Abstract
Background and aims
Evidence suggests that both the nicotinic receptor α5 subunit (CHRNA5) and Cytochrome P450 2A6 (CYP2A6) genotypes influence smoking cessation success and response to pharmacotherapy. We examine the effect of CYP2A6 genotype on smoking cessation success and response to cessation pharmacotherapy, and combine these effects with those of CHRNA5 genotypes.
Design
Placebo-controlled randomized smoking cessation trial
Setting
Ambulatory care facility in Wisconsin, USA.
Participants
Smokers (N=709) of European ancestry were randomized to placebo, bupropion, nicotine replacement therapy, or combined bupropion and nicotine replacement therapy.
Measurements
Survival analysis was used to model time to relapse using nicotine metabolism derived from CYP2A6 genotype-based estimates. Slow metabolism is defined as the lowest quartile of estimated metabolic function.
Findings
CYP2A6-defined nicotine metabolic function moderated the effect of smoking cessation pharmacotherapy on smoking relapse over 90 days (Hazard Ratio (HR) = 2.81, 95%CI=1.32-5.99, p=0.0075), with pharmacotherapy significantly slowing relapse in fast (HR=0.39, 95%CI=0.28-0.55, p=1.97×10-8), but not slow, metabolizers (HR=1.09, 95%CI=0.55-2.17, p=0.80). Further, only the effect of nicotine replacement, and not bupropion, varies with CYP2A6-defined metabolic function. The effect of nicotine replacement on continuous abstinence is moderated by the combined genetic risks from CYP2A6 and CHRNA5 (interaction effect size=0.74, 95%CI=0.59-0.94, p=0.013).
Conclusions
Nicotine replacement therapy is effective amongst individuals with fast, but not slow, CYP2A6-defined nicotine metabolism. The effect of bupropion on relapse likelihood is unlikely affected by nicotine metabolism as estimated from CYP2A6 genotype. The variation in treatment responses amongst smokers with genes may guide future personalized smoking cessation interventions.
Keywords: Smoking Cessation, Nicotine, Metabolism, Pharmacogenetics
INTRODUCTION
Key goals of genetic studies of smoking behaviors are to identify the genes that confer a vulnerability to nicotine dependence and that guide the development of effective, ‘personalized’ treatment strategies for smoking cessation. We recently demonstrated that pharmacologic treatment affects cessation differently depending on genotype of the nicotinic receptor subunit gene, CHRNA5 (1), a locus strongly associated with nicotine dependence (2-5); a similar association between this locus and cessation has been reported recently (6). Variation in nicotine metabolism efficiency, and variation in the gene that encodes the primary nicotine metabolism enzyme, cytochrome P450 2A6 (CYP2A6), are also robustly associated with smoking phenotypes, especially cigarette consumption (5, 7-9). CYP2A6 is highly polymorphic, with reduced function alleles producing significantly slower rates of nicotine metabolism. Relatively common variants define multiple CYP2A6 haplotypes in European populations (10), and the large majority of inter-individual variation in metabolism of nicotine to cotinine can be explained by seven polymorphisms among European Americans (11). Several studies have reported an influence of nicotine metabolic rate upon cessation (12-14), although the relation between metabolism and different treatment regimens remains unclear.
Previous studies of nicotine metabolism and cessation treatment have examined a proxy for CYP2A6 activity, Nicotine Metabolite Ratio (NMR), the ratio of two stable nicotine metabolites, cotinine: 3-hydroxycotine, measured in the blood of current smokers (12-16). We have recently developed another predictive model of nicotine metabolism based on CYP2A6 genotype. CYP2A6 haplotypes included in this model explained 70% (R2=0.7) of the variance in metabolism of oral nicotine among European Americans. Metabolism estimates predicted by the model were significantly correlated with self-reported cigarettes smoked per day (CPD) (7, 11) and exhaled carbon monoxide (unpublished data).
Previous studies demonstrated that treatment success with nicotine replacement therapy (NRT) is associated with markers of slower nicotine metabolism (12, 14). However, because some of these studies did not include a placebo control group, the interaction between treatment and metabolic rate could not be determined. Another study showed more successful cessation among slow nicotine metabolizers than among fast metabolizers when both received placebo treatment; both groups had equivalent quit rates with bupropion treatment (13). In the current research, we will determine if the effect of cessation pharmacotherapy varies with nicotine metabolism in the context of different active pharmacotherapy conditions and placebo.
Using data from a multi-armed cessation trial that includes NRT, bupropion, combination pharmacotherapies, and placebo controls, we tested the hypotheses that: 1) individuals with CYP2A6 genotype-based fast nicotine metabolism are more likely to relapse sooner than individuals with slow metabolism when given placebo intervention, 2) the effect of active pharmacotherapy vs. placebo will vary (i.e., interact) with CYP2A6 genotype, and 3) the effects of NRT will differ (interact) with CYP2A6 genotype but the effects of bupropion will not. In addition, we examined whether the effects of CYP2A6 on smoking cessation outcome, and therapeutic response to NRT, are independent from those of CHRNA5, another gene associated with cessation outcomes and response to smoking cessation pharmacotherapy (1). This research was designed to reveal the genetic conditions under which the tested pharmacotherapies exert their optimal effects, a topic of clear relevance to a genetically informed, personalized approach to smoking cessation pharmacotherapy.
METHODS
Participants were from a University of Wisconsin Transdisciplinary Tobacco Use Research Center (UW-TTURC) randomized, placebo-controlled smoking cessation trial (17), 18 years of age or older, smoked 10 or more cigarettes per day, and were motivated to quit smoking. The University of Wisconsin-Madison IRB approved this trial, and all subjects provided written informed consent. Prior to randomization, participants completed baseline assessments of demographics, smoking history (including CPD), and tobacco dependence including the Fagerström Test for Nicotine Dependence (FTND)(18). Participants provided a breath sample for alveolar carbon monoxide (CO) analysis to verify their smoking status and to estimate their smoking heaviness. The treatment phase lasted 8 weeks. Participants (European-American N=709) were randomly assigned to: placebo (n = 79), bupropion SR (n =118), nicotine replacement therapy (n = 377), or combined bupropion and nicotine replacement therapy (n =135). The pharmacotherapies were: (1) placebo; (2) bupropion SR (150mg twice daily for 9 weeks total: 1 week prior to the quit date and 8 weeks post-quit); (3) nicotine replacement therapy including nicotine lozenge (2 or 4 mg based on the package insert instructions for 12 weeks post-quit), nicotine patch (24-hour patch; 21, 14, and 7 mg; titrated down during 8 weeks post-quit), or nicotine patch + nicotine lozenge combination therapy (dosed as listed above), and (4) combined bupropion SR and nicotine lozenge therapy (dosed as listed above). In addition, all participants received six brief (10 minute) individual counseling sessions.
Biochemically confirmed 7-day point prevalence abstinence was assessed at end-of-treatment (8 weeks post-quit). All of participants’ self-reports of abstinence during study visits were confirmed by an expired CO (abstinence = CO < 10 ppm). Follow-up telephone calls permitted the determination of time of relapse via timeline follow-up assessment (19, 20) up to 90 days after the quit date. Relapse was defined as smoking for 7 consecutive days.
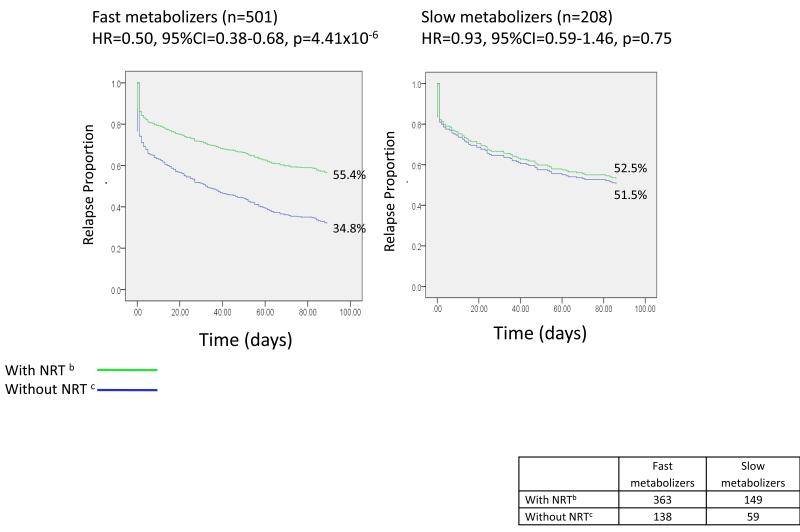
Genotyping was performed by the Center for Inherited Disease Research at Johns Hopkins University using the Illumina Omni2.5 microarray (www.illumina.com). Data cleaning was led by the GENEVA Coordinating Center at the University of Washington. Additional CYP2A6 genotyping, and application of the predictive model of CYP2A6 activity, were conducted as previously described (7, 11). The predicted nicotine metabolism metric for all subjects was calculated from CYP2A6 diplotype. Briefly, all analyses of measured metabolism are performed on a metabolism metric, the ratio of deuterated (D2) cotinine / (D2cotinine+ D2nicotine), determined 30 minutes following oral administration of D2nicotine. The original model parameters were derived from the regression, log (1 −metabolism metric) = α + βH1 + βH2 where α is the intercept, H1 represents the first CYP2A6 haplotype, and H2 represents the second CYP2A6 haplotype for each subject. Slow nicotine metabolism function is defined as the lowest quartile of metabolism function as used in previous research on nicotine metabolism(12-14). Based on the distribution of the metabolism metric, the cut point closest to the lowest quartile defines 29.3% of participants with slower metabolism. The frequencies of slow vs. fast metabolizers, stratified by treatment group, are in Table S1 and Figure 2.
Figure 2.
Effect of nicotine replacement therapy (NRT) on smoking relapse differs in fast vs. slow nicotine metabolizers defined by CYP2A6 genotype a
There is a significant interaction between NRT and CYP2A6 (interaction effect size=1.82, 95%CI=1.07-3.10, p=0.028).
NRT: Nicotine Replacement Therapy
a Time to relapse over 90 days. b The group with NRT includes all treatment arms with NRT (the NRT arms and the arm receiving both NRT and bupropion. c The group without NRT includes all treatment arms without NRT (the placebo arm and the arm receiving bupropion alone).
ANALYSIS
We used Cox proportional hazard regression models to analyze smoking relapse likelihood (smoking on 7 consecutive days) over the 90 day period after the quit date, the primary outcome. Secondary outcomes include: 1) smoking quantity (self-reported cigarettes smoked per day, CPD) for post-treatment weeks 1-8, analyzed with growth curve mixed models for repeated measures per subject, 2) biochemically confirmed 7-day abstinence at 8 weeks analyzed with logistic regression, and 3) continuous abstinence (complete abstinence for 90 days post quit), also analyzed with logistic regression. The primary predictor was CYP2A6 genotype-based metabolic function, which was examined for interaction with treatment (active pharmacotherapy vs. placebo). We tested whether the hazard ratio for relapse associated with treatments differed across predicted metabolic function groups by including a product interaction term in the Cox proportional hazard regression. Covariates included gender, age, and cigarettes per day (in 4 levels: ≤10, 11-20, 21-30, ≥31, while specific cigarette counts were used as the dependent variable for the smoking quantity analysis).
We created a binary variable representing high vs. low risk of relapse based on the diplotype of rs16969968 and rs680244 in CHRNA5 (low risk: GG_CC, GG_CT, GA_CC; high-risk: GG_TT, GA_CT, AA_ CC) and our previous findings (1). Next, we combined the genetic risks defined by CYP2A6 and CHRNA5 into a four-category variable representing the combined genetic risks from CYP2A6 and CHRNA5. We tested whether the hazard ratio for relapse associated with treatments differed across the genetic risk categories by including a product interaction term in the Cox proportional hazard regression.
RESULTS
Subjects of European ancestry enrolled in the UW-TTURC trial with genotype and relapse data were included in this analysis (N=709, Table S1 for demographics). The CYP2A6 genotype-based metabolism function distribution in the UW-TTURC sample was: fast metabolizers (70.7%) and slow metabolizers (29.3%). In this treatment-seeking sample, fast nicotine metabolism was associated with more cigarettes smoked per day (CPD) at baseline, adjusted for age and gender (β=0.19, df=1, p=0.0024).
Metabolism based on CYP2A6 Genotype Predicts Smoking Relapse in the Placebo Group
In this trial, 49.6% of participants relapsed to smoking during the 90-day post-quit follow-up. In the placebo group, slow metabolism based on CYP2A6 genotype predicted decreased relapse risk in comparison to fast metabolism (Hazard Ratio (HR)=0.40, 95%CI=0.19-0.83, p=0.013), adjusted for age and gender. Age and gender did not predict relapse.
Pharmacotherapy Effects Vary with Metabolism based on CYP2A6 Genotype
Active pharmacotherapy decreased the rate of relapse across all participants by almost half in comparison to placebo, adjusted for age, gender, and metabolism based on CYP2A6 genotype (HR=0.51, 95%CI=0.38-0.68, p=4.3x10-6). However, the association of metabolism and relapse was significantly moderated by medication status (placebo vs. active pharmacotherapy) (interaction effect size=2.81, 95%CI=1.32-5.99, p=0.0075; Table 1A). Pharmacotherapy was highly effective in fast metabolizers (HR=0.39, 95%CI=0.28-0.55, p=1.97x10-8) but not in slow metabolizers (HR=1.09, 95%CI=0.55-2.17, p=0.80). Figure 1 illustrates the effect of pharmacotherapy on relapse in fast and slow metabolizers.
Table 1.
Interaction of CYP2A6 and Pharmacotherapy on Time to Smoking Relapse up to 90days (N=709)
| A. Interaction of CYP2A6 and Pharmacotherapy (Placebo vs. Active Pharmacotherapy) | |||
|---|---|---|---|
|
| |||
| Smoking Relapse at 90 Days |
|||
| Predictors | Hazard Ratio | 95% C.I. | P |
| CYP2A6 Activitya | |||
| Fast metabolism | reference | ||
| Slow metabolism | 0.39 | (0.19, 0.79) | 0.0093 |
| Medication Status | |||
| Placebo | reference | ||
| Active Pharmacotherapy | 0.39 | (0.28, 0.54) | 1.4×10−8 |
| Interaction of CYP2A6 and Medication | |||
| Slow metabolism* Active Pharmacotherapy | 2.81 | (1.32, 5.99) | 0.0075 |
| B.Interaction of CYP2A6 and Pharmacotherapy (Placebo vs. NRT vs. Bupropion)b | |||
|---|---|---|---|
|
| |||
| Model 1. Testing Interactions | Smoking Relapse at 90 Days |
||
| Predictors | Hazard Ratio | 95% C.I. | P |
| CYP2A6 Activitya | |||
| Fast metabolism | reference | ||
| Slow metabolism | 0.67 | (0.40, 1.11) | 0.12 |
| Use of NRT | |||
| No | reference | ||
| Yes | 0.50 | (0.37, 0.67) | 2.6×10−6 |
| Use of Bupropion | |||
| No | reference | ||
| Yes | 0.77 | (0.57, 1.03) | 0.082 |
| Interaction of CYP2A6 and NRT | 1.82 | (1.07, 3.10) | 0.028 |
| Interaction of CYP2A6 and Bupropion | 0.95 | (0.58, 1.58) | 0.85 |
| Model 2. Final Model | Smoking Relapse at 90 Days |
||
|---|---|---|---|
| Predictors | Hazard Ratio | 95% C.I. | P |
| CYP2A6 Activitya | |||
| Fast metabolism | reference | ||
| Slow metabolism | 0.65 | (0.42, 0.99) | 0.045 |
| Use of NRT | |||
| No | reference | ||
| Yes | 0.49 | (0.37, 0.66) | 9.0×10−7 |
| Use of Bupropion | |||
| No | reference | ||
| Yes | 0.76 | (0.60, 0.96) | 0.022 |
| Interaction of CYP2A6 and NRT | 1.85 | (1.11, 3.08) | 0.019 |
All models were adjusted for age and gender.
Slow metabolism is defined as the lowest quartile of genotyped-defined CYP2A6 metabolism; fast metabolism is defined as the higher three quartiles of metabolism.
Age and gender are not significant predictors for smoking relapses (HR=1.01, 95%CI=0.99-1.02, p=0.23 for age; HR=1.14, 95%CI=0.92-1.42, p=0.24 for female).
NRT: nicotine replacement therapy.
Figure 1.
Pharmacotherapy reduces relapse in fast nicotine metabolizers, but not in slow metabolizers defined by CYP2A6 genotype a
a Time to relapse over 90 days
There is a significant interaction between medication and CYP2A6 (interaction effect size=2.81, 95%CI=1.32-5.99, p=0.0075)
Effects of NRT, but Not Bupropion, Vary with Metabolism based on CYP2A6 Genotype
The effect of NRT differed by CYP2A6-defined metabolism (interaction effect size=1.82, 95%CI=1.07-3.10, p=0.028) while the effect of bupropion did not (interaction effect size=0.95, 95%CI=0.58-1.58, p=0.85, Table 1B). NRT was effective in fast (HR=0.50, 95%CI=0.38-0.68, p=4.41x10-6), but not slow, metabolizers (HR=0.93, 95%CI=0.59-1.46,p=0.75, Fig 2). Buproprion was effective in both slow and fast metabolizers (HR=0.76, 95%CI=0.60-0.96, p=0.022, Table 1B, Model 2), its effect not varying with metabolism (Fig S1).
Because metabolism was related to baseline smoking quantity, we examined whether the relations amongst metabolism, NRT condition, and relapse depended on smoking quantity. Heavier smoking at baseline was associated with higher likelihood of relapse (HR=1.34, 95% CI=1.17-1.53, p =2.5x10-5), but this relation did not differ by treatment status (interaction effect size=1.34, 95%CI=0.94-1.92, p=0.11). The interaction between CYP2A6-based metabolism and treatment remained significant (interaction effect size=2.55, 95%CI=1.19-5.46, p=0.016; Table S2), after adjusting for CPD. Similar results were found when FTND was used as a covariate.
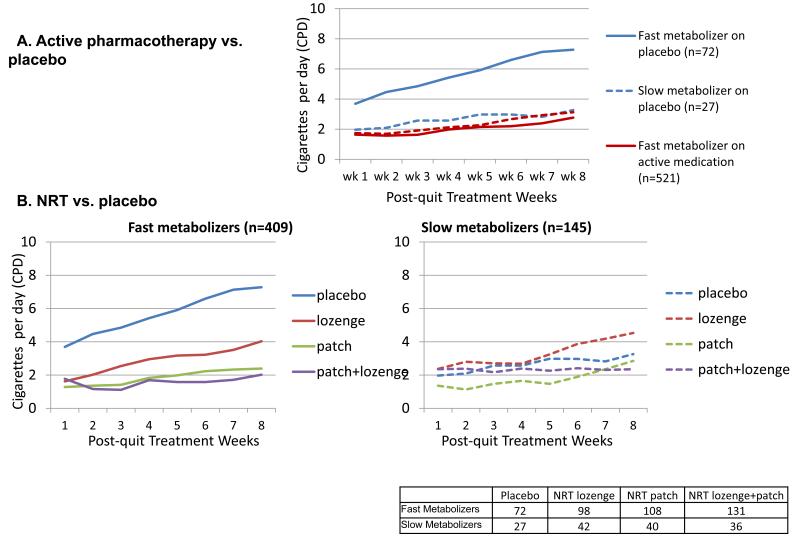
We found similar results when modeling secondary cessation outcomes (smoking quantity across 8 weeks, point-prevalent abstinence at 8 weeks, and continuous abstinence over 90 days). Fast metabolizers receiving placebo escalate their smoking significantly more quickly than do fast metabolizers on active medication and slow metabolizers on active medication or placebo (β=0.14, t=3.13, df=1, p=0.0020, Figure 3A). When comparing the four subgroups of subjects formed by crossing NRT vs. placebo with CYP2A6 estimated fast vs. slow metabolizer conditions, we found a 3-way interaction of NRT condition, CYP2A6 activity, and time (interaction effect size=-0.17, 95%CI=-0.33-0.0025, p=0.053, Figure 3B) reflecting that smoking escalated especially quickly amongst fast metabolizers receiving no NRT. The interaction between CYP2A6 and NRT only approached significance for point-prevalent abstinence at 8 weeks (interaction effect size=0.69, 95%CI=0.33-1.46, p=0.33), but was significant for continuous abstinence over 90 days (interaction effect size=0.37, 95%CI=0.18-0.78, p=0.0091; Table S3), reflecting that fast metabolizers on placebo were especially unlikely to be abstinent from smoking for the whole 90-day period.
Figure 3.
Trajectory of post-quit smoking quantity varies by treatment and CYP2A6: Fast metabolizers on placebo treatment have a significantly faster escalation into heavy smoking over time (nicotine replacement therapy (NRT) vs. placebo)
(3A) A significant interaction between active medication and CYP2A6 on the smoking rate escalation (t=3.13, df=1, p=0.0020).
(3B) A trend interaction between NRT combination and CYP2A6 on smoking quantity over time (F=3.75, df =1; p=0.053)
The number needed to treat (NNT) is the average number of patients who need to be treated for one patient to benefit with active treatment versus with placebo treatment. In our study, the NNT for NRT was 2.9 for fast metabolizers (70.7% of the sample) vs. >1000 for slow metabolizers (29.3% of the sample). The NNT was 4.2 across all individuals regardless of their genotype status, supporting the established effect of NRT. However, the NNT varied widely depending on the individual’s CYP2A6 genotype (Figure S2).
Exploratory analyses showed that amongst fast metabolizers, combination NRT (patch + lozenge) produces lower relapse rates than does NRT monotherapy (HR=0.61, 95%CI=0.42-0.89, p=0.011). The lozenge and patch did not significantly differ from one another (HR=0.69, 95%CI=0.45-1.05, p=0.083). The effects of the NRT subtypes did not differ amongst the slow metabolizers.
Combined Genetic Effects of CYP2A6 (chromosome 19) and CHRNA5 (chromosome 15)
Because CHRNA5 was previously shown to predict smoking cessation and to interact with pharmacotherapy condition, we studied the joint effects of CYP2A6 and CHRNA5 on smoking relapse and the interactions between each gene and pharmacotherapy. The interaction of CYP2A6 and NRT remained significant, even after adjusting for the effect of CHRNA5 (interaction effect size=1.89, 95CI=1.09-3.29, p=0.025; Table S4). Similarly, the interaction effect of CHRNA5 and pharmacotherapy remained consistent with our previous findings (interaction effect size is 0.49, Wald=4.59, df=1, p=0.032, unadjusted for CYP2A6 and 0.48, Wald=3.57, df=1, p=0.059, adjusted for CYP2A6). There was no significant interaction between CHRNA5 and CYP2A6 on relapse (interaction effect size=1.15, 95%CI=0.62-2.17, p=0.66).
Next, we combined the genetic risks defined by CYP2A6 and CHRNA5 genotypes into a variable representing the combined genetic risk with four levels. There was a nonlinear effect for NRT as a function of the four-level combined genetic risk (interaction effect size=0.75, 95%CI=0.58-0.96, p=0.021), but not for bupropion (interaction effect size=1.03, 95%CI=0.82-1.31, p=0.79, Table S5).
To illustrate the interaction between the combined genetic risk and pharmacotherapy (NRT vs. placebo), the absolute rates of continuous abstinence over 90 days are shown in Figure 4. In this study, the NNT for NRT varied with the four combined genetic risk levels: 2.6 for smokers with high-risk status based on CYP2A6 and CHRNA5 (44.5% of the sample), 3.7 for smokers with high-risk status based on CYP2A6 & low-risk status based on CHRNA5 (27.5% of the sample), 16.6 for smokers with low-risk status based on CYP2A6 and high-risk status on CHRNA5 (18.2% of the sample), and >1000 for smokers with low-risk status based on CYP2A6 and CHRNA5 (9.81% of the sample). The last very high value indicates the lack of any treatment effect in the lowest risk group. These NNT values may be contrasted with an overall NNT of 4.2 if NRT is given to everyone regardless of the genetic risk.
Figure 4.
Nicotine replacement therapy (NRT) vs. placebo effect on smoking abstinencea varies with the combined genetic effects of CYP2A6 and CHRNA5
There is a significant interaction between pharmacotherapy (NRT vs. Placebo) and 4 genetic groups (wald=7.44, df=1, p=0.0064). Blue vertical lines are 95% confidence intervals.
a Continued Abstinence for 3 months. b Low vs. risk for CYP2A6 indicates risk for smoking relapse defined by slow vs. fast metabolism. c Low vs. risk for CHRNA5 indicates risk for smoking relapse defined by CHRNA5 (rs16969968, rs680244) (low-risk diplotypes: GG_CC, GG_CT, GA_CC and high-risk diplotypes: GG_TT, GA_CT, AA_ CC).
DISCUSSION
Nicotine metabolism as estimated from CYP2A6 genotype predicts both smoking cessation success and differential response to cessation pharmacotherapy. Specifically, fast nicotine metabolism is associated with heightened relapse likelihood with placebo and counseling, and this increased genetic risk was “treated” by cessation pharmacotherapy. Response to NRT differs based on nicotine metabolism. Specifically, active NRT pharmacotherapy is effective among individuals with fast, but not slow estimated nicotine metabolism thereby reducing the risk of faster metabolism with regards to relapse rate. The effect of bupropion on relapse rate, on the other hand, does not differ with estimated nicotine metabolism. We also demonstrated that the effect of CYP2A6 on relapse likelihood remains significant after statistically adjusting for CHRNA5 (a previously reported genetic predictor of cessation (1)). When both genetic risks are combined, how much an individual benefits from NRT depends on his/her combined genetic risk levels of both CYP2A6 and CHRNA5. In our study, the wide variation in number needed to treat (NNT) between smokers with different genetic risks supports the further exploration of pharmacogenetic approaches to smoking treatment.
These findings extend the existing research on CYP2A6 and the Nicotine Metabolite Ratio (NMR). The NMR is a direct biomarker of nicotine metabolism that reflects both genetic and environmental influences on nicotine metabolism and clearance (21). In general, faster nicotine metabolism as estimated by NMR has predicted reduced smoking cessation success when individuals were given the nicotine patch, gum, or placebo, but not when given nicotine nasal spray or bupropion (14, 22). Using estimated nicotine metabolism based on CYP2A6 genotypes, we provide additional evidence regarding the effects of specific pharmacotherapies from a large scale trial. We confirm that faster nicotine metabolism is associated with greater relapse likelihood in the placebo condition, and we also find that nicotine metabolism is unrelated to response to bupropion treatment. The latter observation is consistent with existing evidence (13) and the fact that bupropion is primarily metabolized by the CYP2B6 enzyme (23, 24). Instead of using NMR, this study presents the first genotype by NRT interaction with a proper placebo-control arm. Our findings differ from earlier reports primarily because we found that nicotine metabolism did not predict cessation outcome amongst persons given NRT which appears to neutralize the relapse risk associated with faster nicotine metabolism. At present, it is difficult to resolve the differences between study findings due to differences between subjects and experimental conditions. These differences highlight important methodology considerations: 1) Some of the prior studies did not include a placebo arm, which is needed to determine a gene × medication interaction, 2) NRT and bupropion were often not included in the same trial, and 3)In the current trial the same behavioral counseling was used in all medication conditions, whereas the effects of counseling could vary across trials which is a possibility based on observed differing abstinence rates in placebo arms of different trials. While future meta-analyses can be helpful, caution should be used as differences in ascertainment, treatment intensity, assessment, and treatment comparisons across trials can result in problematic interpretations (25, 26).
Personalized medicine for smoking cessation will require the optimal combination of multiple genetic predictors. We previously reported genetic variants in CHRNA5 as robust predictors of cessation success and response to pharmacotherapy (1), and similar associations have been reported by a large pharmacogenetic consortium (6) which includes 8 trials including the current study. This current study shows that CYP2A6 on chromosome 19 has an effect independent of CHRNA5 on chromosome 15, suggesting that these two genetic markers represent distinct biological pathways that influence smoking behaviors. Our findings extend the recent report of an additive effect of these two genes on smoking quantity (cigarettes smoked per day) and risk for lung cancer (27).
This study has several limitations. First, the sample is limited in several ways. When multiple genetic markers are analyzed, the sample size in certain conditions becomes small, so these effect size estimates should be considered as preliminary. This sample is the same as that used in the Chen et al. study(1), thus this research does not provide new evidence to support the relation of CHRNA5 with cessation outcomes. This study focuses on only European-Americans. Second, one could surmise that smoking quantity plays a mediating role in our reported association between CYP2A6 and cessation (28). We showed that CYP2A6 remained significantly associated with smoking outcomes, suggesting that it could influence biological processes underlying both smoking quantity and smoking cessation (22, 29, 30). However, self-reported smoking rate is an imperfect measure of actual smoking heaviness (31, 32) and future research would benefit from the use of sensitive biomarkers of tobacco exposure. Future studies of mechanisms require biomarkers such as cotinine levels. The mechanisms underlying CYP2A6 and smoking cessation is not entirely clear, the complex genetic architecture in this chromosomal region and other metabolic pathways in addition to nicotine metabolism could play a role. Third, the CYP2A6 gene is highly polymorphic (33), including many variants in Europeans (10). Its complex genetic architecture challenges the examination of this gene. We may not have captured other important genetic variation in this region which could have contributed to the results. We used a CYP2A6 genotype-based nicotine metabolism estimate derived from an experiment performed in an independent sample (7, 11). Our findings using a genetic metric of CYP2A6 activity provide a complementary research paradigm, but it is certainly possible that different results would have been obtained through use of the NMR. Finally, given such limitations, further work is clearly needed to develop a treatment algorithm that enhances the effectiveness and cost-effectiveness of smoking treatment.
Keeping in mind the above limitations, this study extends previous work on CHRNA5, CYP2A6, and cessation to reach the following conclusions. 1) Nicotine metabolic function estimated via CYP2A6 genotype predicts relapse likelihood in individuals using placebo medication. 2) The effect of pharmacotherapy differs as a function of CYP2A6-based nicotine metabolism. In particular, NRT significantly benefits smokers with fast but not slow nicotine metabolism. The effect of bupropion on relapse likelihood is largely unrelated to CYP2A6 status. 3) The effects of CYP2A6-estimated nicotine metabolism on response to NRT are independent from those of CHRNA5 genotype, with the effects of the two genes being additive. That is, likelihood of benefit from NRT may increase as a function of the combined genetic risks from CYP2A6 and CHRNA5. An important clarification of these pharmacogenetic findings will rely on validation across populations and with different cessation processes such as natural vs. assisted cessation. A larger study or meta-analysis is required to examine the effect of different pharmacotherapies in the combined genetic risk groups. Many other genes have been nominated as predictive of smoking cessation (34), and we anticipate more genes will be identified as playing a role in smoking cessation. Risk prediction modeling to incorporate multiple genetic markers and non-genetic predictors will lay the foundation for a personalized treatment algorithm (35).
Supplementary Material
ACKNOWLEDGMENTS
Glaxo Wellcome provided bupropion at no cost in the UW-TTURC clinical trial. The Wisconsin State Laboratory of Hygiene provided considerable technical assistance in this research effort in the form of DNA extraction, and this research was also aided by the Wisconsin Partnership Program.
FUNDING SUPPORT
This research was supported by NIH grants P01 CA089392 (LJB), P50 CA84724 (TBB), and K05CA139871 (TBB) from the National Cancer Institute, P50 DA19706 (TBB), R01 DA026911 (NLS), K02 DA021237 (LJB), and K08 DA030398 (LSC) from the National Institute on Drug Abuse, U01 HG004422 (LJB) from the National Human Genome Research Institute, sub-award KL2 RR024994 (LSC) from the National Center for Research Resources, 5T32MH014677-33 from the National Institute of Mental Health (AJB), and the Wisconsin Partnership Program. Genotyping services for the UW-TTURC sample were provided by the Center for Inherited Disease Research (CIDR) and DNA extraction supported by the Wisconsin State Laboratory of Hygiene. Funding support for CIDR was provided by NIH grant U01HG004438 and NIH contract HHSN268200782096C to The Johns Hopkins University. Assistance with genotype cleaning was provided by the Gene Environment Association Studies (GENEVA) Coordinating Center (U01 HG004446).
Footnotes
Conflict of Interest
Dr. Bierut, and Dr. Goate are listed as inventors on issued U.S. patent 8,080,371, “Markers for Addiction” covering the use of certain SNPs in determining the diagnosis, prognosis, and treatment of addiction. Dr. Saccone is the spouse of Dr. S. Saccone who is also listed as an inventor on the above patent. Dr. Smith has served in the past 3 years as a co-investigator on an investigator-initiated research study at the University of Wisconsin-Madison that was supported by Pfizer. Drs. Chen, Bloom, Baker, Smith, Piper, Saccone, Hatsukami and Ms. Martinez declare no potential conflict of interest.
REFERENCES
- 1.Chen LS, Baker TB, Piper ME, et al. Interplay of genetic risk factors (CHRNA5-CHRNA3-CHRNB4) and cessation treatments in smoking cessation success. Am J Psychiatry. 2012;169:735–42. doi: 10.1176/appi.ajp.2012.11101545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu JZ, Tozzi F, Waterworth DM, et al. Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nat Genet. 2010;42:436–40. doi: 10.1038/ng.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saccone NL, Culverhouse RC, Schwantes-An TH, et al. Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD. PLoS Genet. 2010:6. doi: 10.1371/journal.pgen.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.TAG Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet. 2010;42:441–7. doi: 10.1038/ng.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thorgeirsson TE, Gudbjartsson DF, Surakka I, et al. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat Genet. 2010;42:448–53. doi: 10.1038/ng.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergen AW, Javitz HS, Krasnow R, et al. Nicotinic acetylcholine receptor variation and response to smoking cessation therapies. Pharmacogenet Genomics. 2013;23:94–103. doi: 10.1097/FPC.0b013e32835cdabd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bloom AJ, Harari O, Martinez M, et al. Use of a predictive model derived from in vivo endophenotype measurements to demonstrate associations with a complex locus, CYP2A6. Hum Mol Genet. 2012;21:3050–62. doi: 10.1093/hmg/dds114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujieda M, Yamazaki H, Saito T, et al. Evaluation of CYP2A6 genetic polymorphisms as determinants of smoking behavior and tobacco-related lung cancer risk in male Japanese smokers. Carcinogenesis. 2004;25:2451–8. doi: 10.1093/carcin/bgh258. [DOI] [PubMed] [Google Scholar]
- 9.Schoedel KA, Hoffmann EB, Rao Y, Sellers EM, Tyndale RF. Ethnic variation in CYP2A6 and association of genetically slow nicotine metabolism and smoking in adult Caucasians. Pharmacogenetics. 2004;14:615–26. doi: 10.1097/00008571-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Haberl M, Anwald B, Klein K, et al. Three haplotypes associated with CYP2A6 phenotypes in Caucasians. Pharmacogenet Genomics. 2005;15:609–24. doi: 10.1097/01.fpc.0000171517.22258.f1. [DOI] [PubMed] [Google Scholar]
- 11.Bloom J, Hinrichs AL, Wang JC, et al. The contribution of common CYP2A6 alleles to variation in nicotine metabolism among European-Americans. Pharmacogenet Genomics. 2011;21:403–16. doi: 10.1097/FPC.0b013e328346e8c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lerman C, Jepson C, Wileyto EP, et al. Genetic variation in nicotine metabolism predicts the efficacy of extended-duration transdermal nicotine therapy. Clin Pharmacol Ther. 2010;87:553–7. doi: 10.1038/clpt.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patterson F, Schnoll RA, Wileyto EP, et al. Toward personalized therapy for smoking cessation: a randomized placebo-controlled trial of bupropion. Clin Pharmacol Ther. 2008;84:320–5. doi: 10.1038/clpt.2008.57. [DOI] [PubMed] [Google Scholar]
- 14.Schnoll RA, Patterson F, Wileyto EP, et al. Nicotine metabolic rate predicts successful smoking cessation with transdermal nicotine: a validation study. Pharmacol Biochem Behav. 2009;92:6–11. doi: 10.1016/j.pbb.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benowitz NL, Pomerleau OF, Pomerleau CS, Jacob P., 3rd Nicotine metabolite ratio as a predictor of cigarette consumption. Nicotine Tob Res. 2003;5:621–4. doi: 10.1080/1462220031000158717. [DOI] [PubMed] [Google Scholar]
- 16.Ray R, Tyndale RF, Lerman C. Nicotine dependence pharmacogenetics: role of genetic variation in nicotine-metabolizing enzymes. J Neurogenet. 2009;23:252–61. doi: 10.1080/01677060802572887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piper ME, Smith SS, Schlam TR, et al. A randomized placebo-controlled clinical trial of 5 smoking cessation pharmacotherapies. Arch Gen Psychiatry. 2009;66:1253–62. doi: 10.1001/archgenpsychiatry.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 19.Piper ME, Federman EB, McCarthy DE, et al. Efficacy of bupropion alone and in combination with nicotine gum. Nicotine Tob Res. 2007;9:947–54. doi: 10.1080/14622200701540820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sobell LC, Sobell MB. Timeline Follow-Back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen J, editors. Measuring alcohol consumption: Psychosocial and biological methods. Human Press; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- 21.St Helen G, Novalen M, Heitjan DF, et al. Reproducibility of the nicotine metabolite ratio in cigarette smokers. Cancer Epidemiol Biomarkers Prev. 2012;21:1105–14. doi: 10.1158/1055-9965.EPI-12-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lerman C, Tyndale R, Patterson F, et al. Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clin Pharmacol Ther. 2006;79:600–8. doi: 10.1016/j.clpt.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Faucette SR, Hawke RL, Lecluyse EL, et al. Validation of bupropion hydroxylation as a selective marker of human cytochrome P450 2B6 catalytic activity. Drug Metab Dispos. 2000;28:1222–30. [PubMed] [Google Scholar]
- 24.Benowitz NL, Zhu AZ, Tyndale RF, Dempsey D, Jacob P., 3rd Influence of CYP2B6 genetic variants on plasma and urine concentrations of bupropion and metabolites at steady state. Pharmacogenet Genomics. 2013;23:135–41. doi: 10.1097/FPC.0b013e32835d9ab0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pogue J, Yusuf S. Overcoming the limitations of current meta-analysis of randomised controlled trials. Lancet. 1998;351:47–52. doi: 10.1016/S0140-6736(97)08461-4. [DOI] [PubMed] [Google Scholar]
- 26.Riet GT, Bachmann LM, Kessels AG, Khan KS. Individual patient data meta-analysis of diagnostic studies: opportunities and challenges. Evid Based Med. 2013 doi: 10.1136/eb-2012-101145. [DOI] [PubMed] [Google Scholar]
- 27.Wassenaar CA, Dong Q, Wei Q, et al. Relationship between CYP2A6 and CHRNA5-CHRNA3-CHRNB4 variation and smoking behaviors and lung cancer risk. J Natl Cancer Inst. 2011;103:1342–6. doi: 10.1093/jnci/djr237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baker TB, Breslau N, Covey L, Shiffman S. DSM criteria for tobacco use disorder and tobacco withdrawal: a critique and proposed revisions for DSM-5. Addiction. 2012;107:263–75. doi: 10.1111/j.1360-0443.2011.03657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kubota T, Nakajima-Taniguchi C, Fukuda T, et al. CYP2A6 polymorphisms are associated with nicotine dependence and influence withdrawal symptoms in smoking cessation. Pharmacogenomics J. 2006;6:115–9. doi: 10.1038/sj.tpj.6500348. [DOI] [PubMed] [Google Scholar]
- 30.Rubinstein ML, Benowitz NL, Auerback GM, Moscicki AB. Rate of nicotine metabolism and withdrawal symptoms in adolescent light smokers. Pediatrics. 2008;122:e643–7. doi: 10.1542/peds.2007-3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Etter JF, Perneger TV. Measurement of self reported active exposure to cigarette smoke. J Epidemiol Community Health. 2001;55:674–80. doi: 10.1136/jech.55.9.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez-Stable EJ, Benowitz NL, Marin G. Is serum cotinine a better measure of cigarette smoking than self-report? Prev Med. 1995;24:171–9. doi: 10.1006/pmed.1995.1031. [DOI] [PubMed] [Google Scholar]
- 33.McDonagh EM, Wassenaar C, David SP, et al. PharmGKB summary: very important pharmacogene information for cytochrome P-450, family 2, subfamily A, polypeptide 6. Pharmacogenet Genomics. 2012;22:695–708. doi: 10.1097/FPC.0b013e3283540217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uhl GR, Liu QR, Drgon T, et al. Molecular genetics of successful smoking cessation: convergent genome-wide association study results. Arch Gen Psychiatry. 2008;65:683–93. doi: 10.1001/archpsyc.65.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lotrich FE. The emerging potential of pharmacogenetics in psychiatry. Am J Psychiatry. 2012;169:681–3. doi: 10.1176/appi.ajp.2012.12040457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.