Abstract
Alcohol exposure affects neuronal plasticity in the adult and developing brain. Astrocytes play a major role in modulating neuronal plasticity and are a target of ethanol. Tissue plasminogen activator (tPA) plays a major role in modulating neuronal plasticity by degrading the extracellular matrix proteins including fibronectin and laminin and is upregulated by ethanol in vivo. In this study we explored the hypothesis that ethanol affects DNA methylation in astrocytes thereby increasing expression and release of tPA. It was found that ethanol increased tPA mRNA levels, an effect mimicked by an inhibitor of DNA methyltransferase (DNMT) activity. Ethanol also increased tPA protein expression and release, inhibited DNMT activity with a corresponding decrease in DNA methylation levels of the tPA promoter. Furthermore, it was observed that protein levels of DNMT3A, but not DNMT1, were reduced in astrocytes after ethanol exposure. These novel studies show that ethanol inhibits DNA methylation in astrocytes leading to increased tPA expression and release; this effect may be involved in astrocyte-mediated inhibition of neuronal plasticity by alcohol.
Keywords: Astrocytes, DNA methyltransferase (DNMT), DNA methylation, ethanol, tissue plasminogen activator (tPA)
Introduction
Alcohol affects epigenetic mechanisms, including histone acetylation, DNA methylation, and non-coding micro RNAs, in the developing and adult brain and in embryo and neural stem cell cultures (Liu et al. 2009, Moonat et al. 2013, Sathyan et al. 2007, Zhou et al. 2011, Govorko et al. 2012) indicating that the epigenetic dysregulation of gene expression plays a role in the pathophysiology of alcoholism and fetal alcohol spectrum disorders (FASD). Both these conditions, caused by ethanol abuse, result in structural brain abnormalities associated with behavioral anomalies and psychiatric disorders (Boden and Fergusson 2011, Harper and Matsumoto 2005, He et al. 2005, Lebel et al. 2012, Riley et al. 2011, Streissguth and O'Malley 2000).
Methylation of cytosine residues at the position C5 of CpG sites in the DNA is associated with condensed chromatin and subsequent inhibition of gene transcription whereas low DNA methylation is associated with open chromatin and increased transcription. DNA methylation is catalyzed by DNA methyltransferases (DNMTs), which include DNMT1, 3A, and 3B (MacDonald and Roskams 2009).
Ethanol delays the DNA methylation program, decreases promoter methylation, and increases expression of genes involved in development in mouse embryos and neural stem cells in vitro (Liu et al. 2009, Zhou et al. 2011).
Astrocytes play a major role in regulating neuronal plasticity both in the developing and adult brain as they release factors that promote or inhibit neuronal development (Asher et al. 2000, Hamel et al. 2005, Tom et al. 2004). Our group and others have reported that neurite outgrowth is modulated by the release of the neuritogenic extracellular matrix (ECM) proteins laminin and fibronectin from astrocytes (Guizzetti et al. 2008, Martinez and Gomes 2002, Tom et al. 2004). We have also reported that ethanol reduces the extracellular levels of laminin and fibronectin in astrocytes and inhibits neuritogenesis (Guizzetti et al. 2010). Interestingly, alcohol abuse and prenatal alcohol exposure are associated with reduced neuronal plasticity (Harper and Matsumoto 2005, He et al. 2005, Lebel et al. 2012).
The serine protease tissue plasminogen activator (tPA) promotes the formation of the proteolytic enzyme plasmin from its zymogen plasminogen; plasmin is an extracellular protease, which degrades ECM components including fibronectin and laminin (Dellas and Loskutoff 2005, Irigoyen et al. 1999). Tissue-PA is upregulated by alcohol in the brain of animal models of both alcoholism and FASD, where it reduces the levels of laminin and causes neurodegeneration (Noel et al. 2011, Skrzypiec et al. 2009). Tissue-PA is highly expressed by astrocytes and is upregulated by astrocyte activation (Ganesh and Chintala 2011).
In the present study we investigated the hypothesis that ethanol inhibits DNA methylation in astrocytes and increases the expression and the release of tPA.
Materials and Methods
Materials
Tissue culture medium, fetal bovine serum (FBS), MethylMiner™ methylated DNA kit, High-Capacity cDNA Reverse Transcription Kits were from Life Technology (Carlsbad, CA). EpiQuik™ Nuclear Extraction Kit I and EpiQuik™ DNA Methyltransferase Activity/Inhibition Assay Ultra Kit were from Epigentek (Brooklyn, NY). Time-pregnant Sprague-Dawley rats were purchased from Charles River (Wilmington, MA). Rat tPA Total Antigen Assay ELISA kit was from Innovative Research (Novi, MI). DNeasy Blood & Tissue Kit, QIAquick PCR Purification Kit, and RNeasy Plus Mini Kit were purchased from Qiagen (Valencia, CA). The protease inhibitor cocktail was from Roche (Indianapolis, IN). The BCA Protein Assay Reagent was from Thermo Scientific (Rockford, IL). DNMT1, DNMT3A, DNMT3B antibody were from Santa Cruz (Santa Cruz, CA). All other chemicals were from Sigma Chemical Co. (St. Louis, MO).
Animals
Time-pregnant Sprague-Dawley rats were housed in a temperature-controlled room with a 12/12 hr light/dark cycle with food and water provided ad libitum. All rat procedures were performed in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee.
Cell culture
Primary cortical astrocytes were prepared from E21 Sprague-Dawley fetuses, as previously described (Guizzetti et al. 1996) and were shown to be >95% GFAP positive. Astrocytes were grown in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% FBS, 100units/ml penicillin, and 100µg/ml streptomycin (FBS/DMEM medium). The treatments were carried out in serum-free DMEM supplemented with 0.1% Bovine Serum Albumin (BSA) and antibiotics.
Ethanol and 5-Aza-2′-deoxycytidine treatments
To reduce ethanol evaporation, ethanol incubations were carried out in sealed chambers, under an atmosphere of 5% CO2 and 95% air as previously described (Guizzetti et al. 2007).
The alcohol concentrations used in this study (25, 50, and 75 mM corresponding to 0.115, 0.23, and 0.35 g/dl, respectively) are clinically relevant as they can be found in the blood of individuals with problems of alcohol dependence (Adachi et al. 1991) and are within the range of concentrations recommended for in vitro studies (Deitrich and Harris 1996).
Ethanol incubations were carried out continuously for 24 or 48 hours, which, in differentiated cells in vitro, are considered protracted incubations resulting in homeostatic responses (Lindsley and Mazurkiewicz 2013).
To inhibit DNMT activity, astrocytes were treated with 2.5 µM 5-Aza-2′-deoxycytidine (5-AzaC) for 40 h.
DNA Methylation assay
DNA was isolated from cortical rat astrocytes using the DNeasy Blood & Tissue Kit, fragmented by sonication, and subjected to MethylMiner™ methylated DNA kit enrichment according to the manufacturer's protocol to precipitate methylated DNA and input samples were collected before the precipitation. The precipitated, methylated DNA was further purified using the QIAquick PCR Purification Kit. DNA methylation levels in the promoter region of tPA were determined by quantitative PCR using the Stratagene MxPro-Mx3000P Systems and SYBR green assay. Tissue-PA primers include forward, AGCTTAGAGCCGCACATCCTTACA, and reverse, CTTGGCTTGACGCCAGCTTGATTA. Data were calculated as 2−ΔΔCT (ΔCT= target gene CT – input CT) and expressed as fold change over control as previously described (Livak and Schmittgen 2001).
RNA extraction and gene expression
RNA was isolated from astrocytes using the RNeasy Plus Mini Kit and transcribed into cDNA using High-Capacity cDNA Reverse Transcription Kits and gene expression was determined by quantitative real time PCR as previously described (Chen et al. 2013). Primers for DNMT1 were: forward, CCATGTTGCCGGGGGCTGAG; reverse, TCGGCTGGGTCTTGGGTGGG; for DNMT3A were: forward, AGGAAGCCCATCCGGGTGCTA; reverse, AGCGGTCCACTTGGATGCCC; for DNMT3B were: forward, TACCAAGTCTCGGAGGCGGCG; reverse, AGCTCGATGCTGGCAGGGGA. Tissue- PA primers were: forward, AGCAAGGCACGGGACACGGA; reverse, GGTCAGGCAACGTGAACGCC. Reference gene β-actin primers were: GCGTCCACCCGCGAGTACAA (forward) and TCCATGGCGAACTGGTGGCG (reverse). Data were calculated as 2−ΔΔCT (ΔCT= target gene CT –β-actin CT) and expressed as fold change over control as previously described (Livak and Schmittgen 2001).
DNMT Activity
Nuclear protein fractions were prepared using a nuclear extraction kit and nuclear proteins were quantified using the BCA Protein Assay measurement; 10 µg nuclear proteins were used to measure DNMT activity by the EpiQuik™ DNA Methyltransferase Activity/Inhibition Assay Ultra Kit following the manufacturer’s protocol. The results were calculated as O.D./ µg/hr.
tPA ELISA
Tissue-PA protein levels were determined in the cell extracts and in the media of control and ethanol-treated astrocytes using the rat tPA Total Antigen Assay kit following the manufacture’s protocol.
Western Blot Analysis
After ethanol treatment, cells were lysed in cell lysis buffer supplemented with a protease inhibitor cocktail; Western blot analysis was carried out as previously described (Guizzetti et al. 2007). DNMT1, 3A, and 3B antibodies (dilution 1:500) were incubated overnight at 4°C; the HRP-conjugated secondary antibody (dilution 1:5,000) was incubated for 1 h at room temperature.
Statistical Analysis
Student’s t-test (for one-to-one comparisons) or one-way ANOVA followed by Dunnett’s Post Hoc test (for multiple comparisons to one control) were used to determine significant differences from controls.
Results
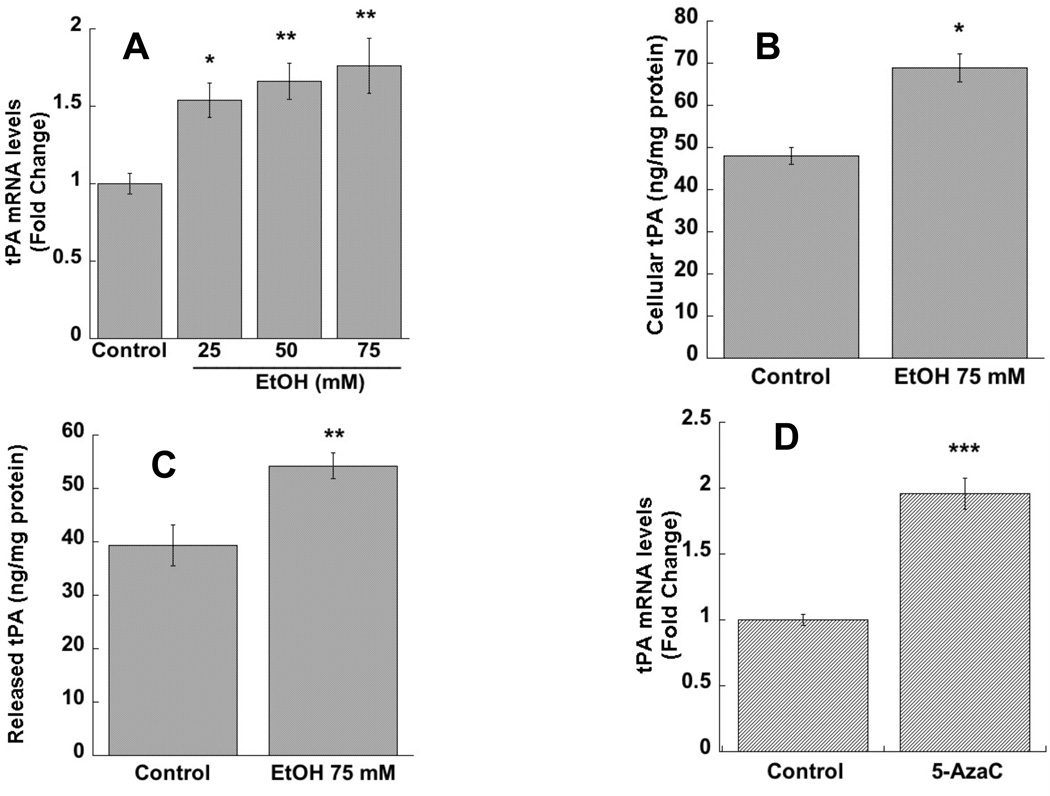
In the present study we investigated the effect of ethanol on tPA mRNA and protein levels in astrocytes. Ethanol treatment (24h) increased tPA mRNA levels in a dose-dependent manner with the lowest effective concentration at 25 mM (Fig. 1 A). The subsequent experiments were therefore carried out using a single ethanol concentration of 75 mM. Alcohol significantly increased cellular levels of tPA protein expression, measured by ELISA in cell lysate extracts (Fig. 1 B). Interestingly, ethanol also increased the levels of released tPA quantified in astrocyte-conditioned medium (Fig. 1 C), a biologically relevant finding since extracellular tPA can access and proteolytically activate the extracellular protease plasmin.
Figure 1. Effect of ethanol and 5-AzaC on tPA expression in astrocytes.
Primary rat cortical astrocytes were incubated in the presence or absence of 25, 50, or 75 mM ethanol for 24 h (A) or 2.5 µm 5-AzaC for 40 h (D). Total RNA was extracted and tPA mRNA levels were quantified by qPCR. The results were normalized to β-actin mRNA and expressed as fold change relatively to control. *: p < 0.05; **: p < 0.01; ***p < 0.001 compared with control by the Dunnett’s post-hoc test (A) or Student’s t-test (D) (n=4-6). The cellular and released levels of tPA were measured by ELISA in astrocyte cell lysate (B) and in astrocyte-conditioned medium (C) after 24 h incubations in the presence and in the absence of 75 mM ethanol. *: p< 0.05; **: p< 0.01; compared with control by Student’s t test (n=6).
Similarly to what was found with ethanol, the DNMT inhibitor 5-AzaC significantly increased tPA mRNA levels in astrocytes (Fig. 1 D).
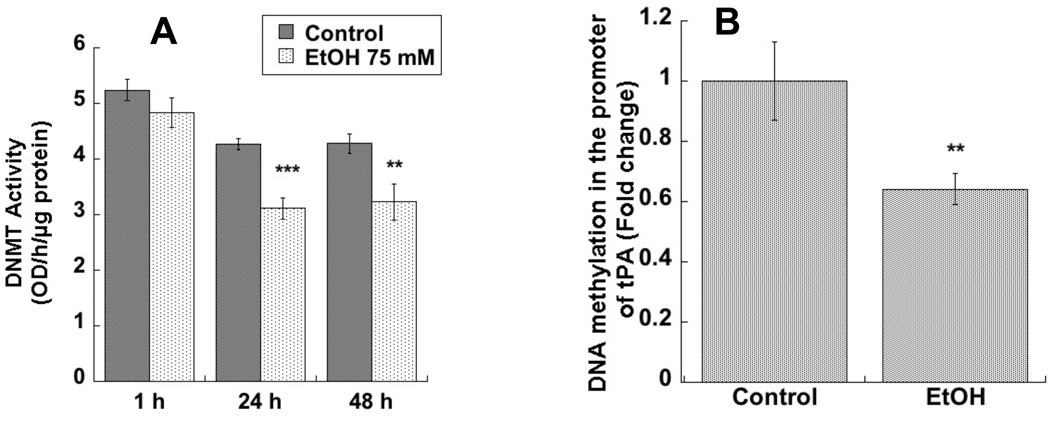
In order to investigate whether the effect of ethanol on tPA expression was indeed mediated by DNA methylation, we measured DNMT activity in astrocytes treated with 75 mM ethanol. DNMT activity was significantly inhibited after 24 and 48 h incubations, but not after acute ethanol treatments (1 h incubations) (Fig. 2 A).
Figure 2. Effect of ethanol on nuclear DNMT activity and on DNA methylation in the promoter region of tPA in astrocytes.
A: DNMT activity was measured in the nuclear extracts of astrocytes treated with 75mM ethanol for 1h, 24h, or 48 h (n=6). B: DNA was extracted from astrocytes treated with or without 75 mM ethanol for 24 h. DNA methylation was determined by qPCR after MethyMiner™ precipitation using specific primers described in the text, normalized to DNA input, and expressed as fold change (n=5). **p< 0.0; ***p< 0.001 compared with control by Student’s t test.
Furthermore, we tested the effect of ethanol on DNA methylation in the promoter region of tPA, where we identified a region containing a high frequency of putative CpG sites (CpG island) located from −295 to −60 with respect to the transcription initiation site. We therefore designed a set of primers amplifying a 145 bp region from position −293 to −148 within the CpG island that included 5 CpG sites and also a cyclic adenosine monophosphate response element (CRE). Quantification of DNA methylation levels was carried out by using the MethylMiner™ assay followed by qPCR quantification. We found that DNA methylation in the promoter region of tPA was significantly reduced in ethanol-treated astrocytes compared to control cells (Fig. 2 B).
These data indicate that ethanol increased the levels of tPA by inhibiting DNMT activity and decreasing DNA methylation in the tPA promoter region.
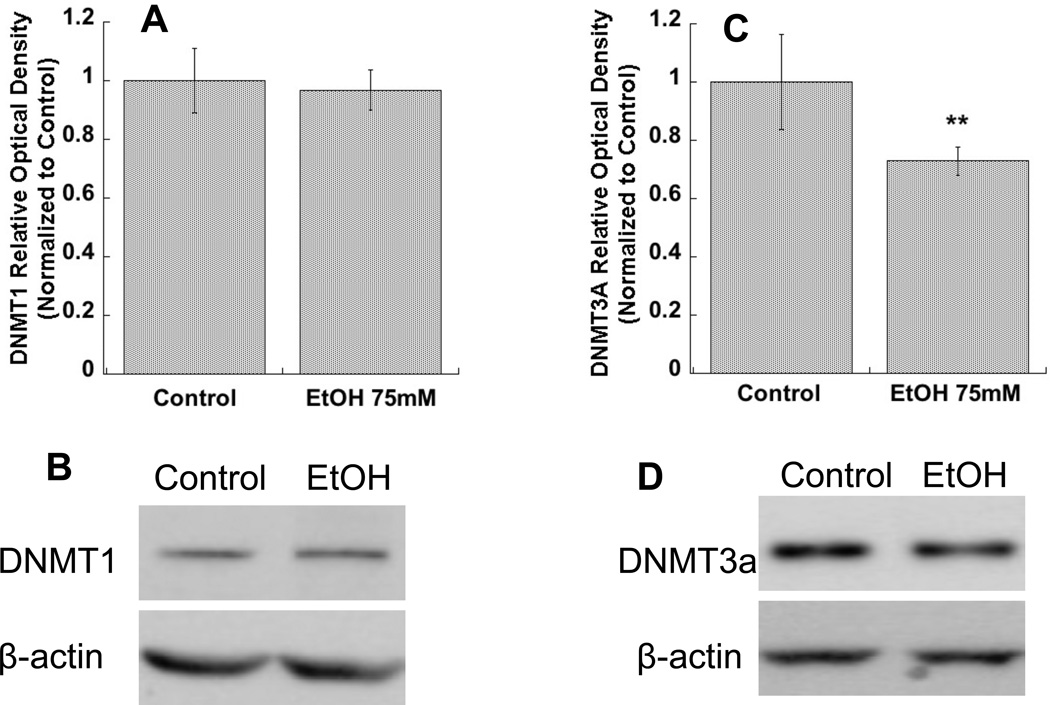
We carried out Western blot analyses to investigate changes in DNMT isoform protein levels in astrocyte cell lysates after 24 h exposure to ethanol (75 mM). DNMT1 (Fig. 3 A, B) and DNMT3A (Fig. 3 C, D), but not DNMT3B (not shown) were detected by this method in astrocytes. The protein levels of DNMT3A (Fig. 3 C, D), but not DNMT1 (Fig. 3 A, B), were significantly reduced in astrocytes by ethanol exposure.
Figure 3. Effect of ethanol on DNMT1 and DNMT3A proteins level in astrocytes.
Primary rat cortical astrocytes were incubated for 24 h in the presence or absence of 75 mM ethanol. Western blot analysis was carried out in the cell lysate of treated astrocytes. A, C: Average optical density of DNMT1 and DNMT3A, respectively, normalized to β-actin and expressed as percent of control. *p< 0.05 compared with control by Student’s t test (n=6). Representative immunoblots of DNMT1 (upper image) and β-actin (lower image) (B) and DNMT3A (upper image) and β-actin (lower image) (D) are shown.
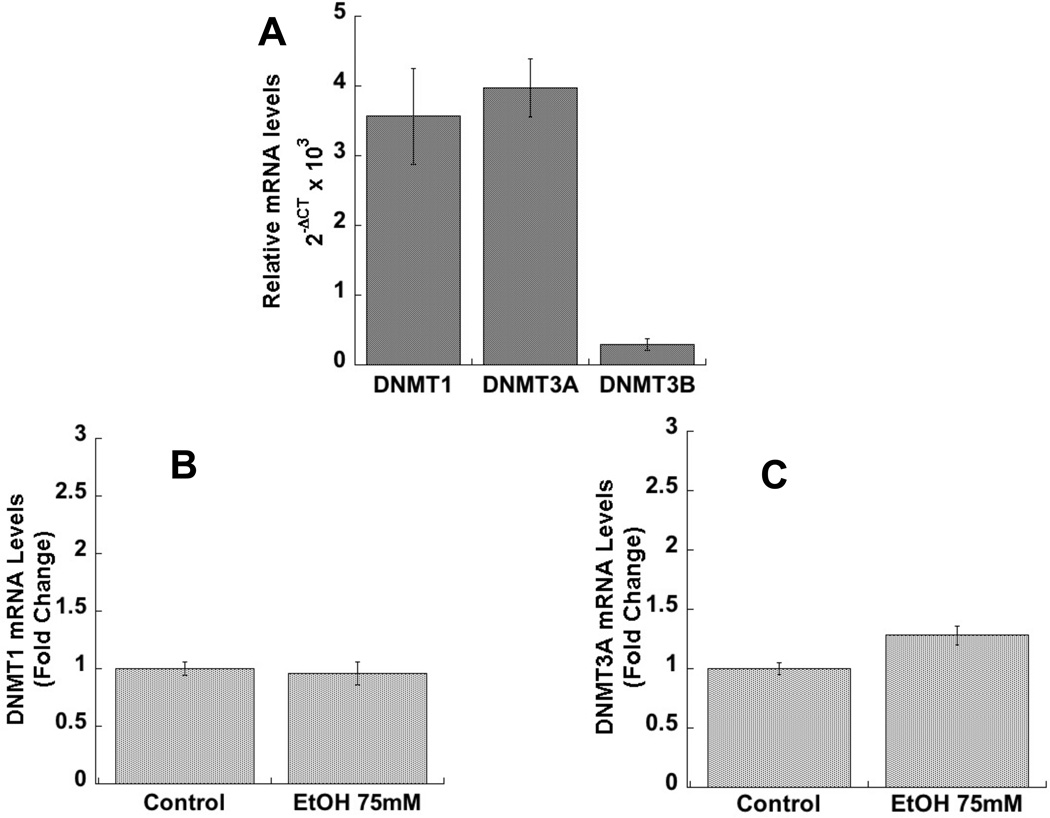
Lastly, we investigated the relative mRNA expression of the three DNMT isoforms. We found that DNMT1 and DNMT3A were highly expressed in astrocytes, while DNMT3B was expressed at a much lower level (Fig. 4 A), confirming the Western blot observation. The three sets of primers used were over 90% efficient, therefore allowing for the comparison of relative levels of expression of these genes in astrocytes. DNMT1 and 3A mRNA levels were not affected by ethanol (Fig. 4 B-C).
Figure 4. Relative expression of DNMT1, 3A, and 3B and effect of ethanol on DNMT1 and 3A mRNA levels in astrocytes.
A: Relative mRNA expression of DNMT1, 3A, and 3B in astrocytes. Data are expressed as 2−ΔCT (ΔCT= target gene CT -β-actin CT); shown is the mean (± SEM) of 6 independent determinations. B: Primary rat cortical astrocytes were incubated for 24 h in the presence or absence of 75 mM ethanol. Total RNA was extracted and DNMT1 and 3A mRNA levels were quantified by qPCR. The results were normalized to β-actin mRNA and expressed as fold change relatively to control (n=6).
Discussion
Astrocytes play a major role in regulating the composition of the ECM and in neuronal plasticity (Asher et al. 2000, Hamel et al. 2005, Tom et al. 2004). Our working hypothesis is that astrocytes respond to endogenous and exogenous stimuli by altering the release of pro-neuritogenic and anti-neuritogenic factors and therefore have the ability to promote or inhibit neuronal development. We have shown that ethanol inhibits carbachol-treated astrocyte-mediated neuritogenesis in an astrocyte-neuron “sandwich” co-culture system in which the two cell populations face each other without touching (Guizzetti et al. 2010); ethanol also inhibits neuritogenesis mediated by astrocyte-neuron adhesion in a co-culture model in which neurons are plated on top of treated astrocytes and the two cell types are touching (Zhang et al, submitted). We also reported lower levels of neuritogenic extracellular laminin, fibronectin, and PAI-1 levels in astrocytes after ethanol treatments (Guizzetti et al. 2010).
In the present study, we investigated the effect of alcohol on the expression and release of tPA, an activator of the extracellular proteolytic enzyme plasmin, which triggers the degradation of ECM proteins (Dellas and Loskutoff 2005, Irigoyen et al. 1999). We found that ethanol increased tPA expression and release through the inhibition of DNMT activity and the decrease in DNA methylation at the tPA promoter in astrocyte cultures. The involvement of DNMT inhibition in the regulation of tPA expression was corroborated by the finding that the DNMT inhibitor 5-AzaC increased tPA mRNA levels in astrocytes (Fig. 1 D). The fact that tPA expression is regulated by DNA methylation in human cells (Dunoyer-Geindre and Kruithof 2011) makes our studies translationally relevant.
The current study also characterized the DNMT isoforms expressed by astrocytes and identified DNMT3A as the likely target of ethanol inhibition (Fig. 3 C, D). The effect of ethanol on DNMT activity and DNMT3A protein levels does not appear to be mediated by altered gene expression, as neither DNMT1 nor DNMT3A mRNA levels are affected (Fig. 4 A, B), and it may be due to increased DNMT3A protein degradation.
DNMT isoforms have overlapping roles in maintaining DNA methylation involved in synaptic plasticity (Feng et al. 2010) and the silencing of a DNMT isoform may lead to compensatory upregulation of other isoforms (Kundakovic et al. 2009); however, ethanol-induced reduction in DNMT3A protein levels did not cause a compensatory increase in other DNMT isoforms therefore resulting in an overall decrease in DNMT activity, as reported here.
In vivo, ethanol is reported to increase tPA levels and decrease laminin in the brain parenchyma of adult mice following chronic exposure and in a mouse model of FASD (Noel et al. 2011, Skrzypiec et al. 2009). The effect of alcohol on tPA and laminin has been implicated in alcohol-induced neurodegeneration (Noel et al. 2011, Skrzypiec et al. 2009). Recently, it became apparent that major proteolytic systems, including the plasminogen activator system and matrix metalloproteases, are present in the brain parenchyma and, because of their ability to modulate brain ECM, are involved in several brain pathologies (Bonneh-Barkay and Wiley 2009).
In conclusion, this is the first study demonstrating that ethanol decreases DNMT activity and DNA methylation in the promoter region of tPA, thereby increasing tPA expression in astrocytes. Epigenetic changes induced by ethanol in astrocytes are likely to be involved in the deleterious effects of alcohol in the developing and adult brain given the important role played by these cells in regulating neuronal functions (Barres 2008).
Acknowledgements
This work was supported in part by grant AA017180 to MG and grants AA016690 and AA-019971 to SCP from the National Institute of Alcoholism and Alcohol Abuse. We thank Dr. David Gavin for insightful discussions, Dr. Sumit Bhattacharyya for his help with the tPA ELISA assay, and Dr. Harish Krishnan for critically reading the manuscript.
Abbreviation used
- DNMT
DNA methyltransferase
- ECM
extracellular matrix
- FASD
fetal alcohol spectrum disorders
- PAI-1
plasminogen activator inhibitor-1
- tPA
tissue plasminogen activator
Footnotes
Disclosure:
No conflict of interest to declare.
References
- Adachi J, Mizoi Y, Fukunaga T, Ogawa Y, Ueno Y, Imamichi H. Degrees of alcohol intoxication in 117 hospitalized cases. J Stud Alcohol. 1991;52:448–453. doi: 10.15288/jsa.1991.52.448. [DOI] [PubMed] [Google Scholar]
- Asher RA, Morgenstern DA, Fidler PS, et al. Neurocan is upregulated in injured brain and in cytokine-treated astrocytes. J Neurosci. 2000;20:2427–2438. doi: 10.1523/JNEUROSCI.20-07-02427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Boden JM, Fergusson DM. Alcohol and depression. Addiction. 2011;106:906–914. doi: 10.1111/j.1360-0443.2010.03351.x. [DOI] [PubMed] [Google Scholar]
- Bonneh-Barkay D, Wiley CA. Brain extracellular matrix in neurodegeneration. Brain Pathol. 2009;19:573–585. doi: 10.1111/j.1750-3639.2008.00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang X, Kusumo H, Costa LG, Guizzetti M. Cholesterol efflux is differentially regulated in neurons and astrocytes: Implications for brain cholesterol homeostasis. Biochim Biophys Acta. 2013;1831:263–275. doi: 10.1016/j.bbalip.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitrich RA, Harris RA. How much alcohol should I use in my experiments? Alcohol Clin Exp Res. 1996;20:1–2. doi: 10.1111/j.1530-0277.1996.tb01033.x. [DOI] [PubMed] [Google Scholar]
- Dellas C, Loskutoff DJ. Historical analysis of PAI-1 from its discovery to its potential role in cell motility and disease. Thromb Haemost. 2005;93:631–640. doi: 10.1160/TH05-01-0033. [DOI] [PubMed] [Google Scholar]
- Dunoyer-Geindre S, Kruithof EK. Epigenetic control of tissue-type plasminogen activator synthesis in human endothelial cells. Cardiovasc Res. 2011;90:457–463. doi: 10.1093/cvr/cvr028. [DOI] [PubMed] [Google Scholar]
- Feng J, Zhou Y, Campbell SL, Le T, Li E, Sweatt JD, Silva AJ, Fan G. Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nat Neurosci. 2010;13:423–430. doi: 10.1038/nn.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh BS, Chintala SK. Inhibition of reactive gliosis attenuates excitotoxicity-mediated death of retinal ganglion cells. PLoS One. 2011;6:e18305. doi: 10.1371/journal.pone.0018305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govorko D, Bekdash RA, Zhang C, Sarkar DK. Male germline transmits fetal alcohol adverse effect on hypothalamic proopiomelanocortin gene across generations. Biol Psychiatry. 2012;72:378–388. doi: 10.1016/j.biopsych.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guizzetti M, Chen J, Oram JF, Tsuji R, Dao K, Moller T, Costa LG. Ethanol induces cholesterol efflux and up-regulates ATP-binding cassette cholesterol transporters in fetal astrocytes. J Biol Chem. 2007;282:18740–18749. doi: 10.1074/jbc.M702398200. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Costa P, Peters J, Costa LG. Acetylcholine as a mitogen: muscarinic receptor-mediated proliferation of rat astrocytes and human astrocytoma cells. Eur J Pharmacol. 1996;297:265–273. doi: 10.1016/0014-2999(95)00746-6. [DOI] [PubMed] [Google Scholar]
- Guizzetti M, Moore NH, Giordano G, Costa LG. Modulation of neuritogenesis by astrocyte muscarinic receptors. J Biol Chem. 2008;283:31884–31897. doi: 10.1074/jbc.M801316200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guizzetti M, Moore NH, Giordano G, VanDeMark KL, Costa LG. Ethanol inhibits neuritogenesis induced by astrocyte muscarinic receptors. Glia. 2010;58:1395–1406. doi: 10.1002/glia.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel MG, Mayer J, Gottschall PE. Altered production and proteolytic processing of brevican by transforming growth factor beta in cultured astrocytes. J Neurochem. 2005;93:1533–1541. doi: 10.1111/j.1471-4159.2005.03144.x. [DOI] [PubMed] [Google Scholar]
- Harper C, Matsumoto I. Ethanol and brain damage. Curr Opin Pharmacol. 2005;5:73–78. doi: 10.1016/j.coph.2004.06.011. [DOI] [PubMed] [Google Scholar]
- He J, Nixon K, Shetty AK, Crews FT. Chronic alcohol exposure reduces hippocampal neurogenesis and dendritic growth of newborn neurons. Eur J Neurosci. 2005;21:2711–2720. doi: 10.1111/j.1460-9568.2005.04120.x. [DOI] [PubMed] [Google Scholar]
- Irigoyen JP, Munoz-Canoves P, Montero L, Koziczak M, Nagamine Y. The plasminogen activator system: biology and regulation. Cell Mol Life Sci. 1999;56:104–132. doi: 10.1007/PL00000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundakovic M, Chen Y, Guidotti A, Grayson DR. The reelin and GAD67 promoters are activated by epigenetic drugs that facilitate the disruption of local repressor complexes. Mol Pharmacol. 2009;75:342–354. doi: 10.1124/mol.108.051763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Mattson SN, Riley EP, et al. A longitudinal study of the long-term consequences of drinking during pregnancy: heavy in utero alcohol exposure disrupts the normal processes of brain development. J Neurosci. 2012;32:15243–15251. doi: 10.1523/JNEUROSCI.1161-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley TA, Mazurkiewicz JE. Ethanol Modulates Spontaneous Calcium Waves in Axonal Growth Cones in Vitro. Barin Sci. 2013;3:615–626. doi: 10.3390/brainsci3020615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Balaraman Y, Wang G, Nephew KP, Zhou FC. Alcohol exposure alters DNA methylation profiles in mouse embryos at early neurulation. Epigenetics. 2009;4:500–511. doi: 10.4161/epi.4.7.9925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- MacDonald JL, Roskams AJ. Epigenetic regulation of nervous system development by DNA methylation and histone deacetylation. Prog Neurobiol. 2009;88:170–183. doi: 10.1016/j.pneurobio.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Martinez R, Gomes FC. Neuritogenesis induced by thyroid hormonetreated astrocytes is mediated by epidermal growth factor/mitogen-activated protein kinase-phosphatidylinositol 3-kinase pathways and involves modulation of extracellular matrix proteins. J Biol Chem. 2002;277:49311–49318. doi: 10.1074/jbc.M209284200. [DOI] [PubMed] [Google Scholar]
- Moonat S, Sakharkar AJ, Zhang H, Tang L, Pandey SC. Aberrant Histone Deacetylase2-Mediated Histone Modifications and Synaptic Plasticity in the Amygdala Predisposes to Anxiety and Alcoholism. Biol Psychiatry. 2013;15:763–773. doi: 10.1016/j.biopsych.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel M, Norris EH, Strickland S. Tissue plasminogen activator is required for the development of fetal alcohol syndrome in mice. Proc Natl Acad Sci U S A. 2011;108:5069–5074. doi: 10.1073/pnas.1017608108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley EP, Infante MA, Warren KR. Fetal alcohol spectrum disorders: an overview. Neuropsychol Rev. 2011;21:73–80. doi: 10.1007/s11065-011-9166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyan P, Golden HB, Miranda RC. Competing interactions between micro-RNAs determine neural progenitor survival and proliferation after ethanol exposure: evidence from an ex vivo model of the fetal cerebral cortical neuroepithelium. J Neurosci. 2007;27:8546–8557. doi: 10.1523/JNEUROSCI.1269-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrzypiec AE, Maiya R, Chen Z, Pawlak R, Strickland S. Plasmin-mediated degradation of laminin gamma-1 is critical for ethanol-induced neurodegeneration. Biol Psychiatry. 2009;66:785–794. doi: 10.1016/j.biopsych.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streissguth AP, O'Malley K. Neuropsychiatric implications and long-term consequences of fetal alcohol spectrum disorders. Semin Clin Neuropsychiatry. 2000;5:177–190. doi: 10.1053/scnp.2000.6729. [DOI] [PubMed] [Google Scholar]
- Tom VJ, Doller CM, Malouf AT, Silver J. Astrocyte-associated fibronectin is critical for axonal regeneration in adult white matter. J Neurosci. 2004;24:9282–9290. doi: 10.1523/JNEUROSCI.2120-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FC, Balaraman Y, Teng M, Liu Y, Singh RP, Nephew KP. Alcohol alters DNA methylation patterns and inhibits neural stem cell differentiation. Alcohol Clin Exp Res. 2011;35:735–746. doi: 10.1111/j.1530-0277.2010.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]