Abstract
Atherosclerosis and osteoporosis are chronic diseases that progress with age, and studies suggest aortic calcification, an indicator of atherosclerosis, is inversely associated with BMD. The OPG/RANK/RANKL system has been proposed as a shared regulatory system for bone and vasculature. Denosumab (DMAb), a monoclonal antibody against RANKL, improved BMD and reduced fracture risk in the FREEDOM trial. We evaluated whether or not treatment with DMAb influenced progression of aortic calcification (AC) and incidence of cardiovascular (CV) adverse events. We included 2,363 postmenopausal women with osteoporosis (1,142 placebo, 1,221 DMAb), selected from 7,808 participants in the FREEDOM trial (3,906 placebo, 3,902 DMAb), at high risk of CV events according to modified Raloxifene Use for the Heart (RUTH) criteria. CV adverse events were reported by participants. AC scores were assessed using a semi-quantitative method from lateral spine x-rays. Change in AC score from baseline to 12 (N = 1,377), 24 (N = 1,231) and 36 months (N = 1,045) was calculated as AC score at follow-up minus AC score at baseline. AC progression was defined as change in AC score > 0. Baseline characteristics, CV risk factors, and AC scores were similar between treatment groups. Mean age of participants was 74 years (range, 60–90), 88% were white, and 77% had AC score > 0 at baseline. Frequency of AC progression over 3 years did not differ between women in placebo (22%) and DMAb (22%) groups (p = 0.98). AC progression did not differ between treatment groups when analyzed by baseline estimated glomerular filtration rate or by baseline AC scores. Frequency of CV adverse events did not differ between placebo (40%) and DMAb (38%) groups (p = 0.26). In conclusion, DMAb treatment had no effect on progression of AC or incidence of CV adverse events compared to placebo.
Keywords: Aortic calcification, osteoporosis, RANKL/OPG, denosumab, cardiovascular
Introduction
The presence of aortic calcification (AC) is strongly associated with risk of cardiovascular events (CV).(1) Epidemiologic studies suggest aortic calcification is also associated with decreased bone mineral density (BMD), greater bone loss, and higher risk of fracture.(2–11) Findings can differ by study design, the population and skeletal site studied, as well as the approach used to evaluate aortic calcification and measure bone density. In the search for a common mediator capable of influencing bone remodeling and vascular calcification, the Osteoprotegerin (OPG)/ Receptor activator of NF-κB (RANK)/RANK Ligand (RANKL) system has received recent attention.(12)
In the bone compartment, RANKL stimulates osteoclast formation, activation, and survival.(13) OPG acts as a decoy receptor preventing RANKL to bind to its receptor RANK on osteoclast and osteoclast precursors and inhibiting bone resorption.(14) Blocking RANKL in preclinical and clinical studies by OPG, and later by denosumab (DMAb), a monoclonal antibody against RANKL, decreases bone resorption and increases bone mineral density.(15) In the randomized, double-blinded, FREEDOM trial (Fracture Reduction Evaluation of Denosumab in Osteoporosis every 6 Months), women assigned to treatment with DMAb compared to placebo had 68% reduction in risk of vertebral fracture and 40% reduction in hip fracture.(16)
In the vascular compartment, the exact role of RANKL and OPG is more controversial, as pre-clinical findings are not consistent with human epidemiological observations.(12,17–19) Genetically modified animals that lack the gene for OPG have increased vascular calcification and osteoporosis, indicating a potential protective role of OPG.(20) Moreover, exogenous OPG has been shown to have mitigating effects on calcification in animal models of atherosclerosis and calcific arteriopathy. (21–24) In contrast, in humans, a large body of epidemiologic literature suggests a positive association between serum OPG and CV outcomes such as atherosclerosis, arterial calcification, or CV disease.(19,25–28) Low serum RANKL was associated with an increased risk of CV disease in older women and men in the Bruneck Study.(29–30) Although these findings from observational studies may provide compelling evidence for a link between OPG, RANKL and cardiovascular disease, randomized trials are needed to help establish causation.
We assessed the effect of RANKL inhibition, by treatment with DMAb, on progression of AC and incidence of CV adverse events in women participating in the FREEDOM trial, a double-blinded, placebo-controlled, randomized trial.(31–32)
Materials and Methods
Participants
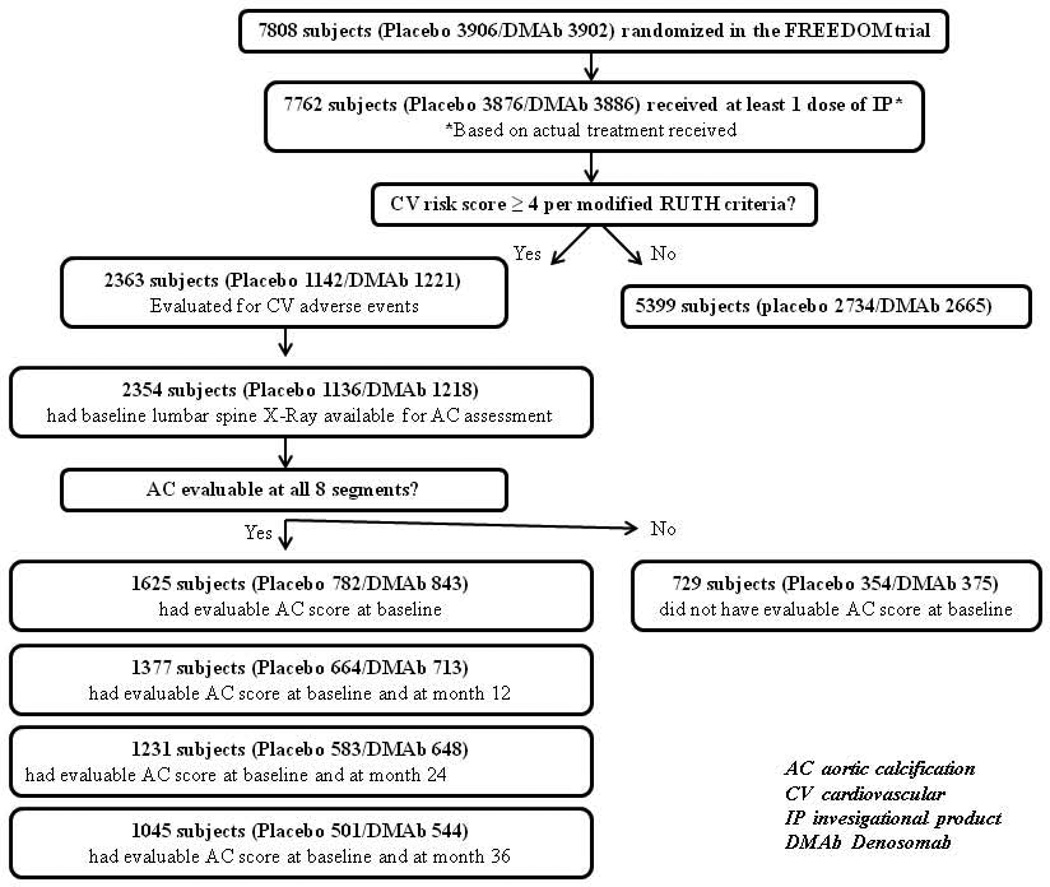
The FREEDOM Trial randomized 7,808 postmenopausal women (3,906 placebo, 3,902 DMAb) aged 60 to 90 years, with osteoporosis (baseline BMD T-score < –2.5 at the lumbar spine or total hip and ≥ –4.0 at both sites) to receive 60 mg of DMAb subcutaneously or placebo every 6 months (Q6M) for 3 years (Figure 1). The inclusion and exclusion criteria have been described by Cummings et al, 2009 (ClinicalTrials.gov number, NCT00089791). (16)
Figure 1.
Flow diagram of study participants
For the current study, we identified 2,363 women from the FREEDOM trial (1,142 placebo, 1,221 DMAb,), who received at least one dose of DMAb and were at increased risk of CV events, defined as a score of ≥ 4 points according to criteria used in the Raloxifene Use for the Heart (RUTH) trial. (31–32) We modified the hyperlipidemia criterion, relying on a history of hyperlipidemia, instead of serum lipid measurements, which were not available. The score was determined as follows: prior myocardial infarction, percutaneous coronary intervention, or coronary artery bypass surgery (4 points), diabetes mellitus (3 points), age ≥ 70 years (2 points), age 65 to 69 years (1 point), current or former smoker (1 point), hypertension (1 point), hyperlipidemia (1 point). (31–32) Information on CV risk factors was obtained using a CV-specific medical history questionnaire administered to participants by study staff. These 2,363 participants were included in the analysis of DMAb treatment on incidence of CV adverse events (Figure 1).
Due to inadequate imaging, AC scores could not be determined for 729 of 2,354 participants who had a baseline x-ray, so that 1,625 women were included in the analysis of DMAb treatment and AC (Figure 1). The number of women with both baseline and follow-up AC scores was 1,377 women (664 placebo, 713 treatment) at 12 months, 1,231 women (583 placebo, 648 treatment) at 24 months, and 1,045 women (501 placebo, 544 treatment) at 36 months.
Treatment
Women participating in the FREEDOM trial were randomized to receive placebo or DMAb 60 mg (Q6M) subcutaneously for 36 months. Participants were instructed to take daily calcium (at least 1g) and vitamin D (400 – 800 IU).
Aortic Calcification
Two readers, who completed standardized training, independently graded each individual set of four radiographs (baseline, 12, 24, and 36 months) in a temporal sequence. Calcific deposits in the abdominal aorta, adjacent to each lumbar vertebra (L1-L4), were assessed separately for the posterior and anterior walls of the aorta (8 segments in total). Each aortic segment was graded according to a 4-point severity scale: 0 (no calcification), 1 (less than 1/3 artery wall with calcification), 2 (1/3 to 2/3 artery wall with calcification) or 3 (more than 2/3 of the artery wall with calcification), as described for the Framingham Heart Study. (33) Readers were instructed that, for each aortic segment, the grade could not be lower at a subsequent time point (indicating improvement) than the grade assigned at a previous time point.
AC scores for each individual were calculated by summing the grades for the 8 aortic segments. AC scores ranged from a minimum of 0, indicating no aortic calcification, and increasing in severity to a maximum of 24. If any one of the eight aortic segments could not be graded (due to inadequate visualization of soft tissue anterior to spine), then the AC score was considered missing. About 30% of individuals had at least one aortic segment that was not evaluable at each time point and therefore set to missing.
To evaluate reader reliability, the 2 readers evaluated AC scores for the same 30 radiographs. The intra-class correlation coefficient (ICC) between readers was ≥ 0.93, indicating excellent agreement. Change in AC score was calculated as the AC score at follow-up minus the AC score at baseline. The methods used to score AC on the baseline and follow-up radiographs in a temporal sequence prohibited any negative change scores. We defined (any) AC progression as change in AC score > 0.
Cardiovascular Adverse Events
Cardiovascular (CV) adverse events and CV serious adverse events were reported by participants and/or physicians and were not adjudicated using medical records. These were coded as preferred terms in the Medical Dictionary for Regulatory Activities (MedDRA, Version 11.0). CV adverse events and serious CV adverse events included vascular and cardiac events as categorized by MedDRA that occurred after the first dose of investigational product.
Renal Function
Renal function was estimated using the Cockcroft-Gault (mL/min) equation [(140 – age [years]) × (weight [kg]) × (0.850 if female) / (72 × plasma creatinine [mg/dL])]. Cockroft-Gault estimation of renal function was chosen instead of the MDRD estimating method because the Cockroft-Gault estimation was used primarily in the analysis of the effects of DMAb on fracture and BMD by level of renal function for the whole FREEDOM study population. (34) In addition, the precision of the Cockroft-Gault estimation of renal function for women over the age of 60 years with truly low renal function appears to be slightly better than that obtained with MDRD estimation.(35–39) An estimated glomerular filtration rate (eGFR) above or less than 45 mL/min was selected to examine the influence of renal function on progression of AC as an eGFR below 45 mL/min has been shown to be associated with an increased prevalence of vascular calcification as well as an increased risk of clinical CV events.(40–42)
Statistical Analysis
We compared baseline factors by treatment group in 1,625 women who had evaluable AC scores at baseline, using two-sided Student T-tests or Chi-square tests as appropriate.
We used a two-sided Wilcoxon rank sum test to compare the mean change in AC score (follow-up AC score minus baseline AC score) at each time point in women in placebo and treatment groups. We compared frequency (%) of any progression (AC score change > 0) at each time point by treatment using a two-sided Chi-square test with 1 degree of freedom (df). The number of women included in the analysis of change in AC score at 12, 24, and 36 months was 1,377, 1,231, and 1,045, respectively.
We used logistic regression to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for the association between AC progression (AC score change > 0) over 3 years and treatment group (N = 1,045) adjusted for baseline AC score. We performed stratified analysis to evaluate potential differences in the association between any AC progression at 3 years and treatment group by baseline AC score (< 6 versus ≥ 6) and baseline renal function (< 45, ≥ 45 mL/min).
We calculated incidence (%) as the number of women who had 1 or more CV adverse events divided by 2,363, the total number of participants with high CV risk (≥ 4 points on modified RUTH trial criteria). We compared incidence of CV adverse events by treatment group using a two-sided, Chi-square test (1 df).
Analyses were performed based on the actual treatment received, where subjects who received at least 1 dose of DMAb were analyzed in the DMAb treatment group. Statistical testing was performed at the 5% significance level. Analyses were conducted using SAS software version 9.1.3 (SAS Institute, Cary, NC).
Results
As shown in Table 1, mean age (74 years), race (88% white), BMI (27 kg/m2), and years since menopause (26) were similar in placebo and DMAb groups (all p-values > 0.05). There were no differences at baseline between treatment groups at baseline in prevalence of vertebral fracture (22–24%), mean lumbar spine BMD T-score (−2.8), or eGFR (64 mL/min).
Table 1.
Baseline characteristics, according to treatment group, in 1,625 women in the FREEDOM trial, scoring ≥ 4 points on modified Raloxifene Use for the Heart (RUTH) trial criteria and who had evaluable aortic calcification (AC) score at baseline
| Placebo N = 782 |
Denosumab N = 843 |
Total N = 1,625 |
|
|---|---|---|---|
| n (%) or Mean (SD) | |||
| Age (years) | 74 (5) | 74 (5) | 74 (5) |
| White (%) | 688 (88) | 743 (88) | 1431 (88) |
| Body mass index (kg/m2) | 27 (4) | 27 (4) | 27 (4) |
| Years since menopause (years) | 26 (7) | 26 (7) | 26 (7) |
| Prevalent vertebral fracture (%) | 175 (22) | 201 (24) | 376 (23) |
| Lumbar spine BMD T-score | −2.75 (0.75) | −2.79 (0.71) | −2.77 (0.73) |
| Lumbar spine BMD (g/cm2), Hologic1 | 0.738 (0.077) | 0.733 (0.063) | 0.735 (0.070) |
| Lumbar spine BMD (g/cm2), Lunar2 | 0.855 (0.093) | 0.850 (0.096) | 0.852 (0.094) |
| Total hip BMD T-score | −1.97 (0.82) | −1.89 (0.84) | −1.93 (0.83) |
| Total hip BMD (g/cm2), Hologic1 | 0.715 (0.101) | 0.731 (0.098) | 0.724 (0.100) |
| Total hip BMD (g/cm2), Lunar2 | 0.755 (0.097) | 0.760 (0.101) | 0.757 (0.099) |
| Estimated glomerular filtration rate (mL/min) | 64 (19) | 64 (20) | 64 (20) |
placebo, N=317; denosumab, N=355; total, N=672
placebo, N=465; denosumab, N=487; total, N=952
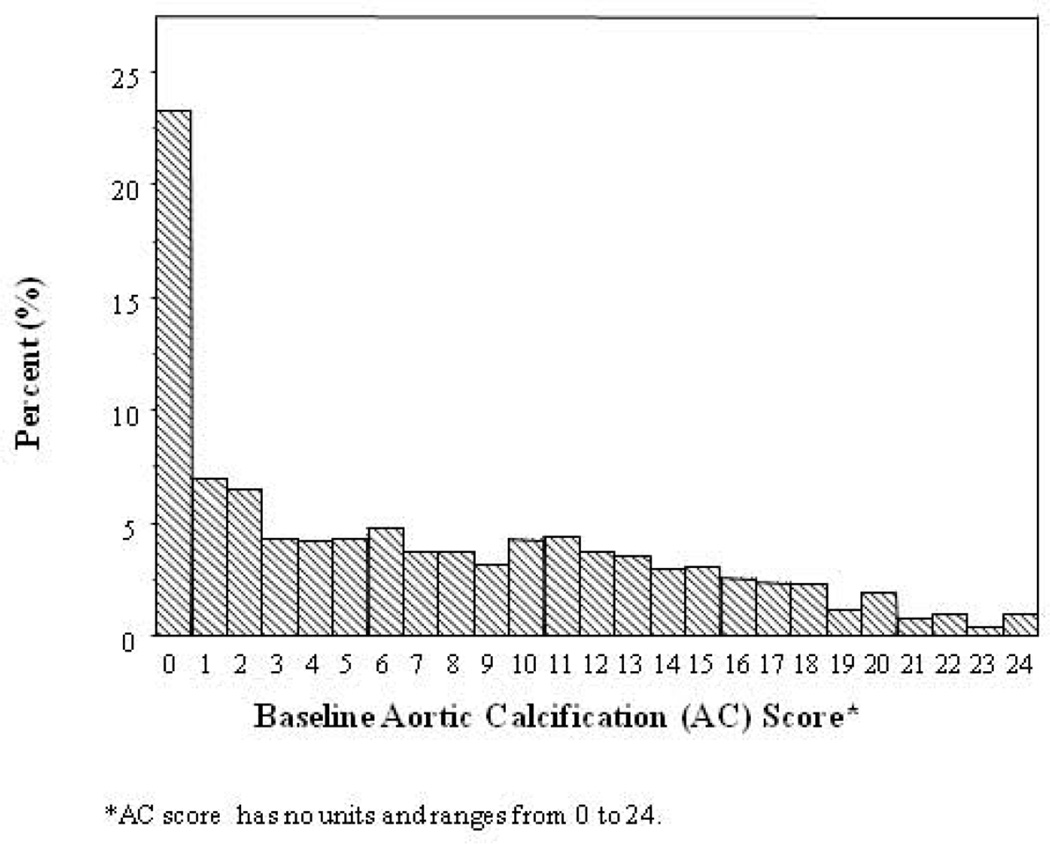
The distibution of baseline AC score was highly skewed (Figure 2) with nearly one-fourth of participants having no evidence of aortic calcification at baseline (AC score = 0: 22% placebo, 24% DMAb; p = 0.24). Median baseline AC scores were similar between placebo (AC = 6) and DMAb (AC = 5) groups (p = 0.20; Table 2). Further, median AC score at baseline was similar between treatment groups in women with low (< 45 ) and high (≥ 45 mL/min) eGFR, and in women with baseline AC scores below the median (< 6) and equal to or above the median (≥ 6); all p-values > 0.05.
Figure 2.
Frequency distribution of baseline aortic calcification (AC) score in 1,625 participants
Table 2.
Baseline aortic calcification (AC) score, according to treatment group, in overall study group and stratified by baseline estimated glomerular filtration rate (eGFR) and by baseline AC score category, in 1,625 women in the FREEDOM trial, scoring ≥ 4 points on modified Raloxifene Use for the Heart (RUTH) trial criteria and who had evaluable AC score at baseline
| Placebo N = 782 |
Denosumab N = 843 |
Total N = 1,625 |
|
|---|---|---|---|
| Median (Interquartile range) | |||
| Aortic calcification (AC) score* | 6 (11) | 5 (11) | 6 (11) |
| AC score by estimated glomerular filtration rate (eGFR) | |||
| eGFR < 45 mL/min | 8 (12) | 8 (13) | 8 (13) |
| eGFR ≥ 45 mL/min | 6 (11) | 5 (11) | 5 (11) |
| AC score by median AC score | |||
| AC score < 6 | 1 (3) | 1 (2) | 1 (3) |
| AC score ≥ 6 | 11 (6) | 12 (7) | 12 (7) |
Aortic calcification score has no units and ranges from 0 to 24.
The distribution of CV risk factors (RUTH criteria) used to select participants for the current study was similar in placebo and DMAb groups (Table 3). Ninety-one percent were 70+ years old, and baseline prevalence of hypertension was 83%, high cholesterol, 66%, and diabetes, 26%. Thirteen percent of women in the DMAb group and 12% in the placebo group had a history of myocardial infarction, coronary artery bypass grafting or percutaneous coronary intervention.
Table 3.
Distribution of cardiovascular risk factors, according to treatment group, in 1,625 women in the FREEDOM trial, scoring ≥ 4 points on modified Raloxifene Use for the Heart (RUTH) trial criteria and who had evaluable aortic calcification (AC) score at baseline
| Points | Placebo N = 782 |
Denosumab N = 843 |
Total N = 1,625 |
|
|---|---|---|---|---|
| Mean (sd) | ||||
| Cardiovascular (RUTH) Score | Range, 4–13 | 5.3 (1.7) | 5.3 (1.7) | 5.3 (1.7) |
| n (%) | ||||
| Myocardial infarction, percutaneous intervention, or coronary bypass | 4 | 94 (12.0) | 113 (13.4) | 207 (12.7) |
| Diabetes mellitus | 3 | 203 (26.0) | 217 (25.7) | 420 (25.8) |
| Age ≥ 70 years | 2 | 705 (90.2) | 766 (90.9) | 1,471 (90.5) |
| Age 65 to 69 years | 1 | 64 (8.2) | 67 (7.9) | 131 (8.1) |
| Former or current smoker | 1 | 351 (44.9) | 404 (47.9) | 755 (46.5) |
| Hypertension | 1 | 652 (83.4) | 692 (82.1) | 1,344 (82.7) |
| High cholesterol | 1 | 525 (67.1) | 542 (64.3) | 1,067 (65.7) |
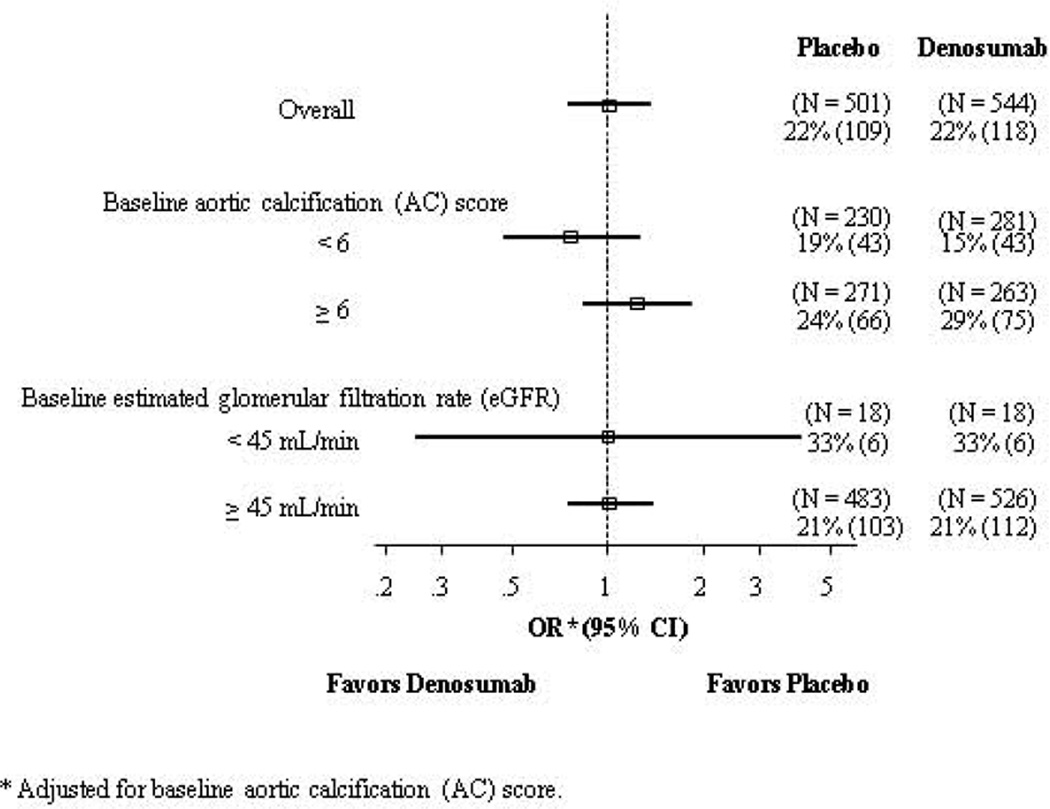
Mean change in AC score was 0.1 at 12 months, 0.2 at 24 months, and 0.4 at 36 months of follow-up in both placebo and DMAb groups (all p-values > 0.05; Table 4). DMAb had no effect on frequency of AC progression (change in AC score > 0) at 12 months (4% placebo, 5% DMAb), 24 months (10% placebo, 12% DMAb), or 36 months (22% placebo, 22% DMAb) of follow-up. The OR for AC progression over 3 years associated with DMAb was 1.00 (95% CI = 0.74–1.34, Figure 3). Further, odds of 3-year progression of AC did not differ according to baseline renal function (eGFR of ≥ 45 mL/min or < 45 mL/min) or baseline AC score (< 6 or ≥ 6).
Table 4.
Comparison of mean change in aortic calcification (AC) score and frequency of any progression in AC score, according to treatment group and months of follow-up, in women in the FREEDOM trial scoring ≥ 4 points on modified Raloxifene Use for the Heart (RUTH) trial criteria
| Change in AC (follow-up minus baseline) |
Frequency of Any Progression (Change in AC > 0) |
Total | |||||
|---|---|---|---|---|---|---|---|
| Follow-up month |
Placebo | Denosumab | P-value | Placebo | Denosumab | P-value | N |
| Mean (SD) | % (n/N) | ||||||
| 12 | 0.1 (0.6) | 0.1 (0.4) | 0.38 | 4% (26/664) | 5% (35/713) | 0.37 | 1,377 |
| 24 | 0.2 (0.7) | 0.2 (0.8) | 0.27 | 10% (60/583) | 12% (78/648) | 0.33 | 1,231 |
| 36 | 0.4 (1.0) | 0.4 (1.1) | 0.97 | 22% (109/501) | 22% (118/544) | 0.98 | 1,045 |
Figure 3.
Odds ratios (OR) and 95% confidence intervals (CI) for association between treatment and any progression of aortic calcification (change in AC score > 0) over 36 months for overall study group and stratified by baseline AC score category and baseline estimated glomerular filtration rate (eGFR) in 1,045 participants
Incidence of CV adverse events (38–40%) and incidence of serious CV adverse events (9–10%) did not differ between women in the placebo and DMAb groups (Table 5).
Table 5.
Comparison of incidence of cardiovascular adverse events* over 36 months of follow-up, according to treatment group, in 2,363 women in the FREEDOM trial scoring ≥ 4 points on modified Raloxifene Use for the Heart (RUTH) trial criteria
| Placebo N = 1,142 |
Denosumab N = 1,221 |
P-value | |
|---|---|---|---|
| n (%) | |||
| Cardiovascular events | 456 (40) | 460 (38) | 0.26 |
| Vascular | 337 (30) | 325 (27) | 0.12 |
| Cardiac | 193 (17) | 214 (18) | 0.69 |
| Serious cardiovascular events | 105 (9) | 122 (10) | 0.51 |
| Vascular | 35 (3) | 36 (3) | 0.87 |
| Cardiac | 79 (7) | 98 (8) | 0.31 |
Number of women with at least 1 cardiovascular adverse event
Discussion
This is the largest study to evaluate the effect of denosumab, an inhibitor of RANKL, on the progression of vascular calcification in a large, randomized clinical trial. We found no evidence that treatment with this drug contributed to the progression of abdominal aortic calcification or to increased risk of CV adverse events in 60–90 year old women with osteoporosis and high cardiovascular risk.
In our study, we used the most conservative criterion for evaluating AC progression defined as change in AC score > 0. Thus, even the smallest increase would be considered as progression in AC score. However, aortic calcification is a slow process, and we cannot rule out that the three-year duration of follow-up may not have been long enough to detect a potential difference between treatment and placebo groups.
Our results are similar to a small, randomized trial of ibandronate in 474 women with osteoporosis 55–80 years old,(43) which reported no differences in 3-year progression of AC (evaluated using the same methods as the current study) between treatment and placebo groups. Although both ibandronate and DMAb are anti-resorptive drugs, the mechanisms of action are distinctly different. Nevertheless, we have demonstrated that three years of DMAb treatment in postmenopausal women, while preventing fractures, does not increase the risk for progression of vascular calcification. As patients with chronic kidney disease are predisposed to vascular calcification, we also studied the effects of treatment on progression of vascular calcification in participants with lower eGFR.(28,44) Even in this higher risk group of women with eGFR below 45 mL/min, there was no evidence that DMAb led to acceleration in the progression of abdominal AC. Moreover, DMAb did not significantly affect eGFR in this study (data not shown).
Multiple reports demonstrate an inverse relationship between the degree of calcification in the aorta and the degree of calcification of the skeleton.(2–11) A biological link has long been hypothesized between bone metabolism pathways and vascular calcification and most recently the OPG/RANK/RANKL system has been proposed as a plausible shared regulatory system.(17–18) While elevated OPG levels have been associated with increased coronary calcification scores in hemodialysis patients, the association has never been causally established. (28)
Numerous epidemiological studies have found a positive association between serum OPG concentrations and CV outcomes, (25–27) however, the biological implications remain unclear. First, it has never been clear whether or not the elevated serum concentrations of OPG cause vascular calcification or represent a response to the calcification. Further, the serum concentrations of OPG might not reflect OPG levels in the vessel wall.
One possible explanation for increased serum OPG levels in women with vascular disease is that they represent an incomplete or insufficient regulatory mechanism to counteract disease progression.(18) This is supported by preclinical findings demonstrating a protective role of RANKL inhibitors on both vascular calcification and bone loss.(20–21,23,45) Of note, low density lipoprotein receptor knock-out mice with atherosclerosis and vascular calcification, induced by an atherogenic diet, had high levels of endogenous OPG. However, treatment of these mice with recombinant OPG resulted in reduced incidence of vascular calcification without changes in atherosclerosis lesion size or number in comparison to a group treated with the vehicle.(22) Additional preclinical evidence indicated that blocking RANKL by DMAb inhibited aortic calcium deposition and prevented bone loss following glucocorticoid exposure in the human RANKL knock-in mice.(46)
Until recently, studies in humans have been limited to measuring circulating OPG (and not RANKL), as OPG is easily measurable and circulates at a higher level than RANKL. OPG levels reflect total OPG (both bound and unbound to RANKL) and cannot be used as a surrogate for either RANKL activity or inhibition. Whereas an inverse association has been observed between levels of circulating RANKL and symptomatic coronary artery disease in small, cross sectional studies,(29,47) high baseline serum concentrations of RANKL increased the risk for incident cardiovascular disease (myocardial infarction, stroke, transient ischemic attack and vascular death) in a large, 15 year population-based study in 909 participants in the Bruneck Study. (30)
Because the radiographs were acquired to assess vertebral fracture, AC scoring was difficult due to the convexity of the lumbar lordosis adjacent to L3 and L4. We conservatively considered AC score as missing if any one of the eight aortic segments could not be graded. Although we used a reliable and validated method of scoring AC based on evaluation of lateral vertebral spine radiographs,(33) 25–30% of women were missing longitudinal AC scores. However, baseline characteristics (age, BMI, years since menopause, BMD T-scores, and eGFR) were similar in women with radiographs that could be assessed and women with radiographs that could not be evaluated. The frequency of women who were white and who had prevalent vertebral fractures was somewhat lower in women with radiographs that could not be assessed (88% white, 23% prevalent vertebral fracture) versus those with radiographs that could be assessed (94% white, 27% fracture). More importantly, there were no differences in baseline characteristics between placebo and treatment groups in women who did not have evaluable radiographs. The amount of missing data also did not differ between treatment groups, although it is possible that the lower sample size could have reduced our ability to detect a potential treatment effect.
In our study, we instructed the radiologist readers to consider only stable or progressively more severe scores in evaluating the longitudinal sets of radiographs, therefore we were unable to exclude the possibility of a small protective effect of DMAb on radiographic progression of vascular calcification. Our imaging approach, while valid for predicting incident cardiovascular events, may not be the most sensitive method for detecting progressive disease. However, we found no treatment effect on incidence of CV adverse events, which occurred in nearly 40% of participants, given the selection criteria to include high risk subjects.
This study provides evidence that DMAb treatment over three years in women with osteoporosis does not affect risk of progression of AC or subject incidence of CV adverse events. The findings are based on a subset of women at high risk of CV disease from a large, randomized clinical trial and robust methods were used to assess AC and CV adverse events. While the relation between OPG/RANK/RANKL and vascular calcification is not yet fully elucidated, findings from the FREEDOM trial demonstrate that the benefits in BMD accrual and fracture risk reduction conferred by DMAb treatment are not accompanied by increased risk of vascular calcification and cardiovascular adverse events.
Acknowledgements
Dr. Samelson’s work on this study was supported by NIH K01 AR053118.
Dr. Cheung’s work is supported by a senior investigator award from the Canadian Institutes of Health Research.
The authors thank the investigators of the FREEDOM trial as well as the participants who participated in the trial.
Footnotes
Disclosures
AM Cheung has served as a consultant to and/or has received grant funding from Amgen, Eli Lilly, Merck, Novartis and Warner Chilcott.
L Grazette has served as a consultant for Otsuka, was an Amgen employee, and owned Amgen Inc. stock and/or stock options at the time of collaboration on the manuscript.
N Franchimont was an Amgen employee and owned Amgen Inc. stock and/or stock options at the time of collaboration on the manuscript. N Franchimont is presently a Biogen Idec employee.
DP Kiel has served as a consultant to Amgen, received grant funding from Amgen, and has served as a consultant for Eli Lilly, Novartis, and Merck, and has received grant funding from Novartis and Merck.
PD Miller has served as a consultant to and received grant funding from Amgen, Lilly, Merck, Radius, and GE-Lunar.
MS Anthony, NS Daizadeh, O Egbuna, S Siddhanti and A Wang are employees at Amgen Inc. and own Amgen Inc. stock and/or stock options.
C Christiansen is an employee of Nordic Bioscience A/S and CCBR/Synarc, has served as consultant to Amgen, Roche, Wyeth-Ayerst, Eli Lilly, Novartis, Novo Nordisk, Proctor and Gamble, Groupe Fournier, Besins EscoVesco, MSD, Chiesi, Boehringer Mannheim, and Pfizer, has received speaking fees from PFIZER and owns stock in Nordic Bioscience and CCBR/Synarc.
This study was funded by Amgen Inc.
Authors’ roles: Study design: SS, LG, AC, PM, NF, CC, OE, ND, AW, DPK Study conduct and data collection: SS, LG Data analysis: AW, ND. Data interpretation: EJS, DPK, SS, MSA. Drafting manuscript: EJS, DPK, NF, SS, OE. Revising manuscript content: EJS, DPK, SS, OE, AW, ND, MSA, LG, AC, PM, CC. Approving final version of manuscript: EJS, PM, CC, ND, LG, MSA, OE, AW, SS, AC, NF, DPK.
References
- 1.Wilson PW, Kauppila LI, O'Donnell CJ, Kiel DP, Hannan M, Polak JM, Cupples LA. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation. 2001;103(11):1529–1534. doi: 10.1161/01.cir.103.11.1529. [DOI] [PubMed] [Google Scholar]
- 2.Kiel DP, Kauppila LI, Cupples LA, Hannan MT, O'Donnell CJ, Wilson PW. Bone loss and the progression of abdominal aortic calcification over a 25 year period: the Framingham Heart Study. Calcif Tissue Int. 2001;68(5):271–276. doi: 10.1007/BF02390833. [DOI] [PubMed] [Google Scholar]
- 3.Naves M, Rodriguez-Garcia M, Diaz-Lopez JB, Gomez-Alonso C, Cannata-Andia JB. Progression of vascular calcifications is associated with greater bone loss and increased bone fractures. Osteoporos Int. 2008;19(8):1161–1166. doi: 10.1007/s00198-007-0539-1. [DOI] [PubMed] [Google Scholar]
- 4.Hak AE, Pols HA, van Hemert AM, Hofman A, Witteman JC. Progression of aortic calcification is associated with metacarpal bone loss during menopause: a population-based longitudinal study. Arterioscler Thromb Vasc Biol. 2000;20(8):1926–1931. doi: 10.1161/01.atv.20.8.1926. [DOI] [PubMed] [Google Scholar]
- 5.Bagger YZ, Tankó LB, Alexandersen P, Qin G, Christiansen C Prospective Epidemiological Risk Factors Study Group. Radiographic measure of aorta calcification is a site-specific predictor of bone loss and fracture risk at the hip. J Intern Med. 2006;259(6):598–605. doi: 10.1111/j.1365-2796.2006.01640.x. [DOI] [PubMed] [Google Scholar]
- 6.Rajzbaum G, Roger VL, Bézie Y, Chauffert M, Bréville P, Roux F, Safar ME, Blacher J. French women, fractures and aortic calcifications. J Intern Med. 2005;257(1):117–119. doi: 10.1111/j.1365-2796.2004.01430.x. [DOI] [PubMed] [Google Scholar]
- 7.Szulc P, Kiel DP, Delmas PD. Calcifications in the abdominal aorta predict fractures in men: MINOS study. J Bone Miner Res. 2008;23(1):95–102. doi: 10.1359/jbmr.070903. [DOI] [PubMed] [Google Scholar]
- 8.Schulz E, Arfai K, Liu X, Sayre J, Gilsanz V. Aortic calcification and the risk of osteoporosis and fractures. J Clin Endocrinol Metab. 2004;89(9):4246–4253. doi: 10.1210/jc.2003-030964. [DOI] [PubMed] [Google Scholar]
- 9.Farhat GN, Cauley JA, Matthews KA, Newman AB, Johnston J, Mackey R, Edmundowicz D, Sutton-Tyrrell K. Volumetric BMD and vascular calcification in middle-aged women: the Study of Women’s Health Across the Nation. J Bone Miner Res. 2006;21(12):1839–1846. doi: 10.1359/jbmr.060903. [DOI] [PubMed] [Google Scholar]
- 10.Hyder JA, Allison MA, Wong N, Papa A, Lang TF, Sirlin C, Gapstur SM, Ouyang P, Carr JJ, Criqui MH. Association of coronary artery and aortic calcium with lumbar bone density: the MESA Abdominal Aortic Calcium Study. Am J Epidemiol. 2009;169(2):186–194. doi: 10.1093/aje/kwn303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwamoto J, Matsumoto H, Takeda T, Sato Y, Uzawa M. A radiographic study on the associations of age and prevalence of vertebral fractures with abdominal aortic calcification in Japanese postmenopausal women and men. J Osteoporos. 2010:748380. doi: 10.4061/2010/748380. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hofbauer LC, Brueck CC, Shanahan CM, Schoppet M, Dobnig H. Vascular calcification and osteoporosis--from clinical observation towards molecular understanding. Osteoporos Int. 2007;18(3):251–259. doi: 10.1007/s00198-006-0282-z. [DOI] [PubMed] [Google Scholar]
- 13.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93(2):165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 14.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89(2):309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 15.Kostenuik PJ. Osteoprotegerin and RANKL regulate bone resorption, density, geometry and strength. Curr Opin Pharmacol. 2005;5(6):618–625. doi: 10.1016/j.coph.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 17.Kiechl S, Werner P, Knoflach M, Furtner M, Willeit J, Schett G. The osteoprotegerin/RANK/RANKL system: a bone key to vascular disease. Expert Rev Cardiovasc Ther. 2006;4(6):801–811. doi: 10.1586/14779072.4.6.801. [DOI] [PubMed] [Google Scholar]
- 18.Schoppet M, Preissner KT, Hofbauer LC. RANK ligand and osteoprotegerin: paracrine regulators of bone metabolism and vascular function. Arterioscler Thromb Vasc Biol. 2002;22(4):549–553. doi: 10.1161/01.atv.0000012303.37971.da. [DOI] [PubMed] [Google Scholar]
- 19.Browner WS, Lui LY, Cummings SR. Associations of serum osteoprotegerin levels with diabetes, stroke, bone density, fractures, and mortality in elderly women. J Clin Endocrinol Metab. 2001;86(2):631–637. doi: 10.1210/jcem.86.2.7192. [DOI] [PubMed] [Google Scholar]
- 20.Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12(9):1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Min H, Morony S, Sarosi I, Dunstan CR, Capparelli C, Scully S, Van G, Kaufman S, Kostenuik PJ, Lacey DL, Boyle WJ, Simonet WS. Osteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesis. J Exp Med. 2000;192(4):463–474. doi: 10.1084/jem.192.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morony S, Tintut Y, Zhang Z, Cattley RC, Van G, Dwyer D, Stolina M, Kostenuik PJ, Demer LL. Osteoprotegerin inhibits vascular calcification without affecting atherosclerosis in ldlr(−/−) mice. Circulation. 2008;117(3):411–420. doi: 10.1161/CIRCULATIONAHA.107.707380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bennett BJ, Scatena M, Kirk EA, Rattazzi M, Varon RM, Averill M, Schwartz SM, Giachelli CM, Rosenfeld ME. Osteoprotegerin inactivation accelerates advanced atherosclerotic lesion progression and calcification in older ApoE−/− mice. Arterioscler Thromb Vasc Biol. 2006;26(9):2117–2124. doi: 10.1161/01.ATV.0000236428.91125.e6. [DOI] [PubMed] [Google Scholar]
- 24.Price PA, June HH, Buckley JR, Williamson MK. Osteoprotegerin inhibits artery calcification induced by warfarin and by vitamin D. Arterioscler Thromb Vasc Biol. 2001;21(10):1610–1616. doi: 10.1161/hq1001.097102. [DOI] [PubMed] [Google Scholar]
- 25.Jono S, Ikari Y, Shioi A, Mori K, Miki T, Hara K, Nishizawa Y. Serum osteoprotegerin levels are associated with the presence and severity of coronary artery disease. Circulation. 2002;106(10):1192–1194. doi: 10.1161/01.cir.0000031524.49139.29. [DOI] [PubMed] [Google Scholar]
- 26.Knudsen ST, Foss CH, Poulsen PL, Andersen NH, Mogensen CE, Rasmussen LM. Increased plasma concentrations of osteoprotegerin in type 2 diabetic patients with microvascular complications. Eur J Endocrinol. 2003;149(1):39–42. doi: 10.1530/eje.0.1490039. [DOI] [PubMed] [Google Scholar]
- 27.Kiechl S, Schett G, Wenning G, Redlich K, Oberhollenzer M, Mayr A, Santer P, Smolen J, Poewe W, Willeit J. Osteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular disease. Circulation. 2004;109(18):2175–2180. doi: 10.1161/01.CIR.0000127957.43874.BB. [DOI] [PubMed] [Google Scholar]
- 28.Ozkok A, Caliskan Y, Sakaci T, Erten G, Karahan G, Ozel A, Unsal A, Yildiz A. Osteoprotegerin/RANKL Axis and Progression of Coronary Artery Calcification in Hemodialysis Patients. Clin J Am Soc Nephrol. 2012;7(6):965–973. doi: 10.2215/CJN.11191111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schoppet M, Schaefer JR, Hofbauer LC. Low serum levels of soluble RANK ligand are associated with the presence of coronary artery disease in men. Circulation. 2003;107(11):e76. doi: 10.1161/01.cir.0000060815.25798.02. author reply e76. [DOI] [PubMed] [Google Scholar]
- 30.Kiechl S, Schett G, Schwaiger J, Seppi K, Eder P, Egger G, Santer P, Mayr A, Xu Q, Willeit J. Soluble receptor activator of nuclear factor-kappa B ligand and risk for cardiovascular disease. Circulation. 2007;116(4):385–391. doi: 10.1161/CIRCULATIONAHA.106.686774. [DOI] [PubMed] [Google Scholar]
- 31.Mosca L, Barrett-Connor E, Wenger NK, Collins P, Grady D, Kornitzer M, Moscarelli E, Paul S, Wright TJ, Helterbrand JD, Anderson PW. Design and methods of the Raloxifene Use for The Heart (RUTH) study. Am J Cardiol. 2001;88(4):392–395. doi: 10.1016/s0002-9149(01)01685-x. [DOI] [PubMed] [Google Scholar]
- 32.Keech CA, Sashegyi A, Barrett-Connor E. Year-by-year analysis of cardiovascular events in the Multiple Outcomes of Raloxifene Evaluation (MORE) trial. Curr Med Res Opin. 2005;21(1):135–140. doi: 10.1185/030079904x18045. [DOI] [PubMed] [Google Scholar]
- 33.Kauppila LI, Polak JF, Cupples LA, Hannan MT, Kiel DP, Wilson PW. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25-year follow-up study. Atherosclerosis. 1997;132(2):245–250. doi: 10.1016/s0021-9150(97)00106-8. [DOI] [PubMed] [Google Scholar]
- 34.Jamal SA, Ljunggren O, Stehman-Breen C, Cummings SR, McClung MR, Goemaere S, Ebeling PR, Franek E, Yang YC, Egbuna OI, Boonen S, Miller PD. Effects of denosumab on fracture and bone mineral density by level of kidney function. J Bone Miner Res. 2011;26(8):1829–1835. doi: 10.1002/jbmr.403. [DOI] [PubMed] [Google Scholar]
- 35.Diamandopoulos A, Goudas P, Arvanitis A. Comparison of estimated creatinine clearance among five formulae (Cockroft-Gault, Jelliffe, Sanaka, simplified 4-variable MDRD and DAF) and the 24hours-urine-collection creatinine clearance. Hippokratia. 2010;14(2):98–104. [PMC free article] [PubMed] [Google Scholar]
- 36.Froissart M, Rossert J, Jacquot C, Paillard M, Houillier P. Predictive performance of the modification of diet in renal disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol. 2005;16(3):763–773. doi: 10.1681/ASN.2004070549. [DOI] [PubMed] [Google Scholar]
- 37.Matsushita K, Mahmoodi BK, Woodward M, Emberson JR, Jafar TH, Jee SH, Polkinghorne KR, Shankar A, Smith DH, Tonelli M, Warnock DG, Wen CP, Coresh J, Gansevoort RT, Hemmelgarn BR, Levey AS. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA. 2012;307(18):1941–1951. doi: 10.1001/jama.2012.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Earley A, Miskulin D, Lamb EJ, Levey AS, Uhlig K. Estimating equations for glomerular filtration rate in the era of creatinine standardization: a systematic review. Ann Intern Med. 2012;156(11):785–795. doi: 10.7326/0003-4819-156-11-201203200-00391. [DOI] [PubMed] [Google Scholar]
- 39.Stevens LA, Zhang Y, Schmid CH. Evaluating the performance of equations for estimating glomerular filtration rate. J Nephrol. 2008;21(6):797–807. [PMC free article] [PubMed] [Google Scholar]
- 40.Nakamura S, Ishibashi-Ueda H, Niizuma S, Yoshihara F, Horio T, Kawano Y. Coronary calcification in patients with chronic kidney disease and coronary artery disease. Clin J Am Soc Nephrol. 2009;4(12):1892–1900. doi: 10.2215/CJN.04320709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 42.Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116(1):85–97. doi: 10.1161/CIRCULATIONAHA.106.678342. [DOI] [PubMed] [Google Scholar]
- 43.Tankó LB, Qin G, Alexandersen P, Bagger YZ, Christiansen C. Effective doses of ibandronate do not influence the 3-year progression of aortic calcification in elderly osteoporotic women. Osteoporosis Int. 2005;16(2):184–190. doi: 10.1007/s00198-004-1662-x. [DOI] [PubMed] [Google Scholar]
- 44.Cannata-Andia JB, Rodriguez-Garcia M, Carrillo-Lopez N, Naves-Diaz M, Diaz-Lopez B. Vascular calcifications: pathogenesis, management, and impact on clinical outcomes. J Am Soc Nephrol. 2006;17(12) Suppl 3:S267–S273. doi: 10.1681/ASN.2006080925. [DOI] [PubMed] [Google Scholar]
- 45.Ominsky MS, Li X, Asuncion FJ, Barrero M, Warmington KS, Dwyer D, Stolina M, Geng Z, Grisanti M, Tan HL, Corbin T, McCabe J, Simonet WS, Ke HZ, Kostenuik PJ. RANKL inhibition with osteoprotegerin increases bone strength by improving cortical and trabecular bone architecture in ovariectomized rats. J Bone Miner Res. 2008;23(5):672–682. doi: 10.1359/jbmr.080109. [DOI] [PubMed] [Google Scholar]
- 46.Helas S, Goettsch C, Schoppet M, Zeitz U, Hempel U, Morawietz H, Kostenuik PJ, Erben RG, Hofbauer LC. Inhibition of receptor activator of NF-kappaB ligand by denosumab attenuates vascular calcium deposition in mice. Am J Pathol. 2009;175(2):473–478. doi: 10.2353/ajpath.2009.080957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crisafulli A, Micari A, Altavilla D, Saporito F, Sardella A, Passaniti M, Raffa S, D'Anneo G, Luca F, Mioni C, Arrigo F, Squadrito F. Serum levels of osteoprotegerin and RANKL in patients with ST elevation acute myocardial infarction. Clin Sci (Lond) 2005;109(4):389–395. doi: 10.1042/CS20050058. [DOI] [PubMed] [Google Scholar]