Abstract
Objective
To characterize pediatricians' and family physicians' (FP) screening practices for type 2 diabetes among adolescents and to examine the impact of the 2010 American Diabetes Association (ADA) guidelines, recommending use of Hemoglobin A1c (HbA1c).
Patients and Methods
We conducted a cross-sectional mail survey of a random sample of 1,400 US pediatricians and FPs and we received 604 eligible responses. Our main outcome measure was the types of tests ordered by physicians, particularly HbA1c, when presented with a hypothetical scenario.
Results
The overall response rate was 52% (57% for pediatricians and 48% for FPs). Fasting glucose and HbA1c were the most commonly ordered tests. Overall, at least 58% of physicians ordered HbA1c; 35% ordered HbA1c in conjunction with fasting tests, and 22% ordered HbA1c alone or with non-fasting tests. Only 38% of providers were aware of the new ADA recommended HbA1c screening guidelines. However, a majority (67%) said they would change their screening practices. In the context of the guidelines, 84% of physicians would now order HbA1c. Furthermore, there was a large increase in the proportion of physicians who would shift to using HbA1c only or with other non-fasting tests.
Conclusions
When screening adolescents for type 2 diabetes, providers are more likely to order HbA1c and order fewer fasting tests in response to the new ADA guidelines. HbA1c has lower sensitivity and higher costs than other testing modalities in children, therefore increasing uptake of this test HbA1c in children may have implications for both detection rates and health care costs.
Keywords: screening, adolescent, diabetes mellitus type 2, Hemoglobin A1c
Introduction
In the 1990's, the well-known epidemic of childhood obesity in the US was accompanied by reports of increasing rates of type 2 diabetes (T2D).1 In response, national organizations including the American Diabetes Association (ADA) and the American Academy of Pediatrics released screening guidelines for identifying children with T2D in 2000. These guidelines recommended that children with body mass index (BMI) ≥ 85th percentile and any two additional risk factors be screened with a fasting plasma glucose (FPG) or a 2-hour glucose tolerance test (OGTT) every 2 years starting at age 10 years, or at onset of puberty.2,3
Although the FPG and the 2-hr OGTT were recommended as screening tests, they are also the gold standard tests for diagnosing diabetes. However, in 2010, the ADA modified its diagnostic guidelines, recommending that HbA1c tests also be used for diagnosing diabetes (HbA1c ≥ 6.5%) and prediabetes (HbA1c 5.7%–6.4%) in both adults and children.4 The rationale for a shift to HbA1c was that it does not require patients to fast prior to testing, has a lower variability,5 and has been linked to the development of diabetes complications in epidemiologic studies.6 However, the guidelines are not without controversy, particularly in the pediatric population, given concerns about nonglycemic test factors impacting HbA1c7,8 and lower test performance of HbA1c for children compared with adults.9–11
While a few studies have evaluated providers' screening practices and tests of choice for identifying adolescents with T2D, these studies were conducted in geographically narrow populations,12 did not include family practitioners,12,13 and were conducted prior to the release of the new guidelines regarding HbA1c.12,13 Therefore, the objectives of our study were to evaluate current screening practices for pediatric T2D among a nationally representative sample of pediatricians and family practitioners, physicians' awareness of the recent ADA guidelines, and the impact of these new guidelines on the adoption of HbA1c as a diabetes screening test.
Research Design and Methods
Study Population
We randomly sampled 700 pediatricians and 700 family physicians (FPs) from the American Medical Association (AMA) Physician Masterfile, through a contracted vendor. We included allopathic (MD) and osteopathic (DO) physicians self-described as a pediatrician or family physician in direct patient care. We excluded physicians who were residents, hospital staff, or retirees, and physicians who were employed at federally owned medical facilities, who had subspecialty board certification, or who were 70 years or older.
Survey Design
We created a 4-page paper survey consisting of 17 items that focused on physicians' screening practices for adolescents at risk for type 2 diabetes. We provided the following hypothetical scenario to respondents: “Imagine that you are seeing a 14-year old female in your clinic for the first time for a well-child visit. She is obese (BMI (≥ 95th percentile) and has at least 2 risk factors (e.g., family history of type 2 diabetes, minority race, or signs of insulin resistance), and therefore meets criteria for type 2 diabetes screening. Currently she has no symptoms of diabetes (e.g., no frequent urination or frequent thirst). She has not been screened previously for diabetes and did not fast before this visit.” Respondents were asked what initial screening tests they would order. After indicating their test choices, respondents were asked if they were aware of the new ADA recommendations for using HbA1c to diagnose diabetes and whether this has changed or would change their screening practices for adolescents. Next, they were asked if they felt test accuracy or patient convenience was more important in screening tests. Finally, respondents were asked what organizations or professional societies they use to guide their decisions regarding T2D screening in in adolescents, whether they manage or refer patients with T2D, and characteristics of their practice setting. Demographic information was retrieved from the Masterfile.
Survey Administration
We pilot tested the survey with a group of local providers in Michigan, to ensure clarity and ease of administration. The initial survey mailing was sent in December 2011 to 700 pediatricians and 700 FPs. The mailing included a personalized cover letter, the survey instrument, a $5 cash incentive, and postage-paid return envelope. We sent non-respondents two additional mailings at 3–4 week intervals. The institutional review board of the University of Michigan Medical School approved this study.
Study definitions
In our analyses we defined “fasting tests” as fasting glucose or 2hr OGTT, and “non-fasting tests” were defined as random glucose, finger stick glucose with glucometer, HbA1c, and urine dipstick. We then divided providers into two groups, those who order only non-fasting tests and those who order at least one fasting test. Insulin was removed from the analysis when comparing fasting and non-fasting tests but was included in the analysis of proportion of tests.
Statistical Analyses
At the provider level, we assessed the number and proportion of physicians who would order a specific type of test. At the test level, we assessed the frequency and proportion of tests that were ordered, with the denominator determined by the total number of tests ordered, since physicians could order multiple tests.
To evaluate the frequency of providers who would use HbA1c after discussion of the guidelines, we included individuals who used HbA1c in the initial scenario and had no intention of changing their screening practices, as well as those who responded that they would run HbA1c either alone or with other tests they typically run.
We generated univariate frequencies for each variable and performed χ2 analyses for categorical variables and t-tests for continuous variables to examine differences between pediatricians and FPs. We also conducted multivariate analyses predicting the likelihood of ordering a non-fasting test for the hypothetical scenario, according to age, sex, preference of test convenience, public vs. private practice setting, whether they had an onsite blood draw station, and whether they used the ADA as their main source of information on diabetes screening in adolescents. A 2-tailed α-level of 0.05 was determined as the threshold for statistical significance. All of the analyses were conducted using Stata 10.0 (StataCorp, College Station, TX).
Results
Respondent Characteristics
Of the 1,400 physicians included in the mailing sample, 2 were excluded because mailing materials were returned as undeliverable (1 pediatrician and 1 family physician). Surveys were returned by 733 (398 pediatricians and 335 FPs) of the remaining 1,398 physicians, providing an overall response rate of 52% (57% pediatricians and 48% FPs).
There were 129 physicians (46 pediatricians and 83 FPs) who returned surveys reporting that they do not provide outpatient primary care to adolescents aged 10–17 years, leaving 604 eligible respondents (352 pediatricians and 252 FPs) and an eligible response rate of 43%. These 604 respondents were the focus of this study.
Respondent and Practice Characteristics
For the overall sample there were slightly more females than males (Table 1). The majority of respondents were 40–55 years of age, board certified, practiced in a solo or group private practice and had an onsite blood drawing station at their practice site (Table 1). Compared with FPs, pediatrician respondents were more likely to be female and have a solo/group practice, but less likely to have an onsite blood drawing station at their practice site.
Table 1.
Respondent & Practice Characteristics
| Respondent/Practice Characteristic | Pediatricians (N=352) % (n) | Family Practitioners (N=252) % (n) | Total (N= 604) % (n) | P |
|---|---|---|---|---|
| Female sex | 63 (222) | 42 (106) | 54 (328) | <0.001 |
| Age (in years) OR add label as shown to each row | NS | |||
| <40 years | 18 (65) | 17 (42) | 18 (107) | |
| 40–55 years | 47 (165) | 55 (138) | 50 (303) | |
| >55 years | 35 (122) | 28 (72) | 32 (194) | |
| Board certified | 93 (327) | 87 (219) | 90 (546) | <0.001 |
| Practice type | ||||
| Solo or group private practice | 72 (252) | 56 (139) | 65 (391) | |
| Hospital/medical center | 11 (39) | 25 (62) | 17 (101) | |
| University health system | 2 (7) | 5 (12) | 3 (19) | |
| Public clinic/community health center | 6 (20) | 8 (19) | 7 (39) | |
| Practice network/HMO | 8 (29) | 6 (14) | 7 (43) | |
| Other practice type | 1 (4) | 1 (2) | 1 (6) | |
| Onsite blood drawing station at practice site | 55 (193) | 85 (214) | 67 (407) | <0.001 |
The average number of patients seen per week was 112 for pediatricians and 98 for FPs. Adolescents (10–17 years) made up 32% of the patient population seen by pediatricians, and only 12% of the patient population seen by FPs.
Management Style and Information Source for T2D
When asked whether they manage adolescents with T2D, only 8% of pediatricians responded affirmatively compared with 60% of FPs (p<0.001). For their main information source related to screening, diagnosis, and management of type 2 diabetes in children, pediatricians overwhelmingly reported that they use the American Academy of Pediatrics (AAP) (93%), whereas the majority of FPs reported using the American Academy of Family Practitioners (70%). A small percentage of providers reported using the ADA as their information source, with a higher percentage among FPs (23%) relative to pediatricians (7%).
Diabetes Screening Tests
Overall, 92% of primary care physicians reported that they would screen the patient in the hypothetical scenario for diabetes. Most physicians (63%) responded that they would initially order at least one fasting test (FPG or 2-hr OGTT) as part of the battery tests that they order). Pediatricians were more likely than FPs to screen the patient (95% vs. 89%, p<0.01) and were more likely to order at least one fasting test to screen the patient (68%) compared with FPs (55%) (p<0.01). Conversely, more FPs than pediatricians ordered non-fasting tests only for their initial screening (44% vs. 32%, p<0.01).
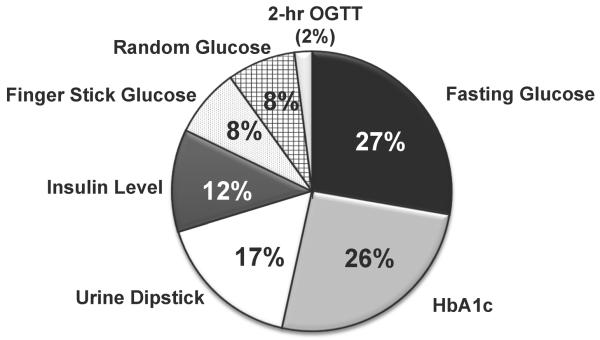
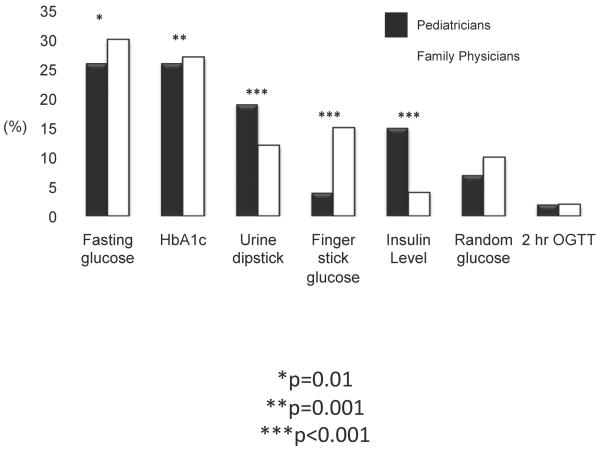
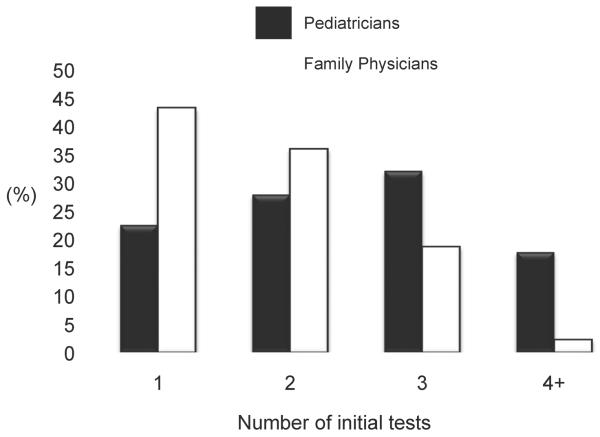
Fasting glucose and HbA1c levels were the screening tests ordered most often, while 2-hr OGTT, finger stick glucose, and random glucose were ordered least often (Figure 1). Only 6.5% of physicians indicated that they would order urine dipstick, fingerstick glucose, or insulin levels only. Fasting glucose, HbA1c, urine dipstick, and insulin levels were more likely to be ordered by pediatricians than FPs (p=0.01, p=0.001, p<0.001, and p<0.001 respectively, Figure 2), while finger stick glucose was more likely to be ordered by FPs (p<0.001). Compared with FPs, pediatricians were more likely to order multiple tests vs. just 1 or 2 (Figure 3, p<0.001). Both pediatricians (85%) and FPs (69%) rated test accuracy as more important than patient convenience for type 2 diabetes screening in adolescents.
Figure 1.
shows the frequency and proportion of the different types of tests that were ordered (denominator is at the test level).
Figure 2.
shows the frequency and proportion of the different types of tests that were ordered (denominator is at the test level) between pediatricians and FPs. *p=0.01, **p=0.001, ***p<0.001
Figure 3.
shows the distribution and number of pediatricians and FPs that order screening tests according to the number of tests ordered (p<0.001)
In the multivariate analysis, physicians were 5.6 times more likely to order non-fasting tests as their initial test of choice if they valued test convenience over accuracy (p≤0.001) and 2.2 times more likely if they did not have an onsite blood draw station at their practice (p=0.002). Age, sex, type of practice setting, and using the ADA as their main information source for diabetes screening in adolescents were not associated with likelihood of ordering non-fasting tests.
Use of Hemoglobin A1c for Initial Screening
The majority of providers (58%) ordered HbA1c as part of their initial battery of tests; 7% ordered HbA1c only, 15% ordered HbA1c plus another non-fasting test, and 35% ordered HbA1c plus a fasting test. More pediatricians included HbA1c as part of their initial screening compared with FPs (63% vs. 49%, p=0.001).
2010 ADA Guidelines Regarding HbA1c: Awareness and Response
Only 38% of physicians reported being aware of the new ADA guidelines for HbA1c screening, with FPs reporting greater awareness than pediatricians (52% vs. 28%, p<0.001). The majority of physicians (67%) reported that they have changed or will change their screening practices in response to new ADA recommendations, mainly by incorporating HbA1c testing along with the typical tests they currently order (48%) or by doing HbA1c testing only (15%). Very few physicians reported that they would discontinue ordering glucose measurements (3%) or make other changes (3%) in response to the new guidelines.
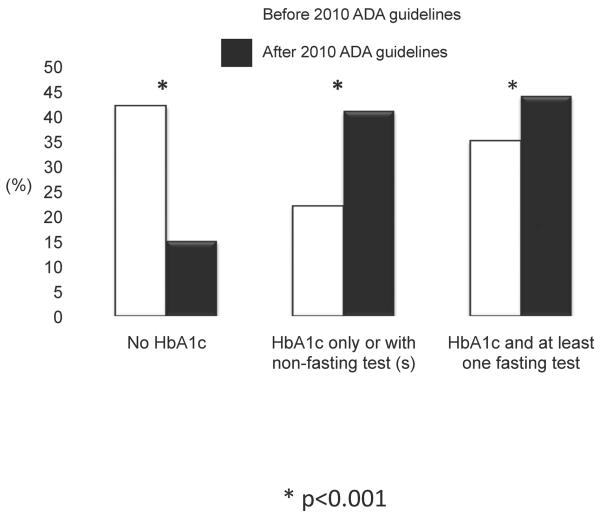
Based on these responses, the percentage of providers who reported that they would now order HbA1c increased from 57% to 84%. Again, pediatricians were more likely than FPs to order HbA1c as part of their initial screen (88% vs 79%, p=0.003). This resulted in a greater proportion of physicians ordering HbA1c with or without additional fasting tests (Figure 4, p<0.001), but there was a greater increase in the proportion of physicians who would shift to using HbA1c only or with other nonfasting tests.
Figure 4.
shows the percentage of primary care providers who responded that they would order Hba1c with at least one fasting test, HbA1c only or with non-fasting test(s) or not order HbA1c in their typical battery of tests before and after the 2010 ADA guidelines. *p<0.001
Discussion
In a national sample of US physicians who provide primary care to adolescents, we found that a majority (58%) of providers ordered HbA1c as part of their battery of initial tests for screening adolescents with T2D. We also found that those who ordered HbA1c were more likely to order them in combination with fasting tests, instead of alone or with nonfasting tests. When presented with the recent revised ADA guidelines on diagnosing diabetes, an even greater percentage of providers reported that they would order HbA1c (84%). This potential for increased uptake of HbA1c has implications for both screening effectiveness and the overall costs of type 2 diabetes screening in children. Regarding effectiveness, a number of studies have shown that HbA1c has lower test performance in pediatric compared with adult populations, and as a result, increased uptake of HbA1c alone or in combination with nonfasting tests could lead to missed diagnoses of type 2 diabetes in the pediatric population.10,11,14 Furthermore, a recent cost effectiveness analysis of screening strategies found that HbA1c is much less cost-effective than other screening tests, which would result in higher overall costs for screening.15
We are unaware of previous studies that have explored differences in T2D screening practices between pediatricians and FPs. For the baseline scenario, pediatricians appeared to be more vigorous testers than FPs given their higher rates of ordering fasting tests and their greater number of initial screening tests ordered. Studies have reported that pediatricians are more likely to assess weight status and provide behavioral counseling in children with obesity compared with FPs; our finding suggests that this may extend to T2D screening as well.13 However this extensive amount of testing could be considered overzealous, leading to excess costs without much additional benefit.
Overall awareness of the 2010 ADA guidelines was relatively low (38%). FPs had greater awareness of the guideline compared with pediatricians, which may be related to the fact that a larger percentage use the ADA as their main information source for diabetes guidelines and work with an adult as well as pediatric population. However after discussion of the guidelines, there were no differences in the most commonly ordered tests (fasting glucose and HbA1c) or in the percentage of providers who changed their screening practices in in response to the ADA guidelines.
The percentage of providers who chose to screen for diabetes in our study was much higher (92%) than in previous surveys conducted in 2002 and 2006, which reported rates of screening closer to 30–50%.12,13 This variation could be due to differences in the study populations and the clinical scenarios presented. However we also speculate that this could be due to greater awareness among providers regarding the risks of childhood obesity. We found higher rates of HbA1c use for our baseline scenario (58%) compared with just 6% reported by Barlow et al in 2002.13 In addition, physicians in our sample reported higher rates of ordering at least one fasting test (63%), compared with results reported by Rhodes et al in 2006.12
Strengths of our study include our study design, which was based on a national random sample of both pediatricians and FPs, and our response rate of >50%. We are unaware of other studies that have evaluated both awareness and screening test choice related to the 2010 ADA guidelines. Furthermore, we identified that physician beliefs, about test convenience over accuracy, and structural characteristics of a practice, i.e. having a laboratory on site, does have an impact on the likelihood of ordering non-fasting tests for T2D screening, which has not been previously reported.
Limitations include the potential response bias that can occur with mailed surveys. We also recognize that the case scenario for evaluating screening practices may not be representative of all children considered for T2D screening in everyday clinical practice.
Conclusion
Greater awareness of the 2010 ADA guidelines will likely lead to increased uptake of HbA1c and a shift to use of non-fasting tests to screen for adolescents with type 2 diabetes. This may have implications for detection rates for diabetes and overall costs of screening. It remains to be seen whether the AAP will endorse the ADA guidelines; future screening policy should consider effectiveness, costs, and provider knowledge about limitations of HbA1c.
What's Known on This Subject.
We are unaware of studies that have evaluated awareness of and the potential impact of the 2010 American Diabetes Association recommendations for type 2 diabetes screening in adolescents.
What This Study Adds.
This study shows that the 2010 ADA recommendations would lead to increased uptake of HbA1c as a screening test for identifying adolescent patients for type 2 diabetes, which may impact detection rates and the cost-effectiveness of screening.
Acknowledgments
Dr. Lee was funded by grant NIDDK K08-DK-082386 and the Clinical Sciences Scholars Program at the University of Michigan.
Courtney Nelson was supported by the 2012 NIDDK Medical Student Research Program in Diabetes.
Dr. Tarini was supported by the Clinical Sciences Scholars Program at the University of Michigan and a K23 Mentored Patient-Oriented Research Career Development Award from the National Institute for Child Health and Human Development (K23HD057994).
Funding Source: Dr. Lee was supported by NIDDK K08 DK082386 and the Clinical Sciences Scholars Program at the University of Michigan.
Abbreviations
- (ADA)
American Diabetes Association
- (HbA1c)
Hemoglobin A1c
- (T2D)
type 2 diabetes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: All of the authors have nothing to disclose.
Conflict of Interest: All of the authors have no conflicts of interest to disclose.
Contributors Statement: Joyce M. Lee: conceptualized and designed the study, designed the data collection instrument, carried out the statistical analyses, drafted the initial manuscript, and approved the final manuscript as submitted.
Ashley Eason: coordinated and supervised data input, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Courtney Nelson: carried out statistical analyses, input data, drafted the initial manuscript, and approved the final manuscript as submitted.
Nayla G. Kazzi: carried out the initial statistical analyses, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Anne E. Cowan: designed the data collection instrument, reviewed and revised the manuscript and approved the final manuscript as submitted.
Beth A. Tarini: conceptualized and designed the study, designed the data collection instrument, reviewed and revised the manuscript, and approved the final manuscript as submitted.
References
- 1.Pinhas-Hamiel O, Dolan LM, Daniels SR, Standiford D, Khoury PR, Zeitler P. Increased incidence of non-insulin-dependent diabetes mellitus among adolescents. J Pediatr. 1996 May;128(5 Pt 1):608–615. doi: 10.1016/s0022-3476(96)80124-7. [DOI] [PubMed] [Google Scholar]
- 2.Type 2 diabetes in children and adolescents. American Diabetes Association. Pediatrics. 2000 Mar;105(3 Pt 1):671–680. doi: 10.1542/peds.105.3.671. [DOI] [PubMed] [Google Scholar]
- 3.Standards of medical care in diabetes--2006. Diabetes Care. 2006 Jan;29(Suppl 1):S4–42. [PubMed] [Google Scholar]
- 4.Standards of medical care in diabetes--2010. Diabetes Care. 2010 Jan;33(Suppl 1):S11–61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selvin E, Crainiceanu CM, Brancati FL, Coresh J. Short-term variability in measures of glycemia and implications for the classification of diabetes. Arch Intern Med. 2007 Jul 23;167(14):1545–1551. doi: 10.1001/archinte.167.14.1545. [DOI] [PubMed] [Google Scholar]
- 6.Gavin JR, Alberti K, Davidson MB. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 7.Hardikar PS, Joshi SM, Bhat DS, et al. Spuriously high prevalence of prediabetes diagnosed by HbA(1c) in young indians partly explained by hematological factors and iron deficiency anemia. Diabetes Care. 2012 Apr;35(4):797–802. doi: 10.2337/dc11-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim C, Bullard KM, Herman WH, Beckles GL. Association between iron deficiency and A1C Levels among adults without diabetes in the National Health and Nutrition Examination Survey, 1999–2006. Diabetes Care. 2010 Apr;33(4):780–785. doi: 10.2337/dc09-0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JM, Gebremariam A, Wu EL, LaRose J, Gurney JG. Evaluation of nonfasting tests to screen for childhood and adolescent dysglycemia. Diabetes Care. 2011 Dec;34(12):2597–2602. doi: 10.2337/dc11-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nowicka P, Santoro N, Liu H, et al. Utility of hemoglobin A(1c) for diagnosing prediabetes and diabetes in obese children and adolescents. Diabetes Care. 2011 Jun;34(6):1306–1311. doi: 10.2337/dc10-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JM, Wu EL, Tarini B, Herman WH, Yoon E. Diagnosis of diabetes using hemoglobin A1c: should recommendations in adults be extrapolated to adolescents? J Pediatr. 2011 Jun;158(6):947–952. e941–943. doi: 10.1016/j.jpeds.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhodes ET, Finkelstein JA, Marshall R, Allen C, Gillman MW, Ludwig DS. Screening for type 2 diabetes mellitus in children and adolescents: attitudes, barriers, and practices among pediatric clinicians. Ambul Pediatr. 2006 Mar-Apr;6(2):110–114. doi: 10.1016/j.ambp.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Barlow SE, Dietz WH, Klish WJ, Trowbridge FL. Medical evaluation of overweight children and adolescents: reports from pediatricians, pediatric nurse practitioners, and registered dietitians. Pediatrics. 2002 Jul;110(1 Pt 2):222–228. [PubMed] [Google Scholar]
- 14.Lee JM, Gebremariam A, Wu EL, Larose J, Gurney JG. Evaluation of Nonfasting Tests to Screen for Childhood and Adolescent Dysglycemia. Diabetes Care. 2011 Sep 27;34:2597–2602. doi: 10.2337/dc11-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu E-L, Kazzi NG, Lee JM. Cost-effectiveness of screening strategies for identifying pediatric diabetes and dysglycemia. In Press. 2012 doi: 10.1001/jamapediatrics.2013.419. [DOI] [PMC free article] [PubMed] [Google Scholar]