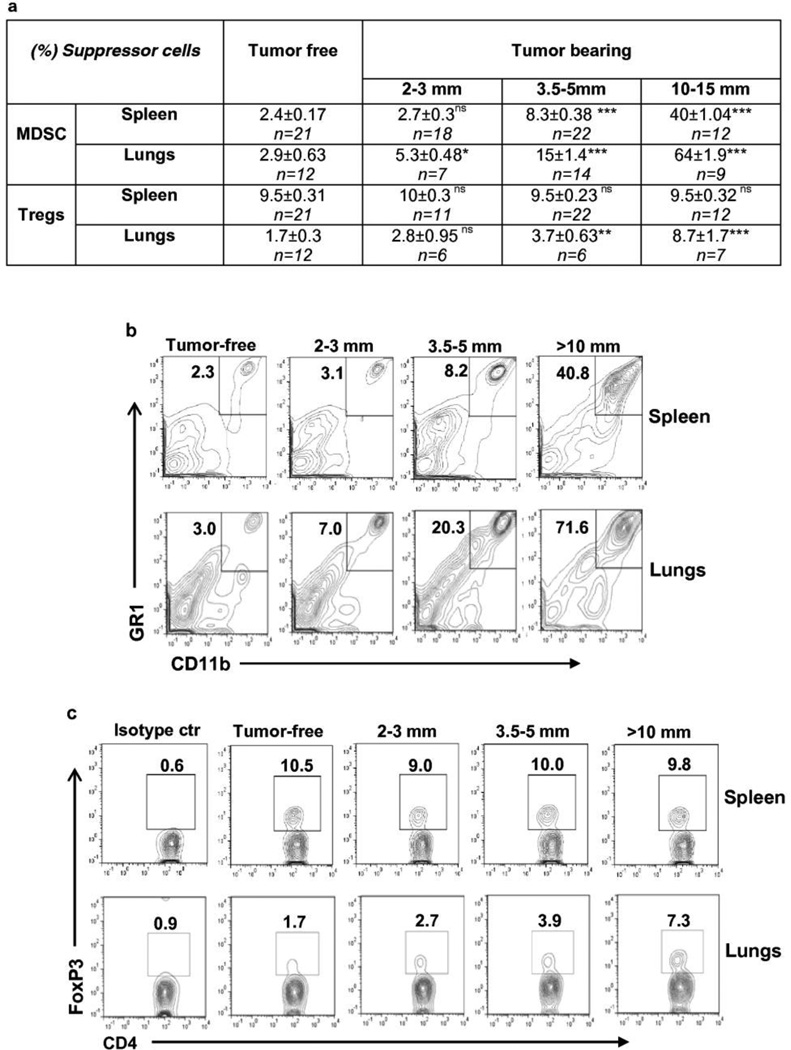
To have an ability to evaluate treatment efficacy of metastatic disease, it is important to establish conditions (e.g. tumor size, numbers of immunosuppressive and effector cells) where essentially all mice develop metastases. As was expected from previous studies [19], in our experiments with 4T1 cells we determined that the numbers of clonogenic lung tumor cells were associated with the primary tumor size. As shown in Fig. 1, only 50% of mice bearing tumor 2–3mm in diameter had metastases, whereas 93% of mice having 3–5mm tumor and 100% of mice with tumor >10mm developed metastases in lungs. We observed a correlation between the tumor size and the frequency and absolute numbers of MDSCs in spleens (R=0.9813, ***P<0.001 and R=0.94, *** P<0.001, respectively) as well as in lungs (R=0.97, *** P<0.001 and ***P<0.001, respectively) of mice (Figure 2 and Supplementary Table 1), as did others [21]. In mice bearing 2–3mm tumors, the frequency of MDSC population in spleens was still at the level of tumor-free mice, whereas the level of this population of cells was elevated in lungs. In mice with 3.5–5mm tumors, the frequency of MDSC in spleens and lungs was increased almost 3.5× and 5×, respectively. It continued to increase with tumor growth and reached 40% of splenocytes and 64% of non-parenchymal lung cells, respectively (Fig. 2) when mice were euthanized due to tumor size (10–15mm). The absolute number of MDSC was also significantly increased in spleens and lungs of tumor-bearing mice (Supplementary Table 1), showing the same profile as the frequency of these cells. No changes were observed in the frequency of Tregs in the spleens of tumor-bearing mice compared with tumor-free mice (Fig.2). However, the absolute number of Treg cells in this organ was significantly increased in mice bearing 2–3mm and 3.5–5mm tumors, but declined when tumors became larger than 10mm (*** P<0.001 for groups 2–3mm v.s. 10–15mm) remaining still higher than that in tumor-free mice (Supplementary Table 1a). In contrast, the frequency of Tregs in the lungs increased with increasing tumor size, reaching up to 8.7% in mice with large tumors (10–15 mm diameter) (Fig. 2), which yielded high levels of metastases in the lungs (Fig. 1). Analysis of dynamic changes in absolute numbers of Tregs in lungs of tumor-bearing mice showed significant increase in mice with tumors of 10–15mm (Supplementary Table 1b).
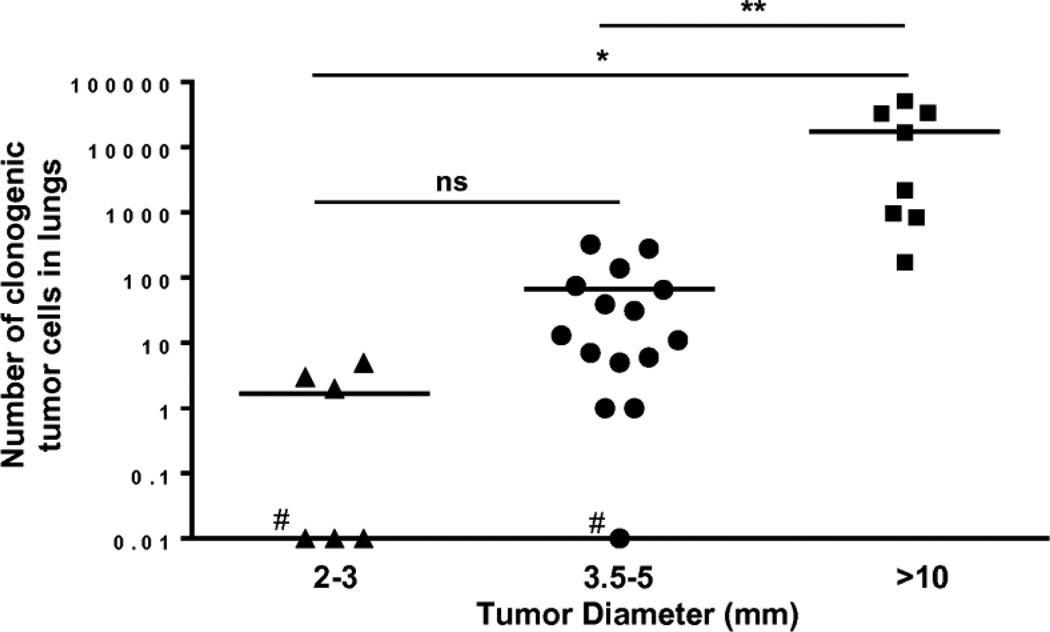
Fig. 1.
The number of clonogenic tumor cells in lungs of tumor-bearing mice was increased with increase of tumor size. Only 50% of mice bearing 2–3mm tumors had metastases in lungs compared to 93% and 100% of mice bearing tumors of 3.5–5 mm and >10mm, respectively, (#indicates animals that had not generated metastatic disease). Statistical significance was calculated using one-way Anova post Tukey comparison test (*P≤0.05, **P≤ 0.01, ***P≤ 0.001 ns = non significant).
Fig. 2.
The frequency of MDSCs in spleens (R=0.9813, P<0.0001) and lungs (R=0.97, P<0.001) positively correlated with the tumor size, whereas the frequency of Treg increased in lungs, but not in spleens of tumor-bearing mice.
(a) The frequencies of GR1+CD11b+ MDSC and FoxP3+ Tregs cells in CD4+ cell population in spleens and lungs of mice bearing tumors of different sizes. Statistical significance was calculated against a group of tumor-free mice using two-tailed t-test (*P≤0.05, **P≤0.01, ***P≤ 0.001, ns = non significant).
(b, c) Representative plots of flow cytometric analyses of MDSC (B) and Treg (C) in spleens and lungs.
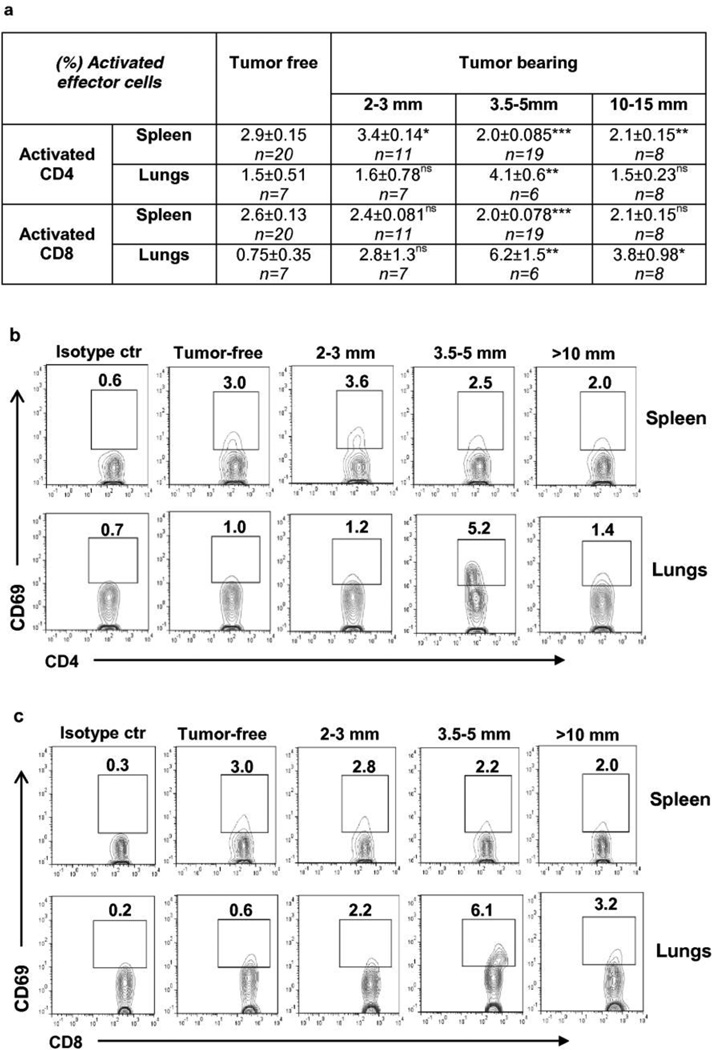
We assessed the impact of tumor size on the proportion of total and activated T cells in the lungs and spleens (Fig. 3 and Supplementary Table 2). Activated CD4+ cells were detected by surface marker CD69, which is the earliest inducible cell surface glycoprotein acquired during lymphocyte activation [31]. The absolute numbers of total CD4+ cells were significantly increased in the spleens of mice bearing 2–3mm tumor compared with tumor-free mice. We did not see significant changes in the group of mice with 3.5–5mm tumor, likely due to a high variability among the mice in this group. In the mice with a large tumor size, the absolute numbers of total CD4+ cells were decreased compared to mice bearing 2–3mm tumors (**P<0.01), but still were significantly higher than that in the spleens of tumor-free mice (Supplementary Table 2a). The absolute numbers of CD69+CD4+ cells and the frequencies of these cells in CD4+ cell population were slightly, but significantly increased in the spleens of mice bearing 2–3mm tumors. In mice with larger tumors, the absolute numbers of these cells were reversed to the level of cells in tumor-free mice, and the frequencies were decreased to a level that had been significantly lower than that in tumor-free mice (Fig.3, Supplementary Table 2a). We observed an inverse correlation between the primary tumor size and the frequency of splenic activated CD4+ cells (R=−0.66, ***P=0.0002). In the lungs, the longitudinal changes were kinetically dissimilar. The absolute numbers of total CD4+ cells in lungs were decreased significantly in mice with small tumors (2–3mm) compared with tumor-free mice, but reversed to the level seen in tumor-free mice with continued tumor growth (Supplementary Table 2b). Small tumors yielded no change from control animals in frequency of activated CD4+ cells, but we observed an increased infiltration of these cells in lungs of animals bearing tumors in the mid-size range. However, with further growth of the primary tumor and increase in metastatic burden, the frequency of this population decreased and returned to the level of these cells in tumor-free mice (Fig.3). The profile of changes in the absolute numbers of activated CD4+ cells infiltrating the lungs was similar to that in the frequency of these cells, although was not statistically significant (Supplementary Table 2b). The timing of increase in the level of activated CD4+ T cells coincided with the capacity to detect pulmonary metastases. Although at this point we also saw the elevated level of Tregs, it was probably not sufficient for complete suppression of activated CD4+ cells.
Fig. 3.
The activated cells in CD4+ and CD8+ populations were suppressed in spleens of mice bearing tumors >3.5–5mm in diameter, whereas in lungs they were transiently activated in mice bearing 3.5–5mm tumors and started to decrease with growth of tumor.
(a) Percent of activated effector cells in spleens and lungs of mice bearing tumors of different sizes. Statistical significance was calculated against tumor-free mice using two-tailed t-test (*P≤0.05, **P≤ 0.01, ***P≤ 0.001, ns = non significant).
(b,c) Representative plots of flow cytometric analyses of activated CD4+ cells (b) and CD8+ cells (c) in spleens and lungs.
Though the absolute numbers of total CD8+ cells were increased in spleens of mice bearing 2–3mm tumors, in mice with 3.5–5mm tumors the changes were not significant. In contrast to CD4+ cells, in mice with large tumors (>10mm), the absolute numbers of CD8+ cells were significantly decreased (Supplementary Table 2a) compared to that in tumor-free mice. No increase was observed either in frequency or in absolute numbers of activated CD8+ cells in the spleens of mice bearing 2–3mm tumors and yet, in mice with 3.5–5mm tumors, the frequency was significantly decreased relative to tumor-free mice of similar age. Correlation between the tumor size and the frequency of activated CD8+ cells in spleens was not significant (R=−0.121, ns P=0.54). The absolute numbers of activated CD8+ cells were significantly decreased in mice with large tumors (10–15mm) compared to that in tumor-free mice (Fig. 3, Supplementary Table 2a).
Similar to activated CD4+ cells, the frequency of activated CD8+ cells infiltrating the lungs was increased when tumors reached 3.5–5mm in diameter compared to control mice. The downward trend in the frequency of activated CD8+ cell population in lungs of mice with >10mm tumor was observed, however in contrast to activated CD4+ cells, it was still significantly higher as compared to that in tumor-free mice. Changes in the absolute numbers of activated CD8+ cells in lungs of tumor-bearing mice were not statistically significant, although the tendency to increase was seen in mice with 3.5–5mm tumor.
Tumor resection decreased immunosuppression, but did not significantly inhibit metastatic disease development
Based on the data above, we decided that a tumor at size of 3.5–5mm diameter, when 93% of mice have lung metastases, was an appropriate model for evaluation of the effect of resection of primary tumor on both tumor-associated immune suppression and development of metastatic disease. This tumor size corresponds to the clinical situation for many women with advanced breast cancer [17].
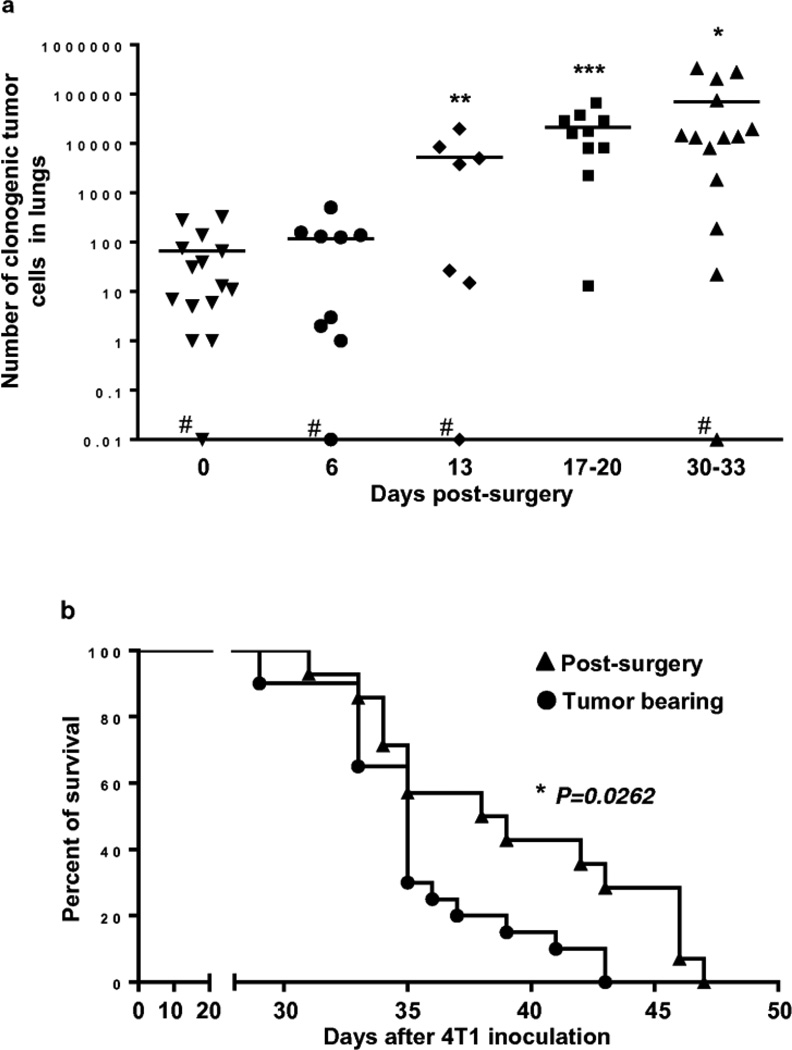
The number of clonogenic tumor cells in the lungs was analyzed at the day of surgery (day 0) and at days 6, 13, 17 to 20 and 30 to 33 after tumor resection (Fig. 4). The number of 4T1 clonogenic tumor cells increased gradually over time indicating that primary tumor resection even at this relatively small tumor size did not prevent from a development of metastases and suggesting that an immune system is not capable of identifying and eliminating an occult micrometastatic tumor. The impact of tumor resection on overall survival of mice with distant metastases resulted in a modest shift in median survival (Fig. 4b). The median survival was 35 days for tumor-bearing mice and 38.5 days for mice whose primary tumors were resected, resulting in a small, but statistically significant difference (p < 0.05). Tumor-bearing mice started to die at day 29 after tumor inoculation and none were alive at day 43. Mice whose tumor was resected once it reached the 3.5–5 mm size, started to die at day 31 and none were alive at day 47 after inoculation of 4T1 cells.
Fig. 4.
Tumor resection did not prevent a development of metastatic disease while slightly, but significantly prolonged the survival of mice.
(a) Number of 4T1 clonogenic tumor cells in lungs increased gradually even after surgical removal of tumors at size of 3.5–5mm (#one mouse per group at days 0, 6, 13 and 30–33 had no metastases). Statistical significance was calculated against day 0 (day of surgery) using two-tailed t-test (*P≤0.05, **P≤ 0.01, ***P≤ 0.001, ns = non significant)
(b) Survival of 4T1 tumor-bearing (n=20) and post-surgery mice (n=14). Tumor was removed at size 3.5–5mm of tumor diameter (9–11days after 4T1 inoculation). At day 43, when tumor-bearing mice had died, 28% of post-surgery mice were still alive. Survival curves were compared using Log-rank (Mantel-Cox) test (*P=0.0262). Experiment was performed twice.
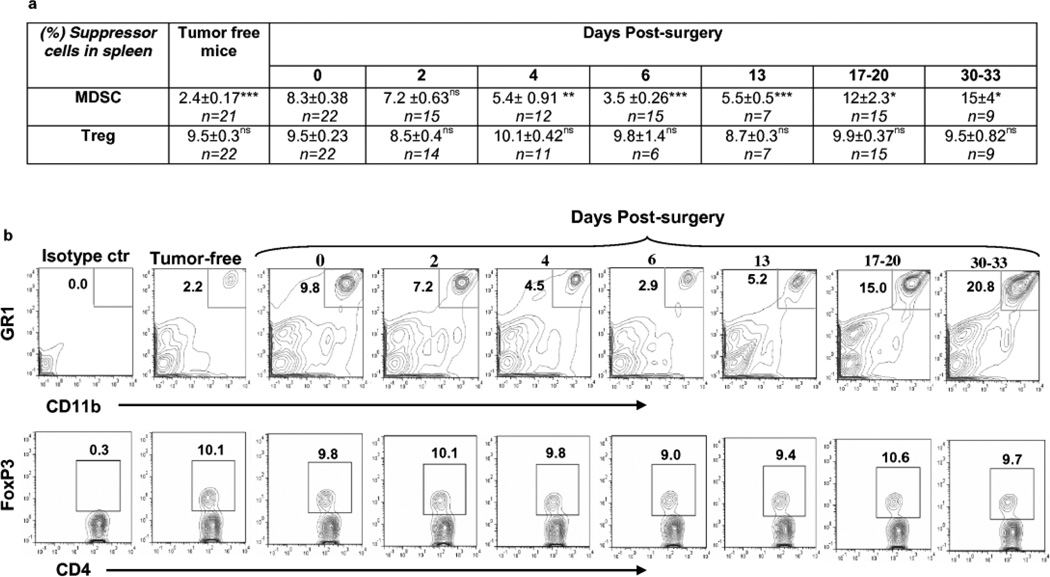
The MDSC population was analyzed at different time points after primary tumor resection (Fig.5, Supplementary Fig.1, Table 3 and 4), in the spleens, and at day 0 and days 17–20 in lungs of mice. At day 0 (day of surgery) the frequency and absolute number of MDSC in spleens of mice were significantly higher than that in spleens of tumor-free mice, as noted in earlier experiments (Supplementary Table 3, 4). Following primary resection, MDSC population was gradually decreased. The frequency and the absolute number of MDSCs reached a minimum on the sixth day and then began to rise again (Fig.5, Supplementary Fig.1). At this time point, 53% of the mice had splenic MDSC levels equal to that in spleens of tumor-free mice (data not shown). At day 13, the frequency of MDSC was still significantly lower than at day 0, but reached and surpassed the day 0 (surgical resection) level at days 17–20 and beyond, yielding values that were significantly higher than at day 0. Of note, despite the increase in the frequency of splenic MDSC population in post-surgery mice, it was still significantly lower at day 30, than level of MDSC in mice with tumors equal to or over 10mm (15±4% vs 40±1.04%, *** P<0.001). The direct correlation between the numbers of clonogenic tumor cells in the lungs and the frequency and the absolute numbers of splenic MDSC (R=0.77, *** P<0.001 and R=0.98, *** P<0.001, respectively) was observed in post-surgery mice.
Fig. 5.
Surgical removal of tumors led to temporary decrease in the frequency of MDSC without changing of the levels of Treg in spleens of mice.
(a) Table presents percent of GR1+CD11b+ MDSC and FoxP3+ Tregs cells in CD4+ cell population in spleens of mice at indicated days after surgical removal of tumor. Statistical significance was calculated against day of surgery (day 0 in the table) using two-tailed t-test (*P≤0.05, **P≤ 0.01, ***P≤ 0.001, ns = non significant).
(b,c) Representative plots of flow cytometric analyses of MDSC (b) and Tregs (c) in spleens.
The frequency of MDSC population was significantly higher in lungs of mice at day of surgery when tumor was 3.5–5mm compared with tumor-free mice (15±1.4% vs 2.9±0.63%, ***P<0.001) and it was almost unchanged by 17–20 days after tumor removal (15±1.4% vs 17.1±3.4%, nsP>0.05).
The Treg population was analyzed in the spleen at different time points after primary tumor resection. Because of the relatively small magnitude of the shift in this population in the lungs, even in the presence of very large tumors, we did not expend animal resources to evaluate the longitudinal impact on Treg cells within the lungs when moderate sized primary tumors were resected, instead opting to examine this population at 17–20 days post tumor resection. Similar to studies with tumor-bearing mice, no changes were observed in the percentage of splenic Tregs in post-surgery mice at any time point, compared with either tumor-free mice (Supplementary Table 4) or mice at the time of primary tumor resection (Fig. 5, Supplementary Fig.1). However, the absolute number of splenic Treg cells was significantly increased in mice bearing tumor of 3.5–5mm (at the day of surgery) (Supplementary Table 3) and was temporary decreased reaching to the level in tumor-free mice at days 2, 4, and 6 post-surgery, as was observed for MDSC (Supplementary Table 3, Supplementary Fig.1). Interestingly, while the primary tumor was intact (3–5mm, day 0), the frequency of Treg cells in lungs was significantly higher than in lungs of tumor-free mice (3.7±0.63% vs 1.7±0.3%, **P<0.01), and this level was almost unchanged at 17–20 days post-surgery (3.7±0.63% vs 4.24±0.9%, nsP>0.05). These data and the data of pulmonary MDSCs suggest that there were no significant, sustained modulation of these populations in the lungs of mice over the 17–20 days post tumor resection.
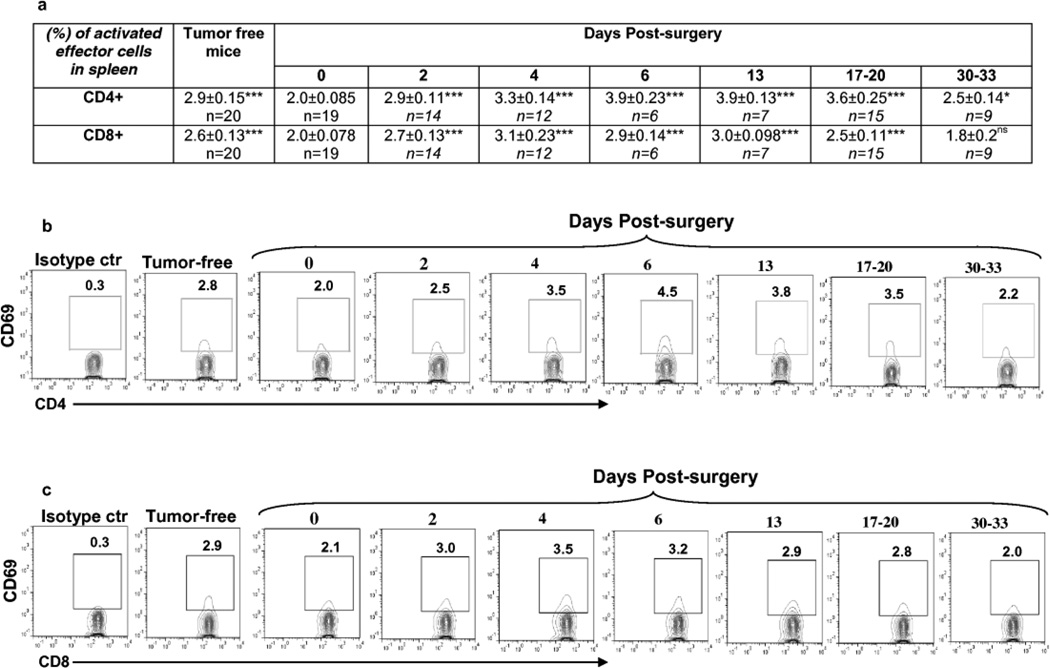
Importantly, we observed an increase in frequency of activated CD4+ and CD8+ cells in the spleens of mice after primary tumor resection (Fig. 6, Supplementary Fig.1 and Table 5). At the day of surgery (0 day, 3.5–5mm tumor-bearing mice), the frequency of activated CD4+ cells in total CD4+ cell population was significantly lower than that in tumor-free mice (Supplementary Table 5). Two days after tumor resection, the frequency of activated CD4+ cells matched that of tumor-free control mice and continued to rise reaching a maximum level at days 6 to 13. At days 17–20, the proportion of activated CD4+ cells remained significantly higher than in tumor-free mice. A similar pattern was observed in the case of activated CD8+ cells with the difference that the frequency of these cells was significantly higher compared to the tumor-free mice only at day 4 post-surgery. At days 6, 13 and 17–20 post-surgery, the frequency of activated CD8+ cell population decreased, returning to the level of activated CD8+ cells in the spleens of tumor-free mice, and by days 30–33, to the day 0 level (Fig. 6, Supplementary Fig.1). Analysis of absolute numbers of total CD4+ and CD8+ cells in spleen showed that these populations are expanded in mice bearing 3.5–5mm tumors at the day of surgery compared with the tumor-free mice,, though this expansion is not statistically significant (P=0.068 for CD4+ cells, P=0.16 for CD8+ cells) (Supplementary Table 3). The absolute numbers of CD4+ and CD8+ T cells at days 2, 4, 6 post-surgery were significantly decreased compared to that at day 0 (i.e. the day of surgery) (Supplementary Fig 1). At day 30 post-surgery the absolute numbers of CD4+ and CD8+ T cells reversed to the level of these T cells at day 0 (Supplementary Fig 1). However, no significant changes were observed in the absolute numbers of both activated CD4+ and CD8+ cells in mice at all time points after the tumor-resection compared with that in tumor-free mice (Supplementary Table 3), or mice at the day of surgery (Supplementary Fig 1), indicating that the shift in frequency of these cells is associated with observed changes in absolute numbers of total CD4+ and CD8+ cell populations.
Fig. 6.
Surgical removal of tumors led to temporary increase of the frequency of activated CD69+CD4+ and CD69+CD8+ T cells in spleens of mice.
(a) Percent of CD69+ cells in CD4+ and CD8+ T cell populations in spleens of mice at indicated days after surgical removal of tumor. Statistical significance was calculated against day 0 using two-tailed t-test (*P≤0.05, **P≤ 0.01, ***P≤ 0.001, ns = non significant).
(b,c) Representative plots of flow cytometric analyses of CD69+CD4+ (b) and CD69+CD8+ (c) cells in spleens.
The frequency of activated CD4+ and CD8+ cells were significantly higher in lungs of mice bearing 3.5–5mm tumor at the day of surgery than the level of these cells in lungs of tumor-free mice [4.1±0.6% (n=6) vs 1.5±0.51% (n=7), **P<0.01 for CD4+ cells and 6.2±1.5% (n=6) vs 0.75±0.35% (n=7), **P<0.01 for CD8+ cells]. This level declined by day 17–20 post-surgery [1.83±0.27 (n=7), ***P<0.001 for CD4+ cells and 4.54±1.13 (n=7), *P<0.05 for CD8+ cells] in association with the development of a high number of clonogenic lung tumor cells although the level of activated CD8+ cells was still significantly higher than in lungs of tumor-free mice (**P<0.01).
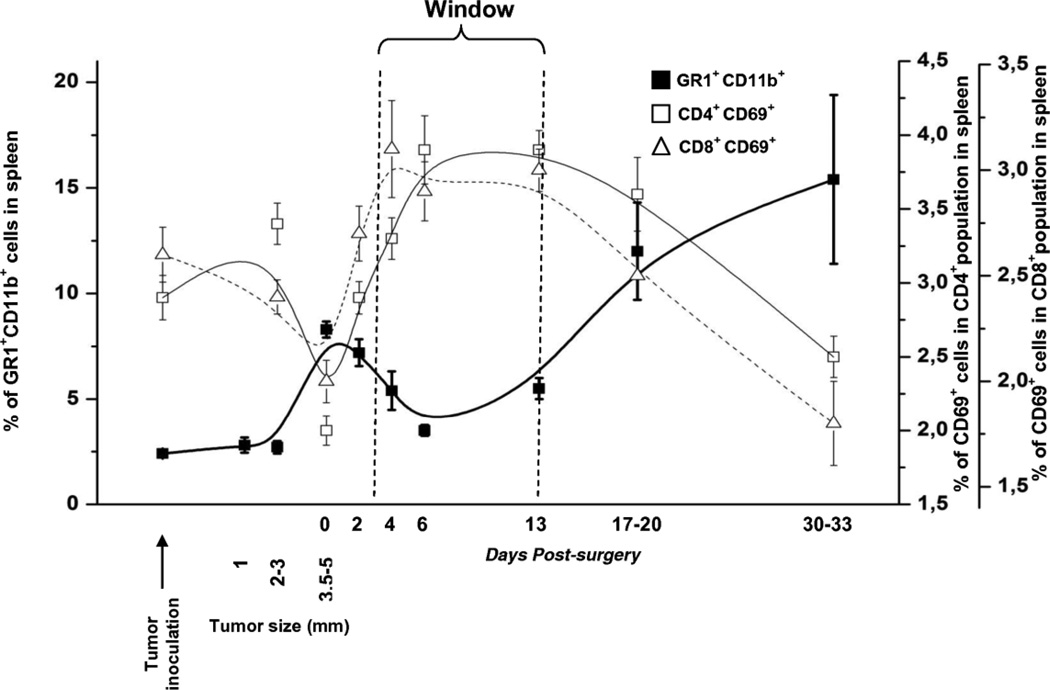
"Window of opportunity" for immunotherapy
To better understand the relationship between suppressor and effector components of immune system we outlined and overlaid the changes in the levels of MDSC and activated effector cells frequency in a spleens of mice at different time points before and after the tumor removal (Fig. 7). To define an appropriate region that meets the requirements of the lowest level of MDSC and the highest percent of activated T cells in the spleen, these data were mathematically analyzed (see detailed description in the Supplementary Methods). The biological region (“window”) was defined according to the statistically significant difference between experimental values measured after removal of the tumor (days 2 to 31) in comparison to those measured on day 0, right before removal of the tumor. We mathematically calculated the 95% confidence interval which corresponds to the decrease of MDSC counts and the increase of the activated T cells counts. The 95% confidence intervals of these functions, which correspond to their significant change after the surgery, were defined between the days 2.4 to 13.3; 1.8 to 26.1; 2.1 to 16.8 for the MDSC, CD4+ and CD8+ cells, respectively. The global overlap region for the significant changes in all three functions was defined between the days 2.4 and 13.3 (Supplementary Methods). According to these data, we may conclude that the time period between days 3 to 13 could be optimal for immunotherapeutic intervention ("window of opportunity") with the most optimal period being between 7 and 10 days post resection of the primary tumor (Fig. 7). Interestingly, when we calculated the longitudinal changes in absolute numbers of MDSC and Treg cells, we found the same "window of opportunity" (data not shown), although the absolute numbers of activated CD4+ and CD8+ cells remained unchanged (Supplementary Fig.1 and Table 3).
Fig. 7.
Surgical removal of tumors led to temporary decrease in frequency of MDSC in spleens of mice, whereas the frequencies of activated CD4+ and CD8+ T cells were temporary increased creating a "critical window" for optimal immunotherapeutic intervention.
Data show experimental mean values with no SD and extrapolating lines (B-spline).
Discussion
The 4T1 mammary carcinoma was isolated from mice of H2d haplotype (BALB/cfC3H), which are prone to develop certain cancers and in some cases metastatic disease [15,17,32,33]. Several groups in addition to ours believe that the 4T1 model is very clinically relevant [15,16–18,34]. It was shown that the resection of the primary 4T1 tumor alone is insufficient to have a prolonged effect on the development of metastatic disease [19,35]. As with humans who develop metastatic disease, the rate of success in the treatment of 4T1 tumor-bearing or even post-surgery mice that develop metastatic disease is exceptionally low. The failure of immunotherapeutic strategies in the 4T1 mouse model of breast cancer is likely due to both the poor-immunogenic nature of this tumor and the tumor-associated immune suppression. Two major populations of cells, MDSC [21,35] and Tregs [22,23,36] have been connected with tumor-associated immune suppression, and both have been described as having contributions in the 4T1 model. The involvement of these cellular subsets in tumor associated immune suppression and potentially in tumor progression is not restricted to the 4T1 model. Expansion of MDSC has been reported in a broad array of different types of tumors in mice including EL-4, DA3, 4T1, CT26, MC38, C3, ANV, LLC, B16, MethA [37] as well as in spontaneous breast cancer model Balb-neuT transgenic mice [38]. Interestingly, several studies have demonstrated an increased level of immature myeloid cells endowed with suppressive activity in cancer patients, suggesting that this biological activity can be generalized across species. For example, in breast cancer patients, the level of circulating MDSC was significantly higher compared with matched healthy controls, and the level of these cell subpopulations was directly proportional to the clinical cancer stage [39,40]. Moreover, the level of MDSC was higher in patients with widely metastatic disease compared to the patients with more limited metastatic disease.
Here we have demonstrated that an increasing metastatic tumor burden in post-surgery mice (days 17–20 and 30–33) leads to increases in the frequency of MDSCs, in both the spleen (Fig.5, Supplementary Fig.1 and Table 4) and in the lungs (see Results section). The tendency to increase was seen also in the absolute numbers of the MDSC in spleens of these mice (Supplementary Fig.1 and Table 3). After primary tumor resection, the splenic population of MDSC was transiently reduced, but rebounded with the increase of pulmonary metastases (Fig. 4a). The frequency of MDSC population did not return to the levels detected in non-tumor bearing animals, but was significantly lower than at the time of resection (day 0) starting from day 4 until days 13 post resection (Fig.5 and Supplementary Fig.1). Of note, our data with MDSC support the previous results demonstrating the partial decrease of MDSC population in mice on 9–10 days after the resection of primary 4T1 tumors [24]. Tumor-derived MDSC, in the absence of tumor-derived factors have been reported to differentiate into mature DCs and macrophages [41–43]. These data provide a potential mechanism by which removal of 4T1 primary tumor could result in significant and rapid shifts in the MDSC population and create favorable conditions for immune mediated affects on tumor progression and the development of metastases.
The evaluation of the Treg suppressor cell subset is slightly more complicated. We did not observe any significant changes in the frequency of Tregs in the spleen with growth of the metastatic tumor (Fig.5). However, the increasing leukomoid reaction with increasing tumor burden and this stable frequency drives an overall increase in absolute numbers of Tregs in the spleen with increasing tumor burden (Supplementary Table 1). The absolute numbers of Treg cells after the primary tumor resection reversed to the numbers seen in the tumor-free mice, but again showed a trend towards the increase at day 30–33 post-surgery associated with the development of increasing pulmonary metastases (Supplementary Fig.1 and Table 3). At the same time, we did note an upturn in the frequency of Treg population in the lungs of tumor-bearing animals (Fig.2). The difference in frequency of Treg populations observed in spleen vs lung, suggests that occult micro-metastatic 4T1 cells in the lungs may support differential local Treg infiltration, which is not manifested in a systemic manner. Alternatively, it is also possible that primary tumor and metastatic tumor may differ in their ability to recruit the T lymphocytes, both activated and Treg cells. Perhaps cells from primary tumor and metastatic sites secrete different soluble factors, or the microenvironments may be somewhat different. Interestingly, it has been reported that metastatic tumor cells may recruit Treg cells through CCR4/MDC, and depletion of Treg cells inhibits dissemination of metastases in this anatomical site [36]. The longitudinal modulation of absolute numbers of Treg cells had a similar profile as that observed for both the absolute number and the frequency of MDSCs in the spleen (Supplementary Fig.1). Data pertaining to the frequency of MDSC and Treg populations suggest that MDSC appear to play a more significant role and are affected to a greater degree by resection of the primary tumor than Treg. Of note, another group also reported that depletion of Treg does not affect the tumor growth and survival of mice in this tumor model [44].
In this study we also characterized the longitudinal modulation of total and activated (CD69+) T lymphocyte populations in the spleens after surgical resection of the primary orthotopic tumor. As was expected from published data of others [21], we observed a trend toward the increase in the absolute numbers of T lymphocytes (both CD4+ and CD8+) in the spleens of tumor-bearing mice at the day of surgery (day 0) possibly due to the tumor-associated leukomoid response or leukocytic hyperplasia (Supplementary table 3). After resection of the primary tumor, these numbers decreased, to the level observed in tumor-free mice, but increased again at days 30–33 when metastatic disease was full-blown (Supplementary Fig 1). Although the absolute numbers of activated CD4+ and CD8+ T lymphocytes in spleens were not significantly changed at the day 0 and after the surgery compared with tumor-free mice (Supplementary Fig 1, Table 3), we observed longitudinal changes in the frequency of these populations in the spleens (Fig.6). The frequency of activated T lymphocytes in spleens was depressed in tumor-bearing mice (at day 0) and rebounded after resection of primary tumors while it declined with the growth of metastatic disease in lungs, exhibiting the expected parabolic trajectory with the development of an increasing metastatic tumor burden.
We mathematically analyzed our experimental data to describe a “window of opportunity” in which the 4T1 tumor-associated immune suppression is at least partially abrogated (Supplementary Methods and Fig.7). This window of opportunity is characterized by shifts in subsets of immune cells in the spleen that reflect a period of decreased tumor associated immune suppression, regardless of whether absolute numbers or frequency of cellular subsets are examined (Supplementary Figure 1). Both the total MDSC population and the frequency of MDSCs decrease with primary tumor resection. The data for Tregs support a similar trajectory of the total Treg splenic populations, but due to an overall decrease in lymphocyte population after primary tumor resection, the frequency remains essentially unchanged. In support of this “window of opportunity”, the longitudinal changes in the frequency of activated T cell populations also varied in a parabolic manner, but were inverted relative to that seen with the MDSC population. Resection of the primary tumor resulted in a transient rebound in the frequencies of activated CD4+ and CD8+ T cells from the levels present at the time of primary tumor resection. The absolute numbers of both activated CD4+ and CD8+ T cells in the spleen remained unchanged.
In contrast to what would be expected from the frequency data, the total splenic populations of CD4 and CD8 positive T cells showed a tendency to increase in the presence of a tumor, primary or metastatic, and transiently decreased after the primary tumor resection (Supplementary Fig 1). Thus, the frequency of activated CD4+ and CD8+ cells inversely correlated with both the frequency (R=−0.35, **P<0.01 for CD4+ cells and R=−0.33, *P<0.05 for CD8+ cells) and the absolute numbers of MDSC (R=−0.43, *P<0.05 for CD4+ cells and R=−0.58, ***P≤ 0.001 CD8+ cells), while the absolute numbers of these T cell subpopulations remained steady. On the other hand, the frequencies of activated CD4+ (R=−0.5, **P<0.01) and CD8+ (R=−0.51, **P<0.01) cells inversely correlated with absolute numbers of Treg cells. These data are entirely consistent with the concept of a “window of opportunity”, which we have characterized as a period of decreased tumor-associated immune suppression, when there is a biological rationale for inducing or amplifying anti-tumor immune responses (Fig.7). Our study cannot address whether a proportion (frequency), or a total population size is most important for the shifts in tumor-associated immune suppression, a question that will await specially-designed studies in the future to characterize which would more accurately reflect the effect of tumor resection on the immunological status of the host. In any case, it seems to us that data presented in this study show that, in general, the primary tumor resection helps reduce the level of suppressor cells and can create favorable conditions for the effector cells of the immune system to fight metastatic disease.
There are human data to support generalization of this concept. It has been reported that MDSC are elevated in patients with breast cancer, decreased to the level of healthy individuals after the removal of the tumor mass, and then again augmented with recurrence [45,46]. More recently, these results have been supported by another group [47], whose data showed that not only MDSC, but also Treg cells were increased within the PBMCs of breast cancer patients. Of note, when evaluated after surgery, the patients who responded to neoadjuvant chemotherapy had a significant reduction of suppressor cells. In other words, the breast cancer patients also have temporary decline of suppressor cells after removal or successful treatment of primary tumors, which provides hope that additional activation of the immune system by immunotherapeutic agents during this time period may be more effective in the eradication of micro-metastatic disease. Interestingly, the data from a recent immunotherapeutic clinical trial of patients with a history of premalignant lesions directly demonstrated that significantly lower levels of pre-existing circulating MDSCs were observed in responders as compared with non-responding subjects [48]. This “window of opportunity” could define a period of maximal efficacy for immunotherapeutic strategies and could potentially be further expanded by using a combination of anti-immunosuppressive agents with immunotherapy, increasing the effect of immunotherapy in post-surgery patients with occult micro-metastatic disease. We believe that the data presented in this report provide additional support for the BALB/c 4T1 mammary carcinoma model as a model for evaluating such therapeutic strategies, particularly immunotherapeutic strategies, in a setting comparable to the human clinical situation where occult micro-metastatic disease is the target.
Supplementary Material
Acknowledgment
This work was supported by Susan Komen Foundation BCTR0707720 for E.L.N. and M.G.A., and in part by Award Number P30CA062203 from the National Cancer Institute. H.D. was supported by T32 training grant (AG000096) from the National Institute of Aging. We would like to thank Dr. Amanda Laust Anderson and Mr. Tigran Tiraturyan for help in some experimental procedures, Dr. Annette Marleau and Dr. Irina Petrushina for their valuable suggestions and editing of the manuscript.
Abbreviations
- MDSC
myeloid derived suppressor cells
- TAA
tumor associated antigen
- Treg
regulatory T cells
- TD
tumor diameter
- PMC
pulmonary mononuclear cells
- DC
dendritic cells
- TAM
tumor associated macrophages
Footnotes
Declaration of competing interests
Author(s) declare that they have no competing interests.
References
- 1.American Cancer Society. Cancer Facts & Figures. 2013 [Google Scholar]
- 2.Hutchinson L, Kirk R. New paradigms to explain metastasis. Nat Rev Clin Oncol. 2011;8(6):313. doi: 10.1038/nrclinonc.2011.68. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Yang JC, Restifo NP. Reply to "Cancer vaccines: pessimism in check". Nat Med. 2004;10(12):1279–1280. doi: 10.1038/nm1204-1279a. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10(9):909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tarhini AA. Tremelimumab: a review of development to date in solid tumors. Immunotherapy. 2013;5(3):215–229. doi: 10.2217/imt.13.9. [DOI] [PubMed] [Google Scholar]
- 6.Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res. 2013;19(5):1021–1034. doi: 10.1158/1078-0432.CCR-12-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montero AJ, Diaz-Montero CM, Kyriakopoulos CE, Bronte V, Mandruzzato S. Myeloid-derived suppressor cells in cancer patients: a clinical perspective. J Immunother. 2012;35(2):107–115. doi: 10.1097/CJI.0b013e318242169f. [DOI] [PubMed] [Google Scholar]
- 9.Bronte V, Mocellin S. Suppressive influences in the immune response to cancer. J Immunother. 2009;32(1):1–11. doi: 10.1097/CJI.0b013e3181837276. [DOI] [PubMed] [Google Scholar]
- 10.Nagaraj S, Gabrilovich DI. Myeloid-derived suppressor cells in human cancer. Cancer J. 2010;16(4):348–353. doi: 10.1097/PPO.0b013e3181eb3358. [DOI] [PubMed] [Google Scholar]
- 11.Ostrand-Rosenberg S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother. 2010;59(10):1593–1600. doi: 10.1007/s00262-010-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rudensky AY. Regulatory T cells and Foxp3. Immunol Rev. 2011;241(1):260–268. doi: 10.1111/j.1600-065X.2011.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 14.Begley CG, Ellis LM. Drug development: Raise standards for preclinical cancer research. Nature. 2012;483(7391):531–533. doi: 10.1038/483531a. [DOI] [PubMed] [Google Scholar]
- 15.Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;52(6):1399–1405. [PubMed] [Google Scholar]
- 16.Heppner GH, Miller FR, Shekhar PM. Nontransgenic models of breast cancer. Breast Cancer Res. 2000;2(5):331–334. doi: 10.1186/bcr77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pulaski BA, Ostrand-Rosenberg S. Mouse 4T1 breast tumor model. In: Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W, editors. Current protocols in immunology. vol 4. New York: John Wiley; 2001. 20.22.21. [DOI] [PubMed] [Google Scholar]
- 18.Pulaski BA, Terman DS, Khan S, Muller E, Ostrand-Rosenberg S. Cooperativity of Staphylococcal aureus enterotoxin B superantigen, major histocompatibility complex class II, CD80 for immunotherapy of advanced spontaneous metastases in a clinically relevant postoperative mouse breast cancer model. Cancer Res. 2000;60(10):2710–2715. [PubMed] [Google Scholar]
- 19.Pulaski BA, Ostrand-Rosenberg S. Reduction of established spontaneous mammary carcinoma metastases following immunotherapy with major histocompatibility complex class II and B7.1 cell-based tumor vaccines. Cancer Res. 1998;58(7):1486–1493. [PubMed] [Google Scholar]
- 20.Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67(20):10019–10026. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Younos I, Donkor M, Hoke T, Dafferner A, Samson H, Westphal S, Talmadge J. Tumor- and organ-dependent infiltration by myeloid-derived suppressor cells. Int Immunopharmacol. 2011;11(7):816–826. doi: 10.1016/j.intimp.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 22.Chaput N, Darrasse-Jeze G, Bergot AS, Cordier C, Ngo-Abdalla S, Klatzmann D, Azogui O. Regulatory T cells prevent CD8 T cell maturation by inhibiting CD4 Th cells at tumor sites. J Immunol. 2007;179(8):4969–4978. doi: 10.4049/jimmunol.179.8.4969. [DOI] [PubMed] [Google Scholar]
- 23.Darrasse-Jeze G, Bergot AS, Durgeau A, Billiard F, Salomon BL, Cohen JL, Bellier B, Podsypanina K, Klatzmann D. Tumor emergence is sensed by self-specific CD44hi memory Tregs that create a dominant tolerogenic environment for tumors in mice. J Clin Invest. 2009;119(9):2648–2662. doi: 10.1172/JCI36628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danna EA, Sinha P, Gilbert M, Clements VK, Pulaski BA, Ostrand-Rosenberg S. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res. 2004;64(6):2205–2211. doi: 10.1158/0008-5472.can-03-2646. [DOI] [PubMed] [Google Scholar]
- 25.Ruiterkamp J, Ernst MF. The role of surgery in metastatic breast cancer. Eur J Cancer. 2011;47(Suppl 3):S6–S22. doi: 10.1016/S0959-8049(11)70142-3. [DOI] [PubMed] [Google Scholar]
- 26.Kurosawa S, Kato M. Anesthetics, immune cells, and immune responses. J Anesth. 2008;22(3):263–277. doi: 10.1007/s00540-008-0626-2. [DOI] [PubMed] [Google Scholar]
- 27.Vasilevko V, Ghochikyan A, Sadzikava N, Petrushina I, Tran M, Cohen EP, Kesslak PJ, Cribbs DH, Nicolson GL, Agadjanyan MG. Immunization with a vaccine that combines the expression of MUC1 and B7 co-stimulatory molecules prolongs the survival of mice and delays the appearance of mouse mammary tumors. Clin Exp Metastasis. 2003;20(6):489–498. doi: 10.1023/a:1025802610724. [DOI] [PubMed] [Google Scholar]
- 28.Loukinov D, Ghochikyan A, Mkrtichyan M, Ichim TE, Lobanenkov VV, Cribbs DH, Agadjanyan MG. Antitumor efficacy of DNA vaccination to the epigenetically acting tumor promoting transcription factor BORIS and CD80 molecular adjuvant. J Cell Biochem. 2006;98(5):1037–1043. doi: 10.1002/jcb.20953. [DOI] [PubMed] [Google Scholar]
- 29.Mkrtichyan M, Ghochikyan A, Loukinov D, Davtyan H, Ichim TE, Cribbs DH, Lobanenkov VV, Agadjanyan MG. DNA, but not protein vaccine based on mutated BORIS antigen significantly inhibits tumor growth and prolongs the survival of mice. Gene Ther. 2008;15(1):61–64. doi: 10.1038/sj.gt.3303044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monzavi-Karbassi B, Whitehead TL, Jousheghany F, Artaud C, Hennings L, Shaaf S, Slaughter A, Korourian S, Kelly T, Blaszczyk-Thurin M, Kieber-Emmons T. Deficiency in surface expression of E-selectin ligand promotes lung colonization in a mouse model of breast cancer. Int J Cancer. 2005;117(3):398–408. doi: 10.1002/ijc.21192. [DOI] [PubMed] [Google Scholar]
- 31.Ziegler SF, Ramsdell F, Hjerrild KA, Armitage RJ, Grabstein KH, Hennen KB, Farrah T, Fanslow WC, Shevach EM, Alderson MR. Molecular characterization of the early activation antigen CD69: a type II membrane glycoprotein related to a family of natural killer cell activation antigens. Eur J Immunol. 1993;23(7):1643–1648. doi: 10.1002/eji.1830230737. [DOI] [PubMed] [Google Scholar]
- 32.Dexter DL, Kowalski HM, Blazar BA, Fligiel Z, Vogel R, Heppner GH. Heterogeneity of tumor cells from a single mouse mammary tumor. Cancer Res. 1978;38:3174–3181. [PubMed] [Google Scholar]
- 33.Miller BE, Miller FR, Leith J, Heppner GH. Growth interaction in vivo between tumor subpopulations derived from a single mouse mammary tumor. Cancer Res. 1980;40(11):3977–3981. [PubMed] [Google Scholar]
- 34.Lelekakis M, Moseley JM, Martin TJ, Hards D, Williams E, Ho P, Lowen D, Javni J, Miller FR, Slavin J, Anderson RL. A novel orthotopic model of breast cancer metastasis to bone. Clin Exp Metastasis. 1999;17(2):163–170. doi: 10.1023/a:1006689719505. [DOI] [PubMed] [Google Scholar]
- 35.Sinha P, Clements VK, Ostrand-Rosenberg S. Reduction of myeloid-derived suppressor cells and induction of m1 macrophages facilitate the rejection of established metastatic disease. J Immunol. 2005;174(2):636–645. doi: 10.4049/jimmunol.174.2.636. [DOI] [PubMed] [Google Scholar]
- 36.Olkhanud PB, Baatar D, Bodogai M, Hakim F, Gress R, Anderson RL, Deng J, Xu M, Briest S, Biragyn A. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res. 2009;69(14):5996–6004. doi: 10.1158/0008-5472.CAN-08-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181(8):5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melani C, Chiodoni C, Forni G, Colombo MP. Myeloid cell expansion elicited by the progression of spontaneous mammary carcinomas in c-erbB-2 transgenic BALB/c mice suppresses immune reactivity. Blood. 2003;102(6):2138–2145. doi: 10.1182/blood-2003-01-0190. [DOI] [PubMed] [Google Scholar]
- 39.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58(1):49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu J, Du W, Yan F, Wang Y, Li H, Cao S, Yu W, Shen C, Liu J, Ren X. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol. 2013;190(7):3783–3797. doi: 10.4049/jimmunol.1201449. [DOI] [PubMed] [Google Scholar]
- 41.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Q, Pan PY, Gu P, Xu D, Chen SH. Role of immature myeloid Gr-1+ cells in the development of antitumor immunity. Cancer Res. 2004;64(3):1130–1139. doi: 10.1158/0008-5472.can-03-1715. [DOI] [PubMed] [Google Scholar]
- 43.Narita Y, Wakita D, Ohkur T, Chamoto K, Nishimura T. Potential differentiation of tumor bearing mouse CD11b+Gr-1+ immature myeloid cells into both suppressor macrophages and immunostimulatory dendritic cells. Biomed Res. 2009;30(1):7–15. doi: 10.2220/biomedres.30.7. [DOI] [PubMed] [Google Scholar]
- 44.Ostrand-Rosenberg S, Clements VK, Terabe M, Park JM, Berzofsky JA, Dissanayake SK. Resistance to metastatic disease in STAT6-deficient mice requires hemopoietic and nonhemopoietic cells and is IFN-gamma dependent. J Immunol. 2002;169(10):5796–5804. doi: 10.4049/jimmunol.169.10.5796. [DOI] [PubMed] [Google Scholar]
- 45.Gonda K, Shibata M, Ohtake T, Yasuda M, Abe N, Watanabe K, Ando J, Okano M, Onozawa H, Tachibana K, Ohto H, Takenoshita S. Myeloid-derived suppressor cells in patients with breast cancer. Gan To Kagaku Ryoho. 2012;39(9):1363–1368. [PubMed] [Google Scholar]
- 46.Ohki S, Shibata M, Gonda K, Machida T, Shimura T, Nakamura I, Ohtake T, Koyama Y, Suzuki S, Ohto H, Takenoshita S. Circulating myeloid-derived suppressor cells are increased and correlate to immune suppression, inflammation and hypoproteinemia in patients with cancer. Oncol Rep. 2012;28(2):453–458. doi: 10.3892/or.2012.1812. [DOI] [PubMed] [Google Scholar]
- 47.Verma C, Eremin JM, Robins A, Bennett AJ, Cowley GP, El-Sheemy MA, Jibril JA, Eremin O. Abnormal T regulatory cells (Tregs: FOXP3+, CTLA-4+), myeloid-derived suppressor cells (MDSCs: monocytic, granulocytic) and polarised T helper cell profiles (Th1, Th2, Th17) in women with large and locally advanced breast cancers undergoing neoadjuvant chemotherapy (NAC) and surgery: failure of abolition of abnormal treg profile with treatment and correlation of treg levels with pathological response to NAC. J Transl Med. 2013;11:16. doi: 10.1186/1479-5876-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kimura T, McKolanis JR, Dzubinski LA, Islam K, Potter DM, Salazar AM, Schoen RE, Finn OJ. MUC1 Vaccine for Individuals with Advanced Adenoma of the Colon: A Cancer Immunoprevention Feasibility Study. Cancer Prev Res (Phila) 2013;6(1):18–26. doi: 10.1158/1940-6207.CAPR-12-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.