INTRODUCTION
The integrated lacrimal functional unit regulates tear production and clearance to maintain an adequate tear volume of appropriate composition to support and protect the ocular surface.1,2 Disease or dysfunction of components of the lacrimal functional unit results in deficiency and/or compositional changes in tears. Lacrimal gland disease causes aqueous tear deficiency, while meibomian gland disease results in lipid tear deficiency and increased tear evaporation.
Diagnosis of aqueous tear deficiency has traditionally relied on the Schirmer test that may induce reflex tearing. Meibomian gland disease is generally diagnosed by presence of clinical signs. Interferometry can be used to image the lipid tear layer.3,4 Lipid tear deficiency has been found to increase tear evaporation5; however, measurement of tear evaporation rate is not a routine clinical test.
Optical coherence tomography (OCT) is an objective, non-invasive technology that has been used to indirectly measure tear volume by measuring tear meniscus parameters.6-13 Previous studies have used OCT to compare tear volume to clinical ocular surface disease parameters and suggested that OCT has the potential to distinguish subgroups of dry eye.14,15
The objective of this study was to compare tear meniscus height and area measured by OCT in a variety of tear dysfunction conditions, including meibomian gland disease, aqueous tear deficiency, and Sjögren syndrome, and examine correlations between tear meniscus and clinical parameters, with the goal of more accurately stratifying and classifying tear dysfunction for clinical trials and therapeutic decision making.
METHODS
This study protocol to prospectively evaluate the utility of the FDA-approved anterior segment OCT device to measure tear meniscus dimensions for diagnostic classification and severity grading of tear dysfunction conditions was approved by the Baylor College of Medicine Institutional Review Board (IRB). It adheres to the tenets of the Declaration of Helsinki for clinical research, complies with the Health Insurance Portability and Accountability Act, and written informed consent was obtained from all participants after explanation of the purpose and possible consequences of the study. This is a single-institution prospective observational study.
Subjects
One hundred twenty-eight eyes from 64 subjects were included in this prospective observational study. Tear dysfunction was stratified into the following groups: meibomian gland disease (MGD), aqueous tear deficiency (ATD), and Sjögren syndrome ATD, both primary and secondary forms. The American College of Rheumatology Classification Criteria were used for diagnosis of Sjögren syndrome.15 Normal subjects with no symptoms of dry eye or tear deficiency were also evaluated.
Dry eye questionnaire
On entry to the study, each subject completed an Ocular Surface Disease Index (OSDI) questionnaire that contains 12 questions that evaluate the character and severity of dry eye symptoms, including vision-related function, ocular symptoms, and environmental triggers.16 The subject was asked to score the frequency of symptoms from none to all the time. The questionnaire scores ranged from 12 (no symptoms) to a maximum of 59.
Ocular surface clinical parameters
Table 1 displays the inclusion and exclusion criteria for each group.17 The ocular surface clinical parameters were all measured by the same observer (S.C.P.). Parameters included tear break-up time (TBUT), corneal fluorescein staining, conjunctival lissamine green staining, and Schirmer I test. TBUT was measured by instilling fluorescein into the lower fornix with a wetted fluorescein strip (BioGlo, HUB, Rancho Cucamonga, CA). The patient was allowed to blink at a spontaneous rate, and the time elapsed from the last blink to the appearance of the first break in the continuous layer of fluorescein, as observed under cobalt blue light, was recorded to be the TBUT. Corneal fluorescein staining was graded 0 to 6 in each of 5 zones (central, temporal, nasal, superior, and inferior), as reported in Rao and associates. Conjunctival lissamine green staining was graded on a scale of 0 to 3 exposed in the nasal and temporal bulbar conjunctiva with a total maximum score of 6. Schirmer I test without anesthesia was used to measure tear production, by inserting a dry Schirmer test strip over the outer one-third of the lower eyelid margin. The distance that the tears traveled along the test strip at 5 minutes was recorded as the Schirmer I score.
Table 1.
Criteria used to define tear dysfunction groups and the control group.
| Groups | OSDIa | TBUTb ≤ 7 sec | Schirmer < 10 mm | MGDc |
|---|---|---|---|---|
| All Tear | ||||
| Dysfunction | >20 | + | ||
| MGDc | >20 | + | - | + |
| ATDd | >20 | + | + | - |
| SSATDe,f | >20 | + | + | - |
| non-SSATDe | >20 | + | + | - |
| Controls | ≤ 20 | - | - | - |
OSDI: Ocular Surface Disease Index questionnaire
TBUT: Tear break-up time
MGD: Meibomian gland disease – diagnostic criteria are provided in the Methods.17
ATD: Aqueous tear deficiency
SSATD: Sjögren syndrome aqueous tear deficiency
SS: Sjögren syndrome. The American College of Rheumatology Classification Criteria were used for diagnosis of Sjögren syndrome.15
Subtypes of dry eye
Criteria for classifying normal tear function or subtypes of tear dysfunction are provided in Table 1. Subjects were excluded if they had prior corneal transplantation surgery, prior surgery of the lacrimal system, a history of contact lens wear, use of topical medications other than preservative-free artificial tears, or chronic use of systemic medications known to reduce tear production. In addition, subjects were excluded if they had active ocular surface or corneal inflammation or infection, eyelid disorders causing exposure of the ocular surface, or clinically significant conjunctivochalasis greater than grade 1 by LIPCOF (Lid-parallel conjunctival folds) criteria as defined by Hoh and associates.18
OCT-defined tear meniscus parameters
: OCT measurement of the lower tear meniscus was performed as described by Gumus and associates.19 All subjects underwent cross-sectional imaging of the lower tear meniscus prior to instilling drops or measuring any clinical parameters, using the RTVue-100 (Ver. 4.0.7.5; Optovue Inc, Fremont, California, USA) with a corneal adaptor. The system takes 26,000 axial scans per second and has a 5-um axial resolution and 15-um transverse resolution. The Cornea-Anterior Module (CAM) software was added to the device for anterior segment imaging. The long-CAM (13 mm, wide field) lens adapter was used to take images. The subject was asked to fixate on a target, but was allowed to blink freely throughout the duration of the measurement, and an image of the vertical cross section through the center of the lower tear meniscus was recorded. The scan was repeated if artifact due to eye or eyelid movement was noted. Tear meniscus height and tear meniscus area were defined as the height and area of the triangular-shaped cross section between the lower eyelid margin and the cornea, and were measured with RTVue-100 image analysis software. (Figure 1)
Figure 1.
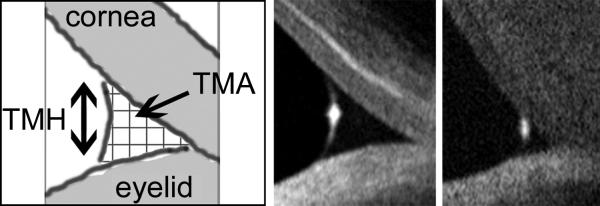
Cross-sectional optical coherence tomography (OCT) images of lower tear meniscus. Measurements of tear meniscus height (TMH) and area (TMA) were performed using Optovue RTVue-100 software. Shown are (left) schematic diagram of tear meniscus cross-sectional image, (middle) sample image of normal tear meniscus (tear meniscus height = 290 μm; tear meniscus area = 0.024 μm2), and (right) sample image of aqueous tear deficient tear meniscus (tear menscius height = 148 μm; tear meniscus area = 0.004 μm2).
Statistical analysis
Tear meniscus dimensions and clinical parameters were recorded in a database. Statistical analysis was performed using GraphPad Prism 5.04 for Windows (GraphPad software, Inc., La Jolla, CA, USA) and Microsoft Excel 14.0 for Windows (Microsoft Corporation, Redmond, WA, USA).
Mean values of tear meniscus dimensions were compared between subtypes using Student's t-test. A value of P ≤ 0.05 was considered to be statistically significant, and 95% confidence intervals were used. Pearson's correlation coefficient (R) was calculated to assess the relationship between tear meniscus dimensions and clinical parameters within the entire tear dysfunction cohort and in each subtype.
RESULTS
Study population
The demographic information for tear dysfunction and control subjects is presented in Table 2. When comparing mean age, there was a statistically significant difference between ATD (64.7 years) and controls (51.1 years) (p = 0.028), and between non-Sjögren syndrome ATD (65.5 years) and controls (51.1 years) (p = 0.036), but not for any other group. There was no significant difference in mean age when each study group was compared to other study groups. There was significant difference in mean age found between controls ≥ 50 years (65.4 years) and controls < 50 years (22.3 years) (p = 6.64 × 10−10).
Table 2.
Demographics of each tear dysfunction group and the control group.
| Groups | N (eyes) | N (subjects) | Age-mean (years) | Age-range (years) | Male-to-female ratio |
|---|---|---|---|---|---|
| All subjects | 128 | 64 | 56 | 19-87 | 0.37 |
| All Tear Dysfunction | 68 | 34 | 60 | 25-87 | 0.13 |
| MGDa | 23 | 13 | 57 | 25-85 | 0.18 |
| ATDb | 41 | 21 | 65 | 31-87 | 0.14 |
| SSATDc | 17 | 9 | 64 | 31-76 | 0.29 |
| non-SSATDd | 16 | 10 | 65 | 49-87 | 0.10 |
| All controls | 33 | 20 | 51 | 19-82 | 2.17 |
| Controls ≥ 50 years | 17 | 12 | 65 | 50-82 | 1.40 |
| Controls < 50 years | 16 | 8 | 22 | 19-27 | 3.00 |
MGD: meibomian gland disease
ATD: aqueous tear deficiency
SSATD: Sjögren syndrome aqueous tear deficiency
non-SSATD: non- Sjögren syndrome aqueous tear deficiency
Summary of tear dysfunction parameters for each group
For each group, the mean and standard deviation values for clinical parameters of tear dysfunction and for OCT-defined tear meniscus parameters are summarized in Table 3.
Table 3.
Summary of mean values of clinical ocular surface parameters and optical coherence tomography-defined tear meniscus dimensions for each tear dysfunction group.
| Groups | OSDIa | Corneal staining | Conjunctival Staining | Schirmer (mm) | TBUTb (sec) | TMHc (μm) | TMAd (μm2) |
|---|---|---|---|---|---|---|---|
| All subjects | |||||||
| Mean (SDe) | 27.1 (±13.1) | 2.7 (±4.4) | 1.8 (±2.2) | 14.0 (±11.0) | 6.6 (±5.3) | 280.68 (±147.53) | 0.0395 (±0.081) |
| All controls | |||||||
| Mean (SDe) | 13.7 (±2.1) | 0.1 (±0.4) | 0.4 (±0.4) | 19.3 (±8.6) | 12.4 (±4.9) | 347.92 (±181.1) | 0 .0621 (±0.134) |
| All tear dysfunction | |||||||
| Mean (SDe) | 36.0* (±9.7) | 4.7* (±5.2) | 2.8* (±2.5) | 11.7* (±10.3) | 2.6* (±1.6) | 234.45* (±124.02) | 0.0271 (±0.033) |
| p value | < 0.01 | < 0.01 | < 0.01 | 0.01 | < 0.01 | < 0.01 | 0.12 |
| MGDf | |||||||
| Mean (SDe) | 39.2* (±11.4) | 0.9* (±1.2) | 2.2* (±2.1) | 17.7 (±11.4) | 3.9* (±1.2) | 313.42 (±157.72) | 0.0465 (±0.044) |
| p value | < 0.01 | < 0.01 | < 0.01 | 0.63 | < 0.01 | 0.43 | 0.50 |
| All ATDg | |||||||
| Mean (SDe) | 32.1* (±8.8) | 6.1* (±5.4) | 2.7* (±2.5) | 5.0* (±2.7) | 2.3* (±1.4) | 204.78* (±88.5) | 0.0198 (±0.0218) |
| p value | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | 0.06 |
| SSATDh | |||||||
| Mean (SDe) | 33.6* (±10.3) | 8.9* (±5.4) | 4.6* (±3.2) | 3.1* (±2.2) | 1.3* (±1.0) | 170.35* (±50.66) | 0.0106* (±0.0061) |
| p value | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | 0.02 |
| Non-SSATDi | |||||||
| Mean (SDe) | 29.6* (±7.2) | 2.9* (±3.2) | 1.8* (±1.4) | 6.8* (±2.3) | 3.3* (±1.4) | 255.38* (±110.78) | 0.0304 (±0.0284) |
| p value | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | 0.03 | 0.17 |
Denotes statistically significance difference (p ≤ 0.05) compared to controls. P values are compared to controls.
OSDI: Ocular Surface Disease Index questionnaire
TBUT: Tear break-up time
TMH: Tear meniscus height
TMA: Tear meniscus height
SD: Standard deviation
MGD: Meibomian gland disease -- diagnostic criteria are provided in the Methods.17
ATD: Aqueous tear deficiency
SSATD: Sjögren syndrome aqueous tear deficiency
non-SSATD: Non-Sjögren syndrome aqueous tear deficiency
Mean values comparison for OCT lower meniscus measurements
Each subject was assigned a mean tear meniscus height that was calculated by averaging values for the two eyes. If only one eye was included, then the value for the one eye was used to represent mean tear meniscus height. Then, the mean tear meniscus height values for all subjects were averaged and this mean value was compared between groups. Mean tear meniscus area was calculated in the same fashion. (Figure 2)
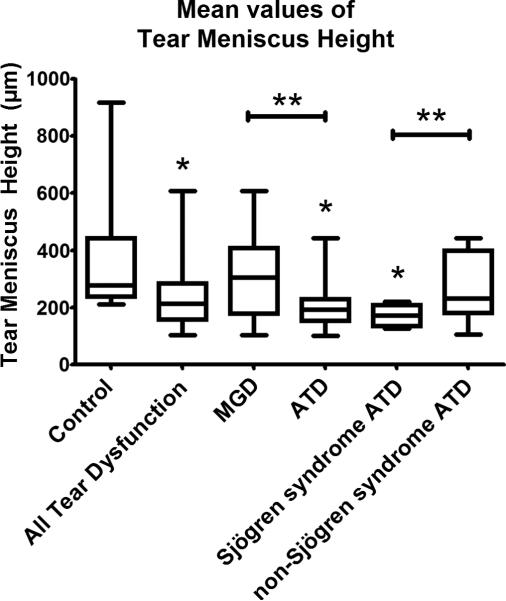
Figure 2.
Mean values of tear meniscus height for each group. *Denotes significance compared to normal (p ≤ 0.05). **Denotes significance between groups indicated (p value ≤ 0.05). Groups include control (345 μm), all tear dysfunction (234 μm), meibomian gland disease (MGD) (302 μm), aqueous tear deficiency (ATD) (210 μm), Sjögren syndrome (171 μm), and non-Sjögren syndrome ATD (262 μm). Significant comparisons include all tear dysfunction vs. control (p = 0.0057); ATD vs. control (p = 0.0016); Sjögren syndrome ATD vs. control (p = 0.0054); and MGD vs. ATD (p = 0.0235); and Sjögren syndrome ATD vs. non-Sjögren syndrome ATD (p = 0.0358). Thick error bars indicate the 25% percentile and 75% percentile. Thin error bars indicate minimum and maximum values. Confidence intervals for each group are listed in the Results section.
When compared with mean tear meniscus height in controls (345 μm; 95% confidence interval (CI) = 266 μm to 423 μm), there was significantly lower mean tear meniscus height for all tear dysfunction (234 μm; CI = 195 μm to 273 μm; p = 0.0057), ATD (210 μm; CI = 172 μm to 248 μm; p = 0.0016), and Sjögren syndrome ATD (171 μm; CI = 143 μm to 200 μm; p = 0.0054). Also, MGD (302 μm; CI = 213 μm to 392 μm) had lower mean tear meniscus height values than control (p = 0.486), and non-Sjögren syndrome ATD (262 μm; CI = 181 μm to 344 μm) had lower mean tear meniscus height than control (p = 0.1763) but these values were not significant. There was significant difference between MGD and ATD groups (p = 0.0235), and there was significant difference between Sjögren syndrome ATD and non-Sjögren syndrome ATD groups (p = 0.0358). There was no statistical difference in tear meniscus height or tear meniscus area when a subgroup of severe Sjögren syndrome ATD (Schirmer < 5) was compared to the entire Sjögren syndrome ATD group (Schirmer < 10). There was no significant difference when mean tear meniscus height of older controls ≥ 50 years of age (344 μm; CI = 268 μm to 419 μm) was compared to younger controls < 50 years of age (346 μm; 150 μm to 542 μm) (p = 0.976).
Among all subjects, a tear meniscus height ≤ 210 μm (the mean tear meniscus height for the ATD group) carried a relative risk ratio for developing corneal staining ≥10 of 4.65 and an odds ratio of 5.59.
OCT-defined tear meniscus parameters correlated with clinical signs and symptoms
Correlation data is presented in Table 4 and Figures 3-5. Significant correlations were found between corneal staining and tear meniscus height, between corneal staining and tear meniscus area, and between TBUT and tear meniscus height in several study groups (all subjects, all tear dysfunction, MGD, and ATD). However, there was no significant correlation between TBUT and tear meniscus area in any subgroup of tear dysfunction (Table 4). Comparisons between tear meniscus parameters and conjunctival staining or OSDI score were not significant for any group. None of the correlations within the non-Sjögren syndrome ATD group were significant.
Table 4.
Correlation data between tear meniscus dimensions in tear dysfunction and clinical parameters of tear dysfunction.
| All subjects | All tear dysfunction | MGDa | ATDb | |||||
|---|---|---|---|---|---|---|---|---|
| R value | p value | R value | p value | R value | p value | R value | p value | |
| Corneal staining vs. TMHa | −0.322* | 0.0008 | −0.31* | 0.015 | 0.400 | 0.059 | −0.36* | 0.04 |
| Corneal staining vs. TMAb | −0.248 | 0.0106 | −0.29* | 0.024 | 0.553* | 0.006 | −0.40* | 0.018 |
| TBUTc vs. TMHa | 0.391* | < 0.0001 | 0.422* | 0.0003 | 0.173 | 0.48 | 0.374* | 0.018 |
| TBUTc vs. TMAb | 0.338* | 0.0004 | 0.405* | 0.0013 | 0.123 | 0.616 | 0.458* | 0.0065 |
| Conjunctival staining vs. TMHa | −0.232 | 0.0549 | −0.15 | 0.338 | −0.02 | 0.931 | −0.40 | 0.052 |
| Conjunctival staining vs. TMAb | −0.156 | 0.201 | −0.11 | 0.485 | 0.044 | 0.841 | −0.36 | 0.085 |
| Schirmer vs. TMHa | +0.385* | 0.0002 | +0.424* | 0.0009 | 0.188 | 0.39 | 0.0686 | 0.691 |
| Schirmer vs. TMAb | +0.354* | 0.001 | +0.382* | 0.004 | 0.198 | 0.36 | 0.0795 | 0.665 |
For each category of tear dysfunction, Pearson's correlation coefficient (R value) was calculated for each comparison. Confidence intervals are listed in the Results section. Sjögren syndrome aqueous tear deficiency (SSATD) and non-SSATD are not listed because their clinical parameters were not found to have significant correlation with tear meniscus height or tear meniscus area. Likewise, ocular surface disease index (OSDI) is not listed as a clinical parameter in the left column because there was no significant correlation found.
Denotes statistically significance difference (p value ≤ 0.05).
MGD: Meibomian gland disease
ATD: Aqueous tear deficiency
TMH: Tear meniscus height
dTMA: Tear meniscus area
Figure 3.
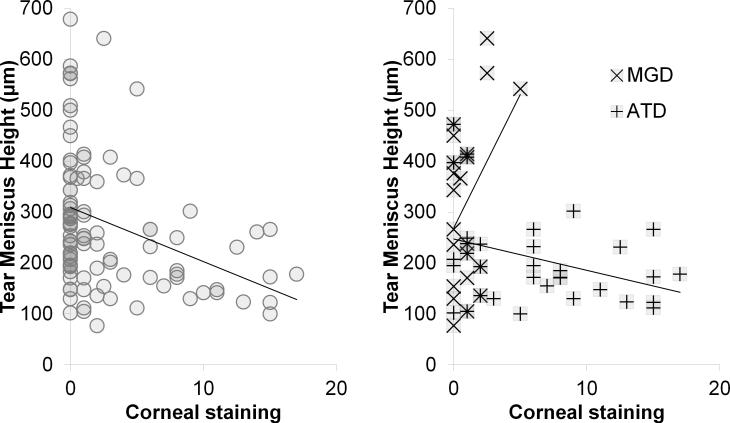
Correlation graphs: Tear meniscus height vs. corneal staining. (left) All subjects (R = −0.32; p = 0.0008), (right) meibomian gland disease (MGD) (R = +0.40; p = 0.059) and aqueous tear deficient dry eye (ATD) (R = −0.36; p = 0.04).
Figure 5.
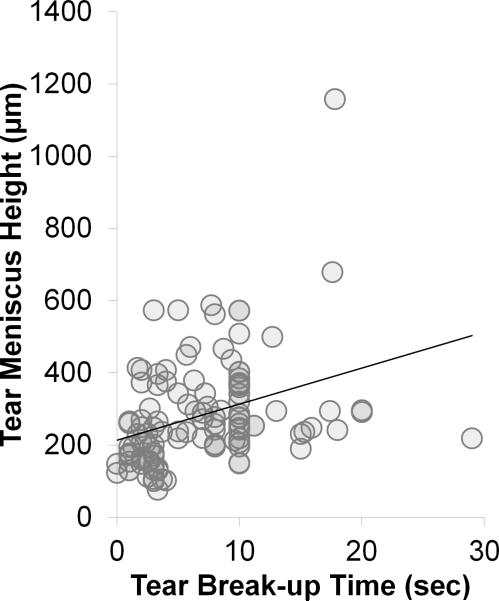
Correlation graphs: Tear meniscus height vs. tear break-up time (sec). All subjects (R = +0.391; p < 0.0001).
For correlation between corneal staining versus tear meniscus height, Pearson's R correlation coefficient was negative for all subjects (R = −0.32; CI = −0.484 μm to −0.139 μm; p = 0.0008) and negative for all tear dysfunction (R = −0.31; CI = −0.519 μm to −0.0637 μm; p = 0.015), positive for MGD (R = +0.40; CI = −0.0147 μm to 0.697 μm; p = 0.059), and negative in ATD (R = −0.36; CI = −0.619 μm to −0.0188 μm; p = 0.04) (Figure 3).
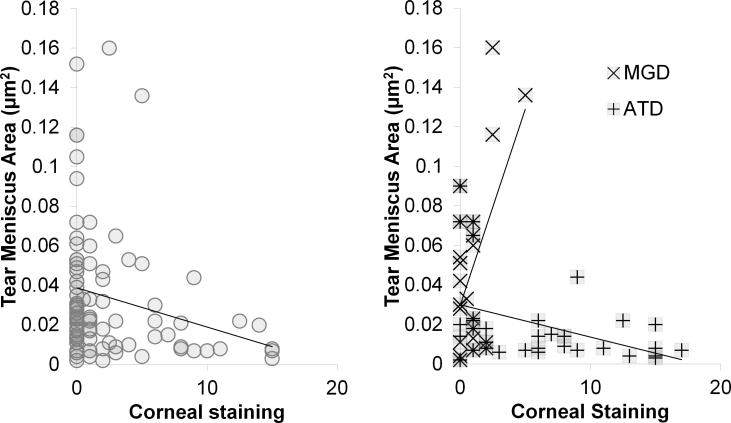
Correlation between corneal staining and tear meniscus area showed stronger R values and similar trends to those seen for tear meniscus height. Specifically, there was positive correlation in MGD (R = +0.55; CI = +0.183 μm to +0.786 μm; p = 0.006), negative correlation in ATD (R = −0.40; CI = −0.653 μm to −0.0755 μm; p = 0.018), and negative correlation in Sjögren syndrome ATD, although the latter correlation did not reach significance (R = −0.43; CI = −0.755 μm to +0.0636 μm; p = 0.085) (Figure 4).
Figure 4.
Correlation graphs: Tear meniscus area vs. corneal staining. (left) All subjects (R = −0.25; p = 0.011), (right) meibomian gland disease (MGD) (R = +0.553; p = 0.006) and aqueous tear deficient dry eye (ATD) (R = −0.40; p = 0.018).
When TBUT and tear meniscus height were compared, correlation was positive for all subjects (R = +0.41; CI = +0.223 μm to +0.536 μm; p < 0.0001) (Figure 5), positive for all tear dysfunction (R = +0.42; CI = +0.205 μm to +0.599 μm; p = 0.0003), and positive for ATD (R = +0.37; CI = +0.0702 μm to +0.614 μm; p = 0.018). No correlation was noted in the MGD group
Significant positive correlations were found between Schirmer I test and tear meniscus dimensions (tear meniscus height, tear meniscus area) in all subjects (tear meniscus height: R = +0.385; CI = 0.189 μm to 0.551 μm; p = 0.0002) (tear meniscus area: R = +0.354; CI = 0.150 μm to 0.529 μm; p = 0.001) and all tear dysfunction (tear meniscus height: R = +0.424; CI = 0.186 μm to 0.615 μm; p = 0.0009) (tear meniscus area: R = +0.382; CI = 0.127 μm to 0.590 μm; p = 0.0044).
DISCUSSION
This study evaluated the relationship between clinical ocular surface/tear parameters and OCT-defined tear meniscus dimensions within a variety of tear dysfunction conditions.
It is not surprising to find that the mean tear meniscus height values for ATD and Sjögren syndrome ATD were significantly lower than mean tear meniscus height in the normal group, since these two groups were defined by deficient tear production.
Similar difference in lower tear meniscus dimensions (tear meniscus height, tear meniscus area) were noted between dry eye and normal control groups in previously reported studies.12,20 Chen and colleagues also found significant decrease in tear meniscus height and tear meniscus area in Sjögren syndrome ATD dry eye compared to normal subjects.21
There may be important clinical implications to the finding that mean tear meniscus height and tear meniscus area are significantly lower in Sjögren syndrome ATD compared to those with non-Sjögren syndrome ATD. It is possible that the lower tear volume in Sjögren syndrome ATD eyes is due to the greater compromise in lacrimal gland function due to lymphocyte infiltration of the gland, impairment of acinar secretion by cytokines such as IL-122 and reduced sensory stimulation due to disease or degeneration of the corneal nerves.23,24 Given that there was a significant negative correlation between tear meniscus dimensions with corneal epithelial disease in the ATD group (R = −0.40; p = 0.018), patients with low tear meniscus height or tear meniscus area should be advised that they have a higher risk for developing more severe corneal epithelial disease. Treatments to maintain or increase tear volume should be considered for tear dysfunction patients with tear meniscus height values ≤ 210 μm because they have a 4.65 relative risk ratio of developing severe corneal epithelial disease (corneal fluorescein staining ≥ 10) (odds ratio = 5.59). The sensitivity and specificity of OCT measured tear meniscus dimensions for determining the risk for developing corneal epithelial disease should be confirmed in a multicenter study evaluating a larger cohort of patients with tear dysfunction.
It is interesting to note that there was no significant difference in mean tear meniscus height and mean tear meniscus area between younger and older asymptomatic control subjects. While the prevalence of dry eye has been found to increase with age, those older individuals who test normal for ocular surface parameters may not exhibit any physiologic decrease in tear volume.
Our findings for all subjects and for all dry eyes were similar to the correlations reported in previously published studies: there was significant positive correlation between TBUT and tear meniscus dimensions, there was significant negative correlation between corneal fluorescein staining and tear meniscus dimensions11,20,25,26, and significant positive correlation between Schirmer I test and tear meniscus dimensions.11,14,20 Our study was unique in that it explored the relationships between tear meniscus and clinical parameters (TBUT, corneal fluorescein staining, Schirmer I) within subtypes of tear dysfunction, namely, MGD, ATD, Sjögren syndrome ATD, and non-Sjögren syndrome ATD. As discussed below, only comparisons within the ATD and MGD groups exhibited significant correlations.
The observation that lower tear volume is associated with significantly worse corneal epithelial disease in ATD eyes is not surprising. The low tear volume in ATD may cause corneal disease from a variety of mechanisms, including increased lid/ocular surface friction, increased tear osmolarity27-31, increased inflammatory mediators32, and greater tear instability. Additionally it is thought that the greater corneal damage observed in this group is multifactorial, including increased hyperosmolarity, unstable tear lipid layer leading to greater evaporation33, and altered composition.
It was surprising to find in MGD that higher tear volume correlates with worse ocular surface disease (tear meniscus height: R = +0.40; p = 0.059), (tear meniscus area: R = +0.55; p = 0.006). We hypothesize possible mechanisms for this finding.
MGD, along with other forms of evaporative dry eye, is characterized by normal tear production and unstable tear lipid layer. It has been previously postulated that an unstable lipid layer leads to greater evaporation rates, and the resulting loss of tear volume is associated with damage of the ocular surface.34 However, our study showed that greater corneal damage is seen in the setting of larger tear volume in the MGD group, implying that loss of tear volume (due to evaporation, etc.) alone cannot explain the development of ocular surface damage. The fact that higher tear volume correlates with greater corneal epithelial damage in this group suggests that changes in tear composition (ie, hyperosmolarity, lactoferrin concentration, inflammatory mediators) may play a greater role than evaporation in causing corneal epithelial damage in MGD. Delayed tear clearance associated with higher levels of matrix metalloproteinases (MMPs) and epidermal growth factors (EGF) have previously been reported in eyes with MGD.35,36 Therefore, higher tear volume in eyes with MGD may prove to be a sign that potentially damaging mediators could be retained on the ocular surface and require therapies to improve tear clearance (punctal enlargement, removal of conjunctivochalasis) or treatment of inflammation.
Conversely, corneal epithelial disease in MGD may cause stimulation of the corneal nerves, signaling reflex tear secretion by a functional lacrimal gland. It may be that MGD and other forms of evaporative dry eye disease cause a sufficient level of irritation to stimulate the tear reflex loop, but not damage the afferent arm of the reflex loop, resulting in a net increase in tear production.
Gumus and Pflugfelder previously reported an age-related increase in OCT-measured conjunctivochalasis and tear meniscus height that was most pronounced in subjects over age 60.37 The mean ages of the control and all tear dysfunction groups was 51 and 60, respectively. Subjects with clinically significant conjunctivochalasis were excluded from this study; however, it is likely that age-related conjunctival changes may have contributed to tear meniscus dimensions in all groups based on the previous findings. This suggests that the correlations between corneal epithelial disease and tear meniscus dimensions observed in the tear dysfunction subsets in this study appear to be independent of conjunctivochalasis.
Objective, non-invasive methods to measure tear volume are essential for accurate classification of tear dysfunction. Clinical tests, such as the Schirmer I test, phenol red cotton thread test, among others have been used as surrogates for tear volume.38-42 The Schirmer test has been favored as a clinical standard for measuring tear production because of its convenience and low cost. However, this test is inherently invasive, causes reflex tear secretion, and has poor reproducibility. Furthermore, these tests do not directly measure tear volume.
Our findings suggest that OCT is a much better measure of tear volume than the Schirmer I test because it is non-invasive, reproducible, comfortable, and directly measures the tear meniscus. This method is limited in that it images only a cross section of the meniscus and assumes constant dimensions over the length of the eyelid. The reliability and repeatability of OCT has been studied and it has been validated by several groups as an objective, non-invasive method to indirectly measure tear volume.7,20,43-46 Several groups have studied the upper and lower tear meniscus dimensions.8,10,14,43,47 Li et al. found that lower tear menisci in dry eye patients correlated more strongly than upper tear menisci when compared with clinical parameters (TBUT, corneal fluorescein staining, non-invasive TBUT, and Schirmer with anesthesia)48, and Wang et al. found that lower tear mensci correlated with TBUT14.
Other objective, non-invasive tests to evaluate tear meniscus include: interferometry4, reflective meniscometry6, high-resolution OCT49,50, strip meniscometry20, noninvasive interference tear meniscometry51, and video-assessment of tear meniscus height52. None of these are widely available at this time.
In summary, OCT is useful as an objective, non-invasive modality to evaluate tear meniscus parameters in subtypes of dry eye. In ATD, lower tear volume carries a markedly elevated risk for development of severe corneal epithelial disease. Treatments aimed at increasing tear volume should be considered for patients with a tear meniscus height ≤ 210 μm, particularly those with Sjögren syndrome ATD who were found to have significantly lower tear volume than their non-Sjögren syndrome ATD counterparts.
In contrast to ATD, greater corneal epithelial disease was associated with higher tear volume in MGD, a counterintuitive observation that may be explained by an altered tear composition due to poor tear clearance or greater stimulation of reflex tearing triggered by evaporative loss and eye irritation. Further studies are indicated to measure tear components in MGD to establish correlation with tear volume measurements.
ACKNOWLEDGEMENTS
a. Funding/Support (including none): Financial Support: NIH Grant EY11915 (SCP), an unrestricted grant from Research to Prevent Blindness, New York, NY (SCP), the Oshman Foundation, Houston, TX (SCP), the William Stamps Farish Fund, Houston, TX (SCP), Hamill Foundation, Houston, TX (SCP)
d. Other Acknowledgments: None.
Biography
 Cynthia I. Tung, MD, received her medical degree from the University of Rochester School of Medicine, Rochester, New York, where she was awarded a research fellowship to study the tear film. She currently serves as chief ophthalmology resident in the Department of Ophthalmology at the University of Texas Medical Branch, Galveston, Texas. Dr. Tung continues to pursue her research interests in ocular surface disease and advanced imaging at the Baylor College of Medicine, Houston, Texas.
Cynthia I. Tung, MD, received her medical degree from the University of Rochester School of Medicine, Rochester, New York, where she was awarded a research fellowship to study the tear film. She currently serves as chief ophthalmology resident in the Department of Ophthalmology at the University of Texas Medical Branch, Galveston, Texas. Dr. Tung continues to pursue her research interests in ocular surface disease and advanced imaging at the Baylor College of Medicine, Houston, Texas.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
c. Contributions to Authors in each of these areas:
Design and conduct of the study: CIT, AFP, KG, SCP
Collection, management, analysis, and interpretation of the data: CIT, AFP, KG, SCP
Preparation of manuscript: CIT, SCP
Review and approval of the manuscript: CIT, AFP, KG, SCP
b. Financial Disclosures (including none):
CIT: none
AFP: none
KG: none
SCP: Allergan:Code C (Consultant); GlaxoSmithKline:Code C (Consultant); Bausch and Lomb:Code C (Consultant)
REFERENCES
- 1.Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC. The pathology of dry eye: The interaction between ocular surface and lacrimal glands. Cornea. 1998;17(6):584–589. doi: 10.1097/00003226-199811000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Stern ME, Schaumburg CS, Pflugfelder SC. Dry eye as a mucosal autoimmune disease. Int Rev Immunol. 2013;32(1):19–41. doi: 10.3109/08830185.2012.748052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yokoi N, Takehisa Y, Kinoshita S. Correlation of tear lipid layer interference patterns with the diagnosis and severity of dry eye. Am J Ophthalmol. 1996;122(6):818–24. doi: 10.1016/s0002-9394(14)70378-2. [DOI] [PubMed] [Google Scholar]
- 4.Goto E, Tseng SC. Differentiation of lipid tear deficiency dry eye by kinetic analysis of tear interference images. Arch Ophthalmol. 2003;121(2):173–80. doi: 10.1001/archopht.121.2.173. [DOI] [PubMed] [Google Scholar]
- 5.Bron AJ, Tiffany JM. The contribution of meibomian disease to dry eye. Ocul Surf. 2004;2(2):149–65. doi: 10.1016/s1542-0124(12)70150-7. [DOI] [PubMed] [Google Scholar]
- 6.Yokoi N, Komuro A. Non-invasive methods of assessing the tear film. Exp Eye Res. 2004;78(3):399–407. doi: 10.1016/j.exer.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 7.Koh S, Tung C, Aquavella J, Yadav R, Zavislan J, Yoon G. Simultaneous measurement of tear film dynamics using wavefront sensor and optical coherence tomography. Invest Ophthalmol Vis Sci. 2010;51(7):3441–3448. doi: 10.1167/iovs.09-4430. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Aquavella J, Palakuru J, Chung S, Feng C. Relationships between central tear film thickness and tear menisci of the upper and lower eyelids. Invest Ophthalmol Vis Sci. 2006;47(10):4349–4355. doi: 10.1167/iovs.05-1654. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y. Dynamic changes in the lower tear meniscus after instillation of artificial tears. Cornea. 2010;29(4):404–408. doi: 10.1097/ICO.0b013e3181bd476c. [DOI] [PubMed] [Google Scholar]
- 10.Mainstone JC, Bruce AS, Golding TR. Tear meniscus measurement in the diagnosis of dry eye. Curr Eye Res. 1996;15(6):653–661. doi: 10.3109/02713689609008906. [DOI] [PubMed] [Google Scholar]
- 11.Qiu X, Gong L, Lu Y, Huan J, Robitaille M. The diagnostic significance of Fourier-domain optical coherence tomography in Sjögren syndrome, aqueous tear deficiency and lipid tear deficiency patients. Acta Ophthalmol. 2012;90(5):e359–e366. doi: 10.1111/j.1755-3768.2012.02413.x. [DOI] [PubMed] [Google Scholar]
- 12.Shen M, Li J, Wang J, et al. Upper and lower tear menisci in the diagnosis of dry eye. Invest Ophthalmol Vis Sci. 2009;50(6):2722–2726. doi: 10.1167/iovs.08-2704. [DOI] [PubMed] [Google Scholar]
- 13.Gumus K, Crockett CH, Rao K, et al. Noninvasive assessment of tear stability with the tear stability analysis system in tear dysfunction patients. Invest Ophthalmol Vis Sci. 2011;52(1):456–61. doi: 10.1167/iovs.10-5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Palakuru JR, Aquavella JV. Correlations among upper and lower tear menisci, nonivasive tear break-up time, and the Schirmer test. Am J Ophthalmol. 2008;145(5):795–800. doi: 10.1016/j.ajo.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shiboski SC, Shiboski CH, Criswell LA, et al. American College of Rheumatology Classification Criteria for Sjögren's Syndrome: A Data-Driven, Expert Consensus Approach in the Sjögren's International Collaborative Clinical Alliance Cohort. Arthritis Care Res (Hoboken) 2012;64(4):475–487. doi: 10.1002/acr.21591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schiffmann RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118(5):615–21. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 17.Rao K, Farley WJ, Pflugfelder SC. Association between high tear epidermal growth factor levels and corneal subepithelial fibrosis in dry eye conditions. Invest Ophthalmol Vis Sci. 2010;51(2):844–9. doi: 10.1167/iovs.09-3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoh H, Schirra F, Kienecker C, Ruprecht KW. [Lid-parallel conjunctival folds are a sure diagnostic sign of dry eye]. Ophthalmologe. 1995;92(6):802–8. [German] [PubMed] [Google Scholar]
- 19.Gumus K, Crockett CH, Pflugfelder SC. Anterior Segment Optical Coherence Tomography: A Diagnostic Instrument for Conjunctivochalasis. Am J Ophthalmol. 2010;150(6):798–806. doi: 10.1016/j.ajo.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ibrahim OM, Dogru M, Takano Y, et al. Application of visante optical coherence tomography tear meniscus height measurement in the diagnosis of dry eye disease. Ophthalmology. 2010;117(10):1923–1929. doi: 10.1016/j.ophtha.2010.01.057. [DOI] [PubMed] [Google Scholar]
- 21.Chen Q, Zhang X, Cui L, et al. Upper and lower tear menisci in Sjögren syndrome dry eye. Invest Ophthalmol Vis Sci. 2011;52(13):9373–8. doi: 10.1167/iovs.11-7431. [DOI] [PubMed] [Google Scholar]
- 22.Zoukhri D, Fix A, Alroy J, Kublin CL. Mechanisms of murine lacrimal gland repair after experimentally induced inflammation. Invest Ophthalmol Vis Sci. 2008;49(10):4399–4406. doi: 10.1167/iovs.08-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benítez del Castillo JM, Wasfy MA, Fernandez C, Garcia-Sanchez J. An in vivo confocal masked study on corneal epithelium and subbasal nerves in patients with dry eye. Invest Ophthalmol Vis Sci. 2004;45(9):3030–5. doi: 10.1167/iovs.04-0251. [DOI] [PubMed] [Google Scholar]
- 24.Villani E, Galimberti D, Viola F, Mapelli C, Ratiglia R. The cornea in Sjögren's syndrome: an in vivo confocal study. Invest Ophthalmol Vis Sci. 2007;48(5):2017–22. doi: 10.1167/iovs.06-1129. [DOI] [PubMed] [Google Scholar]
- 25.Savini G, Barboni P, Zanini M. Tear meniscus evaluation by optical coherence tomography. Ophthalmic Surg Lasers Imaging. 2006;37(2):112–8. [PubMed] [Google Scholar]
- 26.Khurana AK, Chaudhary R, Ahluwalia BK, Gupta S. Tear film profile in dry eye. Acta Ophthalmol (Copenh) 1991;69(1):79–86. doi: 10.1111/j.1755-3768.1991.tb01997.x. [DOI] [PubMed] [Google Scholar]
- 27.Bron AJ, Tokoi N, Gafney E, Tiffany JM. Predicted phenotypes of dry eye: proposed consequences of its natural history. Ocul Surf. 2009;7(2):78–92. doi: 10.1016/s1542-0124(12)70299-9. [DOI] [PubMed] [Google Scholar]
- 28.Stahl U, Willcox M, Stapleton F. Osmolality and tear film dynamics. Clin Exp Optom. 2012;95(1):3–11. doi: 10.1111/j.1444-0938.2011.00634.x. [DOI] [PubMed] [Google Scholar]
- 29.McGinnigle S, Naroo SA, Eperjesi F. Evaluation of Dry Eye. Surv Ophthalmol. 2012;57(4):293–316. doi: 10.1016/j.survophthal.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Lemp MA, Bron AJ, Baudouin C, et al. Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol. 2011;151(5):792–798. e1. doi: 10.1016/j.ajo.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan BD, Whitmer D, Nichols KK, et al. An objective approach to dry eye disease severity. Invest Ophthalmol Vis Sci. 2010;51(12):6125–30. doi: 10.1167/iovs.10-5390. [DOI] [PubMed] [Google Scholar]
- 32.Lam H, Bleiden L, de Paiva CS, Farley W, Stern ME, Pflugfelder SC. Tear cytokine profiles in dysfunctional tear syndrome. Am J Ophthalmol. 2009;147(2):198–205. doi: 10.1016/j.ajo.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathers W. Evaporation from the ocular surface. Exp Eye Res. 2004;78(3):389–94. doi: 10.1016/s0014-4835(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 34.Foulks GN. The correlation between the tear film lipid layer and dry eye disease. Surv Ophthalmol. 2007;52(4):369–74. doi: 10.1016/j.survophthal.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 35.Afonso AA, Sobrin L, Monroy DC, Selzer M, Lokeshwar B, Pflugfelder SC. Tear fluid gelatinase B activity correlates with IL-1alpha concentration and fluorescein clearance in ocular rosacea. Invest Ophthalmol Vis Sci. 1999;40(11):2506–12. [PubMed] [Google Scholar]
- 36.Nava A, Barton K, Monroy DC, Pflugfelder SC. The effects of age, gender, and fluid dynamics on the concentration of tear film epidermal growth factor. Cornea. 1997;16(4):430–8. [PubMed] [Google Scholar]
- 37.Gumus K, Pflugfelder SC. Increasing prevalence and severity of conjunctivochalasis with aging detected by anterior segment optical coherence tomography. Am J Ophthalmol. 2013;155(2):238–242. e2. doi: 10.1016/j.ajo.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 38.Bron A, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22(7):640–650. doi: 10.1097/00003226-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Kurihashi K, Yanagihara N, Honda Y. A modified Schirmer test: the fine-thread method for measuring lacrimation. J Pediatr Ophthalmol. 1977;14(6):390–397. [PubMed] [Google Scholar]
- 40.Hamano H, Hori M, Hamano T, et al. A new method for measuring tears. CLAO J. 1983;9(3):281–289. [PubMed] [Google Scholar]
- 41.Yokoi N, Kinoshita S, Bron AJ, Tiffany JM, Sugita J, Inatomi T. Tear meniscus changes during cotton thread and Schirmer testing. Invest Ophthalmol Vis Sci. 2000;41(12):3748–3753. [PubMed] [Google Scholar]
- 42.Tomlinson A, Blades K, Pearce E. What does the phenol red thread test actually measure? Optom Vis Sci. 2001;78(3):142–146. doi: 10.1097/00006324-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Wang J, Aquavella J, Palakuru J, Chung S. Repeated measurements of dynamic tear distribution on the ocular surface after instillation of artificial tears. Invest Ophthalmol Vis Sci. 2006;47(8):3325–3329. doi: 10.1167/iovs.06-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou S, Li Y, Lu AT, et al. Reproducibility of tear meniscus measurement by Fourier-domain optical coherence tomography: a pilot study. Ophthalmic Surg Lasers Imaging. 2009;40(5):442–447. doi: 10.3928/15428877-20090901-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tung CI, Kottaiyan R, Koh S, et al. Noninvasive, objective, multimodal tear dynamics evaluation of 5 over-the-counter tear drops in a randomized controlled trial. Cornea. 2012;31(2):108–14. doi: 10.1097/ICO.0b013e31821ea667. [DOI] [PubMed] [Google Scholar]
- 46.Kottaiyan R, Yoon G, Wang Q, Yadav R, Zavislan JM, Aquavella JV. Integrated multimodal metrology for objective and noninvasive tear evaluation. Ocul Surf. 2012;10(1):43–50. doi: 10.1016/j.jtos.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 47.Palakuru JR, Wang J, Aquavella JV. Effect of blinking on tear dynamics. Invest Ophthalmol Vis Sci. 2007;48(7):3032–7. doi: 10.1167/iovs.06-1507. [DOI] [PubMed] [Google Scholar]
- 48.Li J, Shen M, Wang J, et al. Clinical significance of tear menisci in dry eye. Eye Contact Lens. 2012;38(3):183–7. doi: 10.1097/ICL.0b013e318252ce0c. [DOI] [PubMed] [Google Scholar]
- 49.Yadav R, Lee KS, Rolland JP, Zavislan JM, Aquavella JV, Yoon G. Micrometer axial resolution OCT for corneal imaging. Biomed Opt Express. 2011;2(11):3037–46. doi: 10.1364/BOE.2.003037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yadav R, Ahmad K, Yoon G. Scanning system design for large scan depth anterior segment optical coherence tomography. Opt Lett. 2010;35(11):1774–6. doi: 10.1364/OL.35.001774. [DOI] [PubMed] [Google Scholar]
- 51.Uchida A, Uchino M, Goto E, et al. Noninvasive interference tear meniscometry in dry eye patients with Sjögren syndrome. Am J Ophthalmol. 2007;144(2):232–237. doi: 10.1016/j.ajo.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 52.Doughty MJ, Laiquzzaman M, Button NF. Video-assessment of tear meniscus height in elderly Caucasians and its relationship to the exposed ocular surface. Curr Eye Res. 2001;22(6):420–6. doi: 10.1076/ceyr.22.6.420.5487. [DOI] [PubMed] [Google Scholar]