Abstract
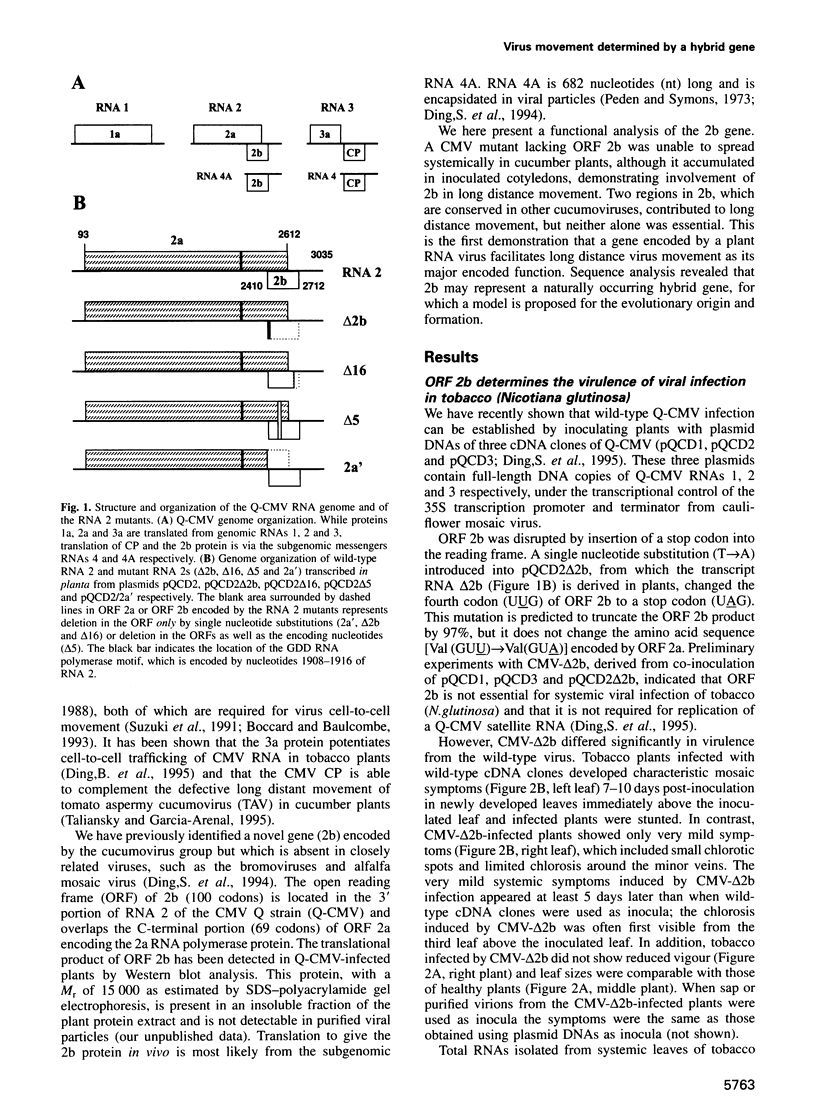
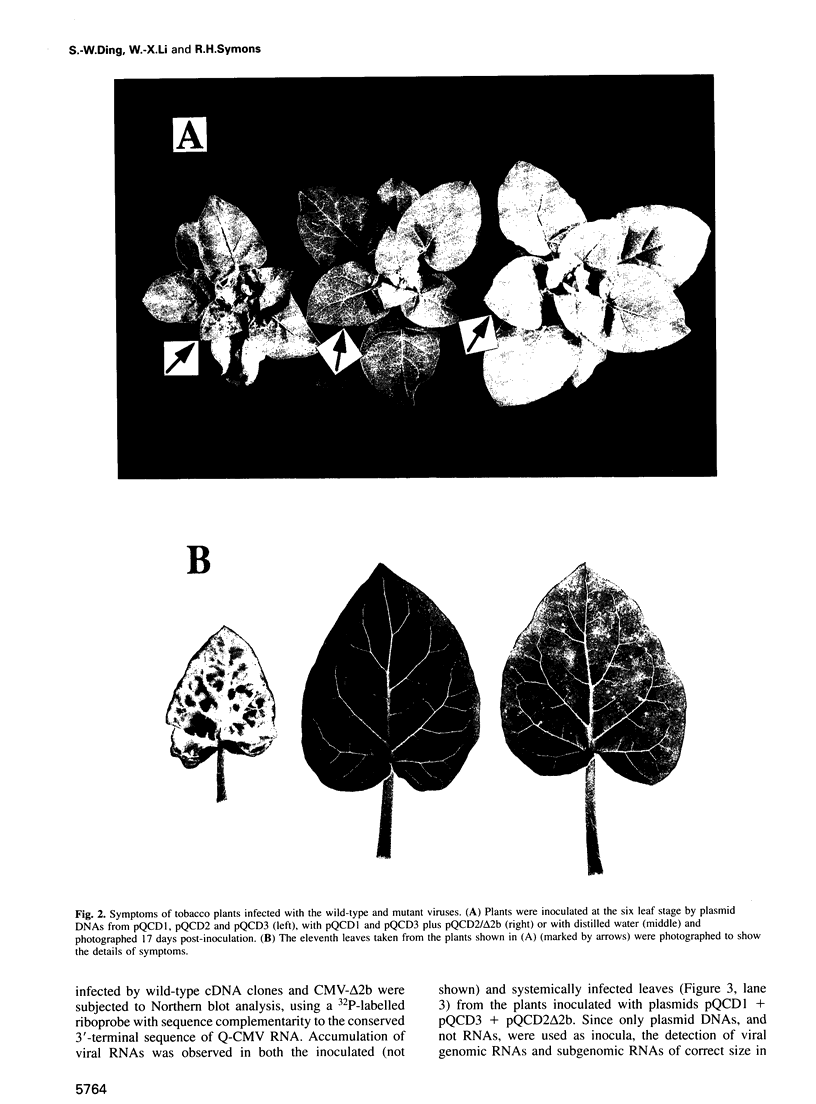
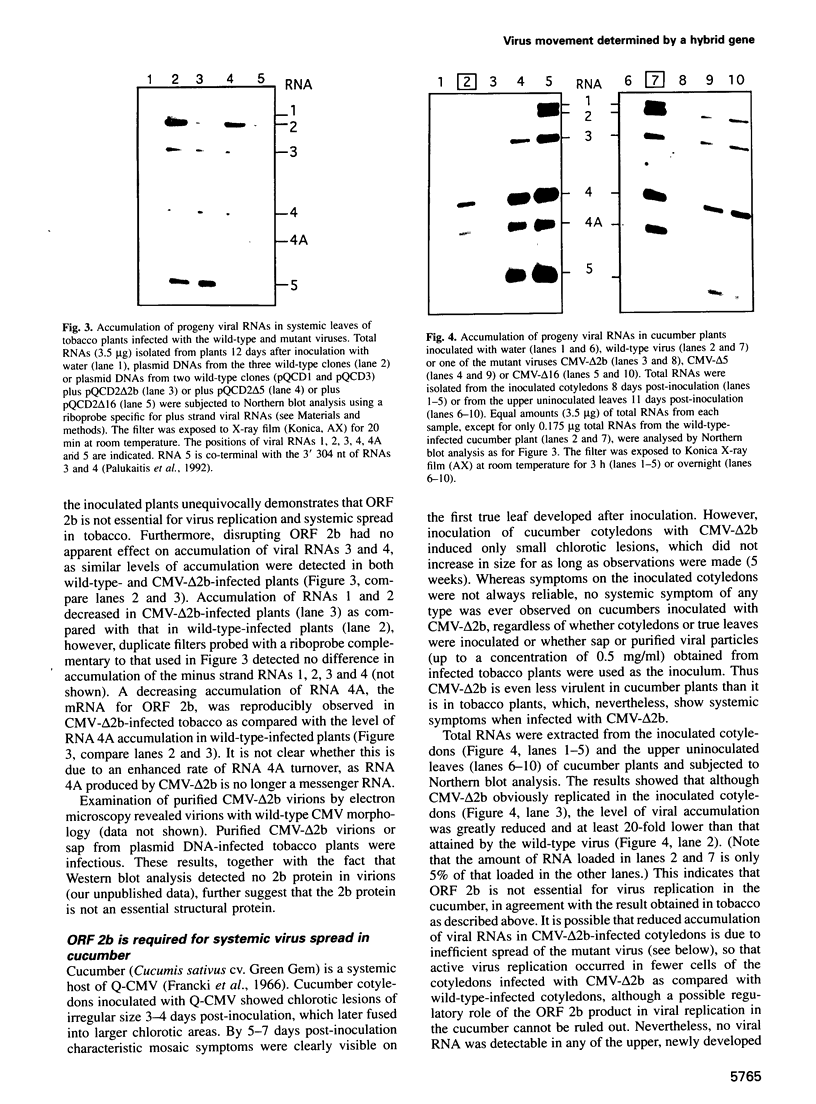
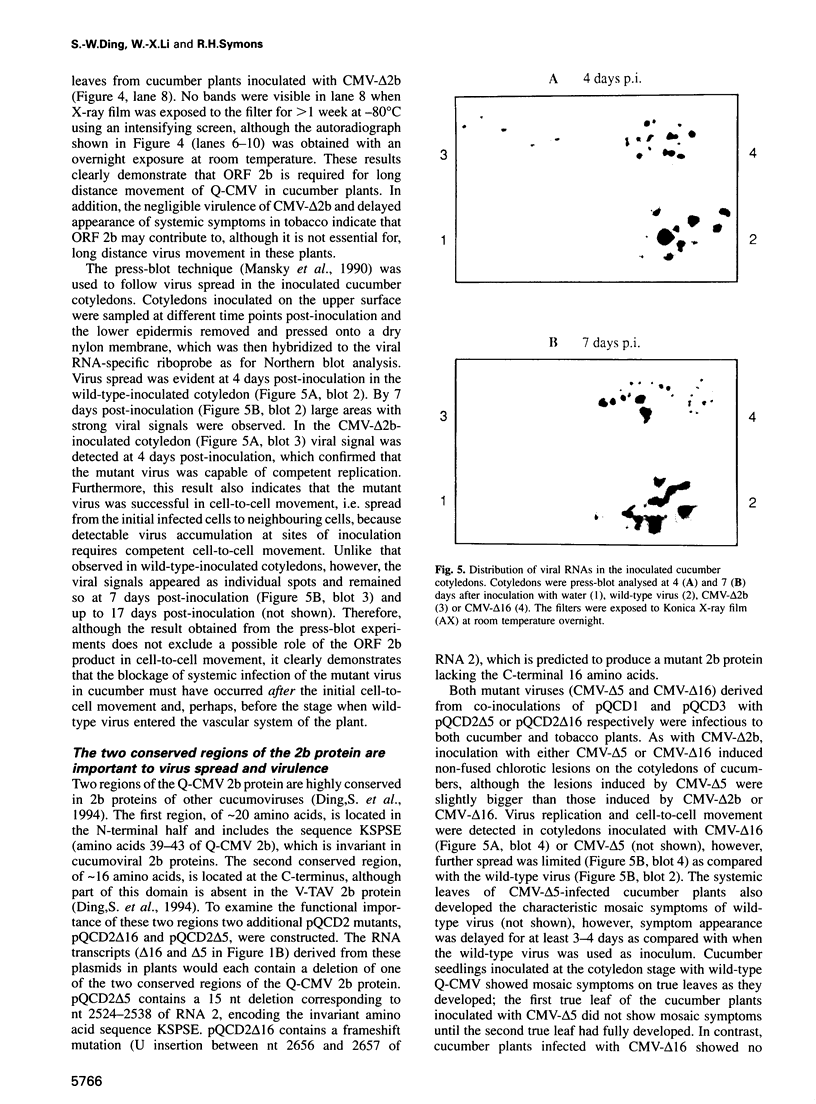
We recently identified a new cucumovirus-specific gene (2b) which is encoded by RNA 2 of the cucumber mosaic cucumovirus (CMV) tripartite RNA genome and whose coding sequence overlaps the C-terminal 69 codons of ORF 2a encoding the RNA polymerase protein. We have now found that although a CMV mutant lacking ORF 2b accumulated in the inoculated cotyledons of cucumber plants, it was unable to spread systemically, demonstrating involvement of 2b in long distance movement. The same mutant infected tobacco systemically with a much reduced virulence and delayed appearance of symptoms, indicating that 2b may contribute to long distance movement in this host. Deletion of the overlapping C-terminal part of ORF 2a did not change infectivity of the mutant in either host species, ruling out 2a mutation as the reason for the change of phenotype. Further infectivity studies with mutants containing partial deletions in ORF 2b further supported the conclusion that 2b encodes a host-specific long distance movement function. Sequence analysis revealed that 2b may represent a novel naturally occurring hybrid gene important to the evolutionary formation of the cucumovirus group and that it could provide a genetic basis for the wide host range of these viruses.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison R. F., Janda M., Ahlquist P. Sequence of cowpea chlorotic mottle virus RNAs 2 and 3 and evidence of a recombination event during bromovirus evolution. Virology. 1989 Sep;172(1):321–330. doi: 10.1016/0042-6822(89)90134-7. [DOI] [PubMed] [Google Scholar]
- Allison R., Thompson C., Ahlquist P. Regeneration of a functional RNA virus genome by recombination between deletion mutants and requirement for cowpea chlorotic mottle virus 3a and coat genes for systemic infection. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1820–1824. doi: 10.1073/pnas.87.5.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball L. A. Requirements for the self-directed replication of flock house virus RNA 1. J Virol. 1995 Feb;69(2):720–727. doi: 10.1128/jvi.69.2.720-727.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccard F., Baulcombe D. Mutational analysis of cis-acting sequences and gene function in RNA3 of cucumber mosaic virus. Virology. 1993 Apr;193(2):563–578. doi: 10.1006/viro.1993.1165. [DOI] [PubMed] [Google Scholar]
- Chapman S., Hills G., Watts J., Baulcombe D. Mutational analysis of the coat protein gene of potato virus X: effects on virion morphology and viral pathogenicity. Virology. 1992 Nov;191(1):223–230. doi: 10.1016/0042-6822(92)90183-p. [DOI] [PubMed] [Google Scholar]
- Davies C., Symons R. H. Further implications for the evolutionary relationships between tripartite plant viruses based on cucumber mosaic virus RNA 3. Virology. 1988 Jul;165(1):216–224. doi: 10.1016/0042-6822(88)90675-7. [DOI] [PubMed] [Google Scholar]
- Deom C. M., Lapidot M., Beachy R. N. Plant virus movement proteins. Cell. 1992 Apr 17;69(2):221–224. doi: 10.1016/0092-8674(92)90403-y. [DOI] [PubMed] [Google Scholar]
- Deom C. M., Oliver M. J., Beachy R. N. The 30-kilodalton gene product of tobacco mosaic virus potentiates virus movement. Science. 1987 Jul 24;237(4813):389–394. doi: 10.1126/science.237.4813.389. [DOI] [PubMed] [Google Scholar]
- Deom C. M., Schubert K. R., Wolf S., Holt C. A., Lucas W. J., Beachy R. N. Molecular characterization and biological function of the movement protein of tobacco mosaic virus in transgenic plants. Proc Natl Acad Sci U S A. 1990 May;87(9):3284–3288. doi: 10.1073/pnas.87.9.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding B., Haudenshield J. S., Hull R. J., Wolf S., Beachy R. N., Lucas W. J. Secondary plasmodesmata are specific sites of localization of the tobacco mosaic virus movement protein in transgenic tobacco plants. Plant Cell. 1992 Aug;4(8):915–928. doi: 10.1105/tpc.4.8.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding B., Li Q., Nguyen L., Palukaitis P., Lucas W. J. Cucumber mosaic virus 3a protein potentiates cell-to-cell trafficking of CMV RNA in tobacco plants. Virology. 1995 Mar 10;207(2):345–353. doi: 10.1006/viro.1995.1093. [DOI] [PubMed] [Google Scholar]
- Ding S. W., Anderson B. J., Haase H. R., Symons R. H. New overlapping gene encoded by the cucumber mosaic virus genome. Virology. 1994 Feb;198(2):593–601. doi: 10.1006/viro.1994.1071. [DOI] [PubMed] [Google Scholar]
- Ding S. W., Rathjen J. P., Li W. X., Swanson R., Healy H., Symons R. H. Efficient infection from cDNA clones of cucumber mosaic cucumovirus RNAs in a new plasmid vector. J Gen Virol. 1995 Feb;76(Pt 2):459–464. doi: 10.1099/0022-1317-76-2-459. [DOI] [PubMed] [Google Scholar]
- Dolja V. V., Haldeman-Cahill R., Montgomery A. E., Vandenbosch K. A., Carrington J. C. Capsid protein determinants involved in cell-to-cell and long distance movement of tobacco etch potyvirus. Virology. 1995 Feb 1;206(2):1007–1016. doi: 10.1006/viro.1995.1023. [DOI] [PubMed] [Google Scholar]
- Fichot O., Girard M. An improved method for sequencing of RNA templates. Nucleic Acids Res. 1990 Oct 25;18(20):6162–6162. doi: 10.1093/nar/18.20.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster R. L., Beck D. L., Guilford P. J., Voot D. M., Van Dolleweerd C. J., Andersen M. T. The coat protein of white clover mosaic potexvirus has a role in facilitating cell-to-cell transport in plants. Virology. 1992 Nov;191(1):480–484. doi: 10.1016/0042-6822(92)90215-b. [DOI] [PubMed] [Google Scholar]
- Francki R. I., Randles J. W., Chambers T. C., Wilson S. B. Some properties of purified cucumber mosaic virus (Q strain). Virology. 1966 Apr;28(4):729–741. doi: 10.1016/0042-6822(66)90257-1. [DOI] [PubMed] [Google Scholar]
- Fujiwara T., Giesman-Cookmeyer D., Ding B., Lommel S. A., Lucas W. J. Cell-to-Cell Trafficking of Macromolecules through Plasmodesmata Potentiated by the Red Clover Necrotic Mosaic Virus Movement Protein. Plant Cell. 1993 Dec;5(12):1783–1794. doi: 10.1105/tpc.5.12.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal-On A., Kaplan I., Roossinck M. J., Palukaitis P. The kinetics of infection of zucchini squash by cucumber mosaic virus indicate a function for RNA 1 in virus movement. Virology. 1994 Nov 15;205(1):280–289. doi: 10.1006/viro.1994.1644. [DOI] [PubMed] [Google Scholar]
- Ge X., Scott S. W. The nucleotide sequence of citrus leaf rugose ilarvirus RNA-2. J Gen Virol. 1994 Oct;75(Pt 10):2841–2846. doi: 10.1099/0022-1317-75-10-2841. [DOI] [PubMed] [Google Scholar]
- Hacker D. L., Petty I. T., Wei N., Morris T. J. Turnip crinkle virus genes required for RNA replication and virus movement. Virology. 1992 Jan;186(1):1–8. doi: 10.1016/0042-6822(92)90055-t. [DOI] [PubMed] [Google Scholar]
- Hayes R. J., Buck K. W. Complete replication of a eukaryotic virus RNA in vitro by a purified RNA-dependent RNA polymerase. Cell. 1990 Oct 19;63(2):363–368. doi: 10.1016/0092-8674(90)90169-f. [DOI] [PubMed] [Google Scholar]
- Hayes R. J., Pereira V. C., McQuillin A., Buck K. W. Localization of functional regions of the cucumber mosaic virus RNA replicase using monoclonal and polyclonal antibodies. J Gen Virol. 1994 Nov;75(Pt 11):3177–3184. doi: 10.1099/0022-1317-75-11-3177. [DOI] [PubMed] [Google Scholar]
- Heaton L. A., Lee T. C., Wei N., Morris T. J. Point mutations in the turnip crinkle virus capsid protein affect the symptoms expressed by Nicotiana benthamiana. Virology. 1991 Jul;183(1):143–150. doi: 10.1016/0042-6822(91)90127-w. [DOI] [PubMed] [Google Scholar]
- Higuchi R., Krummel B., Saiki R. K. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988 Aug 11;16(15):7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilf M. E., Dawson W. O. The tobamovirus capsid protein functions as a host-specific determinant of long-distance movement. Virology. 1993 Mar;193(1):106–114. doi: 10.1006/viro.1993.1107. [DOI] [PubMed] [Google Scholar]
- Karasawa A., Nakaho K., Kakutani T., Minobe Y., Ehara Y. Nucleotide sequence analyses of peanut stunt cucumovirus RNAs 1 and 2. J Gen Virol. 1992 Mar;73(Pt 3):701–707. doi: 10.1099/0022-1317-73-3-701. [DOI] [PubMed] [Google Scholar]
- Keese P. K., Gibbs A. Origins of genes: "big bang" or continuous creation? Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9489–9493. doi: 10.1073/pnas.89.20.9489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriones E., Roossinck M. J., García-Arenal F. Nucleotide sequence of tomato aspermy virus RNA 2. J Gen Virol. 1991 Apr;72(Pt 4):779–783. doi: 10.1099/0022-1317-72-4-779. [DOI] [PubMed] [Google Scholar]
- Nathanson S. D., Nelson L. T., Lee M. A spontaneous subcutaneous tumor in C57BL/6 mice that metastasizes to the liver. Clin Exp Metastasis. 1993 Jan;11(1):45–54. doi: 10.1007/BF00880065. [DOI] [PubMed] [Google Scholar]
- Noueiry A. O., Lucas W. J., Gilbertson R. L. Two proteins of a plant DNA virus coordinate nuclear and plasmodesmal transport. Cell. 1994 Mar 11;76(5):925–932. doi: 10.1016/0092-8674(94)90366-2. [DOI] [PubMed] [Google Scholar]
- Palukaitis P., Roossinck M. J., Dietzgen R. G., Francki R. I. Cucumber mosaic virus. Adv Virus Res. 1992;41:281–348. doi: 10.1016/s0065-3527(08)60039-1. [DOI] [PubMed] [Google Scholar]
- Peden K. W., Symons R. H. Cucumber mosaic virus contains a functionally divided genome. Virology. 1973 Jun;53(2):487–492. doi: 10.1016/0042-6822(73)90232-8. [DOI] [PubMed] [Google Scholar]
- Petty I. T., Edwards M. C., Jackson A. O. Systemic movement of an RNA plant virus determined by a point substitution in a 5' leader sequence. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8894–8897. doi: 10.1073/pnas.87.22.8894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty I. T., Jackson A. O. Mutational analysis of barley stripe mosaic virus RNA beta. Virology. 1990 Dec;179(2):712–718. doi: 10.1016/0042-6822(90)90138-h. [DOI] [PubMed] [Google Scholar]
- Rezaian M. A., Williams R. H., Gordon K. H., Gould A. R., Symons R. H. Nucleotide sequence of cucumber-mosaic-virus RNA 2 reveals a translation product significantly homologous to corresponding proteins of other viruses. Eur J Biochem. 1984 Sep 3;143(2):277–284. doi: 10.1111/j.1432-1033.1984.tb08370.x. [DOI] [PubMed] [Google Scholar]
- Rezaian M. A., Williams R. H., Symons R. H. Nucleotide sequence of cucumber mosaic virus RNA. 1. Presence of a sequence complementary to part of the viral satellite RNA and homologies with other viral RNAs. Eur J Biochem. 1985 Jul 15;150(2):331–339. doi: 10.1111/j.1432-1033.1985.tb09025.x. [DOI] [PubMed] [Google Scholar]
- Romero J., Dzianott A. M., Bujarski J. J. The nucleotide sequence and genome organization of the RNA2 and RNA3 segments in broad bean mottle virus. Virology. 1992 Apr;187(2):671–681. doi: 10.1016/0042-6822(92)90470-a. [DOI] [PubMed] [Google Scholar]
- Saito T., Yamanaka K., Okada Y. Long-distance movement and viral assembly of tobacco mosaic virus mutants. Virology. 1990 Jun;176(2):329–336. doi: 10.1016/0042-6822(90)90002-9. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Kuwata S., Kataoka J., Masuta C., Nitta N., Takanami Y. Functional analysis of deletion mutants of cucumber mosaic virus RNA3 using an in vitro transcription system. Virology. 1991 Jul;183(1):106–113. doi: 10.1016/0042-6822(91)90123-s. [DOI] [PubMed] [Google Scholar]
- Takamatsu N., Ishikawa M., Meshi T., Okada Y. Expression of bacterial chloramphenicol acetyltransferase gene in tobacco plants mediated by TMV-RNA. EMBO J. 1987 Feb;6(2):307–311. doi: 10.1002/j.1460-2075.1987.tb04755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taliansky M. E., García-Arenal F. Role of cucumovirus capsid protein in long-distance movement within the infected plant. J Virol. 1995 Feb;69(2):916–922. doi: 10.1128/jvi.69.2.916-922.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynor P., Young B. M., Ahlquist P. Deletion analysis of brome mosaic virus 2a protein: effects on RNA replication and systemic spread. J Virol. 1991 Jun;65(6):2807–2815. doi: 10.1128/jvi.65.6.2807-2815.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwoerd T. C., Dekker B. M., Hoekema A. A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res. 1989 Mar 25;17(6):2362–2362. doi: 10.1093/nar/17.6.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland J. J., Edwards M. C. Evidence that the alpha a gene of barley stripe mosaic virus encodes determinants of pathogenicity to oat (Avena sativa). Virology. 1994 May 15;201(1):116–126. doi: 10.1006/viro.1994.1271. [DOI] [PubMed] [Google Scholar]
- Wellink J., van Lent J. W., Verver J., Sijen T., Goldbach R. W., van Kammen A. The cowpea mosaic virus M RNA-encoded 48-kilodalton protein is responsible for induction of tubular structures in protoplasts. J Virol. 1993 Jun;67(6):3660–3664. doi: 10.1128/jvi.67.6.3660-3664.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S., Deom C. M., Beachy R. N., Lucas W. J. Movement protein of tobacco mosaic virus modifies plasmodesmatal size exclusion limit. Science. 1989 Oct 20;246(4928):377–379. doi: 10.1126/science.246.4928.377. [DOI] [PubMed] [Google Scholar]
- Xiong Z., Kim K. H., Giesman-Cookmeyer D., Lommel S. A. The roles of the red clover necrotic mosaic virus capsid and cell-to-cell movement proteins in systemic infection. Virology. 1993 Jan;192(1):27–32. doi: 10.1006/viro.1993.1004. [DOI] [PubMed] [Google Scholar]
- Ziegler A., Natsuaki T., Mayo M. A., Jolly C. A., Murant A. F. The nucleotide sequence of RNA-1 of raspberry bushy dwarf virus. J Gen Virol. 1992 Dec;73(Pt 12):3213–3218. doi: 10.1099/0022-1317-73-12-3213. [DOI] [PubMed] [Google Scholar]