Abstract
Despite the important role for epidermal growth factor (EGF) in epithelial homeostasis and wound healing, it has not been investigated in atopic dermatitis (AD). We used AD animal models to explore the role of EGF in AD. In an acute AD model, skin trans-epidermal water loss (TEWL) was significantly attenuated in EGF treated mice. Blockade of EGFR signaling genetically or pharmacologically confirms a protective role for EGFR signaling in AD. In a chronic/relapsing AD model, EGF treatment of mice with established AD resulted in attenuation of AD exacerbation (skin epithelial thickness, cutaneous inflammation, and total and allergen specific IgE) following cutaneous allergen re-challenge. EGF treatment didn’t alter expression of skin barrier junction proteins or antimicrobial peptides in the AD model. However, EGF treatment attenuated allergen-induced expression of IL-17A, CXCL1 and CXCL2, and neutrophil accumulation in AD skin following cutaneous allergen exposure. IL-17A production was decreased in the in vitro re-stimulated skin-draining lymph node cells from the EGF-treated mice. Similarly, IL-17A was increased in waved-2 mice skin following allergen exposure. While IL-6 and IL-1β expression were attenuated in the skin of EGF-treated mice, EGF treatment also suppressed allergen-induced IL-6 production by keratinocytes. Given the central role of IL-6 in priming Th17 differentiation in the skin, this effect of EGF on keratinocytes may contribute to the protective roles for EGFR in AD pathogenesis. In conclusion, our study provides evidence for a previously unrecognized protective role for EGF in AD and a new role for EGF in modulating IL-17 responses in the skin.
Introduction
Atopic Dermatitis (AD) has a prevalence of up to 25% among children (1, 2). Whereas symptoms of early-onset AD usually improve during adolescence, as many as 33% of cases persist into adulthood. Furthermore, nearly 80% of the children with AD also subsequently develop asthma or allergic rhinitis later in life, underscoring the public health impact of this disorder (3). Accumulated evidence reveals that AD has a multifactorial pathogenesis, being influenced by genetic susceptibility, environmental factors and immunologic responses to common antigens (4–6).
AD is characterized by elevated IgE and mixed Th1, Th2 and Th17 cytokine expression (7–12). The percentage of Th17 cells is increased in peripheral blood of AD patients and associated with disease severity suggesting a possible pathogenic role of Th17 cells in AD (13). In mice, epicutaneous sensitization of mouse skin with OVA results in local and systemic Th17 as well as Th2 responses (14, 15). Filaggrin-deficient mice develop spontaneous eczematous inflammation with age and this inflammation is characterized by a local Th17 response, as evidenced by increased skin mRNA levels of IL17A, whereas elevated skin levels of Th2 cytokines were only observed months later (16).
The EGFR signaling pathway is essential in skin development and homeostasis (17–19). EGFR signaling exerts a major impact on keratinocyte proliferation and differentiation and, eventually, on the process of wound healing (20–22). Epidermal keratinocytes are a rich source of EGFR ligands, including transforming growth factor (TGF)-α, amphiregulin, heparin binding (HB)-EGF, and epiregulin (23, 24). In recent years, several studies have revealed the importance of EGF in maintaining intestinal epithelial health and as a potential therapeutic target to induce restitution of the damaged epithelium (17, 19). Furthermore, local administration of EGF induced remission in patients with ulcerative colitis (18). Up-regulation of EGFR and its ligands expression were found in chronic inflammatory skin disorders including psoriasis and AD (24). However, it is still unclear which EGFR ligand is most relevant and whether it acts through effects on resident and/or immigrated cells. For example, while basal or suprabasal expression of amphiregulin in the skin of transgenic mice leads to severe psoriasis-like hyperplasia with skin inflammation (25, 26); dermatitis developed after bone marrow transplantation of amphiregulin−/− bone marrow into wild type recipient C57BL/6 mice (27). Furthermore, epidermis targeted expression of TGF-α does not result in cutaneous inflammatory phenotypes (28, 29) and deficiency of epiregulin results in chronic skin inflammation (30). Other studies revealed that the EGFR signaling pathway may contribute to the pathogenesis of inflammatory skin disorders by regulating cytokine and chemokine secretion by keratinocytes, such as up-regulation of TLR5, TLR9, some antimicrobial peptides, GM-CSF and IL-8 as well as down-regulation of chemokines (CCL2, CCL5, CCL27 and CXCL10) (31–33).
No skin abnormalities have been detected in mice lacking EGF (34), but the roles of EGF and EGFR have not been evaluated in AD. In the present study, we evaluated the role of EGF and EGFR in AD development and subsequent relapse in experimental AD models. Our findings demonstrate that EGF attenuates AD symptom severity and down regulates IL-17 expression in the skin and this may due to alteration of the cutaneous immune system caused by EGF/EGFR repressed IL-6 induction in keratinocytes.
Methods
Mice
Wild type of C57B1/6, BALB/c (Jackson Laboratory, Bar Harbor, ME) and waved-2 (C57B1/6 background, generously provided by Tim Le Cras, CCHMC) mice were kept in a specific pathogen-free environment. All procedures were performed in accordance with the ethical guidelines in the Guide for the Care and Use of Laboratory Animals of the Institutional Animal care and Use committee approved by the Veterinary Service Department of the Cincinnati children’s Hospital Medical Center Research foundation.
Experimental AD model and EGF and erlotinib treatment
Two different experimental models were used to study the development of AD and to study exacerbation. For the acute model described previously (35), mice were anesthetized with Isoflurane (IsoFlo; Abbott Laboratories, North Chicago, IL) and their backs were shaved one day before the first allergen exposure. Either 200μl of saline solution or A. fumigatus extract (Greer Laboratories, Lenior, NC) resuspended in saline at a concentration of 1mg/ml, was applied to a 2 by 2 cm patch of sterile gauze. The patch was secured by TegaDerm and the mouse was wrapped with a band aid and waterproof tape. After 6 days, the patch was remove and 24 hours later, a new patch applied for a total three patches over a three weeks period as shown in Figure 1A. For the experiments utilizing EGF (PeproTech, Rocky Hill, NJ) or erlotinib (Tarceva®, generously provided by Genentech, San Francisco, CA, dissolved in 0.5% wt/vol methylcellulose), EGF (40μg per mice, ip) or erlotinib (100mg/kg, gavage) was given one day before the first patch and then repeated every other day. Control mice received treatment with vehicle alone.
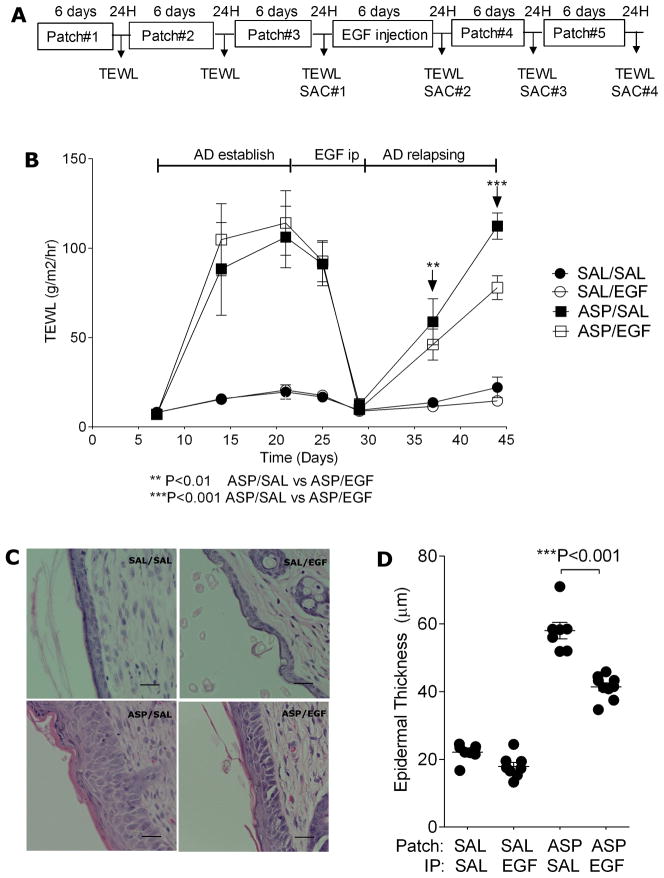
Figure 1.
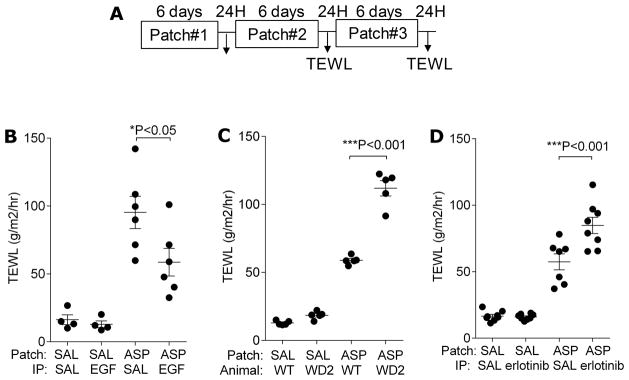
EGFR signaling protects from skin barrier function impairment following cutaneous allergen exposure. A. Overview of the acute AD sensitization protocol. Mice were exposed to A. fumigatus (ASP) or saline (SAL) patch 3 times as indicated and TEWL was assessed 24 hours after the last patch was removed in B. BALB/c mice treated with EGF as indicated; C. Waved-2 (WD2) mice and C57Bl/6 wild type mice; D. BALB/c mice treated with erlotinib as indicated. n=4–8 mice/group *P<0.05, ***P<0.001. Each experiment was performed a minimum of 3 times.
For the chronic/relapsing model (Figure 2A), AD was established as outlined in Figure 1A, and then mice were rested for one week during which time cutaneous inflammation resolves and TEWL normalizes. Then, mice were re-challenged with two additional allergen patches. EGF (40μg/mice) was injected ip every other day (4 injections) as indicated. Endotoxin levels in A. fumigatus extracts were assessed using the Cambrex QCL1000 assay and were very low (1mg contains 1.5–2 EU/ml or 0.3–0.4 ng/ml of endotoxins, resulting 0.06–0.08ng of endotoxins exposure per patch).
Figure 2.
EGF treatment attenuates AD exacerbation following cutaneous allergen re-challenge. A. Overview of the chronic AD sensitization and re-challenge protocol. Twenty four hours after the last patch was removed, the following were assessed: B. TEWL; C. H&E staining of patched skin; D. Epidermal thickness of patched skin; Values are expressed as mean±SEM (n=6–10 mice/group). *P<0.05, **P<0.01, and ***P<0.001. Each experiment was performed a minimum of 3 times. Scale bars, 25μm.
Cell and lymph node cultures
HaCat, an immortalized human keratinocyte cell line, cells (generously provided by Dr. T. Bowden, University of Arizona, Tucson, AZ) were maintained in DMEM (Life Technologies, Grand Island, NY), supplemented with 10% FBS and antibiotics. Cells were serum starved overnight prior to treatment with EGF (50ng/ml). A. fumigatus extract (300μg/ml) was added for the indicated times. RNA or protein samples were collected for further analysis.
Primary human foreskin keratinocytes were prepared and maintained as described previously (36).
Lymph nodes (axillary and accessory) from the AD (cutaneous allergen exposed) mice were collected and cultured in RPMI1640 medium (Life Technologies) at 5×105 per well in 96 well plate. Twenty-four hours later, A. fumigatus extract (25ug/ml) was added to re-stimulate the cells and the supernatant was collected after 5 days and assessed for cytokines by ELISA according to manufacturer instructions (BioLegend, San Diego, CA).
Measurement of TEWL
TEWL was measured by using DermaLab’s instrument (DermaLab USB module; Cortex Technology, Hadsund, Denmark) as previously described (35). Briefly, TEWL was assessed over a one-minute period by placing the probe against the skin surface in the center of area exposed to the saline/allergen soaked patch. An average of two readings per mouse was used and TEWL measurements were recorded as grams per meter squared per hour.
Histology and Immunohistochemistry
Patched skin tissues were fixed in 10% formalin immediately after mice were euthanized. Paraffin-embedded tissues were cut into 5μm sections and stained with H&E to assess skin thickness. Epidermal thickness was quantified using morphometric software (Metamorph, Molecular Devices, Sunnyvale, CA) and an average of 10 random fields (X200) was measured for each sample. T cells and neutrophils were assessed by immunohistochemistry using anti-CD3, anti-CD4, anti-CD8, and Ly6G Abs (Biolegend). Positive cells counted under microscope for 10 to 15 random fields (X200) and results were expressed as cells per field.
RNA isolation and quantitative real time PCR
Total RNA was isolated from homogenized mouse skin using RNeasy Microarray Tissue Mini Kit (QIAGEN, Valencia, CA) according to manufactures’ instructions and DNAse treatment was performed before the reverse transcription reaction with the Ready-To-Go T-Primed First-Stand Kit (Amersham Biosciences, Piscataway, NJ). Quantitative real-time PCR analysis of genes expression was done using LightCycler® FastStart DNA master SYBR green I (Roche, South San Francisco, CA). cDNA were amplified using the following primers: murine Murine IL-4 (forward, 5′-CTGTAGGGCTTCCAAGGTGCTTCG-3′, and reverse, 5′-CCATTTGCATGATGCTCTTTAGGC-3′); IFNγ (forward, 5′-CAGCAACAGCAAGGCGAAAAAGG-3′, and reverse, 5′-TTTCCGCTTCCTGAGGCTGGAT-3′); IL-17A (forward, 5′-ACTACCTCAACCGTTCCACG-3′, and reverse, 5′-AGAATTCATGTGGTGGTCCA-3′); IL-6 (forward, 5′-TGATGCACTTGCAGAAAACA-3′, and reverse, 5′-ACCAGAGGAAATTTTCAATAGGC-3′); CXCL2 (forward, 5′-CAGATAAGGCTCCAGTCAC-3′, and reverse, 5′-GGGTCTACACAGAGAGACA-3′); CXCL1 (forward, 5′-CCACACTCAAGAATGGTCGC-3′, and reverse, 5′-TCTCCGTTACTTGGGGACAC-3′); IL-22 (forward, 5′-GCAATCAGCTCAGCTCCTGT-3′, and reverse, 5′-CGCCTTGATCTCTCCACTCT-3′); IL-23/p19 (forward, 5′-GACCCACAAGGACTCAAGGA-3′, and reverse, 5′-GCTCCCCTTTGAAGATGTCA-3′); TGFβ1 (forward, 5′-GCTGAACCAAGGAGACGGAAT-3′, and reverse, 5′-GCTGATCCCGTTGATTTCCA-3′). Gene expression was normalized to HPRT (forward, 5′-TGCCGAGGATTTGGAAAAAG-3′, and reverse, 5′-CCCCCCTTGAGCACACAG-3′). Primers for human IL-6 are: forward, 5′-GAAAGCAGCAAAGAGGCACT-3′, and reverse, 5′-TTTCACCAGGCAAGTCTCCT-3′.
Statistical analysis
Reported values are expressed as mean±SEM. Statistical analysis was performed using Prism 5. One-way ANOVA followed by Bonferroni’s multiple comparison tests was performed on all experiments unless stated otherwise. Significance was set at a p value of 0.05.
Results
EGFR signaling protects from skin barrier function impairment following cutaneous allergen exposure
To evaluate the impact of EGF on the development of AD, we used the experimental AD model outlined in Figure 1A. This model, described previously (35), yields key features of atopic dermatitis including erythema, pruritis, excoriations, and epidermal thickening, and is associated with increased expression of Th1, Th2 and Th17 cytokines in skin lesions (Supplementary Figure 1). In order to determine the most optimal dose of EGF to use, we gave doses ranging from 4–50μg EGF ip to BALB/c mice and then analyzed skin RNA from treated mice for Egr1 expression (known to be induced downstream of EGF signaling). Egr-1 mRNA expression was maximally increased in the skin of mice treated with 40μg after 30 min and decreased to baseline after 6 hours (Supplementary Figure 2A), thus, we used this EGF dose for our subsequent studies. Mice were treated with EGF 40 μg or saline ip one day prior to the application of the first allergen patch, and then ip every other day throughout the rest of the protocol in Figure 1A. Twenty-four hours after the last allergen patch was removed, TEWL was assessed as an indicator of skin barrier function. EGF treatment resulted in attenuation of the AD phenotype that developed as evidenced by attenuated TEWL (Figure 1B).
In order to further explore the role of EGF in AD, we utilized waved-2 mice, which have a spontaneous loss-of-function mutation in EGFR, and pharmacologic inhibition of EGFR kinase activity with erlotinib, a specific EGFR inhibitor (37, 38). Based on our observations, we predicted that genetic (waved-2 mice) or pharmacologic inhibition (erlotinib) of EGFR would result in an enhanced AD phenotype. Waved-2 mice, repeatedly exposed to cutaneous A. fumigatus according to the AD model outlined in Figure 1A, developed more severe AD compared to strain matched C57Bl/6 mice as evidenced by increased TEWL (Figure 1C).
BALB/c mice were treated with erlotinib (100 mg/kg) by gavage six times a week during the three-week A. fumigatus treatment period (Figure 1A) while control animals were given the same volume of the gavage vehicle (0.5% wt/vol methylcellulose) as previously described (37, 39). Consistent with our previous findings, mice treated with erlotinib had higher TEWL (Figure 1D) compared to saline treated mice. These data collectively strongly support a protective role for EGFR in AD.
EGF treatment attenuates AD exacerbation following cutaneous allergen re-challenge
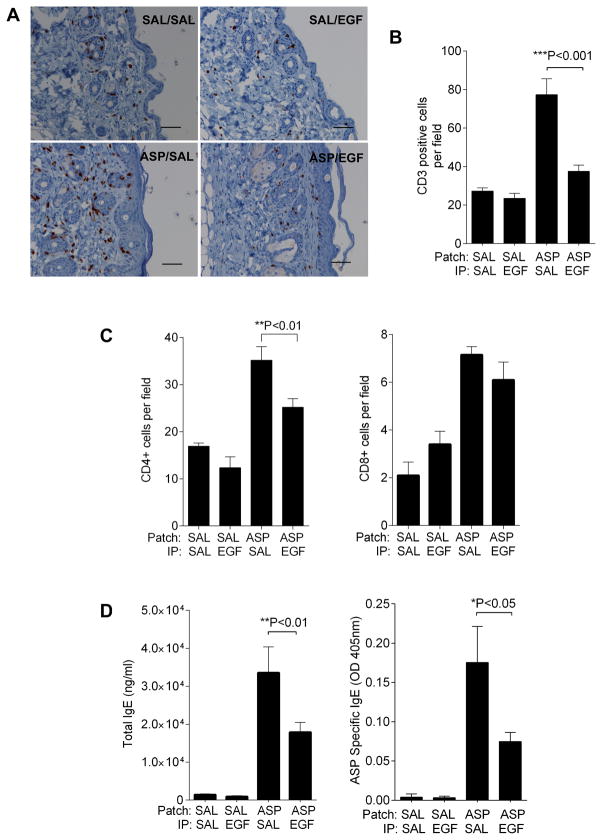
AD is a chronic disease characterized by frequent clinical relapses. We next investigated whether EGF treatment could block or attenuate subsequent relapse following AD development. For this purpose, we used a modified AD model (Figure 2A) in which mice were treated with EGF after AD was established and then re-challenged with allergen to mimic a relapsing phase. As shown in Figure 2B, compared to control treatment, the EGF treated group displayed attenuated TEWL after the allergen re-exposure. Histological analysis of patched skin revealed that A. fumigatus induced a two-to-four-fold increase in epidermal thickness and increased inflammation while the EGF treatment group showed attenuated epidermal thickness (Figure 2C, D). There was significant local infiltration by CD3+ cells in skin of the allergen-exposed animals, which was significantly decreased in EGF treated mice (Figure 3A, B). Infiltrating CD4+ and CD8+ cells were identified and quantified by immunohistochemistry. As shown in Figure 3C, both CD4+ and CD8+ infiltrated the skin following allergen exposure, but EGF treatment blunted infiltration of only the CD4+ cells.
Figure 3.
EGF treatment attenuates skin inflammation and serum total and allergen-specific IgE levels in allergen-exposed mice. A. CD3 staining and B. CD3+ cells counts of the patched skin samples from mice subjected to protocol in Figure 2A; C. CD4+ and CD8+ cells counts of the patched skin samples as above. D. Total and A. fumigatus-specific IgE levels were determined in serum from mice subjected to protocol in Figure 2A. Values are expressed as mean±SEM (n=6–10 mice/group). *P<0.05, **P<0.01, and ***P<0.001. Each experiment was performed a minimum of 3 times. Scale bars, 50μm.
Approximately 80% of AD patients have elevated levels of serum IgE and evidence of IgE against allergens, and there is a strong correlation with the disease severity (6). We measured serum total and A. fumigatus-specific IgE levels. As showed in Figure 3D, the EGF treated group demonstrated significantly lower levels of total and Asp-specific IgE.
Expression of EGFR and EGFR ligands in the skin of mice following the acute and chronic allergen exposure
Given the important role for the EGF pathway, we evaluated the expression levels of EGFR and EGFR ligands in skin tissue following acute and chronic allergen exposure. The effect of exogenous administration of EGF on the expression of EGFR and EGFR ligands was also evaluated. As shown in Supplementary Figure 2B, 2C and 2D, TGF-α expression was not significantly changed either by A. fumigates exposure or exogenous EGF treatment; epiregulin expression was decreased in A. fumigates-exposed mice and exogenous EGF treatment did not alter its expression. In contrast, HB-EGF and amphiregulin were induced following A. fumigates exposure supporting that EGFR ligands are naturally produced in this AD model. Induction of HB-EGF and amphiregulin, was blunted by exogenous EGF treatment. This may be due to a negative feedback loop. EGFR expression level was not affected by A. fumigates exposure or exogenous EGF treatment in the chronic model.
EGF treatment results in attenuated skin IL-17A levels in allergen re-challenged mice
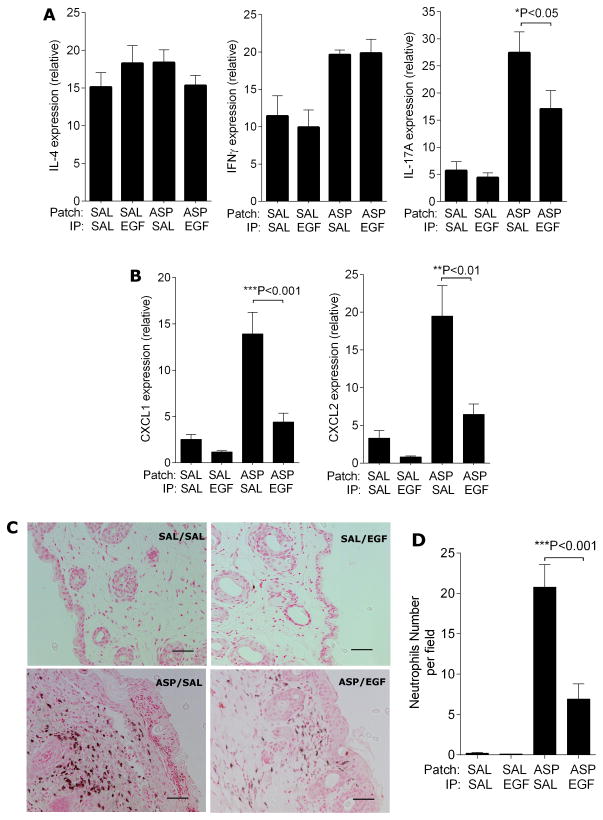
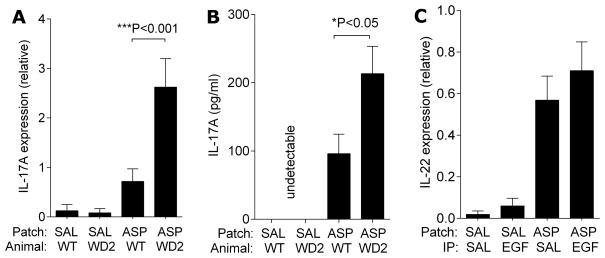
To assess the nature of the immune response, we assessed mRNA expression of Th1, Th2 and Th17 in the skin beneath the patched area in this chronic model (model shown in Figure 2A). While there were no changes in the expression of Th1 and Th2 related cytokines (IL-4 and IFNγ shown in Figure 4A and TSLP, CCL17, CCL20 and CCL27, data not shown), IL-17A expression was dramatically decreased in the EGF-treated AD skin (Figure 4A). Other members of the IL-17 family were also evaluated by q-PCR. As shown in Supplementary Figure 3, IL-17B and IL-17F, but not IL-17C or IL-17E, were induced following allergen exposure, and induction was unaffected by EGF treatment.
Figure 4.
EGF treatment results in attenuated skin IL-17A levels in allergen-exposed mice. Skin RNA was isolated from the skin of mice subjected to the chronic AD model shown in Figure 2A after the last patch. Expression of indicated genes was determined by q-PCR (A and B); C. Ly6G staining of neutrophils in the patched skin and D. neutrophil counts; n=6–10 mice/group. Values are expressed as mean±SEM. *P<0.05, **P<0.01, and ***P<0.001. Each experiment was performed a minimum of 3 times. Scale bars, 50μm.
Since tight junction abnormalities play an important roles during AD development (4, 5), expression of claudin-1, claudin-4, zonulae occludens (ZO)-1, and filaggrin was also assessed in the inflammatory skin. Their expression levels were not affected by EGF treatment (Supplemental Figure 4) and this was confirmed by immunohistochemistry (data not shown). As EGF is known to stimulate keratinocyte growth and differentiation (40–42), we also measured the keratinocyte proliferation in the skin tissues. Ki67 staining revealed no significant change in the positive keratinocyte percentage during the further allergen challenger after EGF treatment in this model (data not shown).
We next examined expression of chemokines downstream of IL-17A including CXCL-1, CXCL-2, CXCL-10, and CCL2 (Figure 4B and data not shown). EGF treatment resulted in attenuated expression of these genes consistent with the observed decrease in IL-17A. Accordingly, neutrophil infiltration was also diminished in EGF treated mice compared to controls (Figure 4C, D).
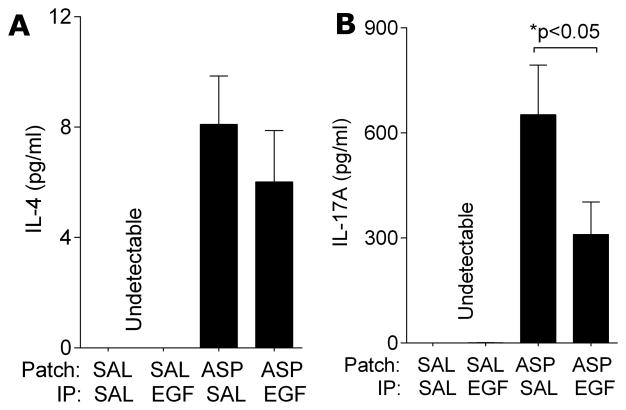
In order to further evaluate the nature of the local T cell response, we assessed cytokine production in the skin-draining lymph nodes. As shown in Figure 5, IL-17A was decreased in the supernatants taken from allergen-stimulated LN cells from EGF treated mice compared to saline treated mice. IL-4 and IFNγ were unchanged (Figure 5 and data not shown). These data suggest that less IL-17A is produced by LN cells of EGF treated mice and there may be fewer Th17 cells in the draining lymph nodes of EGF treated mice. These findings are consistent with our previous data showing decreased expression of IL-17A and IL-17A-induced chemokines in the skin from EGF treated AD mice.
Figure 5.
Cytokine production by restimulated skin draining lymph node cells following repeated cutaneous allergen exposure. Skin draining LN cells isolated from the chronic AD model shown in Figure 2A were cultured and re-stimulated with allergen in vitro. IL-4 (A), IL-17A (B) and IFNγ (undetectable) levels in the cultured medium were quantified by ELISA (n=6–10 mice/group). Values are expressed as mean±SEM. *P<0.05. Each experiment was performed a minimum of 3 times.
EGFR signaling blunts IL-17A, but not IL-22 response in AD model
In order to evaluate the impact of EGFR deficiency on IL-17A induction, we examined IL-17A expression in the patched skin of waved-2 mice. As shown in Figure 1A, waved-2 mice, repeatedly exposed to cutaneous A. fumigatus developed more severe AD. The patched skin of waved-2 mice also had higher expression of IL-17A (Figure 6A). Furthermore, assessment of cytokine production by skin-draining lymph node also revealed increased levels of IL-17A in the waved-2 mice (Figure 6B). These data confirm a role for EGFR in the modulation of IL-17 responses. These data collectively support a protective role for EGF and EGFR in the development and exacerbation of AD, and suggest that EGF attenuates Th17 responses.
Figure 6.
IL-17A levels are further enhanced in waved-2 mice compared to wild type mice following repeated cutaneous challenge. A. RNA was isolated from patched skin of waved-2 (WD2) and wild type mice after the last patch of the acute AD model shown in Figure 1A. Expression of IL-17A was determined; B. Skin draining LN cells isolated from the same mice were cultured and re-stimulated with allergen in vitro and IL-17A levels in the medium were assessed by ELISA. Values are expressed as mean±SEM; C. Expression of IL-22 was determined by q-PCR. (n=4–6 mice/group). *P<0.05, ***P<0.001. Each experiment was performed a minimum of 3 times.
In chronic lesions, increased expression of IL-22 has been reported (43). As shown in Figure 6C, IL-22 expression was increased in chronic AD lesions in exposed mice (assessed 24 hours after the 5th allergen patch was removed per the model depicted in Figure 2A), however, we did not observe any effect of EGF on IL-22 skin mRNA levels at this same time point. Changes in the induction of Th17 responses by EGF may impact IL-22 in more chronic lesions.
Th17 cell differentiation environment altered by EGF signaling
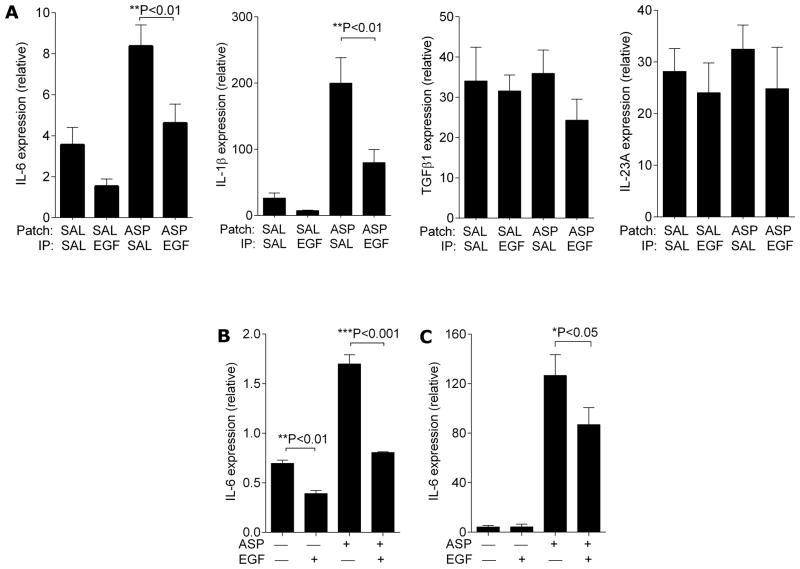
Given the crucial role of IL-6, TGFβ, IL-23 and IL-1β in the differentiation and maintenance of Th17 cells (44, 45), we measured mRNA levels of these cytokines in the skin beneath the patched area of mice after the mice were subjected to the AD protocol in Figure 2A. While TGFβ1 and IL-23/p19 expression were not altered by EGF treatment, IL-6 and IL-1β expression were attenuated in the skin of EGF-treated mice (Figure 7A). IL-6 and IL-1β have been reported to be up-regulated after IL-17 stimulation of macrophages and keratinocytes (46, 47). In order to determine if EGF modified IL-6 expression by keratinocytes independent of IL-17A, we utilized a reductionist approach using the HaCat keratinocyte cell line (Figure 7B) as well as human primary keratinocytes (Figure 7C). Keratinocytes exposed to A. fumigatus demonstrated an induction in IL-6 expression, which was nearly abrogated by EGF pre-treatment. EGF treatment even decreased IL-6 expression from baseline in unexposed keratinocytes (Figure 7C). Expression of IL-1β was not altered by EGF treatment (data not shown).
Figure 7.
EGF treatment alters expression of IL-6 and IL-1β but not TGFβ1 or IL-23/p19 in patched skin following cutaneous allergen exposure. RNA was isolated from patched skin from the mice from the chronic AD model shown in Figure 2A and expression of IL-6, IL-1β, TGFβ1, and IL-23/p19 was determined by q-PCR (A, n=6–10 mice/group). RNA was isolated from cultured (B) HaCat cell and (C) primary human keratinocytes treated as indicated and IL-6 expression was determined by q-PCR (n=3). Values are expressed as mean±SEM. *P<0.05, **P<0.01, ***P<0.001. Each experiment was performed a minimum of 3 times.
Discussion
In this article, we demonstrated for the first time a protective role of EGFR signaling in an experimental model of AD and provided data demonstrating that EGFR ligands are naturally produced in AD skin and EGFR signaling attenuates IL-17A expression in the skin following cutaneous allergen exposure. Further, our data revealed that EGF negatively regulates IL-6 expression by epidermal keratinocytes and this may contribute to the observed suppressive effect of EGF on Th17 cell differentiation in the cutaneous immune system.
Prior studies regarding the role of EGFR signaling in the innate immune response have focused mainly on the acute wound healing process including early recruitment of neutrophils into the wound site, an increase in the expression of antimicrobial proteins, and finally the reestablishment of the physical barrier (23, 24, 48). We assessed each of these possibilities as a potential mechanism for the observed actions of EGF in our model but did not observe any changes following EGF treatment. We also did not observe a change in Ki67 staining in the skin of AD mice treated with EGF, however, it is possible that that there was an early acute effect of EGFR activation on keratinocyte growth and we missed it because we are using a chronic model and only examined this at later time points. There was also no change in the expression of the antimicrobial protein, β2-defensin, following EGF treatment (data not shown). Disturbance of the epithelial barrier is a common feature of AD development (4, 5, 7). A defect in the barrier has been argued to favor the penetration and/or reactivity to microbes and allergens into the dermis. Expression of tight junction proteins including occludin, claudin-1, claudin-4, claudin-23, ZO-1, E-cadherin, and filaggrin (Supplemental Figure 4 and data not shown) were unaffected by EGFR signaling in the skin in our model.
EGF treatment had a striking and unexpected down regulatory effect on the expression of IL-17A in the skin. These data supports that EGF may have a previously unrecognized immunomodulatory role in AD development through regulating IL-17 levels in the inflammatory skin tissue. Interestingly, a common adverse effect of long term use of EGFR tyrosine kinase inhibitors in cancer patients is a cutaneous inflammatory rash characterized histologically by a moderate-to-severe inflammatory reaction dominated by neutrophils (49, 50). Studies of these patients have revealed that EGFR activation potently downregulates expression of chemokines, which promotes the migration of neutrophils and T lymphocytes into the skin, including CXCL10, CCL5, and CCL2 from keratinocytes. Similarly, our data revealed that expression of these chemokines is decreased in the skin tissues following EGF treatment. Based on our data, we hypothesize that EGFR inhibitors used in cancer treatment regimens leads to increased IL-17 levels in the skin as we observed in the waved-2 mice (Figure 6A and B). IL-17 is known to stimulate keratinocyte to secret CXCL10, CCL5, and CCL2 (13, 51) in vivo and in vitro so this is a likely mechanism for the observed skin rash.
The mechanism by which Th17 cells differentiate from naïve CD4+ T cells has been the subject of much attention because these cells have been shown to involved in a variety of immune diseases. The differentiation of Th17 cells in vivo and in many in vitro culture conditions requires the presence of IL-6 and several other factors (52–54). IL-6 can induce expression of IL-21 and IL-23 receptor in naïve T cells and these factors act with IL-1β and TGF-β during the Th17 cell priming process (55). Recent data suggests that Th17 cell lineage commitment has differential cytokine requirements depending on the site of priming (56). The priming microenvironment for Th17 cells in the skin and mucosal tissues required IL-6 while IL-6 was not essential for Th17 cell priming in the spleen. The investigators found that 20% of the DCs in the skin and other mucosal tissues were CD103+ and made high quantities of TGFβ and retinoic acid, both of which have been shown to suppress Th17 cell differentiation. IL-6 may be required to overcome these suppressive effects (56). In our animal model, IL-6 expression by keratinocytes was induced by A. fumigatus exposure and this induction was abrogated by EGF treatment in vivo and in vitro (Figure 7). Altered IL-6 expression in the skin microenvironment may impact Th17 cell priming in this model.
In patients with AD, a significant increase in skin IL-6 expression has been observed (57). Herein, we find that EGF treatment results in decreased IL-17A expression in the skin and IL-6 expression is also decreased in the inflammatory skin tissue after EGF treatment. In primary human keratinocytes and HaCat keratinocytes, EGF treatment attenuated IL-6 expression at baseline and following allergen exposure. In vivo, EGF may decrease IL-6 production in the skin environment resulting in a diminished local Th17 response. IL-6 receptor blocking mAb, tocilizumab, has been used to treat three patients with severe AD and was associated with relief in clinical symptoms although it associated with bacterial superinfection (58). EGF has been successfully used topically to promote wound healing (20) and our data suggest that topical EGF may be beneficial in preventing AD exacerbation. However, despite solid demonstrations of efficacy of topical EGF in experimental conditions and clinical trials (20), there are obvious unresolved concerns about the potential cancer-enhancing properties of exogenously administered EGF. In our studies, EGF is protective in AD and we have found a novel role for EGF in modulating IL-17 responses. Dissecting and delineating this previously unrecognized EGF pathway will aid in the development of therapies that specifically target this immunomodulatory pathway without affecting cellular proliferation. These findings may have important implications for other inflammatory skin disorders as well.
Supplementary Material
Acknowledgments
We thank Eric Brandt and Umasundari Sivaprasad for critical review of this manuscript, Brandy Day for technical assistance, and Cynthia Chappell for editorial assistance.
This work was supported by U19AI70235 (GKKH) and NIEHS T32 ES010957 (ZZ).
References
- 1.Bieber T. Atopic dermatitis. Ann Dermatol. 2010;22:125–137. doi: 10.5021/ad.2010.22.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009;124:1251–1258. e1223. doi: 10.1016/j.jaci.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Bos JD, Schram ME, Mekkes JR. Dermatologists are essential for quality of care in the general practice of medicine. Actas Dermosifiliogr. 2009;100(Suppl 1):101–105. doi: 10.1016/s0001-7310(09)73174-6. [DOI] [PubMed] [Google Scholar]
- 4.Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242:233–246. doi: 10.1111/j.1600-065X.2011.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Benedetto A, Rafaels NM, McGirt LY, Ivanov AI, Georas SN, Cheadle C, Berger AE, Zhang K, Vidyasagar S, Yoshida T, Boguniewicz M, Hata T, Schneider LC, Hanifin JM, Gallo RL, Novak N, Weidinger S, Beaty TH, Leung DY, Barnes KC, Beck LA. Tight junction defects in patients with atopic dermatitis. J Allergy Clin Immunol. 2011;127:773–786. e771–777. doi: 10.1016/j.jaci.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Werfel T. The role of leukocytes, keratinocytes, and allergen-specific IgE in the development of atopic dermatitis. J Invest Dermatol. 2009;129:1878–1891. doi: 10.1038/jid.2009.71. [DOI] [PubMed] [Google Scholar]
- 7.Brandt EB, Sivaprasad U. Th2 Cytokines and Atopic Dermatitis. J Clin Cell Immunol. 2011:2. doi: 10.4172/2155-9899.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dokmeci E, Herrick CA. The immune system and atopic dermatitis. Semin Cutan Med Surg. 2008;27:138–143. doi: 10.1016/j.sder.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Leung DY, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New insights into atopic dermatitis. J Clin Invest. 2004;113:651–657. doi: 10.1172/JCI21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipozencic J, Pastar Z, Kulisic SM, Pavic I. Immunologic aspects of atopic dermatitis. Acta Dermatovenerol Croat. 2009;17:226–234. [PubMed] [Google Scholar]
- 11.Spergel JM, Mizoguchi E, Brewer JP, Martin TR, Bhan AK, Geha RS. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest. 1998;101:1614–1622. doi: 10.1172/JCI1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spergel JM, Mizoguchi E, Oettgen H, Bhan AK, Geha RS. Roles of TH1 and TH2 cytokines in a murine model of allergic dermatitis. J Clin Invest. 1999;103:1103–1111. doi: 10.1172/JCI5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koga C, Kabashima K, Shiraishi N, Kobayashi M, Tokura Y. Possible pathogenic role of Th17 cells for atopic dermatitis. J Invest Dermatol. 2008;128:2625–2630. doi: 10.1038/jid.2008.111. [DOI] [PubMed] [Google Scholar]
- 14.He R, Kim HY, Yoon J, Oyoshi MK, MacGinnitie A, Goya S, Freyschmidt EJ, Bryce P, McKenzie AN, Umetsu DT, Oettgen HC, Geha RS. Exaggerated IL-17 response to epicutaneous sensitization mediates airway inflammation in the absence of IL-4 and IL-13. J Allergy Clin Immunol. 2009;124:761–770. e761. doi: 10.1016/j.jaci.2009.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He R, Oyoshi MK, Jin H, Geha RS. Epicutaneous antigen exposure induces a Th17 response that drives airway inflammation after inhalation challenge. Proc Natl Acad Sci U S A. 2007;104:15817–15822. doi: 10.1073/pnas.0706942104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oyoshi MK, Murphy GF, Geha RS. Filaggrin-deficient mice exhibit TH17-dominated skin inflammation and permissiveness to epicutaneous sensitization with protein antigen. J Allergy Clin Immunol. 2009;124:485–493. 493 e481. doi: 10.1016/j.jaci.2009.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egger B, Buchler MW, Lakshmanan J, Moore P, Eysselein VE. Mice harboring a defective epidermal growth factor receptor (waved-2) have an increased susceptibility to acute dextran sulfate-induced colitis. Scand J Gastroenterol. 2000;35:1181–1187. doi: 10.1080/003655200750056664. [DOI] [PubMed] [Google Scholar]
- 18.Egger B, Carey HV, Procaccino F, Chai NN, Sandgren EP, Lakshmanan J, Buslon VS, French SW, Buchler MW, Eysselein VE. Reduced susceptibility of mice overexpressing transforming growth factor alpha to dextran sodium sulphate induced colitis. Gut. 1998;43:64–70. doi: 10.1136/gut.43.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Troyer KL, Luetteke NC, Saxon ML, Qiu TH, Xian CJ, Lee DC. Growth retardation, duodenal lesions, and aberrant ileum architecture in triple null mice lacking EGF, amphiregulin, and TGF-alpha. Gastroenterology. 2001;121:68–78. doi: 10.1053/gast.2001.25478. [DOI] [PubMed] [Google Scholar]
- 20.Berlanga-Acosta J, Gavilondo-Cowley J, Lopez-Saura P, Gonzalez-Lopez T, Castro-Santana MD, Lopez-Mola E, Guillen-Nieto G, Herrera-Martinez L. Epidermal growth factor in clinical practice - a review of its biological actions, clinical indications and safety implications. Int Wound J. 2009;6:331–346. doi: 10.1111/j.1742-481X.2009.00622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Repertinger SK, Campagnaro E, Fuhrman J, El-Abaseri T, Yuspa SH, Hansen LA. EGFR enhances early healing after cutaneous incisional wounding. J Invest Dermatol. 2004;123:982–989. doi: 10.1111/j.0022-202X.2004.23478.x. [DOI] [PubMed] [Google Scholar]
- 22.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 23.Pastore S, Mascia F. Novel acquisitions on the immunoprotective roles of the EGF receptor in the skin. Expert Rev Dermatol. 2008;3:525–527. doi: 10.1586/17469872.3.5.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pastore S, Mascia F, Mariani V, Girolomoni G. The epidermal growth factor receptor system in skin repair and inflammation. J Invest Dermatol. 2008;128:1365–1374. doi: 10.1038/sj.jid.5701184. [DOI] [PubMed] [Google Scholar]
- 25.Cook PW, Brown JR, Cornell KA, Pittelkow MR. Suprabasal expression of human amphiregulin in the epidermis of transgenic mice induces a severe, early-onset, psoriasis-like skin pathology: expression of amphiregulin in the basal epidermis is also associated with synovitis. Exp Dermatol. 2004;13:347–356. doi: 10.1111/j.0906-6705.2004.00183.x. [DOI] [PubMed] [Google Scholar]
- 26.Cook PW, Piepkorn M, Clegg CH, Plowman GD, DeMay JM, Brown JR, Pittelkow MR. Transgenic expression of the human amphiregulin gene induces a psoriasis-like phenotype. J Clin Invest. 1997;100:2286–2294. doi: 10.1172/JCI119766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zaiss DM, van Loosdregt J, Gorlani A, Bekker CP, Grone A, Sibilia M, van Bergen PM, Henegouwen En, Roovers RC, Coffer PJ, Sijts AJ. Amphiregulin Enhances Regulatory T Cell-Suppressive Function via the Epidermal Growth Factor Receptor. Immunity. 2013;38:275–284. doi: 10.1016/j.immuni.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dominey AM, Wang XJ, King LE, Jr, Nanney LB, Gagne TA, Sellheyer K, Bundman DS, Longley MA, Rothnagel JA, Greenhalgh DA, et al. Targeted overexpression of transforming growth factor alpha in the epidermis of transgenic mice elicits hyperplasia, hyperkeratosis, and spontaneous, squamous papillomas. Cell Growth Differ. 1993;4:1071–1082. [PubMed] [Google Scholar]
- 29.Vassar R, Fuchs E. Transgenic mice provide new insights into the role of TGF-alpha during epidermal development and differentiation. Genes Dev. 1991;5:714–727. doi: 10.1101/gad.5.5.714. [DOI] [PubMed] [Google Scholar]
- 30.Shirasawa S, Sugiyama S, Baba I, Inokuchi J, Sekine S, Ogino K, Kawamura Y, Dohi T, Fujimoto M, Sasazuki T. Dermatitis due to epiregulin deficiency and a critical role of epiregulin in immune-related responses of keratinocyte and macrophage. Proc Natl Acad Sci U S A. 2004;101:13921–13926. doi: 10.1073/pnas.0404217101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mascia F, Mariani V, Girolomoni G, Pastore S. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am J Pathol. 2003;163:303–312. doi: 10.1016/S0002-9440(10)63654-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pastore S, Mascia F, Girolomoni G. The contribution of keratinocytes to the pathogenesis of atopic dermatitis. Eur J Dermatol. 2006;16:125–131. [PubMed] [Google Scholar]
- 33.Yamaki M, Sugiura K, Muro Y, Shimoyama Y, Tomita Y. Epidermal growth factor receptor tyrosine kinase inhibitors induce CCL2 and CCL5 via reduction in IL-1R2 in keratinocytes. Exp Dermatol. 2010;19:730–735. doi: 10.1111/j.1600-0625.2010.01108.x. [DOI] [PubMed] [Google Scholar]
- 34.Luetteke NC, Qiu TH, Fenton SE, Troyer KL, Riedel RF, Chang A, Lee DC. Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development. 1999;126:2739–2750. doi: 10.1242/dev.126.12.2739. [DOI] [PubMed] [Google Scholar]
- 35.Sivaprasad U, Warrier MR, Gibson AM, Chen W, Tabata Y, Bass SA, Rothenberg ME, Khurana Hershey GK. IL-13Ralpha2 has a protective role in a mouse model of cutaneous inflammation. J Immunol. 2010;185:6802–6808. doi: 10.4049/jimmunol.1002118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wise-Draper TM, Morreale RJ, Morris TA, Mintz-Cole RA, Hoskins EE, Balsitis SJ, Husseinzadeh N, Witte DP, Wikenheiser-Brokamp KA, Lambert PF, Wells SI. DEK proto-oncogene expression interferes with the normal epithelial differentiation program. Am J Pathol. 2009;174:71–81. doi: 10.2353/ajpath.2009.080330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Cras TD, Acciani TH, Mushaben EM, Kramer EL, Pastura PA, Hardie WD, Korfhagen TR, Sivaprasad U, Ericksen M, Gibson AM, Holtzman MJ, Whitsett JA, Hershey GK. Epithelial EGF receptor signaling mediates airway hyperreactivity and remodeling in a mouse model of chronic asthma. Am J Physiol Lung Cell Mol Physiol. 2011;300:L414–421. doi: 10.1152/ajplung.00346.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luetteke NC, Phillips HK, Qiu TH, Copeland NG, Earp HS, Jenkins NA, Lee DC. The mouse waved-2 phenotype results from a point mutation in the EGF receptor tyrosine kinase. Genes Dev. 1994;8:399–413. doi: 10.1101/gad.8.4.399. [DOI] [PubMed] [Google Scholar]
- 39.Hegymegi-Barakonyi B, Eros D, Szantai-Kis C, Breza N, Banhegyi P, Szabo GV, Varkondi E, Petak I, Orfi L, Keri G. Tyrosine kinase inhibitors - small molecular weight compounds inhibiting EGFR. Curr Opin Mol Ther. 2009;11:308–321. [PubMed] [Google Scholar]
- 40.Peus D, Hamacher L, Pittelkow MR. EGF-receptor tyrosine kinase inhibition induces keratinocyte growth arrest and terminal differentiation. J Invest Dermatol. 1997;109:751–756. doi: 10.1111/1523-1747.ep12340759. [DOI] [PubMed] [Google Scholar]
- 41.Stoll S, Garner W, Elder J. Heparin-binding ligands mediate autocrine epidermal growth factor receptor activation In skin organ culture. J Clin Invest. 1997;100:1271–1281. doi: 10.1172/JCI119641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stoll SW, Benedict M, Mitra R, Hiniker A, Elder JT, Nunez G. EGF receptor signaling inhibits keratinocyte apoptosis: evidence for mediation by Bcl-XL. Oncogene. 1998;16:1493–1499. doi: 10.1038/sj.onc.1201657. [DOI] [PubMed] [Google Scholar]
- 43.Guttman-Yassky E, Nograles KE, Krueger JG. Contrasting pathogenesis of atopic dermatitis and psoriasis--part II: immune cell subsets and therapeutic concepts. J Allergy Clin Immunol. 2011;127:1420–1432. doi: 10.1016/j.jaci.2011.01.054. [DOI] [PubMed] [Google Scholar]
- 44.Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N, Grainger JR, Chen Q, Kanno Y, Watford WT, Sun HW, Eberl G, Shevach EM, Belkaid Y, Cua DJ, Chen W, O’Shea JJ. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang YH, Wills-Karp M. The potential role of interleukin-17 in severe asthma. Curr Allergy Asthma Rep. 2011;11:388–394. doi: 10.1007/s11882-011-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 47.Reynolds JM, Angkasekwinai P, Dong C. IL-17 family member cytokines: regulation and function in innate immunity. Cytokine Growth Factor Rev. 2010;21:413–423. doi: 10.1016/j.cytogfr.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnston A, Gudjonsson JE, Aphale A, Guzman AM, Stoll SW, Elder JT. EGFR and IL-1 signaling synergistically promote keratinocyte antimicrobial defenses in a differentiation-dependent manner. J Invest Dermatol. 2011;131:329–337. doi: 10.1038/jid.2010.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agero AL, Dusza SW, Benvenuto-Andrade C, Busam KJ, Myskowski P, Halpern AC. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol. 2006;55:657–670. doi: 10.1016/j.jaad.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 50.Hu JC, Sadeghi P, Pinter-Brown LC, Yashar S, Chiu MW. Cutaneous side effects of epidermal growth factor receptor inhibitors: clinical presentation, pathogenesis, and management. J Am Acad Dermatol. 2007;56:317–326. doi: 10.1016/j.jaad.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 51.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 52.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008;28:445–453. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 54.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 55.Zhou L, Ivanov, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 56.Hu W, Troutman TD, Edukulla R, Pasare C. Priming microenvironments dictate cytokine requirements for T helper 17 cell lineage commitment. Immunity. 2011;35:1010–1022. doi: 10.1016/j.immuni.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fedenko ES, Elisyutina OG, Filimonova TM, Boldyreva MN, Burmenskaya OV, Rebrova OY, Yarilin AA, Khaitov RM. Cytokine gene expression in the skin and peripheral blood of atopic dermatitis patients and healthy individuals. Self Nonself. 2011;2:120–124. doi: 10.4161/self.2.2.16939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Navarini AA, French LE, Hofbauer GF. Interrupting IL-6-receptor signaling improves atopic dermatitis but associates with bacterial superinfection. J Allergy Clin Immunol. 2011;128:1128–1130. doi: 10.1016/j.jaci.2011.09.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.