Abstract
Schizophrenia is a devastating neurodevelopmental disorder that affects approximately 1% of the population. Reduced expression of the 67-kD a protein isoform of glutamic acid decarboxylase (GAD67), is a hallmark of the disease, and is encoded by the GAD1 gene. In schizophrenia, GAD67 downregulation occurs in multiple interneuronal subpopulations, including the cannabinoid receptor type 1 positive (CNR1+) cells, but the functional consequences of these disturbances are not well understood. To investigate the role of the CNR1-positive GABA-ergic interneurons in behavioral and molecular processes, we employed a novel, miRNA-mediated transgenic mouse approach. We silenced the Gad1 transcript using a miRNA engineered to specifically target Gad1 mRNA under the control of Cnr1 bacterial artificial chromosome. Behavioral characterization of our transgenic mice showed elevated and persistent conditioned fear associated with an auditory cue and a significantly altered response to an amphetamine challenge. These deficits could not be attributed to sensory deficits or changes in baseline learning and memory. Furthermore, HPLC analyses revealed that Cnr1/Gad1 mice have enhanced serotonin levels, but not dopamine levels in response to amphetamine. Our findings demonstrate that dysfunction of a small subset of interneurons can have a profound effect on behavior and that the GABA-ergic, monoamine, and cannabinoid systems are functionally interconnected. The results also suggest that understanding the function of various interneuronal subclasses might be essential to develop knowledge-based treatment strategies for various mental disorders including schizophrenia and substance abuse.
Keywords: Cnr1, Gad1, GAD67, transgenic, miRNA, dopamine, serotonin, amphetamine, behavior, interneuron, schizophrenia
INTRODUCTION
Genetic and pharmacological studies have demonstrated that endocannabinoids (eCBs) and drugs targeting the eCB system can affect neuronal development and differentiation (Galve-Roperh et al., 2008; Harkany et al., 2007). Several recent epidemiological studies have associated increased psychotic episodes and a higher probability to develop schizophrenia as a result of adolescent cannabis abuse (Henquet et al., 2005; Matheson et al., 2011; Muller-Vahl and Emrich, 2008; van Os et al., 2002; Veen et al., 2004) Furthermore, cannabis abuse can also induce acute psychosis (Morrison et al., 2009).
The eCB system regulates emotion, stress, memory and cognition. The eCBs N-arachidonylethanolamine (AEA) and 2-arachidonoylglycerol (2-AG) are synthesized and released postsynaptically to act as retrograde messengers at presynaptic cannabinoid receptor 1 (CNR1, also known as CB1R or CB1A) located on both glutamatergic and gamma-aminobutyric acid (GABA)-ergic axon terminals (Katona et al., 2001; Katona et al., 2006). They suppress neurotransmitter release (Wilson et al., 2001) and are very effective modulators of synaptic plasticity (Katona and Freund, 2012). In the adult brain, CNR1 is expressed at high levels in the neocortex, hippocampus, striatum, amygdala and cerebellum (Pettit et al., 1998; Tsou et al., 1998). On GABAergic interneurons, CNR1 is primarily expressed on basket cells and represent a smaller subpopulation of cholecystokinin (CCK) expressing interneurons (Eggan et al., 2010a; Katona et al., 2001; Katona et al., 2000). They are slowly adapting and are coupled to 3–8 Hz theta oscillations (Klausberger and Somogyi, 2008).
Previous animal studies have focused on genetic inactivation of Cnr1 receptors. These animals showed increased mortality, hypoactivity, hypoalgesia (Zimmer et al., 1999), as well as elevated arousal/anxiety that might promote enhanced social discrimination memory (Litvin et al., 2013; Martin et al., 2002). However, the majority of these experiments were not able to differentiate between the CNR1 effects mediated through glutamatergic and GABAergic terminals. To directly address the role of inhibitory interneurons in endocannabinoid circuitry, we silenced Gad1, the gene encoding the primary enzyme responsible for producing GABA in the brain, using a Cnr1 bacterial artificial chromosome (BAC) driven system in transgenic mice. After validating the expression specificity and efficacy, we performed comprehensive behavioral and neurochemical assessments of these animals. These experiments demonstrate the importance of Cnr1+ GABAergic interneuron population and provide insight into the specific role of cannabinoid systems in inhibitory circuitry.
MATERIALS AND METHODS
Mouse Generation
RP24-370M5 BAC, containing the mouse cannabinoid receptor1 (mCnr1) locus (Chr4: 33,837,634 – 33,989,366, NCBI Build 38.1), was purchased from the BACPAC Resource at the Children’s Hospital of Oakland Research Institute (http://bacpac.chori.org/). The mCnr1 gene itself is mapped at Chr4: 33,924,632 – 33,948,831, + strand. The BAC was isolated from the original DH10B E. coli strain via standard alkaline lysis protocol (available upon request) and transformed into EL250 E. coli cells (kind gift of Dr. Neil Copeland, NCI). EL250 cells were instrumental for our BAC modifications as they are capable heat-inducible expression of recombination proteins and arabinose-inducible FLP recombination. The presence of the mCnr1 locus in RP24-370M5 was verified by restriction enzyme digest mapping. A BAC targeting construct was generated using previously engineered targeting constructs for the mNPY gene, described in detail by (Garbett et al., 2010). In essence, the mNPY homology arms were swapped with the mCnr1 homology arms in pSTBlue-1 plasmid vector (Novagen, Madison). The mCnr1 targeting construct carried Cnr1 5′ (205 bp) and 3′ (260 bp) homology arms, surrounding eGFP, β-globin minigene and an FRT-flanked neomycin resistance cassette. The β-globin minigene contained a Gad1 targeted miRNA in an intronic location (allowing the in vivo release of functional miRNA) which effectively reduced the GAD67 protein to undetectable levels in cell cultures (Garbett et al., 2010). Digestion with EcoRI released the targeting fragment from the base plasmid; the targeting fragment was then used for homologous recombination into the mCnr1 containing BAC RP24-370M5, heat shock of the EL250 cells was used to induce homologous recombination. The resulting BACs were screened by PCR and confirmed with restriction mapping and sequence analysis for correct modifications. Finally, the E. coli strain containing the modified BAC was treated with arabinose to induce the expression of FLP recombinase, which removed the FRT-flanked neomycin resistance cassette. Proper recombination was confirmed with restriction mapping and sequence analysis of the region of interest. The modified RP24-370M5 BAC was isolated with alkaline lysis and purified with Sepharose CL-4B chromatography as described previously (Gong and Yang, 2005). Transgenic mice were generated by injection of circular modified BAC into fertilized C57Bl/6 mouse oocytes by the University of California Irvine Transgenic Mouse Facility). Transgenic founder mice were identified by PCR using construct-specific primer pairs.
Mouse Genotyping
Tail samples of 2 mm were taken at P21. The tissue was digested over night at 55C in 245ul Direct PCR (Tail) (Viagen Biotech, Cat# 102-T) and 5ul Proteinase K (Clontech, Cat# 740506), then incubated at 85C for 45 min. Primers used to genotype the samples had the following sequences: ACGACGGCAACTACAAGACC (GFP.k.geno.F1) and ACCTTGATGCCGTTCTTCTG (GFP.k.geno.R1). The annealing temperature for these primers is 60°C (30 sec), and the amplification yields a product size of 184 base pairs.
Immunohistochemistry
For immunolocation studies, animals were deeply anesthetized with isoflurane and transcardially perfused with ice cold 1 × phosphate buffer (PB) solution followed by 4% paraformaldehyde in 0.1 M PB (pH 7.4) at room temperature. All procedures were performed in accordance with the guidelines of the American Association for Laboratory Animal Science and the Vanderbilt University Institutional Animal Care. Brains were then removed and postfixed for 4 h at room temperature in 4% paraformaldehyde. Coronal sections, 40-μm thick, were prepared with a vibratome (VT 1000S, Leica Microsystems, Bannockburn, IL, USA), and then washed several times in 0.1 M PB.
Brain sections were incubated for 1 h in 10% normal donkey serum in 0.1 m PB (pH 7.4). Immunostaining for eGFP was performed either with a rabbit anti-GFP (Invitrogen; 1:2000) or chicken anti-GFP (Abcam; 1:2000). Immunostaining for CNR1 was performed using 1:2000 dilution of affinity-purified polyclonal guinea pig anti-CNR1 antibody raised against the 31aa C-terminus of the mouse CNR1 (Frontier Science Co. Ltd, Hokkaido, Japan). For PV immunostaining, a 1:5000 dilution of rabbit anti-parvalbumin antiserum (Swant Ltd., Switzerland) was used. For GAD67 immunostaining, sections were pre-incubated with 70 μg ml−1 of a monovalent Fab′ fragment of donkey anti-mouse immunoglobulin G (Jackson Immunoresearch, West Grove, PA, USA) to block endogenous mouse immunoglobulins before proceeding with the standard protocol for immunolabeling with mouse anti-GAD67 (Millipore; 1:2000). The following secondary antibodies (Jackson Immunoresearch, West Grove, PA, USA) were used for fluorescence detection: donkey anti-guinea pig Cy3, donkey anti-rabbit AMCA, donkey anti-chicken DyLight488 and donkey anti-mouse Cy3 (all diluted 1:250). All sections were incubated with primary antibodies for 72 h at 4 °C, washed extensively and incubated in secondary antibodies for 3 h at room temperature. Immunolabeled sections were mounted in ProLong® Gold Antifade Reagent (Life Technologies, NY, USA) and examined using an Olympus BX51 fluorescence microscope with DP21 digital camera (Olympus Corporation, Tokyo, Japan). Images were stored and analyzed using ImageJ for Windows scientific imaging software (NIH, Bethesda, MD, United States) with Microscopy plugins (http://rsbweb.nih.gov/ij/). Brightness and contrast were adjusted for the whole image using Adobe Photoshop CS5 software (Adobe Systems, Inc, San Jose, CA, USA).
Animals
All animal procedures were performed according to Vanderbilt University Institutional Animal Care and Use Committee approved procedures. Male C57Bl/6J mice (3–5 months of age at start of testing), were used for all experiments. All animals were housed in groups of two to five. Food and water were available ad libitum. All mice were kept on a 12-hr light-dark cycle.
Behavioral experiments
Behavioral testing was performed in the Vanderbilt Murine Neurobehavioral Laboratory. Adult male mice were handled for 5 days prior to the beginning of the battery. Prior to each testing session, mice were brought from the animal housing room into an anteroom outside each testing room and acclimated for 1 hour under red light. Consecutive tests were at least 24 hours apart. Experimenters were blinded to genotypes. All equipment was cleaned with Vimoba solution between animals to reduce odor contamination. Mice were evaluated behaviorally on the following tests described below in greater detail: (i) 10 min open field exploration, (ii) Irwin screen and battery of sensorimotor measures (grip strength, rotor rod and swim speed), (iii) fear conditioning, (iv) 0-maze and y-maze, (v) prepulse inhibition (PPI), (vi) social interaction and social odor investigation, and (vii) response to CNR1 agonist and amphetamine (AMPH) challenge.
(i) Open field
Mice were placed in the center of a white plastic box (50cm × 50cm × 40cm) lighting in the room was 600 lux and allowed to explore for 10min on two consecutive days. Video was recorded and locomotor activity was analyzed by ANY-maze (Stoelting Co., Wood Dale, IL).
(ii) Irwin screen/sensorimotor battery
A modified Irwin screen was used to assess general health and neuromuscular function.
(iii) Fear conditioning
Analysis was performed using the 3 day protocol outlined by (Smith et al., 2007) with the day one habituation phase for 12 min, day two training phase with 6 tone- shock pairs, and day three context testing phase in the familiar environment with no tones over a 15 min period, followed by novel environment for 3 min, and concluded with 10 cue trials separated by 80 second intervals. We tested a main effect of trial for repeated measures ANOVA done on the data for each group. Extinction was defined as a statistically significant reduction in freezing across subsequent cue presentations in the absence of the foot shock. Thus, the null-hypothesis was that neither the Tg or the Wt animals will change their freezing behavior over the extinction experiment.
(iv) 0-maze and y-maze
A white plastic zero maze apparatus was placed in the center of a brightly lit room (600 lux), at an elevation of 60 cm above the floor. The runway was 5 cm wide and divided into four quadrants: two open to the room and two closed with 15cm high walls. Mice were placed in the center of one open area and allowed to explore freely for a single 6min session. Video was recorded and time spent in each zone and locomotor activity were analyzed by ANY-maze (Stoelting Co., Wood Dale, IL). For Y-maze mice were placed into a clear plastic y-maze (35cm × 5cm) with a clear plastic top to prevent escape and placed on a table in the center of a room lit to 25 luv and allowed to explore freely for 5min. Video was recorded and ANY-maze software (Stoelting Co., Wood Dale, IL) set to score an arm entry when >90% of the mouse’s body moved into the arm. Alternations were defined as entering each of the three arms without entering a previously entered arm in a sliding window of three entries. Percent alternation was determined by calculating the number of alternations out of a total number of possible alternations for each mouse (= total arm entries − 2) (Drew et al., 1973).
(v) Prepulse inhibition
For acoustic startle reflex, sound-attenuating acoustic startle was performed as described before (Galici et al., 2005). Briefly, for each animal the session started with a 5-min acclimatization period, during which a 65dB background noise was continuously present. Every session included a total of 54 trials. Six different trial types were randomly assigned and delivered (every 15–20s on average) for nine times throughout the session: 40ms broadband 120dB burst (pulse only), 65dB background noise (noise only), and 20ms prepulse of 70, 76, 82, and 88 dB followed by 100ms of 120dB pulse. Responses were analyzed using MED Startle Reflex software (MED Associates Inc., St Albans, VT).
(vi) Social interaction and odor recognition
In this task a three-chambered social interaction box was used as previously described (Yang et al., 2011) with minor modifications. The three chambered, clear plastic box (4″ sliding gates separating the 7″ × 9″ chambers) was placed on a table in the center of the room. This test involved three 10min trials. Naïve adult male C57Bl/6 wild type mice were used as social stimulus. In the first trial, mice explored all three empty chambers freely. In the second trial, two wire pencil cups were placed in the side chambers. In one cup, a novel mouse (social stimulus) was placed while the second cup remained empty. In the third trial, a second novel mouse was placed in the cup that had previously been empty. Cup and social stimuli order were counterbalanced between groups. ANY-maze software (Stoelting Co., Wood Dale, IL) tracked the head position of the test mouse and scored interaction time when the head was <1cm from the cups. Odor recognition experiments were performed as previously described (Yang and Crawley, 2009). Nonsocial odors included water, almond, and orange extract (McCormick, Baltimore, MD), while social odors were swabs of dirty cages housing non-littermate male mice.
(vii) Drug challenge experiments
Locomotor and exploratory activity was evaluated over a 90 min period by placing individual mice into transparent (30cm×30cm×20cm) polystyrene enclosures. Each enclosure was surrounded by a frame containing a 4 × 8 matrix of photocell pairs, the output of which was fed to an online computer, using MED Activity software (MED Associates Inc., St Albans, VT) Total amount of ambulation (whole body movements) was assessed using MED Activity Monitor. Mice were allowed to habituate inside the chamber for 15 min. AMPH was dissolved in sterile saline solution containing 0.9% NaCl. Transgenic mice and wild-type control littermates received single intraperitoneal injections of AMPH (3 mg/kg; Sigma, St Louis, MO, USA) and immediately placed in beam-break chambers for evaluation. For the CNR1/CNR2-receptor agonist challenge mice received intraperitoneal injections of CP55940 at a dose of 25 mg/kg dissolved in ethanol:cremophor:saline (1 : 1 : 18; cremophor; Sigma Aldrich) immediately prior to testing. Animals were injected with AMPH 75 minutes or CP55940 30 minutes prior to testing.
Statistical analyses
All behavioral experiments were analyzed in a blinded fashion using Any-maze or Graph pad software (Stoelting Co., Wood Dale, IL & GraphPad Software, Inc., La Jolla, CA.). ANOVA models were used to analyze the behavioral data, and contained one between-subjects variable, such as “genotype” and “trial number” (e.g., Cnr1/Gad1 mice versus control littermates), and at least one within-subjects variable, such as blocks of trials, or test sessions. The appropriateness of ANOVA models was evaluated by considering the distributional properties of the variables studied and by the adequacy of the homogeneity of variance assumption. The Greenhouse-Geisser (or Huynh-Feldt) adjustment was used for all within-subjects effects containing more than two levels in order to protect against violations of the sphericity/compound symmetry assumptions when repeated measures ANOVAs are used. Multivariate analyses (e.g., Hotelling-Lawley trace statistic) were also utilized for this purpose. Bonferroni correction or Tukey’s test was used as appropriate to maintain prescribed alpha levels (e.g., p=0.05) when multiple comparisons were conducted. For studies involving several behavioral tests, we also performed principle component analyses (PCA) within a given study to control against alpha inflation. Briefly, behavioral measures were converted to z-scores, and PCA performed on a covariance matrix of these data utilizing a varimax rotation. Natural breaks occurring in the scree-plot analyses were used to determine the number of components, and this was employed for subsequent analyses.
HPLC of Monoamines
Monoamine levels were determined 30 min after IP injection of CP55940 (25mg/kg) and 35 min after IP injection of AMPH (3mg/kg). The left striata of transgenic mice and control littermates were rapidly dissected, immediately snap frozen in liquid nitrogen and kept at −80°C for high-performance liquid chromatography (HPLC) analysis of dopamine, serotonin, noradrenaline, adrenaline, 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), and 3-methoxytyramine (3-MT) levels. Samples were diluted 1:20 in HPLC buffer (0.1 M NaH2PO4, 0.25 g/l heptanesulfonic acid, 0.08 g/l EDTA, 6% methanol, pH 2.5), separated on a catecholamine ESA HR-80 column (Chelmsford, MA, USA), using the same HPLC buffer for the mobile phase, and analyzed by electrochemical detection. Samples were analyzed in triplicate by the Vanderbilt Kennedy Center & Vanderbilt Brain Institute Neurochemistry Core Laboratory.
RESULTS
Validation of Tg(Cnr1-eGFP/miRNA:Gad1) transgenic mouse line
The generation of a hybrid reporter/miRNA knockdown transgenic mouse Tg(Cnr1-eGFP/miRNA:Gad1) (abbreviated as Cnr1/Gad1 in further text) was accomplished using a multi-step cloning process described in detail previously (Garbett et al., 2010). In creating the Cnr1/Gad1 transgenic mouse line, a centrally located minigene containing a truncated part of the first exon, intervening intron, and second exon of the β-globin gene provided the necessary sequence elements for expression modulation. This non-coding, but transcribed sequence served as a ‘carrier’ for a synthetic miRNA directed against the Gad1 mRNA. 5′ to the silencing minigene, an eGFP sequence was inserted in frame with the start codon of the BAC-encoded Cnr1 gene, thus eliminating Cnr1 expression from the transgene itself: the Cnr1 BAC served only as a driver for the construct. We flanked the minigene with LoxP sites to allow excision of the gene-silencing of the construct while retaining its eGFP expression for future experiments.
The targeting construct was activated by endogenous Cnr1 transcription-controlling elements through the Cnr1 BAC, which triggered the transcription of the Gfp marker, the beta-globin-embedded Gad1 miRNA and the SV40 polyadenylation signal sequence (Figure 1A). Of these, the Gfp sequence was “in-frame” and translated, marking the targeted cells with green fluorescence. In contrast, the transcribed beta-globin/Gad1 was not translated due to the truncated exons, but was spliced, and the Gad1 miRNA was subsequently liberated from the intron by the Dicer-Drosha cascade (Wilson and Doudna, 2013). Next, the Gad1 miRNA targeted the endogenous Gad1 mRNA, and degraded it through the RNA-induced silencing complex (Hammond, 2005). The end result of the process was that that CNR1+ cells were GFP-fluorescent, with diminished levels of GAD67 expression.
Figure 1. Generation and validation of Cnr1/Gad1 transgenic mouse line.

(A) Schematic representation of the construct used to generate the Cnr1/Gad1 transgenic mouse line. A centrally located minigene, containing a truncated part of the first exon, intervening intron, and second exon of the β-globin gene were the non-coding, but transcribed backbone which served as a ‘carrier’ for a synthetic miRNA directed against the Gad1 mRNA. The transcribed-translated part of the construct was the Gfp sequence inserted in frame with the start codon of the BAC-encoded Cnr1 gene, marking the cells in which the construct was activated. (B–C–D) Immunohistochemical assessment of Tg mice. (B) Triple immunohistochemistry showing the near-perfect co-localization of GFP with CNR1 in the hippocampus, stratum, substantia nigra, amygdala and cerebellum. Larger images on the left denote merged CNR1-GFP-DAPI triple-labeled images, small image stripes on the right denote enlarged part of the image on the left with signge-channel label (CNR1, GFP, DAPI and combined). DAPI co-labeling was performed to reveal overall tissue structure. Note that the GFP (produced from our transgene) and the co-expressed CNR1 (from the endogenous source) showed a staining pattern that is consistent with the previously described CNR1 expression across the rodent brain(Pettit et al., 1998; Tsou et al., 1998), suggesting that our transgene achieved the right spatial targeting. (C–D) To examine the cell-type specificity of the construct effects, we performed GFP-GAD67-PV triple-labeling of neocortical (C) and hippocampal (D) tissue sections. Larger pictures represent merged triple-labeled images, smaller monochrome images denote single channel fluorescence, higher magnification images (PV, GAD67 and GFP) originating from the larger picture (denoted by the white rectangle). As expected, the GFP expressing neurons (thus CNR1-expressing neurons) lacked GAD67 expression (yellow circles), suggesting that we achieved our goal of eliminating GAD67 production in the CNR1+ neuronal population. In contrast, GAD67 expression in the parvalbumin+ interneurons (blue circles) was not affected (retained both GAD67-PV immunostaining), suggesting that our construct specifically affected the CNR1-GAD67 subpopulation of interneurons. Abbreviations. Neocortex: Roman numerals denote neocortical laminae; WM - white matter; Hippocampus: so – stratum oriens, sp - stratum pyramidale, sr - stratum radiatum, slm - stratum lacunosum moleculare; Striatum: LGP - lateral globus pallidus ; CPu - caudate putamen; Substantia nigra: SNR - pars reticulata, SNC - pars compacta ; Amygdala: La – lateral amygdaloid nucleus, BLA - basolateral amygdaloid nucleus, ec - external capsule, En – endopiriform nucleus; Cerebellum: ml – molecular layer, plc – Purkinje cell layer, igl - internal granule layer. Calibration bar=100μm.
Validation of the basic construct and its specificity in transgenic animals was already presented for the neuropeptide Y BAC driven Npy/Gad1 mice, which used the same Gad1 miRNA silencing construct (Garbett et al., 2010). While our Cnr1/Gad1 construct was active in all CNR1-expression cells, it was designed to have functional consequences only in the CNR1/GAD67 co-expressing neurons. To prove that the construct performed as expected, we performed a series of standard validation experiments {Garbett, 2010 #20;MJ Schmidt, 2013 #150} using double- and triple-immunohistochemistry (Figure 1B–D). In brain sections from transgenic animals, strong eGFP fluorescence was detected even without immunostaining, indicating that the construct was active across multiple brain regions. In a regional survey of the brains derived from Cnr1/Gad1 transgenic animals, we found that eGFP expression correlated with previous descriptions of CNR1 distribution in wild-type rodent brains {Pettit, 1998 #99;Tsou, 1998 #97}. Furthermore, eGFP and CNR1 expression were co-localized across all of the investigated brain regions (Figure 1B) (cortex, hippocampus, substantia nigra, amygdala and cerebellum), with strong labeling of neuronal somata and synaptic terminals. Our transgene achieved its functional purpose and acted only on its intended interneuronal targets: GAD67 was not detected in CNR1+ interneurons, but was normally expressed in the parvalbumin-positive (PV+) subpopulation (Figure 1C–D). Furthermore, in our previous experiments using the same miRNA directed against Gad1, we did not observe any obvious off-target effects on glutamatergic projection neurons (Garbett et al., 2010).
After the molecular/cellular validation of the construct expression and effect on GAD67 expression, we performed a panel of standard behavioral tests on our mice {MJ Schmidt, 2013 #150}, which included assessment of open field exploration, Irwin screen and battery of sensorimotor measures (grip strength, rotor rod and swim speed), fear conditioning, 0-maze and y-maze, prepulse inhibition (PPI), social interaction and social odor investigation. Furthermore, we also investigated response to CNR1 agonist and amphetamine challenge.
Cnr1/Gad1 transgenic mice show altered amygdala-based memory and social interaction
A cohort of littermates composed of 13 WT and 11 Cnr1/Gad1 transgenic (Tg) male mice were assayed in a broad battery of behavioral tests. The transgenic mice showed no obvious neurological phenotype and performed similarly to the control littermates on the basic neurological tests including Irwin Screen, grip strength, rotorod, open field, y-maze and 0 maze.
Fear conditioning
In the training phase (Figure 2A), 6 cue learning trials were performed with conditioned stimulus (CS) (audible tone) presented 18 sec before delivery of the unconditioned stimulus (US) (i.e., foot shock). During this phase, both Wt and Tg animals learned at the same rate and spent comparable amount of time freezing (Figure 2B).
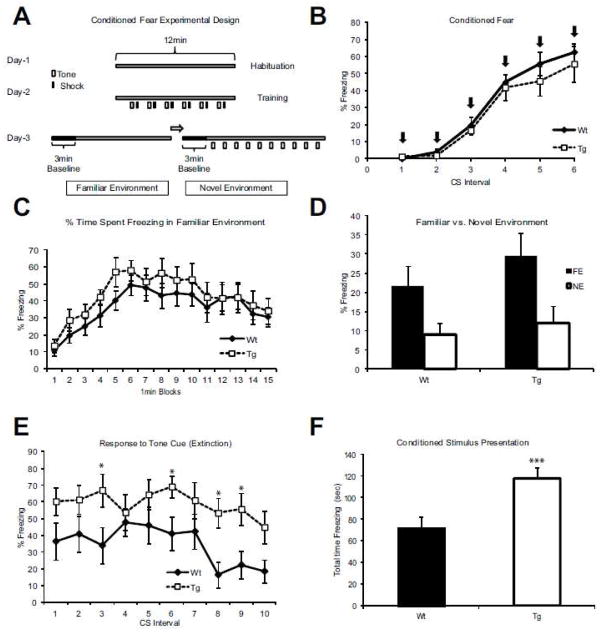
Figure 2. Altered fear attenuation in Cnr1/Gad1 transgenic mice.
(A) Experimental design. The experiment was performed over 3 days (Smith et al., 2007). Day one: habituation phase for 12 min. Day two: training phase with 6 tone- shock pairs. Day three: context testing phase in the familiar environment with no tones over a 15 min period, followed by novel environment for 3 min, and concluded with 10 cue trials separated by 80 second intervals. (B) After a day of environment habituation on day two mice were conditioned to associate a tone followed by a foot shock. Over the course of 6 shock pairings (X-axis) both the Wt and the Tg learned the task equally well judged by the % of freezing response (Y-axis). Arrows denote the presentation of the conditioning stimulus. (C) Percent of time spent freezing in familiar environment was comparable between Wt and Tg mice. X axis denotes 1-minute intervals following the presentation of the condition stimulus (tone), Y-axis denotes % of freezing for each of the 15 intervals. None of the data points showed a significant difference between the Wt and Tg animals. (D) To assess hippocampus-dependent associative memory, we measured freezing % in the same familiar environment (FE) where training occurred, compared to a new novel environment (NE). This type of memory appears to be unaffected in our Tg line compared to Wt littermates. (E) In the novel environment, where a tone (but no shock) is presented, Wt mice display relatively little fear/freezing (Y-axis). In contrast, Tg are still freezing almost as much as at the end of the training day even after 10 trials with no shock. Asterisk denotes statistically significant differences for the cycles (p<0.05). (F) Total time spent freezing in response to tone alone on day 3, showing a significant increase in the time spent freezing in Tg animals in comparison to Wt littermates (p =0.001) (F-value =21.8). n= 13 Wt; n=11 Tg in all panels.
Contextual and cue-based memory assessment
Tg and WT animals showed no difference in freezing behavior in the familiar environment (Figure 2C) or novel environment. On the day after training, contextual memory was assessed by comparing freezing behavior in the environment associated with the unconditioned stimulus (i.e., the familiar environment [FE] versus the novel environment [NE]). This form of memory has been closely associated the hippocampal function (Phillips and LeDoux, 1992) and was unperturbed in our Tg mouse line. Both Wt and Tg mice show significantly more freezing in the FE than in the NE (p= 0.001) (Figure 2D). However, when assessing cue-based memory, which has a strong amygdala-dependent component (Phillips and LeDoux, 1992), Tg mice showed an increased fear retention over 10 cue trials (Figure 2E) (p<0.001), compared to Wt animals. In addition, Wt animals showed normal extinction across trials (p=0.02), while, Tg mice did not extinguish their freezing behavior (p=0.12). Thus, the null-hypothesis (which stated that neither the Tg or the Wt animals will change their freezing behavior over the extinction experiment) was rejected. When comparing total time spent freezing in response to the conditioning stimuli, Tg mice showed significant enhancement of cue based memory (p= 0.001) (Figure 2F). Taken together, these results revealed a selective disruption of cue based memory extinction, even while learning and contextual memory remained intact.
Motor tests
One concern when evaluating behavior performance is that almost all measures of cognitive performance rely on motor output, and sensory motor deficits could be incorrectly identified as learning or memory perturbation. To rule out this potential confound, we performed a battery of sensory motor tests to evaluate physical ability and sensation. No significant differences in body weight or the Irwin Screen were observed (data not shown). Swim speed, a measure of activity and physical ability, also did not distinguish the Tg and Wt littermates (Supplemental Figure 1A). Accelerating rotarod, which tests balance and motor coordination (Supplemental Figure 1B), and grip strength, which tests neuromuscular function, (Supplemental Figure 1C) were also unaltered. Based on these tests, we can conclude that the Tg mice have no discernible motor deficits that would confound the interpretation of other higher-order cognitive tests. Furthermore, we did not observe seizures in our Tg mice (data not shown).
Exploratory behavior and cognitive tests
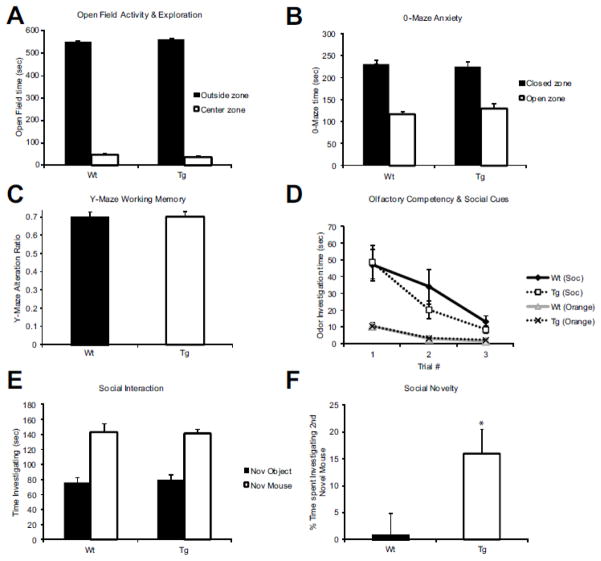
Next we investigated basic exploratory behavior, anxiety, working memory, and olfactory sensory function. In a brightly lit open field paradigm, both Wt and Tg showed not only the same level of exploration (i.e., no hyper- or hypoactivity) (data not shown), but also the same preference for periphery vs. the central region of the field (p= 0.001) (Figure 3A). In 0-maze exploration, Tg and Wt mice showed the same preference for closed vs. open arms, suggesting that their baseline anxiety is similar (Figure 3B). Furthermore, assessing working memory with the Y-maze spontaneous alternation method, we found that Wt and Tg both preformed at the level previously reported as the standard for C57B6 mice (Figure 3C). Olfaction is the primary sensory modality for a mouse and often drives its behavior to novel environments, objects, and social cues (Misslin and Ropartz, 1981). To assess whether our Tg mice have normal olfactory sensation, we presented them with 3 distinct odors on cotton swabs: water, almond, and orange, as well as two cotton swabs taken from conspecific cage bedding (data not shown). For both a simple odor (orange) and a socially relevant one (novel male bedding) Wt and Tg habituated over time and showed significantly greater investigation of the socially relevant scent (p= 0.001) (Figure 3D). These data suggest that inactivation of GAD67 in CNR1 expressing interneurons does not lead to sweeping changes in exploration, memory, or anxiety. This, combined with normal locomotor activity and sensory perception, suggests that the differences that we uncovered are the result of specific cognitive defects.
Figure 3. Baseline learning, memory, and anxiety are normal in Cnr1/Gad1 transgenic mice.
(A) Comparison of time spent in the center or periphery of an open field box (Y-axis) as a measure of exploration. Tg mice show normal preference for the periphery that is similar to that seen in Wt mice. (B) Zero-maze evacuation of anxiety also shows that Tg and Wt mice both preferred to spend time in closed arms over open arm. (C) Y-maze evaluation of hippocampus-dependent memory shows that Tg and Wt mice were indistinguishable. (D) Assessment of order recognition revealed that both Wt and Tg spent less time investigating non-socially relevant odor (orange extract) then socially relevant odor (bedding from unfamiliar male cage) that decreased over time. (E) Social interaction assessment was measured by time spent investigating a novel mouse. Both Tg and Wt mice spent significantly greater time investigating a novel mouse than a novel object (an empty pencil cup). (F) When a novel object was replaced with a novel mouse while simultaneously presenting the now familiar mouse, Tg mice showed a significant preference (p= 0.01) for increased interaction with the novel mouse over the Wt littermates. n= 13 Wt; n=11 Tg in all panels.
Social interaction
In a social interaction test utilizing a 3 chamber box, mice were first allowed to explore the box and habituate to the new environment. Then, an empty pencil cup was placed in one side and a pencil cup housing a novel mouse was placed in the other. Interaction was scored when the head of the test mouse was ≤ 2cm from either cup. Both Wt and Tg demonstrated a strong preference for novel mouse over novel object (p= 0.001) (Figure 3E). However, when a second novel mouse cup was presented in place of the novel object cup, Tg mice showed a prolonged preference of this new mouse over the familiar mouse indicating an increased preference for social novelty (p= 0.01) (Figure 3F).
In summary, the Cnr1/Gad1 Tg mice have normal sensory and motor performance, activity, olfaction, baseline anxiety, learning and hippocampal-based memory. However, in the social interaction and fear conditioned paradigms, Tg mice showed heightened preference for social novelty and displayed defects in amygdala-dependent memory in cue habituation.
Pharmacological challenge of Cnr1/Gad1 transgenic mice reveals robust behavioral abnormalities and elevated serotonin levels
Suppressing Gad1 expression in CNR1+ interneurons resulted in a distinct behavioral phenotype. However, the mechanisms by which behavioral deficits arise are usually best revealed by specific pharmacological challenges. To better understand the molecular changes that might give rise to the observed behavioral changes, we decided to challenge the Tg mice with a CNR1 agonist (CP55940) and the psychostimulant amphetamine (AMPH), which increases monoamine and excitatory neurotransmitter activity in the brain (Khoshbouei et al., 2004). As patients with schizophrenia have well-documented subcortical deficits in the catecholamine system function (de Bartolomeis et al., 2013; Navailles and De Deurwaerdere, 2011; Tritsch and Sabatini, 2012), we hypothesized that exposure to both of these challenges would reveal an altered catecholamine release, which in turn might lead to further behavioral alterations.
CP55940 challenge
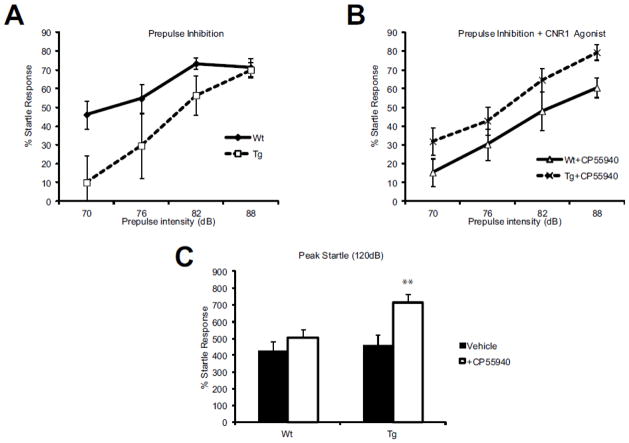
CNR1 is expressed on both CCK+ GABAergic interneurons and excitatory pyramidal cells. As our transgenic mouse only blocks the production of GAD67, the excitatory pyramidal cells are unaffected, allowing us to determine the contribution of GABAergic interneurons to the response to CNR1 receptor activation. To establish this, we assessed the startle response under a pharmacological challenge with CNR1 agonist. In unchallenged animals, the response to each individual noise burst was comparable between Wt and Tg animals (Figure 4A), whereby animals inhibited their startle response in correspondence with the intensity of the prepulse being presented. When animals where administered 25μg/kg CP55940 (a CNR1 agonist), pre-pulse inhibition was not significantly altered for the individual intensities (Figure 4B). However, when the means were compared across the 4 tone intensities (70, 76, 82, 88 dB), the Tg animals showed significantly increased startle (p=0.005). Furthermore, the peak startle was also significantly elevated in the Tg vs. the Wt mice (Figure 4C).
Figure 4. Effect of a CNR1 agonist, CP55490 on prepulse inhibition (PPI) and acoustic startle response (ASR).
(A) Percent prepulse inhibition (Y-axis) was positively correlated to acoustic sound intensity (X-axis) in both Wt and Tg animals. (B) This response was not significantly affected by IP administration of 25mg/kg CP55940 for any of the individual sound intensities, however, when the average group responses were compared across the 4 tone intensities (70, 76, 82, 88 dB), Tg animals showed a significantly increased startle (p=0.005).(C) Acoustic startle response was also significantly elevated after IP injection of CP55940 in Tg animals (p=0.01) (F-value =16.4). n= 13 Wt; n=11 Tg in all panels.
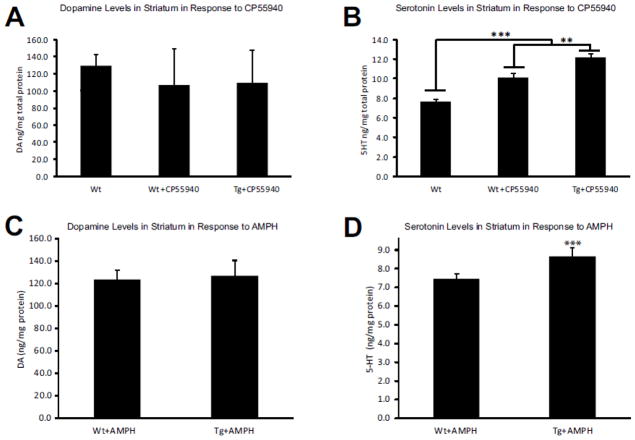
To further elucidate the causes of the different responses to the CNR1 agonist, we evaluated striatal tissue concentration of a panel of catecholamines using HPLC. While dopamine (DA) levels did increase as a result of CP55940 administration, there was no difference between Wt and Tg animals (Figure 6A), arguing that the previously reported release of DA in response to activation of CNR1 is predominantly driven by the pyramidal cells, not the interneurons. However, we observed significantly increased serotonin (5-HT) levels in Tg mice over Wt littermates receiving the same treatment (Figure 6B) (p= 0.02) suggesting that CNR1 expressing GABAergic interneurons are involved in the regulation of serotonin homeostasis and response.
Figure 6. Serotonin levels in the striatum are increased after CNR1 receptor activation and AMPH challenge.
(A) Activation of CNR1+ neurons with CP55940 did not significantly alter dopamine levels in the striatum in the Wt or Tg animals. However, activation of CNR1+ neurons with CP55940 significantly increased serotonin levels in both Tg and Wt animals (p=0.001) (B). Furthermore, this elevation of 5-HT levels was significantly higher in the Tg mice than in the Wt littermates (p=0.01). A similar experiment was performed on the AMPH-treated Tg and Wt mice (C–D), with a comparable outcome: striatal response to AMPH was similar in both Wt and Tg mice, but Tg animals responded with increased levels of 5-HT in the stratum (p= 0.001). n=10 Wt; n=10 Tg in all panels.
Amphetamine challenge
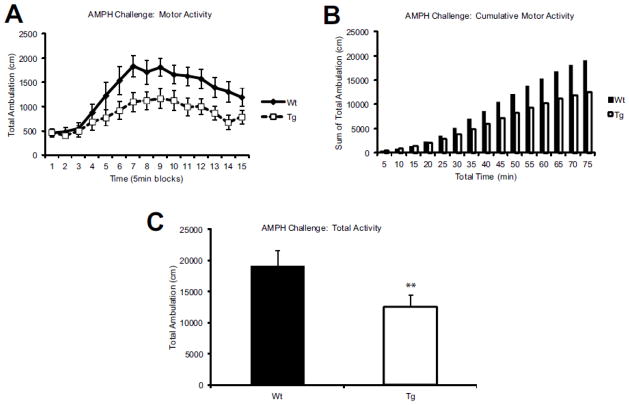
Next, to further elucidate the status of the catecholamine system responsiveness in the Cnr1/Gad1 Tg mice, we performed an amphetamine (AMPH) challenge using 3mg/kg AMPH. In the 15 min prior to AMPH administration, locomotor activity was indistinguishable between Wt and Tg animals. In response to the AMPH challenge, Tg mice reported significantly attenuated response compared to the Wt littermates (Figure 5A) (p= 0.001) and this blunted response persisted over the course of 75 minutes (Figure 5B): over the course of the entire trial, Tg mice had significantly fewer ambulations (p= 0.01) (Figure 5C). A follow-up HPLC analysis of striata of animals treated with 3mg/kg AMPH 35min after injection revealed that DA and its metabolite levels were not different between Wt and Tg cohorts (Figure 6C). However, 5-HT levels and its metabolite 5-hyroxyindoleacetic acid (5-HIAA) were significantly higher in Tg than in Wt animals (p= 0.001) (Figure 6D). As previous reports suggest that the serotonin system is also involved in response to APMH administration (Buchmayer et al., 2013), we interpret our findings that the attenuated response of Cnr1/Gad1 Tg animals to AMPH was not mediated through the dopamine transporter (DAT), but through the serotonin transporter (SERT). These findings are also in concert with the outcome of the CNR1 agonist challenge, which also reported significantly increased serotonin (5-HT) levels in our Tg mice.
Figure 5. Cnr1/Gad1 transgenic mice have decreased response to amphetamine (AMPH) challenge.
(A) AMPH challenge with 3mg/kg IP injection and evaluation of motor activity in beam break cage in 5 min bins (Y-axis) show significant and long lasting suppression of AMPH -induced motor activity (p= 0.01). (B–C) Cumulative ambulations as well as total ambulation is significantly reduced in AMPH treated Tg mice compared to WT littermate controls (p= 0.001) (F-value =14). n= 13 Wt; n=11 Tg in all panels.
DISCUSSION
Leveraging a novel transgenic mouse created in our laboratory (Garbett et al., 2010), we show that genetic inactivation of the GABAergic signaling capacity in CNR1-expressing inhibitory interneurons leads to complex behavioral and molecular disturbances as well as altered responses to cannabinoid and psychostimulant drugs: 1) The Cnr1/Gad1 transgenic mice are viable, without major neurological, sensory, motor or developmental deficits; 2) Tg mice show strong prolonged enhancement of cue based fear memory lacking extinction, even while learning and contextual memory remain intact; 3) in the social interaction paradigm, Cnr1/Gad1 mice show heightened preference for social novelty; 4) loss of GABA-ergic inhibition in CNR1+ neurons results in increased sensorimotor gating in response to cannabinoid receptor activation by CP55940; 5) the genetically altered mice show a blunted response to a psychostimulant AMPH challenge; and 6) both CP55940 and AMPH challenge result in increased 5-HT levels, but without changes in DA release or turnover in the Cnr1/Gad1 mice compared to Wt.
Schizophrenia is a complex and debilitating neurodevelopmental disorder with genetic and environmental susceptibility factors (Horvath and Mirnics, 2009). Importantly, cannabis and stimulants are the most common substances of abuse among individuals suffering from schizophrenia (Dixon et al., 1991; Mueser et al., 1990). Whether drug use is an environmental risk factor predisposing to disease states (Andreasson et al., 1987; Arseneault et al., 2004; Evins et al., 2013; Henquet et al., 2005; Horvath and Mirnics, 2009) or is a self-medication attempt to improve neurological imbalances (Bizzarri et al., 2009; Dixon et al., 1991; Goswami et al., 2004; Khantzian, 1997) is still debated, but not mutually exclusive (Bhattacharyya et al., 2010; Bloomfield et al., 2013; Evins et al., 2013). However, it is well established that interactions of cannabinoids and stimulants with susceptible genetic backgrounds is strongly interlinked with the disease state of schizophrenia (Andreasson et al., 1987; Arseneault et al., 2004; Henquet et al., 2005; Pelayo-Teran et al., 2012; Saito et al., 2013; van Os et al., 2002; Veen et al., 2004).
Studies for the last 25 years consistently suggest that the critical, brain-specific GABA synthesizing gene, GAD1, is downregulated in the postmortem brain of subjects with schizophrenia and perhaps other mental disorders (Curley et al., 2011; Guidotti et al., 2000; Hashimoto et al., 2003; Impagnatiello et al., 1998; Mirnics et al., 2006). In schizophrenia, this GAD1 downregulation occurs in various subpopulations of interneurons. One of these subpopulations, the CNR1/GAD1 co-expressing interneurons, might hold special relevance with regard to mental health (Eggan et al., 2010b; Seillier et al., 2013; Volk and Lewis, 2005): CNR1 shows genetic both evidence for association with schizophrenia (Ujike et al., 2002; Xu et al., 2009) and a decreased in expression in postmortem brain from subjects with schizophrenia (Eggan et al., 2008).Furthermore, disturbed endocannabinoid signaling might play an important role in the pathophysiology of depression, anxiety, eating disorders, attention deficit/hyperactivity disorder, posttraumatic stress disorder and substance abuse (Hillard et al., 2012). However, since CNR1 is expressed on both GABA-ergic and glutamatergic neurons (Marsicano and Lutz, 1999), understanding the contributions of CNR1+ principal cells vs. CNR1+ interneurons to the disease has been challenging. Still, it has been demonstrated that interneurons can release endocannabinoids through metabotropic glutamate receptor- and NMDAR-dependent mechanisms and contribute to activity-dependent modulation of circuit properties (Beierlein and Regehr, 2006).
Complete knockout of Cnr1 on both interneurons and principle cells has been shown to alter anxiety and social interaction (Litvin et al., 2013; Martin et al., 2002; Zimmer et al., 1999). We found that while sensory and motor functions were unperturbed, extinction of cue based memory was reduced, suggesting that CNR1+ interneurons are important for inhibition of the previously learned repose to the conditioned stimulus. Interestingly, this deficit was both specific and restricted, since learning and contextual memory were unaffected. Previously published data suggest that this effect of the CNR1+ interneurons might be related to dysfunction of both the cortical and the amygdalar circuitry: CNR1 is highly expressed in the amygdala (Katona et al., 2001) and it is known that the amygdala significantly contributes to cue based memory, while contextual memory is largely hippocampal-dependent (Amano et al., 2011; Phillips and LeDoux, 1992). Furthermore, both CNR1 antagonists (Ganon-Elazar and Akirav, 2009) or disruption of GABA in the amygdala also lead to loss of extinction (Ehrlich et al., 2009; Pereira et al., 2013). Our work suggests that inactivation of CNR1+ interneurons is sufficient to block extinction, highlighting the important role of this interneuronal subclass in the response to cannabinoid activation and subsequent behavior. However, at this time we cannot exclude the possibility that these behavioral effects are a result of complex changes that may involve both the amygdala and the prefrontal cortex, either through interneuronal regulation of circuitry or their interconnectivity (Sah and Westbrook, 2008).
Social interaction is one of the most difficult challenges facing individuals suffering from schizophrenia (Kirkpatrick et al., 2006; Mueser et al., 1991). Perturbations in social interaction are important, complex, and diverse in many mental disorders. Unfortunately, mimicking it in animal models represents a continuous challenge to the neuroscience community. While human social interaction cannot be modeled in a mouse (and true mouse models of schizophrenia will likely never exist), it is reasonable to believe that the networks governing and regulating social interaction have significant similarities between species. In fact, much of the neurobiology of social attachment has been discovered in rodents (Insel, 1997) and pharmacological manipulation or genetic ablation of Cnr1 have been shown to both increase or decrease social interaction depending on the drug used and dose (Litvin et al., 2013; Seillier et al., 2013). Yet, we observed an increase in preference for social novelty: Cnr1/Gad1 mice persisted in their investigation of a second novel mouse whereas Wt littermates showed reduced interest to this second and presumably less novel presentation. These behavioral findings are quite different between the Cnr1−/− and Cnr1/Gad1 mice, but can be easily be explained by the targeted inactivation of the CNR1+ inhibitory interneurons, revealing their isolated contribution to behavior. It is also noteworthy that the Cnr1/Gad1 mice responses across multiple behavioral tasks suggest that these mice might have a limited ability to adapt to new circumstances, which could be related to behavioral aspects of the disease (Waltz et al., 2007; Waltz and Gold, 2007).
At baseline, Gad1 suppression in CNR1+ interneurons did not result in altered anxiety levels measured by open field and elevated 0-maze. However when they were pharmacologically stimulated with CNR1 agonist at anxiogenic doses (Rey et al., 2012), Cnr1/Gad1 mice demonstrated a heighted acoustic startle response. Yet, previous studies reported no effects of CNR1 agonist on PPI in Wt mice (Bortolato et al., 2005). This suggests a complex interplay between the glutamatergic and GABA-ergic networks in response to cannabinoid agents, where the baseline tone is glutamate-dependent, with an additional GABA-ergic response being recruited with more robust simulation of the CNR1 signaling system. Furthermore, it is also plausible that CNR1 stimulation of principal glutamatergic and GABA-ergic interneurons have opposing effects on neural networks (Monory et al., 2007; Rey et al., 2012), and that the net effect on the balance of the excitation/inhibition is both dose- and region-dependent. However, these studies focused on deletion of CNR1 receptors in the different cell types, and did not assess the effects of GABA inactivation in the CNR1+ interneurons.
Cnr1/Gad1 mice showed a significantly attenuated locomotor response to AMPH. Two major catecholamines, dopamine (DA) and serotonin (5-HT) are thought to regulate response to amphetamine (Hernandez et al., 1987; Kuczenski et al., 1995). In the case of CP55940 it has also been demonstrated to alter release of both DA and 5-HT (Arevalo et al., 2001), suggesting a mechanistic convergence of between the effects of CP55940 and AMPH in our model. Assessment of the striatum of CP55940 and AMPH challenged animals showed comparable responses in both Wt and Cnr1/Gad1 mice with regard to DA levels and its breakdown products. However, 5-HT and its proximal breakdown product 5-HIAA were significantly increased in Cnr1/Gad1 mice compared to Wt. These data argue that there is a strong interaction between the CNR1-diven GABA-ergic system and the 5-HT system and that the observed behavioral changes are at least partially 5-HT system driven. This is also relevant in the context of schizophrenia pathophysiology as increasing evidence implicates serotonin and dopamine system interaction in both positive and negative symptoms (Abi-Dargham et al., 1997; Iqbal and van Praag, 1995; Kapur and Remington, 1996).
It is also important to notice that inactivation of Gad1 in different interneuronal subtypes results in quite different, and often opposite phenotypes (Schmidt and Mirnics, 2012). Cnr1/Gad1 transgenic mice show prolonged enhancement of cue based fear memory lacking extinction, heightened preference for social novelty, increased sensorimotor gating in response to cannabinoid receptor activation, a blunted response to a psychostimulant challenge and increased 5-HT levels in the striatum. In contrast, neuropeptide Y(NPY)/Gad1 mice, exhibited an extreme hyper responsiveness to amphetamine, and reduced anxiety-like behavior in both light-dark box and elevated zero-maze paradigms {MJ Schmidt, 2013 #150}. Thus, inactivation of the same transcript (Gad1), using the same methodology, shows strikingly different patterns of behavior which can be explained by the unique actions and connectivity of the various interneuronal subpopulations, suggesting that these cells might control distinct domains of behavior.
In conclusion, while we did not create a schizophrenic mouse or a schizophrenia mouse model, our results do provide a strong link between the GABA-ergic, cannabinoid, serotonergic systems and specific behavioral changes that is relevant to our understanding of schizophrenia. Further studies are needed to understand how these disturbances emerge on the developmental timeline, what pharmacological agents will be able to reverse the deficits, as well as assessing the interaction between the genetic Cnr1/Gad1 deficits and environmental challenges such as maternal immune activation. Furthermore, the specificity of our results underscore the utility and importance of mimicking well established postmortem findings in transgenic mouse models, and establishing the link to the various disease symptom domains. Such approaches might lead to developing novel, knowledge-based drug targets that can be comprehensively assessed across the various in vivo, disease-related experimental models.
Supplementary Material
Highlights.
Inactivation of Gad1 in CNR1+ GABA-ergic interneurons affects behavior.
Loss of Gad1 in CNR1+ cells leads to impaired amygdalar memory extinction.
Cnr1/Gad1 transgenic mice show heightened preference for social novelty.
These mice react adversely to CNR1 activation and amphetamine challenge.
Tg mice show increased 5-HT levels in the striatum in response to drug challenge.
Acknowledgments
This work was supported by National Institutes of Health R01 MH067234 (KM) and by the NICHD Grant P30 HD15052 awarded to the Vanderbilt Kennedy Center for Research on Human Development. JAB is supported by the 2T32MH065215-11 T32 NIH fellowship, while MJS was supported by the Vanderbilt Neuroscience Scholars Award. The authors would like to thank the Vanderbilt Murine Neurobehavioral Laboratory, especially Gregg Stanwood and John Allison, for consultation on behavioral tasks and equipment. We are also grateful for the VBI/VKC Neurochemistry Core Laboratory for generating the HPLC data.
Footnotes
Conflict of Interest: The authors declare no competing financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abi-Dargham A, et al. The role of serotonin in the pathophysiology and treatment of schizophrenia. J Neuropsychiatry Clin Neurosci. 1997;9:1–17. doi: 10.1176/jnp.9.1.1. [DOI] [PubMed] [Google Scholar]
- Amano T, et al. The fear circuit revisited: contributions of the basal amygdala nuclei to conditioned fear. J Neurosci. 2011;31:15481–9. doi: 10.1523/JNEUROSCI.3410-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasson S, et al. Cannabis and schizophrenia. A longitudinal study of Swedish conscripts. Lancet. 1987;2:1483–6. doi: 10.1016/s0140-6736(87)92620-1. [DOI] [PubMed] [Google Scholar]
- Arevalo C, et al. Cannabinoid effects on anxiety-related behaviours and hypothalamic neurotransmitters. Pharmacol Biochem Behav. 2001;70:123–31. doi: 10.1016/s0091-3057(01)00578-0. [DOI] [PubMed] [Google Scholar]
- Arseneault L, et al. Causal association between cannabis and psychosis: examination of the evidence. The British journal of psychiatry : the journal of mental science. 2004;184:110–7. doi: 10.1192/bjp.184.2.110. [DOI] [PubMed] [Google Scholar]
- Beierlein M, Regehr WG. Local interneurons regulate synaptic strength by retrograde release of endocannabinoids. J Neurosci. 2006;26:9935–43. doi: 10.1523/JNEUROSCI.0958-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, et al. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35:764–74. doi: 10.1038/npp.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzarri JV, et al. Substance use in severe mental illness: self-medication and vulnerability factors. Psychiatry Res. 2009;165:88–95. doi: 10.1016/j.psychres.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Bloomfield MA, et al. Dopaminergic Function in Cannabis Users and Its Relationship to Cannabis-Induced Psychotic Symptoms. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.05.027. [DOI] [PubMed] [Google Scholar]
- Bortolato M, et al. The CB receptor agonist WIN 55,212-2 fails to elicit disruption of prepulse inhibition of the startle in Sprague-Dawley rats. Psychopharmacology. 2005;177:264–71. doi: 10.1007/s00213-004-1941-4. [DOI] [PubMed] [Google Scholar]
- Buchmayer F, et al. Amphetamine actions at the serotonin transporter rely on the availability of phosphatidylinositol-4,5-bisphosphate. Proc Natl Acad Sci U S A. 2013;110:11642–7. doi: 10.1073/pnas.1220552110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley AA, et al. Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features. Am J Psychiatry. 2011;168:921–9. doi: 10.1176/appi.ajp.2011.11010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bartolomeis A, et al. Serotonin-glutamate and serotonin-dopamine reciprocal interactions as putative molecular targets for novel antipsychotic treatments: from receptor heterodimers to postsynaptic scaffolding and effector proteins. Psychopharmacology (Berl) 2013;225:1–19. doi: 10.1007/s00213-012-2921-8. [DOI] [PubMed] [Google Scholar]
- Dixon L, et al. Drug abuse in schizophrenic patients: clinical correlates and reasons for use. Am J Psychiatry. 1991;148:224–30. doi: 10.1176/ajp.148.2.224. [DOI] [PubMed] [Google Scholar]
- Drew WG, et al. Effects of delta9-THC, LSD-25 and scopolamine on continuous, spontaneous alternation in the Y-maze. Psychopharmacologia. 1973;32:171–82. doi: 10.1007/BF00428688. [DOI] [PubMed] [Google Scholar]
- Eggan SM, et al. Reduced cortical cannabinoid 1 receptor messenger RNA and protein expression in schizophrenia. Arch Gen Psychiatry. 2008;65:772–84. doi: 10.1001/archpsyc.65.7.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggan SM, et al. Relationship of cannabinoid CB1 receptor and cholecystokinin immunoreactivity in monkey dorsolateral prefrontal cortex. Neuroscience. 2010a;169:1651–61. doi: 10.1016/j.neuroscience.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggan SM, et al. Cannabinoid CB1 receptor immunoreactivity in the prefrontal cortex: Comparison of schizophrenia and major depressive disorder. Neuropsychopharmacology. 2010b;35:2060–71. doi: 10.1038/npp.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, et al. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–71. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Evins AE, et al. Does using marijuana increase the risk for developing schizophrenia? J Clin Psychiatry. 2013;74:e08. doi: 10.4088/JCP.12012tx2c. [DOI] [PubMed] [Google Scholar]
- Galici R, et al. A selective allosteric potentiator of metabotropic glutamate (mGlu) 2 receptors has effects similar to an orthosteric mGlu2/3 receptor agonist in mouse models predictive of antipsychotic activity. J Pharmacol Exp Ther. 2005;315:1181–7. doi: 10.1124/jpet.105.091074. [DOI] [PubMed] [Google Scholar]
- Galve-Roperh I, et al. Mechanisms of control of neuron survival by the endocannabinoid system. Curr Pharm Des. 2008;14:2279–88. doi: 10.2174/138161208785740117. [DOI] [PubMed] [Google Scholar]
- Ganon-Elazar E, Akirav I. Cannabinoid receptor activation in the basolateral amygdala blocks the effects of stress on the conditioning and extinction of inhibitory avoidance. J Neurosci. 2009;29:11078–88. doi: 10.1523/JNEUROSCI.1223-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbett KA, et al. Novel animal models for studying complex brain disorders: BAC-driven miRNA-mediated in vivo silencing of gene expression. Mol Psychiatry. 2010;15:987–95. doi: 10.1038/mp.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Yang XW. Modification of bacterial artificial chromosomes (BACs) and preparation of intact BAC DNA for generation of transgenic mice. Curr Protoc Neurosci. 2005;Chapter 5(Unit 5):21. doi: 10.1002/0471142301.ns0521s31. [DOI] [PubMed] [Google Scholar]
- Goswami S, et al. Substance-abusing schizophrenics: do they self-medicate? The American journal on addictions/American Academy of Psychiatrists in Alcoholism and Addictions. 2004;13:139–50. doi: 10.1080/10550490490435795. [DOI] [PubMed] [Google Scholar]
- Guidotti A, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–9. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Hammond SM. Dicing and slicing: the core machinery of the RNA interference pathway. FEBS Lett. 2005;579:5822–9. doi: 10.1016/j.febslet.2005.08.079. [DOI] [PubMed] [Google Scholar]
- Harkany T, et al. The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol Sci. 2007;28:83–92. doi: 10.1016/j.tips.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–26. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquet C, et al. Prospective cohort study of cannabis use, predisposition for psychosis, and psychotic symptoms in young people. BMJ. 2005;330:11. doi: 10.1136/bmj.38267.664086.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez L, et al. Simultaneous microdialysis and amphetamine infusion in the nucleus accumbens and striatum of freely moving rats: increase in extracellular dopamine and serotonin. Brain research bulletin. 1987;19:623–8. doi: 10.1016/0361-9230(87)90047-5. [DOI] [PubMed] [Google Scholar]
- Hillard CJ, et al. Contributions of endocannabinoid signaling to psychiatric disorders in humans: genetic and biochemical evidence. Neuroscience. 2012;204:207–29. doi: 10.1016/j.neuroscience.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Mirnics K. Breaking the gene barrier in schizophrenia. Nature medicine. 2009;15:488–90. doi: 10.1038/nm0509-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impagnatiello F, et al. A decrease of reelin expression as a putative vulnerability factor in schizophrenia. Proc Natl Acad Sci U S A. 1998;95:15718–23. doi: 10.1073/pnas.95.26.15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. A neurobiological basis of social attachment. Am J Psychiatry. 1997;154:726–35. doi: 10.1176/ajp.154.6.726. [DOI] [PubMed] [Google Scholar]
- Iqbal N, van Praag HM. The role of serotonin in schizophrenia. Eur Neuropsychopharmacol. 1995;5(Suppl):11–23. doi: 10.1016/0924-977x(95)00027-m. [DOI] [PubMed] [Google Scholar]
- Kapur S, Remington G. Serotonin-dopamine interaction and its relevance to schizophrenia. The American journal of psychiatry. 1996;153:466–76. doi: 10.1176/ajp.153.4.466. [DOI] [PubMed] [Google Scholar]
- Katona I, Freund TF. Multiple functions of endocannabinoid signaling in the brain. Annu Rev Neurosci. 2012;35:529–58. doi: 10.1146/annurev-neuro-062111-150420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, et al. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci. 2001;21:9506–18. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, et al. GABAergic interneurons are the targets of cannabinoid actions in the human hippocampus. Neuroscience. 2000;100:797–804. doi: 10.1016/s0306-4522(00)00286-4. [DOI] [PubMed] [Google Scholar]
- Katona I, et al. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci. 2006;26:5628–37. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harv Rev Psychiatry. 1997;4:231–44. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- Khoshbouei H, et al. N-terminal phosphorylation of the dopamine transporter is required for amphetamine-induced efflux. PLoS Biol. 2004;2:E78. doi: 10.1371/journal.pbio.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, et al. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32:214–9. doi: 10.1093/schbul/sbj053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–7. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, et al. Hippocampus norepinephrine, caudate dopamine and serotonin, and behavioral responses to the stereoisomers of amphetamine and methamphetamine. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15:1308–17. doi: 10.1523/JNEUROSCI.15-02-01308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvin Y, et al. CB1 receptor signaling regulates social anxiety and memory. Genes Brain Behav. 2013;12:479–89. doi: 10.1111/gbb.12045. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci. 1999;11:4213–25. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- Martin M, et al. Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology. 2002;159:379–87. doi: 10.1007/s00213-001-0946-5. [DOI] [PubMed] [Google Scholar]
- Matheson SL, et al. A systematic meta-review grading the evidence for non-genetic risk factors and putative antecedents of schizophrenia. Schizophr Res. 2011;133:133–42. doi: 10.1016/j.schres.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Mirnics K, et al. Critical appraisal of DNA microarrays in psychiatric genomics. Biol Psychiatry. 2006;60:163–76. doi: 10.1016/j.biopsych.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Misslin R, Ropartz P. Olfactory regulation of responsiveness to novelty in mice. Behav Neural Biol. 1981;33:230–6. doi: 10.1016/s0163-1047(81)91677-0. [DOI] [PubMed] [Google Scholar]
- Monory K, et al. Genetic dissection of behavioural and autonomic effects of Delta(9)-tetrahydrocannabinol in mice. PLoS Biol. 2007;5:e269. doi: 10.1371/journal.pbio.0050269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison PD, et al. The acute effects of synthetic intravenous Delta9-tetrahydrocannabinol on psychosis, mood and cognitive functioning. Psychol Med. 2009;39:1607–16. doi: 10.1017/S0033291709005522. [DOI] [PubMed] [Google Scholar]
- Mueser KT, et al. Prevalence and stability of social skill deficits in schizophrenia. Schizophrenia research. 1991;5:167–76. doi: 10.1016/0920-9964(91)90044-r. [DOI] [PubMed] [Google Scholar]
- Mueser KT, et al. Prevalence of substance abuse in schizophrenia: demographic and clinical correlates. Schizophr Bull. 1990;16:31–56. doi: 10.1093/schbul/16.1.31. [DOI] [PubMed] [Google Scholar]
- Muller-Vahl KR, Emrich HM. Cannabis and schizophrenia: towards a cannabinoid hypothesis of schizophrenia. Expert Rev Neurother. 2008;8:1037–48. doi: 10.1586/14737175.8.7.1037. [DOI] [PubMed] [Google Scholar]
- Navailles S, De Deurwaerdere P. Presynaptic control of serotonin on striatal dopamine function. Psychopharmacology (Berl) 2011;213:213–42. doi: 10.1007/s00213-010-2029-y. [DOI] [PubMed] [Google Scholar]
- Pelayo-Teran JM, et al. Gene-environment interactions underlying the effect of cannabis in first episode psychosis. Curr Pharm Des. 2012;18:5024–35. doi: 10.2174/138161212802884609. [DOI] [PubMed] [Google Scholar]
- Pereira CW, et al. Electrolytic lesion of the nucleus incertus retards extinction of auditory conditioned fear. Behav Brain Res. 2013;247:201–10. doi: 10.1016/j.bbr.2013.03.025. [DOI] [PubMed] [Google Scholar]
- Pettit DA, et al. Immunohistochemical localization of the neural cannabinoid receptor in rat brain. J Neurosci Res. 1998;51:391–402. doi: 10.1002/(SICI)1097-4547(19980201)51:3<391::AID-JNR12>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–85. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Rey AA, et al. Biphasic effects of cannabinoids in anxiety responses: CB1 and GABA(B) receptors in the balance of GABAergic and glutamatergic neurotransmission. Neuropsychopharmacology. 2012;37:2624–34. doi: 10.1038/npp.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Westbrook RF. Behavioural neuroscience: The circuit of fear. Nature. 2008;454:589–90. doi: 10.1038/454589a. [DOI] [PubMed] [Google Scholar]
- Saito A, et al. Endocannabinoid system: potential novel targets for treatment of schizophrenia. Neurobiol Dis. 2013;53:10–7. doi: 10.1016/j.nbd.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MJ, Mirnics K. Modeling interneuron dysfunction in schizophrenia. Dev Neurosci. 2012;34:152–8. doi: 10.1159/000336731. [DOI] [PubMed] [Google Scholar]
- Seillier A, et al. Phencyclidine-Induced Social Withdrawal Results from Deficient Stimulation of Cannabinoid CB1 Receptors: Implications for Schizophrenia. Neuropsychopharmacology. 2013;38:1816–24. doi: 10.1038/npp.2013.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DR, et al. Genetic background differences and nonassociative effects in mouse trace fear conditioning. Learn Mem. 2007;14:597–605. doi: 10.1101/lm.614807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Sabatini BL. Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron. 2012;76:33–50. doi: 10.1016/j.neuron.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou K, et al. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Ujike H, et al. CNR1, central cannabinoid receptor gene, associated with susceptibility to hebephrenic schizophrenia. Mol Psychiatry. 2002;7:515–8. doi: 10.1038/sj.mp.4001029. [DOI] [PubMed] [Google Scholar]
- van Os J, et al. Cannabis use and psychosis: a longitudinal population-based study. American journal of epidemiology. 2002;156:319–27. doi: 10.1093/aje/kwf043. [DOI] [PubMed] [Google Scholar]
- Veen ND, et al. Cannabis use and age at onset of schizophrenia. Am J Psychiatry. 2004;161:501–6. doi: 10.1176/appi.ajp.161.3.501. [DOI] [PubMed] [Google Scholar]
- Volk DW, Lewis DA. GABA Targets for the Treatment of Cognitive Dysfunction in Schizophrenia. Current neuropharmacology. 2005;3:45–62. doi: 10.2174/1570159052773396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz JA, et al. Selective reinforcement learning deficits in schizophrenia support predictions from computational models of striatal-cortical dysfunction. Biol Psychiatry. 2007;62:756–64. doi: 10.1016/j.biopsych.2006.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz JA, Gold JM. Probabilistic reversal learning impairments in schizophrenia: further evidence of orbitofrontal dysfunction. Schizophr Res. 2007;93:296–303. doi: 10.1016/j.schres.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RC, Doudna JA. Molecular mechanisms of RNA interference. Annu Rev Biophys. 2013;42:217–39. doi: 10.1146/annurev-biophys-083012-130404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, et al. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron. 2001;31:453–62. doi: 10.1016/s0896-6273(01)00372-5. [DOI] [PubMed] [Google Scholar]
- Xu B, et al. Elucidating the genetic architecture of familial schizophrenia using rare copy number variant and linkage scans. Proc Natl Acad Sci U S A. 2009;106:16746–51. doi: 10.1073/pnas.0908584106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Crawley JN. Simple behavioral assessment of mouse olfaction. In: Crawley Jacqueline N, et al., editors. Current protocols in neuroscience/editorial board. Unit 8. Chapter 8. 2009. p. 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, et al. Automated three-chambered social approach task for mice. In: Crawley Jacqueline N, et al., editors. Current protocols in neuroscience/editorial board. Unit 8. Chapter 8. 2011. p. 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer A, et al. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci U S A. 1999;96:5780–5. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.