Abstract
BACKGROUND
We have shown previously that honokiol (HNK), a bioactive component of the medicinal plant Magnolia officinalis, inhibits growth of human prostate cancer cells in vitro and in vivo. However, the effect of HNK on androgen receptor (AR) signaling is not known.
METHODS
LNCaP, C4-2, and TRAMP-C1 cells were used for various assays. Trypan blue dye exclusion assay or clonogenic assay was performed for determination of cell viability. The effects of HNK and/or its analogs on protein levels of AR and its target gene product prostate specific antigen (PSA) were determined by western blotting. RNA interference of p53 was achieved by transient transfection. Reverse transcription-polymerase chain reaction was performed for mRNA expression of AR. Nuclear translocation of AR was visualized by microscopy. Apoptosis was quantified by DNA fragmentation assay or flow cytometry after Annexin V-propidium iodide staining.
RESULTS
HNK and its dichloroacetate analog (HDCA) were relatively more effective in suppressing cell viability and AR protein level than honokiol epoxide or biseugenol. Nuclear translocation of AR stimulated by a synthetic androgen (R1881) was markedly suppressed in the presence of HNK. Downregulation of AR protein resulting from HNK exposure was attributable to transcriptional repression as well as proteasomal degradation. HNK-mediated suppression of AR protein was maintained in LNCaP cells after knockdown of p53 protein. HNK-induced apoptosis was not affected by R1881 treatment.
CONCLUSIONS
The present study demonstrates, for the first time, that HNK inhibits activity of AR in prostate cancer cells regardless of the p53 status.
Keywords: Honokiol, Alternative medicine, Androgen receptor, Prostate cancer
INTRODUCTION
Men with prostate cancer experience substantial loss in quality of life from the disease and treatments. Despite a much deeper grasp of the prostate cancer biology, this disease continues to be a leading cause of cancer-related deaths among men in western countries including the United States [1, 2]. Effective and safer preventive interventions are needed to diminish morbidity and mortality in men with prostate cancer. Plants used in complementary and alternative medicine are a rich source of phytochemicals potentially useful for prevention and treatment of cancers [3–5]. Honokiol (HNK) is one such small-molecule isolated from the bark of Magnolia officinalis, and exhibits a variety of pharmacological properties including in vivo anti-cancer effect [6–8]. For example, previous work from our own laboratory has revealed in vivo growth inhibitory effect of orally administered HNK against PC-3 human prostate cancer cells [9]. Bone metastatic growth of an androgen-independent human prostate cancer cell line (C4-2) was inhibited significantly by daily intraperitoneal administration of HNK, and this inhibitory effect was potentiated by docetaxel [10]. The in vivo antitumor effect of cetuximab, an epidermal growth factor receptor inhibitor, was enhanced by HNK treatment in a human head and neck cancer cell line [11]. HNK increased the efficacy of other commonly used cancer chemotherapeutic agents such as cisplatin and gemcitabine [12, 13]. Other notable anti-cancer effects of HNK include prevention of UVB-induced skin carcinogenesis, and inhibition of angiogenesis and metastasis [14–16].
Significant progress has been made in our understanding of the mechanisms underlying anti-cancer effects of HNK [17–20]. For instance, growth inhibitory effect of HNK in PC-3 and LNCaP human prostate cancer cells was associated with G0–G1 phase cell cycle arrest due to suppression of E2F1 transcriptional activity [17]. In addition, HNK treatment caused apoptosis in human prostate cancer cells in association with induction of proapoptotic proteins (Bax, Bak, and Bad) and down-regulation of anti-apoptotic proteins Bcl-xL and Mcl-1 [9].
Previous studies have shown that HNK administration to C4-2 tumor bearing mice causes a decrease in serum prostate specific antigen (PSA) level [10]. Because PSA is a well-accepted target of androgen receptor (AR), which plays an important role in prostate cancer development and progression of the disease to castration-resistant state [21], it was of interest to determine if HNK inhibits AR activity.
MATERIALS AND METHODS
Reagents
HNK (purity ≥ 98%) was purchased from LKT Laboratories (St. Paul, MN) whereas its analogs [honokiol dichloroacetate (HDCA), honokiol epoxide, and biseugenol] were synthesized as described below. The stock solution of each compound was prepared in dimethyl sulfoxide (DMSO) at 50 mM concentration, and diluted with culture media immediately before use. Final concentration of DMSO was ≤ 0.08%. The proteasomal inhibitor MG132 and anti-p53 antibody were purchased from Calbiochem-EMD Chemicals (Gibbstown, NJ); the 4’,6-diamidino-2-phenylindole (DAPI), anti-α-tubulin antibody, and anti-actin antibody were purchased from Sigma-Aldrich (St. Louis, MO); and anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody was purchased from GeneTex (Irvine, CA). Synthetic androgen R1881 was a gift from Dr. Zhou Wang (Department of Urology, University of Pittsburgh, Pittsburgh, PA). Charcoal/dextran-treated fetal bovine serum (cFBS) was purchased from HyClone-Thermo Fisher Scientific (Waltham, MA); phenol red-free RPMI1640 medium, antibiotic mixture, and phosphate-buffered saline (PBS) were from Invitrogen-Life Technologies (Grand Island, NY); and DMEM and heat-inactivated FBS were from Mediatech (Manassas, VA). Antibody against AR was from Santa Cruz Biotechnology (Dallas, TX). Anti-PSA antibody was purchased from Dako-Agilent Technologies (Carpinteria, CA). FuGENE 6, Dual-Luciferase Reporter Assay kit, and pRL-CMV vector were purchased from Promega (Madison, WI), whereas the pARLUC plasmid was a gift from Dr. William H. Walker (Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh, Pittsburgh, PA) [22]. Alexa Fluor 488 goat anti-rabbit antibody was from Molecular Probes-Life Technologies. A control nonspecific siRNA and a p53-specific siRNA were purchased from Qiagen (Germantown, MD) and Santa Cruz Biotechnology, respectively. Anti-phospho-(S15)-p53 antibody was from Cell Signaling Technology (Danvers, MA). Annexin V Apoptosis Detection kit was from BD Pharmingen (San Jose, CA).
Synthesis of Honokiol Analogs
NMR spectra were recorded in deuterated chloroform (CDCl3) with a Varian INOVA 400 MHz instrument, calibrated using residual undeuterated chloroform (1H: δ = 7.24 ppm) as internal standard. The following abbreviations, or a combination thereof, are used to explain the multiplicities: s = singlet, d = doublet, t = triplet. High resolution mass spectrometry (HRMS) analysis was performed with a Thermo Scientific LTQ FT Ultra Hybrid mass spectrometer set on positive ionization.
Biseugenol was synthesized by dimerization of eugenol according to the procedure described by de Farias [23]. Clove oil from Matheson Coleman & Bell (Gardena, CA) was used as the source for eugenol. Clove oil (1.0 g, 5.5 mmol, 90% eugenol) was dissolved in acetone/H2O 2:1 (30 mL), NH4OH (aq, 18 mL, 29%) was added and the mixture was stirred at room temperature for 10 minutes. A saturated aqueous solution of K3Fe(CN)6 (2.0 g, 6.1 mmol) was added drop wise over a period of 4 hours, followed by another addition of NH4OH (aq, 18 mL, 29%). The mixture was stirred at room temperature for an additional 18 hours and then neutralized by drop wise addition of HCl (aq, 10%). A precipitate was formed, which was filtered off, dissolved in acetone, dried over Na2SO4, filtered, and concentrated under reduced pressure. The crude product was purified by column chromatography on silica gel (ethyl acetate/hexanes 1:3), which afforded the product as a white powder (0.67 g, 75%). The obtained 1H NMR was according to the literature [24].
For the synthesis of honokiol epoxide, HNK (2.7 g, 10 mmol) was dissolved in dry dichloromethane (40 mL) from Sigma-Aldrich and the solution was cooled to 0° C followed by addition of meta-chloroperoxybenzoic acid (6.7 g, 40 mmol) from Sigma-Aldrich in portions over 30 minutes. After stirring for another 4 hours at room temperature all starting material was consumed according to thin-layer chromatography. The reaction mixture was diluted with dichloromethane and washed three times with 5% NaHCO3, and once with H2O. The organic layer was dried over Na2SO4, filtered and concentrated under reduced pressure. The crude product was purified by column chromatography on silica gel (ethyl acetate/hexanes 1:4), which yielded the desired product (1.3 g, 44%). 1H NMR (CDCl3) δ: 7.28-7.17 (m, 2H), 7.11-7.05 (2H), 7.01 (d, 1H, J = 8.0 Hz), 6.99 (d, 1H, J = 8.0 Hz), 3.34-3.29 (m, 1H), 3.26 (dd, 1H, J1 = 15.2 Hz, J2= 1.6 Hz), 3.16-3.11 (m, 1H), 2.96 (t, 1H, J = 4.4 Hz), 2.68–2.88 (m, 5H), 2.57-2.53 (m, 1H).
For the synthesis of honokiol dichloroacetate (HDCA), HNK (1.0 g, 3.7 mmol) was dissolved in dry dichloromethane (200 mL), followed by addition of 4-dimethylaminopyridine (200 mg) from Sigma-Aldrich. The reaction was heated to 40°C while stirring and dicholoacetylchloride (1.45 mL, 15 mmol) was added drop wise over 10 minutes. Next, the reaction mixture was refluxed for 5 hours, after which all starting material was consumed according to thin-layer chromatography. After cooling the solution was washed with brine, dried over Na2SO4, filtered and concentrated under reduced pressure. The crude product was purified by column chromatography on silica gel (ethyl acetate/hexanes 1:9), which resulted in the wanted product (1.6 g, 76%). 1H NMR (CDCl3) δ: 7.31-7.20 (m, 4H), 7.15-7.09 (m, 2H), 6.16 (s, 1H), 6.02-5.84 (m, 3H), 5.14-5.04 (m, 4H), 3.43 (d, 2H, J = 6.8 Hz), 3.35 (d, 2H, J = 6.4 Hz). HRMS calculated for C22H19Cl4O4 487.00320, found 487.00339.
Cell Lines and Cell Viability Assay
LNCaP and C4-2 cell lines were obtained from the American Type Culture Collection (Manassas, VA) and UroCor (Oklahoma City, OK), respectively, and maintained as described previously [25, 26]. TRAMP-C1 cells (a generous gift from Dr. Barbara Foster, Roswell Park Cancer Institute, Buffalo, NY) were cultured in DMEM supplemented with 10% heat-inactivated FBS, 10 nM 5α-androstan-17β-ol-3-one, and antibiotics. The effect of HNK and its analogs on cell viability was determined by trypan blue dye exclusion assay essentially as described by us previously [27]. Briefly, the cells were seeded in triplicate at a density of 7.5×104 cells per well in 12-well plates, allowed to attach overnight, and then treated with DMSO or desired concentrations of the agent. The cells were trypsinized and stained with trypan blue solution. The live cells were examined under an inverted microscope. In some experiments, the cells were plated in phenol red-free media containing cFBS and allowed to attach overnight. The cells were treated with ethanol (control) or 1 nM R1881 and/or 40 µM HNK in phenol red-free media containing cFBS.
Colony Formation Assay
Initial experiments focused on optimization of cell number for the colony formation assay, and in subsequent experiments 500 cells/well were used. Twenty four hours after plating, the cells were exposed to DMSO or desired concentrations of HNK or its analogs. After 10 days of treatment with replacement of culture medium containing DMSO or the test agent every 3rd day, the cells were rinsed with PBS, fixed with 100% methanol for 5 minutes, and stained with a 0.5% crystal violet solution in 20% methanol for 30 minutes at room temperature. The cells were rinsed with water and air-dried. Colonies of more than 50 cells were counted using GelCount (Oxford Optronix, Abingdon, United Kingdom).
RNA Interference of p53
LNCaP cells at 50% confluency were transiently transfected with 100 nM of a control nonspecific siRNA or 100 nM of a p53-targeted siRNA. Twenty four hours after transfection, the cells were treated for 24 hours with either DMSO or 40 µM HNK. The cells were collected and used for western blot analysis for p53 (to confirm knockdown) or AR protein.
Western Blotting
The cells (4×105 cells per dish in 60-mm dish) were plated in phenol red-free media containing cFBS and allowed to attach overnight, and then treated with DMSO or desired concentrations of the agents. Western blot analysis was done as described by us previously [28]. Enhanced chemiluminescence reagent was used for detection of the bands. Change in protein expression was determined by densitometric scanning. In some experiments, the cells were pretreated with 1.5 µM MG132 for 2 hours and then treated with DMSO or 40 µM HNK in the absence or presence of MG132 for an additional 24 hours.
Microscopic Analysis for Nuclear Translocation of AR
LNCaP and C4-2 cells (7×104 cells per well) were plated on coverslips in 12-well plates in phenol red-free media containing cFBS, allowed to attach overnight, and then pre-treated with DMSO (control) or 40 µM HNK. After 3 hours of incubation at 37°C, ethanol or R1881 (1 nM) was added to designated plates. After treatment, the cells were washed with PBS, fixed with 2% paraformaldehyde, permeabilized with 0.5% Triton X-100, and incubated with BSA buffer (PBS containing 0.5% bovine serum albumin and 0.15% glycine). After blocking with BSA buffer for 1 hour, the cells were exposed to the anti-AR antibody (1:200 dilution) overnight at 4°C. Subsequently, the cells were treated with Alexa Fluor 488-conjugated secondary antibody (1:1000 dilution) for 1 hour at room temperature, and then counterstained with DAPI (50 ng/mL). The stained cells were examined under a Leica DC 300F fluorescence microscope.
Quantitation of PSA in Cell Culture Medium
The cells (7.5×104 cells per well in 12-well plates) were plated in triplicate in phenol red-free media containing cFBS, allowed to attach overnight, and treated with DMSO or desired concentrations of HNK for 24 hours. After treatment, media were collected and centrifuged at 3,500 rpm for 15 minutes. Equal volume of the resultant supernatant from each group was processed to measure PSA level using the Quantikine Human KLK3/PSA Immunoassay kit from R&D Systems (Minneapolis, MN).
Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Analysis
Total RNA from cells was extracted using RNeasy Mini Kit (Qiagen, Germantown, MD) according to the manufacturer's instructions. Complementary DNA was synthesized from 1 µg of total RNA using reverse transcriptase and oligo(dT)20. PCR reaction was carried out using GoTaq qPCR Master Mix (Promega), gene-specific primers, and cDNA. PCR products were resolved by 1.5% agarose gel electrophoresis prestained with ethidium bromide. PCR primers and amplification conditions were as follows: AR (25 cycles): forward: 5’-ATGGTGAGCAGAGTGCCCTA-3’, reverse: 5’-GTGGTGCTGGAAGCCTCTCCT-3’; 95°C for 1 minute, 57°C for 1 minute, 72°C for 1 minute. GAPDH (25 cycles): forward: 5’-GGACCTGACCTGCCGTCTAGAA-3’, reverse: 5’-GGTGTCGCTGTTGAAGTCAGAG-3′; 95°C for 30 seconds, 55°C for 30 seconds, 72°C for 30 seconds.
Luciferase Reporter Assay
The cells (5×104 cells per well in 12-well plates) were plated in triplicate in phenol red-free media containing cFBS, allowed to attach overnight, co-transfected with 2 µg of pARLUC and 0.5 µg of pCMV-RL plasmids for 24 hours, and then treated with DMSO or desired concentrations of HNK for 24 hours. The transient transfection was achieved using FuGENE6 (Roche Applied Science). Luciferase activity was measured using Dual-Luciferase Reporter Assay System (Promega) following the manufacturer’s protocol.
Apoptosis Assays
Apoptosis was quantified by analysis of histone-associated DNA fragment release into the cytosol or flow cytometry after staining the cells with Annexin V/propidium iodide (PI). The cells were plated in triplicate in phenol red-free media containing cFBS and allowed to attach overnight. The cells were then treated with DMSO (control), 1 nM R1881 and/or 40 µM HNK for 24 hours. Histone-associated DNA fragment release into the cytosol was determined using Cell Death Detection ELISAPLUS kit (Roche Diagnostics, Indianapolis, IN) as suggested by the supplier. For flow cytometric quantitation of apoptosis, LNCaP cells (2×105 cells/well) were plated in 6-well plates in triplicate and allowed to attach by overnight incubation at 37°C. Subsequently, the cells were treated with DMSO or HNK in the absence or presence of R1881. The cells were then treated with Annexin V-FITC/PI for 15 minutes in the dark at room temperature. The stained cells were analyzed using a BD Accuri flow cytometer.
Statistical Analysis
Statistical tests were performed using Prism GraphPad (version 4.03). Statistical significance of difference in measured parameters between groups was determined by one-way analysis of variance (ANOVA) followed by Dunnett’s or Bonferroni’s multiple comparison test. A P value < 0.05 was considered statistically significant.
RESULTS
Effect of HNK and Its Analogs on Viability of Human Prostate Cancer Cells
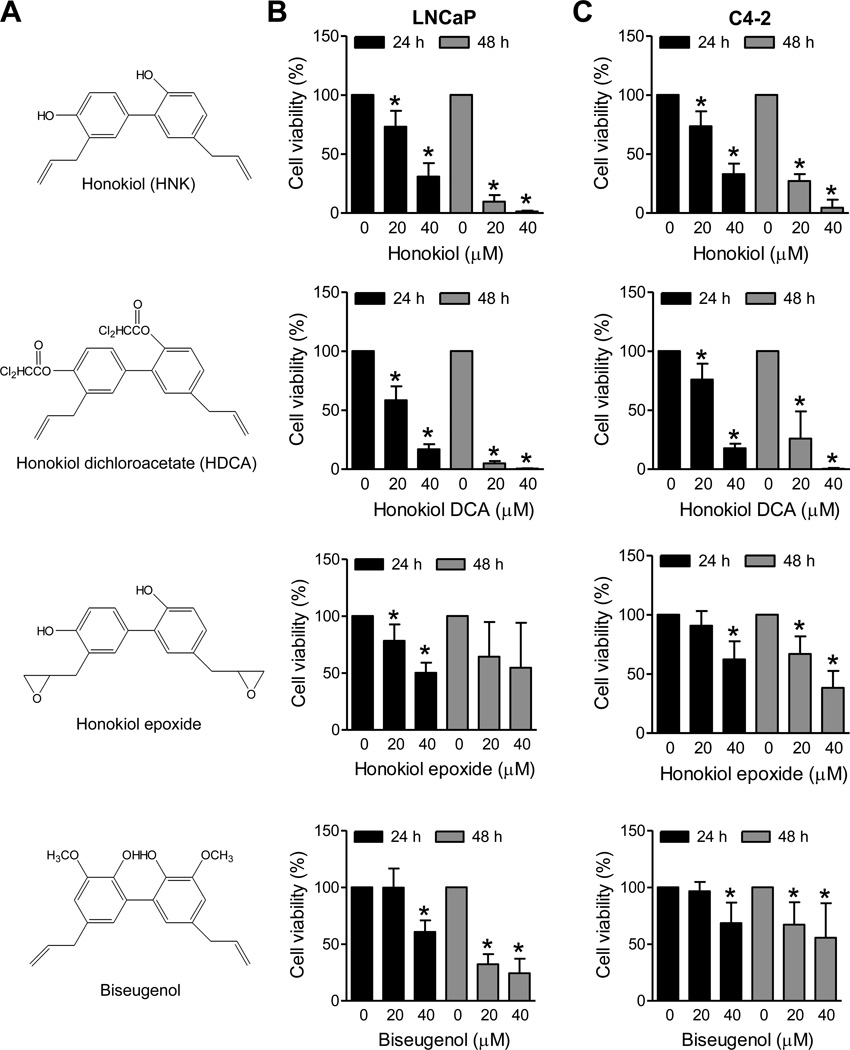
Initially, we determined the growth inhibitory effects of HNK and its analogs (chemical structures of HNK and its analogs are shown in Fig. 1A) against LNCaP (an androgen-responsive prostate cancer cell line with wild-type p53) and its androgen-independent variant (C4-2). The viability of LNCaP (Fig. 1B) and C4-2 cells (Fig. 1C) was inhibited in a dose-and time-dependent manner. The growth inhibitory effect of HNK was comparable in both cells suggesting that the cytotoxicity of this agent is not impacted by the androgen-responsiveness. The order of potency for the compounds was HDCA > HNK > honokiol epoxide > biseugenol.
Fig. 1.
Effects of HNK and its analogs on viability of LNCaP and C4-2 human prostate cancer cells. A: Chemical structures of HNK and its analogs. Effects of HNK and its analogs on viability of (B) LNCaP and (C) C4-2 cells after 24 hour and 48 hour treatments as determined by trypan blue dye exclusion assay. Combined results from two independent experiments are shown as mean ± SD (n=5–6). *Significantly different (P<0.05) compared with DMSO-treated control by one-way ANOVA followed by Dunnett’s test.
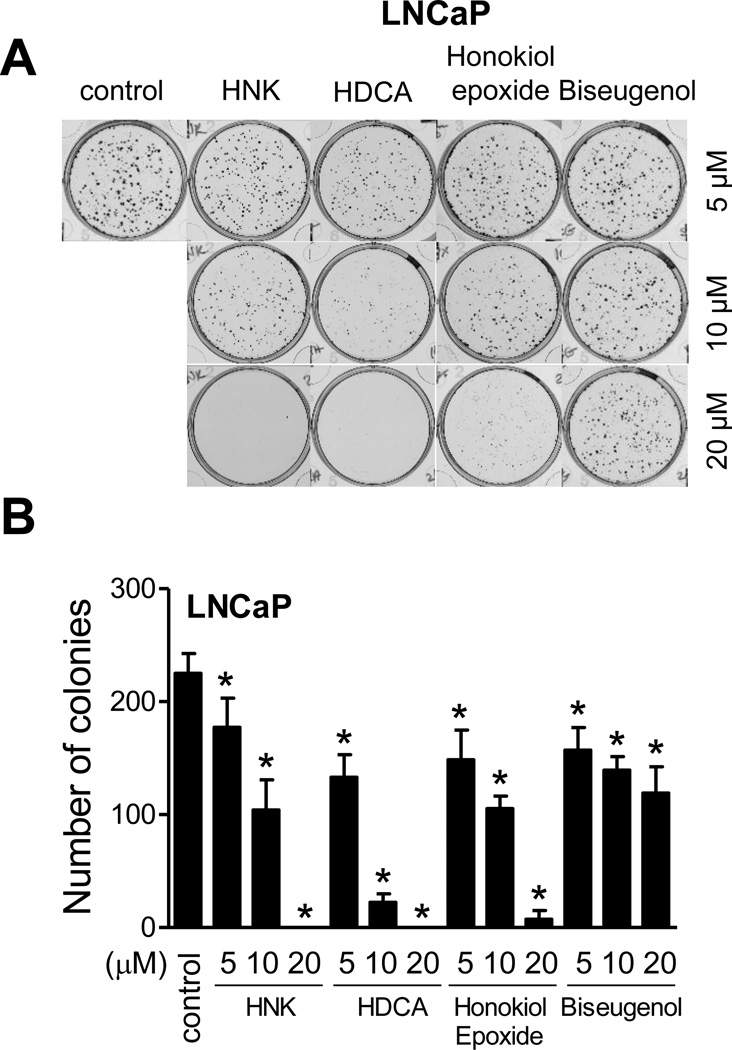
Relative potency of HNK and its analogs was compared by colony formation assay after 10 days of treatment using LNCaP (Fig. 2A). Similar to trypan blue dye exclusion assay, biseugenol was least effective among different compounds. The HDCA appeared most effective at least at the 10 µM dose when compared with other compounds (Fig. 2B). Collectively, these studies indicated that HNK treatment inhibited cell viability and clonogenicity of prostate cancer cells.
Fig. 2.
Effects of HNK and its analogs on clonogenicity of LNCaP cells. A: Clonogenicity of LNCaP cells after 10 days of treatment with HNK or its analogs. B: Quantitation of the effects of HNK and its analogs on colony formation in LNCaP cells. Results shown are mean ± SD (n=3). *Significant (P<0.05) by one-way ANOVA followed by Dunnett’s test.
Effect of HNK and Its Analogs on AR Protein Level in Prostate Cancer Cells
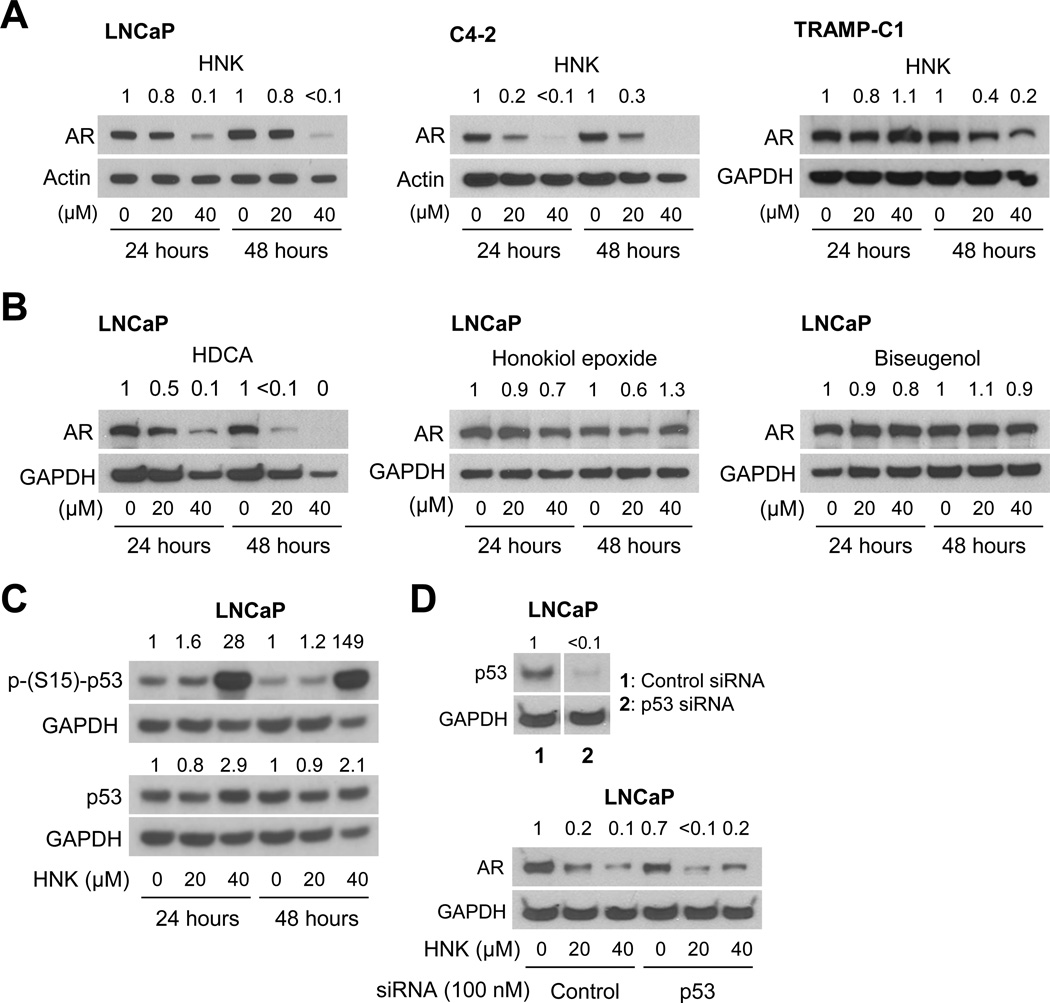
The protein level of AR was decreased markedly after 24 hour and 48 hour treatment with HNK in a dose-dependent manner in LNCaP cells as well as in its androgen-independent variant C4-2 (Fig. 3A). The level of AR protein was also decreased after 48-hour treatment, but not at the 24 hour time point, with HNK in a cell line derived from spontaneously developing prostate tumor of transgenic adenocarcinoma of the mouse prostate mice (Fig. 3A). Consistent with the cell viability data (Fig. 1B–C), HDCA treatment resulted in a dramatic decrease in AR protein level, whereas honokiol epoxide and biseugenol treatments marginally affected its protein expression (Fig. 3B).
Fig. 3.
HNK suppresses AR protein expression in human (LNCaP and C4-2) and mouse (TRAMP-C1) prostate tumor cells. A: Western blotting for AR protein using lysates from LNCaP, C4-2, and TRAMP-C1 cells after 24 hour or 48 hour treatment with DMSO or the indicated doses of HNK. B: Western blotting for AR protein using lysates from LNCaP cells after 24 hour or 48 hour treatment with DMSO or the indicated doses of HNK analogs. The numbers on top of the bands indicate the change in AR protein level compared to corresponding DMSO-treated control. C: Western blots showing the effect of HNK treatment (24 hour and 48 hour) on protein level and S15 phosphorylation of p53 in LNCaP cells. D: Immunoblotting for p53 or AR using cellular lysates from LNCaP cells transiently transfected with a control nonspecific siRNA or a p53-targeted siRNA and treated for 24 hours with DMSO or the specified concentrations of HNK. Each experiment was repeated at least twice with comparable results.
We raised the question of whether HNK-mediated downregulation of AR protein was affected by the p53 status. We addressed this question using LNCaP cells. Treatment of LNCaP cells with 40 µM HNK, but not the lower dose, resulted in a marked increase in protein level as well as S15 phosphorylation of p53 (Fig. 3C). We performed knockdown experiments to directly explore the role of p53, if any, in AR suppression by HNK. The level of p53 protein was decreased by >99% upon its RNA interference (Fig. 3D). The level of AR protein was modestly decreased (about 30% decrease) in LNCaP cells transfected with the p53-targeted siRNA (Fig. 3D). However, HNK treatment resulted in suppression of AR protein level to nearly similar extent in cells transfected with the control siRNA and p53-targeted siRNA (Fig. 3D). Based on these findings, we conclude that p53 status has minimal impact on AR protein downregulation by HNK.
Effect of HNK on Androgen-Stimulated Nuclear Translocation of AR
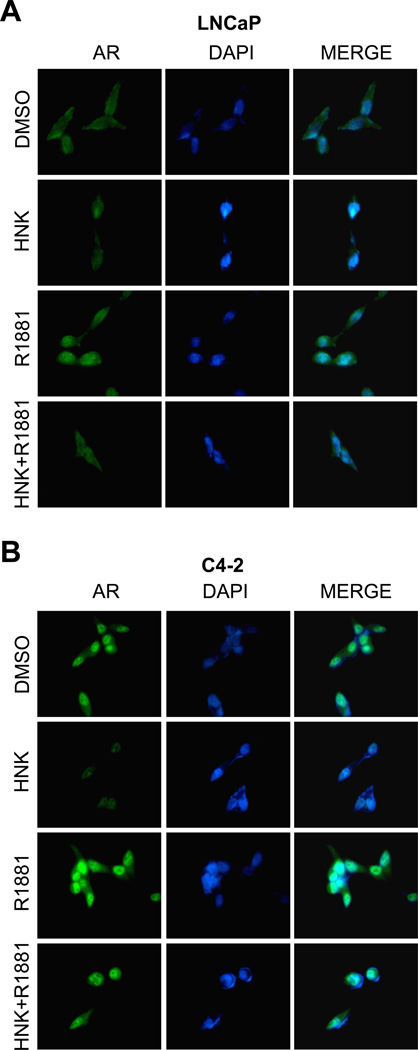
The ligand-bound AR translocates to the nucleus and binds to androgen-responsive elements in the regulatory regions of its target genes for regulation of their expression [21]. We designed experiments to determine the effect of HNK on nuclear levels of AR with or without stimulation with a synthetic androgen (R1881). In DMSO-treated control LNCaP cells that were cultured in phenol-red free medium supplemented with cFBS (without R1881), the immunostaining for AR was visible in both the cytosol and the nucleus (Fig. 4A). As expected, exposure of LNCaP cells to 1 nM R1881 resulted in nuclear enrichment of the AR protein as evidenced by merging of the AR-associated green fluorescence and blue-color nuclear DAPI signal (Fig. 4A). The basal level as well as R1881-stimulated nuclear translocation of AR was diminished markedly in the presence of HNK (Fig. 4A). The C4-2 cell line (Fig. 4B) displayed a much higher basal nuclear level of AR compared with LNCaP cells (Fig. 4A). Nevertheless, the R1881-stimulated nuclear translocation of AR was markedly suppressed by HNK in the C4-2 cells. These results demonstrated HNK-mediated inhibition of nuclear localization of AR in prostate cancer cells. These results indicated that HNK treatment inhibited nuclear translocation of AR with or without stimulation with R1881.
Fig. 4.
HNK inhibits R1881-stimulated nuclear translocation of AR in human prostate cancer cells. A–B: Immunocytochemistry for AR (green) in (A) LNCaP and (B) C4-2 cells detected by fluorescence microscopy (100× objective magnification). The cells were pretreated with DMSO or 40 µM HNK for 3 hours and then incubated with ethanol or R1881 (1 nM) for an additional 3 hours. Each experiment was repeated at least twice with comparable results.
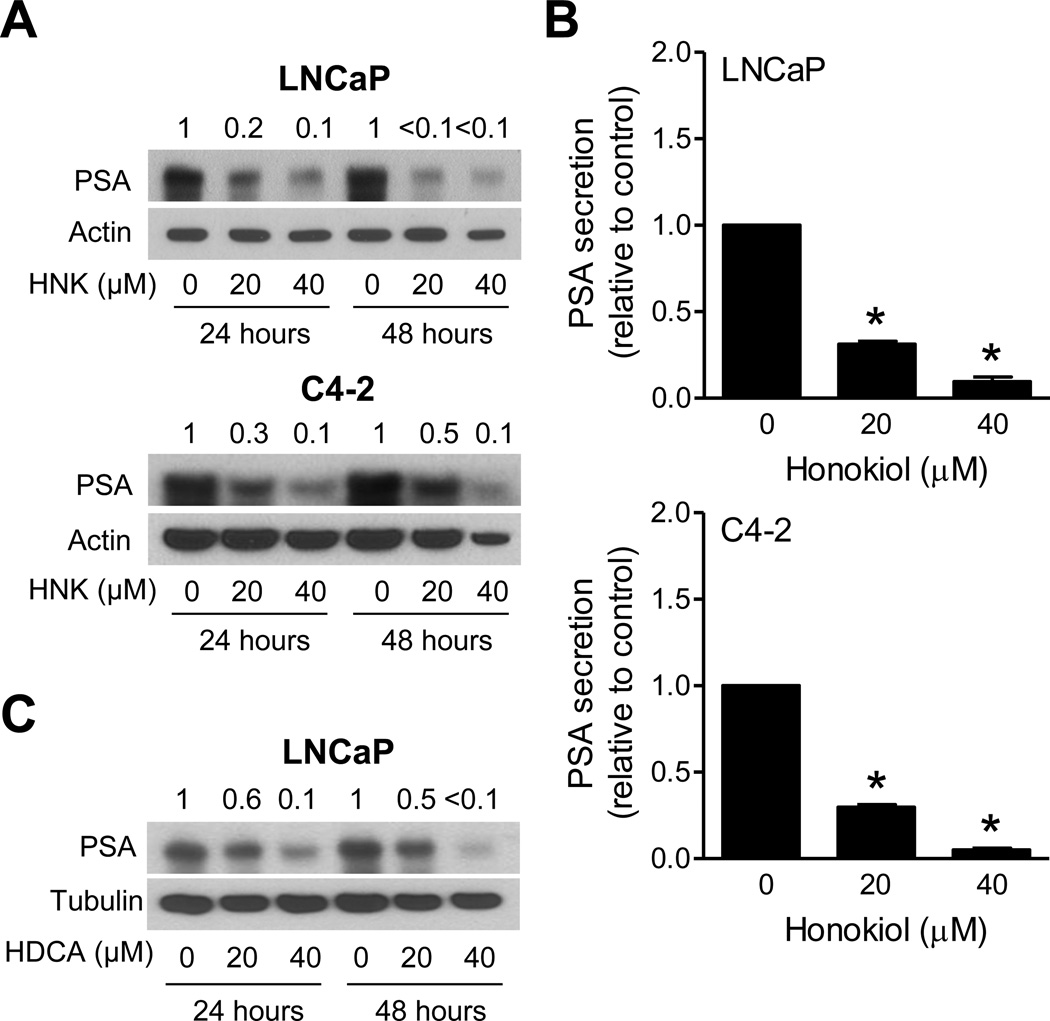
Effect of HNK and Its Analogs on PSA Expression and Secretion
We next raised the question of whether HNK-mediated downregulation of AR protein was accompanied by inhibition of its activity. We addressed this question by determining the effect of HNK on expression and secretion of AR-regulated gene product PSA. As can be seen in Fig. 5A, the HNK treatment downregulated PSA protein level in LNCaP and C4-2 cells in a dose-dependent manner. PSA secretion is considered a useful marker of prostate cancer progression [21]. HNK treatment dose-dependently inhibited the level of secreted PSA in LNCaP and C4-2 cells (Fig. 5B). Consistent with the AR western blotting data, the protein level of PSA was also inhibited after treatment with HDCA (Fig. 5C). These results indicated inhibition of AR activity upon exposure of prostate cancer cells to HNK and HDCA. The effects of the remaining analogs on PSA expression or secretion were not determined.
Fig.5.
HNK and HDCA inhibit expression and/or secretion of PSA in prostate cancer cells. A: Western blotting for PSA using lysates from LNCaP and C4-2 cells after 24 hour or 48 hour treatment with DMSO or HNK. The numbers on top of bands indicate the change in PSA protein level compared to corresponding DMSO-treated control. B: Secreted levels of PSA in LNCaP and C4-2 cells after 24 hour treatment with DMSO or specified doses of HNK. Results shown are mean ± SD (n=3 for each column). *Significantly different (P<0.05) compared to DMSO-treated control by one-way ANOVA followed by Dunnett’s test. C: Western blotting for PSA using lysates from LNCaP cells after 24 hour or 48 hour treatment with DMSO or the indicated doses of HDCA. The numbers on top of bands indicate the change in PSA protein level compared to DMSO-treated control. Each experiment was repeated at least twice with comparable results.
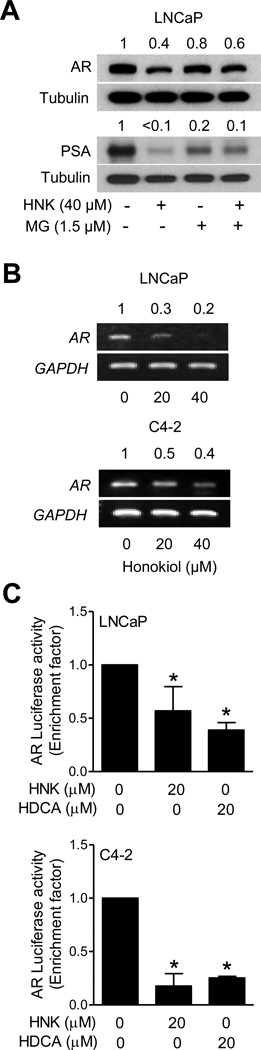
Mechanism Underlying HNK-Mediated Downregulation of AR Protein
Initially, we considered the possibility that HNK promotes proteasomal degradation of AR. Suppression of AR and PSA protein level after HNK exposure was marginally restored in the presence of proteasomal inhibitor MG132 (Fig. 6A). On the other hand, HNK treatment causes a marked decrease in mRNA level of AR (Fig. 6B). We determined the effect of HNK and HDCA on promoter activity of AR using luciferase reporter assay (Fig. 6C). The pARLUC plasmid used in the present study is a modification of the pAR1.1LUC plasmid containing the proximal 1047 bp rat AR promoter fragment inserted upstream of the luciferase gene in the pGL2-enhancer vector [22]. Exposure of LNCaP and C4-2 cells to HNK or HDCA for 24 hours resulted in statistically significant decrease in AR promoter activity (Fig. 6C). The HDCA was slightly superior to HNK at least in the LNCaP cell line (Fig. 6C). These results indicated that the HNK-mediated decline in AR protein level was due to its transcriptional repression (Fig. 6C). Based on these results, we conclude that both transcriptional repression and proteasomal degradation mechanisms likely contribute to HNK-mediated downregulation of the AR protein expression. Also the effect of HNK on AR protein and mRNA was modestly different between LNCaP and C4-2 cells for reasons not yet clear.
Fig.6.
HNK decreases AR mRNA level in human prostate cancer cells. A: Western blotting for AR and PSA proteins using lysates from LNCaP cells after treatment with 1.5 µM MG132 (2 hour pretreatment) and/or 40 µM HNK (24 hour treatment). The numbers on top of bands indicate the change in proteins level compared to DMSO-treated control. B: mRNA level of AR in LNCaP and C4-2 cells after 24 hour treatment with DMSO or HNK. The numbers on top of bands indicate the change in AR mRNA level compared to DMSO-treated control. C: Luciferase activity in LNCaP and C4-2 cells after 24 hour treatment with DMSO, HNK or HDCA. Results shown are mean ± SD (n=3). *Significantly different (P<0.05) compared to DMSO-treated control by one-way ANOVA followed by Bonferroni’s test. Similar results were observed in replicate experiments.
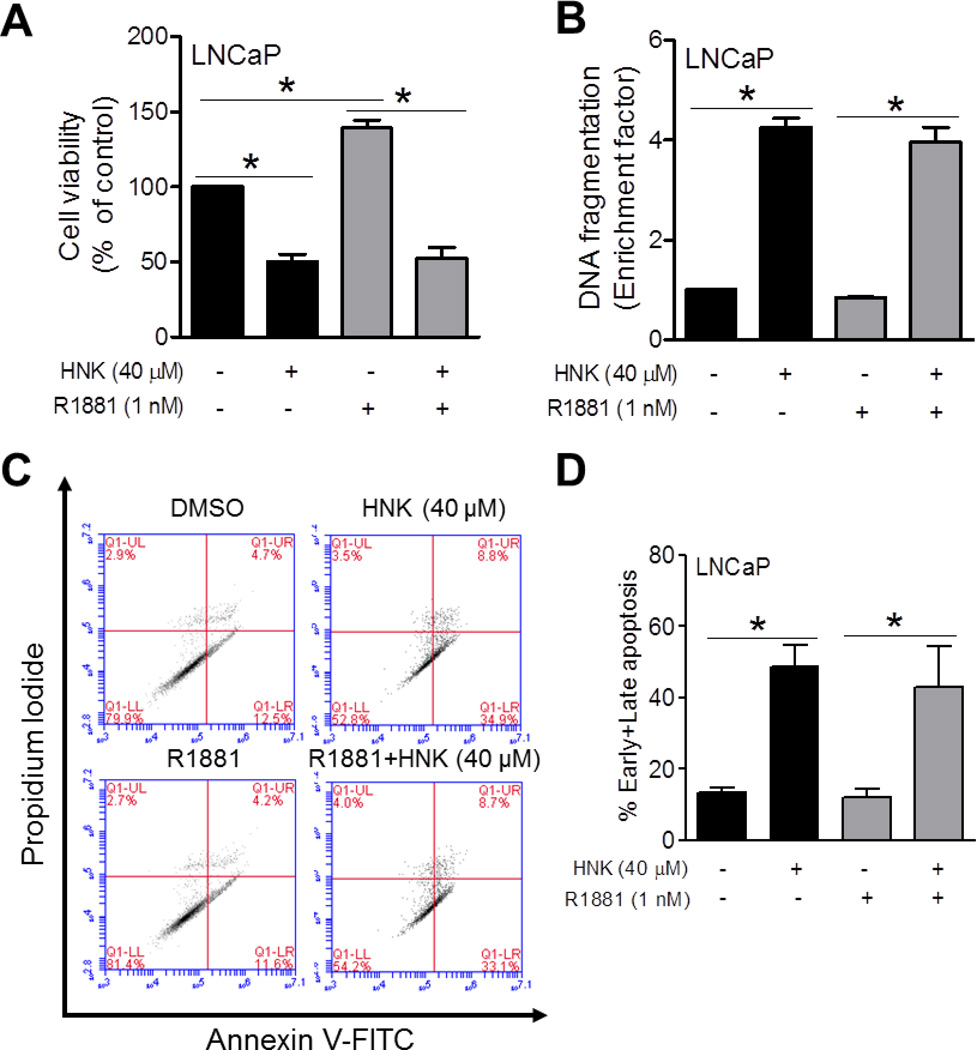
Effects of HNK and/or R1881 Treatments on Apoptosis in LNCaP Cells
Next, we determined the effect of HNK (24 hour exposure) on cell viability and apoptosis induction in the presence or absence of synthetic androgen R1881 using LNCaP cells cultured for 24 hours in phenol-red free medium supplemented with 10% cFBS. The R1881 treatment caused a significant increase in viability of LNCaP cells as judged by trypan blue dye exclusion assay (Fig. 7A). The viability of LNCaP cells was decreased by about 50% by a 24 h exposure to 40 µM HNK. The HNK-mediated suppression of LNCaP cell viability was maintained even in the presence of R1881. Consistent with these results, a 24 hour exposure of LNCaP cells to 40 µM HNK resulted in increased cytoplasmic histone-associated apoptotic DNA fragmentation compared with DMSO-treated control regardless of the R1881 stimulation (Fig. 7B). A different method of apoptosis detection was used to confirm these results. Flow histograms for early (Annexin V-high, PI-low) and late (Annexin V-high, PI-high) apoptotic fraction after 24 h treatment of LNCaP cells with DMSO, HNK and/or R1881 are shown in Figure 7C. Similar to the histone-associated DNA fragment release into the cytosol results (Fig. 7B), the HNK-induced apoptosis was not affected by R1881 in the Annexin V-PI assay (Fig. 7D). Collectively, these results indicated that HNK-induced apoptosis was not affected by R1881.
Fig. 7.
Effects of HNK and/or R1881 treatments on cell viability and apoptosis induction in LNCaP cells. A: Viability of LNCaP cells after exposure to 1 nM of R1881 alone or in combination with 40 µM HNK (24 hour treatment). B: Quantitation of histone-associated DNA fragment release into the cytosol in LNCaP cells after 24 hour treatment with 1 nM of R1881 alone or in combination with 40 µM HNK. C: Representative flow histograms depicting early (Annexin V-high, PI-low) and late apoptotic fraction (Annexin V-high, PI-high) in LNCaP cells after 24 h treatment with DMSO, HNK and/or R1881. D: Quantitation of apoptotic fraction in LNCaP cells after 24 hour treatment with 1 nM of R1881 alone or in combination with 40 µM HNK. Quantitative results (panels A, B, and D) are mean ± SD (n=3). *Significantly different (P<0.05) between the indicated groups by one-way ANOVA followed by Bonferroni’s multiple comparison test. Each experiment was repeated at least twice with comparable results.
DISCUSSION
Mechanisms underlying prostate cancer development are not fully understood but age, race, dietary habits, and androgen secretion and metabolism are some of the risk factors associated with this devastating disease malignancy [1, 29]. AR, which is a ligand activated transcription factor belonging to the steroid receptor superfamily, plays an important role in the development and maintenance of prostate cancer [29–31]. Furthermore, AR signaling is implicated in transition of prostate cancer from hormone-sensitive state to castration-resistant (androgen-independent) form [21, 32]. Consequently, novel strategies for inhibition of AR activity are desirable. Previous work from our laboratory has revealed that HNK inhibits the growth of human prostate cancer cells by causing cell cycle arrest and apoptosis induction regardless of the androgen-responsiveness or the p53 status [9, 17]. This study now demonstrates, for the first time, that HNK treatment decreases protein level of AR in both androgen-responsive and androgen-independent prostate cancer cells. The HNK-mediated downregulation of AR protein is due to modest proteasomal degradation as well as reduction in AR message.
The inhibitory effect of HNK on AR signaling is evident at 40 µM concentration, which is pharmacologically achievable based on rodent pharmacokinetic studies. For example, the pharmacokinetic studies in female BALB/c mice after intraperitoneal administration of 250 mg/kg HNK revealed peak plasma concentration of 1,100 µg/mL [33]. Notably, the plasma concentration of HNK exceeded the concentrations used in the present study even after 12 hours of administration [33]. The area under the curve in rat plasma after bolus intravenous administrations of 5 mg/kg and 10 mg/kg HNK were about 22 and 50 µM, respectively [34]. A biphasic pharmacokinetic behavior involving a rapid distribution phase followed by a slow elimination phase were observed in the plasma concentration time curve [34]. However, further studies are needed to determine the peak plasma concentration of HNK in humans after oral administration. In this context, it is important to mention that oral administration of HNK significantly inhibits growth of subcutaneous PC-3 xenografts in male athymic mice [9].
The AR protein predominantly localizes in the cytoplasm in the absence of the ligand but complexed with chaperone proteins in a conformation susceptible to ligand binding [30]. Ligand-activated regulation of AR target gene expression is achieved by nuclear translocation and dimerization of the receptor [30, 31]. The present study reveals that the transcriptional activity of AR is inhibited by HNK because: (a) the R1881-stimulated nuclear translocation of AR is suppressed markedly in the presence of HNK; (b) HNK treatment causes a significant decrease in expression as well as secretion of AR regulated gene product PSA; and (c) HNK is able to inhibit R1881-stimulated growth of LNCaP cells. The present study also indicates that pro-apoptotic response to HNK is not dependent on AR signaling.
Androgen deprivation therapy is the standard of care for prostate. The tumors initially respond to androgen deprivation therapy, but a large fraction become resistant to this strategy and currently available options have side effects [35–37]. For example, Casodex, an androgen receptor antagonist, is commonly used for the treatment of prostate cancer in combination with GnRH agonists, but this combination results in osteoporosis [38]. On the other hand, HNK has been demonstrated to promote bone stability in part by inhibiting RANKL signaling [39]. Recent work on androgen resistance has shown that many advanced tumors are resistant to androgen blockade, they still require AR activation, which is often constitutive and ligand independent [35–37]. Downregulating AR with HNK and its analogs may be an effective strategy to treat AR-dependent, but androgen independent tumors. Finally, HNK might also have additional beneficial effects, in that it lowers expression of epidermal growth factor receptor [11].
In conclusion the present study reveals that, analogous to certain other diet-derived agents [25, 26], HNK is highly effective in reducing protein level of AR, androgen-stimulated nuclear translocation of AR, and transcriptional activity of AR in human prostate cancer cells.
ACKNOWLEDGMENTS
The authors thank Priya Deshmukh for technical assistance. This study was supported in part by the grants CA113363, CA115498, and CA101753 (to S.V.S.) awarded by the National Cancer Institute, National Institutes of Health, grant AR47901 awarded by the National Institutes of Health, and grants awarded to J.L.A. by the Rabinowitch-Davis Foundation, Minsk Foundation and Margolis Foundation.
Abbreviations
- HNK
honokiol
- HDCA
honokiol dichloroacetate
- AR
androgen receptor
- PSA
prostate specific antigen
- cFBS
charcoal/dextran-treated fetal bovine serum
- DMSO
dimethyl sulfoxide
- DAPI
4’,6-diamidino-2-phenylindole
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- PBS
phosphate-buffered saline
- RT-PCR
reverse transcription-polymerase chain reaction
- PI
propidium iodide
- ANOVA
analysis of variance
Footnotes
Disclosure Statement: None.
REFERENCES
- 1.Drudge-Coates L, Turner B. Prostate cancer overview. Part 1: non-metastatic disease. Br J Nurs. 2012;21(9):S23–S28. [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Garodia P, Ichikawa H, Malani N, Sethi G, Aggarwal BB. From ancient medicine to modern medicine: Ayurvedic concepts of health and their role in inflammation and cancer. J Soc Integr Oncol. 2007;5(1):25–37. doi: 10.2310/7200.2006.029. [DOI] [PubMed] [Google Scholar]
- 4.Li X, Yang G, Li X, Zhang Y, Yang J, Chang J, Sun X, Zhou X, Guo Y, Xu Y, Liu J, Bensoussan A. Traditional Chinese medicine in cancer care: A review of controlled clinical studies published in Chinese. PLoS One. 2013;8(4):e60338. doi: 10.1371/journal.pone.0060338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang G, Li X, Li X, Wang L, Li J, Song X, Chen J, Guo Y, Sun X, Wang S, Zhang Z, Zhou X, Liu J. Traditional Chinese medicine in cancer care: A review of case series published in the Chinese literature. Evid Based Complement Alternat Med. 2012;2012:751046. doi: 10.1155/2012/751046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maruyama Y, Kuribara H. Overview of the pharmacological features of Honokiol. CNS Drugs Reviews. 2000;6(1):35–44. [Google Scholar]
- 7.Fried LE, Arbiser JL. Honokiol, a multifunctional antiangiogenic and antitumor agent. Antioxid Redox Signal. 2009;11(5):1139–1148. doi: 10.1089/ars.2009.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arora S, Singh S, Piazza GA, Contreras CM, Panyam J, Singh AP. Honokiol: a novel natural agent for cancer prevention and therapy. Curr Mol Med. 2012;12(10):1244–1252. doi: 10.2174/156652412803833508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hahm ER, Arlotti JA, Marynowski SW, Singh SV. Honokiol, a constituent of Oriental medicinal herb Magnolia officinalis, inhibits growth of PC-3 xenografts in vivo in association with apoptosis induction. Clin Cancer Res. 2008;14(4):1248–1257. doi: 10.1158/1078-0432.CCR-07-1926. [DOI] [PubMed] [Google Scholar]
- 10.Shigemura K, Arbiser JL, Sun SY, Zayzafoon M, Johnstone PA, Fujisawa M, Gotoh A, Weksler B, Zhau HE, Chung LW. Honokiol, a natural plant product, inhibits the bone metastatic growth of human prostate cancer cells. Cancer. 2007;109(7):1279–1289. doi: 10.1002/cncr.22551. [DOI] [PubMed] [Google Scholar]
- 11.Leeman-Neill RJ, Cai Q, Joyce SC, Thomas SM, Bhola NE, Neill DB, Arbiser JL, Grandis JR. Honokiol inhibits epidermal growth factor receptor signaling and enhances the antitumor effects of epidermal growth factor receptor inhibitors. Clin Cancer Res. 2010;16(9):2571–2579. doi: 10.1158/1078-0432.CCR-10-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Chen L, He X, Fan L, Yang G, Chen X, Lin X, DU L, Li Z, Ye H, Mao Y, Zhao X, Wei Y. Enhancement of therapeutic effectiveness by combining liposomal honokiol with cisplatin in ovarian carcinoma. Int J Gynecol Cancer. 2008;18(4):652–659. doi: 10.1111/j.1525-1438.2007.01070.x. [DOI] [PubMed] [Google Scholar]
- 13.Arora S, Bhardwaj A, Srivastava SK, Singh S, McClellan S, Wang B, Singh AP. Honokiol arrests cell cycle, induces apoptosis, and potentiates the cytotoxic effect of gemcitabine in human pancreatic cancer cells. PLoS One. 2011;6(6):e21573. doi: 10.1371/journal.pone.0021573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guillermo RF, Chilampalli C, Zhang X, Zeman D, Fahmy H, Dwivedi C. Time and dose-response effects of honokiol on UVB-induced skin cancer development. Drug Discov Ther. 2012;6(3):140–146. [PubMed] [Google Scholar]
- 15.Bai X, Cerimele F, Ushio-Fukai M, Waqas M, Campbell PM, Govindarajan B, Der CJ, Battle T, Frank DA, Ye K, Murad E, Dubiel W, Soff G, Arbiser JL. Honokiol, a small molecular weight natural product, inhibits angiogenesis in vitro and tumor growth in vivo. J Biol Chem. 2003;278(37):35501–35507. doi: 10.1074/jbc.M302967200. [DOI] [PubMed] [Google Scholar]
- 16.Wen J, Fu AF, Chen LJ, Xie XJ, Yang GL, Chen XC, Wang YS, Li J, Chen P, Tang MH, Shao XM, Lu Y, Zhao X, Wei YQ. Liposomal honokiol inhibits VEGF-D-induced lymphangiogenesis and metastasis in xenograft tumor model. Int J Cancer. 2009;124(11):2709–2718. doi: 10.1002/ijc.24244. [DOI] [PubMed] [Google Scholar]
- 17.Hahm ER, Singh SV. Honokiol causes G0–G1 phase cell cycle arrest in human prostate cancer cells in association with suppression of retinoblastoma protein level/phosphorylation and inhibition of E2F1 transcriptional activity. Mol Cancer Ther. 2007;6(10):2686–2695. doi: 10.1158/1535-7163.MCT-07-0217. [DOI] [PubMed] [Google Scholar]
- 18.Crane C, Panner A, Pieper RO, Arbiser J, Parsa AT. Honokiol-mediated inhibition of PI3K/mTOR pathway: a potential strategy to overcome immunoresistance in glioma, breast, and prostate carcinoma without impacting T cell function. J Immunother. 2009;32(6):585–592. doi: 10.1097/CJI.0b013e3181a8efe6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu C, Zhang Q, Zhang HY, Zhang X, Huo X, Cheng E, Wang DH, Arbiser JL, Spechler SJ, Souza RF. Targeting the intrinsic inflammatory pathway: honokiol exerts proapoptotic effects through STAT3 inhibition in transformed Barrett's cells. Am J Physiol Gastrointest Liver Physiol. 2012;303(5):G561–G569. doi: 10.1152/ajpgi.00033.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahn KS, Sethi G, Shishodia S, Sung B, Arbiser JL, Aggarwal BB. Honokiol potentiates apoptosis, suppresses osteoclastogenesis, and inhibits invasion through modulation of nuclear factor-κB activation pathway. Mol Cancer Res. 2006;4(9):621–633. doi: 10.1158/1541-7786.MCR-06-0076. [DOI] [PubMed] [Google Scholar]
- 21.Yuan X, Cai C, Chen S, Chen S, Yu Z, Balk SP. Androgen receptor functions in castration-resistant prostate cancer and mechanisms of resistance to new agents targeting the androgen axis. Oncogene. 2013 doi: 10.1038/onc.2013.235. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delfino FJ, Boustead JN, Fix C, Walker WH. NF-κB and TNF-α stimulate androgen receptor expression in Sertoli cells. Mol Cell Endocrinol. 2003;201(1–2):1–12. doi: 10.1016/s0303-7207(03)00005-4. [DOI] [PubMed] [Google Scholar]
- 23.de Farias Dias A. An improved high yield synthesis of dehydrodieugenol. Phytochemistry. 1988;27(9):3008–3009. [Google Scholar]
- 24.Bortolomeazzi R, Verardo G, Liessi A, Callea A. Formation of dehydrodiisoeugenol and dehydrodieugenol from the reaction of isoeugenol and eugenol with DPPH radical and their role in the radical scavenging activity. Food Chemistry. 2010;118(2):256–265. [Google Scholar]
- 25.Stan SD, Singh SV. Transcriptional repression and inhibition of nuclear translocation of androgen receptor by diallyl trisulfide in human prostate cancer cells. Clin Cancer Res. 2009;15(15):4895–4903. doi: 10.1158/1078-0432.CCR-09-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SH, Singh SV. D,L-Sulforaphane causes transcriptional repression of androgen receptor in human prostate cancer cells. Mol Cancer Ther. 2009;8(7):1946–1954. doi: 10.1158/1535-7163.MCT-09-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao D, Choi S, Johnson DE, Vogel VG, Johnson CS, Trump DL, Lee YJ, Singh SV. Diallyl trisulfide-induced apoptosis in human prostate cancer cells involves c-Jun N-terminal kinase and extracellular-signal regulated kinase-mediated phosphorylation of Bcl-2. Oncogene. 2004;23(33):5594–5606. doi: 10.1038/sj.onc.1207747. [DOI] [PubMed] [Google Scholar]
- 28.Xiao D, Srivastava SK, Lew KL, Zeng Y, Hershberger P, Johnson CS, Trump DL, Singh SV. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis. 2003;24(5):891–897. doi: 10.1093/carcin/bgg023. [DOI] [PubMed] [Google Scholar]
- 29.Nelson WG, De Marzo AM, Isaacs WB. Mechanisms of disease: Prostate cancer. N Engl J Med. 2003;349(4):366–381. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- 30.Burnstein KL. Regulation of androgen receptor levels: Implications for prostate cancer progression and therapy. J Cell Biochem. 2005;95(4):657–669. doi: 10.1002/jcb.20460. [DOI] [PubMed] [Google Scholar]
- 31.Kaarbø M, Klokk TI, Saatcioglu F. Androgen signaling and its interactions with other signaling pathways in prostate cancer. BioEssays. 2007;29(12):1227–1238. doi: 10.1002/bies.20676. [DOI] [PubMed] [Google Scholar]
- 32.Tamura K, Furihata M, Tsunoda T, Ashida S, Takata R, Obara W, Yoshioka H, Daigo Y, Nasu Y, Kumon H, Konaka H, Namiki M, Tozawa K, Kohri K, Tanji N, Yokoyama M, Shimazui T, Akaza H, Mizutani Y, Miki T, Fujioka T, Shuin T, Nakamura Y, Nakagawa H. Molecular features of hormone-refractory prostate cancer cells by genome-wide gene expression profiles. Cancer Res. 2007;67(11):5117–5125. doi: 10.1158/0008-5472.CAN-06-4040. [DOI] [PubMed] [Google Scholar]
- 33.Chen F, Wang T, Wu Y-F, Gu Y, Xu X-L, Zheng S, Hu X. Honokiol: A potent chemotherapy candidate for human colorectal carcinoma. World J Gastroenterol. 2004;10(23):3459–3463. doi: 10.3748/wjg.v10.i23.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsai T-H, Chou C-J, Cheng F-C, Chen C-F. Pharmacokinetics of honokiol after intravenous administration in rats assessed using high-performance liquid chromatography. J Chromatography B. 1994;655(1):41–45. doi: 10.1016/0378-4347(94)00031-x. [DOI] [PubMed] [Google Scholar]
- 35.Leibowitz-Amit R, Joshua AM. Targeting the androgen receptor in the management of castration-resistant prostate cancer: rationale, progress, and future directions. Curr Oncol. 2012;19(suppl. 3):S22–S31. doi: 10.3747/co.19.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grossmann M, Cheung AS, Zajac JD. Androgens and prostate cancer; pathogenesis and deprivation therapy. Best Pract Res Clin Endocrinol Metab. 2013;27(4):603–616. doi: 10.1016/j.beem.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 37.Shafi AA, Yen AE, Weigel NL. Androgen receptors in hormone-dependent and castration-resistant prostate cancer. Pharmacol Ther. 2013;140(3):223–238. doi: 10.1016/j.pharmthera.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 38.Smith MR, Fallon MA, Goode MJ. Cross-sectional study of bone turnover during bicalutamide monotherapy for prostate cancer. Urology. 2003;61(1):127–131. doi: 10.1016/s0090-4295(02)02006-x. [DOI] [PubMed] [Google Scholar]
- 39.Yamaguchi M, Arbiser JL, Weitzmann MN. Honokiol stimulates osteoblastogenesis by suppressing NF-κB activation. Int J Mol Med. 2011;28(6):1049–1053. doi: 10.3892/ijmm.2011.786. [DOI] [PubMed] [Google Scholar]