Abstract
Background
The duration and exclusivity of breastfeeding in infancy have been inversely associated with future cardiometabolic risk. We investigated the effects of an experimental intervention to promote increased duration of exclusive breastfeeding on cardiometabolic risk factors in childhood.
Methods and results
We followed-up children in the Promotion of Breastfeeding Intervention Trial, a cluster-randomized trial of a breastfeeding promotion intervention based on the World Health Organization/United Nations Children’s Fund Baby-Friendly Hospital Initiative. 17,046 breastfeeding mother-infant pairs were enrolled in 1996/7 from 31 Belarussian maternity hospitals and affiliated polyclinics (16 intervention vs 15 control sites); 13,879 (81.4%) children were followed-up at 11.5 years, with 13,616 (79.9%) fasted and without diabetes. The outcomes were blood pressure; fasting insulin, adiponectin, glucose and apolipoprotein A1; and presence of metabolic syndrome. Analysis was by intention to treat, accounting for clustering within hospitals/clinics. The intervention substantially increased breastfeeding duration and exclusivity compared with the control arm (43% vs. 6% and 7.9% vs. 0.6% exclusively breastfed at 3 and 6 months, respectively). Cluster-adjusted mean differences at 11.5 years between experimental vs control groups were: 1.0mmHg (95% CI: −1.1, 3.1) for systolic and 0.8mmHg (−0.6, 2.3) for diastolic blood pressure; −0.1mmol/l (−0.2, 0.1) for glucose; 8% (−3%, 34%) for insulin; −0.33μ/ml (−1.5, 0.9) for adiponectin; and 0.0g/l (−0.1, 0.1) for ApoA1. The cluster-adjusted odds ratio for metabolic syndrome, comparing experimental vs control groups, was 1.21 (0.85, 1.72).
Conclusions
An intervention to improve breastfeeding duration and exclusivity among healthy term infants did not influence cardiometabolic risk factors in childhood.
Clinical Trial Registration Information
Current Controlled Trials: ISRCTN37687716 (http://www.controlled-trials.com/ISRCTN37687716); Clinicaltrials.gov. Identifier: NCT01561612.
Keywords: Breastfeeding, lactation, blood pressure, fasting insulin, glucose, adiponectin, lipids, randomized controlled trial, childhood
Introduction
Observational studies suggest that greater duration and exclusivity of having been breastfed is associated with lower levels of cardio-metabolic risk factors in later life, including blood pressure,1 insulin resistance,2 blood glucose,2 lipids,3 carotid intima media thickness4 and type 2 diabetes,2 although associations with cardiovascular disease endpoints have been inconsistent.5
Breastfeeding6 and metabolic factors,7 however, are both highly socially patterned in high-income countries, where most of the studies cited above were set. Even if observational associations of breastfeeding with cardiometabolic risk are adjusted for maternal or socioeconomic confounders, residual confounding by unmeasured or poorly measured factors may explain the observed associations.6 Inference from observational studies is also hampered by other problems: i) reverse causality, since the relationship of breastfeeding with infant growth is bi-directional such that the direction of cause and effect may be the reverse - poor growth (itself associated with later adiposity8 and metabolic risk9) may be the cause of formula supplementation or weaning;10 ii) selective publication;1 and iii) limited information on the exclusivity of breastfeeding, of particular relevance for high-income countries where truly exclusive breastfeeding is rare and robust information on any benefits could help promote it. The causal effects of breastfeeding, therefore, can best be investigated in a large randomized controlled trial.11
To overcome the limitations inherent in observational studies of the long-term effects of breastfeeding,12 we designed a follow-up of 17,046 children participating in the Promotion of Breastfeeding Intervention Trial (PROBIT), a cluster-randomized controlled trial in the Republic of Belarus.13 The intervention resulted in two groups with substantially different exposure to breastfeeding, providing a unique opportunity to test, in an intention-to-treat (ITT) analysis, the extent to which greater duration and exclusivity of breastfeeding causally influences cardiometabolic risk factors. We previously reported that the breastfeeding promotion intervention had no measurable effect on adiposity or blood pressure at age 6.5 years (PROBIT II)14 or on adiposity, height, and insulin-like growth factor at age 11.5 years (PROBIT III).15 The current study (also part of PROBIT III) provides experimental evidence on whether increased duration and exclusivity of breastfeeding has beneficial effects on cardiometabolic risk factors at 11.5 years.
Methods
A detailed description of the cluster-based randomisation, experimental intervention, sample size calculations and participant eligibility in PROBIT has been published.13 Briefly, the units of randomization (clusters) were maternity hospitals and their associated polyclinics (outpatient health clinics following up both well and ill children). These units were randomized to a control group, consisting of continuation of the breastfeeding practices and policies in effect at the time of randomisation, or an experimental intervention, based on the Baby Friendly Hospital Initiative developed by the World Health Organization (WHO) and United Nations Children’s Fund (UNICEF) to promote and support breastfeeding, particularly among mothers chosing to initiate breastfeeding.16 The trial results are based on 17 046 healthy breastfed infants from 31 maternity hospitals/polyclinics, born at term (≥37 weeks gestation) in 1996–7 and enrolled during their postpartum stay. Trial inclusion criteria required the infants to be healthy, singleton, with birth weight ≥2500g and Apgar score ≥5 at 5 minutes, and their mothers to have initiated breastfeeding and have no condition that would interfere with breastfeeding.13 The mother-infant pairs were followed up regularly within the first 12 months of life for the occurrence of 1 or more episodes of gastrointestinal tract infection (the primary outcome and basis for the original sample size calculation), 2 or more episodes of respiratory tract infection, and atopic eczema during infancy, compared between the intervention and control groups. The results reporting on these outcomes have been published.13
A long-term follow-up of the trial (PROBIT II) was conducted between 2002–2005 when the children were a mean age of 6.5 years, for the following a priori defined secondary outcomes that have been reported: anthropometry, blood pressure, cognition, behavior, asthma/allergies, and dental caries.17 The focus of the current paper is a further follow-up when the children were 11.5 years (PROBIT III) for the following a priori defined secondary outcomes: adiposity and insulin-like growth factor-I (reported previously15); blood pressure; fasting insulin, adiponectin (a marker of insulin resistance18), glucose and apolipoprotein A1 (the main protein constituent of high density lipoprotein cholesterol (HDL-C), strongly correlated with HDL-C (r ~ 0.80) and inversely associated with coronary events at a similar magnitude to HDL-C19); and metabolic syndrome, defined by the European Group for the Study of Insulin Resistance (EGIR20).
PROBIT III follow-up was approved by the Belarusian Ministry of Health and received ethical approval from the McGill University Health Centre Research Ethics Board; the Human Subjects Committee at Harvard Pilgrim Health Care; and the Avon Longitudinal Study of Parents and Children (ALSPAC) Law and Ethics Committee. A parent or legal guardian provided written informed consent in Russian at enrollment and at the follow-up visits, and all children provided written assent at the 11.5 year visit.
Follow-up at 11.5 years
Between January 2008 and December 2010 the children were followed-up at dedicated research visits by 39 specially-trained pediatricians at 31 polyclinics.17 Training and quality assurance procedures have been fully described previously and included a 3-day initial training workshop and practical sessions, retraining workshops every 6 months, and ongoing data monitoring.21 We asked children to fast for at least eight hours prior to the visit, which included the following measurements21: whole blood fasting glucose (Roche ACCU-CHEK Advantage meter system, Basel, Switzerland); systolic and diastolic blood pressure in triplicate using OMRON 705IT and an appropriate sized cuff; Tanner pubertal stage by direct physician examination; and waist circumference in duplicate. Pediatricians also obtained single measurements of maternal systolic and diastolic blood pressure, height and body mass index and asked mothers whether they had a diagnosis of hypertension, type 2 diabetes, or gestational diabetes.
Dried blood spot sampling
At the visit, finger-prick whole-blood spot samples were collected by the 39 pediatricians, who had received special training, onto Whatman 903 filter paper cards as described previously.22 To maintain sample stability, the dried blood spot cards were air-dried, placed in sealed low gas-permeable plastic bags containing dry silica gel and stored in a −20°C freezer at each of the 31 polyclinic sites until transport to the laboratory at the National Mother and Child Centre in Minsk, where they were stored at −80°C. Paediatricians collected a median of 8 blood spots from 13,487 (97%) children22; the samples used in the current analysis were stored at −20 °C for a median of 1.7 months (interquartile range, IQR 1.0–5.1 months) and at −80 °C for a median of 18.6 (IQR 13.8–22.2) months.
Assays
All samples were analysed after a single thaw. Discs were punched from the centre of the blood spot cards directly into 96-well microtitre plates using an automated hole puncher (Wallac DBS (Dried Blood Spot) Puncher: 1296-071, Perkin Elmer, USA). We quantified circulating insulin from two 3-mm diameter discs (≈ 6 μL of blood) per child, using methods described previously22 that are based on an adaptation of an existing commercial kit (Mercodia Human Insulin ELISA 10-1113-01, Mercodia AB, Sweden). Circulating levels of adiponectin and apolipoprotein A1 (apoA1) were each quantified from one 3-mm diameter disc (≈ 3 μL of blood) per child using existing assay kit reagents (adiponectin human ELISA, EIA4177, DRG International Inc, New Jersey, and Turbox APO A1 catalogue number 67561, Orion Diagnostica, Finland, respectively) and validated methods for blood spots.23, 24 We did not assay HDL-C directly, as the direct measurement of cholesterol from dried blood spots is not straightforward or reliable.25 The assay performance characteristics are given in Supplemental Table 1. All bloodspot analytes were stable for at least 30 months at −80 °C.22 Insulin was assayed from two reagent lots (production batches), adiponectin from four lots, and Apo A1 from three lots between December 2009 and May 2012. To remove the potential effect of storage time and between-lot or between-run variation, we adjusted regression models containing these analytes for the time between sampling and assay date.
We calculated the homeostasis model assessment of β-cell function (HOMA-B) and HOMA of insulin resistance (HOMA-IR) using the calculator available at http://www.dtu.ox.ac.uk. We defined a binary outcome for the presence or absence of metabolic syndrome according to recommendations of the European Group for the Study of Insulin Resistance (EGIR):20 raised insulin levels (fasting values ≥75th sample percentiles for sex and pubertal stage, as in other studies.26) and at least two of the following metabolic abnormalities based on population reference values: hyperglycemia (whole blood fasting values ≥5.6 mmol/l, hypertension (systolic blood pressure ≥90th percentile for age, sex and height 27), dyslipidemia (ApoA values ≤10th percentile for age, sex28) and abdominal obesity (waist circumference ≥90th percentile for age, sex29).
Analysis
All analyses used SAS version 9.3 (SAS Institute, Cary, NC), unless stated. We excluded children who had fasted for less than 8 hours before sampling (n= 130) or with known diabetes (n = 39). Continuous measures of insulin, HOMA-B and HOMA-IR were natural log-transformed. Comparisons between experimental and control groups were based on intention-to-treat analysis. We accounted for possible non-independence of measurements within individual hospital/polyclinic sites (clustering) using random effects models, which permit inference at the level of the individual child rather than the cluster. The MIXED procedure was used for continuous outcomes (to estimate mean differences and 95% CIs) and the GLIMMIX procedure for binary outcomes (to estimate odds ratios and 95% CIs) in SAS. The results are presented for the simple cluster-adjusted model, as well as after additional adjustment for stratum-level variables (urban versus rural and East versus West Belarus residence) and for child age at follow-up, sex, birth weight, and maternal and paternal education (further controlling for maternal age and smoking made little difference to the results). Differences in mean insulin, HOMA-IR and HOMA-B were based on the natural log of their values. Hence we report exponentiated estimates from the model using these logged variables, which are interpreted as the ratio of geometric means in intervention versus control group; the confidence intervals were back transformed from the log scale. To determine whether results differed in boys versus girls, we analyzed mixed models that included terms for the sex of each child and a multiplicative sex*treatment interaction term.
In a sensitivity analysis, we investigated whether loss to follow-up influenced the results by undertaking multiple imputation to generate plausible values of missing 11.5 year outcomes and thereby including all 17 046 randomized participants (assuming data missing at random).30 We used SAS imputations (Proc MI) to impute 5 values for each missing observation and combined multivariable modeling estimates using Proc MI ANALYZE in SAS (using 10 and 25 imputations gave almost identical results).
The intention-to-treat analysis underestimates the effect of the true exposure of interest (breastfeeding exclusivity and duration), owing to overlap in breastfeeding between the randomized groups (many intervention mothers did not exclusively breastfeed for 3 or 6 months, whereas some control mothers did). In a secondary analysis, we used instrumental variable methods to estimate the causal effect of the duration of exclusive breastfeeding on our outcomes,31 comparing < 3 months (reference), ≥3 to <6 months and ≥6 months. We used randomization status as the ‘instrument,’ because it is independent of any confounders of the exposure-outcome relationship and is related to the outcomes only via the exposure (breastfeeding duration and exclusivity). We performed instrumental variable estimation of continuous outcomes using the generalized two-stage least squares estimator implemented in the xtivreg command in Stata version 12.1 (Stata Corp., College Station, TX), while accounting for clustering by study site. Instrumental variable estimation of the binary ‘metabolic syndrome’ outcome used a random effects version of the ratio estimator of the causal odds ratio.32
To assess whether we could reproduce the inverse associations of increased duration and exclusivity of breastfeeding reported in previous observational studies, we conducted observational analyses (i.e., disregarding randomization status), in which we estimated the effects of the duration of any and exclusive breastfeeding on the same outcomes, accounting for clustering and the same baseline characteristics as in the expanded mixed models described above, using multiple linear regression for continuous outcomes and multiple logistic regression for binary outcomes. Duration of breastfeeding (any or exclusive) was classified as < 3 months (reference), ≥3 to <6 months and ≥6 months.
Results
As previously reported,13 the randomization produced two groups with similar distributions of baseline sociodemographic and potential confounding factors, including birth weight and maternal and paternal education, smoking, and alcohol intake. The intervention substantially increased breastfeeding duration and exclusivity, based on WHO definitions,16 versus the control arm: e.g., at 3 months, intervention infants were 7 times more likely to be exclusively (43.3% vs. 6.4%) and twice as likely to be predominantly (51.9 vs. 28.3%) breastfed, and were breastfed to any degree at higher rates throughout infancy.13
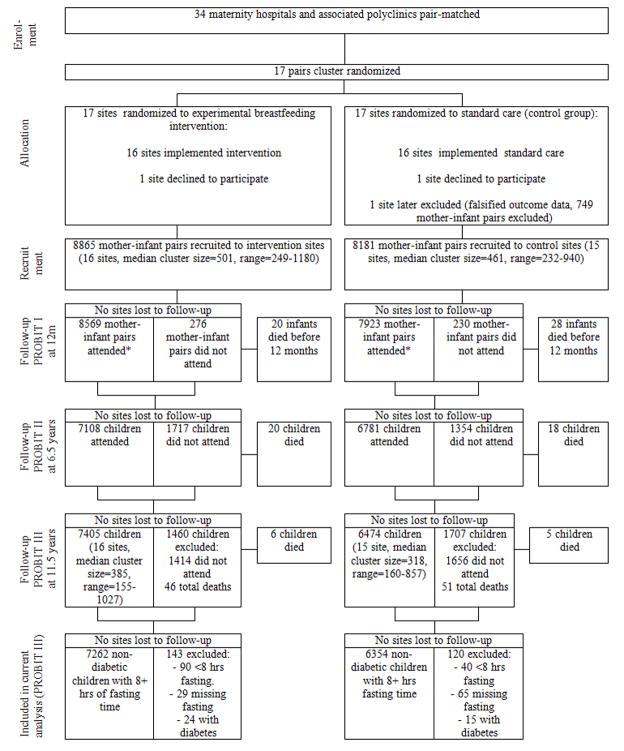
A total of 13,879 children were examined at a median age of 11.5 years (standard deviation: 0.50, interquartile range: 11.3–11.8 years), representing 81.4% of the 17,046 originally randomized (Figure 1). Of the 3,167 children randomized but not followed up at 11.5 years, 2,645 were lost to follow-up, 425 were unable or unwilling to come for their visit and 97 had died since randomisation. Follow-up rates were similar in the experimental (83.5%) and control (79.1%) polyclinics, although they varied between 48 and 98%. Of the 13,879 children who were followed up at age 11.5, 13,616 (98.1%) fasted at least 8 hours overnight and did not have diabetes. Included children in the experimental and control groups had similar baseline characteristics, with small differences paralleling those seen (and previously reported13) at randomisation (Table 1). The groups were also virtually identical in mean parental height and BMI and maternal systolic and diastolic blood pressure, reported hypertension, type 2 diabetes and gestational diabetes, as measured during follow-up.
Figure 1.
Flow diagram of progress of clusters and individuals through PROBIT recruitment and follow-up phases I, II and III. Footnote: Of the 3,167 not seen at PROBIT III; 913 were seen at both 12 months and PROBIT II, 483 were not seen at either 12 months or PROBIT II, 1768 were seen at 12 months but not seen at PROBIT II and 3 were seen at PROBIT II but not seen at 12 months. Data shown in Figure 1 vary minimally from previously published data due to the updating of some variables during PROBIT III data collection and cleaning
Table 1.
Baseline and follow-up characteristics of PROBIT children at 11.5 years who were fasted at least 8 hours and did not have diabetes (N=13,616)
| Variable | Intervention arm (n=7262) | Control arm (n=6354) |
|---|---|---|
| Measured at child’s birth | ||
| Maternal age, years (%) | ||
| < 20 | 13.8 | 12.6 |
| 20–34 | 81.8 | 83.2 |
| ≥ 34 | 4.3 | 4.2 |
| Maternal education (%) | ||
| Completed university | 14.1 | 13.0 |
| Advanced secondary or partial university | 48.2 | 55.2 |
| Common secondary | 33.5 | 29.1 |
| Incompleted secondary | 4.2 | 2.8 |
| Paternal education (%) | ||
| Completed university | 13.7 | 12.5 |
| Advanced secondary or partial university | 42.9 | 52.9 |
| Common secondary | 40.8 | 32.7 |
| Incompleted secondary | 2.5 | 1.9 |
| Stratum-level variable (%) | ||
| East/Urban | 33.8 | 29.8 |
| East/Rural | 14.8 | 16.0 |
| West/Urban | 31.5 | 19.5 |
| West/Rural | 19.9 | 34.7 |
| Older siblings living in household (%) | ||
| 0 | 59.0 | 54.5 |
| 1 | 33.3 | 36.4 |
| ≥ 2 | 7.7 | 9.1 |
| Maternal smoking in pregnancy (%) | 2.5 | 1.6 |
| Male (%) | 50.9 | 52.1 |
| Mean (SD) birthweight (g) | 3444 (417) | 3443 (423) |
| Measured at age 6.5 years: | ||
| Mean (SD) paternal height (cm)a | 176 (7) | 176 (7) |
| Mean (SD) paternal BMI (kg/m2)b | 25.7 (3.3) | 25.7 (3.3) |
| Measured at age 11.5 years: | ||
| Mean (SD) maternal height (cm)c | 164 (5.7) | 164 (5.8) |
| Mean (SD) maternal BMI (kg/m2)d | 26.3 (5.5) | 26.7 (5.6) |
| Mean (SD) maternal systolic BP (mmHg)e | 124 (14) | 125 (15) |
| Mean (SD) maternal diastolic BP (mmHg)e | 77 (10) | 78 (11) |
| Reported hypertension (%)f | 30.2% | 34.9% |
| Reported type 2 diabetes (%)g | 0.24% | 0.33% |
| Reported gestational diabetes (%)h | 0.06% | 0.09% |
SD = standard deviation; BMI= body mass index;
based on n =5785 experimental and 5742 control measured in PROBIT II;
based on n = 5778 experimental and 5724 control measured in PROBIT II
based on n = 7208 experimental and 6314control measured in PROBIT III;
based on n = 6119 experimental and 5433 control measured in PROBIT III;
based on n=4694 experimental and n=4541 control measured in PROBIT III;
based on n=5247 experimental and n=5073 control measured in PROBIT III;
based on n=7225 experimental and n=6311 control measured in PROBIT III;
based on n=7225 experimental and n=6318 control measured in PROBIT III.
The main results are shown in Table 2. Within-polyclinic clustering (the tendency for measurements on children attending the same polyclinic to be more similar to each other than to children attending other polyclinics21) was moderate for fasting glucose and ApoA1 (intraclass correlation coefficients, ICCs: 0.11 and 0.15, respectively), but low (ICCs ≤ 0.10) for the other measures. Mean values of all the continuous outcomes, and the prevalence of metabolic syndrome, were similar in the experimental versus control groups. Cluster-adjusted mean differences at 11.5 years between experimental versus control groups were: 1.0 mmHg (95% CI: −1.1, 3.1) for systolic and 0.8 mmHg (95% CI: −0.6, 2.3) for diastolic blood pressure; 8% (−3%, 34%) for insulin; 8% (−3%, 33%) for HOMA-IR; 7% (−9%, 27%) for HOMA-B; −0.3 3μ/ml (−1.5, 0.9) for adiponectin; and 0.0 g/l (−0.1, 0.1) for ApoA1. The cluster-adjusted odds ratio for metabolic syndrome, comparing experimental versus control groups, was 1.21 (0.85, 1.72). These findings were little altered after adjusting for baseline potential confounders (Table 2) or using multiply imputed outcomes (Supplemental Table 2), apart from the emergence of a positive association of the intervention with systolic blood pressure using multiple imputation. We observed little evidence of interaction by sex (all interaction p-values > 0.06), except for metabolic syndrome (OR = 1.49; 95% CI, 1.01 to 2.22 in males and 0.94; 95% CI, 0.63 to 1.42 in females; p for interaction = 0.01).
Table 2.
Differences in cardiometabolic measurements at 11.5 years in experimental versus control group: intention to treat analysis without imputation from the PROBIT trial (N=13,616)
| Outcome | Intervention | Control | ICC | Difference in mean (ratio of geometric means where stated) (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| N | Mean | 95% CI | N | Mean | 95% CI | Cluster adjusted | Further adjusted for baseline factorsa | ||
| Systolic blood pressure (mmHg) | 7261 | 108.89 | 108.68, 109.10 | 6350 | 107.01 | 106.79, 107.23 | 0.10 | 1.01 (−1.05, 3.08) | 1.23 (−0.58, 3.05) |
| Diastolic blood pressure (mmHg) | 7261 | 62.54 | 62.38, 62.71 | 6350 | 61.52 | 61.36, 61.69 | 0.08 | 0.82 (−0.61, 2.25) | 0.77 (−0.69, 2.23) |
| Glucose (mmol/l) | 7236 | 5.32 | 5.31, 5.34 | 6330 | 5.36 | 5.34, 5.37 | 0.11 | −0.05 (−0.18, 0.07) | −0.03 (−0.16, 0.10) |
| Insulin (mU/L)b,c | 6864 | 3.37 | 3.28, 3.46 | 6003 | 3.51 | 3.42, 3.61 | 0.07 | 1.08 (0.97, 1.34) | 1.06 (0.85, 1.31) |
| HOMA-IRb,c | 6859 | 0.44 | 0.43, 0.46 | 5995 | 0.46 | 0.45, 0.48 | 0.07 | 1.08 (0.97, 1.33) | 1.05 (0.85, 1.30) |
| HOMA-Bb,c | 6859 | 48.62 | 47.77, 49.48 | 5995 | 49.38 | 48.51, 50.28 | 0.10 | 1.07 (0.91, 1.27) | 1.05 (0.88, 1.25) |
| Adiponectin (μg/ml)b | 7069 | 17.58 | 17.40, 17.76 | 6225 | 18.18 | 17.98, 18.38 | 0.05 | −0.29 (−1.51, 0.94) | −0.24 (−1.54, 1.05) |
| Apo A1 (g/L)b | 6991 | 1.61 | 1.60, 1.62 | 6140 | 1.62 | 1.60, 1.63 | 0.15 | 0.03 (−0.11, 0.11) | 0.03 (−0.08, 0.14) |
| N | % | N | % |
Odds ratio (95% CI)
|
|||||
| Cluster adjusted | Further adjusted for baseline factorsa,b | ||||||||
|
|
|||||||||
| Metabolic syndrome | 6752 | 3.92% | 5881 | 3.57% | 1.21 (0.85, 1.72) | 1.16 (0.81, 1.66) | |||
CI = confidence interval; HOMA-IR = Homeostasis Model of Assessment: Insulin Resistance; HOMA-B = Homeostasis Model of Assessment: Beta Cell Function; ICC = intraclass correlation coefficient;
Adjusted for stratum-level variables (urban versus rural and East versus West Belarus), and for child age at follow-up, sex, birthweight and both maternal and paternal education;
Assay coefficients additionally controlled for time between sampling and assay analysis date.
geometric means, back-transformed confidence intervals and ratio of geometric means based on the natural log of insulin, HOMA-IR and HOMA-B: hence those estimates are exp(x) of the estimates from the model using these logged variables, the confidence intervals were back transformed from the log scale and the (ratio of geometric means minus 1)×100 gives the % change in insulin, HOMA-IR and HOMA-B between intervention vs control arms.
The results of the instrumental variable analyses (Table 3), which provide estimates of the causal effects of exclusive breastfeeding for ≥ 3 to < 6 months and ≥ 6 months versus < 3 months (and therefore directly comparable to estimates from observational studies), are in line with the intention-to-treat inference that increased duration and exclusivity of breastfeeding provides no important beneficial effects on the study outcomes.
Table 3.
Instrumental variable estimates of the causal effect of duration of exclusive breastfeeding on cardiometabolic factors at 11.5 years using randomized treatment as the instrumental variable (N=13,616)
| Cluster adjusted | Further adjusted for baseline factorsa | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| <3 mo | 3–<6 mo | ≥6 mo | <3 mo | 3–<6 mo | ≥6 mo | |
| Difference in mean (ratio of means where stated) (95% CI) | Difference in mean (ratio of means where stated) 95% CI) | |||||
| Systolic blood pressure (mmHg) | 0.0 (ref) | 2.89 (−3.60, 9.39) | 4.46 (−5.14, 14.05) | 0.0 (ref) | 3.32 (−2.28, 8.92) | 4.71 (−2.86, 12.28) |
| Diastolic blood pressure (mmHg) | 0.0 (ref) | 2.23 (−2.16, 6.63) | 3.37 (−3.49, 10.24) | 0.0 (ref) | 2.03 (−2.79, 6.84) | 2.85 (−3.93, 9.63) |
| Glucose (mmol/l) | 0.0 (ref) | −0.14 (−0.48, 0.20) | −0.22 (−0.76, 0.31) | 0.0 (ref) | −0.09 (−0.46, 0.29) | −0.15 (−0.72, 0.42) |
| Insulin (mU/L)b,c | 0.0 (ref) | 1.26 (0.67, 2.39) | 1.48 (0.55, 3.96) | 0.0 (ref) | 1.18 (0.58, 2.42) | 1.30 (0.44, 3.85) |
| HOMA-IRb,c | 0.0 (ref) | 1.25 (0.67, 2.34) | 1.45 (0.55, 3.83) | 0.0 (ref) | 1.17 (0.58, 2.37) | 1.28 (0.44, 3.76) |
| HOMA-Bb,c | 0.0 (ref) | 1.23 (0.75, 2.00) | 1.41 (0.66, 3.03) | 0.0 (ref) | 1.15 (0.67, 1.97) | 1.26 (0.57, 2.79) |
| Adiponectin (μg/ml)b | 0.0 (ref) | −0.87 (−4.23, 2.49) | −1.47 (−6.76, 3.82) | 0.0 (ref) | −0.74 (−4.75, 3.26) | −1.29 (−7.05, 4.48) |
| Apo A1 (g/L)b | 0.0 (ref) | 0.01 (−0.32, 0.35) | 0.02 (−0.47, 0.51) | 0.0 (ref) | 0.02 (−0.34, 0.37) | 0.02 (−0.47, 0.51) |
| Odds ratio (95% CI) | Odds ratio (95% CI) | |||||
| Metabolic syndrome | 1.0 (ref) | 1.84 (0.66, 5.15) | 2.32 (0.47, 11.43) | 1.0 (ref) | 1.91 (0.72, 5.05) | 2.23 (0.52, 9.68) |
CI = confidence interval; HOMA-IR = Homeostasis Model of Assessment: Insulin Resistance; HOMA-B = Homeostasis Model of Assessment: Beta Cell Function; ICC = intraclass correlation coefficient;
Adjusted for stratum-level variables (urban versus rural and East versus West Belarus), and for child age at follow-up, sex, birthweight and both maternal and paternal education;
Assay coefficients additionally controlled for time between sampling and assay analysis date;
ratio of geometric means based on the natural log of insulin, HOMA-IR and HOMA-B: hence those effect-estimates are exp(x) of the estimates from the model using these logged variables, the confidence intervals were back transformed from the log scale and the (ratio of geometric means minus 1)×100 gives the % change in insulin, HOMA-IR and HOMA-B between categories of exposure.
In observational analyses (Table 4), increased duration of exclusive breastfeeding was associated with higher diastolic blood pressure, but there was little evidence of any associations with the other cardiometabolic outcomes. The results were similarly null for all outcomes when the exposure was duration of any breastfeeding (Supplemental Table 3).
Table 4.
Observational associations of duration of exclusive breastfeeding with cardiometabolic factors at 11.5 years (n = 13,616)
| Cluster adjusted
|
Trend p | Further adjusted for baseline factorsa
|
Trend p | |||||
|---|---|---|---|---|---|---|---|---|
| <3 mo | 3–<6 mo | ≥6 mo | <3 mo | 3–<6 mo | ≥6 mo | |||
| Difference in mean (ratio where stated) (95% CI) | Difference in mean (ratio where stated) (95% CI) | |||||||
| Systolic blood pressure (mmHg) | 0 (ref) | 0.14 (−0.25, 0.53) | 0.62 (−0.18, 1.43) | 0.16 | 0 (ref) | 0.09 (−0.31, 0.48) | 0.73 (−0.09, 1.54) | 0.17 |
| Diastolic blood pressure (mmHg) | 0 (ref) | 0.29 (−0.01, 0.60) | 0.56 (−0.07, 1.19) | 0.01 | 0 (ref) | 0.24 (−0.07, 0.55) | 0.58 (−0.06, 1.22) | 0.03 |
| Glucose (mmol/l) | 0 (ref) | 0.02 (0.00, 0.04) | 0.00 (−0.04, 0.05) | 0.23 | 0 (ref) | 0.02 (0.01, 0.04) | 0.00 (−0.05, 0.04) | 0.38 |
| Insulin (mU/L)b,c | 0 (ref) | 1.00 (0.95, 1.06) | 1.02 (0.91, 1.13) | 0.72 | 0 (ref) | 1.00 (0.95, 1.05) | 1.01 (0.91, 1.12) | 0.95 |
| HOMA-IRb,c | 0 (ref) | 1.01 (0.96, 1.06) | 1.02 (0.92, 1.13) | 0.70 | 0 (ref) | 1.00 (0.95, 1.05) | 1.01 (0.91, 1.12) | 0.94 |
| HOMA-Bb,c | 0 (ref) | 0.99 (0.96, 1.03) | 1.01 (0.94, 1.08) | 0.98 | 0 (ref) | 0.99 (0.96, 1.02) | 1.00 (0.94, 1.07) | 0.74 |
| Adiponectin (μg/ml)b | 0 (ref) | −0.19 (−0.55, 0.16) | −0.24 (−0.98, 0.50) | 0.27 | 0 (ref) | −0.25 (−0.61, 0.11) | −0.30 (−1.05, 0.44) | 0.16 |
| Apo A1 (g/L)b | 0 (ref) | 0.00 (−0.02, 0.01) | −0.01 (−0.05, 0.02) | 0.45 | 0 (ref) | 0.00 (−0.02, 0.01) | −0.03 (−0.06, 0.01) | 0.19 |
|
Odds ratio (95% CI)
|
Odds ratio (95% CI)
|
|||||||
| Metabolic syndrome | 0 (ref) | 1.08 (0.85, 1.37) | 1.09 (0.65, 1.81) | 0.52 | 0 (ref) | 1.09 (0.86, 1.39) | 1.14 (0.68, 1.89) | 0.43 |
CI = confidence interval; HOMA-IR = Homeostasis Model of Assessment: Insulin Resistance; HOMA-B = Homeostasis Model of Assessment: Beta Cell Function; ICC = intraclass correlation coefficient;
Adjusted for stratum-level variables (urban versus rural and East versus West Belarus), and for child age at follow-up, sex, birthweight and both maternal and paternal education;
Assay coefficients additionally controlled for time between sampling and assay analysis date;
ratio of geometric means based on the natural log of insulin, HOMA-IR and HOMA-B: hence those effect-estimates are exp(x) of the estimates from the model using these logged variables, the confidence intervals were back transformed from the log scale and the (ratio of geometric means minus 1)×100 gives the % change in insulin, HOMA-IR and HOMA-B between categories of exposure.
Discussion
The results from this large cluster-randomized trial indicate that the experimental intervention, despite large improvements in the duration and exclusivity of breastfeeding, reduced neither measures of insulin resistance nor cardiometabolic risk at 11.5 years. The point estimates were generally in the opposite direction to the hypothesised protective effects and the confidence intervals were consistent with chance or small intervention effects, thus ruling out an important beneficial impact of the intervention on cardiometabolic risk factors. The absence of a favourable effect on blood pressure is similar to the results we obtained at age 6.5 years14 and consistent with the recently reported lack of impact on stature or measures of general and peripheral adiposity at age 11.5 years.15 The data reported herein extend our observations to older children and include additional measures of cardiometabolic risk biomarkers.
Strengths and weaknesses
The substantial differences in duration and exclusivity of breastfeeding between the two trial arms were created by randomization at the time of birth, rather than by the mother’s choice. Coupled with our high rates of follow-up over 11.5 years, the intention-to-treat analysis therefore minimizes the problems of confounding and reverse causality that plague observational studies.6 We minimized measurement bias by assessing infant feeding regularly during the first 12 months of life, strictly adhering to WHO definitions of breastfeeding duration and exclusivity;16 at 11.5 years we incorporated biomarker measures of insulin resistance, cardiometabolic risk and metabolic syndrome. Several definitions exist for the metabolic syndrome in childhood.33 Our approach was twofold: i) we evaluated individual components of the metabolic syndrome separately;7 and ii) used the European Group for the Study of Insulin Resistance (EGIR) definition of metabolic syndrome20 because this definition has high sensitivity and specificity in predicting type 2 diabetes33 and cardiovascular disease34 in prospective studies. In contrast, the clinically-focused definition of the metabolic syndrome proposed by the National Cholesterol Education Program (NCEP) Expert Group is relatively insensitive in predicting diabetes33 and cardiovascular disease mortality.34
To estimate the unbiased effects of the experimental breastfeeding promotion intervention, we used an intention-to-treat analysis. The estimates provided by the intention-to-treat analysis are the most robustly estimated expected average effects on the metabolic outcomes of the experimental (breastfeeding promotion) intervention. However, because of the substantial overlap in breastfeeding duration and exclusivity in the 2 randomized groups, these average effects may underestimate the differences in outcome caused by increased duration and exclusivity of breastfeeding. We used instrumental variables analysis to estimate the magnitude of the causal effects on our outcomes attributable to breastfeeding duration and exclusivity, which supported our inference that increased duration and exclusivity of breastfeeding was not inversely associated with the outcomes of interest. The confidence intervals of the instrumental variables analyses were wider than those provided by the intention-to-treat analysis, and thus do not exclude protective estimates reported in observational studies.1–4
PROBIT was carried out in Belarus, because at the time of randomization maternity hospital practices in Belarus and other former Soviet republics were similar to those in North America and Western Europe 30–40 years ago and thus provided a greater potential contrast between intervention and control study sites. Although different in many socioeconomic, cultural and economic respects from North America and Western Europe, Belarus is a relatively developed country with strict hygienic standards, high immunization rates, low incidence of infection, low rates of infant and child mortality, similar types of formula feeds and accessible health care services. Our null results may not generalize to settings with childhood cardiometabolic levels that differ from those in Belarus (characterized, for example, by heavy paternal smoking35 but relatively low rates of child obesity15).
We excluded mothers who were unable to breastfeed and preterm or low birth weight infants, characteristics that may predict later-life metabolic syndrome. Thus, this healthy population at birth may have been at lower overall risk compared with a general population sample.
Over such long-term follow-up, imbalances in determinants of cardiometabolic risk in childhood could have arisen between trial arms. Because any such imbalances would have occurred after randomization and thus could be effects (mediators) of the randomized intervention, they should not be considered as confounding variables and should not be controlled for. Although imbalances can lead to selection bias when follow-up rates are low, over 80% of randomized children in PROBIT were followed-up at 11.5 years, and no important differences in measured baseline characteristics were observed between the two randomized groups. The intervention may have altered the diet after weaning, given observational evidence that later-life food choices are influenced by whether a child was breast- or formula-fed, providing a potential mechanism by which promoting exclusive breastfeeding could exert later effects.36 We observed no effect of the intervention on cardiometabolic risk, however, so if there had been an intervention effect on later diet, this could only explain a false-negative result if the breastfeeding promotion led to an unhealthy diet that obscured (countered) a beneficial effect on cardiometabolic risk factors, which seems unlikely.36
The observed sex-interaction of the intervention on presence/absence of ‘metabolic syndrome’, with boys in the intervention group having a higher risk, was observed in the context of multiple hypothesis tests and may have arisen by chance (Bonferroni corrected p-value for nine tests: 0.01*9 = 0.09). Our assays were measured from dried blood spots; in addition to our validation data, the metabolites presented here have previously been reported to be stable and validly and reliably measured from dried blood spots.22–24
Comparison with other studies
Our findings are in line with a cross-cohort observational study6 comparing results from the ALSPAC cohort in Bristol, UK (a setting with strong socioeconomic patterning of breastfeeding), with those from Pelotas, Brazil, and a meta-analysis of five cohorts in low- and middle-income countries (settings with weak socioeconomic patterning of breastfeeding). In ALSPAC, breastfeeding was strongly associated with lower blood pressure and lower BMI in the directions expected if due to socioeconomic patterning (even after adjustment for measured confounders), but in Pelotas and the low- and middle-income countries, breastfeeding was not strongly associated with these outcomes. Taken together with our experimental evidence, these findings suggest that previously reported inverse associations of breastfeeding duration and/or exclusivity with cardiometabolic risk factors (most of which were set in high-income countries with strong socioeconomic patterning of breastfeeding), are likely to reflect residual confounding.
Implications
Among healthy term infants in Belarus, an intervention to improve the duration and exclusivity of breastfeeding did not alter levels of cardiometabolic risk factors among these children when aged 11.5 years. Nevertheless, the PROBIT trial, the largest randomized trial ever conducted in the field of human lactation, has provided robust evidence that promoting increased duration and exclusivity of breastfeeding prevents gastro-intestinal infections and eczema in infancy13 and improves cognitive development.37 The intervention was designed to increase the duration and exclusivity of breastfeeding among women initiating breastfeeding. Our findings do not, therefore, apply to comparisons of breast- versus formula feeding, the comparisons most frequently described in the literature. Nonetheless, our findings do not support a number of previous studies suggesting inverse associations of the exclusivity and/or duration of breastfeeding with cardiometabolic risk factors.1–4
Supplementary Material
Acknowledgments
We are grateful to the cohort members, their parents and the study pediatricians and auditors who participated so willingly in the study. We thank Sheryl Rifas-Shiman and Ken Kleinman for statistical advice. RMM had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. RMM, MSK, GDS and MWG developed the hypotheses and secured funding; RP, NB, and NS ran the fieldwork under the direction of RMM, MSK, EO and KV. RP and NB were responsible for the database and data cleaning. NG and YF developed the methods for the dried blood spot assays and NG directed the laboratory analyses. RP, TP and JT undertook statistical analyses. RMM wrote the first draft of the paper. All authors critically commented on and approved the final submitted version of the paper. RMM is the guarantor.
Funding Sources: This study was supported by the EuropeanUnion, Early Nutrition Programming Long-term Efficacyand Safety Trials grant no. FOOD-DT-2005-007036; Canadian Institutes of Health Research (MOP - 53155); and the USA National Institutes of Health (R01 HD050758). Dr. Oken was supported by US National Institute of Child Health and Development (K24 HD069408). Professor Martin is executive member of the National Institute for Health Research Bristol Nutrition Biomedical Research Unit. No funding bodies played any role in the design, writing or decision to publish this manuscript.
Footnotes
Conflict of Interest Disclosures: EO has given an invited talk for Nestle Nutrition Institute in 2011 on secular trends in birth weight. RMM gave an invited talk for the Nestle Nutrition Institute in 2010 on the role of the insulin-like growth factor system in growth and chronic disease risk. MSK has received unrelated meeting expenses from the Nestle Nutrition Institute. The remaining authors do not declare any competing interests.
References
- 1.Owen CG, Whincup PH, Gilg JA. Effect of breast feeding in infancy on blood pressure in later life: Systematic review and meta-analysis. BMJ. 2003;327:1189. doi: 10.1136/bmj.327.7425.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Does breastfeeding influence risk of type 2 diabetes in later life? A quantitative analysis of published evidence. Am J Clin Nutr. 2006;84:1043–54. doi: 10.1093/ajcn/84.5.1043. [DOI] [PubMed] [Google Scholar]
- 3.Owen CG, Whincup PH, Kaye SJ, Martin RM, vey Smith G, Cook DG, Bergstrom E, Black S, Wadsworth ME, Fall CH, Freudenheim JL, Nie J, Huxley RR, Kolacek S, Leeson CP, Pearce MS, Raitakari OT, Lisinen I, Viikari JS, Ravelli AC, Rudnicka AR, Strachan DP, Williams SM. Does initial breastfeeding lead to lower blood cholesterol in adult life? A quantitative review of the evidence. Am J Clin Nutr. 2008;88:305–14. doi: 10.1093/ajcn/88.2.305. [DOI] [PubMed] [Google Scholar]
- 4.Evelein AM, Geerts CC, Visseren FL, Bots ML, van der Ent CK, Grobbee DE, Uiterwaal CS. The association between breastfeeding and the cardiovascular system in early childhood. Am J Clin Nutr. 2011;93:712–8. doi: 10.3945/ajcn.110.002980. [DOI] [PubMed] [Google Scholar]
- 5.Martin RM, Davey Smith G, Tilling K, Frankel S, Gunnell D. Breastfeeding and cardiovascular mortality: the Boyd Orr cohort and a systematic review with meta-analysis. Eur Heart J. 2004;25:778–86. doi: 10.1016/j.ehj.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Brion MJ, Lawlor DA, Matijasevich A, Horta B, Anselmi L, Arajo CL, Menezes AM, Victora CG, Davey Smith G. What are the causal effects of breastfeeding on IQ, obesity and blood pressure? Evidence from comparing high-income with middle-income cohorts. Int J Epidemiol. 2011;40:670–80. doi: 10.1093/ije/dyr020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawlor DA, Ebrahim S, Davey Smith G British women’s heart and health study. Socioeconomic position in childhood and adulthood and insulin resistance: cross sectional survey using data from British women’s heart and health study. BMJ. 2002;325:805–7. doi: 10.1136/bmj.325.7368.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCarthy A, Hughes R, Tilling K, Davies D, Smith GD, Ben-Shlomo Y. Birth weight; postnatal, infant, and childhood growth; and obesity in young adulthood: evidence from the Barry Caerphilly Growth Study. Am J Clin Nutr. 2007;86:907–13. doi: 10.1093/ajcn/86.4.907. [DOI] [PubMed] [Google Scholar]
- 9.Tilling K, Davies N, Windmeijer F, Kramer MS, Bogdanovich N, Matush L, Patel R, Davey Smith G, Ben-Shlomo Y, Martin RM for the Promotion of Breastfeeding Intervention Trial (PROBIT) study group. Is infant weight associated with childhood blood pressure? Analysis of the Promotion of Breastfeeding Intervention Trial (PROBIT) cohort. Int J Epidemiol. 2011;40:1227–37. doi: 10.1093/ije/dyr119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kramer MS, Moodie EEM, Dahhou M, Platt RW. Breastfeeding and Infant Size: Evidence of Reverse Causality. Am J Epidemiol. 2011;173:978–83. doi: 10.1093/aje/kwq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lucas A. Programming by early nutrition: an experimental approach. J Nutr. 1998;128:401S–6S. doi: 10.1093/jn/128.2.401S. [DOI] [PubMed] [Google Scholar]
- 12.Kramer MS, Guo T, Platt RW, Shapiro S, Collet JP, Chalmers B, Hodnett E, Sevkovskaya Z, Dzikovich I, Vanilovich I PROBIT Study Group. Breastfeeding and infant growth: biology or bias? Pediatrics. 2002;110:343–7. doi: 10.1542/peds.110.2.343. [DOI] [PubMed] [Google Scholar]
- 13.Kramer MS, Chalmers B, Hodnett ED, Sevkovskaya Z, Dzikovich I, Shapiro S, Collet JP, Vanilovich I, Mezen I, Ducruet T, Shishko G, Zubovich V, Mknuik D, Gluchanina E, Dombrovskiy V, Ustinovitch A, Kot T, Bogdanovich N, Ovchinikova L, Helsing E PROBIT Study Group (Promotion of Breastfeeding Intervention Trial) Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285:413–20. doi: 10.1001/jama.285.4.413. [DOI] [PubMed] [Google Scholar]
- 14.Kramer MS, Matush L, Vanilovich I, Platt RW, Bogdanovich N, Sevkovskaya Z, Dzikovich I, Shishko G, Collet JP, Martin RM, vey Smith G, Gillman MW, Chalmers B, Hodnett E, Shapiro S for the Promotion of Breastfeeding Intervention Trial (PROBIT) Study Group. Effects of prolonged and exclusive breastfeeding on child height, weight, adiposity, and blood pressure at age 6.5 y: evidence from a large randomized trial. Am J Clin Nutr. 2007;86:1717–21. doi: 10.1093/ajcn/86.5.1717. [DOI] [PubMed] [Google Scholar]
- 15.Martin RM, Patel R, Kramer MS, Guthrie LB, Vilchuck K, Bogdanovich N, Sergeichick N, Gusina N, Foo Y, Palmer T, Rifas-Shiman SL, Gillman MW, Davey Smith G, Oken E. Effects of promoting longer-term and exclusive breastfeeding on adiposity and insulin-like growth factor-i at age 11.5 years: A randomized trial. JAMA. 2013;309:1005–13. doi: 10.1001/jama.2013.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO/UNICEF. Protecting, Promoting and Supporting Breastfeeding: The Special Role of Maternity Services. Geneva, Switzerland: World Health Organisation; 1989. [Google Scholar]
- 17.Patel R, Oken E, Bogdanovich N, Matush L, Sevkovskaya ZCB, Hodnett E, Vilchuck K, Kramer MS, Martin RM. Cohort Profile: The Promotion of Breastfeeding Intervention Trial (PROBIT) Int J Epidemiol. 2013 doi: 10.1093/ije/dyt003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawlor DA, Davey Smith G, Ebrahim S, Thompson C, Sattar N. Plasma Adiponectin Levels Are Associated with Insulin Resistance, But Do Not Predict Future Risk of Coronary Heart Disease in Women. J Clin Endocrinol Metab. 2005;90:5677–83. doi: 10.1210/jc.2005-0825. [DOI] [PubMed] [Google Scholar]
- 19.Parish S, Offer A, Clarke R, Hopewell JC, Hill MR, Otvos JD, Armitage J, Collins R. Lipids and Lipoproteins and Risk of Different Vascular Events in the MRC/BHF Heart Protection Study. Circulation. 2012;125:2469–78. doi: 10.1161/CIRCULATIONAHA.111.073684. [DOI] [PubMed] [Google Scholar]
- 20.Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. Diabet Med. 1999;16:442–3. doi: 10.1046/j.1464-5491.1999.00059.x. [DOI] [PubMed] [Google Scholar]
- 21.Guthrie LB, Oken E, Sterne JAC, Gillman MW, Patel R, Vilchuck K, Bogdanovich N, Kramer MS, Martin RM. Ongoing monitoring of data clustering in multicenter studies. BMC Med Res Methodol. 2012;12 doi: 10.1186/1471-2288-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin RM, Patel R, Zinovik A, Kramer MS, Oken E, Vilchuck K, Bogdanovich N, Sergeichick N, Gunnarsson R, Grufman L, Foo Y, Gusina N. Filter Paper Blood Spot Enzyme Linked Immunoassay for Insulin and Application in the Evaluation of Determinants of Child Insulin Resistance. PLoS One. 2012;7:e46752. doi: 10.1371/journal.pone.0046752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong N, Beeso J, Sherwood RA, Peters TJ. An improved method for the quantitation of apolipoprotein B and apolipoprotein A-1 in dried blood spots. Ann Clin Biochem. 1995;32:175–80. doi: 10.1177/000456329503200207. [DOI] [PubMed] [Google Scholar]
- 24.Martin RM, Patel R, Oken E, Thompson J, Zinovik A, Kramer MS, Vilchuck K, Bogdanovich N, Sergeichick N, Foo Y, Gusina N. Filter Paper Blood Spot Enzyme Linked Immunoassay for Adiponectin and Application in the Evaluation of Determinants of Child Insulin Sensitivity. PLoS One. 2013;8:e71315. doi: 10.1371/journal.pone.0071315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradbury WH, Forrest AR. Screening for hypercholesterolemia by use of blood spotted on filter paper. Clin Chem. 1985;31:648–9. [PubMed] [Google Scholar]
- 26.Chen W, Bao W, Begum S, Elkasabany A, Srinivasan SR, Berenson GS. Age-related patterns of the clustering of cardiovascular risk variables of syndrome X from childhood to young adulthood in a population made up of black and white subjects: the Bogalusa Heart Study. Diabetes. 2000;49:1042–8. doi: 10.2337/diabetes.49.6.1042. [DOI] [PubMed] [Google Scholar]
- 27.US Department of Health and Human Services. The Fourth Report on the Diagnosis, Evaluation and Treatment of High Blood Pressure in Children and Adolescents. National Institutes of Health. National Heart, Lung, and Blood Institute; 2005. [Google Scholar]
- 28.Bachorik PS, Lovejoy KL, Carroll MD, Johnson CL. Apolipoprotein B and AI distributions in the United States, 1988–1991: results of the National Health and Nutrition Examination Survey III (NHANES III) Clin Chem. 1997;43:2364–78. [PubMed] [Google Scholar]
- 29.Fernandez JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr. 2004;145:439–44. doi: 10.1016/j.jpeds.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 30.Rubin DB. Multiple Imputation for Non Response In Surveys. New York: J. Wiley & Sons; 1987. [Google Scholar]
- 31.Angrist JD, Imbens GW, Rubin DB. Identification of causal effects using instrumental variables. J Am Stat Assoc. 1996;91:444–72. [Google Scholar]
- 32.Palmer TM, Sterne JAC, Harbord RM, Lawlor DA, Sheehan NA, Meng S, Granell R, Smith GD, Didelez V. Instrumental Variable Estimation of Causal Risk Ratios and Causal Odds Ratios in Mendelian Randomization Analyses. Am J Epidemiol. 2011;173:1392–403. doi: 10.1093/aje/kwr026. [DOI] [PubMed] [Google Scholar]
- 33.Laaksonen DE, Lakka HM, Niskanen LK, Kaplan GA, Salonen JT, Lakka TA. Metabolic syndrome and development of diabetes mellitus: application and validation of recently suggested definitions of the metabolic syndrome in a prospective cohort study. Am J Epidemiol. 2002;156:1070–7. doi: 10.1093/aje/kwf145. [DOI] [PubMed] [Google Scholar]
- 34.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–16. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 35.Yang S, Decker A, Kramer M. Exposure to parental smoking and child growth and development: a cohort study. BMC Pediatr. 2013;13:104. doi: 10.1186/1471-2431-13-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson S, Ntani G, Simmonds S, Syddall H, Dennison E, Sayer AA, Barker D, Cooper C. Type of milk feeding in infancy and health behaviours in adult life: findings from the Hertfordshire Cohort Study. Br J Nutr. 2013;109:1114–22. doi: 10.1017/S000711451200267X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kramer MS, Aboud F, Mironova E, Vanilovich I, Platt RW, Matush L, Igumnov S, Fombonne E, Bogdanovich N, Ducruet T, Collet JP, Chalmers B, Hodnett E, Davidovsky S, Skugarevsky O, Trofimovich O, Kozlova L, Shapiro S for the Promotion of Breastfeeding Intervention Trial (PROBIT) Study Group. Breastfeeding and Child Cognitive Development: New Evidence From a Large Randomized Trial. Arch Gen Psychiatry. 2008;65:578–84. doi: 10.1001/archpsyc.65.5.578. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.