Abstract
Rationale
Brain orexin 1 receptors (OX1Rs) are involved in food-motivated behavior. Most research has focused on fore-brain OX1R populations, but hindbrain OX1Rs affect feeding. We hypothesized that hindbrain OX1Rs affect the reward value of food.
Objectives
We examined the effects of hindbrain OX1R stimulation or blockade on motivation for food, palatable high-fat (HF) food intake, and food-conditioned place preference.
Methods
Rats trained to lever press for sucrose on a progressive ratio (PR) schedule received fourth intracerebroventricular (icv) injections of vehicle, orexin-A (0.1–1 nmol), or the OX1R antagonist SB334867 (10–20 nmol) before operant test sessions. Effects of these treatments on HF food intake during daily 1-h tests were assessed with fourth icv and nucleus of the solitary tract (NTS) injections. We conditioned a place preference by pairing HF food with one side of a two-sided chamber and then examined the effect of 20 nmol fourth icv SB334867 on the expression of that preference.
Results
In ad lib fed rats on the PR schedule, fourth icv orexin-A significantly increased responding and breakpoint relative to the vehicle. In 24-h food-deprived rats, fourth icv SB334867 significantly decreased responding and breakpoint. Orexin-A delivered to the fourth ventricle (0.1 nmol) or NTS (0.01 nmol) increased HF diet intake. Fourth icv SB334867 did not affect HF food intake, but SB334867 delivered either fourth icv (20 nmol) or intra-NTS (5–10 nmol) suppressed chow intake. Expression of HF food-conditioned place preference was inhibited by fourth icv SB334867.
Conclusions
Hindbrain OX1R activity affects food-motivated operant behavior and may play a role in responding to cues that predict palatable food.
Keywords: Orexin, Hindbrain, Nucleus of the solitary tract, High-fat food, Progressive ratio, Conditioned place preference
Introduction
Food intake is governed not only by homeostatic need but also by reward-related factors such as palatability of food, motivation to obtain food, and learned associations and cues that predict food availability. Eating driven by food reward, sometimes called “hedonic” eating, is thought to be a major contributor to overeating and obesity (Volkow et al. 2013). It is, therefore, critical that we improve our understanding of brain mechanisms that control hedonic eating. In the studies presented here, we explored the involvement of caudal brainstem orexin 1 receptors (OX1Rs) in food reward. Orexin-A and orexin-B, also known as hypocretin-1 and hypocretin-2, are the cleavage products of prepro-orexin (Sakurai et al. 1998). Orexin-A binds to the OX1R and OX2R, while orexin-B binds with much higher affinity to OX2R than to OX1R (Ammoun et al. 2003). Here, we focus on orexin-A's action at the OX1R for several reasons: (1) orexin-A treatment increases food intake (Haynes et al. 1999), (2) the OX1R-selective antagonist SB334867 reduces feeding without causing behaviors that indicate malaise or distress (Ishii et al. 2004), and (3) activation of the OX1R by orexin-B has little effect on feeding (Haynes et al. 1999). Orexin cell bodies reside in the lateral hypothalamus and perifornical area and project widely (Peyron et al. 1998) to many food intake-relevant areas where OX1Rs are expressed (Hervieu et al. 2001; Marcus et al. 2001). These neurons are activated by cues that predict highly rewarding food stimuli (Choi et al. 2010) and can also be activated by pharmacologic treatments that elicit hyperphagia for palatable food (Zheng et al. 2003). Injection of the mu-opioid receptor agonist DAMGO into the nucleus accumbens (NAc) strongly stimulates high-fat (HF) food intake in the absence of any homeostatic need to eat, and this manipulation induces c-fos expression in orexin neurons (Zheng et al. 2003). Blockade of OX1R in the ventral tegmental area (VTA) prevents intra-NAc DAMGO from increasing HF intake (Zheng et al. 2007), suggesting that endogenous orexin-A release at this site is relevant for this sort of hedonic eating. Motivation to obtain palatable food has often been assessed through the measurement of operant responding for palatable food reinforcement on a progressive ratio (PR) schedule of reinforcement. On a PR schedule, the rat must make an increasing number of operant responses to obtain each successive reinforcement. Injection of orexin-A into the third cerebral ventricle increases responding for sucrose pellet reinforcers on a PR schedule (Choi et al. 2010), and conversely, intraperitoneal (IP) injection of the OX1R antagonist SB334867 reduces PR responding for both palatable (sweetened HF or sucrose) and standard food pellets (Cason and Aston-Jones 2013; Choi et al. 2010; Sharf et al. 2010).
Research on orexin involvement in food reward has focused largely on the VTA as a mediatory site. A recent paper also implicates OX1R in the paraventricular nucleus of the thalamus (PVT) which contributes to hedonic eating of HF food (Choi et al. 2012). We hypothesized that caudal brainstem OX1R play a role in food reward, as well. The nucleus of the solitary tract (NTS) and area postrema (AP) are well established to play a role in the control of food intake through the detection of within-meal gastrointestinal satiation signals as well as the adiposity hormone leptin (Grill 2010). The orexin neuron projection to the dorsal vagal complex (NTS, AP, and the dorsal motor nucleus of the vagus nerve) has been demonstrated by retrograde tracing and immunohis-tochemical studies (Peyron et al. 1998; Zheng et al. 2005), and OX1Rs are expressed in these nuclei (Hervieu et al. 2001; Marcus et al. 2001). Orexin-A increases meal size when delivered to the hindbrain via fourth intracerebroventricular (icv) injection (Baird et al. 2009; Parise et al. 2011), and we have demonstrated that endogenous activation of hindbrain OX1Rs do the same: meal size is reduced by fourth icv injection of SB334867 (Parise et al. 2011). We also showed that doses of fourth icv orexin-A and SB334867 that affect meal size have no effects on spontaneous locomotor activity, so their feeding effects are not secondary to general changes in arousal or motor behavior (Parise et al. 2011). We hypothesize that these food intake effects of hindbrain OX1R stimulation are, at least in part, due to the influence of these receptors on food reward. Here, we investigated the effects of fourth icv orexin-A and the OX1R antagonist on HF food intake, PR responding for sucrose pellets, and expression of a place preference conditioned by HF diet. We also targeted injections of orexin-A and SB334867 directly to the NTS to determine whether OX1R at this site play a role in the control of feeding.
Materials and methods
Subjects
Adult male Wistar rats (Harlan, Prattville, AL) with a mean body weight of 345 g at the time of surgery were individually housed in Plexiglas cages with food hoppers in temperature-controlled vivariums maintained on a 12:12-h light–dark cycle. Distilled water and rat chow (5001; Purina, St. Louis, MO) were available ad libitum except where otherwise noted. All subjects were handled daily and habituated to experimental procedures before the studies. All experimental procedures were approved by the Florida State University Institutional Animal Care and Use Committee.
Surgery
Rats were implanted with 26-G guide cannulae (Plastics One, Roanoke, VA) targeting either the fourth ventricle or the NTS as described previously (Williams et al. 2009). Stereotaxic coordinates for fourth icv cannulae were on the midline, 2.5 mm anterior to the occipital suture and 5.2 mm ventral to the skull surface. Unilateral NTS cannulae were implanted at 0.6 mm to the right of the midline, on the occipital suture, and 7 mm ventral to surface of the skull. Carprofen (5 mg/kg sc) (Butler Schein Animal Health Supply, Dublin, OH) was administered with the onset of surgery and, again, if rats showed signs of distress (e.g., weight loss, poor grooming). Body weight and food intake were monitored while rats recovered for at least 5 days before experimental procedures began. Fourth icv and NTS cannula placements were verified before the start of experiments through the measurement of a sympathetically mediated increase in plasma glucose 45– 60 min after injection of 5-thio-D-glucose (MP Biomedicals, Santa Ana, CA) at a dose of 210 μg/2 μl for the fourth ventricle or 50 μg/0.5 μl for the NTS (Ritter et al. 1981, 2000).
NTS cannula placements were also verified histologically after behavioral experiments were completed. Rats were anesthetized (180 mg/kg ketamine and 30 mg/kg xylazine, i.p.) and received intra-NTS injections of 0.5 μl of 2 % True Blue (Sigma-Aldrich, St. Louis, MO) dissolved in sterile water. Thirty minutes later, they were transcardially perfused with 10 mM phosphate-buffered saline (PBS) and 4 % paraformal-dehyde (Electron Microscopy Sciences, Hatfield, PA). Brains were removed and sunk in 30 % sucrose in PBS and then frozen in isopentane on dry ice. Coronal cryostat sections (20 μm) through the NTS were slide mounted and stored at −80 °C. Sections were examined with the Olympus BX41 fluorescence microscope, and monochromatic digital images were acquired with the Retiga EXI Aqua camera and Q-Capture software (Hunt Optics, Pittsburgh, PA). Injection sites were identified by injection-related damage and the presence of tracer labeling. Injection sites within the boundaries of the NTS as drawn in the atlas of Paxinos and Watson (2007) were considered correct.
Drugs and injections
Orexin-A (Bachem, Torrance, CA) was dissolved in sterile 0.9 % saline. The OX1R antagonist SB334867 was obtained from Tocris Bioscience (Ellisville, MO) and was dissolved in 66 % DMSO and 3.4 % hydroxypropyl-β cyclodextrin in sterile deionized water for the PR operant responding and conditioned place preference experiments and 100 % DMSO for the food intake experiments. In all experiments, the corresponding vehicle control was used for comparison with drug. The vehicles for SB334867 differed across these studies because 100% DMSO was required to dissolve the compound at sufficiently high concentration to deliver the desired dose intraparenchymally. Blevins and colleagues (2002) demonstrated that 75 % DMSO injected into the paraventricular nucleus of the hypothalamus has no detectable impact on food intake relative to artificial cerebrospinal fluid (acsf), and brains of rats injected with 75 % DMSO showed no gross histological differences from those of rats injected with acsf. We confirm this in the present studies for fourth icv and NTS injections of 66–100 % DMSO (see Supplemental Figs.1 and 2). Injections were made with a 10-μl syringe (Hamilton, Reno, NV) connected to a 33-G injector (Plastics One, Roanoke, VA) via the Tygon tubing (VWR, Radnor, PA). Injectors extended 2 mm beyond the end of the guide cannu-lae. Injections were delivered at a rate of 1 μl/min to the fourth ventricle or 0.25 μl/min to the NTS. Fourth icv injection volume was 2 μl, and NTS injections were 0.5 μl.
Effects of fourth icv orexin-A or SB334867 on PR operant responding for sucrose
Naïve male rats with fourth icv cannulae were trained to lever press for sucrose in operant conditioning chambers (Coulbourn Instruments, Allentown, PA) fitted with two response levers, one active and one inactive. Presses on the active lever were reinforced, whereas inactive lever presses were not reinforced. During operant training sessions, a cue light was illuminated above the active lever. The location of the active and inactive levers was counterbalanced across subjects. During 2-h sessions, rats were trained to receive a 45-mg sucrose pellet reinforcer (TestDiet, Richmond, IN) on a fixed ratio continuous reinforcement schedule with a 5-s time- out after each reinforcement. When rats showed stable responding (less than 10 % day-to-day variation), they were switched to a PR schedule. Increases in the ratio requirement followed the algorithm of Richardson and Roberts (1996): 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 52, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492, 603… lever presses for reinforcement. PR sessions were terminated if the rat failed to press the active lever for 30 min, with a maximum session length of 2 h. In the experiments presented here, rats required 10–12 sessions on a PR schedule before responding is stabilized. After responding was stable, they were habituated to the fourth icv injection procedure by receiving a 2-μl saline injection 20 min before the start of the test session in the operant chambers. We chose to use sucrose reinforcers in these experiments based on previous findings that third icv orexin-A increases PR responding for sucrose (Choi et al. 2010) and that peripheral injection of SB334867 decreases PR responding for sucrose (Cason and Aston-Jones 2013; España et al. 2010).
On experimental treatment days, one group of rats (n =8) received a fourth icv injection of saline vehicle, 0.1 nmol orexin-A, or 1.0 nmol orexin-A. These doses were chosen based on our previous observation that they significantly affect dark-phase chow intake (Parise et al. 2011). All subjects received all treatment conditions in counterbalanced order with injections separated by at least 48 h. Between drug treatment days, these rats were given identical 2-h sessions in the operant chambers with no injections. A second group of rats (n =16) were food deprived overnight before operant responding test sessions, and they were injected with vehicle, 10 nmol SB334867, or 20 nmol SB334867 prior to the start of the sessions. We chose these doses based on our previous observation that 20 nmol SB334867 suppresses dark-phase chow intake (Parise et al. 2011). We chose to food deprive the rats in this experiment because previous studies have shown that peripheral injection of SB334867 does not suppress op-erant responding in non-deprived rats (Cason et al. 2010; Cason and Aston-Jones 2013). After test sessions, rats received ad lib access to chow in their home cages and were monitored for recovery of lost body weight before the next test session. Rats received all drug conditions in counterbalanced order separated by at least 72 h and were not given operant responding test sessions on days between drug conditions.
Effects of fourth icv orexin-A or SB334867 on HF diet intake
The effects of hindbrain orexin-A on sucrose intake have been explored previously (Baird et al. 2009), so we examined effects on intake of HF food, another type of palatable food that is readily consumed even in the absence of homeostatic need. Naïve male rats (n =11) implanted with fourth icv cannulae were habituated to receive daily 1-h access to a pre-weighed hopper filled with HF diet (60 % fat; Research Diets, New Brunswick, NJ) in their home cages during the mid-light phase. Chow was removed from the cage, but water was available ad lib during these test sessions. After the 1-h test period, HF food was removed from the cages and weighed, and chow was returned. When HF food intakes were stable (less than 10 % variation over three consecutive days), rats received a fourth icv saline injection 20 min prior to HF access to habituate them to the injection procedure. Chow hoppers were removed at the time of injection. On experimental treatment days, rats received fourth icv injections of either vehicle or 0.1 nmol orexin-A 20 min before the HF intake test. All rats received both conditions in counterbalanced order separated by at least 48 h. Daily 1-h HF access continued between drug treatment days.
After the conclusion of orexin-A vs. vehicle treatments, the same 11 rats were used to examine the effect of the OX1R antagonist. Rats were given 2 weeks of no drug treatment between experiments, and we verified that they showed normal baseline HF intake during 1-h test sessions before beginning the next study. On experimental treatment days, rats received fourth icv injections of either vehicle, 10 nmol SB334867, or 20 nmol SB334867 20 min before the HF intake test. Each rat received all conditions in counterbalanced order separated by at least 48 h, and daily 1-h HF access continued on days between drug treatments. As a control for the effectiveness of the SB334867, we examined the effect of fourth icv SB334867 on chow intake in a subset (n =7) of these rats 1 week after the HF intake experiment was complete. During that week off, chow intake and body weight were measured, and we verified that rats showed no lingering effects of previous drug treatments. They received either vehicle or 20 nmol SB334867 20 min before the onset of the dark phase, and chow intake was measured 30 min later.
Effect of NTS orexin-A or SB334867 on food intake
Naïve male rats (n =10) with cannulae targeting NTS were trained to receive daily 1-h access to HF diet as described above. On experimental treatment days, they received intraNTS injections of either vehicle or 0.01 nmol orexin-A. We chose this dose based on a pilot study in which we determined that it is subthreshold for effect when delivered to the fourth ventricle.
Because fourth icv SB334867 failed to suppress HF diet intake but does suppress chow intake during the first meal of the dark phase (Parise et al. 2011), we examined the effects of NTS OX1R blockade on chow intake. Naïve male rats (n =17) with cannulae targeting NTS were habituated to the intra-NTS injection procedure and dark cycle chow intake measurement. On treatment days, they received intra-NTS injections 20 min before dark cycle onset. One group (n =8) received either vehicle or 5 nmol SB334867, and the other group (n =9) received either vehicle or 10 nmol SB334967. These doses were chosen based on a pilot study in which we observed that they were subthreshold for effect when delivered to the fourth ventricle. Each rat received both the vehicle and drug condition in counterbalanced order separated by at least 48 h.
Effect of fourth icv SB334867 on HF food-conditioned place preference
In this conditioned place preference (CPP) procedure, we trained naïve male rats (n =15) with fourth icv cannulae to associate a location with access to HF diet (60 % fat; Research Diets, New Brunswick, NJ) using an approach similar to that reported previously (Figlewicz et al. 2004). We used HF food as the unconditioned stimulus as opposed to sucrose in this experiment because it has been reported that substantial food restriction is required to obtain CPP using sucrose (Figlewicz et al. 2001). The CPP system (Coulbourn Instruments, Allentown, PA) consists of two distinct chambers connected by a computer-controlled gate that can either prevent or allow the rat to travel between chambers. One chamber has black Plexiglass walls and a smooth floor, and the other has clear Plexiglass walls and a textured floor. Photobeams located on either side of the gate allow computer measurement of rats' movements between chambers when the gate is open. The system is housed in a sound-attenuating cabinet. Before CPP training began, rats were given 1 g HF diet in their home cages to minimize any potential effect of novelty of the food during CPP training. Subsequently, rats were given pretraining tests in which they were placed in the chamber with the gate opened for 10 min, to establish baseline preference. This was done twice on consecutive days, with the rats starting in the clear-walled chamber on 1 day and in the black-walled chamber on the other. After the 2 days of pretraining tests, CPP training began. Rats were randomly assigned to receive 5 g HF diet in one chamber or the other with the gate closed, so that each rat had a “paired” and “non-paired” chamber. Each day, rats received a 20-min training session, with paired and non-paired sessions alternating for 10 days (e.g., paired sessions on days 1, 3, 5, 7, and 9 and non-paired on days 2, 4, 6, 8, and 10). Order of training sessions was counterbalanced across rats. Rats consumed the entire 5 g HF diet on all occasions. After 10 days of training, we conducted a preference test by placing the rats into the chambers with the gates opened for 10 min. No food was present during the post-training preference test. Rats were randomly assigned to receive fourth icv injections of either vehicle (n =8) or 20 nmol SB334867 (n =7) 15 min before this preference test, and the starting side was counterbalanced across rats. The time spent in either chamber was recorded and compared with the average time spent during the two pretraining tests. Percentage change in preference for the HF-paired chamber was calculated as [(posttest-pretest)/(test duration-pre-test)]×100. Number of crossings from one chamber to the other was also recorded.
Statistical analysis
The effects of fourth icv orexin-A or SB334867 were analyzed with repeated measures ANOVA and Tukey's honestly significant difference (HSD) post hoc test where appropriate. NTS orexin-A and SB334867 effects were assessed via paired samples Student's t tests. CPP data were analyzed by mixed design two-way ANOVA (factors: pretest vs. posttest, drug) with Tukey's HSD post hoc test, and independent samples Student's t test for percent change in preference. p values of <0.05 were considered significant.
Results
Effect of fourth icv orexin-A or SB334867 on PR operant responding for sucrose
Hindbrain ventricle administration of orexin-A significantly increased active lever presses for sucrose reinforcement (F (2, 14)=11.32, p <0.01) (Fig. 1a). Presses on the active lever were increased by both 0.1 nmol orexin-A (p <0.05) and 1 nmol orexin-A (p <0.001) relative to vehicle, and responding after 1 nmol was significantly higher than after 0.1 nmol (p <0.05). There was no drug effect on inactive lever presses (mean, 11.0±3.3). Breakpoint, the highest completed ratio requirement, was significantly increased by fourth icv orexin-A, as well (F (2, 14)=11.24, p <0.01) (Fig. 1b). Here, only breakpoint after the 1 nmol orexin-A dose differed significantly from vehicle (p <0.01), while differences between vehicle and the 0.1 nmol dose and between 0.1 and 1 nmol orexin-A doses failed to reach significance (p = 0.06). Number of rein-forcers obtained was significantly increased by orexin-A in a dose-responsive manner (F (2, 14)= 19.12, p <0.001), with each condition differing significantly from the others (p <0.05) (Fig. 1c). Orexin-A also increased the length of the operant responding test session in a dose-responsive manner (F (2, 14)=83.90, p <0.001), from a mean of 45.4±2.7 min after vehicle, 73.4±6.7 min after 0.1 nmol orexin-A, and 124.9±8.5 min after 1 nmol orexin-A (each vs. the others, p < 0.01).
Fig. 1.
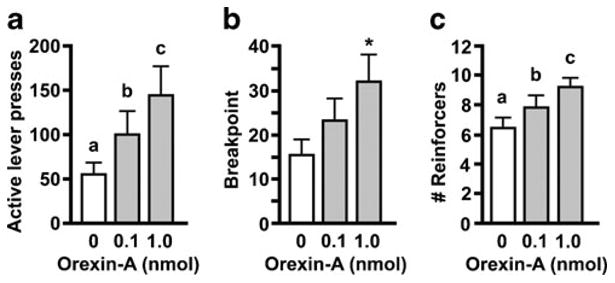
Effect of fourth icv orexin-A on active lever presses (a), breakpoint (b), and number of reinforcers earned (c) while responding for sucrose on a PR schedule. Data are mean±standard error of the mean (SEM). a, c, Significant differences (p <0.05) among conditions are denoted by different letters above bars. b *p <0.05, compared to vehicle
SB334867 administered to the fourth ventricle had a smaller but significant effect on PR responding. Active lever presses were significantly reduced by SB334867 (F (2, 30)=9.57, p < 0.001), with both the 10 and 20 nmol doses significantly differing from vehicle (p <0.01) (Fig. 2a). There was a significant reduction in breakpoint (F (2, 30)=4.99, p <0.05), with the 20 nmol dose significantly differing from vehicle (Fig. 2b). Number of reinforcers obtained was also significantly suppressed by fourth icv SB334867 (F (2, 30)= 6.07, p < 0.01) (Fig. 2c), with both the 10 and 20 nmol doses differing significantly from vehicle. There were no effects on inactive lever presses or session duration.
Fig. 2.
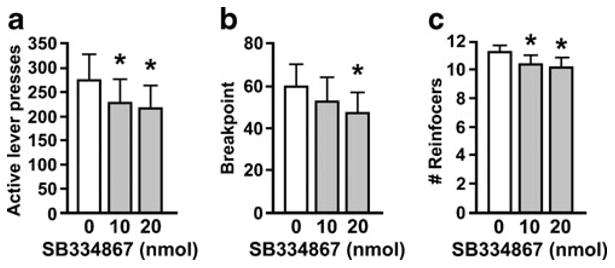
Effect of fourth icv SB334867 on active lever presses (a), breakpoint (b), and number of reinforcers earned (c) while responding for sucrose on a PR schedule. Data are mean±SEM. *p <0.05, compared to vehicle
Effects of fourth icv orexin-A or SB334867 on HF intake
Hindbrain ventricular injection of orexin-A strongly and significantly elevated 1-h HF food intake (t (10)=7.48, p <0.001) (Fig. 3a). However, administration of SB334867 to the fourth ventricle had no significant effect on 1-h HF intake (Fig. 3b). The same rats that failed to suppress HF intake after SB334867 did show a significant reduction in 30-min chow intake (t (6)=2.45, p <0.05) with drug treatment (Fig. 3c) similar to that which we previously reported (Parise et al. 2011), confirming that the lack of effect on HF intake was not due to poor quality or inactivity of the drug.
Fig. 3.
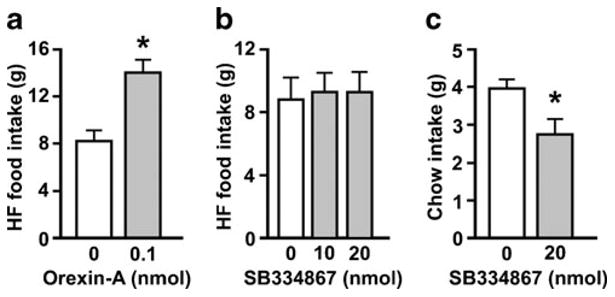
1-h HF diet intake is increased by fourth icv orexin-A (a) but is not affected by fourth icv SB334867 (b). Fourth icv SB334867 suppresses chow intake during the first 30 min of the dark phase (c). *p <0.05, compared to vehicle
Effect of NTS orexin-A or SB334867 on food intake
Intra-NTS orexin-A, delivered at a dose that is subthreshold for effect when injected into the ventricle, significantly increased HF diet intake (t (9)=2.88, p <0.01) (Fig. 4a). NTS injection of SB334867 significantly suppressed 30-min chow intake relative to vehicle at both the 5 nmol (t (7)=3.07, p <0.01) and 10 nmol (t (8)=2.34, p <0.05) doses (Fig. 4b). Representative images of NTS injection spread and injection sites are shown in Fig. 4c-g.
Fig. 4.
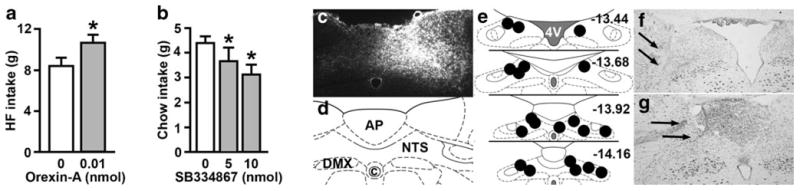
NTS injection of orexin-A increases 1-h HF food intake (a), and NTS injection of SB334867 suppresses chow intake during the first 30 min of the dark phase (b). *p <0.05, compared to vehicle. c A representative image of a NTS injection site and spread of 0.5 μl True Blue. d A corresponding diagram of the area based on the atlas of Paxinos and Watson (2007). e Also based on the atlas of Paxinos and Watson (2007), a representative image of NTS injection sites for the rats used in these studies is shown, ranging from −13.44 to −14.16 mm relative to bregma. Additional rats' injections were identified in similar locations at points between the anterior and posterior levels shown here. f, g Representative images of thionine-stained sections showing representative NTS injection sites, identified by black arrows, in rats that were injected with 100 % DMSO
Effect of fourth icv SB334867 on HF food-conditioned place preference
Vehicle-treated rats showed a significant increase in time spent in HF-paired chamber from pre- to post-training test (p <0.01), whereas SB334867-treated rats showed no change (interaction, F (1, 13)=10.03, p <0.01) (Fig. 5a). Percent increase in preference for HF-paired chamber differed significantly across groups (t (13)=3.54, p <0.01) (Fig. 5b). There was no drug effect on the number of crossings between chambers (vehicle, 29.7±3.5; SB334867, 27.2±2.3).
Fig. 5.
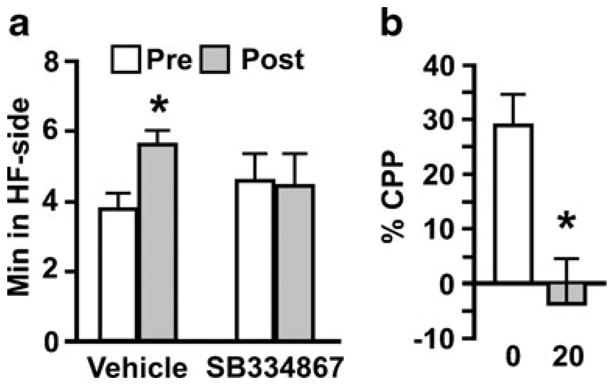
Effect of fourth icv SB334867 on CPP induced by HF food. Time spent in the HF-paired side of the chamber during the pretest vs. the post-training test (a). *p <0.05, compared to pretest time. Percent change in preference from the pretest to posttest (b). *p <0.05, compared to vehicle
Discussion
Our results suggest that hindbrain OX1Rs play a role in motivation for palatable food and that hindbrain OX1R stimulation can increase palatable, energy-dense food intake. Orexin-A injection into the fourth ventricle robustly increased operant responding for sucrose pellets on a PR schedule, significantly increasing breakpoint, while fourth icv delivery of the OX1R antagonist SB334867 significantly suppressed PR responding and breakpoint for sucrose reinforcement. We also observed that hindbrain orexin-A treatment potently increased HF diet intake during 1-h access tests, and others have previously shown that fourth icv orexin-A injection stimulates licking for sucrose solutions (Baird et al. 2009). In addition, blockade of hindbrain OX1Rs completely prevented the expression of a place preference conditioned by HF food. Together, these data highlight the ability of caudal brainstem OX1Rs to influence food reward and intake of palatable foods.
The effects of orexin-A injection are pharmacologic in nature, because even the lowest doses used most likely provide significantly more agonist to receptors than would be endogenously released. While those experiments support the hypothesis that OX1R stimulation can drive palatable food intake and operant responding for food, they do not provide insight into the role of endogenous OX1R stimulation. In addition, it is possible that some of the observed effects of orexin-A are mediated by OX2R, which is expressed in the dorsomedial hindbrain (Marcus et al. 2001). For this reason, we examined responses to hindbrain injection of the selective OX1R antagonist SB334867. An effect of the antagonist can be interpreted as resulting from a loss of endogenous OX1R activation. Based on the effects of fourth icv injection of orexin-A, we expected that fourth icv SB334867 would suppress progressive ratio responding and HF food intake. The OX1R antagonist did significantly suppress operant responding and breakpoint for sucrose, so we conclude that endogenous stimulation of hindbrain OX1Rs contributes to motivation for sucrose. However, fourth icv SB334867 failed to affect HF food intake. We believe that this lack of effect is not due to inactivity of the drug itself, because fourth icv SB334867 was able to significantly suppress chow intake in a subset of the same rats that showed no response to the drug when ingesting HF food. Although hindbrain orexin-A can drive HF food intake, endogenous ligand action at hindbrain OX1Rs does not appear to make a significant contribution to ingestion of HF food, at least under the specific conditions of our experiment.
The results of our CPP study also suggest that endogenous stimulation of OX1Rs in the hindbrain contributes to at least some aspects of food reward. Rats in the vehicle group showed a significant increase in preference for the HF-paired chamber, as expected (Figlewicz et al. 2004), but rats given fourth icv injection of SB334867 prior to the preference test showed no preference for one side vs. the other. We are confident that this lack of preference is not due to suppression of locomotor activity, because we have previously shown that this dose delivered via fourth icv injection has no effect on locomotor activity (Parise et al. 2011), and in this study, rats treated with vehicle and SB334867 crossed between chambers with equivalent frequency. It is particularly surprising that blockade of OX1Rs had a large impact on place preference conditioned by HF food, but no effect on ingestion of the same HF food. However, it is not unprecedented to find drug effects on one feeding-related behavior, but not another. For example, antagonism of D1 and D2 receptors in the NAc suppresses operant responding for food but does not reduce food intake (Nowend et al. 2001). Another recent study showed that dopamine receptors in the NAc mediate the ability of intra-VTA ghrelin treatment to increase operant responding for sucrose on a PR schedule but are not involved in ghrelin's stimulatory effect on chow intake (Skibicka et al. 2013). Specifically with respect to brain OX1R involvement in food intake vs. food reward, Choi and colleagues (2012) have reported a dissociation in the opposite direction from our findings: knockdown of OX1R in PVT reduces HF food intake in satiated rats but has no impact on operant responding for sucrose on a PR schedule. These differential effects underscore the point that while food consumption may often be influenced by food-related motivation/reward, these are distinct and can be measured and manipulated independently. Further research will be needed to clarify exactly what aspects of food intake and reward are influenced by endogenous activation of hindbrain and other populations of OX1Rs.
Most previous investigations of orexin-A and OX1R effects on food reward have not targeted specific brain sites. Choi and colleagues (2010) showed that third icv orexin-A increased lever pressing for sucrose on a PR schedule in an experiment similar to that reported here. Injection into the third ventricle effectively delivers the drug to hypothalamic areas as well as more caudal sites through the ventricular system, so our finding that fourth icv orexin-A stimulates this same behavior raises the possibility that some portion of the effects of forebrain ventricular orexin-A application are, in fact, mediated by hindbrain receptor populations. A number of other groups have previously shown that peripheral injection of SB334867 suppresses operant responding for sucrose pellets (Cason and Aston-Jones 2013; España et al. 2010) and for sweet HF reinforcers (Choi et al. 2010; Borgland et al. 2009; Nair et al. 2008). One study showed no effect of SB334867 to suppress responding for sucrose (Richards et al. 2008), but this may have been due to the lack of food restriction in that study or related to their use of low-concentration sucrose solution as opposed to sucrose pellets as a reinforcer. SB334867 crosses the blood–brain barrier, and its effects on behavior are generally assumed to be mediated centrally (Porter et al. 2001), but IP injection studies allow no conclusions about specific brain areas to be drawn. Here, too, our findings raise the possibility that some of the effects of peripheral SB334867 administration on responding for sucrose reflect the action of OX1Rs in the hindbrain. Because we did not assess operant responding for HF food reinforcers here, we cannot say whether hindbrain OX1R may be involved in any part of the suppressive effects of IP SB334867 on responding for HF food observed by several laboratories (Choi et al. 2010; Borgland et al. 2009; Nair et al. 2008). It is notable that the effect sizes reported in those studies using HF reinforcers are large relative to those seen in studies using sucrose, but it is impossible to say whether this is due to the differential involvement of OX1R in responding for sucrose vs. HF food or to other differences in experimental paradigms across laboratories.
Because cerebrospinal fluid flows in a rostro-caudal direction in the ventricular system, we can be confident that fourth icv injections delivered orexin-A or SB334867 to the caudal brainstem and not to more rostral brain regions. However, ventricular administration does not allow the identification of the specific brain nuclei that mediate the observed effects. We focused on the NTS as a candidate site because NTS neurons express OX1Rs (Marcus et al. 2001), orexin-A-positive fibers are found within the NTS (Peyron et al. 1998; Zheng et al. 2005; Parise et al. 2011), and fourth icv injection of either orexin-A (Zheng et al. 2005) or SB334867 (Parise et al. 2011) induces c-fos expression in NTS. Zheng and colleagues (2005) examined the effects of intra-NTS orexin-A on feeding previously and reported no effect on chow intake. When they examined effects on HF food intake, they found that a dose of 0.4 nmol orexin-A increased 30-min intake, while raising the dose to 1 nmol caused a significant suppression of HF intake. In our hands, these doses are above threshold for intake-stimulatory effects when delivered to the fourth ventricle, so we examined the effect of 0.01 nmol orexin-A, which we determined to be below threshold for effect when delivered via fourth icv injection. Here, we report that this dose significantly increases HF food intake during a short mid-light-phase test. Although we did not observe effects of SB334867 on HF food intake, we did find that intra-NTS injection of the OX1R antagonist suppressed 30-min dark-phase chow intake, again at doses that are subthreshold for effect in the ventricle. While our findings do not rule out contributions of other nearby structures where OX1Rs are expressed, such as the area postrema, our data support the hypothesis that OX1Rs in the NTS are involved in food intake control. These results raise the possibility that NTS OX1Rs also underlie the observed effects of fourth icv orexin-A and SB334867 on food reward-related behaviors, although this remains to be investigated.
Orexin neurons have previously been implicated in food reward, but a contribution of hindbrain OX1Rs to this function had not previously been considered. Our data support the hypothesis that hindbrain OX1Rs play a role in food reward-related behaviors, but OX1Rs in the other brain regions, such as the VTA, are most likely involved in these behaviors, as well. Research on caudal brainstem control of feeding has focused primarily on mediation of gastrointestinal satiation signals and, to a lesser extent, adiposity signals such as leptin. Here, we provide evidence that hindbrain-targeted manipulations can influence food reward. Although the present studies focused on orexin-A and OX1R action in hindbrain, we speculate that additional research will reveal other mechanisms through which caudal brainstem nuclei contribute to food reward.
Supplementary Material
Acknowledgments
This work was funded by NIH grants DK078779 and DK095757 to D.L.W.
Footnotes
Electronic supplementary material: The online version of this article (doi:10.1007/s00213-013-3248-9) contains supplementary material, which is available to authorized users.
Conflict of interest: The authors have no conflicts of interest to disclose.
References
- Ammoun S, Holmqvist T, Shariatmadari R, Oonk HB, Detheux M, Parmentier M, Akerman KE, Kukkonen JP. Distinct recognition of OX1 and OX2 receptors by orexin peptides. J Pharmacol Exp Ther. 2003;305:507–514. doi: 10.1124/jpet.102.048025. [DOI] [PubMed] [Google Scholar]
- Baird JP, Choe A, Loveland JL, Beck J, Mahoney CE, Lord JS, Grigg LA. Orexin-A hyperphagia: hindbrain participation in consum-matory feeding responses. Endocrinology. 2009;150:1202–1216. doi: 10.1210/en.2008-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins JE, Stanley BG, Reidelberger RD. DMSO as a vehicle for central injections: tests with feeding elicited by norepinephrine injected into the paraventricular nucleus. Pharmacol Biochem Behav. 2002;71:277–282. doi: 10.1016/s0091-3057(01)00659-1. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci A. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29:11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason AM, Aston-Jones G. Role of orexin/hypocretin in conditioned sucrose-seeking in rats. Psychopharmacology (Berl) 2013;226:155–165. doi: 10.1007/s00213-012-2902-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason AM, Smith RJ, Tahsili-Fahadan P, Moorman DE, Sartor GC, Aston-Jones G. Role of orexin/hypocretin in reward-seeking and addiction: implications for obesity. Physiol Behav. 2010;100:419–428. doi: 10.1016/j.physbeh.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DL, Davis JF, Fitzgerald ME, Benoit SC. The role of orexin-A in food motivation, reward-based feeding behavior and food-induced neuronal activation in rats. Neuroscience. 2010;167:11–20. doi: 10.1016/j.neuroscience.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Choi DL, Davis JF, Magrisso IJ, Fitzgerald ME, Lipton JW, Benoit SC. Orexin signaling in the paraventricular thalamic nucleus modulates mesolimbic dopamine and hedonic feeding in the rat. Neuroscience. 2012;210:243–248. doi: 10.1016/j.neuroscience.2012.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- España RA, Oleson EB, Locke JL, Brookshire BR, Roberts DC, Jones SR. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci. 2010;31:336–348. doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figlewicz DP, Higgins MS, Ng-Evans SB, Havel PJ. Leptin reverses sucrose-conditioned place preference in food-restricted rats. Physiol Behav. 2001;73:229–234. doi: 10.1016/s0031-9384(01)00486-3. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Bennett J, Evans SB, Kaiyala K, Sipols AJ, Benoit SC. Intraventricular insulin and leptin reverse place preference conditioned with high-fat diet in rats. Behav Neurosci. 2004;118:479–487. doi: 10.1037/0735-7044.118.3.479. [DOI] [PubMed] [Google Scholar]
- Grill HJ. Leptin and the systems neuroscience of meal size control. Front Neuroendocrinol. 2010;31:61–78. doi: 10.1016/j.yfrne.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes AC, Jackson B, Overend P, Buckingham RE, Wilson S, Tadayyon M, Arch JR. Effects of single and chronic intra-cerebroventricular administration of the orexins on feeding in the rat. Peptides. 1999;20:1099–1105. doi: 10.1016/s0196-9781(99)00105-9. [DOI] [PubMed] [Google Scholar]
- Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA. Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience. 2001;103:777–797. doi: 10.1016/s0306-4522(01)00033-1. [DOI] [PubMed] [Google Scholar]
- Ishii Y, Blundell JE, Halford JC, Upton N, Porter R, Johns A, Rodgers RJ. Differential effects of the selective orexin-1 receptor antagonist SB-334867 and lithium chloride on the behavioural satiety sequence in rats. Physiol Behav. 2004;81:129–140. doi: 10.1016/j.physbeh.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- Nair SG, Golden SA, Shaham Y. Differential effects of the hypocretin 1 receptor antagonist SB 334867 on high-fat food self-administration and reinstatement of food seeking in rats. Br J Pharmacol. 2008;154:406–416. doi: 10.1038/bjp.2008.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowend KL, Arizzi M, Carlson BB, Salamone JD. D1 or D2 antagonism in nucleus accumbens core or dorsomedial shell suppresses lever pressing for food but leads to compensatory increases in chow consumption. Pharmacol Biochem Behav. 2001;69:373–382. doi: 10.1016/s0091-3057(01)00524-x. [DOI] [PubMed] [Google Scholar]
- Parise EM, Lilly N, Kay K, Dossat AM, Seth R, Overton JM, Williams DL. Evidence for the role of hindbrain orexin-1 receptors in the control of meal size. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1692–R1699. doi: 10.1152/ajpregu.00044.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; New York: 2007. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RA, Chan WN, Coulton S, Johns A, Hadley MS, Widdowson K, Jerman JC, Brough SJ, Coldwell M, Smart D, Jewitt F, Jeffrey P, Austin N. 1,3-Biarylureas as selective non-peptide antagonists of the orexin-1 receptor. Bioorg Med Chem Lett. 2001;11:1907–1910. doi: 10.1016/s0960-894x(01)00343-2. [DOI] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, Bartlett SE. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology (Berl) 2008;199:109–117. doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Ritter RC, Slusser PG, Stone S. Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science. 1981;213:451–452. doi: 10.1126/science.6264602. [DOI] [PubMed] [Google Scholar]
- Ritter S, Dinh TT, Zhang Y. Localization of hindbrain glucoreceptive sites controlling food intake and blood glucose. Brain Res. 2000;856:37–47. doi: 10.1016/s0006-8993(99)02327-6. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:1. doi: 10.1016/s0092-8674(02)09256-5. page following 696. [DOI] [PubMed] [Google Scholar]
- Sharf R, Sarhan M, Brayton CE, Guarnieri DJ, Taylor JR, DiLeone RJ. Orexin signaling via the orexin 1 receptor mediates operant responding for food reinforcement. Biol Psychiatry. 2010;67:753–760. doi: 10.1016/j.biopsych.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibicka KP, Shirazi RH, Rabasa-Papio C, Alvarez-Crespo M, Neuber C, Vogel H, Dickson SL. Divergent circuitry underlying food reward and intake effects of ghrelin: dopaminergic VTA-accumbens projection mediates ghrelin's effect on food reward but not food intake. Neuropharmacology. 2013;73C:274–283. doi: 10.1016/j.neuropharm.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Baler RD. The addictive dimensionality of obesity. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, Baskin DG, Schwartz MW. Hindbrain leptin receptor stimulation enhances the anorexic response to cholecystokinin. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1238–R1246. doi: 10.1152/ajpregu.00182.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Corkern M, Stoyanova I, Patterson LM, Tian R, Berthoud HR. Peptides that regulate food intake: appetite-inducing accumbens manipulation activates hypothalamic orexin neurons and inhibits POMC neurons. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1436–R1444. doi: 10.1152/ajpregu.00781.2002. [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Berthoud HR. Orexin-A projections to the caudal medulla and orexin-induced c-Fos expression, food intake, and autonomic function. J Comp Neurol. 2005;485:127–142. doi: 10.1002/cne.20515. [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007;27:11075–11082. doi: 10.1523/JNEUROSCI.3542-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


