Abstract
Objective
Current adjuvant therapy for advanced-stage, recurrent, and high-risk endometrial cancer (EC) has not reduced mortality from this malignancy, and novel systemic therapies are imperative. Oncolytic viral therapy has been shown to be effective in the treatment of gynecologic cancers, and we investigated the in vitro and in vivo efficacy of the Edmonston strain of measles virus (MV) and vesicular stomatitis virus (VSV) on EC.
Methods
Human EC cell lines (HEC-1-A, Ishikawa, KLE, RL95-2, AN3 CA, ARK-1, ARK-2, and SPEC-2) were infected with Edmonston strain MV expressing the thyroidal sodium iodide symporter, VSV expressing either human or murine IFN-β, or recombinant VSV with a methionine deletion at residue 51 of the matrix protein and expressing the sodium iodide symporter. Xenografts of HEC-1-A and AN3 CA generated in athymic mice were treated with intratumoral MV or VSV or intravenous VSV.
Results
In vitro, all cell lines were susceptible to infection and cell killing by all 3 VSV strains except KLE. In addition, the majority of EC cell lines were defective in their ability to respond to type I IFN. Intratumoral VSV–treated tumors regressed more rapidly than MV-treated tumors, and intravenous VSV resulted in effective tumor control in 100% of mice. Survival was significantly longer for mice treated with any of the 3 VSV strains compared with saline.
Conclusion
VSV is clearly more potent in EC oncolysis than MV. A phase 1 clinical trial of VSV in EC is warranted.
Introduction
While low-risk stage I endometrial cancer (EC) has a 5-year overall survival of 96% with surgical extirpation alone, 5-year overall survival for stage III and IV disease is 67% and 16%, respectively [1]. In addition, optimal therapy for high-risk earlystage and advanced-stage disease remains unclear. External beam radiotherapy [2–4], systemic chemotherapy [5, 6], combined chemotherapy and radiation [7, 8], and most recently, biologics [9, 10] have been and continue to be investigated as adjuvant therapies for EC after surgical staging. However, despite nearly 3 decades of randomized controlled trials of adjuvant therapy, 5-year overall survival in metastatic EC continues to decline [1, 11].
While isolated EC vaginal recurrences can be treated with pelvic radiation and brachytherapy [12], multisite and distant recurrence often portends death from disease [1]. Numerous systemic cytotoxic therapies have been investigated for recurrent EC [13–17], with response rates ranging from 0% for oral etoposide [17] to 27.3% for single-agent paclitaxel [15]. However, the response rate for most single-agent chemotherapies investigated in patients with recurrent disease remains in the single-digit percentages [14, 16]. Additionally, in the past decade, biologic agents have emerged, and their activity as single agents in the patients with recurrent EC has been similar to that of cytotoxic agents, with response rates ranging from 3.3% for lapatinib [18] to 13.5% for bevacizumab [9]. And while progestin therapy for recurrent EC may extend progressionfree survival, it does not improve overall survival [19].
Taken together, the substantial risk of recurrence in advanced-stage EC and the modest response to systemic therapies in patients with recurrence suggest that novel approaches are imperative in mitigating the high risk of death from advanced-stage and recurrent EC.
It has been a century since the first published account of viral oncolytic activity in gynecologic cancer when an advanced cervical cancer regressed in response to rabies vaccination [20]. Early clinical trials used wild-type viruses; however, bioengineering of oncolytic viruses (OVs) has reenergized them as potentially potent therapies for malignancy. Ovarian cancer is the gynecologic malignancy that has garnered the most OV therapy focus. Measles virus (MV), vaccinia virus, vesicular stomatitis virus (VSV), herpes simplex virus, reovirus, and several adenoviruses all have shown activity against ovarian cancer in preclinical cancer models [21–25], and ovarian cancer clinical trials have been performed using MV, herpes simplex virus, reovirus, and adenoviruses [24, 26–28]. However, OV activity in other gynecologic cancers is not well studied, and to our knowledge, only 3 cases of EC treated with an OV have been published. This small cohort treated on a phase 1 adenoviral trial had promising results, with 2 recurrent ECs maintaining stable disease [27].
A highly promising OV, VSV is a negative-stranded RNA virus of the Rhabdoviridae family, which is nonpathogenic in humans and has recently entered clinical testing with human IFN-β (VSV-hIFNβ) for patients with hepatocellular carcinoma at Mayo Clinic in Arizona. Mucous membrane lesions develop in the mouth and nose of infected domestic and farm animals and infection is nonlethal. VSV infects cells through the low density lipid receptor (LDLR) and replicates in the cytoplasm without integrating the viral genome into the host genome [29]. The VSV genome can be manipulated to insert and express transgenes, but it does not have transforming activity [30]. VSV infection initiates a cascade that begins with innate immune system activation and IFN-β production [31]. IFN-β activates genes, upregulates antigen processing machinery, and activates antigen-presenting cells, which stimulates leukocytes to clear the infected cells [32]. Malignant cells have dysfunctional translational and defective immune signaling. And while the normal cellular response to viral infection involves cessation of translation through IFN-dependent pathways, malignant cells have defective IFN pathways, allowing high levels of viral loads that lead to cell death [33]. Additionally, engineering VSV to code for IFN-β attenuates the effect of the virus by inducing an antiviral state in normal cells surrounding malignant cells [34]. VSV antitumor activity has been demonstrated in cancers of the ovary [23], prostate [35], head and neck [36], and colon [37], melanoma [38], and hepatocellular carcinoma [39].
Given the high mortality associated with advanced-stage and recurrent EC, efficacy limitations of currently available therapies, and promising antitumor potency of OVs, we elected to investigate their activity in EC. We present preclinical efficacy of recombinant Edmonston strain MV expressing the thyroidal sodium iodide symporter (MV-NIS) and recombinant VSV expressing either human IFN-β (VSV-hIFNβ) or murine IFN-β (VSV-mIFNβ) in EC. To our knowledge, this is the first published report of MV and VSV activity in EC.
Methods
Cells and Viruses
Type I human EC cell lines HEC-1-A, Ishikawa, KLE, RL95-2, and AN3 CA and type II cell lines ARK-1, ARK-2, and SPEC-2 cells were used. KLE and RL95-2 were cultured in Dulbecco Modified Eagle Medium (DMEM) and Ham F-12 Nutrient Mixture (DMEM/F12; Mediatech, Herndon, Virginia) supplemented with 10% FBS (Life Technologies, Grand Island, New York). AN3 CA and Ishikawa were cultured in 10% FBS DMEM (Mediatech). HEC-1-A, ARK-1, ARK-2, and SPEC-2 were maintained in 10% FBS RPMI-1640 (Mediatech). African green monkey kidney Vero cells (CCL-81; American Type Culture Collection, Manassas, Virginia) was maintained in 5% FBS DMEM.
Expression levels of MV and VSV receptors on all EC cell lines were determined by flow cytometry using R-phycoerythrin (PE)-conjugated antibodies specifically against the MV receptors CD46 (624048, BD, Franklin Lakes, NJ) and PVRL4 (FAB2659P, R&D), and the VSV receptor, LDLR (FAB2148P, R&D).
The following viruses were propagated as previously described: Edmonston strain MV expressing the thyroidal sodium iodide symporter (MV-NIS) [40, 41], VSV expressing hIFNβ (VSV-hIFNβ) or mIFNβ (VSV-mIFNβ) [42] and VSV expressing NIS and with a methionine deletion at residue 51 of the matrix protein (VSV-M51-NIS), which abolishes the functions of the M protein to block the nuclear to cytoplasmic transport of IFN-β mRNA [43]. Viral titers were determined by 50% tissue culture infective dose (TCID50) assay on Vero cells.
Virus Infection, Cell Viability, and Progeny Production
For the virus infection assays, cells in 96 well plates were exposed to MV-NIS, VSV-hIFNβ, VSV-mIFNβ, or VSV-M51-NIS at multiplicity of infection (MOI; 0, 0.001, 0.01, 0.1, 1, and 10). Cell viability was assessed at 48 hours (VSV) or 120 hours (MV) post infection using the MTS cell proliferation assay according to manufacturer’s instructions (Promega, Madison, WI).
For viral progeny propagation assays, cells (2×105/well) were seeded in 6 well plates and infected with viruses (MOI 0.02). Two hours later, the virus inoculum was removed, cells were washed with PBS and growth media was replaced. Media (for VSV) or cells (for MV) were collected at the indicated time points. Viral titers were determined by TCID50 assay on Vero cells.
Sensitivity of ECs to IFN
To evaluate whether EC cells have a functional IFN antiviral response pathway, the panel of EC cell lines in 96 well plates were incubated overnight with increasing concentrations of human IFN-α (Universal Type I Interferon; R&D, Piscataway, New Jersey). The next day, VSV expressing green fluorescent protein (VSVGFP) (MOI 0.1 or 1.0) was added to the cells. Cell viability was determined by the cell proliferation MTS assay 48 hours later.
Animal Experiments
All animal experiments were approved by and performed according to the guidelines of the Mayo Clinic Institutional Animal Care and Use Committee. Four- to 5-week-old female athymic mice were purchased from Harlan (Indianapolis, Indiana). Mice were implanted subcutaneously in the right flank with 2×106 HEC-1-A or AN3 CA cells. When tumors reached 0.3 to 0.5 cm in diameter, 100 µL of virus or saline was injected intratumorally (107 TCID50/mouse) or intravenously through the tail vein (106 TCID50/mouse). Of note, IV MV-NIS was not evaluated in these preclinical studies as immunocompetent humans vaccinated against MV have neutralizing antibodies that rapidly inactivate the virus and, as such, IV MV human clinical trials will not be planned. Tumors were measured twice per week, and mice were euthanized when tumor mass reached 10% of the mouse’s body weight, ulcerated or interfered with a mouse’s ability to reach food or water.
Tumor RNA Extraction and Quantitative RT-PCR for VSV-Nucleocapsid Protein
Tumors were preserved in RNAlater® (Applied Biosystems, Carlsbad, CA) at the time of necropsy and were homogenized in a TissueLyser II instrument with stainless steel beads (Qiagen, Valencia, California). Sample RNA was extracted using an RNA purification kit (RNeasy Plus Universal Mini Kit; Qiagen) and was diluted to 0.2 µg per reaction (total sample volume of 5 µL). Samples were quantified by comparison with a standard curve generated by amplification of 432-bp in vitro–transcribed RNA (MAXIscript SP6 kit; Applied Biosystems) encoding a 298-base portion of the VSV nucleoprotein gene (bases 972–1269) cloned in pCR®II-TOPO® (Invitrogen, Carlsbad, CA). All samples and standards were run in triplicate.
Statistical Analysis
Statistical analyses and graphing were done using JMP software (SAS Institute Inc, Cary, North Carolina). The statistical significance of difference between the survival of mice treated with viruses or buffer was compared using the log-rank (Mantel-Cox) method. Unpaired Student t test and ANOVA were applied to determine the statistical significance of differences between the fractional tumor volumes of animals treated with viruses or buffer at each time point. P values <.05 were considered significant.
Results
Susceptibility of Type I EC to MV and VSV
The panel of type I EC cell lines (Ishikawa, HEC-1-A, KLE, RL95-2, and ANC 3A) was infected with MV-NIS, VSV-mIFNβ, VSV-hIFNβ, or VSV-ΔM51-NIS, and the extent of virus-associated cell killing was compared. There was no correlation between receptor levels and the cellular susceptibility to MV or VSV infection (data not shown).
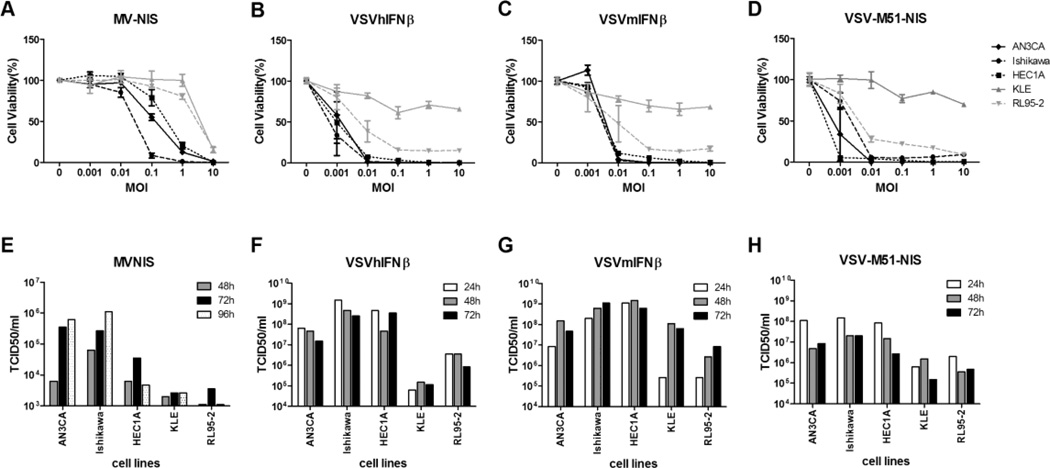
MV-NIS infection induced the cytopathic effects of intercellular fusion or syncytia formation while VSV-infected EC cells rounded up and lifted from the plates (data not shown). As shown in Figure 1A, type I EC cells were susceptible to infection and killing by MV-NIS. Ishikawa, ANC 3A, and HEC-1-A were more susceptible to MV-NIS compared with KLE and RL95-2. The extent of cell killing correlated well with the extent of MV replication and progeny production in the cells (Figure 1E). There was robust MV replication in ANC 3A and Ishikawa cells, reaching titers that were comparable to Vero cells (which replicate viruses very well and are used to produce viral stocks; data not shown). In contrast, MV amplification was substantially lower in RL95-2 and KLE cells.
Figure 1.
Cell-killing ability and progeny propagation of MV and VSV variants in type I EC cell lines. A, B, C, and D, AN3CA, Ishikawa, HEC1A, KLE, and RL95-2 cell lines were evaluated for MV-NIS–, VSV-hIFNβ-, VSV-mIFNβ-, and VSV-M51-NIS–induced cytotoxicity as determined by the MTS cell viability assay. Cells were infected with viruses at the indicated MOI. MTS assays were performed 48 hours after VSV infections and 120 hours after MV infection. E, F, G, and H, Cells were treated with MV-NIS, VSV-hIFNβ, VSV-mIFNβ, and VSV-M51-NIS under multiple-cycle (MOI 0.02) infection conditions. At various times after infection, cells or small aliquots of supernatant were collected, and the amount of viral progeny was determined by TCID50 assay. Abbreviations are defined in the text.
All type I EC lines, except for KLE, were highly susceptible to infection and cell killing induced by all 3 recombinant VSVs (Figure 1B–1D). At the low MOI of 0.01 (1 virus/100 cells), almost 90% of Ishikawa, ANC 3A, and HEC-1-A cells and about 60% of RL95-2 cells were killed by VSV. As was seen with MV-NIS, the KLE cells were more resistant to virus infection, and only 25% of cells were killed at MOI of 10. In contrast to MV-NIS, VSV is a much faster virus, and viral amplification peaked at 24 hours compared with 72 to 96 hours for MV-NIS (Figure 1F–1H). VSV titers reached 108 to 109 TCID50/mL. Since input virus was 4×103 infectious virus particles per 2×105 cells (MOI 0.02), this represents a robust production of about 25,000 to 250,000 viral particles per infected cell over 24 hours.
Susceptibility of Type II ECs to MV and VSV
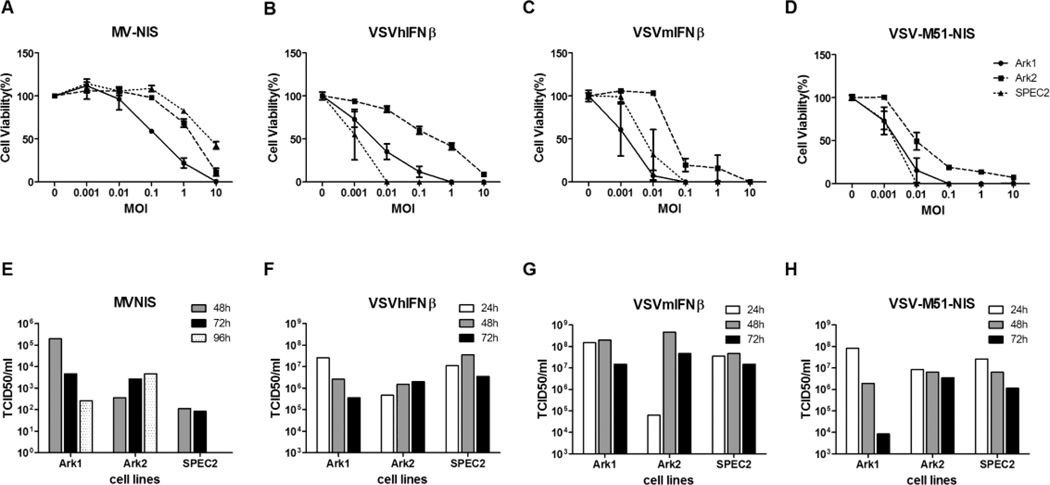
Type II EC cell lines were also susceptible to infection and killing by MV and VSV. ARK-1 was more susceptible to MV-NIS killing than ARK-2 and SPEC-2 cells (Figure 2A). MV replication was negligible in ARK-2 and SPEC-2 cells. There was also no correlation between receptor levels and the cellular susceptibility to MV or VSV infection among type II EC (data not shown). Overall, it appears that type II EC was less susceptible to MV-NIS than type I EC.
Figure 2.
Cell-killing ability and progeny propagation of MV and VSV variants in type II EC cell lines. A, B, C, and D, Ark1, Ark2, and SPEC2 cell lines were evaluated for MV-NIS–, VSV-hIFNβ-, VSV-mIFNβ-, and VSV-M51-NIS–induced cytotoxicity as determined by the MTS cell viability assay. Cells were infected with viruses at the indicated MOI. MTS assays were performed 48 hours after VSV infections and 120 hours after MV infection. E, F, G, and H, Cells were treated with MV-NIS, VSV-hIFNβ, VSV-mIFN β, and VSV-M51-NIS under multiple-cycle (MOI 0.02) infection conditions. At various times after infection, cells or small aliquots of supernatant were collected, and the amount of viral progeny was determined by TCID50 assay. Abbreviations are defined in the text.
The type II EC cell lines were susceptible to infection by the 3 different recombinant VSVs. By 48 hours, 75% to 95% of ARK-1 and SPEC-2 cells were killed by VSV at MOI 0.01 (Figure 2B–2D). ARK-2 cells were less efficiently killed by VSV compared with the other lines. Viral replication was robust in the cell lines, yielding about 107 to 108 TCID50/mL of VSV. These cells supported 10-fold less VSV replication compared with type I EC cells (Figure 1).
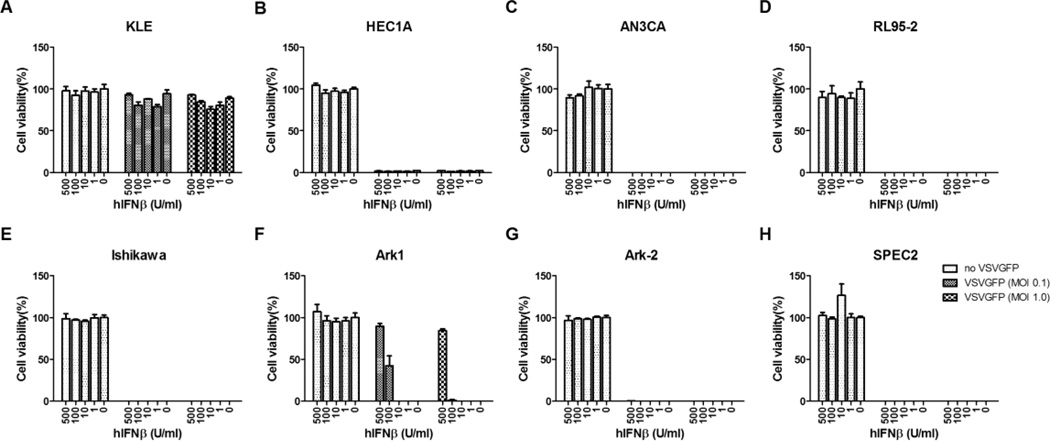
Sensitivity of EC Cells to IFN
Viral expression of hIFNβ protects normal cells from infection and killing by VSV, but it might also restrict viral replication in tumor cells that have retained functional IFN response pathways. To assess whether EC cell lines are responsive to IFN, cells were incubated overnight with increasing concentrations of type I IFN, from 1 to 500 units, and infected with VSV the next day. The majority of EC cell lines were defective in their ability to respond to type I IFN (Figure 3). While type I IFN has been used as an antiproliferative drug for some cancers because of its ability to induce apoptosis [44–46], EC cell lines were not killed by IFN in the absence of virus. In the presence of virus, VSV-GFP induced 100% of cell killing despite the addition of high concentrations of IFN in 4 of 5 type I EC cell lines. Interestingly, KLE was highly resistant to VSV oncolysis. Both ARK-2 and SPEC-2 cells were resistant to the protective antiviral effects of type I IFN, and ARK-1 was responsive only when treated with high levels of type I IFN (100–500 units).
Figure 3.
The responsiveness of EC cell lines to type I IFN. All the EC cell lines were incubated with increasing concentrations of universal type I IFN for 24 hours and then challenged with VSV-GFP at either MOI 0.1 or MOI 1.0. Cell viability was measured by MTS assays 48 hours after infection. Abbreviations are defined in the text.
Intratumoral Virotherapy
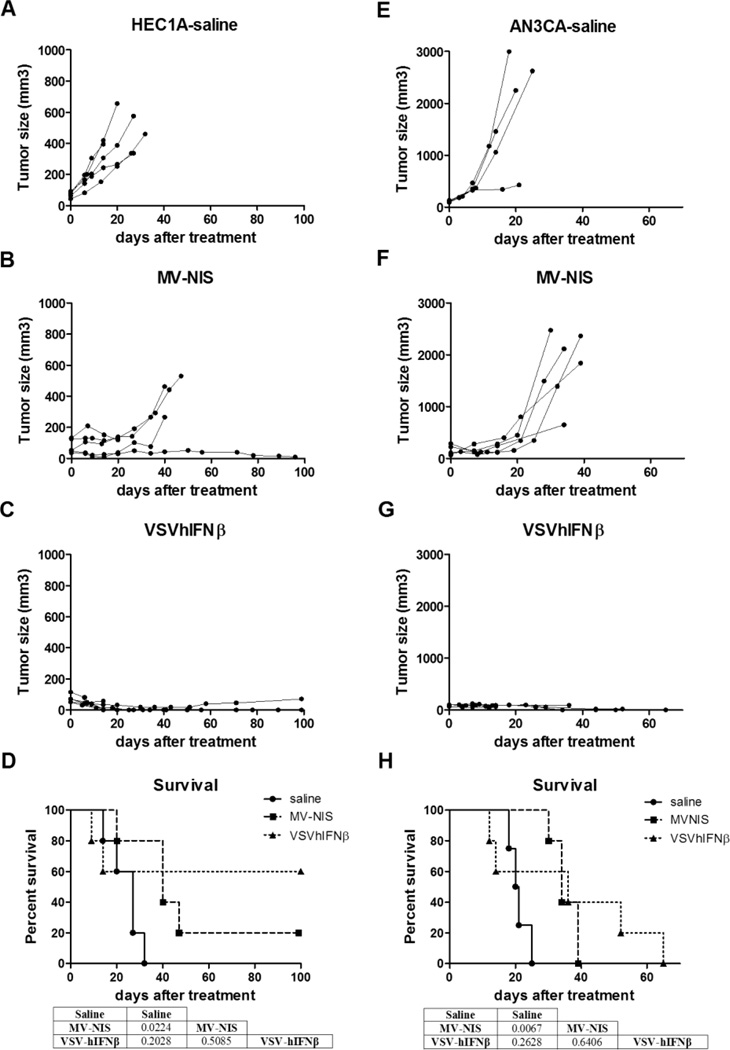
We assessed the antitumor activity of MV-NIS and VSV-hIFNβ in 2 xenograft models of type I EC, HEC-1-A and ANC 3A. We selected cell lines with excellent reputations for tumor xenograft formation for in vivo studies; similar cell killing was observed in both type I and type II cell lines in vitro. When the subcutaneous tumor xenografts reached 0.3 to 0.5 cm in diameter, they were injected directly with 1 dose of 107 TCID50 of MV-NIS or VSV-hIFNβ. For both xenograft models, tumors given saline continued to grow over time, and mice were euthanized because of either tumor burden or ulceration (Figure 4A and 4E; Table). In contrast, virotherapy by MV-NIS (Figure 4B and 4F) or VSV-hIFNβ (Figure 4C and 4G) halted progression in all tumors. In particular, VSV-treated tumors regressed more rapidly than MV-NIS–treated tumors. Three of 10 VSV-hIFNβ mice (2 from HEC-1-A, 1 from AN3 CA) had complete tumor regression. For both xenograft models, both MV-NIS–treated groups had a significantly longer survival when compared with the saline group (P<.05) (Figure 4D and 4H). One MV-NIS–treated mouse with HEC-1-A xenografts had tumor regression and survived until the end of the study. However, for VSV-hIFNβ–treated groups, 5 of 10 mice from both xenograft models were euthanized during the study because of neurotoxic signs (weight loss or paralysis) as the hIFNβ was not biologically active in mice and, thus, not able to protect the animals from VSV-induced neurotoxicity (data not shown). Because of the early euthanization and despite total tumor control, survival was comparable between VSV-hIFNβ–treated and saline groups (Figure 4D and 4H).
Figure 4.
Intratumoral MV-NIS or VSV-hIFNβ treatment of endometrial xenografts derived from HEC1A (A–D) or AN3CA (E–H) cells. Subcutaneous xenografts were established in the right flank of athymic nude mice. Once tumors formed, the mice were assigned randomly to a single IT injection of saline, MV-NIS, or VSV-hIFNβ (1×107 TCID50). A–C and E–G, Individual tumor volumes for the duration of the study for each study group. D and H, Survival of each group was plotted on a Kaplan-Meier survival curve. P values were determined by the log-rank (Mantel-Cox) method and shown as tables underneath the survival curve of each tumor model. P<.05 was considered significant. Study was ended at day 100 after virus injection. Five mice were included in each group, except the AN3CA saline group, which comprised 4 mice.
Intravenous Virotherapy
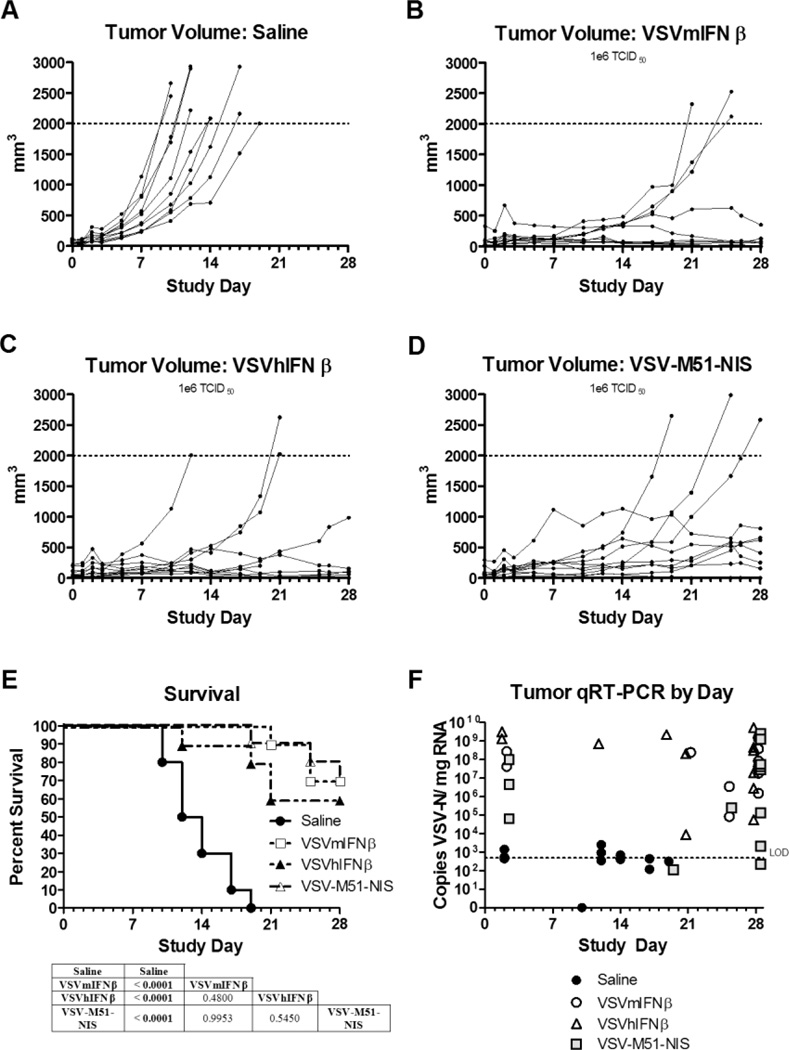
To compare the relative activities of VSV for systemic therapy of EC, athymic mice with established subcutaneous AN3 CA tumors (diameters 0.5 cm) were given 1 intravenous dose of saline or 106 TCID50 of VSV-mIFNβ, VSV-hIFNβ, or VSV-M51-NIS. At the dose of 106 TCID50 VSV, 1 mouse in the VSV-hIFNβ–treated group presented with paralysis at day 19 and was euthanized. Nineteen of 40 total mice were euthanized due to tumor exceeding the limit of 10% of the mouse’s body weight. Ten of those were from the saline-treated group. The remaining 20 mice survived until the end of the study (data not shown). As seen in Figure 5A–5D, therapy with all 3 recombinant VSVs was effective at stopping tumor progression and inducing tumor regression. Tumor control was effective in 100% of mice, and the Kaplan-Meier survival curves of mice treated with all 3 viruses were significantly prolonged compared with the saline controls (Figure 5E). All 3 VSVs were similar in their antitumor activity and were equally effective at prolonging survival (VSV-hIFNβ vs VSV-mIFNβ, P=.48; VSV-hIFNβ vs VSV-ΔM51-NIS, P=.55; VSV-mIFNβ vs VSV-ΔM51-NIS, P>.99). Quantitative real-time (qRT)–PCR of VSV-nucleocapsid protein showed virus levels to be by far the highest in tumor tissue when compared with brain and blood samples (Figure 5F and data not shown). Even at day 28, all 3 recombinant VSVs could still be detected in most of the tumor samples, suggesting sustained replication of the virus in the tumor of these immunocompromised animals.
Figure 5.
Treatment effects of IV VSV on endometrial xenografts derived from the AN3CA cell line. Subcutaneous xenografts were established in the right flank of athymic nude mice. Once tumors formed, the mice were assigned randomly to a single IV injection of saline or different VSV variants (1×106 TCID50). A–D, Individual tumor volumes for the duration of the study for each study group: saline (A), VSV-mIFNβ (B), VSV-hIFNβ (C), and VSV-M51-NIS (D). E, Survival of each group was plotted on a Kaplan-Meier survival curve. P values were determined by the log-rank (Mantel-Cox) method and shown as tables underneath the survival curve of each tumor model. P<.05 was considered significant. F, A qRT-PCR was performed to quantify VSV-N mRNA in tumors on mice necropsied after meeting euthanasia criteria during the efficacy portion of the study or necropsied at the end of the study except for the day 2 samples. Two or 3 mice from each group were euthanized 2 days after treatment to determine the acute effects of viral therapy and spread of virus (safety study). The limit of reliable detection (LOD) for the assay was 500 copies/µg (dotted horizontal line on graphs). Copy numbers less than 500 were interpreted as suspect results. Ten mice were included in the efficacy study and 2 or 3 mice in the safety study.
More detailed toxicity evaluation (no adverse event level (NOAEL) and maximal tolerated dose (MTD)) will be performed in preclinical studies of IV VSV-hIFNβ and VSV-mIFNβ in immunocompetent mice in preparation for a human clinical trial.
Discussion
The high mortality of advanced stage and recurrent EC makes novel systemic therapies imperative. Oncolytic viral therapy has reemerged with potentially potent efficacy in several malignancies [21–25, 35–39]. OVs exploit the replicating machinery of the cancer cell to generate a continuous supply of virions that propagate the apoptotic infection. In addition, OVs are selective for cancer cells and are relatively nonpathogenic toward normal cells. An effective OV that could be administered systemically would be an attractive alternative to the currently available therapies for metastatic EC [3–5, 10, 15, 18]. To that end, we present the first in vitro and in vivo evidence of EC oncolysis secondary to either MV or VSV exposure.
As EC can metastasize or recur as a localized, solitary lesion or as multisite metastases, we approached the assessment of MV and VSV oncolytic activity using intratumoral (IT) MV and VSV injection and intravenous (IV) VSV injection. Both MV and VSV were able to infect type I and type II ECs in vitro and yield high concentrations of virus. While neither MV nor VSV receptor levels correlated with cellular susceptibility, the extent of viral killing is likely more dependent on a combination of the amount of viral replication, progeny production, and anti-apoptotic mechanisms of the host cell. VSV is clearly more potent than MV against EC with near-total tumor regression observed among those treated with IT VSV-hIFNβ. Not only was tumor regression rapid, single-dose IT VSV treatment produced long-term disease suppression, which is especially notable in immunocompromised mice where there is no T-cell–mediated clearance of residual tumor cells.
Despite the potency of VSV cell killing and subsequent improved disease-specific survival, when administered directly into the tumor, VSV-hIFNβ also presents challenging neurotoxicity in immunocompromised mice. VSV infects neuronal cells and causes fatal encephalitis when introduced into the brains of research rodents. VSV is sensitive to the antiviral effects of IFN-α and IFN-β produced by infected cells which limit viral replication and spread to neighboring cells. To attenuate the virus for cancer therapy and protect the host from neurotoxicity, VSV used in our study was engineered to encode the human or mouse IFN-β gene. It is important to note that hIFNβ is not biologically active in mice and is not able to protect the mice from VSV infection of the neurons and its associated neurotoxicity. However, given that the preclinical experiments were intended to assess efficacy for an eventual human clinical trial, we intentionally selected VSV-hIFNβ, expecting to observe neurotoxicity in immunocompromised mice. Additionally, given that xenografts were human EC and that mIFNβ is biologically inactive in human cells, functional IFN pathways and true efficacy could only be evaluated in the setting of hIFNβ exposure.
Observing neurotoxicity with IT VSV-hIFNβ administration suggests leakage of the virus into circulation during tumor injection. Hence, for IV administration we gave a 10-fold lower dose. IV VSV-hIFNβ was well tolerated, with only 1 mouse experiencing neurotoxicity. And despite toxicity, total tumor regression was observed. Among all recombinant VSV strains, IV administration resulted in substantial concentration of the virus within tumor, dramatic tumor regression similar to IT administration, and significantly longer survival (vs. saline). The virus appears to be tumor selective; despite giving nonattenuated VSV, there was minimal viral replication in the brain and neurotoxicity. Extended high levels of intratumoral viral replication after IV infection suggests VSV rapidly infects EC, spreads, and is immunologically protected within the tumor. Prolonged replication in tumor may not happen in humans due to induction of the host immune system in response to viral replication in the tumor. However, the response of the host immune system could further enhance virotherapy as immune-mediated clearance of virally infected cells can induce T-cell response, which primes clearance of residual uninfected tumor cells and generates tumor antigen–specific T-cells [47–49]. Indeed, even in tumors that are not optimally infected by the OV, viral immunotherapy still appears to prolong survival of xenografted mice through induction of tumor-specific cytotoxic T cells secondary to tumor cell infection by the initial input virus.
The clinical relevance of these preclinical findings is several-fold. First, while MV has oncolytic activity in ECs, most humans have been vaccinated against Edmonston strain MV, and rapid neutralization occurs with IV administration [50]. With multisite EC metastases, small recurrences, or solitary tumors inaccessible to IT injection, IT OV injection may not be feasible. Systemic administration is more appealing in the presence of metastatic EC, and VSV is a nonhuman pathogen to which humans are rarely exposed. People who work in proximity to cattle are often seropositive, suggesting subclinical infection. Humans accidentally exposed to wild-type VSV present with only mild influenza-like symptoms. Second, the low rate of central nervous system VSV infection after IV injection of nonattenuated VSV-hIFNβ into athymic mice suggests that in the healthy human immune system, IV VSV may have minimal serious adverse effects. Third, a single dose of IV VSV was able to sustain lasting tumor responses and significantly improved overall survival, suggesting that continued viral production using the EC’s cellular machinery perpetuated the infection and response. In the setting of potentially rapid antibody formation to a new virus, single-dose treatment would be ideal. Fourth, the response rate for all mice treated with recombinant VSV strains was 67% (20/30 mice alive with either negligible or nonmoribund tumor burdens at the end of 28 days). This is a 2.5-fold higher response rate than the current best single-agent chemotherapy in recurrent EC [15]. We have also previously performed extensive preclinical toxicology and pharmacology evaluation of VSV-hIFNβ and shown its safety in mice, rats and rhesus macaques. Nonhuman primates tolerated intrahepatic injection of 1010 TCID50 virus with no adverse events or abnormal hematologic or chemistry changes [51]. IT VSV-hIFNβ is currently being studied in hepatocellular carcinoma in a phase I clinical trial at Mayo Clinic Arizona (PI: Dr. Mitesh Borad, clinical trials.gov identifier NCT01628640). Clinical testing of IV VSV-hIFNβ in EC would be a logical next step in the study of this OV. It is important to note that both type I and type II EC cell lines were killed by VSV. Current therapies for type II EC and advanced disease have limited efficacy, and novel treatments are needed.
Interestingly, only 1 EC cell line (KLE) was intrinsically resistant to VSV. It has been reported that some tumor cells are intrinsically resistant to VSV due to constitutive induction or expression of high levels of antiviral genes such as OAS and MXA [52]. Additionally, the majority of EC cells have defective IFN response pathways, and when treated with high levels of IFN (500 units), they were not protected and were efficiently killed by VSV. Further exploration of the functionality of IFN-stimulated genes in primary samples of EC may shed light on whether EC has reduced expression of IFN-responsive genes and/or dysfunctional mechanism(s) leading to the dysregulated IFN pathways.
In summary, while both MV and VSV have oncolytic activity against EC, VSV is more potent and, given the public health mandate for vaccination against MV, rapid serum antibody neutralization is less likely to occur with IV VSV than with IV MV. Preclinical data support development of a phase 1 clinical trial of IV VSV-hIFNβ in women with recurrent, advanced-stage, and high-risk EC. Given the continued decline in overall survival associated with advanced disease, there is a need for novel and potent systemic antitumor agents in both primary adjuvant therapy and recurrent disease settings. Human safety and efficacy of IV VSV as an oncolytic therapy for EC should be pursued in clinical trials.
Highlights.
Endometrial cancer (EC) cell lines and xenografts undergo oncolysis when exposed to measles virus (MV) and vesicular stomatitis virus (VSV).
VSV is more potent in EC oncolysis than MV.
A phase 1 clinical trial of VSV in EC is warranted.
Acknowledgments
This work was supported by Mayo Clinic Comprehensive Cancer Center (P30CA015083), and grants from the National Institutes of Health National Cancer Institute (R01CA129193, R01CA136547, Mayo Clinic Ovarian SPORE P50CA136393), Office of Women’s Health Research Building Interdisciplinary Careers in Women’s Health (K12 HD065987) and a generous gift from Harry and Lorraine Hammerly.
We thank Suzanne Greiner and Nathan Jenks, Toxicology/Pharmacology Laboratory, Mayo Clinic, for their expert technical help and members of the Viral Vector Production Laboratory for purified stocks of viruses. The human type I cell lines were provided by William Cliby, MD, Mayo Clinic, and the type II cell lines were provided by Sean Dowdy, MD, Mayo Clinic.
Abbreviations
- CD46
cluster of differentiation 46
- DMEM
Dulbecco Modified Eagle Medium
- EC
endometrial cancer
- GFP
green fluorescent protein
- hIFNβ
human IFN-β
- IT
intratumoral
- IV
intravenous
- LDLR
low density lipoprotein receptor
- M51 protein
residue 51 of the matrix protein
- mIFNβ
murine IFN-β
- MOI
multiplicity of infection
- MV
measles virus
- MV-NIS
Edmonston strain MV expressing the thyroidal sodium iodide symporter
- NIS
sodium iodide symporter
- NOAEL
no adverse event level
- OV
oncolytic virus
- PE
R-phycoerythrin
- PVRL4
poliovirus receptor-related 4
- qRT
quantitative real-time
- TCID50
50% tissue culture infective dose
- VSV
vesicular stomatitis virus
- VSV-hIFNβ
vesicular stomatitis virus expressing human IFN-β
- VSV-mIFNβ
vesicular stomatitis virus expressing murine IFN-β
- VSV-M51-NIS
vesicular stomatitis virus with a methionine deletion at residue 51 of the matrix protein and expressing the sodium iodide symporter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: Drs. Federspiel, Russell and Peng are co-founders of Omnis Pharma, an oncolytic VSV company.
References
- 1.Mariani A, Dowdy SC, Cliby WA, Gostout BS, Jones MB, Wilson TO, Podratz KC. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecologic Oncology. 2008;109:11–18. doi: 10.1016/j.ygyno.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blake P, Swart AM, Orton J, Kitchener H, Whelan T, Lukka H, Eisenhauer E, Bacon M, Tu D, Parmar MK, Amos C, Murray C, Qian W. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): pooled trial results, systematic review, and meta-analysis. Lancet. 2009;373:137–146. doi: 10.1016/S0140-6736(08)61767-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Creutzberg CL, van Putten WLJ, Koper PCM, Lybeert MLM, Jobsen JJ, Wárlám-Rodenhuis CC, De Winter KAJ, Lutgens LCHW, van den Bergh ACM, van de Steen-Banasik E, Beerman H, van Lent M. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. The Lancet. 2000;355:1404–1411. doi: 10.1016/s0140-6736(00)02139-5. [DOI] [PubMed] [Google Scholar]
- 4.Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, Pearlman A, Maiman MA, Bell JG. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecologic Oncology. 2004;92:744–751. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 5.Maggi R, Lissoni A, Spina F, Melpignano M, Zola P, Favalli G, Colombo A, Fossati R. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial. Br J Cancer. 2006;95:266–271. doi: 10.1038/sj.bjc.6603279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller D, Filiaci V, Fleming G, Mannel R, Cohn D, Matsumoto T, Tewari K, DiSilvestro P, Pearl M, Zaino R. Late-Breaking Abstract 1: Randomized phase III noninferiority trial of first line chemotherapy for metastatic or recurrent endometrial carcinoma: A Gynecologic Oncology Group study. Gynecologic Oncology. 2012;125:771. [Google Scholar]
- 7.Bock C, Tomazou EM, Brinkman AB, Muller F, Simmer F, Gu H, Jager N, Gnirke A, Stunnenberg HG, Meissner A. Quantitative comparison of genome-wide DNA methylation mapping technologies. Nature biotechnology. 2010;28:1106–1114. doi: 10.1038/nbt.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Homesley HD, Filiaci V, Gibbons SK, Long HJ, Cella D, Spirtos NM, Morris RT, DeGeest K, Lee R, Montag A. A randomized phase III trial in advanced endometrial carcinoma of surgery and volume directed radiation followed by cisplatin and doxorubicin with or without paclitaxel: A Gynecologic Oncology Group study. Gynecologic Oncology. 2009;112:543–552. doi: 10.1016/j.ygyno.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aghajanian C, Sill MW, Darcy KM, Greer B, McMeekin DS, Rose PG, Rotmensch J, Barnes MN, Hanjani P, Leslie KK. Phase II Trial of Bevacizumab in Recurrent or Persistent Endometrial Cancer: A Gynecologic Oncology Group Study. Journal of Clinical Oncology. 2011;29:2259–2265. doi: 10.1200/JCO.2010.32.6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oza A, Elit L, Biagi J, et al. Molecular correlates associated with a phase II study of temsirolimus (CCI-77) in patients with metastatic or recurrent endometrial cancer. J Clin Oncol; ASCO Annual Meeting Proceedings; 2006. p. 18S. [Google Scholar]
- 11.Khuri SF, Daley J, Henderson WG. The comparative assessment and improvement of quality of surgical care in the Department of Veterans Affairs. Archives of surgery. 2002;137:20–27. doi: 10.1001/archsurg.137.1.20. [DOI] [PubMed] [Google Scholar]
- 12.Creutzberg CL, van Putten WLJ, Koper PC, Lybeert MLM, Jobsen JJ, Wárlám-Rodenhuis CC, De Winter KAJ, Lutgens LCHW, van den Bergh ACM, van der Steen-Banasik E, Beerman H, van Lent M. Survival after relapse in patients with endometrial cancer: results from a randomized trial☆. Gynecologic Oncology. 2003;89:201–209. doi: 10.1016/s0090-8258(03)00126-4. [DOI] [PubMed] [Google Scholar]
- 13.Dizon DS, Blessing JA, McMeekin DS, Sharma SK, DiSilvestro P, Alvarez RD. Phase II Trial of Ixabepilone As Second-Line Treatment in Advanced Endometrial Cancer: Gynecologic Oncology Group Trial 129-P. Journal of Clinical Oncology. 2009;27:3104–3108. doi: 10.1200/JCO.2008.20.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia AA, Blessing JA, Nolte S, Mannel RS. A phase II evaluation of weekly docetaxel in the treatment of recurrent or persistent endometrial carcinoma: A study by the Gynecologic Oncology Group. Gynecologic Oncology. 2008;111:22–26. doi: 10.1016/j.ygyno.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 15.Lincoln S, MD, Blessing JA, PhD, Lee RB, MD, Rocereto TF., MD Activity of paclitaxel as second-line chemotherapy in endometrial carcinoma: a gynecologic oncology group study. Gynecologic Oncology. 2003;88:277–281. doi: 10.1016/s0090-8258(02)00068-9. [DOI] [PubMed] [Google Scholar]
- 16.Muggia FM, Blessing JA, Sorosky J, Reid GC. Phase II Trial of the Pegylated Liposomal Doxorubicin in Previously Treated Metastatic Endometrial Cancer: A Gynecologic Oncology Group Study. Journal of Clinical Oncology. 2002;20:2360–2364. doi: 10.1200/JCO.2002.08.171. [DOI] [PubMed] [Google Scholar]
- 17.Rose PG, Blessing JA, Lewandowski GS, Creasman WT, Webster KD. A Phase II Trial of Prolonged Oral Etoposide (VP-16) as Second-Line Therapy for Advanced and Recurrent Endometrial Carcinoma: A Gynecologic Oncology Group Study. Gynecologic Oncology. 1996;63:101–104. doi: 10.1006/gyno.1996.0286. [DOI] [PubMed] [Google Scholar]
- 18.Leslie KK, Sill MW, Lankes HA, Fischer EG, Godwin AK, Gray H, Schilder RJ, Walker JL, Tewari K, Hanjani P, Abulafia O, Rose PG. Lapatinib and potential prognostic value of EGFR mutations in a Gynecologic Oncology Group phase II trial of persistent or recurrent endometrial cancer. Gynecologic Oncology. 2012;127:345–350. doi: 10.1016/j.ygyno.2012.07.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kokka FBE, Oram D, Gallagher C, Bryant A. Hormonal therapy in advanced or recurrent endometrial cancer. Cochrane Database Syst Rev. 2010;8:CD007926. doi: 10.1002/14651858.CD007926.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DePace N. Sulla Scomparsa di un enorme cancro begetante del callo dell'utero senza cura chirurgica. Ginecolgia. 1912;9:82. [Google Scholar]
- 21.Benencia F, Coukos G. Biological Therapy with Oncolytic Herpesvirus. In: Coukos G, Berchuck A, Ozols R, editors. Ovarian Cancer. New York: Springer; 2008. pp. 221–233. [DOI] [PubMed] [Google Scholar]
- 22.Brüning A, Runnebaum IB. The coxsackie adenovirus receptor inhibits cancer cell migration. Experimental Cell Research. 2004;298:624–631. doi: 10.1016/j.yexcr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Capo-chichi CD, Yeasky TM, Heiber JF, Wang Y, Barber GN, Xu X-X. Explicit targeting of transformed cells by VSV in ovarian epithelial tumor-bearing Wv mouse models. Gynecologic Oncology. 2010;116:269–275. doi: 10.1016/j.ygyno.2009.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galanis E, Hartmann LC, Cliby WA, Long HJ, Peethambaram PP, Barrette BA, Kaur JS, Haluska PJ, Aderca I, Zollman PJ, Sloan JA, Keeney G, Atherton PJ, Podratz KC, Dowdy SC, Stanhope CR, Wilson TO, Federspiel MJ, Peng K-W, Russell SJ. Phase I Trial of Intraperitoneal Administration of an Oncolytic Measles Virus Strain Engineered to Express Carcinoembryonic Antigen for Recurrent Ovarian Cancer. Cancer Research. 2010;70:875–882. doi: 10.1158/0008-5472.CAN-09-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirasawa K, Nishikawa SG, Norman KL, Alain T, Kossakowska A, Lee PWK. Oncolytic Reovirus against Ovarian and Colon Cancer. Cancer Research. 2002;62:1696–1701. [PubMed] [Google Scholar]
- 26.Hasenburg A, Tong XW, Fischer PDDC, Rojas-Martinez A, Nyberg-Hoffman C, Kaplan AL, Kaufman RH, Ramzy I, Aguilar-Cordova E, Kieback DG. Adenovirus-Mediated Thymidine Kinase Gene Therapy in Combination with Topotecan for Patients with Recurrent Ovarian Cancer: 2.5-Year Follow-Up. Gynecologic Oncology. 2001;83:549–554. doi: 10.1006/gyno.2001.6442. [DOI] [PubMed] [Google Scholar]
- 27.Kim KH, Dmitriev I, O'Malley JP, Wang M, Saddekni S, You Z, Preuss MA, Harris RD, Aurigemma R, Siegal GP, Zinn KR, Curiel DT, Alvarez RD. A Phase I Clinical Trial of Ad5.SSTR/TK.RGD, a Novel Infectivity-Enhanced Bicistronic Adenovirus, in Patients with Recurrent Gynecologic Cancer. Clinical Cancer Research. 2012;18:3440–3451. doi: 10.1158/1078-0432.CCR-11-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phelps CD, MA, O’Malley DM, et al. REOVIRUS Replication in ovarian and peritoneal tumors after intravenous administration. Proceedings of the 101st Annual Meeting of the American Association for Cancer Research. 2010 Abstract nr 2594. [Google Scholar]
- 29.Finkelshtein D, Werman A, Novick D, Barak S, Rubinstein M. LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:7306–7311. doi: 10.1073/pnas.1214441110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lichty BD, Power AT, Stojdl DF, Bell JC. Vesicular stomatitis virus: re-inventing the bullet. Trends in Molecular Medicine. 2004;10:210–216. doi: 10.1016/j.molmed.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Der SD, Zhou A, Williams BRG, Silverman RH. Identification of genes differentially regulated by interferon α, β, or γ using oligonucleotide arrays. Proceedings of the National Academy of Sciences. 1998;95:15623–15628. doi: 10.1073/pnas.95.26.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heiber JFXX, Barber GN. Potential of vesicular stomatitis virus as an oncolytic therapy for recurrent and drug-resistant ovarian cancer. Chin J Cancer. 2011;30:805–814. doi: 10.5732/cjc.011.10205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balachandran S, Barber GN. Defective translational control facilitates vesicular stomatitis virus oncolysis. Cancer Cell. 2004;5:51–65. doi: 10.1016/s1535-6108(03)00330-1. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y-P, Suksanpaisan L, Steele MB, Russell SJ, Peng K-W. Induction of antiviral genes by the tumor microenvironment confers resistance to virotherapy. Sci. Rep. 2013;3 doi: 10.1038/srep02375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moussavi M, Tearle H, Fazli L, Bell JC, Jia W, Rennie PS. Targeting and Killing of Metastatic Cells in the Transgenic Adenocarcinoma of Mouse Prostate Model With Vesicular Stomatitis Virus. Mol Ther. 2013 doi: 10.1038/mt.2012.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alajez N, Mocanu J, Krushel T, Bell J, Liu F-F. Enhanced vesicular stomatitis virus (VSVDelta51) targeting of head and neck cancer in combination with radiation therapy or ZD6126 vascular disrupting agent. Cancer Cell International. 2012;12:27. doi: 10.1186/1475-2867-12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamaki MSK, Sakaguchi T, Meseck M, Ebert O, Ohdan H, Woo SL. The potential of recombinant vesicular stomatitis virus-mediated virotherapy against metastatic colon cancer. Int J Mol Med. 2013;31:299–306. doi: 10.3892/ijmm.2012.1205. [DOI] [PubMed] [Google Scholar]
- 38.Blackham AU, Northrup SA, Willingham M, D'Agostino RB, Jr, Lyles DS, Stewart Iv JH. Variation in susceptibility of human malignant melanomas to oncolytic vesicular stomatitis virus. Surgery. 2013;153:333–343. doi: 10.1016/j.surg.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ebert O, Shinozaki K, Huang T-G, Savontaus MJ, García-Sastre A, Woo SLC. Oncolytic Vesicular Stomatitis Virus for Treatment of Orthotopic Hepatocellular Carcinoma in Immune-competent Rats. Cancer Research. 2003;63:3605–3611. [PubMed] [Google Scholar]
- 40.Dingli D, Peng KW, Harvey ME, Greipp PR, O'Connor MK, Cattaneo R, Morris JC, Russell SJ. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood. 2004;103:1641–1646. doi: 10.1182/blood-2003-07-2233. [DOI] [PubMed] [Google Scholar]
- 41.Peng K-W, TenEyck CJ, Galanis E, Kalli KR, Hartmann LC, Russell SJ. Intraperitoneal Therapy of Ovarian Cancer Using an Engineered Measles Virus. Cancer Research. 2002;62:4656–4662. [PubMed] [Google Scholar]
- 42.Obuchi M, Fernandez M, Barber GN. Development of recombinant vesicular stomatitis viruses that exploit defects in host defense to augment specific oncolytic activity. Journal of virology. 2003;77:8843–8856. doi: 10.1128/JVI.77.16.8843-8856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goel A, Carlson SK, Classic KL, Greiner S, Naik S, Power AT, Bell JC, Russell SJ. Radioiodide imaging and radiovirotherapy of multiple myeloma using VSV(Delta51)-NIS, an attenuated vesicular stomatitis virus encoding the sodium iodide symporter gene. Blood. 2007;110:2342–2350. doi: 10.1182/blood-2007-01-065573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Almasan A, Ashkenazi A. Apo2L/TRAIL: apoptosis signaling, biology, and potential for cancer therapy. Cytokine & growth factor reviews. 2003;14:337–348. doi: 10.1016/s1359-6101(03)00029-7. [DOI] [PubMed] [Google Scholar]
- 45.Fritz E, Ludwig H. Interferon-alpha treatment in multiple myeloma: meta-analysis of 30 randomised trials among 3948 patients. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2000;11:1427–1436. doi: 10.1023/a:1026548226770. [DOI] [PubMed] [Google Scholar]
- 46.Jelinek DF, Arora T. Effects of interferon alpha on myeloma cells: mechanisms of differential responsiveness. Current topics in microbiology and immunology. 1997;224:261–268. doi: 10.1007/978-3-642-60801-8_27. [DOI] [PubMed] [Google Scholar]
- 47.Bridle BW, Hanson S, Lichty BD. Combining oncolytic virotherapy and tumour vaccination. Cytokine & growth factor reviews. 2010;21:143–148. doi: 10.1016/j.cytogfr.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 48.Naik S, Nace R, Federspiel MJ, Barber GN, Peng KW, Russell SJ. Curative one-shot systemic virotherapy in murine myeloma. Leukemia. 2012;26:1870–1878. doi: 10.1038/leu.2012.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prestwich RJ, Harrington KJ, Pandha HS, Vile RG, Melcher AA, Errington F. Oncolytic viruses: a novel form of immunotherapy. Expert review of anticancer therapy. 2008;8:1581–1588. doi: 10.1586/14737140.8.10.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ong HT, Hasegawa K, Dietz AB, Russell SJ, Peng KW. Evaluation of T cells as carriers for systemic measles virotherapy in the presence of antiviral antibodies. Gene therapy. 2007;14:324–333. doi: 10.1038/sj.gt.3302880. [DOI] [PubMed] [Google Scholar]
- 51.Jenks N, Myers R, Greiner SM, Thompson J, Mader EK, Greenslade A, Griesmann GE, Federspiel MJ, Rakela J, Borad MJ, Vile RG, Barber GN, Meier TR, Blanco MC, Carlson SK, Russell SJ, Peng KW. Safety studies on intrahepatic or intratumoral injection of oncolytic vesicular stomatitis virus expressing interferon-beta in rodents and nonhuman primates. Human gene therapy. 2010;21:451–462. doi: 10.1089/hum.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moerdyk-Schauwecker M, Shah NR, Murphy AM, Hastie E, Mukherjee P, Grdzelishvili VZ. Resistance of pancreatic cancer cells to oncolytic vesicular stomatitis virus: role of type I interferon signaling. Virology. 2013;436:221–234. doi: 10.1016/j.virol.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]