Abstract
Aims
To reconcile an inconsistency in the disordered gambling literature by revisiting a previous study that claimed to find evidence for large gender differences in the magnitude of genetic and environmental influences.
Design
Univariate structural equation twin models were fit to decompose the variation in gambling behavior into additive genetic, shared environmental, and unique environmental influences.
Setting
United States.
Participants
Participants were 1,196 same-sex and unlike-sex twins (18–28 years of age, 49% male, 51% female) from the National Longitudinal Study of Adolescent Health (Add Health).
Measurements
Eight questions about normative and problematic gambling involvement were assessed by in-person interview. Although disordered gambling symptoms were assessed, the number of individuals that were administered these questions precluded twin analysis, including analysis of potential gender differences. Of the eight questions, only three were deemed usable for twin analysis – these were all questions about normative gambling involvement.
Findings
Individual differences in (non-disordered) gambling involvement were completely explained by family (C = 38% [30–46%]) and unique environmental factors (E = 62% [54–70%]). There was no evidence for genetic factors (A = 0), nor was there evidence for sex differences (Δχ2 = 1.23, df = 2, p = .54).
Conclusions
There appears to be no evidence for gender differences in the genetic contributions to disordered gambling. Family environment appears to play a significant role in explaining individual differences in (non-disordered) gambling involvement among emerging adults.
Introduction
Disordered gambling (DG) is an addiction characterized by having difficulty limiting money or time spent on gambling; this leads to adverse consequences for the gambler as well as people in the gambler's social network and the larger community1. Estimates of the past-year prevalence of DG from cross-national meta-analyses of over 200 studies range from 0.2% to 7.6%1,2.
Although there are many studies documenting the rates of DG, there are far fewer inquiries into its causes. For example, compared to other addictive disorders, there is a dearth of behavioral genetic research on DG. For many years, the only investigation was a large twin study based on data collected from the all-male Vietnam Era Twin (VET) registry3. In the VET study, structured diagnostic telephone interviews including assessments of DSM-III-R4 pathological gambling were conducted with 7869 individuals in 1991–1993 when the men were 34–54 years of age. When DG was defined as having one or more DSM-lll-R symptoms, the contribution of genetic, shared, and non-shared environmental factors were 48%, 0, and 52%, respectively3.
It was a full decade before another twin study of DG was conducted5. This new study was based on data collected from the nationally-representative National Longitudinal Study of Adolescent Health (Add Health). In the Add Health study, personal interviews including assessments of eight different gambling behaviors (“designed to measure serious gambling problems5”(p. 538), were conducted with 602 individual twins in 2001–2002 when the twins were 18–24 years of age. This study complemented the VET study nicely in that it focused on an earlier developmental period (emerging adulthood), on a time period when there were more gambling opportunities in the United States (2002 versus 1992), and it also included women.
In the overall Add Health sample, the contribution of genetic, shared, and non-shared environmental factors were 72%, 0, and 28%, respectively. The estimates substantially differed when the analyses were conducted separately for men and women. For men, the estimates of the contribution of genetic, shared, and non-shared environmental factors were 85%, 0, and 15%, respectively; for women the estimates were 0, 45%, and 55%, respectively. This suggests that there are substantially different contributions of genetic and environmental influences to gambling behavior in men versus women.
A subsequent study, however, based on data collected from the Australian Twin Registry reached different conclusions6. In the Australian study, structured diagnostic telephone interviews including assessments of DSM-IV7 pathological gambling were conducted with 4764 individuals in 2004–2007 when the twins were 32–43 years of age. Structural equation models fitted to a broad DG phenotype of one or more pathological gambling symptoms yielded estimates similar to those obtained in the VET study. In the overall sample, the contribution of genetic, shared, and non-shared environmental factors were 49%, 0, and 51%, respectively, and there was no evidence for significant gender differences in these estimates (the estimates of the contribution of genetic influences were 49% among men and 52% among women). This basic finding was recently replicated in a web-based survey of DG symptoms in an age-heterogeneous sample of 573 twin and 303 sibling pairs8.
It is difficult to reconcile the different findings obtained in the Add Health versus the Australian studies; the Add Health study found evidence for large differences between men and women, whereas the Australian study found no evidence for sex differences in the contribution of genetic factors to DG. The main difference between the Add Health and Australian studies was that they focused on twins of different age groupings (emerging adulthood versus middle adulthood). This might lead to the intriguing conclusion that there are age-related differences in the genetic and environmental underpinnings of DG in men and women. Before drawing this conclusion, however, it is worthwhile taking a closer look at the data and gambling assessment used in the Add Health study.
Methods
Methods common to the previous and current studies Participants
Participants were from the National Longitudinal Study of Adolescent Health (Add Health), a study designed to assess adolescent health and risk behaviors. Respondents completed four waves of interviews; variables of interest were obtained in Wave III during in-home interviews that took place in 2001–2002 (N = 15,197, age = 18–28 (M = 22.0, SD = 1.78)). Add Health oversampled for sibling pairs and included 3,139 adolescent pairs of varying genetic relatedness. There were 1,196 twins (476 monozygotic [MZ; 223 male, 253 female] and 720 dizygotic [DZ; 215 same-sex male, 187 same-sex female, and 149 males and 169 females from unlike-sex pairs]) that completed the gambling assessment at Wave III. Participant response rates at Wave III for the genetic sample were excellent9 (92.2% for MZ twins and 88.9% for DZ twins). All participants gave informed consent and the study was approved by the Institutional Review Board of the University of North Carolina at Chapel Hill.
Measures
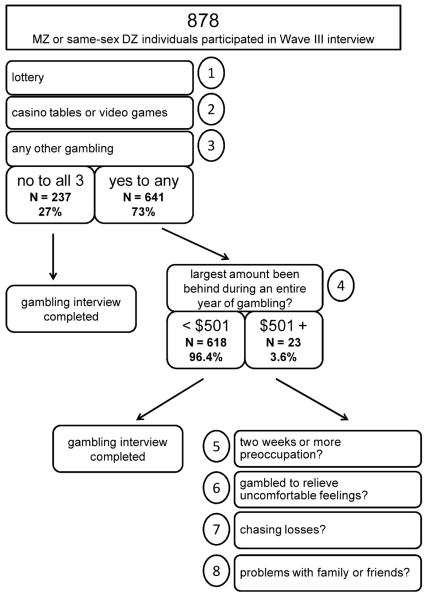
The gambling assessment from the Add Health Wave III interview included eight questions evaluating normative and problematic gambling involvement (see Figure 1). The first three questions pertained to various gambling activities and asked whether individuals had ever: (1) “bought lottery tickets, such as daily, scratch-offs, or lotto?” (2) “played casino tables or video games for money, such games as craps, blackjack, roulette, slot machines, or video poker?” and (3) “played any other games, such as cards or bingo, for money, or bet on horse races or sporting events, or taken part in any other kinds of gambling for money?” If individuals did not endorse any gambling, they were not queried further. If they endorsed at least one of the first three questions, they were asked about the largest amount of money they had ever lost in a year of gambling with the question “In all the time since you first started any type of gambling, what would you say was the largest amount of money that you have ever been behind across an entire year of gambling?”. Only individuals who endorsed losing more than $500 were administered the last four questions aimed to assess problematic gambling behaviors. These questions asked whether individuals had ever: (1) spent at least two weeks thinking about or planning out future gambling ventures, (2) gambled to relieve uncomfortable feelings (e.g., anxiety or depression), (3) lost money one day and returned to get even (“chased losses”), and (4) had gambling cause serious or repeated problems in relationships with family and friends. Table 1 shows the endorsement frequencies of these eight questions for the Add Health twin sample; these were quite similar to the endorsement frequencies obtained in the full Add Health Wave III sample (see online supplemental materials).
Figure 1.
Flow chart illustrating the administration of the eight gambling items in the Wave III Add Health interview. See text for full wording of interview items.
Table 1.
Endorsement frequencies of gambling items.
| Lifetime gambling involvement | Twin sample | ||
|---|---|---|---|
| full sample | men | women | |
| N = 878 | N = 438 | N = 440 | |
| Lottery | 59.5 (521) | 62.1 (270) | 57.1 (251) |
| Casino tables or video games | 47.1 (413) | 52.2 (228) | 42.1 (185) |
| Other gambling | 38.9 (341) | 47.8 (209) | 30.1 (132) |
| Yes to any of the above | 73.3 (641) | 77.0 (335) | 69.6 (306) |
| N = 641 | N = 335 | N = 306 | |
| Gambled > $500 in a year | 3.6 (23) | 6.3 (21) | a |
| N = 23 | N = 21 | a | |
| Preoccupation | 39.1 (9) | 42.7 (9) | a |
| Gambled to relieve feelings | 13.0 (3) | 14.3 (3) | a |
| Chasing losses | 47.8 (11) | 52.4 (11) | a |
| Problems with friends or family | 17.4 (4) | 19.1 (4) | a |
| Yes to any of the above | 60.9 (14) | 66.7 (14) | a |
Note: cells represent percentages (samples sizes in parentheses), Ns represent number of individuals. The twin samples only include twins from like-sex pairs.
Cell values are omitted due to risk of inadvertent disclosure of persons.
Methods specific to the previous study of Beaver et al (2010) of disordered gambling Measures
The eight gambling items were combined into a single scale. Because seven of the items were dichotomous (0,1) and the other item was coded with an eight-point response scale, the items were first standardized. Although it was not explicitly stated, items that had not been administered due to skip-outs were re-coded as zero. Only 14 individuals (all men) had endorsed any of the four problem gambling questions. The consequence of standardizing a variable in which four to 11 individuals had a value of `1' and 500+ individuals had a score of `0' is that those with a `1' received a standardized score as large as 14. The result of summing these eight standardized items can be seen in Table 1 of the original paper5(p.538) in which the maximum score on the gambling scale was 55.44 among men and 5.06 among women. Note that this is the sum of eight standardized items whose means individually were zero.
We repeated these analyses described in the previous study and obtained similar results. The discrepant distributions of the gambling scale in women versus men were explored further. The variance, skewness, and kurtosis of the gambling scale were 8.3, 0.9, and −1.3, respectively, among women; in contrast, they were 37.1, 4.3, and 27.5, respectively, among men. A number of methods were used to identify potential outliers or influential observations on the gambling scale. The influential observations were apparent even when examining the histogram for the two sexes – there were 14 men and no women in the sample with outlying scores on the gambling scale.
Multivariate outliers were identified by examining scatterplots based on plotting the gambling score of the first twin on the X-axis and the gambling score of the cotwin on the Y-axis for the four different zygosity groups (i.e., male MZ and DZ pairs, female MZ and DZ pairs). Again, there were 14 male twin pairs and no female twin pairs that were multivariate outliers. (Due to concerns about risk of inadvertent disclosure of persons or families, information about the actual number and characteristics of outliers included in the original manuscript review has since been removed.) The conclusions reached in the previous paper of Beaver et al (2010) of a substantial genetic contribution to gambling behavior and a large sex difference in the genetic contribution to gambling behavior were based on inadvertently including extreme outliers. This was clear from the twin correlations, but was also confirmed by repeating the regression-based twin analyses used in the previous paper (see online supplemental materials).
Methods specific to the current study of non-disordered gambling Measures
Of the eight gambling questions in the Add Health study, only three were usable for twin analysis. The maximum yearly monetary gambling losses variable was not deemed adequate for twin modeling analyses (see the online supplemental materials for a more thorough description of the analytic options considered for this measure), and as a consequence of the low prevalence of the “skip-out” of ever losing more than $500 in a year, very few participants (N = 23, 3.6%; see Figure 1) were administered the four problem gambling questions. The claim by the authors of the previous paper that the gambling measure was “designed to measure serious gambling problems” was somewhat misleading because the measure included questions about both normative and problematic gambling involvement and very few twins were administered the problem gambling questions. The only usable questions were about normative gambling. Therefore, this study changed from being a study of disordered gambling to a study of non-disordered gambling involvement.
Respondents' overall participation in gambling activities was operationalized in two ways: A dichotomous “any gambling” variable was created assessing whether individuals endorsed any of the first three gambling questions. In addition, gambling involvement was assessed as a continuous variable by summing participants' responses to questions 1–3 into a count variable of the number of gambling activities.
Statistical Analysis
Twin correlations were computed for all of the measures of gambling involvement. Tests of differences between MZ and DZ correlations were performed to evaluate the evidence for genetic influences on gambling involvement. Evidence for genetic influences is present if MZ correlations are significantly larger than the corresponding DZ correlations. In addition, tests of differences between same-sex DZ and unlike-sex DZ correlations were performed to evaluate the evidence for qualitative sex differences. Qualitative sex differences are differences in the genes and/or shared environmental factors that influence a phenotype; evidence for qualitative differences is present if unlike-sex DZ twin correlations are significantly lower than the corresponding same-sex DZ correlations.
Biometric models were fitted using the methods of maximum likelihood (for the gambling activity count) and robust weighted least squares (for the dichotomous “any gambling” variable) directly to the raw twin data using Mplus Version 710. Univariate biometric model fitting was conducted to partition the variation into additive genetic (A), shared environmental (C), and unique environmental (E) influences, and to test for quantitative sex differences in the genetic and environmental contributions to gambling involvement. Quantitative sex differences refer to differences in the magnitude of genetic, shared environmental, and unique environmental influences on a phenotype. Evidence for quantitative sex differences was tested by comparing the fits of models that allowed parameter estimates for men and women to vary with the fits of models that constrained estimates to be the same. Separate models for each gambling phenotype were constructed employing data from only same-sex twins (following from the previous paper5) and data from both same- and unlike-sex twins.
Results
Twin Correlations
None of the MZ twin correlations were significantly larger than the corresponding DZ correlations, indicating limited or no genetic influences on gambling participation (see Table 2). None of the unlike-sex DZ correlations were significantly lower than the corresponding same-sex DZ correlations, indicating no qualitative sex differences in the genetic and/or environmental contributions to gambling involvement (Table 2).
Table 2.
Twin correlations.
| Full sample | Men | Women | Unlike-sex DZ | ||||
|---|---|---|---|---|---|---|---|
| Lifetime gambling involvement | MZ | DZ | MZ | DZ | MZ | DZ | |
| N = 241 | N = 200 | N = 111 | N = 107 | N = 130 | N = 93 | N = 156 | |
| Lottery | .53 | .63 | .62 | .64 | .44 | .62 | .55 |
| test of MZ vs DZa | Δχ2 = .48, p = .49 | Δχ2 = .04, p = .84 | Δχ2 = 1.00, p = .32 | ||||
| test of DZ US vs SSa | Δχ2 = .45, p = .50 | ||||||
| Casino tables or video games | .47 | .65 | .51 | .75 | .42 | .51 | .57 |
| test of MZ versus DZa | Δχ2 = 3.35, p = .07 | Δχ2 = 2.44, p = .12 | Δχ2 = .25, p = .62 | ||||
| test of DZ US vs SSa | Δχ2 = .75, p = .39 | ||||||
| Other gambling | .41 | .29 | .35 | .19 | .46 | .29 | .38 |
| test of MZ versus DZa | Δχ2 = 1.25, p = .26 | Δχ2 = .51, p = .48 | Δχ2 = .57, p = .45 | ||||
| test of DZ US vs SSa | Δχ2 = .71, p = .40 | ||||||
| Any gambling | .46 | .54 | .41 | .44 | .50 | .59 | .58 |
| test of MZ versus DZa | Δχ2 = .22, p = .64 | Δχ2 = .004, p = .95 | Δχ2 = .20, p = .65 | ||||
| test of DZ US vs SSa | Δχ2 = .06, p = .81 | ||||||
| Gambling activity count | .40 | .49 | .40 | .47 | .39 | .49 | .46 |
| test of MZ versus DZa | Δχ2 = 1.05, p = .31 | Δχ2 = .30, p = .58 | Δχ2 = .73, p = .39 | ||||
| test of DZ US vs SSa | Δχ2 = .15, p = .70 | ||||||
Note: The full sample only includes like-sex twin pairs. Ns represent number of twin pairs. MZ = monozygotic, DZ = dizygotic, US = unlike sex, SS = same sex.
Δχ2 tests all have 1 degree of freedom
Biometric model fitting
Additive genetic influences could be dropped from both models without a significant decrement in fit (any gambling: Δχ2 = .003, df = 1, p = .96; activity count: Δχ2 = .00, df = 1, p = 1.00); however, shared environmental influences could not be dropped (any gambling: Δχ2 = 6.48, df = 1, p = .01; activity count: Δχ2 = 11.64, df = 1, p < .001). Therefore, CE models were retained for tests of quantitative sex differences.
Tests of quantitative sex differences were performed within the reduced models. Shared and unique environmental parameters could be constrained across men and women in both models without a significant decrement in fit, indicating no quantitative differences in the environmental influences on gambling involvement (any gambling: Δχ2 = .63, df = 2, p = .73; activity count: Δχ2 = 1.23, df = 2, p = .54). The results of models including unlike-sex twins were nearly identical to those including only same-sex twins. To simplify presentation and following from5, results from models including only same-sex twins are reported here in Table 3 (the other results are included in online supplemental materials).
Table 3.
Full model estimates of the proportion of variation in two gambling phenotypes attributable to additive genetic, shared environmental, and unique environmental factors.
| Proportion of Variation | |||
|---|---|---|---|
| Gambling Phenotype | Additive Genetic | Shared Environment | Unique Environment |
| Full Sample | |||
| Any gambling | .00 (ne) | .50 (.04, .95) | .50 (.31, .70) |
| Gambling Activity Count | .00 (ne) | .38 (.30, .46) | .62 (.54, .70) |
| Men | |||
| Any Gambling | .00 (ne) | .42 (.20, .65) | .58 (.35, .80) |
| Gambling Activity Count | .00 (ne) | .38 (.26, .50) | .62 (.50, .74) |
| Women | |||
| Any Gambling | .00 (ne) | .55 (.37, .73) | .45 (.27, .64) |
| Gambling Activity Count | .00 (ne) | .38 (.26, .49) | .62 (.51, .74) |
Note: Analyses performed using data from like-sex twins only. 95% confidence intervals are in parentheses, `ne' = not estimable (because the monozygotic were smaller than the dizygotic twin correlations).
Discussion
A closer look at the Add Health gambling data and assessments leads to a much different conclusion than that reached in the previous report5. A closer inspection shows that the Add Health study does not have adequate numbers of individuals with DG to draw any conclusions about the relative contributions of genetic and environmental factors. Similarly, the numbers of men and women that were administered the DG questions do not allow one to draw any conclusions about gender differences in the contributions of genetic and environmental factors to DG.
The analyses in a previous report5 were fraught with many problems including: (1) mishandling of the interview data by not attending to sections of questions that were not administered based on responses to prior questions (“skip-outs”), (2) assuming that problem gambling symptoms were absent for individuals who were not assessed, (3) creating extreme scores by standardizing the gambling items before combining them, and (4) failing to conduct standard regression diagnostics to identify potential univariate and multivariate outliers. If the last problem had been avoided, the results of the previous paper would have been much different. The corrected conclusion of the previous paper would have been: no evidence for significant genetic influences and no evidence for sex differences in the magnitude of genetic and environmental influences, but significant evidence for shared environmental influences on individual differences in gambling involvement.
In the Add Health survey, individuals who reported that the maximum amount spent on gambling in any given year was less than $500 were not asked about any DG. Using such a “skip-out” in an interview is premised on the idea that individuals below the threshold chosen would be unlikely to endorse the subsequent questions. There are two problems with the skip-out used in the Add Health survey.
First, respondents differ considerably in how they define a gambling win versus loss, and there are major concerns about the validity of assessments of gambling expenditures11–12. The process of estimating how much money is spent on gambling is an inherently difficult task. Questions about lifetime maximum yearly expenditures would not be expected to fare any better. Second, even if one assumes that one can obtain reliable and valid assessments of lifetime gambling expenditures, this is an extremely high cut-off to use to rule-in and rule-out individuals for further questioning about potential gambling problems. In the gambling assessment included in the National Epidemiologic Survey on Alcohol and Related Conditions13 and in the Australian twin study6, the skip-out question was whether a participant had ever gambled at least five times in a single year. By employing a cut-off as extreme as spending more than $500 in a single year, one risks excluding many people from the DG assessment that may have had problems; furthermore, it would be inappropriate to assume that those who skipped-out would not have endorsed any subsequent questions about DG symptoms. This is especially true in a young adult sample that may have less money with which to gamble, and for whom losing smaller amounts might still be problematic.
Although the Add Health gambling assessment cannot yield useful information about genetic and environmental underpinnings or gender differences in DG, it is informative about non-disordered gambling involvement among emerging young adults. The results of this study are clear in suggesting that variation in gambling involvement is completely explained by environmental factors, both familial factors shared by twins, and non-familial factors that are specific to the individual. Overall, familial factors explained about one-third to one-half of the variation in any lifetime gambling participation. The remaining variation was accounted for by unique environmental factors. There was no evidence for genetic influences explaining individual differences in non-disordered gambling involvement. This is an especially noteworthy finding given that it is extremely rare in the behavioral genetics literature to find that variation in a trait among adults is influenced by family environmental factors and not by genetic factors.
There was also no evidence for either quantitative or qualitative sex differences in the role of genetic, shared, or unique environmental influences in explaining individual differences in gambling involvement. In other words, the proportion of variation in gambling involvement attributable to each of these sources was the same in men and women. Because the results of this study suggested that the predominant contribution to the familial aggregation of gambling involvement were environmental factors, failure to find evidence for qualitative sex differences suggests that the important familial factors relevant to gambling involvement are the same in men and women. The results of the Add Health study are somewhat consistent with two other twin studies of gambling involvement among adolescents14 (tabled in15) and young adults16 conducted in the United States, but are inconsistent with a twin study of gambling involvement among middle-aged adults conducted in Australia15 and a web-based survey of an age-heterogeneous twin sample.
In both the landmark National Merit Scholarship Qualifying Test (NMSQT) twin study14 and another small-scale study of 155 Minnesota-born twins, there was evidence for an important influence of the shared environment and less robust evidence for genetic influences on the frequency of gambling among adolescents15 and young adults16. In the Australian twin study15, previously described above with respect to the DG results, participants were asked whether they had ever participated in 11 different gambling activities (“initiation”). Across the 11 different activities, the mean parameter estimates obtained for the contribution of genetic, shared environment, and unique environmental influences were 43%, 10%, and 46%, respectively, and the estimates for a composite “any gambling” variable were 55%, 21%, and 24%, respectively. In a recent web-based twin survey8, the estimates obtained for the contribution of genetic, shared environment, and unique environmental influences to variation in the number of lifetime gambling episodes (“frequency”) were 32%, 42%, and 25%, respectively.
In sum, the results of these five studies (including the present one) of twins studied during adolescence, emerging, young, and middle adulthood raise the possibility that there may be differences in the contributions of genetic and family environmental factors to variation in gambling involvement across the life span. In particular, genetic factors appear to play an increasing role at later developmental stages and also at later stages of gambling involvement progression (i.e., from initiation to frequency of use to disordered gambling). This aligns nicely with findings from the substance use literature demonstrating that the contribution of genetic influences increase and the contribution of shared environmental influences decrease with age17 and stage of involvement18.
Conclusions
In an on-line summary19 of the original report based on the Add Health study5, the reviewer concluded that “The present findings suggest that there are very different factors contributing to gambling problems for males and females, with genetic factors being the main contributor for males and environmental factors being the main contributor for females. These findings point to the possibility that there may be unique pathways to gambling problems for males and females”. It was this report along with comments from colleagues that prompted this “closer look.” Careful scrutiny of the assessment and data indicates that the Add Health study provides no evidence supporting the conclusion that there are different factors contributing to gambling problems for males and females. Instead, the data provide novel evidence for the importance of family environmental factors equally contributing to variation in (non-disordered) gambling involvement in men and women.
Supplementary Material
Acknowledgements
This work was supported by National Institute on Alcohol Abuse and Alcoholism grant AA13526. This research uses data from Add Health, a program project directed by Kathleen Mullan Harris and designed by J. Richard Udry, Peter S. Bearman, and Kathleen Mullan Harris at the University of North Carolina at Chapel Hill, and funded by grant P01-HD31921 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with cooperative funding from 23 other federal agencies and foundations. Special acknowledgment is due Ronald R. Rindfuss and Barbara Entwisle for assistance in the original design. Information on how to obtain the Add Health data files is available on the Add Health website (http://www.cpc.unc.edu/addhealth). No direct support was received from grant P01-HD31921 for this analysis.
Footnotes
Conflicts of interest: None
References
- 1.Williams RJ, Volberg RA, Stevens RMG. The Population Prevalence of Problem Gambling: Methodological Influences, Standardized Rates, Jurisdictional Differences, and Worldwide Trends. Report prepared for the Ontario Problem Gambling Research Centre and the Ontario Ministry of Health and Long Term Care. 2012 May 8; 2012. http://hdl.handle.net/10133/3068.
- 2.Hodgins DC, Stea JN, Grant JE. Gambling disorders. Lancet. 2011;378:1874–1884. doi: 10.1016/S0140-6736(10)62185-X. [DOI] [PubMed] [Google Scholar]
- 3.Eisen SA, Lin N, Lyons MJ, Scherrer J, Griffith K, True WR, Goldberg J, Tsuang MT. Familial influences on gambling behavior: An analysis of 3,359 twin pairs. Addiction. 1998;93:1375–1384. doi: 10.1046/j.1360-0443.1998.93913758.x. [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: DSM-III-R. rev. ed 3 American Psychiatric Association; Washington, D.C.: 1987. [Google Scholar]
- 5.Beaver KM, Hoffman T, Shields RT, Vaughn MG, DeLisi M, Wright JP. Gender Differences in Genetic and Environmental Influences on Gambling: Results from a Sample of Twins from the National Longitudinal Study of Adolescent Health. Addiction. 2010;105:536–542. doi: 10.1111/j.1360-0443.2009.02803.x. [DOI] [PubMed] [Google Scholar]
- 6.Slutske WS, Zhu G, Meier MH, Martin NG. Genetic and environmental influences on disordered gambling in men and women. Arch Gen Psychiatry. 2010;67(6):624–630. doi: 10.1001/archgenpsychiatry.2010.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. ed 4 American Psychiatric Association; Washington, D.C.: 1994. [Google Scholar]
- 8.Blanco C, Myers J, Kendler KS. Gambling, disordered gambling and their association with major depression and substance use: A web-based cohort and twin-sibling study. Psych Med. 2012;42:497–508. doi: 10.1017/S0033291711001401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris KM, Halpern CT, Smolen A, Haberstick BC. The National Longitudinal Study of Adolescent Health (Add Health) Twin Data. Twin Res Hum Genet. 2006;9(6):988–997. doi: 10.1375/183242706779462787. [DOI] [PubMed] [Google Scholar]
- 10.Muthén LK, Muthén BO. Mplus User's Guide: Seventh Edition. Author; Los Angeles, CA: 1998–2012. [Google Scholar]
- 11.Blaszczynski A, Dumlao V, Lange M. “How much do you spend gambling?” ambiguities in survey questionnaire items. J Gambl Stud. 1997;13:237–52. doi: 10.1023/a:1024931316358. [DOI] [PubMed] [Google Scholar]
- 12.Blaszczynski A, Ladouceur R, Goulet A, Savard C. Differences in monthly versus daily evaluations of money spent on gambling and calculation strategies. Journal of Gambling Issues. 2008;21:98–105. [Google Scholar]
- 13.Slutske WS. Natural recovery and treatment-seeking in pathological gambling: Results of two US national surveys. Am J Psychiatry. 2006;163:297–302. doi: 10.1176/appi.ajp.163.2.297. [DOI] [PubMed] [Google Scholar]
- 14.Loehlin JC, Nichols RC. Heredity, Environment and Personality: A Study of 850 Sets of Twins. University of Texas Press; Austin: 1976. [Google Scholar]
- 15.Slutske WS, Meier MH, Zhu G, Statham DJ, Blaszczynski A, Martin NG. The Australian twin study of gambling (OZ-GAM): rationale, sample description, predictors of participation, and a first look at sources of individual differences in gambling involvement. Twin Res Hum Genet. 2009;12(1):63–78. doi: 10.1375/twin.12.1.63. [DOI] [PubMed] [Google Scholar]
- 16.Winters KC, Rich T. A twin study of adult gambling behavior. J Gambl Stud. 1998;14:213–225. [Google Scholar]
- 17.Kendler KS, Schmitt E, Aggen SH, Prescott CP. Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Arch Gen Psychiat. 2008;65:674–682. doi: 10.1001/archpsyc.65.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Stallings MC. Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Arch Gen Psychiat. 2003;60:1256–1264. doi: 10.1001/archpsyc.60.12.1256. [DOI] [PubMed] [Google Scholar]
- 19.Ontario Problem Gambling Research Center Synopsis Project. 2013 Webcite: http://www.webcitation.org/6E73l5MOO.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.