Abstract
Superfund chemicals such as polychlorinated biphenyls pose a serious human health risk due to their environmental persistence and link to multiple diseases. Selective bioactive food components such as flavonoids have been shown to ameliorate PCB toxicity, but primarily in an in vitro setting. Here, we show that mice fed a green tea-enriched diet and subsequently exposed to environmentally relevant doses of coplanar PCB exhibit decreased overall oxidative stress primarily due to the upregulation of a battery of antioxidant enzymes. C57BL/6 mice were fed a low fat diet supplemented with green tea extract (GTE) for 12 weeks and exposed to 5 μmol PCB 126/kg mouse weight (1.63 mg/kg-day) on weeks 10, 11 and 12 (total body burden: 4.9 mg/kg). F2-Isoprostane and its metabolites, established markers of in vivo oxidative stress, measured in plasma via HPLC-MS/MS exhibited five-fold decreased levels in mice supplemented with GTE and subsequently exposed to PCB compared to animals on a control diet exposed to PCB. Livers were collected and harvested for both mRNA and protein analyses, and it was determined that many genes transcriptionally controlled by AhR and Nrf2 proteins were upregulated in PCB-exposed mice fed the green tea supplemented diet. An increased induction of genes such as SOD1, GSR, NQO1 and GST, key antioxidant enzymes, in these mice (green tea plus PCB) may explain the observed decrease in overall oxidative stress. A diet supplemented with green tea allows for an efficient antioxidant response in the presence of PCB 126 which supports the emerging paradigm that healthful nutrition may be able to bolster and buffer a physiological system against the toxicities of environmental pollutants.
Keywords: Green tea, oxidative stress, PCB toxicity, antioxidant response, AhR, Nrf2
Introduction
The contamination of soil and groundwater aquifers by toxic chlorinated organic compounds at Superfund sites, e.g., polychlorinated biphenyls (PCBs) and trichloroethylene (TCE), is a pervasive environmental problem with serious public health consequences1. PCBs are persistent organic pollutants found in soil, air, and water, and a major source of human exposure to PCBs is dietary intake of contaminated foods2. Because PCBs are lipid soluble, they readily accumulate in human tissues, thus increasing human health concerns3. For example, the recent Aniston Community Health Survey reported a significant correlation between PCB levels and risk of developing diabetes4, and circulating levels of PCBs have also been associated with cardiovascular disease risk5. Prenatal exposure to PCBs also may be associated with increased weight in children6. The liver is particularly vulnerable to PCB-induced toxicity because it is the primary organ associated with detoxification, and there is strong evidence from NHANES data that exposure to PCBs is associated with liver disease in humans7.
There is evidence from cell culture and animal models that nutrition can modulate the toxicity of environmental pollutants8 and thus affect vulnerability to environmental insults and compromised health. For example, PCBs can act as diet-dependent obesogens when administered with a high-fat diet, and thus worsen nonalcoholic fatty liver disease9. We have previously shown that PCB exposure can modify lipid metabolism while dietary fat supplementation can ameliorate these negative effects10. For example, we have shown that PCB exposure increases neutral lipid staining in LDL-R / mice fed a corn oil-enriched diet (i.e., a diet rich in omega-6 fatty acids), which could indicate increased inflammation, while inflammation was decreased in mice fed an olive oil-enriched diet. Omega-3 fatty acids derived from fish oil are protective and reduce PCB-induced toxicity in endothelial cells11;12. Similarly, antioxidant nutrients such as dietary flavonoids can protect against endothelial cell damage mediated by these persistent organic pollutants13;14. This is important since coplanar PCBs (e.g., PCB 126) exert their toxicity primarily through activation of the aryl hydrocarbon receptor (AhR) and subsequent uncoupling of cytochrome P450 1A1 (CYP1A1), which can be a source of oxidative stress15;16.
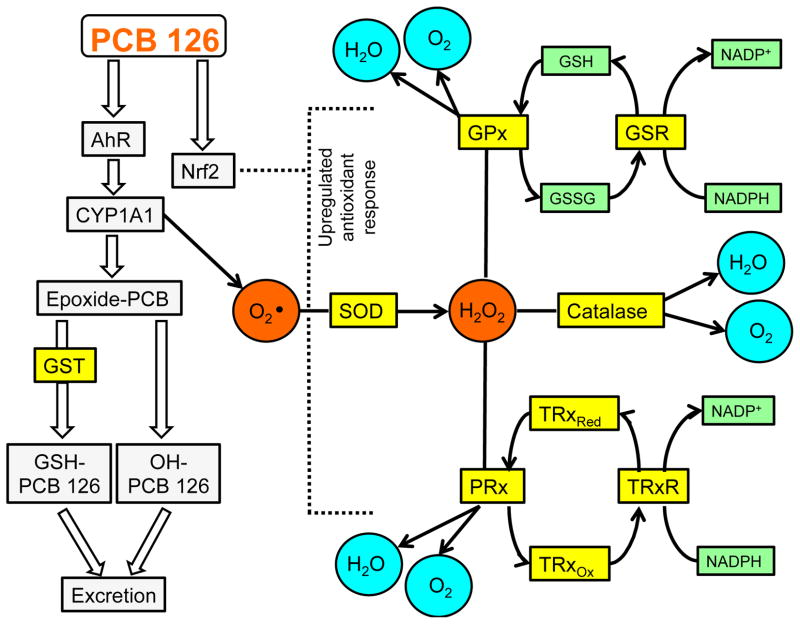
Mammalian cells are constantly exposed to endogenous and exogenous sources of free radicals which tip the cellular balance towards an overall oxidative stress condition. To counteract the ubiquitous nature of reactive oxygen species (ROS), mammalian cells have evolved intricate and interrelated protein defenses that can work efficiently to limit the detrimental effects of these toxic molecules. PCBs have been shown to cause oxidative stress primarily through a CYP1A1 uncoupling mediated mechanism17. Production of superoxide and related ROS triggers an upregulation of a battery of antioxidant enzymes such as superoxide dismutase (SOD), catalase, glutathione reductase (GSR), glutathione transferases (GST), thioredoxins (Trx) and thioredoxin reductases (TrxR)18; 19. These proteins work in concert to either catalyze the transformation of ROS to benign molecules such as water and molecular oxygen or to reactivate enzymes, usually by catalytic reductions (e.g. TrxR reduces oxidized Trx to its active form20). Such an interconnected system requires the crosstalk of multiple regulatory pathways including the aryl hydrocarbon receptor (AhR) and nuclear factor (erythroid-derived 2)-like 2 (Nrf2) transcription factors, which work together to detoxify xenobiotics and to upregulate the antioxidant response (see Fig. 1).
Fig. 1.
Proposed signaling pathway for PCB detoxification in vivo. PCB 126, an AHR ligand and activator of NRF2, causes CYP1A1 upregulation, which leads to superoxide production. Green tea extract (GTE) diet supplementation effectively upregulates redox-related enzymes in the presence of PCB 126 which allows for a more efficient antioxidant response to environmental insult.
Upon activation via endogenous ligands, such as arachidonic acid metabolites, or xenobiotics, such as dioxin (TCDD) or coplanar PCB 126, the AhR translocates to the nucleus, binds consensus cis-acting sequences known as dioxin or xenobiotic response elements (DRE or XRE) and facilitates the upregulation of multiple genes, especially those related to phase I detoxification (e.g. CYP1A1 and UDP-glucuronosyl S-transferases)21. Certain environmental toxicants with relatively long half-lives, such as TCDD and PCB 126, can promote sustained AhR activation resulting in chronic low levels of oxidative stress and inflammation22. The Nrf2 pathway shares many target genes with AhR, but is generally regarded as a redox sensor, because its dissociation with inhibitory proteins (e.g. Keap1) and subsequent transactivation is promoted by ROS and electrophiles23. Binding of Nrf2 to consensus antioxidant response elements (ARE) upregulates a battery of protective genes including cytochrome P450s, GSTs and NAD(P)H dehydrogenase [quinone] 1 (NQO1)24. Nrf2 is a critical mediator of oxidative stress and xenobiotic toxicity as evidenced by multiple studies involving Nrf2 KO mice25,26. It appears that the interrelatedness of Nrf2 and AhR pathways is not a coincidental occurrence as recently intimate cross-talk between the two xenobiotic related proteins has been illustrated27. In fact, AhR and Nrf2 promoter gene sequences contain binding sites for one another and in instances where either is absent (e.g., KO) a decreased protective response occurs28. Importantly, bioactive nutrients such as tea catechins may work through both Nrf2- and AhR-mediated mechanisms to prevent toxicant-induced global inflammation14.
We have demonstrated previously that the tea catechin epigallocatechin-3-gallate (EGCG) can protect against vascular endothelial cell activation by coplanar PCBs14; 29, and that EGCG can inhibit expression of AhR-regulated genes and induce Nrf2-regulated antioxidant enzymes, thus providing protection against PCB-induced inflammatory responses in cultured endothelial cells14. EGCG also can inhibit oxidative damage and attenuate carbon tetrachloride-induced hepatic fibrosis30. Mechanisms responsible for EGCG-induced protection against environmental pollutants are not fully understood. In the current study we provide evidence that green tea extract, composed primarily of EGCG (see Supp. Table 1), can decrease oxidative stress in livers of mice exposed to PCB 126 by a mechanism that, at least in part, is due to induction of antioxidant genes. Thus, diet supplementation with green tea may allow for an efficient antioxidant response to buffer against toxicities of environmental pollutants in humans and protect against PCB-induced liver damage7
2. Materials and Methods
2.1. Animals, diets, and dosing treatments
Forty C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME) at 2 months of age and evenly assigned to the following experimental groups: control diet (10% kcal as fat) + vehicle, control+1% green tea extract (GTE) + vehicle, control + PCB 126, control+1% GTE + PCB 126. The control diet was purchased from Research Diets, Inc. (New Brunswick, NJ, catalog number D12450B). Sunphenon 30S-O organic green tea extract (lot number: 105131, containing 37.4% total polyphenols, 32.0% total catechins, and 5.3% caffeine) was obtained from Taiyo International Inc. (Minneapolis, MN) and incorporated into the control diet formulation, as described in Supp. Table 1. Green tea extract treatment amounts per body weight coincide with approximately 4 cups of tea (~200 ml/cup) per day in humans31. Mice were fed the control and GTE-supplemented diets for 12 weeks and were gavaged with PCB 126 (5 μmol/kg mouse) or vehicle (stripped corn oil; Acros Chemical Company, Pittsburgh, PA) in weeks 10, 11, and 12. The PCB 126 gavage concentration was chosen based on observations in preliminary studies where gavage of 5 μmol PCB 126/kg mouse weight (1.63 mg/kg-day, total body burden: 4.9 mg/kg) showed pro-inflammatory responses in C57/BL6 mice but not wasting syndrome.
2.2 Blood and tissue harvesting
In this study, we examined the role that green tea extract catechins play in altering oxidative stress and inflammation following insult with environmental pollutants (i.e., PCB 126). 24 h after week 12 treatment, mice were euthanized with CO2 and quickly exsanguinated. Ethylenediaminetetraacetic acid (EDTA) was added to collected blood samples, briefly mixed, and centrifuged at 5000 g for 5 min at 4 °C to separate blood plasma. Plasma samples were frozen in liquid nitrogen and stored at −80 °C until processing. Livers were harvested, weighed, divided in half, and frozen in liquid nitrogen for protein studies or stored in RNAlater solution (Life Technologies, Grand Island, NY) at 4 °C for 24 h then −80 °C prior to mRNA analysis.
2.3. Plasma PCB and isoprostane analysis
PCB 126 and its metabolites were extracted from plasma samples to determine systemic PCB and metabolite concentrations and correlate these findings to potential PCB-induced oxidative stress as well as the role of green tea extract in mitigating these effects. PCB 126 and its hydroxy metabolites were isolated from plasma samples (plus 10 μM 13C12-labeled PCB 126 internal standard (IS), Cambridge Isotope Laboratories, Tewksbury, MA) through extraction with acetonitrile and subsequent sonication and centrifugation at 15,000 rpm for 5 min to pellet plasma debris. Supernatants were dried under N2 and reconstituted in 99:1 methanol:dI H2O solvent mixture with 0.5% formic acid and 0.1% 5 M ammonium formate.
Measurement of F2-Isoprostanes (F2-IsoPs) provides one of the most reliable assessment methods for oxidative stress in vivo32. For F2-IsoP analysis, plasma samples were added to 5:1 ethyl acetate: methanol + 0.5% acetic acid (v/v) + 10 μM 8-iso-PGF2α-D4 (internal standard, Cayman Chemical, Ann Arbor, MI), vortexed briefly, and centrifuged to pellet plasma debris. Supernatants were transferred and dried under N2 prior to reconstitution in methanol and addition of acetic acid for subsequent solid phase extraction (SPE).
Reconstituted F2-IsoP samples were loaded onto pre-conditioned Supel-Select HLB SPE columns (Sigma-Aldrich, St. Louis, MO) and washed with 0.5% acetic acid followed by washing with 0.5% acetic acid containing 20% methanol. Columns were eluted with methanol, eluent was evaporated to dryness with N2, and samples were reconstituted with 50:50 methanol:dI H2O.
Plasma PCB 126 and a hydroxy metabolite as well as extracted plasma F2-IsoPs were analyzed using a Shimadzu ultra fast liquid chromatography (UFLC) system coupled with an AB Sciex 4000-Qtrap hybrid linear ion trap quadrupole mass spectrometer in multiple reaction monitoring (MRM) mode. MRM transitions monitored: 325.9/256.1, 325.9/254.1, and 325.9/184 for PCB 126; 338/268.1, 338/196.1, and 338/265.7 for 13C12 PCB126. In the MRM ion transition, the precursor ion represents the M+ and the product ion represents either [M-Cl]+ or [M−2Cl]+. MRM transitions monitored with regard to hydroxy PCB metabolites: 340.8/340.9 for hydroxy PCB126 and 386.8/340.9 for dihydroxy PCB126. The precursor ion of the ion transition is a formic acid adduct: [M+FA-H]− and product ion is [M-H]-. F2-IsoP were analyzed by integrating peak area (area under the curve, AUC) with regard to known internal standard concentrations (AUC/IS). All values were subsequently normalized for sample volume and compared to ion transitions of internal standard (13C12 PCB 126) with known concentration to determine PCB parent and metabolite concentrations (pmol/μL plasma).
2.4. RNA isolation and polymerase chain reaction (PCR) amplification
Liver samples used to analyze oxidative stress and inflammatory mRNA markers were homogenized and mRNA was isolated with TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. mRNA concentrations were then determined using a NanoDrop 2000 spectrophotometer (Thermo Scientific, Waltham, MA). Reverse transcription was performed using the AMV reverse transcription system (Promega, Madison, MI). mRNA levels were determined by quantitative real-time PCR using a 7300 Real-Time PCR system (Applied Biosystems, Foster City, CA) and SYBR Green master mix (Applied Biosystems) as compared to constitutively expressed β-actin (forward primer: 5′-TGTCCACCTTCCAGCAGATGT-3′; reverse primer: 5′-GCTCAGTAACAGTCCGCCTAGAA-3′) using the relative quantification method (ΔΔCt). Primer sequences (see Table 1) for SYBR Green reactions were designed using the Primer Express Software 3.0 for real-time PCR (Applied Biosystems) and synthesized by Integrated DNA Technologies, Inc. (Coralville, IA).
Table 1.
Primers used for qRT-PCR
| Gene name | Forward primer 5′-3′ | Reverse primer 5′-3′ | Fragment size |
|---|---|---|---|
| Inflammatory and xenobiotic-related markers | |||
| AhR | GACCAAACACAAGCTAGACTTCACACC | CAAGAAGCCGGAAAACTGTCATGC | 200 bp |
| CCL3 | CACCCTCTGTCACCTGCTCAA | TGGCGCTGAGAAGACTTGGT | 100 bp |
| CYP1A1 | TGGAGCTTCCCCGATCCT | CATACATGGAAGGCATGATCTAGGT | 100 bp |
| CYP1B1 | TGCATCGGTGAGGAACTGTCT | CTCATGTTTGAGGACTCATTTTGG | 104 bp |
| MCP-1 | GCAGTTAACGCCCCACTCA | CCTACTCATTGGGATCATCTTGCT | 63 bp |
| Nrf2 | GAGTCGCTTGCCCTGGATATC | TCATGGCTGCCTCCAGAGAA | 100 bp |
| Antioxidant markers | |||
| Catalase | CAGAGAGCGGATTCCTGAGAGA | CTTTGCCTTGGAGTATCTGGTGAT | 100 bp |
| Gpx2 | GTGGCGTCACTCTGAGGAACA | CAGTTCTCCTGATGTCCGAACTG | 125 bp |
| Gpx3 | CATACCGGTTATGCGCTGGTA | CCTGCCGCCTCATGTAAGAC | 80 bp |
| Grx2 | CATCCTGCTCTTACTGTTCCATGGCCAA | TCATCTTGTGAAGCGCATCTTGAAACTGG | 123 bp |
| GSR | TCGGAATTCATGCACGATCA | GGCTCACATAGGCATCCCTTT | 100 bp |
| GSTa1 | AAGCCCGTGCTTCACTACTTC | GGGCACTTGGTCAAACATCAAA | 159 bp |
| GSTa4 | TACCTCGCTGCCAAGTACAAC | GAGCCACGGCAATCATCATCA | 109 bp |
| GSTm1 | ATACTGGGATACTGGAACGTCC | AGTCAGGGTTGTAACAGAGCAT | 349 bp |
| GSTm2 | ACACCCGCATACAGTTGGC | TGCTTGCCCAGAAACTCAGAG | 118 bp |
| GSTm3 | CCCCAACTTTGACCGAAGC | GGTGTCCATAACTTGGTTCTCCA | 208 bp |
| NQO1 | GGCATCCAGTCCTCCATCAA | GTTAGTCCCTCGGCCATTGTT | 100 bp |
| SOD1 | GAAACAAGATGACTTGGGCAAAG | TTACTGCGCAATCCCAATCA | 100 bp |
| Trx2 | GCTAGAGAAGATGGTCGCCAAGCAGCA | TCCTCGTCCTTGATCCCCACAAACTTG | 168 bp |
| TrxR1 | GGCCAAAATCGGTGAACACATGGAAG | CGCCAGCAACACTGTGTTAAATTCGCCCT | 175 bp |
2.5. Immunoblotting
Liver samples used for protein analysis were homogenized in extraction RIPA buffer containing protease inhibitors (Pierce, Rockford, IL). Lysed tissue was centrifuged at 10,000 g for 30 min at 4 °C followed by Bradford protein assay (Pierce). Protein samples were separated using 10% SDS-PAGE and subsequently were transferred onto nitrocellulose membranes. Membranes were blocked with 5% non-fat milk buffer and incubated overnight at 4 °C with the following primary antibodies: β-actin (product #A2066, ~42 kD, Sigma, St. Louis, MO), GAPDH (product #sc-20357, ~37 kD, Santa Cruz Biotechnology, Dallas, TX), GSR (product #ab16801, ~58 kD, Abcam, Cambridge, MA), and NQO1 (product #ab34173, ~31 kD, Abcam). After washing, membranes were incubated with secondary antibodies conjugated with horseradish peroxidase and visualized using ECL detection reagents (Thermo, Waltham, MA).
Liver samples used for nuclear translocation assays were prepared and cytoplasmic and nuclear proteins were extracted according to manufacturer protocol (NE-PER® Nuclear and Cytoplasmic Extraction Method, Thermo). Translocation samples subsequently were processed for Western blotting as described above, and probed with the following primary antibodies: lamin (product #sc-7292, ~69 kD, Santa Cruz Biotechnology) and Nrf2 (product #sc-722, ~59 kD, Santa Cruz Biotechnology).
2.6. Data analyses
Data were analyzed using SigmaStat software (Systat Software, Point Richmond, CA). Comparisons between treatments were made by one-way or two-way ANOVA with post-hoc comparisons of the means. Overall, few statistical differences were exhibited between vehicle control diet groups, thus in most cases Student’s t-test was used to analyze differences between PCB treatment groups. qRT-PCR mRNA analysis (n=8–10), Western blot protein analysis (n=8), and nuclear translocation analysis (n=4) represent three experimental replicates. A probability value of p ≤ 0.05 was considered statistically significant.
3. Results
3.1. Systemic toxicity associated with PCBs
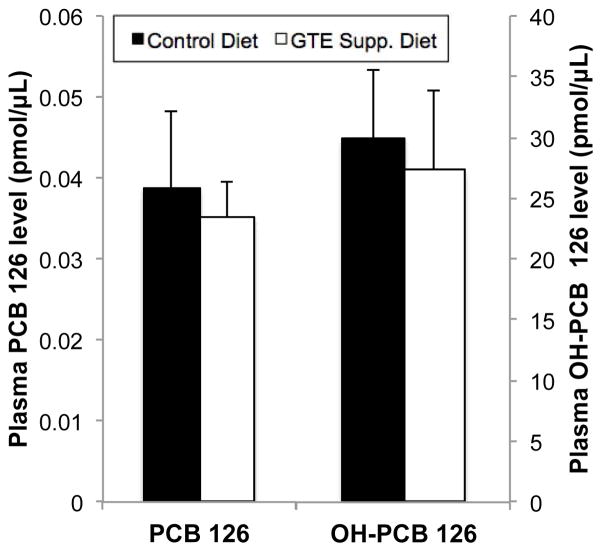
PCB 126 levels in mouse plasma were examined as a measure of systemic PCB body burden and to determine the possible effect of green tea extract (GTE) diet supplementation on PCB metabolism/excretion. As seen in Fig. 2, plasma PCB 126 was found almost completely as its hydroxylated metabolite, OH-PCB 126, with concentrations of approx. 0.04 pmol PCB 126/μL plasma versus approx. 30 pmol OH-PCB 126/μL, respectively. GTE diet supplementation did not modulate PCB metabolism or plasma concentrations 24 h following PCB exposure, indicating that it plays a minimal role in pollutant clearance from the body. Additionally, Supp. Fig. 1 shows that while PCB treatment led to a significant increase in liver/body weight ratio (hepatosomatic index, p<0.001), in both control and GTE-supplemented diets, GTE supplementation did not significantly mitigate this PCB-induced increase.
Fig. 2.
The effect of green tea extract (GTE) diet supplementation on systemic PCB 126 concentration and metabolism. PCB 126 and its hydroxy metabolites were measured in mouse plasma by UFLC/MS MS and normalized to sample volume and internal standard recovery. PCB 126 is heavily metabolized in vivo, as seen by very low levels of parent PCB 126 remaining in plasma samples while its hydroxylated metabolites predominate. GTE supplementation did not significantly modulate systemic PCB or metabolite concentrations. Data are presented as mean±S.E.M (n=5).
3.2. F2-isoprostane levels are significantly reduced in green tea extract-supplemented, PCB-exposed mice
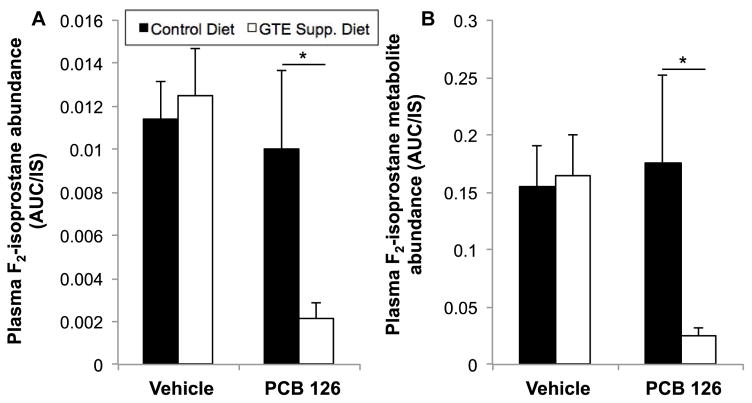
Analysis of F2-isoprostanes (F2-IsoPs), prostaglandin-like eicosanoids formed during fatty acid peroxidation, has emerged as the most reliable method for assessing in vivo oxidative stress32. Plasma samples from mice fed control and GTE-supplemented diets and subsequently treated with vehicle or PCB 126 (n=8–10) were analyzed to determine GTE’s role in modulating environmental toxicant-induced oxidative stress. Plasma F2-IsoP (including PGF2α, 8-iso-PGF2α, iPF2α-III, 8-epiPGF2α, 8-isoprostane, and 15-F2t isoprostanes) and F2-IsoP metabolite (13,14-dihydro-15-ketoPGF2α) concentrations were determined. As seen in Fig. 3, GTE diet supplementation led to drastically decreased F2-IsoP levels (approximately a five-fold reduction, p<0.05) in mice treated with PCB 126, indicating that GTE acts as a strong antioxidant to modulate against environmental toxicant insult. Additionally, GTE drastically decreased PCB-induced F2-IsoP metabolite production (greater than a five-fold reduction, p<0.05); F2-IsoP metabolite analysis is developing as an even more sensitive measure of in vivo oxidative stress because the metabolites do not undergo autoxidation and artificial production as has been seen with parent F2-IsoP33. Interestingly, GTE supplementation led to no significant modulation of F2-IsoP parent or metabolite levels under control situations, indicating that antioxidant modulation occurs primarily when a system is under a secondary stressor.
Fig.3.
PCB 126-induced oxidative stress is modulated by green tea extract (GTE) diet supplementation. Plasma F2-isoprostane (A) and metabolite (B) levels were measured by HPLC/MS MS to assess in vivo oxidative stress induced by PCB 126 that is potentially mitigated by GTE supplementation. Relative levels of combined F-2 IsoPs, including PGF2α, 8-iso-PGF2α, iPF2α-III, 8-epiPGF2α, 8-isoprostane, and 15-F2t isoprostanes, were determined by averaging the AUC integration values from retention times of 8 and 11.3 minutes (Q1 = 353.144, Q2 =193.1). Additionally, the level of 13,14-dihydro-15-ketoPGF2α, an F-2 IsoP metabolite, was determined by integrating its peak at 11.3 minutes (Q1 = 355.2, Q2 = 311.4). Data are presented as mean±S.E.M. (n=8–10). GTE supplementation significantly decreased oxidative stress induced by PCB 126 treatment (*p<0.05).
3.3. Green tea extract increases antioxidant gene expression
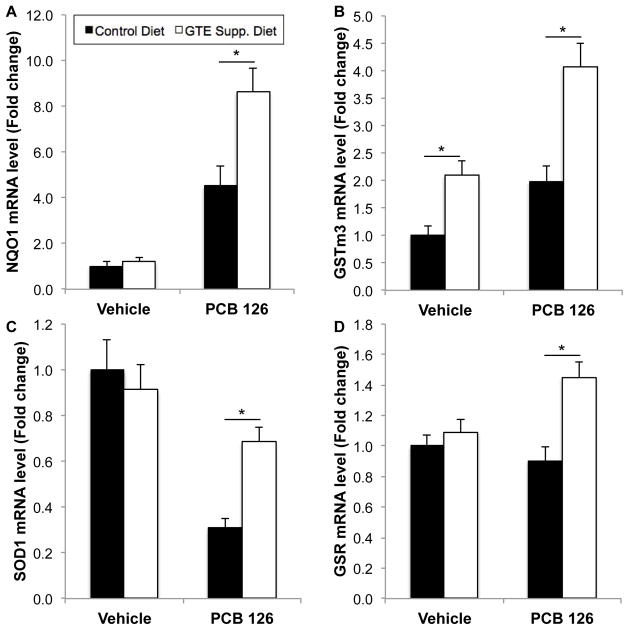
Antioxidant enzyme levels were measured in mouse liver to further develop the role of GTE diet supplementation in modulating environmental insults in vivo. Table 2 highlights antioxidant mRNA markers tested and overall results. qRT-PCR analysis (n=8–10) shows a significant upregulation in catalase, glutathione peroxidase (Gpx3), glutaredoxin (Grx2), glutathione reductase (GSR), glutathione S-transferases (GSTa1, GSTa4, GSTm1, GSTm2, and GSTm3), NAD(P)H dehydrogenase [quinone] 1 (NQO1), superoxide dismutase 1 (SOD1), thioredoxin 2 (Trx2), and thioredoxin reductase 1 (TrxR1) during the concomitant treatment of PCB 126 and GTE diet supplementation. As before, in most cases GTE supplementation did not significantly modulate antioxidant response in the absence of PCBs. NQO1 and GSTm3, enzymes associated with detoxification, exhibited significantly increased mRNA levels above vehicle control diet levels in the presence of PCB 126, while GTE diet supplementation drastically induced antioxidant mRNA expression following PCB insult. mRNA levels of SOD1, critical for modulating harmful superoxide radicals produced during toxicant insult, were significantly decreased following PCB gavage, while GTE supplementation returned mRNA expression to vehicle control diet levels. While PCB administration did not modulate GSR (an important cellular antioxidant) mRNA levels in mice fed vehicle control diets, GTE diet supplementation led to a significantly increased antioxidant response (see Fig. 4, p<0.01). Additional data shown in Supp. Fig. 2 is also consistent with these trends observed in response to GTE supplementation. For example, thioredoxin 2 (Trx2, an important redox protein) mRNA levels are significantly upregulated in the concomitant presence of GTE and PCB 126 although GTE does not induce increased antioxidant activity without the addition of secondary external insult.
Table 2.
The effect of green tea extract (GTE) diet supplementation on PCB 126-induced mRNA Inflammatory, xenobiotic-related and antioxidant markers
| Gene name | 5 μmol PCB 126/kg mouse (fold change)
|
||
|---|---|---|---|
| Control diet | Control + 1% GTE | p-value | |
| Inflammatory and xenobiotic-related markers | |||
| AhR | 0.771±0.096 | 1.506±0.131 | <0.001 |
| CCL3 | 2.051±0.224 | 0.945±0.116 | <0.001 |
| CYP1A1 | 915.208±136.510 | 1169.338±78.900 | N.S. |
| CYP1B1 | 87.504±7.694 | 146.998±12.329 | <0.001 |
| MCP-1 | 1.909±0.478 | 0.745±0.235 | 0.012 |
| Nrf2 | 3.088±0.307 | 3.212±0.234 | N.S. |
| Antioxidant markers | |||
| Catalase | 0.495±0.057 | 0.817±0.071 | 0.003 |
| Gpx2 | 0.339±0.161 | 0.682±0.098 | 0.08 |
| Gpx3 | 0.925±0.167 | 1.411±0.159 | 0.004 |
| Grx2 | 0.213±0.015 | 0.486±0.078 | 0.003 |
| GSR | 0.702±0.074 | 1.245±0.097 | 0.001 |
| GSTa1 | 14.771±2.911 | 22.955±3.975 | 0.034 |
| GSTa4 | 0.896±0.117 | 2.222±0.245 | <0.001 |
| GSTm1 | 2.306±0.450 | 4.024±0.301 | 0.006 |
| GSTm2 | 0.868±0.097 | 1.366±0.114 | 0.004 |
| GSTm3 | 4.469±0.664 | 18.596±1.819 | <0.001 |
| NQO1 | 1.980±0.051 | 6.138±0.031 | 0.006 |
| SOD1 | 0.311±0.041 | 0.684±0.063 | <0.001 |
| Trx2 | 0.731±0.050 | 1.556±0.152 | <0.001 |
| TrxR1 | 1.522±0.143 | 2.673±0.276 | 0.002 |
Fig. 4.
Relative mRNA levels of representative antioxidant enzyme markers, NQO1 (A), GSTm3 (B), SOD1 (C) and GSR (D) in mouse liver samples. Overall GTE supplementation did not significantly increase antioxidant mRNA levels in control diets, but, in the presence of environmental perturbation (i.e. PCB 126 gavage), significantly higher antioxidant levels were seen in mouse liver above non-supplemented diet. All values were determined using the relative quantification method (ΔΔCt) as a fold change from control. Data are presented as mean±S.E.M (*p<0.01, n=8–10). See Table 2 and Supp. Fig. 1. for more information concerning all antioxidant markers tested.
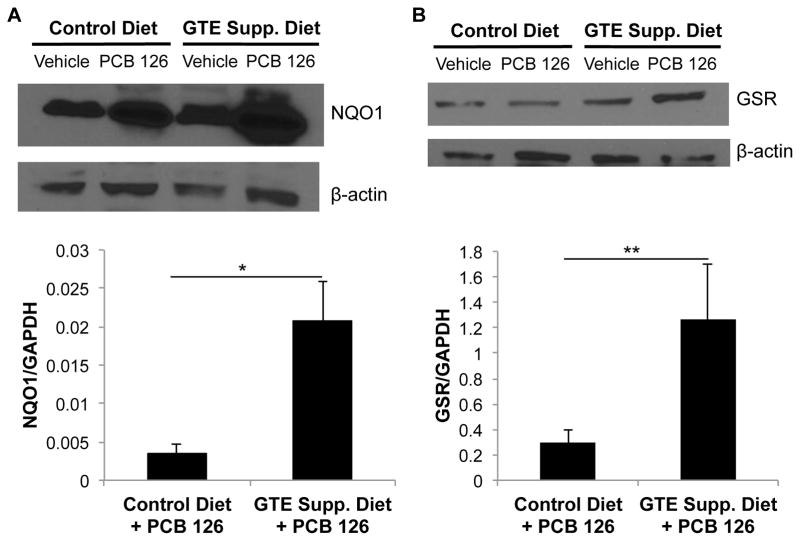
3.4. Green tea extract increases NQO1 and GSR antioxidant protein response against PCB 126
Antioxidant marker protein analysis was performed in order to better understand the role that GTE diet supplementation plays in increasing the body’s defensive mechanisms against toxicant insult. Proteins of interest were normalized to multiple housekeeping genes, β-actin and GAPDH. In PCB 126-treated mouse liver samples, NQO1 protein was significantly upregulated when fed a GTE supplemented diet, as shown in Fig. 5 when quantified against GAPDH (p<0.01). The associated representative blot continues trends seen in mRNA with a large increase in antioxidant protein activity in GTE supplemented mice exposed to PCB insult. GSR protein expression was also statistically increased in response to diet supplementation and continues the trend seen in mRNA analysis in which neither GTE supplementation nor PCB treatment led to modulation of antioxidant response while their concomitant treatment led to significant upregulation (p≤0.05).
Fig. 5.
GTE supplementation leads to increased antioxidant protein expression in the presence of PCB 126. Protein expression of NQO1 (A) and GSR (B) in mouse liver samples was assessed by Western blot analysis. Protein samples were separated through gel electrophoresis and probed with NQO1 and GSR primary antioxidant-related antibodies. Statistically significant increases in antioxidant protein activity were seen in PCB 126-treated mice that were fed a GTE-supplemented diet. In addition to visualized Western blot comparison to β-actin housekeeping gene, samples were compared to GAPDH housekeeping gene for densitometry quantification to further substantiate findings. GTE supplemented mice exposed to PCB showed a significant increase in protein expression, indicating a strengthened antioxidant response due to GTE supplementation (*p<0.01,**p≤0.05, N=8).
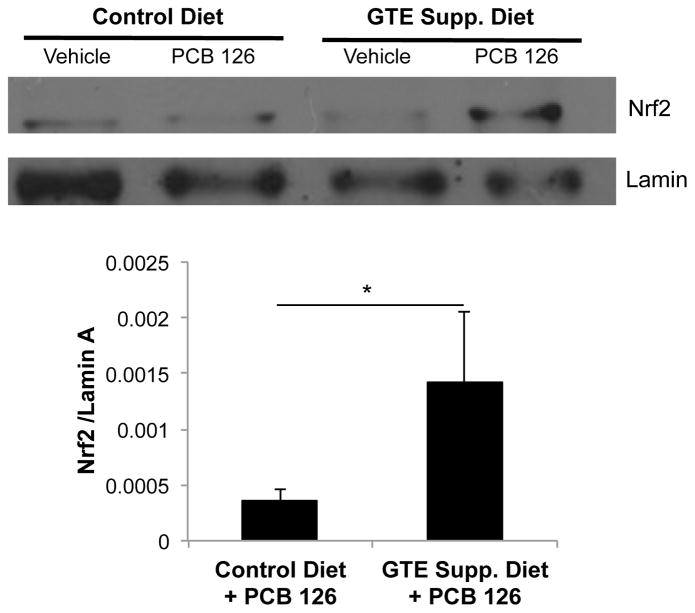
3.5. Green tea extract drives Nrf2 nuclear translocation in the presence of PCB 126
Nuclear translocation assays are commonly used techniques that serve as a representation of cellular transcriptional activation. Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) nuclear translocation was analyzed in comparison to lamin, a nuclear fraction housekeeping gene; liver samples from GTE-supplemented mice exposed to PCB 126 showed a trend toward increased nuclear abundance of Nrf2 (p=0.1, n=4). As in antioxidant mRNA and protein studies, GTE without a concomitant insult from PCB did not modulate Nrf2 activity and translocation. The Nrf2 antioxidant pathway plays a pivotal role in modulating oxidative stress, and therefore its upregulation by GTE diet supplementation, as seen in Fig. 6, is an important indicator of GTE’s ability to increase the body’s responsiveness toward environmental stressors.
Fig. 6.
Nuclear translocation of Nrf2 in mouse liver samples. Mice fed a 1% GTE-supplemented diet and subsequently exposed to PCB 126 displayed increased Nrf2 activation, as evidenced by increased Nrf2 translocation to the nucleus, compared to mice fed 10% fat control diet and exposed to PCB. Lamin was used as a nuclear fraction housekeeping gene for densitometry quantifications. GTE supplemented mice exposed to PCB showed a trend toward increased nuclear abundance of Nrf2 (*p=0.1, n=4).
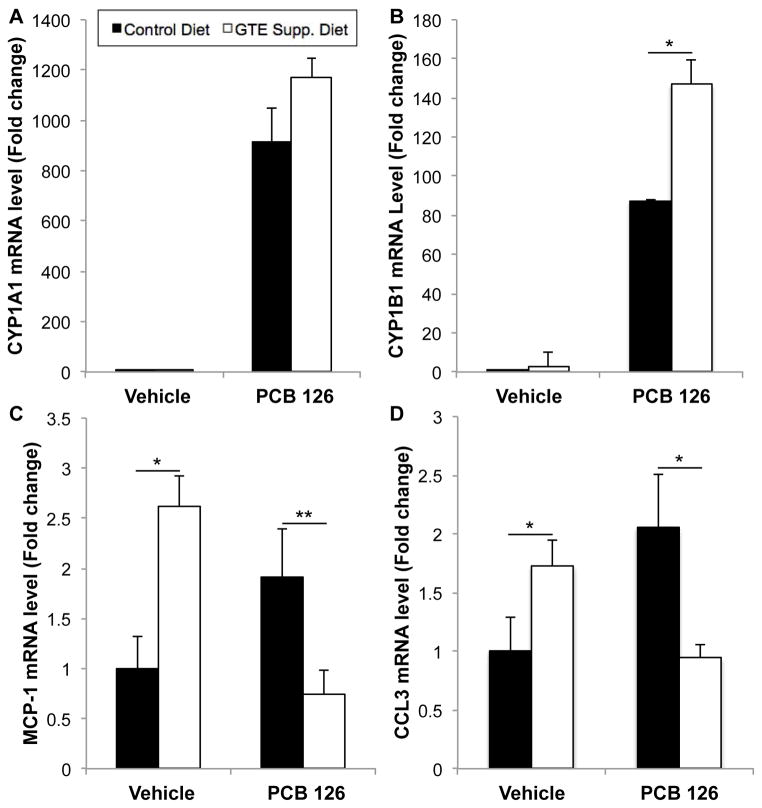
3.6. Green tea extract modulates inflammatory and xenobiotic-related markers in the presence of PCB 126
Cytochrome P450 (e.g., CYP1A1 and CYP1B1), a family of enzymes controlled by the aryl hydrocarbon receptor (AhR) and Nrf2 proteins that are vital for the metabolism of xenobiotic substances, including toxicants, was analyzed from liver samples to determine potential modulation of its activity in response to GTE diet supplementation. Substantial CYP upregulation was seen in the presence of PCB insult, as has been shown previously13, while concurrent GTE supplementation causes a significant upregulation in CYP1B1 mRNA analysis (as seen in Fig. 7). In mice fed GTE-supplemented diets and exposed to PCB, major indicators of inflammation including monocyte chemoattractant protein-1 (MCP-1, also referred to as chemokine (C-C motif) ligand 2, CCL2) and CCL3 (also referred to as macrophage inflammatory protein-1α, MIP-1α) were significantly decreased back to vehicle control levels. Interestingly, GTE supplementation alone led to significant increases in inflammatory marker mRNA levels, although these levels returned to vehicle control levels with concomitant treatment (p<0.05).
Fig. 7.
Relative mRNA levels of inflammatory and xenobiotic-related markers, Cyp1A1 (A), Cyp1B1 (B), MCP-1 (C) and CCL3 (D) in mouse liver samples. GTE supplementation led to increased cytochrome P450 (CYP1A1 and CYP1B1) mRNA expression in the presence of both GTE and environmental toxicant (i.e., PCB 126), indicating increased activity for toxicant degradation and/or excretion. MCP-1 and CCL3 inflammatory markers were statistically increased in GTE-supplemented mice liver samples but toxicant-induced inflammatory markers returned to control levels due to GTE supplementation. All values were determined using the relative quantification method (ΔΔCt) as a fold change from control. Data are presented as mean±S.E.M. (*p<0.01, **p<0.05, n=8–10).
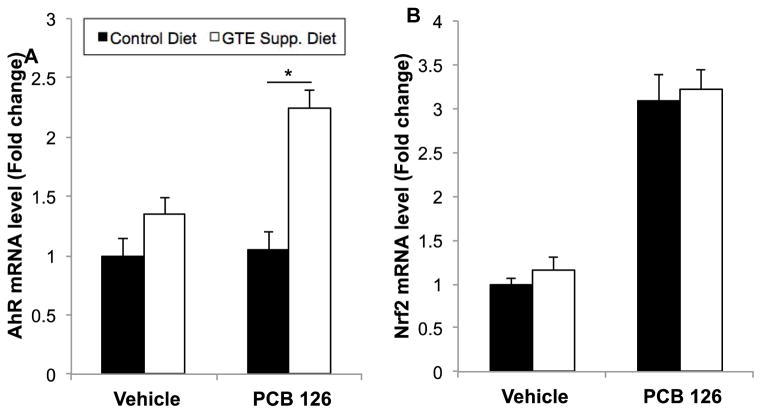
3.7. The AhR is implicated as a control mechanism both in PCB 126 toxicity and antioxidant response
As seen in Fig. 8, GTE diet supplementation led to significant upregulation of AhR mRNA levels in liver in the presence of PCB 126, a dioxin-like AhR ligand. PCB 126 insult did not induce AhR mRNA in vehicle control, vehicle + GTE control, and PCB control settings, but, interestingly, concomitant treatment led to a two-fold upregulation, similarly to that seen in mRNA and protein antioxidant and inflammatory markers, thus allowing for increased in vivo toxicant clearance (p<0.01). Nrf2 mRNA levels were also significantly increased during PCB 126 insult, although GTE supplementation did not cause significant modulation of PCB toxicity. AhR and Nrf2 signaling pathways control both xenobiotic responses and inflammatory cascades, therefore their modulation by GTE diet supplementation implicates further GTE’s role in strengthening antioxidant response toward insult by environmental pollutants.
Fig. 8.
mRNA expression for AhR (A) and Nrf2 (B) genes in mouse liver samples. GTE diet supplementation leads to significant upregulation of AhR mRNA levels in the presence of PCB 126, thus increasing in vivo toxicant clearance. Nrf2 mRNA levels are significantly increased during PCB 126 insult, although GTE supplementation does not cause statistically significant modulation of PCB toxicity. All values were determined using the relative quantification method (ΔΔCt) as a fold change from control. Data are presented as mean±S.E.M (n=8–10). GTE supplementation significantly increased AhR in the presence of PCB 126 treatment (*p<0.01).
4. Discussion
Healthy nutrition can positively influence, or lessen, the human health risks associated with exposure to mixtures of environmental chemicals34. The liver is one tissue particularly vulnerable to environmental pollutants, and especially PCB-induced toxicity 7. One type of liver disease that affects more than 20% of Americans is nonalcoholic fatty liver disease, which can lead to nonalcoholic steatohepatitis35. Industrial toxicants also have been linked to secondary insults that can lead to steatohepatitis35. Our laboratory and others have shown that PCB exposure increases liver/body weight ratio (hepatosomatic index, HSI; see Supp. Fig. 1), which is generally accepted as indicative of an adverse hepatic response to chemical exposure36. Mechanisms defining the involvement of environmental pollutants in the pathology of liver diseases may include a compromised redox status or toxicant-induced increase in oxidative stress.
Lifestyle modifications that include healthful nutrition have been suggested as a powerful means of reducing the vulnerability to environmental insults34 and in reducing the risk to environmental toxicant-induced liver disease35. For example, green tea extract has been shown to protect against hepatic steatosis in obese mice by reducing oxidative stress and enhancing hepatic antioxidant defenses37; 38. In the present study we observed significant protection against PCB 126-induced oxidative stress by dietary supplementation with green tea extract. In fact, mice supplemented with green tea extract and subsequently exposed to PCB displayed a decrease in overall F2-isoprostane and metabolite levels compared to animals on a control diet exposed to PCB. We did not see a large increase in oxidative stress as evidenced by an increase in F2-isoprostane levels in mice fed a control diet and subsequently gavaged with PCB. This may have been due to the fact that the amount of administered PCB 126 reflects more relevant environmental PCB exposure concentrations in humans than previous studies using high concentrations of PCB congeners (e.g., 150 μmol/kg in previous studies versus 5 μmol/kg used herein)39; 40. We hypothesize that levels of PCBs encountered by humans today initiate low levels of chronic oxidative stress and inflammation that, together with multiple other factors including poor diet and exposures to other environmental stressors, leads to augmented or exacerbated human disease. The protective properties of green tea have been studied extensively, and recent human studies suggest that consumption of green tea may protect against cardiovascular disease and some forms of cancer, have anti-hypertensive and anti-obesity effects, and contribute to antibacterial and antiviral activity41.
Certain environmental pollutants, and in particular ligands for the AhR, induce oxidative stress, in part via induction and uncoupling of cytochrome P450 enzymes42. Hepatic changes in lipid composition, altered membrane structure and membrane functions are well-described phenomena of PCB-induced liver damage43–46. The protective properties of green tea extracts against PCB-induced pathologies, and in particular liver damage, may be numerous. For example, green tea may normalize the formation of lipid peroxide products induced by exposure to toxicants to prevent hepatic fibrosis30, or green tea may favorably regulate intestinal tight junction proteins or overall intestinal barrier function47. It has been proposed that green tea can inhibit the intestinal absorption of lipids and highly lipophilic organic compounds48; 49. Induction of antioxidant enzymes by green tea also may contribute to its tissue protective properties50. Our current study support this concept, and we observed an increased induction of antioxidant genes such as SOD1, GSR, NQO1 and GST in mice that were fed a green tea-supplemented diet and subsequently challenged with PCB, compared to animals exposed to the control diet plus PCB. This may explain in part the observed decrease in overall oxidative stress due to green tea supplementation. Of particular interest is our observed induction of NQO1, a Nrf2 target gene, which suggests that green tea protects in part by modulation of the Nrf2/ARE pathway. In fact, it has been shown that food polyphenols, including the green tea polyphenol EGCG, the primary component of GTE tested herein, can modulate Nrf2-mediated antioxidant and detoxifying enzyme induction51; 52. Our own work with vascular endothelial cells further suggests that multiple pathways including lipid raft caveolae and the antioxidant defense controller Nrf2 play a role in nutritional modulation of PCB-induced vascular toxicity17 and that cross-talk between caveolae-related proteins and cellular Nrf2 may be required for optimal cytoprotection by green tea catechins and other diet-derived polyphenols.
Many groups, including ours, have shown that green tea catechins such as EGCG can upregulate basal levels of antioxidant enzymes in vitro41; 53; 54. Interestingly, our overall results show that green tea extract supplemented in the diet acts as an antioxidant only in the presence of a secondary stressor, in this case, the pro-inflammatory coplanar PCB 126. The inconsistencies between in vitro and in vivo studies may be explained by the relatively high doses of tea catechins usually employed in cell culture or the fact that most tea catechins are quickly biotransformed in vivo to metabolites that exhibit differential physiological effects50. There are many other examples of instances where supplementation with GTE or specific catechins is protective in in vivo models of inflammation and oxidative stress. For many of our investigated antioxidant enzymes we saw decreased expression in the presence of PCB when fed a control diet, but levels were upregulated, many returning to control vehicle levels in PCB groups fed a GTE-rich diet (e.g., SOD1 in Fig. 4). These observations are in line with other groups who investigated GTE effects on other stressors including ethanol toxicity and bacterial infection55; 56.
Our past work in cell culture points to the antioxidant controller Nrf2 as a major player in nutritional modulation of PCB toxicity. Many nutrients other than green tea catechins, including resveratrol, found in the skins of grapes, and sulforaphane, which is found in broccoli, have been shown to activate Nrf257–59. Nrf2 can become transcriptionally active through multiple mechanisms including direct phosphorylation by PKC delta and loss of contact between Nrf2 and inhibitory kelch-like ECH-associated protein 1 (Keap1)60. Upon activation, Nrf2 is able to evade ubiquitination, enter the nucleus, and bind cis-acting antioxidant response elements in target genes such as NQO123. Nrf2 activation leads to decreased overall oxidative stress and inflammation, which is a hallmark of PCB toxicity61. In this work we observed a relatively significant trend toward increased NRF2 translocation to the nucleus in animals supplemented with GTE and subsequently exposed to PCB (Fig. 6). More interestingly and novel, we also saw a drastic increase in AhR mRNA expression in this same treatment group (Fig. 8). This upregulation was mirrored in increases in both CYP1A1 and CYP1B1 mRNA levels in mice fed a GTE rich diet and subsequently exposed to PCB. An increase in AhR may help to detoxify the acute exposure to PCB by increasing metabolism-assisted excretion. Although a consistent, steady upregulation of AhR may create a negatively imbalanced redox situation, the GTE’s ability to upregulate AhR only in the presence of a toxicant may in some cases be a protective and positive mechanism. Other groups have shown that different catechins within GTE display either antagonistic or agonist activities against CYP1A162, but to our knowledge no group has reported the mRNA upregulation as seen in Fig. 7. In our analysis of PCB concentrations in plasma we observed a very slight trend towards decreased levels of parent PCB 126 in the plasma of mice supplemented with GTE (Fig. 2). Although plasma levels may be a good overall picture of body-burden of PCBs, in the future, collecting urine and feces may paint a clearer picture of GTE’s involvement with detoxification and excretion. Also, we may have been able to see a more significant decrease in PCB levels and or a modulation of PCB hydroxy metabolite in mice supplemented with GTE if we sacrificed the mice more than 24 hours after the final PCB dose49.
PCBs can induce vascular inflammation by upregulating pro-inflammatory mediators such as MCP-1 and CCL3. We hypothesized that GTE would downregulate basal levels of these inflammatory markers in vehicle treated mice as well as decrease PCB-mediated upregulation in PCB 126 treated mice. Interestingly however, for both of our inflammatory markers we saw a significant increase in mRNA levels in vehicle treated mice supplemented with GTE (Fig. 7). This observation would suggest that the dose of GTE used in this study may not be optimal, and perhaps toxic to some degree, in basal levels of oxidative stress and inflammation. Other groups have shown GTE toxicities at certain doses in vivo63; 64, and interestingly, data illustrating protection seems to be more conclusive in animal models of oxidative stress and inflammation. Importantly, for our study, both MCP-1 and CCL3 mRNA levels return to vehicle treated control diet levels in mice fed GTE and subsequently exposed to PCB. This may point to GTE as exhibiting possible hormetic activity by inducing a slight response by the organism that ultimately primes the protective antioxidant system for a future stressor, i.e., Superfund pollutant exposure. Understanding hormesis and the role that nutrients can play is an extremely interesting scientific discipline and demands much more future investigation65.
In summary, our current study supports our in vitro data that green tea catechins can protect against PCB 126-induced cytotoxicity by reducing oxidative stress14. Our current in vivo data contributes to the overall hypothesis that nutrition can modulate environmental insults. More studies are needed to further understand detailed mechanisms of protective benefits to consume diets high in protective and healthful nutrients such as plant-derived polyphenols and other bioactive compounds.
Supplementary Material
Acknowledgments
Funding: This work was supported by the National Institute of Environmental Health Sciences at the National Institutes of Health [P42ES007380] and the University of Kentucky Agricultural Experiment Station.
We would like to thank Taiyo International Inc. for kindly providing the green tea extract incorporated into the mouse diets used for these studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weber R, Gaus C, Tysklind M, Johnston P, Forter M, Hollert H, Heinisch E, Holoubek I, Lloyd-Smith M, Masunaga S, Moccarelli P, Santillo D, Seike N, Symons R, Torres JP, Verta M, Varbelow G, Vijgen J, Watson A, Costner P, Woelz J, Wycisk P, Zennegg M. Dioxin- and POP-contaminated sites--contemporary and future relevance and challenges: overview on background, aims and scope of the series. Environmental science and pollution research international. 2008;15:363–93. doi: 10.1007/s11356-008-0024-1. [DOI] [PubMed] [Google Scholar]
- 2.ATSDR; HEALTH DO, SERVICES AH, editor. Polychlorinated Biphenyls (PCB) ToxicityExposure Pathways. 2000 http://www.atsdr.cdc.gov/csem/csem.asp?csem=22&po=4.
- 3.Hopf NB, Ruder AM, Succop P. Background levels of polychlorinated biphenyls in the U.S. population. The Science of the total environment. 2009;407:6109–19. doi: 10.1016/j.scitotenv.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 4.Silverstone AE, Rosenbaum PF, Weinstock RS, Bartell SM, Foushee HR, Shelton C, Pavuk M. Polychlorinated biphenyl (PCB) exposure and diabetes: results from the Anniston Community Health Survey. Environmental health perspectives. 2012;120:727–32. doi: 10.1289/ehp.1104247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lind PM, van Bavel B, Salihovic S, Lind L. Circulating levels of persistent organic pollutants (POPs) and carotid atherosclerosis in the elderly. Environmental health perspectives. 2012;120:38–43. doi: 10.1289/ehp.1103563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valvi D, Mendez MA, Martinez D, Grimalt JO, Torrent M, Sunyer J, Vrijheid M. Prenatal concentrations of polychlorinated biphenyls, DDE, and DDT and overweight in children: a prospective birth cohort study. Environmental health perspectives. 2012;120:451–7. doi: 10.1289/ehp.1103862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cave M, Appana S, Patel M, Falkner KC, McClain CJ, Brock G. Polychlorinated biphenyls, lead, and mercury are associated with liver disease in American adults: NHANES 2003–2004. Environmental health perspectives. 2010;118:1735–42. doi: 10.1289/ehp.1002720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hennig B, Toborek M, Bachas LG, Suk WA. Emerging issues: nutritional awareness in environmental toxicology. The Journal of nutritional biochemistry. 2004;15:194–5. doi: 10.1016/j.jnutbio.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Wahlang B, Falkner KC, Gregory B, Ansert D, Young D, Conklin DJ, Bhatnagar A, McClain CJ, Cave M. Polychlorinated biphenyl 153 is a diet-dependent obesogen that worsens nonalcoholic fatty liver disease in male C57BL6/J mice. The Journal of nutritional biochemistry. 2013 doi: 10.1016/j.jnutbio.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hennig B, Reiterer G, Toborek M, Matveev SV, Daugherty A, Smart E, Robertson LW. Dietary fat interacts with PCBs to induce changes in lipid metabolism in mice deficient in low-density lipoprotein receptor. Environmental health perspectives. 2005;113:83–7. doi: 10.1289/ehp.7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, Reiterer G, Toborek M, Hennig B. Changing ratios of omega-6 to omega-3 fatty acids can differentially modulate polychlorinated biphenyl toxicity in endothelial cells. Chemico-biological interactions. 2008;172:27–38. doi: 10.1016/j.cbi.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Majkova Z, Layne J, Sunkara M, Morris AJ, Toborek M, Hennig B. Omega-3 fatty acid oxidation products prevent vascular endothelial cell activation by coplanar polychlorinated biphenyls. Toxicology and applied pharmacology. 2011;251:41–9. doi: 10.1016/j.taap.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi YJ, Arzuaga X, Kluemper CT, Caraballo A, Toborek M, Hennig B. Quercetin blocks caveolae-dependent pro-inflammatory responses induced by co-planar PCBs. Environment international. 2010;36:931–4. doi: 10.1016/j.envint.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han SG, Han SS, Toborek M, Hennig B. EGCG protects endothelial cells against PCB 126-induced inflammation through inhibition of AhR and induction of Nrf2-regulated genes. Toxicology and applied pharmacology. 2012;261:181–8. doi: 10.1016/j.taap.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De S, Ghosh S, Chatterjee R, Chen YQ, Moses L, Kesari A, Hoffman EP, Dutta SK. PCB congener specific oxidative stress response by microarray analysis using human liver cell line. Environment international. 2010;36:907–17. doi: 10.1016/j.envint.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai I, Chai Y, Simmons D, Luthe G, Coleman MC, Spitz D, Haschek WM, Ludewig G, Robertson LW. Acute toxicity of 3,3′,4,4′,5-pentachlorobiphenyl (PCB 126) in male Sprague-Dawley rats: effects on hepatic oxidative stress, glutathione and metals status. Environment international. 2010;36:918–23. doi: 10.1016/j.envint.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petriello MC, Newsome B, Hennig B. Influence of nutrition in PCB-induced vascular inflammation. Environmental science and pollution research international. 2013 doi: 10.1007/s11356-013-1549-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Myers CR. The effects of chromium(VI) on the thioredoxin system: implications for redox regulation. Free radical biology & medicine. 2012;52:2091–107. doi: 10.1016/j.freeradbiomed.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mann GE, Niehueser-Saran J, Watson A, Gao L, Ishii T, de Winter P, Siow RC. Nrf2/ARE regulated antioxidant gene expression in endothelial and smooth muscle cells in oxidative stress: implications for atherosclerosis and preeclampsia. Sheng li xue bao: [Acta physiologica Sinica] 2007;59:117–27. [PubMed] [Google Scholar]
- 20.Nordberg J, Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free radical biology & medicine. 2001;31:1287–312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 21.Savouret JF, Berdeaux A, Casper RF. The aryl hydrocarbon receptor and its xenobiotic ligands: a fundamental trigger for cardiovascular diseases. Nutrition, metabolism, and cardiovascular diseases: NMCD. 2003;13:104–13. doi: 10.1016/s0939-4753(03)80026-1. [DOI] [PubMed] [Google Scholar]
- 22.Kopf PG, Walker MK. 2,3,7,8-tetrachlorodibenzo-p-dioxin increases reactive oxygen species production in human endothelial cells via induction of cytochrome P4501A1. Toxicology and applied pharmacology. 2010;245:91–9. doi: 10.1016/j.taap.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baird L, Dinkova-Kostova AT. The cytoprotective role of the Keap1-Nrf2 pathway. Archives of toxicology. 2011;85:241–72. doi: 10.1007/s00204-011-0674-5. [DOI] [PubMed] [Google Scholar]
- 24.Singh S, Vrishni S, Singh BK, Rahman I, Kakkar P. Nrf2-ARE stress response mechanism: a control point in oxidative stress-mediated dysfunctions and chronic inflammatory diseases. Free radical research. 2010;44:1267–88. doi: 10.3109/10715762.2010.507670. [DOI] [PubMed] [Google Scholar]
- 25.Lee JM, Chan K, Kan YW, Johnson JA. Targeted disruption of Nrf2 causes regenerative immune-mediated hemolytic anemia. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:9751–6. doi: 10.1073/pnas.0403620101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114:1248–59. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayes JD, Dinkova-Kostova AT, McMahon M. Cross-talk between transcription factors AhR and Nrf2: lessons for cancer chemoprevention from dioxin. Toxicological sciences: an official journal of the Society of Toxicology. 2009;111:199–201. doi: 10.1093/toxsci/kfp168. [DOI] [PubMed] [Google Scholar]
- 28.Yeager RL, Reisman SA, Aleksunes LM, Klaassen CD. Introducing the “TCDD-inducible AhR-Nrf2 gene battery”. Toxicological sciences: an official journal of the Society of Toxicology. 2009;111:238–46. doi: 10.1093/toxsci/kfp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramadass P, Meerarani P, Toborek M, Robertson LW, Hennig B. Dietary flavonoids modulate PCB-induced oxidative stress, CYP1A1 induction, and AhR-DNA binding activity in vascular endothelial cells. Toxicological sciences: an official journal of the Society of Toxicology. 2003;76:212–9. doi: 10.1093/toxsci/kfg227. [DOI] [PubMed] [Google Scholar]
- 30.Zhen MC, Wang Q, Huang XH, Cao LQ, Chen XL, Sun K, Liu YJ, Li W, Zhang LJ. Green tea polyphenol epigallocatechin-3-gallate inhibits oxidative damage and preventive effects on carbon tetrachloride-induced hepatic fibrosis. The Journal of nutritional biochemistry. 2007;18:795–805. doi: 10.1016/j.jnutbio.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 31.Bruno RS, Dugan CE, Smyth JA, DiNatale DA, Koo SI. Green tea extract protects leptin-deficient, spontaneously obese mice from hepatic steatosis and injury(1,2) Journal of Nutrition. 2008;138:323–31. doi: 10.1093/jn/138.2.323. [DOI] [PubMed] [Google Scholar]
- 32.Montuschi P, Barnes P, Roberts LJ. Insights into oxidative stress: The isoprostanes. Current Medicinal Chemistry. 2007;14:703–17. doi: 10.2174/092986707780059607. [DOI] [PubMed] [Google Scholar]
- 33.Dorjgochoo T, Gao YT, Chow WH, Shu XO, Yang G, Cai Q, Rothman N, Cai H, Li H, Deng X, Franke A, Roberts LJ, Milne G, Zheng W, Dai Q. Major metabolite of F2-isoprostane in urine may be a more sensitive biomarker of oxidative stress than isoprostane itself. Am J Clin Nutr. 2012;96:405–14. doi: 10.3945/ajcn.112.034918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hennig B, Ettinger AS, Jandacek RJ, Koo S, McClain C, Seifried H, Silverstone A, Watkins B, Suk WA. Using nutrition for intervention and prevention against environmental chemical toxicity and associated diseases. Environmental health perspectives. 2007;115:493–5. doi: 10.1289/ehp.9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cave M, Deaciuc I, Mendez C, Song Z, Joshi-Barve S, Barve S, McClain C. Nonalcoholic fatty liver disease: predisposing factors and the role of nutrition. The Journal of nutritional biochemistry. 2007;18:184–95. doi: 10.1016/j.jnutbio.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Hall AP, Elcombe CR, Foster JR, Harada T, Kaufmann W, Knippel A, Kuttler K, Malarkey DE, Maronpot RR, Nishikawa A, Nolte T, Schulte A, Strauss V, York MJ. Liver hypertrophy: a review of adaptive (adverse and non-adverse) changes--conclusions from the 3rd International ESTP Expert Workshop. Toxicologic pathology. 2012;40:971–94. doi: 10.1177/0192623312448935. [DOI] [PubMed] [Google Scholar]
- 37.Chung MY, Park HJ, Manautou JE, Koo SI, Bruno RS. Green tea extract protects against nonalcoholic steatohepatitis in ob/ob mice by decreasing oxidative and nitrative stress responses induced by proinflammatory enzymes. The Journal of nutritional biochemistry. 2012;23:361–7. doi: 10.1016/j.jnutbio.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park HJ, DiNatale DA, Chung MY, Park YK, Lee JY, Koo SI, O’Connor M, Manautou JE, Bruno RS. Green tea extract attenuates hepatic steatosis by decreasing adipose lipogenesis and enhancing hepatic antioxidant defenses in ob/ob mice. The Journal of nutritional biochemistry. 2011;22:393–400. doi: 10.1016/j.jnutbio.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Sipos E, Chen L, Andras IE, Wrobel J, Zhang B, Pu H, Park M, Eum SY, Toborek M. Proinflammatory adhesion molecules facilitate polychlorinated biphenyl-mediated enhancement of brain metastasis formation. Toxicological sciences: an official journal of the Society of Toxicology. 2012;126:362–71. doi: 10.1093/toxsci/kfr349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seelbach M, Chen L, Powell A, Choi YJ, Zhang B, Hennig B, Toborek M. Polychlorinated biphenyls disrupt blood-brain barrier integrity and promote brain metastasis formation. Environmental health perspectives. 2010;118:479–84. doi: 10.1289/ehp.0901334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cabrera C, Artacho R, Gimenez R. Beneficial effects of green tea--a review. Journal of the American College of Nutrition. 2006;25:79–99. doi: 10.1080/07315724.2006.10719518. [DOI] [PubMed] [Google Scholar]
- 42.Schlezinger JJ, Struntz WD, Goldstone JV, Stegeman JJ. Uncoupling of cytochrome P450 1A and stimulation of reactive oxygen species production by co-planar polychlorinated biphenyl congeners. Aquatic toxicology. 2006;77:422–32. doi: 10.1016/j.aquatox.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 43.Schlezinger JJ, Stegeman JJ. Induction and suppression of cytochrome P450 1A by 3,3′,4,4′,5-pentachlorobiphenyl and its relationship to oxidative stress in the marine fish scup (Stenotomus chrysops) Aquatic toxicology. 2001;52:101–15. doi: 10.1016/s0166-445x(00)00141-7. [DOI] [PubMed] [Google Scholar]
- 44.Twaroski TP, O’Brien ML, Robertson LW. Effects of selected polychlorinated biphenyl (PCB) congeners on hepatic glutathione, glutathione-related enzymes, and selenium status: implications for oxidative stress. Biochemical pharmacology. 2001;62:273–81. doi: 10.1016/s0006-2952(01)00668-2. [DOI] [PubMed] [Google Scholar]
- 45.Katynski AL, Vijayan MM, Kennedy SW, Moon TW. 3,3′,4,4′,5-Pentachlorobiphenyl (PCB 126) impacts hepatic lipid peroxidation, membrane fluidity and beta-adrenoceptor kinetics in chick embryos. Comparative biochemistry and physiology Toxicology & pharmacology: CBP. 2004;137:81–93. doi: 10.1016/j.cca.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 46.Hassoun EA, Li F, Abushaban A, Stohs SJ. The relative abilities of TCDD and its congeners to induce oxidative stress in the hepatic and brain tissues of rats after subchronic exposure. Toxicology. 2000;145:103–13. doi: 10.1016/s0300-483x(99)00221-8. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki T, Hara H. Role of flavonoids in intestinal tight junction regulation. The Journal of nutritional biochemistry. 2011;22:401–8. doi: 10.1016/j.jnutbio.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 48.Koo SI, Noh SK. Green tea as inhibitor of the intestinal absorption of lipids: potential mechanism for its lipid-lowering effect. The Journal of nutritional biochemistry. 2007;18:179–83. doi: 10.1016/j.jnutbio.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim J, Koo SI, Noh SK. Green tea extract markedly lowers the lymphatic absorption and increases the biliary secretion of 14C-benzo[a]pyrene in rats. The Journal of nutritional biochemistry. 2012;23:1007–11. doi: 10.1016/j.jnutbio.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 50.Frei B, Higdon JV. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. J Nutr. 2003;133:3275S–84S. doi: 10.1093/jn/133.10.3275S. [DOI] [PubMed] [Google Scholar]
- 51.Na HK, Surh YJ. Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food Chem Toxicol. 2008;46:1271–8. doi: 10.1016/j.fct.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 52.Scapagnini G, Vasto S, Abraham NG, Caruso C, Zella D, Fabio G. Modulation of Nrf2/ARE pathway by food polyphenols: a nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Molecular neurobiology. 2011;44:192–201. doi: 10.1007/s12035-011-8181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lorenz M, Urban J, Engelhardt U, Baumann G, Stangl K, Stangl V. Green and black tea are equally potent stimuli of NO production and vasodilation: new insights into tea ingredients involved. Basic research in cardiology. 2009;104:100–10. doi: 10.1007/s00395-008-0759-3. [DOI] [PubMed] [Google Scholar]
- 54.Zheng Y, Morris A, Sunkara M, Layne J, Toborek M, Hennig B. Epigallocatechin-gallate stimulates NF-E2-related factor and heme oxygenase-1 via caveolin-1 displacement. The Journal of nutritional biochemistry. 2012 doi: 10.1016/j.jnutbio.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Skrzydlewska E, Ostrowska J, Farbiszewski R, Michalak K. Protective effect of green tea against lipid peroxidation in the rat liver, blood serum and the brain. Phytomedicine: international journal of phytotherapy and phytopharmacology. 2002;9:232–8. doi: 10.1078/0944-7113-00119. [DOI] [PubMed] [Google Scholar]
- 56.Guleria RS, Jain A, Tiwari V, Misra MK. Protective effect of green tea extract against the erythrocytic oxidative stress injury during mycobacterium tuberculosis infection in mice. Molecular and cellular biochemistry. 2002;236:173–81. doi: 10.1023/a:1016119718321. [DOI] [PubMed] [Google Scholar]
- 57.Miao X, Bai Y, Su W, Cui W, Xin Y, Wang Y, Tan Y, Miao L, Fu Y, Su G, Cai L. Sulforaphane prevention of diabetes-induced aortic damage was associated with the up-regulation of Nrf2 and its down-stream antioxidants. Nutrition & metabolism. 2012;9:84. doi: 10.1186/1743-7075-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kang HJ, Hong YB, Kim HJ, Wang A, Bae I. Bioactive food components prevent carcinogenic stress via Nrf2 activation in BRCA1 deficient breast epithelial cells. Toxicology letters. 2012;209:154–60. doi: 10.1016/j.toxlet.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nair S, Barve A, Khor TO, Shen GX, Lin W, Chan JY, Cai L, Kong AN. Regulation of Nrf2- and AP-1-mediated gene expression by epigallocatechin-3-gallate and sulforaphane in prostate of Nrf2-knockout or C57BL/6J mice and PC-3 AP-1 human prostate cancer cells. Acta pharmacologica Sinica. 2010;31:1223–40. doi: 10.1038/aps.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Niture SK, Jain AK, Jaiswal AK. Antioxidant-induced modification of INrf2 cysteine 151 and PKC-delta-mediated phosphorylation of Nrf2 serine 40 are both required for stabilization and nuclear translocation of Nrf2 and increased drug resistance. Journal of cell science. 2009;122:4452–64. doi: 10.1242/jcs.058537. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Kim J, Cha YN, Surh YJ. A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutation research. 2010;690:12–23. doi: 10.1016/j.mrfmmm.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 62.Williams SN, Shih H, Guenette DK, Brackney W, Denison MS, Pickwell GV, Quattrochi LC. Comparative studies on the effects of green tea extracts and individual tea catechins on human CYP1A gene expression. Chemico-biological interactions. 2000;128:211–29. doi: 10.1016/s0009-2797(00)00204-0. [DOI] [PubMed] [Google Scholar]
- 63.Pae M, Ren Z, Meydani M, Shang F, Smith D, Meydani SN, Wu D. Dietary supplementation with high dose of epigallocatechin-3-gallate promotes inflammatory response in mice. The Journal of nutritional biochemistry. 2012;23:526–31. doi: 10.1016/j.jnutbio.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 64.Chan PC, Ramot Y, Malarkey DE, Blackshear P, Kissling GE, Travlos G, Nyska A. Fourteen-week toxicity study of green tea extract in rats and mice. Toxicologic pathology. 2010;38:1070–84. doi: 10.1177/0192623310382437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Birringer M. Hormetics: dietary triggers of an adaptive stress response. Pharmaceutical research. 2011;28:2680–94. doi: 10.1007/s11095-011-0551-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.