Abstract
Objective
To investigate association of scavenger receptor class B, member 1 (SCARB1) genetic variants with serum carotenoid levels of lutein (L) and zeaxanthin (Z) and macular pigment optical density (MPOD).
Design
A cross-sectional study of healthy adults aged 20-70.
Participants
302 participants recruited following local advertisement.
Methods
MPOD was measured by customized heterochromatic flicker photometry. Fasting blood samples were taken for serum L and Z measurement by HPLC and lipoprotein analysis by spectrophotometric assay. Forty-seven single nucleotide polymorphisms (SNPs) across SCARB1 were genotyped using Sequenom technology. Association analyses were performed using PLINK to compare allele and haplotype means, with adjustment for potential confounding and correction for multiple comparisons by permutation testing. Replication analysis was performed in the TwinsUK and CAREDS cohorts.
Main outcome measures
Odds ratios (ORs) for macular pigment optical density area, serum lutein and zeaxanthin concentrations associated with genetic variations in SCARB1 and interactions between SCARB1 and sex.
Results
Following multiple regression analysis with adjustment for age, body mass index, sex, high-density lipoprotein cholesterol (HDLc), low-density lipoprotein cholesterol (LDLc), triglycerides, smoking, dietary L and Z levels, 5 SNPs were significantly associated with serum L concentration and 1 SNP with MPOD (P<0.01). Only the association between rs11057841 and serum L withstood correction for multiple comparisons by permutation testing (P<0.01) and replicated in the TwinsUK cohort (P=0.014). Independent replication was also observed in the CAREDS cohort with rs10846744 (P=2×10−4), a SNP in high linkage disequilibrium with rs11057841 (r2=0.93). No significant interactions by sex were found. Haplotype analysis revealed no stronger association than obtained with single SNP analyses.
Conclusions
Our study has identified association between rs11057841 and serum L concentration (24% increase per T allele) in healthy subjects, independent of potential confounding factors. Our data supports further evaluation of the role for SCARB1 in the transport of macular pigment and the possible modulation of AMD risk through combating the effects of oxidative stress within the retina.
Keywords: Age-related macular degeneration, association study, lutein, macular pigment, macular pigment optical density, SCARB1, zeaxanthin
Introduction
Age-related macular degeneration (AMD; MIM# 603075) is the most common form of visual impairment among older people of European descent,1 accounting for more than half of all new cases of registered blindness.2 The socioeconomic burden associated with AMD continues to challenge our aging society with almost 30% of those aged 75 years and above showing early signs of disease.3 AMD is a common multifactorial disorder of complex etiology with multiple genetic, environmental and lifestyle risk factors, although the specifics of the etiology remain largely unresolved.4 By definition, AMD specifically affects the macular region of the retina, an area responsible for detailed central vision.
Macular pigment (MP) accumulates in the central retina and is composed of the carotenoids, lutein (L), zeaxanthin (Z) and meso-zeaxanthin (meso-Z), which give the macula its characteristic yellow colour. L and Z are not synthesized de novo in humans and are of dietary origin (mostly fruit and vegetables), whereas meso-Z has been reported to be predominantly non-dietary and formed following conversion from L in the retina,5-6 although the exact process of conversion from L to meso-Z has still to be elucidated.7 Dietary L and Z are absorbed with fats in the gut and are transported to the liver, whereupon they form carotenoid–lipoprotein complexes that facilitate their transport through the vascular system.8 MP confers powerful antioxidant protection and also filters actinic short wavelength, blue light, limiting the (photo-)oxidative damage to retinal cells.9 These properties of MP are believed to limit the development and/or progression of AMD.10 Although MP is dietary derived, its concentration at the macula is, in part, determined by genetic factors with heritability estimated at 67-85% in a cross-sectional study of individual MP levels.11 More recently, longitudinal response to L and Z supplementation has estimated genetic influence approximating 27%, although this may have been influenced by the composition of the L supplement used.12
Genetic variation in SCARB1 has previously been reported in association with AMD, implicating a role for cholesterol and MP metabolism in the disease process.13 The gene, located at 12q24.31, is a region of interest for AMD originating from linkage analysis.4, 14 The SCARB1 gene encodes a multi-ligand cell surface receptor that mediates selective cholesterol uptake and efflux.15-16 Reverse cholesterol transport is a major process required for the clearance of excess cholesterol from the body and the high density lipoprotein cholesterol (HDLc) pathway genes, LIPC and CETP, have been implicated in AMD pathogenesis through large genome-wide association studies.17-18 Other studies have also reported common variants in SCARB1 in association with development of coronary heart disease19 and lipid profiles,20-21 with several studies providing evidence of a sex-related effect.22-24 Both coronary heart disease and dyslipidemia have been reported to share common pathogenic pathways with AMD.25-26 In addition, SRB1, the protein encoded by SCARB1, has also been detected in the retinal pigment epithelium,27-28 and in intestine cells where it mediates cholesterol efflux and xanthophyll uptake.29
In light of the strong evidence implicating cholesterol metabolism in AMD pathogenesis, coupled with a relative lack of MP in association with a clinically confirmed family history of AMD,30 we sought to evaluate common genetic polymorphisms within SCARB1 and how they might contribute to variation of this putatively protective pigment.
Materials and Methods
Participants
Three hundred and two subjects were recruited for this study, which was carried out in the Macular Pigment Research Group (MPRG) laboratory at Waterford Institute of Technology, Ireland. Subjects were recruited following local advertisement in various media. This study was approved by the Research Ethics Committee of Waterford Institute of Technology, and subjects gave written informed consent prior to participation. All experimental procedures adhered to the tenets of the Declaration of Helsinki.
Inclusion criteria for participation in this study were: age between 20 and 70 years, no clinical evidence of ocular pathology, no dietary supplementation with the MP carotenoids, visual acuity 20/40 or better. The following information was recorded for each subject: demographic details, family history of AMD (confirmed in writing by the diagnosing ophthalmologist), personal smoking history, dietary intake of L and Z, assessed using a validated 170-item food frequency questionnaire (FFQ). Examination included: visual acuity (Snellen and LogMAR), body mass index [BMI (calculated as kg/m2)], MP optical density (MPOD) measurement by customized heterochromatic flicker photometry (cHFP) using the Macular Densitometer™, non-mydriatic fundus photography using a NIDEK AFC-210 nonmydriatic auto fundus camera to screen for ocular pathology, 12-hour fasting blood samples were taken to quantify serum concentrations of L and Z using high performance liquid chromatography (HPLC) and for genotyping.
Measurement of macular pigment optical density
MPOD was measured psychophysically by cHFP, a technique that has been validated against the absorption spectrum of MP in vitro.31 cHFP is based on the fact that MP absorbs short-wavelength blue light, with peak absorption occurring at a wavelength of 458 nm. The subject is required to make iso-luminance matches between two flickering lights, a green light (not absorbed by MP) and a blue light (maximally absorbed by MP). The log ratio of the amount of blue light absorbed centrally, where MP peaks, to that absorbed at a peripheral retinal locus (the ‘reference point’, where MPOD is assumed to be zero), gives a measure of the subject's MPOD.
In this study, we used the Macular Densitometer™, a cHFP instrument that is slightly modified from a device described previously.32 The subject is required to observe a flickering target, alternating in square-wave counterphase between a green light (with a wavelength of 564 nm) and a blue light (with a wavelength of 460 nm), and to make isoluminance matches between these flickering lights. The luminance of the green and blue lights is varied in a yoked manner, which avoids a change in the overall luminance of the test target. When an iso-luminant (‘null-flicker’) match has been made between these flickering lights, flicker is no longer perceived, and this is the desired endpoint of the test. Different sized targets enable measurement of MPOD at 0.25°, 0.5°, 1°, and 1.75° retinal eccentricity, relative to a reference point at 7° retinal eccentricity (where MPOD is assumed to be zero). Targets are presented on a blue background test field (wavelength 468 nm) that saturates the S-cone pathway. A minimum of three null-flicker readings, with a coefficient of variance ≤10%, were recorded for each subject at each of the test loci (0.25°, 0.5°, 1°, 1.75°, and 7° retinal eccentricity). Measurement of MPOD at these points of retinal eccentricity enabled us to plot the spatial profile of MP across the macula. We then calculated the area of MPOD under the spatial profile (MPODArea), using the trapezium rule which approximates the area by a series of trapeziums constructed by assuming linearity in the relationship between MPOD values and retinal eccentricity between adjacent measurement angles:
The MPODArea, a weighted average of the MPOD value at the various points of retinal eccentricity, should offer improved accuracy of the quantity of MP across the macula compared with a single eccentricity measurement alone. MPOD measurement was performed under conditions of dimmed light (ambient illuminance: 4 lux, as measured with an Iso-Tech ILM 350 Lux Meter) at a viewing distance of 18.5 inches (47cm).
The major advantage of cHFP over standard HFP instruments is that the flicker frequency of each test target is customized for each individual subject, minimizing variance between consecutive measurements and thus increasing the accuracy and ease of use of the test. Further information on the technique and advantages of cHFP has previously been published.33-34
Blood sample collection
A 12-hour fasting blood sample was collected from each subject in a 4 ml Z Serum Sep Clot Activator Vacuette® tube (Greiner Bio-One GmbH, Kremsmünster, Austria) at the beginning of the study visit. This whole blood sample was immediately refrigerated at 2°C to 8°C, prior to centrifugation at approximately 1800g for 10 min. Centrifugation was performed within four hours of phlebotomy. Following centrifugation, the supernatant serum sample was aliquoted into 1.5 ml amber (light-sensitive) microcentrifuge tubes (Brand GmbH, Wertheim, Germany) and stored at minus 70°C prior to analysis.
Serum L and Z analysis
Serum L and Z were quantified using reverse phase HPLC. We used an Agilent 1200 series LC system (Agilent Technologies Ireland Ltd., Dublin, Ireland), with photodiode array detection at 295 nm (detection of the internal standard, alpha tocopherol acetate) and 450 nm (detection of L and Z). A 5 μm analytical/preparative 4.6 × 250 mm 201TP specialty reverse phase column (Vydac, Hesperia, CA, USA) was used with an in-line guard column. The mobile phase consisted of 97% methanol and 3% tetrahydrofuran, and was degassed using an in-line degasser. The flow rate was 1 ml/min, and the total run time was 15 minutes. All carotenoid peaks were integrated and quantified using Agilent Chem Station software. Further detail on the methodology used for this analysis is provided in a separate publication.35
Serum Lipoprotein Analysis
Lipoprotein analysis (HDLc, LDLc, triglycerides [TGs]) was performed using the ACE Clinical Chemistry System (Alfa Wassermann, Woerden, The Netherlands) with reagents and consumables supplied by Randox Laboratories Ltd. (Antrim, UK). Analyses were performed on 200-μL serum samples by spectrophotometric analysis at 37°C. The analysis module uses a holographic diffraction grating spectrophotometer to measure absorbance at 16 different wavelengths. Measurements for each assay were recorded at wavelengths and times preprogrammed for each test with results calculated as specified by the test parameters. The protocol used included daily, two-level quality control assessments and duplicate analyses on 50 (16.6%) samples, to ensure the precision of the results.
SNP selection and genotyping
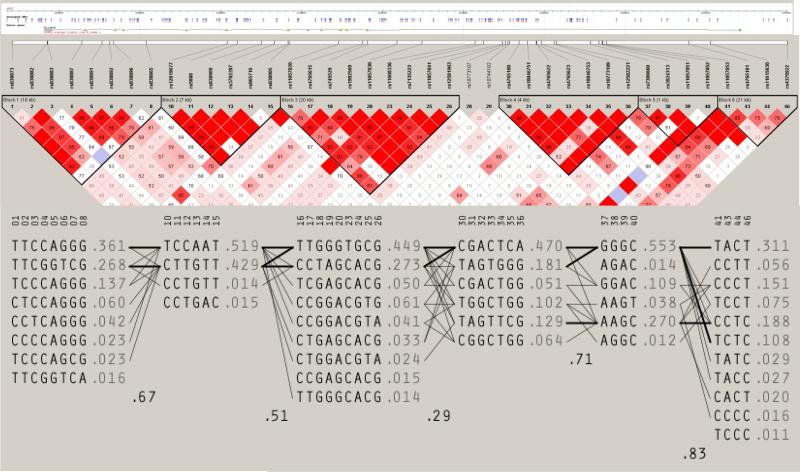
Common variants were selected from Phase III, release 2 HapMap (http://www.hapmap.org) CEPH data (Utah residents with ancestry in northern and western Europe; CEU) using Haploview (http://www.broadinstitute.org/haploview) to visualise linkage disequilibrium (Figure 1). Tag SNPs were selected using multimarker tagging where r2>0.8 (LOD threshold 3.0) for all downloaded SNPs with a minor allele frequency (MAF) ≥5%, genotype call rate ≥95%, and no significant deviation from Hardy–Weinberg equilibrium (HWE).
Figure 1.
Schematic representation of SCARB1, linkage disequilibrium (measured by D’) and putative haplotype block structure across the gene.
Genotyping was performed by MassARRAY iPLEX (Sequenom, San Diego, CA, USA) assays according to the manufacturer's instructions. Quality filters for exclusion of SNPs included call rates below 95% and deviation from HWE (P<0.001). DNA samples were excluded if missing genotypes exceeded 10%. Other quality control measures included duplicates on plates, random sample allocation to plates, independent scoring of problematic genotypes by two individuals and re-sequencing of selected DNAs to validate genotypes.
Replication Cohorts
Replication of the most significant SNP was undertaken in the TwinsUK adult twin cohort (n=199) and in the Carotenoids in Age-Related Eye Disease Study (CAREDS), an ancillary study of the Women's Health Initiative Observational Study (n=1,643). TwinsUK genotyping had been undertaken previously using an Illumina HumanHap 610 Quad array36 and the CAREDS cohort using a customized Illumina array. In CAREDS, MPOD was estimated using cHFP,37 using protocols similar to those implemented in the discovery cohort described above, and serum samples were analyzed for concentrations of trans-lutein and zeaxanthin at Tufts University by a reverse phase HPLC analysis.38 The TwinsUK study used HFP (Maculometer) and an imaging method (2-wavelength fundus autofluorescence) to estimate MPOD with serum analysis in the same laboratory to that implemented in the discovery cohort.12 All procedures conformed to the Declaration of Helsinki and were approved by the institutional review board at each university.
Statistical analysis
Chi-squared tests and one way analysis of variance (ANOVA) tests for trend were used to investigate differences in qualitative and quantitative traits, respectively, between genotype subgroups. Analyses were performed using PLINK (version 1.07)39 under an additive genotypic model. Multiple regression analysis was used to adjust for potential confounders (age, sex, smoking status in pack years, BMI, dietary L and Z estimates, LDLc, HDLc and TGs). Serum L and Z concentrations were log base 10 transformed in light of heavy positive skew in their distributions and were summarized using geometric means and interquartile ranges. The genotype regression coefficients (and their confidence limits) for these transformed quantitative traits were anti-logged and interpreted as proportionate changes in concentration per allele. Given previous evidence suggesting a sex-related effect at SCARB1,22-24 a term for genotype by sex interaction was also included within the regression model. Correction of the quantitative trait comparisons for multiple SNPs was performed in PLINK by permutation testing (n=100,000). The level of statistical significance used to assess the permutation test p values was 5/3=1.67% to allow for the analysis of the three quantitative traits (serum L, serum Z and MPODArea).
Results
Genotype data were available for 47 SNPs from 301 of the 302 (99.7%) subjects included in the study (Table 1). A total of 7 SNPs were excluded for failing to meet the quality filters of call rates below 95% or deviation from HWE (P<0.001). The average call rate for the remaining SNPs was 98.8%. No duplicate inconsistencies were observed.
Table 1.
SNP metrics and genotype counts. HWpval: Hardy Weinberg P value; %Geno: % genotyped; Maj/Min Major/minor alleles; MAF: minor allele frequency; Macular pigment optical density area (MPODArea); serum zeaxanthin (serum Z); serum lutein (serum L); Unadj: unadjusted P value; Adj: Adjusted P value (for age, sex, smoking status, body mass index, high density lipoprotein cholesterol, low density lipoprotein cholesterol, triglycerides, dietary L and Z); Perm: P value following permutation test (n=100,000).
| Maj/Min Alleles | MPOD Area | Serum Z | Serum L | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP | Position | HWpval | %Geno | Genotypes | MAF | Unadj | Adj | Perm | Unadj | Adj | Perm | Unadj | Adj | Perm | |
| rs838873 | 125257551 | 0.456 | 99.7 | 7/67/226 | T:C | 0.138 | 0.299 | 0.381 | 1 | 0.371 | 0.237 | 0.999 | 0.016 | 0.013 | 0.312 |
| rs838882 | 125261813 | 0.543 | 99.7 | 17/119/164 | T:C | 0.255 | 0.749 | 0.656 | 1 | 0.558 | 0.521 | 1 | 0.282 | 0.534 | 1 |
| rs838883 | 125261839 | 0.581 | 100.0 | 1/30/270 | C:T | 0.055 | 0.866 | 0.882 | 1 | 0.662 | 0.491 | 1 | 0.973 | 0.712 | 1 |
| rs838887 | 125265973 | 0.690 | 99.7 | 32/127/141 | C:G | 0.314 | 0.461 | 0.645 | 1 | 0.272 | 0.568 | 1 | 0.734 | 0.966 | 1 |
| rs838891 | 125268432 | 0.356 | 99.7 | 35/124/141 | A:G | 0.319 | 0.729 | 0.999 | 1 | 0.403 | 0.838 | 1 | 0.887 | 0.562 | 1 |
| rs838892 | 125269323 | 0.894 | 99.3 | 30/128/141 | G:T | 0.309 | 0.746 | 0.960 | 1 | 0.467 | 0.887 | 1 | 0.966 | 0.589 | 1 |
| rs838896 | 125269799 | 0.692 | 96.3 | 30/132/128 | G:C | 0.324 | 0.652 | 0.387 | 1 | 0.159 | 0.579 | 1 | 0.960 | 0.337 | 1 |
| rs838865 | 125273767 | 1.000 | 95.3 | 0/19/268 | G:A | 0.033 | 0.168 | 0.259 | 0.999 | 0.675 | 0.361 | 1 | 0.118 | 0.354 | 1 |
| rs1031605 | 125276969 | 0.247 | 80.7 | 9/62/172 | C:T | 0.158 | 0.553 | 0.538 | 1 | 0.268 | 0.134 | 0.971 | 0.520 | 0.458 | 1 |
| rs12819677 | 125283521 | 0.348 | 97.0 | 63/155/74 | T:C | 0.480 | 0.826 | 0.657 | 1 | 0.989 | 0.648 | 1 | 0.736 | 0.935 | 1 |
| rs5888 | 125284748 | 0.293 | 99.3 | 54/157/88 | C:T | 0.440 | 0.871 | 0.771 | 1 | 0.899 | 0.805 | 1 | 0.975 | 0.809 | 1 |
| rs838909 | 125287648 | 0.356 | 99.0 | 64/157/77 | C:T | 0.477 | 0.842 | 0.737 | 1 | 0.880 | 0.643 | 1 | 0.961 | 0.858 | 1 |
| rs3782287 | 125289265 | 0.416 | 98.7 | 64/156/77 | A:G | 0.477 | 0.842 | 0.735 | 1 | 0.885 | 0.638 | 1 | 0.957 | 0.852 | 1 |
| rs865716 | 125290856 | 0.293 | 98.7 | 57/157/83 | A:T | 0.455 | 0.754 | 0.704 | 1 | 0.871 | 0.982 | 1 | 0.994 | 0.865 | 1 |
| rs838905 | 125291006 | 1.000 | 97.3 | 0/14/279 | T:C | 0.023 | 0.931 | 0.841 | 1 | 0.201 | 0.028 | 0.549 | 0.247 | 0.756 | 1 |
| rs11057820 | 125296964 | 0.249 | 99.7 | 62/160/78 | T:C | 0.470 | 0.010 | 0.008 | 0.225 | 0.867 | 0.785 | 1 | 0.119 | 0.023 | 0.478 |
| rs4765615 | 125299830 | 0.563 | 99.7 | 63/155/82 | T:C | 0.465 | 0.105 | 0.092 | 0.915 | 0.617 | 0.307 | 1 | 0.146 | 0.033 | 0.606 |
| rs745529 | 125300472 | 0.783 | 99.3 | 25/127/147 | G:T | 0.291 | 0.101 | 0.117 | 0.956 | 0.312 | 0.733 | 1 | 0.533 | 0.944 | 1 |
| rs1902569 | 125304004 | 1.000 | 99.3 | 46/143/110 | G:A | 0.383 | 0.196 | 0.244 | 1 | 0.868 | 0.578 | 1 | 0.706 | 0.337 | 1 |
| rs11057830 | 125307053 | 1.000 | 99.7 | 4/67/229 | G:A | 0.128 | 0.569 | 0.373 | 1 | 0.319 | 0.341 | 1 | 0.003 | 0.002 | 0.062 |
| rs10846744 | 125312425 | 1.000 | 85.4 | 4/55/198 | G:C | 0.125 | 0.389 | 0.306 | 1 | 0.639 | 0.831 | 1 | 0.011 | 0.011 | 0.269 |
| rs10846745 | 125312612 | 0.214 | 70.4 | 49/96/67 | C:G | 0.464 | 0.047 | 0.039 | 0.650 | 0.048 | 0.022 | 0.464 | 0.004 | 2×10−4 | 0.010 |
| rs11608336 | 125313426 | 0.908 | 99.3 | 64/151/84 | C:T | 0.473 | 0.075 | 0.078 | 0.874 | 0.216 | 0.123 | 0.962 | 0.010 | 0.001 | 0.041 |
| rs7135223 | 125314414 | 0.720 | 99.7 | 47/148/105 | G:A | 0.392 | 0.386 | 0.562 | 1 | 0.674 | 0.563 | 1 | 0.720 | 0.433 | 1 |
| rs11057841 | 125316743 | 0.599 | 99.0 | 6/64/228 | C:T | 0.130 | 0.730 | 0.495 | 1 | 0.228 | 0.239 | 0.999 | 2×10−4 | 2×10−4 | 0.006 |
| rs12581963 | 125317125 | 0.132 | 99.3 | 3/34/262 | G:A | 0.069 | 0.927 | 0.964 | 1 | 0.565 | 0.620 | 1 | 0.001 | 0.003 | 0.098 |
| rs12580803 | 125317247 | 0.010 | 81.4 | 7/38/200 | A:G | 0.111 | 0.043 | 0.049 | 0.732 | 0.303 | 0.689 | 1 | 0.719 | 0.879 | 1 |
| rs10773107 | 125317391 | 0.410 | 96.0 | 67/137/85 | C:A | 0.469 | 0.376 | 0.269 | 1 | 0.580 | 0.385 | 1 | 0.163 | 0.056 | 0.780 |
| rs10744182 | 125317582 | 0.724 | 99.7 | 58/144/98 | T:C | 0.429 | 0.806 | 0.765 | 1 | 0.846 | 0.690 | 1 | 0.369 | 0.298 | 1 |
| rs4765180 | 125318772 | 0.220 | 95.7 | 52/128/108 | C:T | 0.412 | 0.933 | 0.962 | 1 | 0.583 | 0.611 | 1 | 0.149 | 0.111 | 0.947 |
| rs10846751 | 125319771 | 0.500 | 99.7 | 31/123/146 | G:A | 0.312 | 0.571 | 0.574 | 1 | 0.687 | 0.711 | 1 | 0.584 | 0.557 | 1 |
| rs4765622 | 125320828 | 1.000 | 100.0 | 67/150/84 | A:G | 0.479 | 0.661 | 0.671 | 1 | 0.721 | 0.731 | 1 | 0.155 | 0.047 | 0.725 |
| rs4765623 | 125320850 | 0.496 | 98.7 | 31/121/145 | C:T | 0.313 | 0.665 | 0.654 | 1 | 0.668 | 0.735 | 1 | 0.614 | 0.603 | 1 |
| rs10846753 | 125321461 | 0.697 | 99.7 | 11/87/202 | T:G | 0.184 | 0.285 | 0.204 | 0.996 | 0.358 | 0.347 | 1 | 0.493 | 0.127 | 0.966 |
| rs10773109 | 125322995 | 0.716 | 99.7 | 44/147/109 | C:G | 0.401 | 0.707 | 0.568 | 1 | 0.065 | 0.047 | 0.724 | 0.026 | 0.001 | 0.032 |
| rs12582221 | 125323240 | 1.000 | 98.0 | 68/148/79 | G:A | 0.469 | 0.944 | 0.935 | 1 | 0.166 | 0.200 | 0.996 | 0.035 | 0.017 | 0.397 |
| rs7306660 | 125327384 | 0.243 | 99.7 | 28/142/130 | G:A | 0.334 | 0.162 | 0.096 | 0.922 | 0.795 | 0.683 | 1 | 0.127 | 0.228 | 0.998 |
| rs3924313 | 125328375 | 0.496 | 99.0 | 25/133/140 | G:A | 0.311 | 0.612 | 0.432 | 1 | 0.531 | 0.469 | 1 | 0.187 | 0.324 | 1 |
| rs11057851 | 125328779 | 1.000 | 100.0 | 4/64/233 | G:A | 0.123 | 0.108 | 0.186 | 0.994 | 0.050 | 0.037 | 0.643 | 0.680 | 0.605 | 1 |
| rs11057852 | 125328826 | 1.000 | 99.0 | 0/21/277 | C:T | 0.038 | 0.476 | 0.535 | 1 | 0.357 | 0.568 | 1 | 0.015 | 0.120 | 0.959 |
| rs11057853 | 125329313 | 1.000 | 99.3 | 54/146/99 | T:C | 0.434 | 0.027 | 0.031 | 0.572 | 0.374 | 0.295 | 1 | 0.643 | 0.647 | 1 |
| rs7954697 | 125335253 | 0.0004 | 61.5 | 58/68/59 | A:C | 0.488 | 0.156 | 0.137 | 0.974 | 0.159 | 0.129 | 0.967 | 0.743 | 0.600 | 1 |
| rs4765181 | 125339228 | 0.146 | 96.3 | 54/128/108 | C:A | 0.399 | 0.085 | 0.079 | 0.879 | 0.046 | 0.093 | 0.916 | 0.039 | 0.039 | 0.659 |
| rs11615630 | 125341870 | 0.178 | 99.3 | 49/130/120 | C:T | 0.389 | 0.588 | 0.472 | 1 | 0.924 | 0.607 | 1 | 0.935 | 0.876 | 1 |
| rs7139401 | 125345448 | 0.165 | 73.8 | 1/11/210 | G:A | 0.032 | 0.697 | 0.925 | 1 | 0.354 | 0.380 | 1 | 0.147 | 0.164 | 0.987 |
| rs4379922 | 125351116 | 0.265 | 99.0 | 46/130/122 | T:C | 0.380 | 0.296 | 0.221 | 0.998 | 0.698 | 0.566 | 1 | 0.656 | 0.889 | 1 |
| rs6488950 | 125354103 | 0.146 | 79.4 | 0/49/190 | C:T | 0.098 | 0.250 | 0.266 | 1 | 0.517 | 0.247 | 0.999 | 0.705 | 0.231 | 0.998 |
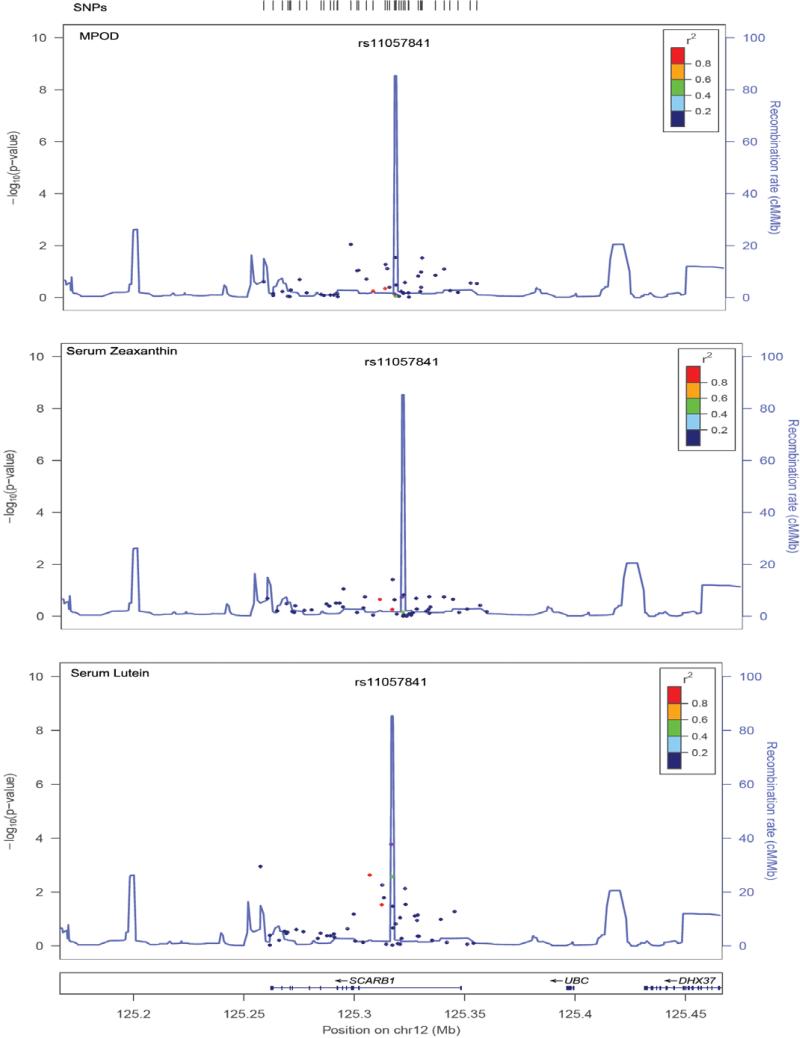
In a univariate analysis, 5 SNPs were significantly associated with serum L (rs11057841, P=2×10−4; rs10773109, P=0.001; rs11057830, P=0.002; rs11608336, P=0.001; rs12581963, P=0.003), although only rs11057841 was significant (P<0.0167) after correction for multiple testing by permutation. Multiple regression analysis was performed on MPOD area, serum L and Z concentrations both before and after adjustment for age, BMI, sex, HDLc, LDLc, TGs, smoking status and dietary L and Z estimates (Table 1, Figure 2). One SNP (rs11057820, P=0.008) was associated with MPOD, although this did not remain significant following correction for multiple testing. No significant interactions by sex were found. Haplotype analysis revealed no stronger associations than were obtained with the single SNP analysis. No significant associations were identified for serum Z.
Figure 2.
Regional association plots for SNPs investigated. Panels represent signals for the outcome measures (1) macular pigment optical density (MPOD), (2) serum zeaxanthin levels (serum Z) and (3) serum lutein levels (serum L). The color of the SNP symbol indicates the linkage disequiliblium (r2) with the index SNP, which is colored purple (http://csg.sph.umich.edu/locuszoom/). Points on the plot relate to the p-values (indicated on the left side scale) and the lines relate to the recombination rates (right side scale).
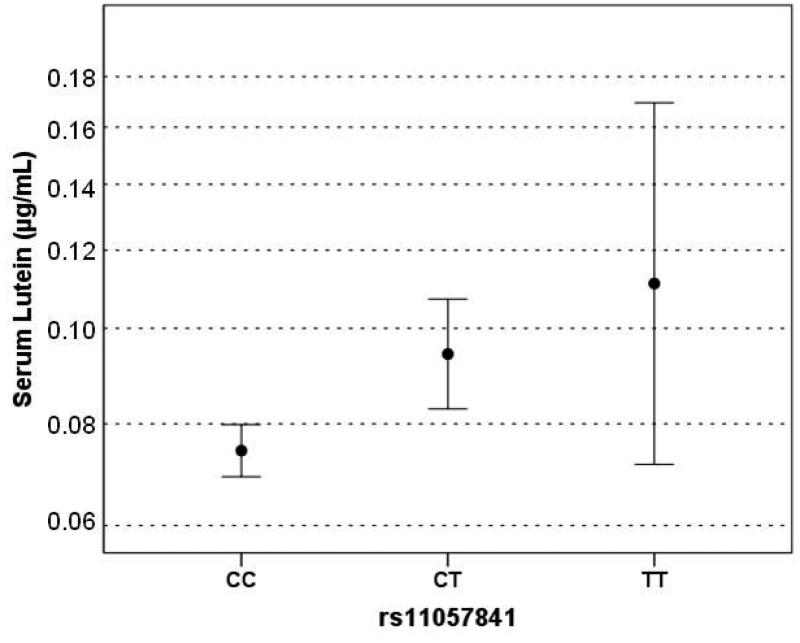
Anthropometric and lifestyle data are presented in relation to rs11057841 genotype for all 298 successfully genotyped subjects (Table 2). The mean ± standard deviation (range) for age was 48 ± 11 (21-66) years and 70% were female. There was no statistically significant difference between rs11057841 genotypes for any of the following variables: age, BMI, cigarette smoking, dietary L intake, dietary Z intake, serum Z concentration, HDLc, LDLc, TGs (one way ANOVA P>0.05). Neither was there any statistically significant difference in the male:female ratio between the genotype groups. Significant differences in serum L concentration (increase in geometric mean per T allele by a factor of 1.24 [95% CI 1.11-1.39]; P=2×10−4) and in the ratio of positive:negative family history of AMD (P=0.028) were detected between the three rs11057841 genotypes (Table 2, Figure 3).
Table 2.
Anthropometric and lifestyle data for all genotyped subjects in the discovery sample for rs11057841; SD, standard deviation; BMI (Body mass index), MPOD, macular pigment optical density; AMD, Age-related Macular Degeneration.
| Characteristic | CC n=228 | CT n=64 | TT n=6 | P value | |||
|---|---|---|---|---|---|---|---|
| Age( years) | 47.8 | (11.4) | 48.4 | (10.2) | 51.2 | (10.4) | 0.28 |
| Sex Male, n (%) | 66 | 29% | 22 | 34% | 3 | 50% | |
| BMI (kg/m2) | 26.6 | (4.5) | 27.3 | (5.0) | 24.7 | (2.4) | 0.24 |
| MPOD Area | 0.713 | (0.436) | 0.734 | (0.411) | 0.735 | (0.507) | 0.70 |
| MPOD 0.25° | 0.477 | (0.208) | 0.494 | (0.201) | 0.500 | (0.231) | 0.85 |
| MPOD 0.5° | 0.376 | (0.171) | 0.392 | (0.185) | 0.348 | (0.255) | 0.15 |
| MPOD 1° | 0.239 | (0.128) | 0.240 | (0.129) | 0.203 | (0.132) | 0.76 |
| MPOD 1.75° | 0.126 | (0.097) | 0.125 | (0.095) | 0.138 | (0.106) | 0.63 |
| Pack years smoked | 5.997 | (11.94) | 4.811 | (9.222) | 5.667 | (9.136) | 0.26 |
| Serum Lutein (μg/mL) | 0.075 | [0.057-0.102] | 0.094 | [0.066-0.127] | 0.111 | [0.076-0.160] | 2×10−4 |
| Serum Zeaxanthin (μg/mL) | 0.013 | [0.009-0.200] | 0.014 | [0.009-0.200] | 0.016 | [0.010-0.021] | 0.85 |
| Dietary Lutein (mg) | 0.939 | [0.681-0.984] | 1.060 | [0.738-1.630] | 0.679 | [0.403-0.922] | 0.07 |
| Dietary Zeaxanthin (mg) | 0.156 | [0.109-0.238] | 0.158 | [0.111-0.215] | 0.127 | [0.087-0.198] | 0.20 |
| LDLc (mmol/L) | 3.211 | (0.910) | 3.271 | (0.968) | 2.780 | (1.017) | 0.83 |
| HDLc (mmol/L) | 1.491 | (0.380) | 1.509 | (0.372) | 1.715 | (0.505) | 0.98 |
| Triglycerides (mmol/L) | 1.167 | [0.087-1.490] | 1.281 | [0.785-1.728] | 0.942 | [0.628-1.238] | 0.17 |
| Total cholesterol (mmol/L) | 5.379 | (1.092) | 5.518 | (1.149) | 5.548 | (0.925) | 0.79 |
| Positive family history of AMD, n (%) | 83 | (36%) | 31 | (48%) | 4 | (67%) | |
rs11057841: data presented as mean (SD), geometric mean [IQR] or n (%).
Figure 3.
Plots of serum lutein (A) showing geometric means and 95% CIs against rs11057841 genotype.
Evaluation of rs11057841 in the independent replication cohorts
Replication of association for rs11057841 with serum L concentration was undertaken in the TwinsUK adult cohort (n=199) using a likelihood ratio chi-squared test accounting for familial relatedness with support for the association detected in the discovery cohort (Table 3; beta coefficient=0.252, P=0.014). Although CAREDS did not genotype rs11057841 directly, there was data available for rs10846744, a SNP in high LD with rs11057841 (r2=0.93, http://www.1000genomes.org/), providing further support to the size and direction of effect observed at this locus (Table 3; beta coefficient=0.0395; P=2×10−4).
Table 3.
Genotype effect size for rs11057841 on serum lutein concentration (log base 10 scale) estimated from an additive genetic model without adjustment for covariates in discovery and replication cohorts.
| Cohort | Allele | MAF | n | Coefficient | SE | Change per allele (CI)2 | P |
|---|---|---|---|---|---|---|---|
| Waterford (Discovery) | T | 0.13 | 298 | 0.094 | 0.025 | 1.24 (1.11-1.39) | 2×10−4 |
| TwinsUK (Replication) | T | 0.09 | 199 | 0.252 | 0.102 | 1.79 (1.13-2.83) | 0.014 |
| 1CAREDS (Replication) | C | 0.15 | 1640 | 0.040 | 0.011 | 1.10 (1.04-1.15) | 2×10−4 |
MAF = minor allele frequency; SE = standard error; CI = 95% confidence intervals.
Discussion
AMD is a common complex disease and one strategy proposed for a reduction in associated risk includes modification of nutrient intake. Several studies have shown that an increased intake of the macular carotenoids L and Z through foods rich in these nutrients (e.g. spinach and egg yolk), tends to reduce the risk of the development and/or progression of late AMD.40-41 This study has investigated the relationship between common variants in SCARB1, MPOD and serum concentrations of L and Z in 302 healthy subjects aged between 21 and 66 years. The mean MPOD of all genotyped subjects recorded at 0.5° retinal eccentricity was 0.38 ± 0.17 optical density units, a value comparable to previous studies that used cHFP to measure MPOD at this eccentricity.10, 30, 34 Genotype data were available on 99.7% of our sample and association of rs11057841 with serum L concentration survived correction by permutation for multiple testing, showing a 24% increase per T allele, further supported following replication in the independent TwinsUK and CAREDS studies. While Wang and colleagues reported transportation of serum L preferentially on HDLc,42 we found association of rs11057841 and serum L independent of HDLc and other factors such as BMI, smoking, sex, LDLc and TGs.
Early-stage AMD is characterized by hallmark lesions of cholesterol and lipid-rich drusen and basal linear deposits, which accumulate with age between the retinal pigment epithelium and the choroid.43-44 Bruch's membrane forms the inner margin of the choroid, effectively acting as a vessel wall.44 During the atherosclerotic process, lipoproteins traverse the vascular endothelium and accumulate in the arterial wall on binding to proteoglycans, culminating in deleterious processes which include inflammation and neovascularization.45-46 In many respects, these atherosclerotic plaques mimic the accumulation of drusen and basal laminar deposits in a manner similar to that observed in early AMD. Most studies of early AMD are often predominated by younger adults and have reported either a protective association or no association with elevated HDLc levels, in contrast to studies of late-stage AMD, characterized by elderly participants, which have tended to report the opposite.47 As such, although HDLc has been widely studied and implicated in AMD disease aetiology, the mechanisms and timings involved with respect to participant age and disease status, remain unresolved.
Previous reported association of SCARB1 SNP rs5888 with AMD implicated a role for cholesterol and antioxidant metabolism, identifying L in particular, in AMD disease etiology.13 Recent identification of the hepatic lipase (LIPC) and cholesterylester transfer protein (CETP) genes 17-18 provide further evidence in support of cholesterol metabolism and AMD pathogenesis, particularly given previous association of these genes with HDLc levels in blood.49-49 While multiple common alleles near CETP and LIPC have been independently associated with HDLc levels,47 they have also shown modest association in a smaller AMD cohort with less power.17
Alternatively, the associations with SCARB1 variants in this and other studies might reflect variation in carotenoid uptake into the body and eye. There is evidence that transport of carotenoids in the retina28 and intestine29 is a facilitated process mediated by the scavenger receptor class B type I (SR-BI). The direct association of rs11057841 genotypes with increasing T-alleles and level of lutein and zeaxanthin in the serum are consistent with this possibility.
Several studies have provided evidence of a sex-related effect at this locus.22-24 In a community-based cohort of post-menopausal women SCARB1 polymorphisms were associated with decreased HDLc and elevated TG levels in an estrogen-dependent manner.22-23 Although the majority of female participants in this study were likely to be pre-menopausal we found no evidence of a sex-specific interaction between serum L concentration or MPOD and the most significantly associated SCARB1 SNPs. Interestingly, analyses originating from the Multi-Ethnic Study of Atherosclerosis, has shown association between rs10846744 and common carotid intimal-medial artery thickness, a surrogate marker for sub-clinical atherosclerosis and increased risk of cardiovascular disease.24 The top SNP identified in our study associated with increased serum L concentration (rs11957841) and rs10846744, tested in the CAREDS replication study, share high linkage disequilibrium (r2=0.93). Manichaikul and colleagues suggest that genetic variants within SCARB1 may exert cis or trans regulatory effects, possibly influencing endothelial function or inflammatory pathways.24 Although the participants in this study were too young to identify symptoms associated with AMD, identification of a positive family history of AMD was undertaken, showing a positive correlation between serum L concentration, family history of AMD and rs11957841. In view of the common pathways shared between AMD and cardiovascular disease,25-26 these data would implicate SCARB1 in both disease processes.
Furthermore, given that genetic variation attenuates cholesterol levels, which may influence drusen formation and modulate AMD risk, and the poor correlation observed between serum L and Z concentration and MPOD in this study and elsewhere,12 it would be important for further studies to explore the influence of rs11957841 and other genetic variants in SCARB1 on cholesterol transport. Accumulation of MP in the central retina is reliant on a complex process originating from ingested foodstuff, digestion, absorption, transport in the serum and ultimately capture by, and stabilization in, the retina.12 While our study examines a key gene implicated in this process, assessment of additional genetic components of this pathway should be undertaken and their potential influence evaluated. Our data indicates an important role for SCARB1 in serum L concentration but this would appear to be a poor surrogate for MPOD. It would be particularly interesting to examine the genetic influence of SCARB1 polymorphisms in an elderly population and their respective influences on serum L concentration and associated AMD risk.
Acknowledgements
We would like to thank the participants who volunteered for this study. This study was supported in part by Bausch and Lomb, Ireland and EU Strand 1 research funding. The TwinsUK authors acknowledge funding from the Wellcome Trust, and Dr SH Melissa Liew who performed the twin phenotyping. Genotyping was supported in part by US National Institutes of Health (NIH)/National Eye Institute (NEI) grant 1RO1EY018246 and performed by the NIH Center for Inherited Disease Research. The study also received support from the National Institute for Health Research comprehensive Biomedical Research Centre award to Guy's and St. Thomas' National Health Service Foundation Trust partnering with King's College London. The CAREDS authors acknowledge funding from the NIH, NEI [grants EY013018, EY016886], Research to Prevent Blindness and the Women's Health Initiative (WHI), of which CAREDS is ancillary to, is supported by contracts from the National Heart, Lung and Blood Institute, NIH. The authors thank the WHI investigators and staff for their dedication, and the study participants for making the program possible. A listing of WHI investigators can be found at https://cleo.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investiga tor%20Short%20List.pdf.
Footnotes
Financial Disclosure(s): The author(s) have made the following disclosure(s): John M. Nolan and Stephen Beatty do consultancy work for nutraceutical companies, in a personal capacity, and as directors of Nutrasight Consultancy Limited.
References
- 1.Centers for Disease Control and Prevention (CDC) Prevalence of visual impairment and selected eye diseases among persons aged >/= 50 years with and without diabetes- United States, 2002. MMWR Morb Mortal Wkly Rep. 2004;53:1069–71. [PubMed] [Google Scholar]
- 2.Bunce C, Xing W, Wormald R. Causes of blind and partial sight certifications in England and Wales: April 2007eMarch 2008. Eye (Lond) 2010;24:1692e9. doi: 10.1038/eye.2010.122. [DOI] [PubMed] [Google Scholar]
- 3.Klein R, Klein B, Linton K. Prevalence of age-related maculopathy: the Beaver Dam Eye study. Ophthalmology. 1992;99:933–43. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 4.Swaroop A, Branham KE, Chen W, Abecasis G. Genetic susceptibility to age-related macular degeneration: a paradigm for dissecting complex disease traits. Hum Mol Genet. 2007;16:174–82. doi: 10.1093/hmg/ddm212. [DOI] [PubMed] [Google Scholar]
- 5.Bone RA, Landrum JT, Hime GW, et al. Stereochemistry of the human macular carotenoids. Invest Ophthalmol Vis Sci. 1993;34:2033–40. [PubMed] [Google Scholar]
- 6.Johnson EJ, Neuringer M, Russell RM, et al. Nutritional manipulation of primate retinas, III: effects of lutein or zeaxanthin supplementation on adipose tissue and retina of xanthophyll-free monkeys. Invest Ophthalmol Vis Sci. 2005;46:692–702. doi: 10.1167/iovs.02-1192. [DOI] [PubMed] [Google Scholar]
- 7.Thurnham DI, Trémel A, Howard AN. A supplementation study in human subjects with a combination of meso-zeaxanthin, (3R,3'R)-zeaxanthin and (3R,3'R,6'R)-lutein. Br J Nutr. 2008;100:1307–14. doi: 10.1017/S0007114508971336. [DOI] [PubMed] [Google Scholar]
- 8.Parker RS. Absorption, metabolism, and transport of carotenoids. FASEB J. 1996;10:542–51. [PubMed] [Google Scholar]
- 9.Snodderly DM. Evidence for protection against Age-Related Macular Degeneration by carotenoids and antioxidant vitamins. Am J Clin Nutr. 1995;62:S1448–61. doi: 10.1093/ajcn/62.6.1448S. [DOI] [PubMed] [Google Scholar]
- 10.Beatty S, Murray IJ, Henson DB, et al. Macular pigment and risk for age-related macular degeneration in subjects from a Northern European population. Invest Ophthalmol Vis Sci. 2001;42(2):439–46. [PubMed] [Google Scholar]
- 11.Liew SHM, Gilbert C, Spector TD, et al. Heritability of Macular Pigment: a Twin Study. Invest Ophthalmol Vis Sci. 2005;46:4430–6. doi: 10.1167/iovs.05-0519. [DOI] [PubMed] [Google Scholar]
- 12.Hammond CJ, Liew SM, Van Kuijk FJ, et al. The Heritability of Macular Response to Supplemental Lutein and Zeaxanthin: a Classical Twin Study. Invest Ophthalmol Vis Sci. 2012;53:4963–8. doi: 10.1167/iovs.12-9618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zerbib J, Seddon JM, Richard F, et al. rs5888 variant of SCARB1 gene is a possible susceptibility factor for age-related macular degeneration. PLoS One. 2009;4(10):e7341. doi: 10.1371/journal.pone.0007341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schick JH, Iyengar SK, Klein BE, et al. A whole-genome screen of a quantitative trait of age-related maculopathy in sibships from the Beaver Dam Eye Study. Am J Hum Genet. 2003;72:1412–24. doi: 10.1086/375500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji Y, Jian B, Wang N, et al. Scavenger receptor BI promotes high density lipoprotein-mediated cellular cholesterol efflux. J Biol Chem. 1997;272:20982–5. doi: 10.1074/jbc.272.34.20982. [DOI] [PubMed] [Google Scholar]
- 16.Acton S, Rigotti A, Landschulz KT, et al. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–20. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 17.Neale BM, Fagerness J, Reynolds R, et al. Genome-wide association study of advanced age-related macular degeneration identifies a role of the hepatic lipase gene (LIPC). Proc Natl Acad Sci U S A. 2010;107:7395–400. doi: 10.1073/pnas.0912019107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen W, Stambolian D, Edwards AO, et al. Genetic variants near TIMP3 and high-density lipoprotein-associated loci influence susceptibility to age-related macular degeneration. Proc Natl Acad Sci U S A. 2010;107:7401–6. doi: 10.1073/pnas.0912702107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodríguez-Esparragón F, Rodríguez-Pérez JC, Hernández-Trujillo Y, et al. Allelic variants of the human scavenger receptor class B type 1 and paraoxonase 1 on coronary heart disease: genotype-phenotype correlations. Arterioscler Thromb Vasc Biol. 2005;25:854–60. doi: 10.1161/01.ATV.0000157581.88838.03. [DOI] [PubMed] [Google Scholar]
- 20.Acton S, Osgood D, Donoghue M, et al. Association of polymorphisms at the SR-BI gene locus with plasma lipid levels and body mass index in a white population. Arterioscler Thromb Vasc Biol. 1999;19:1734–43. doi: 10.1161/01.atv.19.7.1734. [DOI] [PubMed] [Google Scholar]
- 21.Morabia A, Ross BM Costanza MC, et al. Population-based study of SR-BI genetic variation and lipid profile. Atherosclerosis. 2004;175:159–68. doi: 10.1016/j.atherosclerosis.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 22.McCarthy JJ, Somji A, Weiss LA, et al. Polymorphisms of the scavenger receptor class B member 1 are associated with insulin resistance with evidence of gene by sex interaction. J Clin Endocrinol Metab. 2009;94(5):1789–96. doi: 10.1210/jc.2008-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiba-Falek O, Nichols M, Suchindran S, et al. Impact of gene variants on sex-specific regulation of human Scavenger receptor class B type 1 (SR-BI) expression in liver and association with lipid levels in a population-based study. BMC Med Genet. 2010;11:9. doi: 10.1186/1471-2350-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manichaikul A, Naj AC, Herrington D, et al. Association of SCARB1 variants with subclinical atherosclerosis and incident cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:1991–9. doi: 10.1161/ATVBAHA.112.249714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snow KK, Seddon JM. Do age-related macular degeneration and cardiovascular disease share common antecedents? Ophthalmic Epidemiol. 1999;6:125–43. doi: 10.1076/opep.6.2.125.1558. [DOI] [PubMed] [Google Scholar]
- 26.Klein R, Deng Y, Klein BE, Hyman L, Seddon J, et al. Cardiovascular disease, its risk factors and treatment, and age-related macular degeneration: Women's Health Initiative Sight Exam ancillary study. Am J Ophthalmol. 2007;143:473–83. doi: 10.1016/j.ajo.2006.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duncan KG, Bailey KR, Kane JP, Schwartz DM. Human retinal pigment epithelial cells express scavenger receptors BI and BII. Biochem Biophys Res Commun. 2002;292:1017–22. doi: 10.1006/bbrc.2002.6756. [DOI] [PubMed] [Google Scholar]
- 28.During A, Doraiswamy S, Harrison EH. Xanthophylls are preferentially taken up compared with beta-carotene by retinal cells via a SRBI-dependent mechanism. J Lipid Res. 2008;49:1715–24. doi: 10.1194/jlr.M700580-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reboul E, Abou L, Mikail C, et al. Lutein transport by Caco-2 TC-7 cells occurs partly by a facilitated process involving the scavenger receptor class B type I (SR-BI). Biochem J. 2005;387:455–61. doi: 10.1042/BJ20040554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nolan JM, Stack J, O'Donovan O, et al. Risk factors for age-related maculopathy are associated with a relative lack of macular pigment. Exp Eye Res. 2007;84:61–74. doi: 10.1016/j.exer.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 31.Bone RA, Landrum JT, Cains A. Optical density spectra of the macular pigment in vivo and in vitro. Vis Res. 1992;32:105–10. doi: 10.1016/0042-6989(92)90118-3. [DOI] [PubMed] [Google Scholar]
- 32.Wooten BR, Hammond BR, Land RI, Snodderly DM. A practical method for measuring macular pigment optical density. Invest Ophthalmol Vis Sci. 1999;40:2481–9. [PubMed] [Google Scholar]
- 33.Nolan JM, Stringham JM, Beatty S, Snodderly DM. Spatial profile of macular pigment and its relationship to foveal architecture. Invest Ophthalmol Vis Sci. 2008;49:2134–42. doi: 10.1167/iovs.07-0933. [DOI] [PubMed] [Google Scholar]
- 34.Loane E, Stack J, Beatty S, Nolan JM. Measurement of macular pigment optical density using two different heterochromatic flicker photometers. Curr Eye Res. 2007;32:555–64. doi: 10.1080/02713680701418405. [DOI] [PubMed] [Google Scholar]
- 35.Loane E, McKay GJ, Nolan JM, Beatty S. Apolipoprotein E genotype is associated with macular pigment optical density. Invest Ophthalmol Vis Sci. 2010;51:2636–43. doi: 10.1167/iovs.09-4397. [DOI] [PubMed] [Google Scholar]
- 36.Hysi PG, Young TL, Mackey DA, et al. A genome-wide association study for myopia and refractive error identifies a susceptibility locus at 15q25. Nat Genet. 2010;42:902–5. doi: 10.1038/ng.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mares JA, LaRowe TL, Snodderly DM, et al. Predictors of optical density of lutein and zeaxanthin in retinas of older women in the Carotenoids in Age-Related Eye Disease Study, an ancillary study of the Women's Health Initiative. Am J Clin Nutr. 2006;84:1107–22. doi: 10.1093/ajcn/84.5.1107. [DOI] [PubMed] [Google Scholar]
- 38.Yeum KJ, Booth SL, Sadowski JA, et al. Human plasma carotenoid response to the ingestion of controlled diets high in fruits and vegetables. Am J Clin Nutr. 1996;64(4):594–602. doi: 10.1093/ajcn/64.4.594. [DOI] [PubMed] [Google Scholar]
- 39.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seddon JM, Ajani UA, Sperduto RD, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA. 1994;272:1413–20. Erratum in: JAMA 1995; 273:622. [PubMed] [Google Scholar]
- 41.Loane E, Kelliher C, Beatty S, Nolan JM. The rationale and evidence base for a protective role of macular pigment in age-related maculopathy. Br J Ophthalmol. 2008;92:1163–8. doi: 10.1136/bjo.2007.135566. [DOI] [PubMed] [Google Scholar]
- 42.Wang W, Connor SL, Johnson EJ, et al. Effect of dietary lutein and zeaxanthin on plasma carotenoids and their transport in lipoproteins in age-related macular degeneration. Am J Clin Nutr. 2007;85:762–9. doi: 10.1093/ajcn/85.3.762. [DOI] [PubMed] [Google Scholar]
- 43.Mullins RF, Russell SR, Anderson DH, Hageman GS. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000;14:835–46. [PubMed] [Google Scholar]
- 44.Curcio CA, Johnson M, Rudolf M, Huang JD. The oil spill in ageing Bruch membrane. Br J Ophthalmol. 2011;95:1638–45. doi: 10.1136/bjophthalmol-2011-300344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol. 1995;15:551–61. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tabas I, Williams KJ, Borén J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–44. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 47.Klein R, Cruickshanks KJ, Nash SD, et al. The prevalence of age-related macular degeneration and associated risk factors. Arch Ophthalmol. 2010;128:750–8. doi: 10.1001/archophthalmol.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Willer CJ, Sanna S, Jackson AU, et al. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet. 2008;40:161–9. doi: 10.1038/ng.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kathiresan S, Willer CJ, Peloso GM, et al. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat Genet. 2009;41:56–65. doi: 10.1038/ng.291. [DOI] [PMC free article] [PubMed] [Google Scholar]