Abstract
Objective
Platelet-derived growth factor receptor alpha (PDGFRα) is believed to be associated with cell survival. We examined (i) whether PDGFRα blockade enhances the antitumor activity of taxanes in ovarian carcinoma and (ii) potential biomarkers of response to anti-PDGFRα therapy.
Methods
PDGFRα expression in 176 ovarian carcinomas was evaluated with tissue microarray and correlated to survival outcome. Human-specific monoclonal antibody to PDGFRα (IMC-3G3) was used for in vitro and in vivo experiments with or without docetaxel. Gene microarrays and reverse-phase protein arrays with pathway analyses were performed to identify potential predictive biomarkers.
Results
When compared to low or no PDGFRα expression, increased PDGFRα expression was associated with significantly poorer overall survival of patients with ovarian cancer (P = 0.014). Although treatment with IMC-3G3 alone did not affect cell viability or increase apoptosis, concurrent use of IMC-3G3 with docetaxel significantly enhanced sensitization to docetaxel and apoptosis. In an orthotopic mouse model, IMC-3G3 monotherapy had no significant antitumor effects in SKOV3-ip1 (low PDGFRα expression), but showed significant antitumor effects in HeyA8-MDR (high PDGFRα expression). Concurrent use of IMC-3G3 with docetaxel, compared with use of docetaxel alone, significantly reduced tumor weight in all tested cell lines. In protein ontology, the EGFR and AKT pathways were downregulated by IMC-3G3 therapy. MAPK and CCNB1 were downregulated only in the HeyA8-MDR model.
Conclusion
These data identify IMC-3G3 as an attractive therapeutic strategy and identify potential predictive markers for further development.
Keywords: Platelet-derived growth factor receptor alpha, Ovarian cancer, MAPK, EGFR, IMC-3G3
Introduction
Ovarian carcinoma is the most common cause of death among gynecologic malignancies. In 2013, over 22,200 women in the United States will be estimated to be diagnosed with ovarian carcinoma, and over 14,000 will die of this disease [1]. Although most patients with ovarian carcinoma initially respond to primary cytoreductive surgery and platinum-based chemotherapy, most of these women eventually develop recurrences and succumb to their disease [2,3]. These observations drive the urgent need to gain a better understanding of the mechanisms contributing to disease progression and to identify novel therapeutic targets.
Platelet-derived growth factor receptor alpha (PDGFRα) is a class III receptor tyrosine kinase that is physiologically associated with various developmental stages including development of the cardiovascular and central nervous systems [4]. PDGFRα also plays a crucial role in tumor growth and survival via inhibition of apoptosis signaling [5]. In ovarian carcinoma, some studies have examined the biological role of PDGFRα and its efficacy as a therapeutic target. A large percentage of tumors with epithelial ovarian carcinoma have been reported to express PDGFRα (56%–97%) [6,7], but PDGFRα mutations are largely absent [8]. Stimulation of PDGFRα with its ligand, such as PDGF-AA, increases cell proliferation and activation of AKT and MAPK signaling, and neutralizing PDGFRα inhibited these signaling effects [6]. However, the biological roles of PDGFRα in ovarian cancer are not well understood. Here, we demonstrated that PDGFRα blockade with a neutralizing mono-clonal antibody enhances the antitumor activity of taxanes in ovarian carcinoma. Moreover, we identified potential biomarkers to predict response to anti-PDGFRα therapy.
Material and methods
Drugs and reagents
See Supplemental file for description (Method S1). IMC-3G3 is a fully human anti-PDGFRα monoclonal antibody stocked as a 5 mg/mL concentration (ImClone Systems, New York, NY) [9].
Cell lines and cultures
See Supplemental file for description (Method S2).
Polymerase chain reaction
See Supplemental file for description (Method S3).
Apoptosis assay
See Supplemental file for description (Method S4).
Cytotoxicity assays
Cytotoxicity associated with the docetaxel treatment, IMC-3G3 monotherapy, and docetaxel with IMC-3G3 treatment for various ovarian cancer cell lines was assessed with a 3-(4,5-dimethylthiazol-2-yl)-2,5-di-phenyltetrazolium bromide (MTT) uptake assay (Sigma-Aldrich), as described previously [10]. Briefly, 2 × 103 cells with serum-containing medium were plated in each well of a 96-well plate and incubated for 24 h. The medium was then replaced with fresh serum-free medium containing various concentrations of drugs (200 μL). Treatment was stopped at 24-, 48-, and 72-hour time points, and 0.15% MTT (50 μL) was added to each well. After 2 h of incubation (37 °C), the medium was removed from each well, and 100 μL of DMSO (Sigma-Aldrich) was added. The absorbance at 570 nm wavelengths was measured by using a Falcon micro-plate reader (Becton Dickinson Labwave, Franklin Lakes, NJ). Each sample was analyzed in triplicate.
Western blot analysis
Preparation of lysates from cultured cells was performed as previously described [11,12]. Briefly, cells with 80% confluence were harvested and lysed in modified radioimmunoprecipitation (RIPA) assay buffer (50 mmol/L Tris, 150 mmol/L NaCl, 1% Triton X-100, 0.5% deoxycholate, 25 μg/mL leupeptin, 10 μg/mL aprotinin, 2 mmol/L EDTA, 1 mmol/L sodium orthovanadate), as described previously [12]. Protein concentrations were measured with a BCA Protein Assay Reagent Kit (Pierce Bio-technology, Rockford, IL), and 50 μg of lysate protein was mixed with sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) on 6% gels transferred electrophoretically onto a nitrocellulose membrane. Nonfat milk powder (5%) in TBS-T (10 mmol/L Tris [pH 8], 150 mmol/L NaCl, 0.05% Tween 20) was used for protein block for 1 h. The blots were incubated with anti-PDGFRα (1:500) and anti-phospho-PDGFRα (1:1000) antibodies at 4 °C overnight and then washed with TBS-T. Antibody binding was probed by incubating the blots with horseradish peroxidase-conjugated goat anti-rabbit antibodies (GE Healthcare, Waukesha, WI) in 5% milk diluted with TBS-T for 1 h at room temperature. Reactivity was visualized with the use of an enhanced chemiluminescence detection kit (Pierce Biotechnology). Anti-vinculin (1:5000) was used to evaluate an equal protein loading. Densitometry (ImageJ, NIH) was used to interpret the differences in the Western blots.
Reverse-phase protein array analysis
Expression of 182 proteins was evaluated in 2 ovarian cancer cell lines (SKOV3-ip1 and HeyA8-MDR) in 3 different treatment groups (control, PDGF-AA alone, PDGF-AA with IMC-3G3). Briefly, SKOV3-ip1 or HeyA8-MDR cells were prepared and treated in a fashion similar to that used in the microarray analysis. Early (6 h) and late (24 h) time points were used for the treatment periods. Then, protein was isolated by using the lysis buffer as previously described (1% Triton X-100, 50 nM HEPES, pH 7.4, 150 mM MgCl2, 1 mM EDTA, 100 mM Naf, 10 mM Na pyrophosphate, 1 mM Na3VO4, 10% glycerol, and protease/phosphatase inhibitors) [13]. Denaturing of cellular proteins was done with the use of 1% SDS.
Reverse-phase protein array (RPPA) was performed per manufacturer’s instructions, as follows [13,14]: Cell lysates were two-fold-serial diluted for 5 dilutions (from undiluted to 1:16 dilution) and arrayed on nitrocellulose-coated slide in an 11 × 11 format. Samples were probed with antibodies by the CSA amplification approach and visualized by diaminobenzidine (DAB) colorimetric reaction. Slides were scanned on a flatbed scanner to produce 16-bit TIFF image. Spots from TIFF images were identified and the density was quantified by MicroVigene (Vigene Tech, Carlisle, MA). Relative protein levels for each sample were determined by interpolation of each dilution curves from the “standard curve” (supercurve) of the slide (antibody). A supercurve is constructed by a script in R written by Bioinformatics [15]. These values (given as log 2 values) are defined as “supercurve log 2 value.” All of the data points were normalized for protein loading and transformed to a linear value designated as “linear after normalization.” This value was transformed to natural log value and median-centered for Hierarchical Cluster analysis.
Animal care
Nude mice (athymic females, Ncr-nu, 8–12 weeks old) were purchased from the National Cancer Institute/Frederick Cancer Research and Development Center. The mice were quarantined, housed, and maintained in a specific pathogen-free environment in an animal facility approved by the American Association for Accreditation of Laboratory Animal Care in agreement with the current regulations and standards of the United States Department of Agriculture, the Department of Health and Human Services, and the National Institutes of Health. Approval of the study protocols was obtained and supervised by the Institutional Animal Care and Use Committee at The University of Texas MD Anderson Cancer Center.
In vivo therapeutic experiment
Four ovarian carcinoma cell lines were used in the in vivo therapeutic experiments. Cells from ovarian serous carcinoma cell lines SKOV3-ip1 (1 × 106 cells/mouse), HeyA8 (2.5 × 105 cells/mouse), and HeyA8-MDR (1 × 106 cells/mouse) and from ovarian clear cell carcinoma cell line ES2 (2.5 × 105 cells/mouse) were injected into the peritoneal cavity of 40 orthotopic nude mice per cell line. Each group of mice representing each ovarian carcinoma cell line was randomized into 4 subgroups of 10 mice according to type of treatment (control, docetaxel treatment alone, IMC-3G3 treatment alone, and docetaxel with IMC-3G3), and treatment was initiated 1 week after the carcinoma cells were injected. Docetaxel (30 μg/mouse for mice that received SKOV3-ip1, HeyA8, and ES2 cells; 50 μg/mouse for mice that received HeyA8-MDR cells) was given intraperitoneally once per week in docetaxel alone and docetaxel with IMC-3G3 groups after being dissolved in PBS. IMC-3G3 (40 mg/kg/mouse) was given intraperitoneally three times per week in IMC-3G3 monotherapy and docetaxel with IMC-3G3 groups as described previously [16]. Control mice received PBS intraperitoneally three times per week.
Mice were monitored daily and weighed weekly. After approximately 5 to 6 weeks of treatment, the mice were sacrificed with cervical dislocation, and the total body weight of each mouse, tumor locations and weight, and number of tumor nodules were recorded. Tumor samples were fixed with 10% formalin and embedded in paraffin or with OCT compound in liquid nitrogen.
In vivo tumor regression experiment
The luciferase-transfected HeyA8-MDR cell line (HeyA8-MDR-Luc), established with a lentivirus system as previously described, was used for the experiment [17]. Briefly, HeyA8-MDR-Luc cells (1 × 106 cells/mouse) were injected into the peritoneal cavity of 40 nude mice. Mice were randomized into 4 subgroups according to type of treatment as in the therapeutic experiment, and treatment was initiated 17 days after the carcinoma cells were injected. The treatment doses for HeyA8-MDR-injected mice were similar to those used in the therapeutic experiment. The IVIS Imaging System 100 (Caliper Life Sciences, Hopkinton, MA) combined with Living Image software (Xenogen Corporation, Alameda, CA) was used for image-captioning and data acquisition. Each individual mouse was identified with an ear tag and longitudinally assessed for bioluminescence imaging at 17, 21, 23, 25, 27, and 31 days after tumor cell injection. At the end of the experiment, the mice were killed with cervical dislocation, and a necropsy was performed. The total body weight of each mouse, tumor locations and weight, and number of tumor nodules were recorded.
3D tumor vascular imaging
HeyA8-MDR cells (1 × 106 cells/mouse) were injected into the peritoneal cavity of 6 nude mice. Mice with palpable tumors in the abdomen 17 days after injection were randomized to either the control group or treatment group. For the treatment group, the docetaxel and IMC-3G3 regimen was similar to that used in the therapeutic experiment. After 1.5 weeks of treatment, mice were euthanized and the abdominal and thoracic cavities were opened to expose the heart and vascular system. A polyethylene cannula was inserted distally into the left ventricle. The right atrium was opened by incision to serve as a drain. The mouse was perfused with PBS until all of the visceral blood was flushed out. The Microfil agent was injected into the mouse through the cannula by syringe until it flowed from the outlet. The inlet and outlet points were clamped and we allowed the mouse to rest for 90 min until the polymer cured. After adequate polymerization, the tumor and surrounding vascular tissue were carefully harvested. The tumor was imaged with microtomography.
Functional analysis
Microarray and RPPA results were analyzed for functional analysis with gene ontology. Extraction of networks of molecular interactions for each dataset was performed with the use of NetWalk, a random walk-based network retrieval algorithm [18,19]. NetWalk-mediated analyses and visualizations were performed by using NetWalker, an integrated platform for network-based data analyses and visualization [20]. Intra- and inter-cell line comparisons were evaluated for SKOV3-ip1 and HeyA8-MDR cell lines.
Tissue microarray analysis
On approval of the Institutional Review Board at Wayne State University, tissue microarray analysis of formalin-fixed paraffin-embedded ovarian carcinoma tissues was performed to evaluate PDGFRα expression (N = 176). The results were dichotomized into PDGFRα expression (intensity ≥ 1+) versus non-expression and were correlated to clinical variables and survival outcomes.
Statistical analysis
See Supplemental file for detail (Method S5).
Results
PDGFRα expression and survival outcomes in patients with ovarian carcinoma
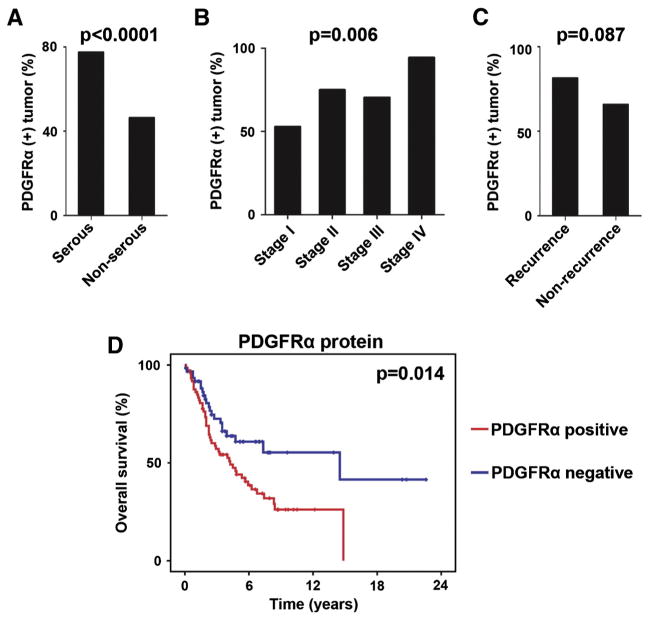
First, we examined the extent of PDGFRα protein expression in 176 human ovarian tumors and correlated the findings to disease severity and survival outcomes (patient clinical demographics are in Table S1). PDGFRα expression was detected in 70% (95%CI 63–77%) of all cases. In logistic regression analysis, PDGFRα expression was significantly associated with serous histology (serous versus non-serous, 77% versus 46%, respectively; OR 4.0, 95%CI 1.9 to 8.3; P < 0.0001; Fig. 1A) and advanced stage (OR 1.7, 95%CI 1.2–2.3; P = 0.006; Fig. 1B). There was a non-significant increase in recurrence rate for PDGFRα-expressing tumors compared with those not expressing PDGFRα (81% versus 66%, respectively; OR 2.3, 95%CI 0.9–6.0; P = 0.087; Fig. 1C). The most common type of histology was high-grade serous. Among high-grade serous ovarian carcinomas in our study, PDGFRα-expressing tumors, compared with PDGFRα-non-expressing tumors, were associated with significantly poorer survival outcomes (median overall survival duration, 51 versus 174 months, respectively; P = 0.014, Fig. 1D). In a multivariate analysis, controlled for other significant variables for survival including age and stage, PDGFRα expression remained a significant variable for overall survival (OR 2.3, 95%CI 1.4–3.9, P = 0.002). Taken together, our results suggest that protein expression of PDGFRα is important in ovarian carcinoma disease progression and survival.
Fig. 1.
PDGFRα expression and clinical outcomes of ovarian carcinoma. A) Proportion of PDGFRα-expressing tumors in serous ovarian carcinoma. B) Proportion of expressed protein PDGFRα and FIGO stage. C) Proportion of protein PDGFRα-expressing tumors in ovarian carcinoma and disease recurrence. D) Overall survival and PDGFRα protein expression. Red, protein PDGFRα-expressing tumors; blue, protein PDGFRα non-expressing tumors. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
PDGFRα blockade and sensitization to docetaxel therapy
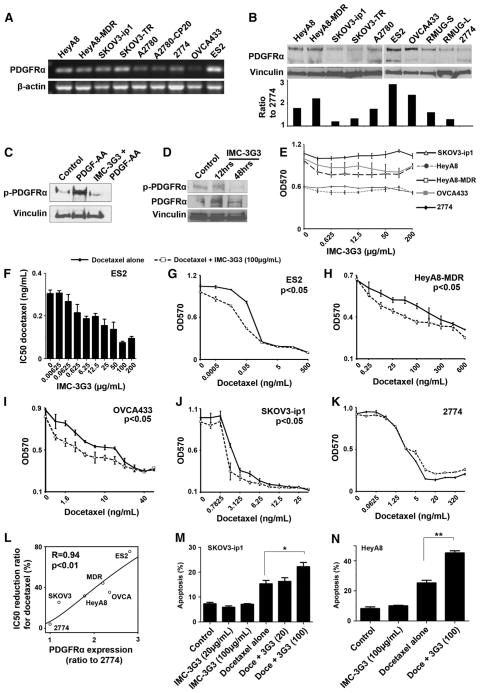
To evaluate the significance of PDGFRα blockade in ovarian carcinoma, we conducted multiple in vitro experiments by using an anti-PDGFRα monoclonal antibody (IMC-3G3). First, PDGFRα mRNA and protein expression was evaluated in various ovarian cancer cell lines. All tested ovarian cancer cell lines had variable expression of PDGFRα (higher mRNA expression in SKOV3-TR, ES2, and HeyA8; and lower mRNA expression in OVCA433, A2780, and A2780-CP20, Fig. 2A). The ligands, PDGF-A, -B, -C, and -D were also expressed at varying amounts in the ovarian cancer cell lines, and PDGF-A was most commonly expressed among the four ligands (Fig. S1). SKOV3-ip1 and 2774 cell lines showed the lowest PDGFRα protein expression, whereas HeyA8-MDR and ES2 had the highest PDGFRα protein expression (Fig. 2B).
Fig. 2.
PDGFRα blockade and docetaxel sensitization in ovarian carcinoma. A) PCR analysis for 9 ovarian carcinoma cell lines. B) Western blot of 10 ovarian carcinoma cell lines. Densitometry was used for quantification of PDGFRα expression. Bars represent the ratio of PDGFRα to 2774, the cell line that showed the least PDGFRα expression among tested cell lines. C) Neutralization of phosphorylation of PDGFRα with IMC-3G3 (100 μg/mL) demonstrated in SKOV3-ip1 cells. D) Neutralization of PDGFRα phosphorylation with IMC-3G3 (40 mg/kg/mouse, i.p.) in HeyA8 tumor-bearing mice (n = 3 per group). Mice were killed at 12 and 48 h after the second injection of IMC-3G3. E) Cell viability assay for IMC-3G3 monotherapy in 5 ovarian carcinoma cell lines. Treatment was given for 72 h. No change in cell viability was seen in any of the cell lines tested. F) Cell viability assay for the combination treatment with docetaxel and IMC-3G3 in the ES2 cell line. Treatment was given for 72 h. Sensitization to docetaxel was shown to be dose-dependent with IMC-3G3. G–K) Cell viability assay results for 5 different cell lines treated with docetaxel alone (solid line) and docetaxel with IMC-3G3 (100 μg/mL, dashed line). All cell lines except 2774 showed statistically significant sensitization to docetaxel with IMC-3G3 co-treatment. L) Correlation between the extent of PDGFRα protein expression and the extent of docetaxel sensitization; the correlation was statistically significant (r = 0.94, P < 0.01). M–N) Apoptosis assay for docetaxel and IMC-3G3 is shown at the 72-hour time point in SKOV3-ip1 (M) and HeyA8 (N). The proportion of total apoptosis was determined by flow cytometry. Although there was no change in the proportion of apoptosis in IMC-3G3 monotherapy, apoptosis in docetaxel used with IMC-3G3, compared with apoptosis in docetaxel used alone, increased significantly (* p < 0.05, ** p < 0.01). The dot and error bar represent the mean with SE in (E) and (G) through (K). The bar represents the mean with SE in (F), (M), and (N).
The efficacy of IMC-3G3 on PDGFRα blockade was also evaluated (Fig. 2C). SKOV3-ip1 cells treated with PDGF-AA showed induction of PDGFRα phosphorylation. IMC-3G3 treatment substantially blocked phosphorylation of PDGFRα in response to PDGF-AA exposure (Fig. 2C). To determine in vivo effects, the efficacy of IMC-3G3 on PDGFRα phosphorylation was examined in HeyA8 tumor-bearing mice (Fig. 2D), which were intraperitoneally injected with IMC-3G3 (40 mg/kg) for 2 doses given 48 h apart. Tumors obtained at 12 and 48 h after the second dose of IMC-3G3 injection were evaluated for phosphorylation of PDGFRα, which showed a significant downregulation of PDGFRα phosphorylation at the 48-hour time point (Fig. 2D).
Next, we determined the in vitro effects of PDGFRα blockade on ovarian cancer cell viability. Five different ovarian cancer cell lines were treated with various doses of IMC-3G3 (Fig. 2E). None of the cell lines tested showed changes in cell viability with IMC-3G3 monotherapy. However, IMC-3G3 treatment with docetaxel resulted in sensitization to docetaxel therapy in a dose-dependent fashion in the ES2 cell line (50% inhibitory concentration [IC50] reduction with IMC-3G3 at 100 μg/mL, 75.2%, Fig. 2F–G). Sensitization to docetaxel therapy after treatment with combined docetaxel plus IMC-3G3 pretreatment was also seen in the HeyA8-MDR (IC50 reduction with IMC-3G3 at 100 μg/mL, 44.2%, Fig. 2H), OVCA433 (35.3%, Fig. 2I), HeyA8 (31.7%), and SKOV3-ip1 (25.5%, Fig. 2J) cells (all, P < 0.05). However, IMC-3G3 treatment did not result in sensitization to docetaxel in the 2774 cells (IC50 reduction with IMC-3G3 at 100 μg/mL, 4.0%, Fig. 2K). Among these 6 cell lines, the extent of PDGFRα protein expression was significantly associated with the extent of sensitization to docetaxel (Spearman’s correlation r = 0.94, P < 0.01, Fig. 2L).
Next, we examined the effects of PDGFRα blockade on cancer cell apoptosis (Fig. 2M–N). The SKOV3-ip1 and HeyA8 cell lines were treated with either IMC-3G3 (100 μg/mL), docetaxel, or a combination of the two drugs for 72 h. Consistent with the cell viability assays, in which none of the cell lines tested showed changes in cell viability with IMC-3G3 monotherapy, the proportion of apoptosis did not increase in the cell lines treated with IMC-3G3 monotherapy. However, treatment with combined IMC-3G3 and docetaxel induced significant apoptosis in the two cell lines; the percent increase in apoptosis with combined treatment compared with the percent increase with docetaxel treatment alone was as follows: SKOV3-ip1, 44.2%, P < 0.05 (Fig. 2M); and HeyA8, 78.7%, P < 0.01 (Fig. 2N). These in vitro data indicate that PDGFRα serves as a survival factor for ovarian cancer cells and PDGFRα blockade sensitizes the cancer cells to chemotherapy.
Differences in antitumor activity with PDGFRα blockade among PDGFRα-expressing tumors
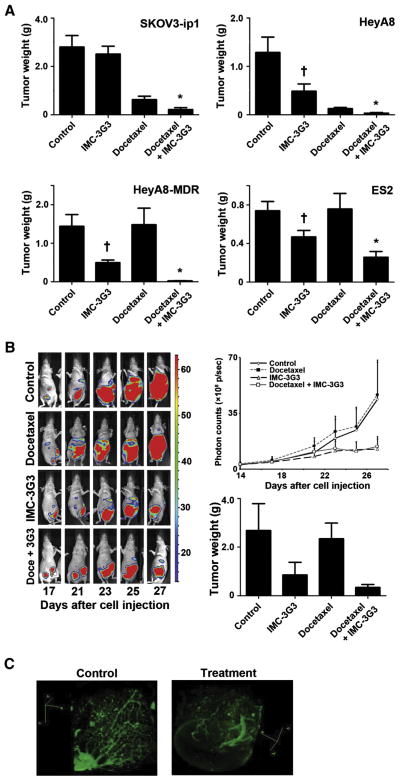
Next, we conducted in vivo therapy experiments to evaluate the anti-tumor effects of IMC-3G3 treatment (Fig. 3A). In SKOV3-ip1 tumor-bearing mice, administration of IMC-3G3 monotherapy had no significant antitumor effects compared with the control group (mean tumor weight, 2.5 ± 0.32 versus 2.9 ± 0.51 g, respectively; P > 0.05). Concurrent use of IMC-3G3 with docetaxel significantly reduced tumor weight compared with use of docetaxel alone (mean tumor weight, 0.22 ± 0.07 versus 0.63 ± 0.14 g, respectively; tumor reduction rate, 65%; P < 0.05). In contrast, in HeyA8 tumor-bearing mice, administration of IMC-3G3 monotherapy showed significant antitumor effects compared with the control group (mean tumor weight, 0.49 ± 0.15 versus 1.29 ± 0.32 g, respectively; tumor reduction rate, 62%; P < 0.05). As in the SKOV3-ip1 tumor-bearing mice, concurrent use of IMC-3G3 with docetaxel significantly reduced tumor weight compared with use of docetaxel alone in HeyA8 tumor-bearing mice (mean tumor weight, 0.033 ± 0.013 versus 0.13 ± 0.025 g, respectively; tumor reduction rate, 74%; P < 0.01). Similar results were seen in the multidrug-resistant HeyA8-MDR model, and IMC-3G3 monotherapy showed significant antitumor effects compared with the control group (mean tumor weight, 0.5 ± 0.07 versus 1.44 ± 0.31 g; respectively; tumor reduction rate, 66%; P < 0.05). Although docetaxel treatment did not result in significant antitumor effects compared with the control group, concurrent use of IMC-3G3 with docetaxel significantly reduced tumor weight compared with use of docetaxel alone (mean tumor weight, 0.03 ± 0.004 versus 1.48 ± 0.43 g, respectively; tumor reduction rate, 98%; P = 0.01). Even in a short-term therapy experiment conducted with the ES2 model (2 weeks of treatment), administration of IMC-3G3 monotherapy showed significant antitumor effects compared with the control group (mean tumor weight, 0.47 ± 0.07 versus 0.74 ± 0.1 g, respectively; tumor reduction rate, 37%; P < 0.05). Our in vivo therapeutic experiments suggest that IMC-3G3 significantly enhances the antitumor effects of docetaxel.
Fig. 3.
Antitumor effects of PDGFRα blockade in ovarian carcinoma. A) In vivo therapy experiments for 4 different ovarian carcinoma cell lines. Treatment was given for 5–6 weeks (weekly docetaxel i.p. and IMC-3G3 three times a week i.p.). A short-term (2-week) experiment was conducted in the ES2 cell line. †Significant antitumor effect with IMC-3G3 monotherapy was seen in HeyA8, HeyA8-MDR, and ES2. No antitumor effect was seen with IMC-3G3 monotherapy in SKOV3-ip1 tumor-bearing mice. *Significant sensitization of docetaxel antitumor effect was seen in all 4 tested cell lines with co-administration of IMC-3G3 and docetaxel. The bar represents mean with SE. B) Salvage therapy experiment with drug-resistant ovarian carcinoma cell line (HeyA8-MDR-Luc). Treatment was initiated 17 days after cell injection. Borderline significance was seen in the IMC-3G3 monotherapy group as well as in the docetaxel with IMC-3G3 treatment group when compared with the control group (P = 0.07 and .054, respectively). Error bars represent SE. C) 3D tumor vascular imaging is shown in HeyA8-MDR tumor. Docetaxel and IMC-3G3 combination therapy was given for treatment tumor.
Ovarian cancer often recurs, and such recurrent tumors are characterized by multidrug resistance. Thus, we asked whether IMC-3G3 could result in regression of established HeyA8-MDR-Luc tumors (Fig. 3B). Combination therapy with docetaxel and IMC-3G3 resulted in significantly smaller tumor weight compared with docetaxel treatment alone (mean tumor weight, 0.34 ± 0.12 versus 2.34 ± 0.65 g, respectively; P < 0.05) or with the control group (mean tumor weight, 0.34 ± 0.12 versus 2.38 ± 1.11 g, respectively; P < 0.05). Although IMC-3G3-based therapy did not show tumor regression, the therapy was associated with suppression of tumor growth. In addition, the effect of IMC-3G3 therapy on tumor vasculature was evaluated in the salvage setting with use of docetaxel-resistant bulky tumors (HeyA8-MDR). Compared with controls, tumors treated with IMC-3G3-based therapy showed a markedly decreased number of microvessels (Fig. 3C).
Relevant biomarkers of anti-PDGFRα therapy
We evaluated the downstream signaling proteins following PDGFRα blockade by using cell lines that showed opposite responses to PDGFRα blockade in the in vivo experiments to identify potential pathways contributing to response to anti-PDGFRα therapy (HeyA8-MDR as the IMC-3G3-responding cell line and SKOV3-ip1 as the IMC-3G3-non-responding cell line, Fig. 3A). In patient tumor samples, protein expression, but not mRNA expression of PDGFRα was associated with patient survival (Fig. 1D). Therefore, we examined protein-to-protein interactions in relation to PDGFRα blockade with RPPA between these two cell lines. Because changes in protein levels generally take longer than changes in gene transcription, we initially set two different time points (early and late time points, 6 and 24 h, respectively, Fig. 4). Among the two experimental time points, the 24-hour time point was chosen for further experiments because the number of proteins changed in response to IMC-3G3 pretreatment followed by PDGF-AA exposure during this period was significantly higher than the number of proteins changed at the 6-hour time point (the proportion of changed proteins at 24 versus 6 h was 32% versus 11%, respectively; OR 3.64, 95%CI 1.9–7.0; P < 0.0001; Fig. 4).
Fig. 4.
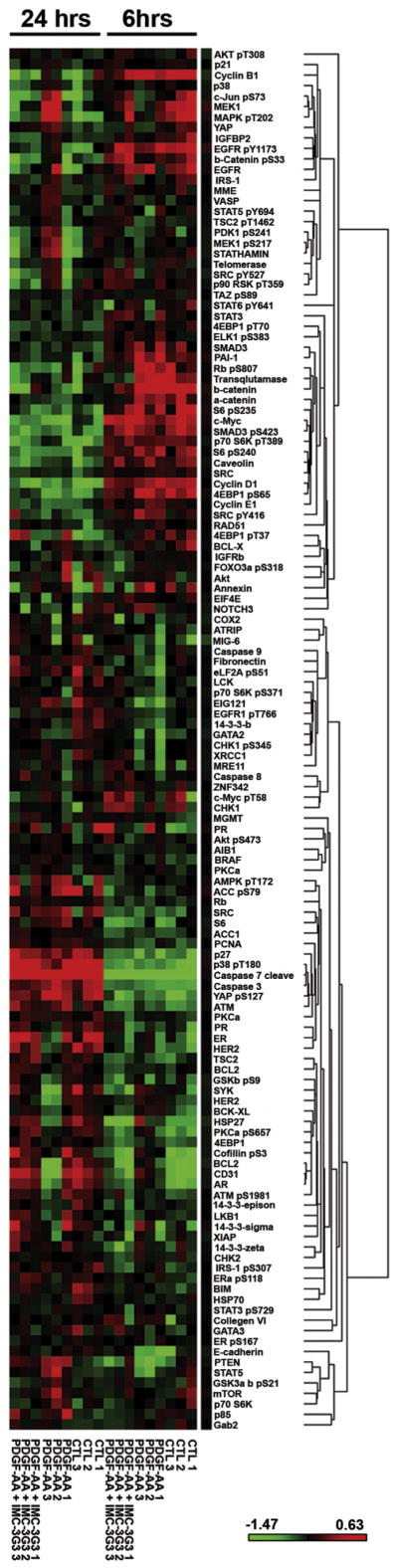
Reverse-phase protein array for PDGFRα blockade in ovarian carcinoma. Heatmap of reverse-phase protein array (RPPA) results in HeyA8-MDR (PDGFRα-sensitive cell line) treated with PDGF-AA and IMC-3G3 at an early time point (6 h) and late time point (24 h).
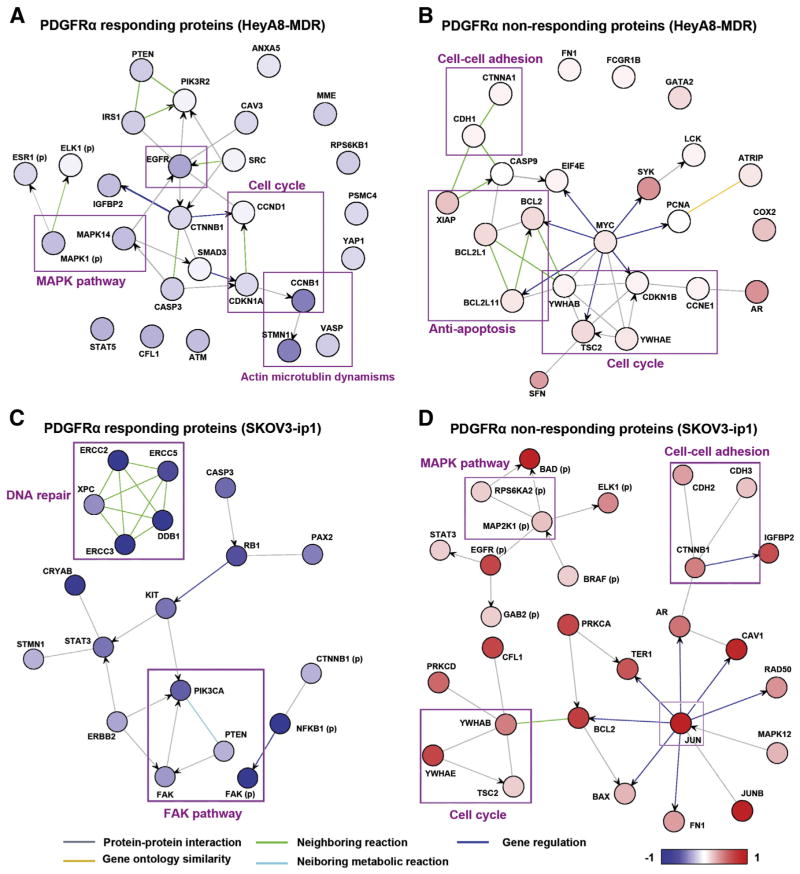
We then conducted a functional analysis of the RPPA results from the 24-hour time point in the HeyA8-MDR and SKOV3-ip1 cell lines (Fig. 5A–D). We examined the proteins that responded to IMC-3G3 treatment in the HeyA8-MDR cells, which showed antitumor activity with IMC-3G3 therapy in vivo. In this analysis, the proteins that showed downregulation of expression with IMC-3G3 treatment included those involved with (i) the MAPK pathway, (ii) EGFR, (iii) cell cycle regulation such as CCNB1, and (iv) actin microtubulin dynamisms such as STMN1 (Fig. 5A). These mechanisms may represent pathways of PDGFRα response. In contrast, groups of proteins that did not respond to IMC-3G3 treatment included those involved with (i) anti-apoptosis such as BCL2 and XIAP, (ii) cell-cell adhesion such as CDH1, and (iii) cell cycle regulation such as TSC2 (Fig. 5B). When the 6-hour time point is combined, cofilin remained the marker that showed a response to IMC-3G3 therapy at the two different time points. There are some markers that showed an initial response to IMC-3G3 therapy at the early time point, but did not respond at the later time point (e.g., BCL2, HER2, and AR).
Fig. 5.
PDGFRα blockade and protein interactions in ovarian carcinoma. A–B) Network ontology of proteins (A) increasing the expression with PDGF-AA treatment and neutralized with IMC-3G3 pretreatment, and (B) not responding to IMC-3G3 in the HeyA8-MDR cell line. C–D) Network ontology of proteins (C) increasing the expression with PDGF-AA treatment and neutralized with IMC-3G3 pretreatment, and (D) not responding to IMC-3G3 in the SKOV3-ip1 cell line. Proteins with parentheses (p) represent phosphoproteins.
We next examined proteins that responded to IMC-3G3 treatment in the SKOV3-ip1 cell line, which did not respond well to IMC-3G3 therapy in vivo. In protein ontology, groups of proteins that changed in response to IMC-3G3 treatment included those involved with (i) the FAK pathway and (ii) damaged DNA repair mechanisms such as the ERCC family (Fig. 5C). In contrast, groups of proteins that did not respond to IMC-3G3 therapy included those involved with (i) the MAPK pathway, (ii) cell cycle regulation, and (iii) cell–cell adhesion dynamism (Fig. 5D). These mechanisms may be associated with lack of response to IMC-3G3 therapy.
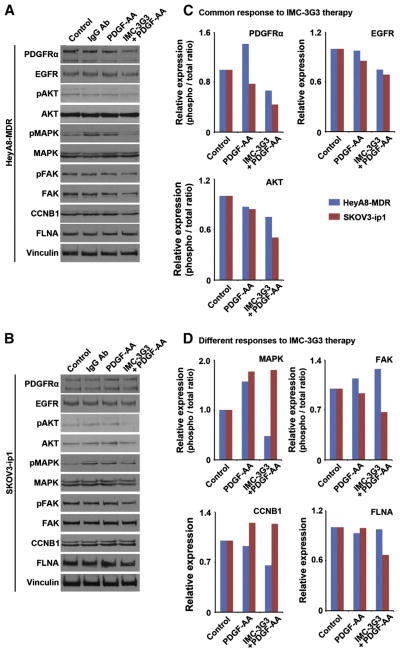
Protein expression in the two cell lines was then examined by Western blotting for the various biomarkers that were identified by the RPPA results (Fig. 6A–B). Decreased activity of EGFR and AKT with IMC-3G3 therapy was commonly seen in the two cell lines (Fig. 6C). AKT was evaluated because SKOV3-ip1 is known to have a mutation in the PIK3CA-AKT pathway. In contrast, as shown in Fig. 6D, MAPK, CCNB1, and FAK showed different responses between the HeyA8-MDR and SKOV3-ip1 cell lines: MAPK and CCNB1 were associated with IMC-3G3 response in HeyA8-MDR cells, but not in the SKOV3-ip1cells.
Fig. 6.
PDGFRα targeting and relevant biomarkers in ovarian carcinoma. A–B) Western blot analysis for 12 antibodies is shown for HeyA8-MDR (A) and SKOV3-ip1 (B). Experiment time point was at 24 h. IgG Ab, human immunoglobulin (100 μg/mL). C–D) Relative expression of protein in Western blot analysis compared with the control group of each targeted biomarker. ImageJ was used for densitometry measurement and standardized with the control group. Protein activity was evaluated for the phosphoprotein/total protein ratio in PDGFRα, AKT, MAPK, and FAK. C) Proteins that showed a common response to IMC-3G3 therapy included PDGFRα, EGFR, and AKT. D) Protein that showed different responses between HeyA8-MDR and SKOV3-ip1 in IMC-3G3 therapy included MAPK, FAK, and CCNB1.
Discussion
Our clinical, in vitro, and in vivo results highlight important features that contribute to our understanding of the mechanisms of PDGFRα targeting in ovarian carcinoma. First, PDGFRα blockade significantly enhanced sensitization to docetaxel therapy in ovarian carcinoma. Second, the differences in antitumor effects with PDGFRα blockade depended on whether MAPK or CCNB1 was downregulated. Several key areas in this notable observation deserve special mention.
In the in vitro experiments, although PDGFRα blockade alone did not affect cell viability or apoptosis in cancer cells, concurrent use of PDGFRα blockade with a cytotoxic agent significantly enhanced sensitization and apoptosis, suggesting that PDGFRα functions as a survival factor [21]. In protein array analyses, we showed that AKT was the commonly down-regulated downstream target of PDGFRα signaling, with the receptor neutralized antibody in multiple ovarian cancer cell lines. These data suggest that AKT likely plays a pivotal role in PDGFRα’s function as a survival factor. In addition, we observed that PDGFRα blockade down-regulated the expression of EGFR in ovarian cancer cells (Fig. 5C). This cross-talk between different members of the tyrosine kinase family was previously known to occur between EGFR and PDGFR [22,23]. Therefore, inter-receptor communication between EGFR and PDGFRα may also play an important role as a survival factor for cancer cells. In this setting, dual targeting of PDGFRα and EGFR may be an attractive approach to further enhance response to cytotoxic agents.
The key observation in our study is that in downstream of PDGFRα signaling, there are significant differences between tumors that respond to anti-PDGFRα therapy and those that showed no response. Representative molecules of this observation included those involved in the cell growth pathway (MAPK) and cell cycle regulation (CCNB1). That is, whereas MAPK and CCNB1 were downregulated with anti-PDGFRα antibody treatment in the cell line responsive to anti-PDGFRα therapy (HeyA8-MDR), MAPK and CCNB1 downregulation was not observed in the non-responsive cell line (SKOV3-ip1). In contrast, the cell line that did not show antitumor effects to anti-PDGFRα therapy showed an opposite response in downstream PDGFRα signaling compared with the responding cell line (FAK pathway). It is possible that signal-transduction of PDGFRα depends on cellular context, and Ras/MAPK pathway may be activated to a greater extent in PDGFRα sensitive cells [5]. Therefore, our results suggest that these biomarkers may be related to differences in the antitumor effects with anti-PDGFRα therapy and may be relevant targets of PDGFRα blockade.
In the RPPA analysis, the protein alterations were examined in cell lines treated with PDGF-AA with or without IMC-3G3 pretreatment. The HeyA8-MDR and SKOV3-ip1 cells were selected based on the in vivo response or lack thereof to IMC-3G3 treatment. Since responses to single agent IMC-3G3 monotherapy were limited under in vitro settings, it is possible that results of the RPPA analysis may vary depending on in vitro versus in vivo testing.
In summary, downstream PDGFRα signaling has an important key role in determining the response to PDGFRα targeting in ovarian carcinoma. Thus, individualized evaluation of PDGFRα signaling may be useful for predicting response to anti-PDGFRα therapy in patients with ovarian carcinoma.
Supplementary Material
HIGHLIGHTS.
Targeting PDGFRα with monoclonal antibody (IMC-3G3) significantly enhanced the efficacy of chemotherapy in ovarian cancer cells both in-vitro and in-vivo.
Ovarian cancer cells with high-PDGFRα expression showed significant antitumor effects with IMC-3G3 monotherapy, whereas those expressing low-PDGFRα did not.
MAPK and CCNB1 were associated with response to IMC-3G3 in high-PDGFRα cells that showed antitumor effects with IMC-3G3 monotherapy.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ygyno.2013.10.027.
Footnotes
Grant support: National Institutes of Health grants (CA 109298, P50 CA083639, P50 CA098258, CA128797, RC2GM092599), Ovarian Cancer Research Fund, Inc. (Program Project Development Grant), the DOD (OC073399, OC093146), Zarrow Foundation, Marcus Foundation, Betty Anne Asche Murray Distinguished Professorship, NCI-DHHS-NIH T32 Training Grant (T32 CA101642) (JNB, RLS), GCF/Gail MacNeil KOH Research Grant (RLS), Meyer and Ida Gordon Foundation #2 (KM), and GCF/OCRF Ann Schreiber Ovarian Cancer Research Grant (KM). This research was also supported, in part, by the MD Anderson’s Cancer Center Support Grant from the NIH (CA016672).
Conflict of interest statement
Dr. Loizos is employed by ImClone Systems, a wholly owned subsidiary of Eli Lilly. The other authors declare that there is no conflict of interest in the study.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Matsuo K, Lin YG, Roman LD, Sood AK. Overcoming platinum resistance in ovarian carcinoma. Expert Opin Investig Drugs. 2010;19(11):1339–54. doi: 10.1517/13543784.2010.515585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsuo K, Bond VK, Eno ML, Im DD, Rosenshein NB. Low drug resistance to both platinum and taxane chemotherapy on an in vitro drug resistance assay predicts improved survival in patients with advanced epithelial ovarian, fallopian and peritoneal cancer. Int J Cancer. 2009;125(11):2721–7. doi: 10.1002/ijc.24654. [DOI] [PubMed] [Google Scholar]
- 4.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22(10):1276–312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oseini AM, Roberts LR. PDGFRalpha: a new therapeutic target in the treatment of hepatocellular carcinoma? Expert Opin Ther Targets. 2009;13(4):443–54. doi: 10.1517/14728220902719233. [DOI] [PubMed] [Google Scholar]
- 6.Matei D, Emerson RE, Lai YC, Baldridge LA, Rao J, Yiannoutsos C, et al. Autocrine activation of PDGFRalpha promotes the progression of ovarian cancer. Oncogene. 2006;25(14):2060–9. doi: 10.1038/sj.onc.1209232. [DOI] [PubMed] [Google Scholar]
- 7.Wilczynski SP, Chen YY, Chen W, Howell SB, Shively JE, Alberts DS. Expression and mutational analysis of tyrosine kinase receptors c-kit, PDGFRalpha, and PDGFRbeta in ovarian cancers. Hum Pathol. 2005;36(3):242–9. doi: 10.1016/j.humpath.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Lassus H, Sihto H, Leminen A, Nordling S, Joensuu H, Nupponen NN, et al. Genetic alterations and protein expression of KIT and PDGFRA in serous ovarian carcinoma. Br J Cancer. 2004;91(12):2048–55. doi: 10.1038/sj.bjc.6602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah GD, Loizos N, Youssoufian H, Schwartz JD, Rowinsky EK. Rationale for the development of IMC-3G3, a fully human immunoglobulin G subclass 1 monoclonal antibody targeting the platelet-derived growth factor receptor alpha. Cancer. 2010;116(4 Suppl):1018–26. doi: 10.1002/cncr.24788. [DOI] [PubMed] [Google Scholar]
- 10.Lee JW, Han HD, Shahzad MM, Kim SW, Mangala LS, Nick AM, et al. EphA2 immunoconjugate as molecularly targeted chemotherapy for ovarian carcinoma. J Natl Cancer Inst. 2009;101(17):1193–205. doi: 10.1093/jnci/djp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halder J, Kamat AA, Landen CN, Jr, Han LY, Lutgendorf SK, Lin YG, et al. Focal adhesion kinase targeting using in vivo short interfering RNA delivery in neutral liposomes for ovarian carcinoma therapy. Clin Cancer Res. 2006;12(16):4916–24. doi: 10.1158/1078-0432.CCR-06-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mangala LS, Zuzel V, Schmandt R, Leshane ES, Halder JB, Armaiz-Pena GN, et al. Therapeutic targeting of ATP7B in ovarian carcinoma. Clin Cancer Res. 2009;15(11):3770–80. doi: 10.1158/1078-0432.CCR-08-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shahzad MM, Lu C, Lee JW, Stone RL, Mitra R, Mangala LS, et al. Dual targeting of EphA2 and FAK in ovarian carcinoma. Cancer Biol Ther. 2009;8(11):1027–34. doi: 10.4161/cbt.8.11.8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.http://inside.mdanderson.org/departments/ccsg/functional-proteomics-rppa-core/reversephase-services.html.
- 15.http://bioinformatics.mdanderson.org/OOMPA.
- 16.Loizos N, Xu Y, Huber J, Liu M, Lu D, Finnerty B, et al. Targeting the platelet-derived growth factor receptor alpha with a neutralizing human monoclonal antibody inhibits the growth of tumor xenografts: implications as a potential therapeutic target. Mol Cancer Ther. 2005;4(3):369–79. doi: 10.1158/1535-7163.MCT-04-0114. [DOI] [PubMed] [Google Scholar]
- 17.Lu C, Bonome T, Li Y, Kamat AA, Han LY, Schmandt R, et al. Gene alterations identified by expression profiling in tumor-associated endothelial cells from invasive ovarian carcinoma. Cancer Res. 2007;67(4):1757–68. doi: 10.1158/0008-5472.CAN-06-3700. [DOI] [PubMed] [Google Scholar]
- 18.Komurov K, Padron D, Cheng T, Roth M, Rosenblatt KP, White MA. Comprehensive mapping of the human kinome to epidermal growth factor receptor signaling. J Biol Chem. 2010;285(27):21134–42. doi: 10.1074/jbc.M110.137828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Komurov K, White MA, Ram PT. Use of data-biased random walks on graphs for the retrieval of context-specific networks from genomic data. PLoS Comput Biol. 6(8) doi: 10.1371/journal.pcbi.1000889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.http://research.cchmc.org/netwalker.
- 21.Kumar SR, Singh J, Xia G, Krasnoperov V, Hassanieh L, Ley EJ, et al. Receptor tyrosine kinase EphB4 is a survival factor in breast cancer. Am J Pathol. 2006;169(1):279–93. doi: 10.2353/ajpath.2006.050889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prenzel N, Zwick E, Leserer M, Ullrich A. Tyrosine kinase signalling in breast cancer. Epidermal growth factor receptor: convergence point for signal integration and diversification. Breast Cancer Res. 2000;2(3):184–90. doi: 10.1186/bcr52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu P, Anderson RG. Spatial organization of EGF receptor transmodulation by PDGF. Biochem Biophys Res Commun. 1999;261(3):695–700. doi: 10.1006/bbrc.1999.1082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.