Abstract
Background
Older patients constitute a growing proportion of U.S. kidney transplant recipients and often have a high burden of comorbidities. A summary measure of health such as functional status might enable transplant professionals to better evaluate and counsel these patients about their prognosis after transplant.
Methods
We linked UNOS registry data about post-transplant survival with pre-transplant functional status data (physical function [PF] scale of the Medical Outcomes Study Short Form-36) among individuals undergoing kidney transplant from 6/1/2000 – 5/31/2006. We examined the relationship between survival and functional status with multivariable Cox regression, adjusted for age. Using logistic regression models for three-year survival, we also estimated the reduction in deaths in the hypothetical scenario that recipients with poor functional status in this cohort experienced modest improvements in function.
Results
The cohort comprised 10,875 kidney transplant recipients (KTRs) with a mean age of 50 years; 14% were ≥65. Differences in three-year mortality between highest and lowest PF groups ranged from 3% among recipients <35 years to 14% among recipients ≥65 years. In multivariable Cox regression, worse PF was associated with higher mortality (HR 1.66 for lowest versus highest PF quartiles; p<0.001). Interactions between PF and age were non-significant. We estimated that 11% fewer deaths would occur if KTRs with the lowest functional status experienced modest improvements in function.
Conclusions
Across a wide age range, functional status was an independent predictor of post-transplant survival. Functional status assessment may be a useful tool with which to counsel patients about post-transplant outcomes.
Keywords: Older age, functional status, kidney transplant, survival
Background
The kidney transplant population is aging rapidly.(1) During the decade from 1999 – 2008, the proportion of incident kidney transplant recipients (KTRs) in the U.S. who are elderly (>65 years) more than doubled from 7% to 16%.(2) Additionally, waiting times have lengthened and transplant candidates commonly carry a heavy burden of comorbidities that worsen with chronic dialysis.(3) Transplant professionals therefore face challenges in evaluating a population of older and increasingly complicated patients about kidney transplantation.
Functional status has potential advantages as a tool to evaluate and counsel patients about transplantation. Adequate functional status depends on physical fitness, sensory function, and the ability to negotiate the external environment.(4) Functional status usually worsens with advanced age, particularly among elderly individuals with chronic disease.(5) Functional status can capture the cumulative impact of chronic health conditions that have a high prevalence in the end-stage renal disease (ESRD) population, such as diabetes, cardiovascular disease, and hepatitis C. Independent of age and comorbidities, ESRD is associated with loss of functional status as well as poor physical performance and the syndrome of frailty.(6–8) This loss of function may be driven by the systemic inflammation, cachexia, and sarcopenia associated with the uremic state.(9–12)
Limited high-quality data are available about functional status among KTRs, particularly elderly recipients.(13–15) Given the extensive medical work-up required to be accepted for transplantation,(16) it is unclear whether substantial variation in function exists across transplant candidates. It is also unknown whether functional status assessments while patients are on the transplant waiting list are associated with survival after transplant for different age groups. Therefore, the aims of this study were to determine 1) whether a self-reported measure of functional status predicts survival after kidney transplantation, and 2) whether this predictive ability differs by age at transplantation.
Results
Study Cohort
We identified 150,843 adults who had received ≥12 consecutive months of hemodialysis or peritoneal dialysis through Fresenius Medical Care (FMC), a provider of chronic dialysis services. Among these, 114,133 (76%) had completed at least one Medical Outcomes Study Short Form-36 questionnaire (SF-36). Using patient identifiers, we linked this file of dialysis patients to a file from the United Network for Organ Sharing (UNOS) and identified 10,994 individuals who had undergone kidney transplantation after SF-36 measurement. 119 (1%) had missing PF responses, leaving 10,875 KTRs at 212 transplant centers.
The cohort had a wide age range and substantial racial/ethnic diversity. We compared our cohort to all adults on dialysis who underwent kidney transplantation in the U.S. during the same period (n=113,675). Compared to KTRs outside the cohort, this cohort had a similar mean age (50 years versus 49 years outside the cohort), a similar proportion of elderly (≥65 years) recipients (14% versus 13% outside the cohort), and similar proportions of male (63% versus 61% outside the cohort), and diabetic patients (36% in both groups). The study cohort had a higher proportion of Black (34% versus 26% outside the cohort) and Hispanic participants (17% versus 14% outside the cohort).
Physical Function Scores and Subject Characteristics
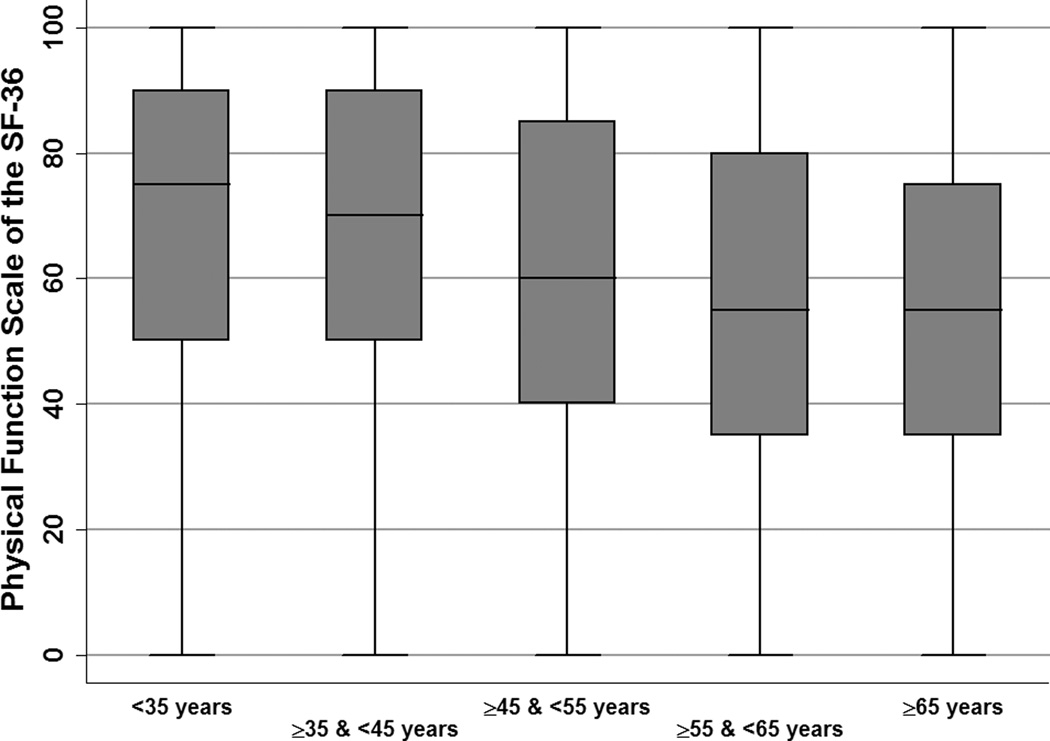
We empirically divided physical function (PF) subscores of the SF-36 into quartiles. The median PF score was 60 (interquartile range [IQR] 40, 85). The median interval between SF-36 completion and transplantation was 228 days (IQR 113, 563) and did not differ between PF quartiles (p=0.58). As in Figure 1, PF scores were highest among patients <45 years and showed a general trend of decline among older age groups.
Figure 1.
Distribution of Physical Function Scores, by Recipient Age
Table 1 presents subject characteristics. Compared to recipients in the lowest PF quartile, recipients in the highest quartile were younger (median 47 versus 55 years), more likely to be male (69% versus 57%), and less likely to be CMV positive (60% versus 67%) or have diabetes (26% versus 49%).
Table 1.
Demographic and Clinical Characteristics of Kidney Transplant Recipients and Donors, by Recipient Physical Function Quartile
| RECIPIENT CHARACTERISTICS | Highest Physical Function (n=2,843) | Near-highest Physical Function (n=2,543) | Near-lowest Physical Function (n=2,957) | Lowest Physical Function (n=2,532) | p-value |
| MEAN AGE (standard deviation) | 47 (13) | 51 (13) | 52 (13) | 55 (12) | <0.001 |
| <35 years (%) | 300 (11) | 170 (7) | 183 (6) | 92 (4) | <0.001 |
| ≥35 & <45 years (%) | 957 (34) | 665 (26) | 714 (24) | 460 (18) | |
| ≥45 & <55 years (%) | 736 (26) | 681 (27) | 832 (28) | 686 (27) | |
| ≥55 & <65 years (%) | 558 (20) | 687 (27) | 818 (28) | 853 (34) | |
| ≥65 years (%) | 292 (10) | 340 (13) | 410 (14) | 441 (17) | |
| MALE (%) | 1,970 (69%) | 1,645 (65) | 1,746 (59) | 1,442 (57) | <0.001 |
| RACE (%) | 0.001 | ||||
| White | 1,200 (42) | 1,100 (43) | 1,202 (41) | 1,183 (47) | |
| Black | 1,006 (35) | 865 (34) | 1,033 (35) | 769 (30) | |
| Hispanic | 488 (17) | 412 (16) | 524 (18) | 448 (18) | |
| Asian | 98 (3) | 113 (4) | 135 (4) | 91 (4) | |
| Other | 51 (2) | 53 (2) | 63 (2) | 41 (2) | |
| CAUSE OF ESRD (%) | <0.001 | ||||
| Diabetes | 588 (21) | 626 (25) | 887 (30) | 964 (38) | |
| Hypertension | 778 (27) | 622 (24) | 735 (25) | 571 (23) | |
| Glomerulonephritis | 637 (22) | 542 (21) | 519 (18) | 352 (14) | |
| Polycystic Kidney Disease | 210 (7) | 177 (7) | 195 (7) | 138 (5) | |
| Congenital/Reflux | 56 (20) | 40 (2) | 35 (1) | 31 (1) | |
| Other | 574 (20) | 536 (21) | 586 (20) | 476 (19) | |
| DIABETES (%) | 742 (26) | 827 (33) | 1,125 (38) | 1,229 (49) | <0.001 |
| HEPATITIS C SEROPOSITIVE (%) | 151 (5.3) | 143 (5.6) | 150 (5.1) | 158 (6.3) | 0.20 |
| PEAK PANEL REACTIVE ANTIBODY >80 (%) | 214 (7.5) | 233 (9.2) | 245 (8.3) | 213 (8.4) | 0.22 |
| YEARS ON DIALYSIS (%) | 0.24 | ||||
| ≥1 & <5 | 1,171 (41) | 983 (39) | 1,155 (39) | 970 (38) | |
| ≥3 & <5 | 831 (29) | 768 (30) | 900 (30) | 811 (32) | |
| ≥5 | 813 (29) | 758 (30) | 877 (30) | 725 (29) | |
| CYTOMEGALOVIRUS SEROPOSITIVE | 1,708 (60) | 1,610 (63) | 1,890 (64) | 1,686 (67) | <0.001 |
| PRIOR ORGAN TRANSPLANT | 304 (11) | 278 (11) | 303 (10) | 254 (10) | 0.70 |
| DONOR CHARACTERISTICS | Highest Physical Function (n=2,843) | Near-highest Physical Function (n=2,543) | Near-lowest Physical Function (n=2,957) | Lowest Physical Function (n=2,532) | p-value |
| MEAN AGE (standard deviation) | 37 (16) | 38 (16) | 37 (16) | 38 (16) | 0.01 |
| <40 years (%) | 1,491 (52) | 1,260 (50) | 1,520 (51) | 1,252 (49) | <0.001 |
| ≥40 & <50 years (%) | 650 (23) | 590 (23) | 662 (22) | 543 (21) | |
| ≥50 & <60 years (%) | 535 (19) | 483 (19) | 551 (19) | 484 (19) | |
| ≥60 years (%) | 167 (6) | 210 (8) | 224 (8) | 253 (10) | |
| MALE | 1,588 (56) | 1,427 (56) | 1,670 (56) | 1,483 (59) | 0.18 |
| BLACK (vs. NON-BLACK) | 430 (15) | 396 (16) | 469 (16) | 404 (16) | 0.83 |
| DONOR TYPE* | <0.001 | ||||
| Live donor | 510 (18) | 420 (17) | 501 (17) | 410 (16) | |
| Deceased, non-ECD | 2,032 (71) | 1,729 (68) | 2,050 (69) | 1,721 (68) | |
| ECD deceased donor | 301 (11) | 394 (15) | 406 (14) | 401 (16) | |
| CYTOMEGALOVIRUS SEROPOSITIVE | 1,476 (52) | 1,352 (53) | 1,547 (52) | 1,335 (53) | 0.82 |
Abbreviations: ECD – Extended criteria donor
Overall Mortality
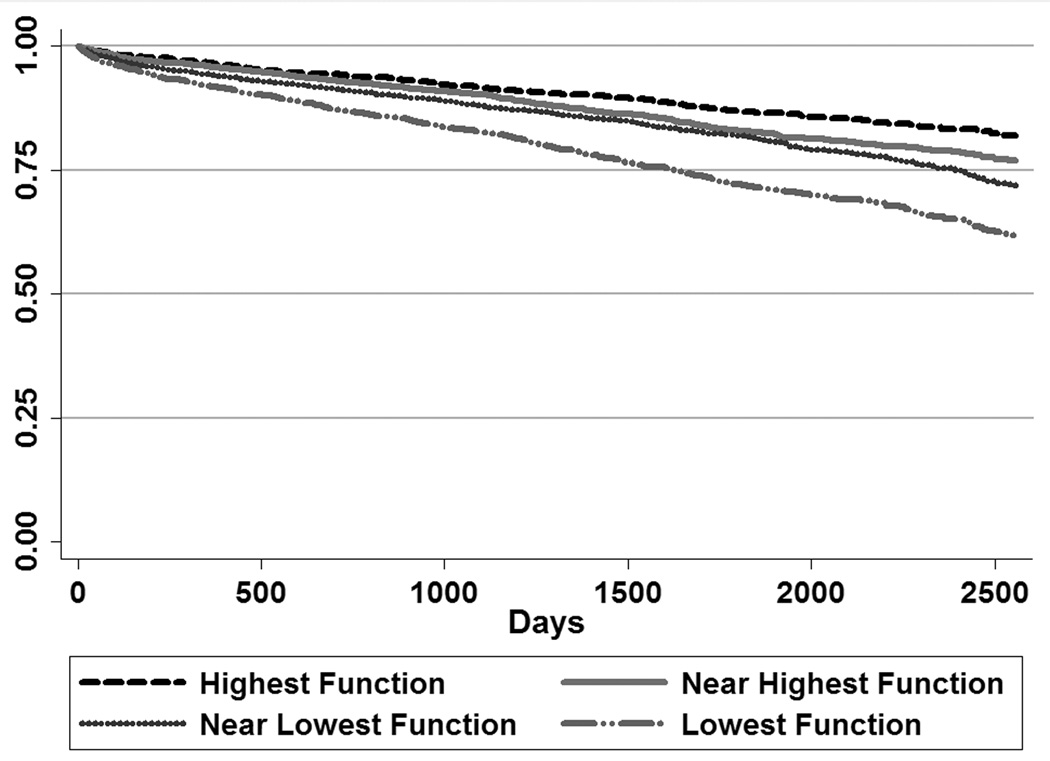
2,089 KTRs (19%) died. Median follow-up time after transplant was 1,631 days (IQR 997, 2218). Figure 2 shows post-transplant survival. Recipients with the highest function had the best survival, and recipients with the lowest function had the worst survival. Survival curves for KTRs in the second and third PF quartiles did not separate consistently until 5 years after transplantation.
Figure 2.
Survival after Kidney Transplantation, by Quartile of Physical Function Scale of the SF-36
Stratified analyses showed that lowest versus highest PF quartile was significantly associated with worse survival for every age group (p<0.01) except KTRs <35 years (p=0.10). A global test for interaction between age and PF quartiles, however, was not significant.
As in Table 3, multivariable Cox regression confirmed that lower functional status was independently associated with higher mortality (hazard ratio [HR] 1.66 for lowest versus highest PF quartiles; 95% CI 1.45, 1.89; p<0.001). SDC Table 1 shows the full model.
Three Year Mortality
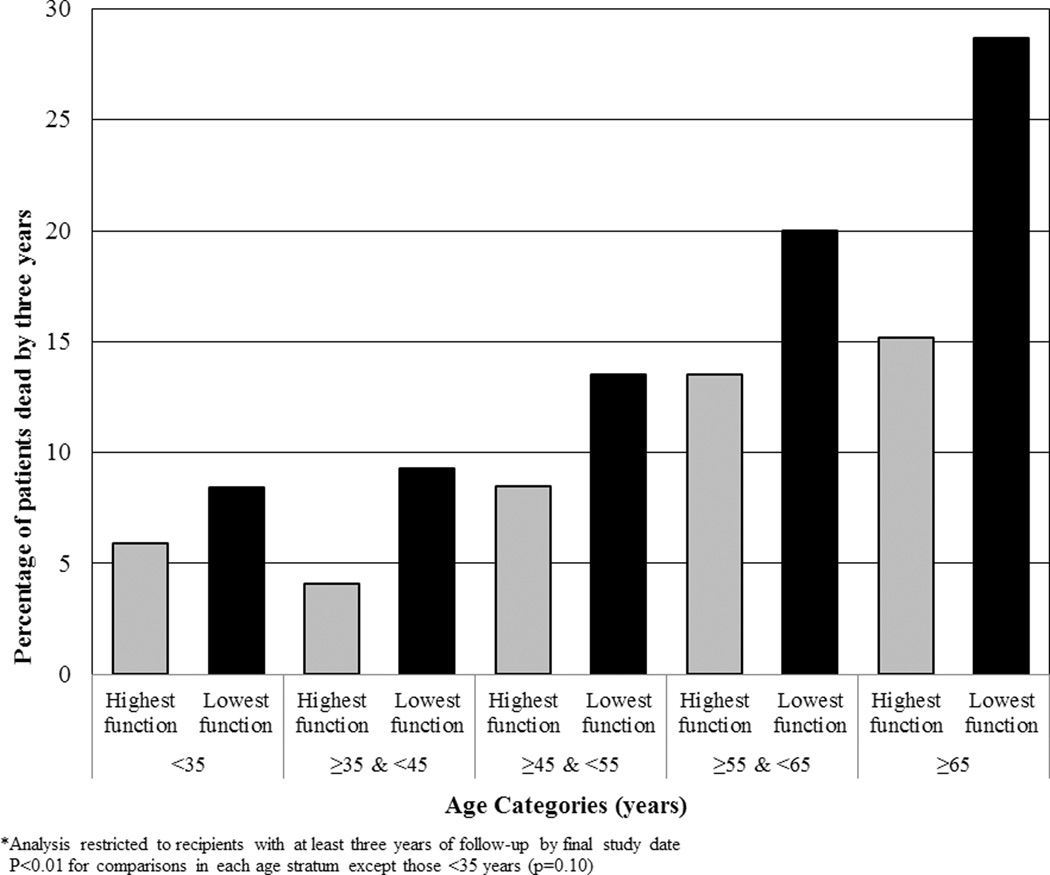
Among recipients with at least three years of follow-up (n=9,307), 12% of the KTRs died (1,089). Figure 3 shows the absolute difference in risk between the highest and lowest PF quartiles, which ranged from 3% among recipients <35 years, to 14% among recipients over 65 years. Notably, younger recipients with poor function had lower survival than recipients with good functional status in the adjacent older age category.
Figure 3.
Three-Year Mortality after Kidney Transplantation Contrasted between Recipients in Highest versus Lowest Physical Function Quartiles, by Recipient Age Strata
We fit a multivariable logistic regression model for the outcome of three-year mortality. The logistic model predicted 1,065 deaths by three years, similar to the number observed. To estimate the deaths that would occur if KTRs in this cohort experienced modest improvements in function, we increased the PF quartile of recipients in the lower half of functional status by one quartile. With this new PF distribution, the model predicted 946 deaths, an 11% decrease in mortality.
Secondary analyses of mortality
In multivariable logistic regression models, KTRs in the highest PF quartile experienced lower death rates than KTRs in the lowest quartile at six months (HR 1.70; p=0.001) and one year (HR 1.74; p<0.001) after transplant.
Delayed graft function and allograft failure
A total of 2,604 (24%) KTRs experienced delayed graft function, while 2,975 (27%) experienced allograft failure. In multivariable logistic regression, only KTRs in the lowest PF quartile had a higher odds of delayed graft function (OR 1.18; CI 1.04, 1.35; p=0.01).
In multivariable Cox regression, the lowest PF quartile was associated with a higher rate of allograft failure (HR 1.35; CI 1.21, 1.50; p<0.001). The association of near-lowest PF quartile with allograft failure did not reach statistical significance (HR 1.11; CI 1.00, 1.23; p=0.057).
Discussion
In a diverse cohort of KTRs across 212 centers, pre-transplant functional status was an independent predictor of post-transplant survival. Differences in three-year mortality across PF quartiles were substantial in all age strata except individuals <35 years. These findings provide evidence that measures of function could prove useful during the evaluation of ESRD patients considering kidney transplantation.
Wide variation in functional status was evident. Despite the multidisciplinary evaluation required for kidney transplantation, a process that commonly includes cardiovascular testing and age-appropriate cancer screening(17), the cohort showed variation in PF scores within all age strata. As shown in Figure 1, PF scores were lower in the older age groups but appeared to level off after age 55 years. Given that function usually decreases with advancing age, and certainly after age 55, this finding could be explained if older patients with worse function were not selected by centers for wait-listing. This finding could also be explained by the self-reported nature of the PF score.(18) Studies in non-transplant populations suggest that after adjustment for comorbidities, older individuals rate their health as better than younger individuals.(5, 19) If the older individuals in this cohort rated their health more highly than younger patients, this would lead our models to underestimate the magnitude of the hazard associated with worse functional status. Thus, it is possible that if the PF scale were compared to observed physical performance, a physical performance measure might show a more consistent pattern of decline with older age than functional status would.
Although functional status had a strong association with mortality in patients across a wide age spectrum, a functional measure may be particularly useful in evaluating older candidates who have the highest rate of death after transplant. As shown in Figure 3, the oldest patients in our cohort had the largest risk difference between low and high PF quartiles at three years. Notably, despite multiple studies showing that the elderly derive a survival benefit from kidney transplant compared to dialysis, elderly patients with ESRD have much lower access to kidney transplantation.(20–22) Alexander et al. studied 7,125 incident dialysis patients and reported that older patients were far less likely to be referred to transplant centers, to complete evaluation, and to receive a kidney transplant.(23) More recently, using United States Renal Data Systems data that allowed limited adjustment for comorbidities, Kucirka et al. reported that older patients were less likely to receive information about kidney transplant options.(24) This diminished transplant access extends to live donor kidney transplant (LDKT). A single center study by Weng et al. showed that older patients were less likely to attract potential donors and undergo LDKT, such that transplant candidates >60 years had an odds ratio of 0.21 for LDKT compared to candidates <40 years.(20) These troubling findings are not easily explained. It is possible that providers, using the “eyeball test” in a clinical evaluation, overestimate the frailty or comorbidities of older patients.(25, 26) Another explanation is that older patients are less willing to accept the short-term risks of peri-operative events and infection with transplant in order to enjoy the long-term benefits of quality of life and survival. In any case, the use of a validated functional status measure may enable providers to assess the global health of an elderly transplant candidate in a more standardized and just way than relying solely on age or a subjective evaluation of physical fitness.
These results complement a growing literature about the relationships of functional status, frailty, and physical performance to transplant outcomes. Our finding that functional status is an independent predictor of post-transplant survival confirms the work of Kutner et al. in a larger, more contemporary cohort that permitted examination of function across multiple age strata.(13) The distribution of PF scores in our cohort was similar to the distribution in the Kutner study. In a single-center study, Garonzik-Wang reported that 24% of KTRs met frailty criteria when evaluated immediately prior to transplant surgery; frailty predicted a higher odds of delayed graft function.(27) Although the relationship of pre-transplant physical performance to survival after kidney transplant has not been reported, Hartmann et al. examined physical performance among 26 renal transplant candidates over 60 years of age using the Short Physical Performance Battery (SPPB), which comprises observed tests of walking speed, standing balance, and chair raises. SPPB scores among these renal transplant candidates were lower than among older individuals with heart failure and chronic obstructive pulmonary disease.(7) Taken together, these findings suggest that KTRs commonly have poor functional status or other evidence of physical deterioration, and that assessing function may help transplant professionals characterize the survival opportunities of these patients. Additionally, it is possible that assessments of functional status could identify wait-listed patients who would benefit from physical therapy or other interventions with the goal of helping these individuals survive to transplant.
The SF-36 and its method of collection in this study offered advantages. The SF-36 has been validated in elderly patients and dialysis patients.(28–30) Individuals in the cohort completed the SF-36 as part of routine data collection in dialysis units. Thus, we have no basis for concern that responses would be affected by patient expectations about transplant candidacy. Because dialysis providers are required by regulation to collect quality of life data (encompassing questions about function), nephrologists already have access to information about functional status for many patients who they might consider for transplant referral or other services.(31) In contrast to binary clinical attributes such as diabetes or hepatitis C (HCV), a continuous measure such as the PF scale may have advantages in that it classifies patients over a spectrum of severity. Our results reveal that differences in functional status as measured by the SF-36 are associated with a large and clinically meaningful difference in mortality. We estimated that 11% fewer deaths would occur in the scenario that the KTRs in our cohort with the lowest functional status experienced modest improvements in function.
These findings should be evaluated in the context of the study’s limitations. The PF scale is a subjective, self-reported measure. An observed measure of function, such as gait speed evaluation or the full SPPB, might better distinguish differences in global health between subgroups, such as those defined by age. Also, functional status can change over time, and the utility of functional status as a tool may depend on regular assessments of it. The study population was also limited to individuals receiving chronic dialysis. Given known associations between ESRD, poor nutrition, and loss of muscle,(9, 10, 32, 33) functional status in our population might be worse than among individuals undergoing pre-emptive transplantation. In this retrospective study, residual confounding is also plausible. Finally, UNOS registry data lack detailed information about important comorbidities such as cardiovascular disease and about the severity of known comorbidities such as diabetes.
Conclusion
Independent of age, pre-transplant functional status predicted mortality after kidney transplantation. Future studies should prospectively examine a range of related measures including functional status, frailty, and physical performance to determine their utility in predicting transplant outcomes. Functional status assessment might also identify individuals on the waiting list who would benefit from physical therapy or more frequent evaluation by the transplant center.
Methods
We performed a retrospective cohort study of adult (age ≥18 years) KTRs in the United States using a linked dataset from the UNOS/Organ Procurement and Transplantation Network and the Fresenius Medical Care Corporation, a provider of chronic dialysis services. The primary outcome was patient survival after transplantation and the primary exposure was the PF subscale of the SF-36. The study was approved by the University of Pennsylvania Institutional Review Board.
Participants had received >12 months of chronic dialysis through Fresenius, were wait-listed for kidney transplantation from 6/1/2000 – 5/31/2006, and had completed the SF-36 at least once before transplantation. Patients wait-listed for multi-organ transplants other than kidney-pancreas, and patients without responses to all 10 PF scale questions were excluded.
FMC administered the SF-36 to patients approximately every year. The PF scale is generated from 10 items that address activities of daily living and more strenuous tasks such as carrying groceries and walking a mile.(18) The PF score collected closest to the transplantation date was used and transformed into a scale from 10 – 100.(34) We empirically divided the population into quartiles of PF scores. The rationale was that these four ordered categories would be more clinically intuitive than a PF variable transformed to a normal distribution and that these four categories would be amenable to graphical representation.
The UNOS dataset provided detailed information about every KTR.(35) Death was ascertained through center reports and linkage to the Social Security Death Master File (SSDMF). Because of the high level of death ascertainment, we performed analyses assuming complete follow-up from transplant until the date of merger of the UNOS file with the SSDMF.
Statistical analysis
We conducted analyses using Stata (version 11.0, Stata Corporation, College Station, TX). We used the t-test to compare PF scores between patients with and without diabetes. We used ANOVA to compare the means of normally distributed variables and Chi-square tests to compare categorical variables across PF quartiles.
We fit multivariable Cox regression models for mortality. Our selection of independent variables was guided by prior studies.(36–38) Recipient variables included age (<35, ≥35 & <45, ≥45 & <55, ≥55 & <65, and ≥65 years), sex, race, prior organ transplant, dialysis vintage (<3, ≥3 and <5, ≥5 years), high peak panel reactive antibody (PRA, defined by UNOS convention as ≥80% versus <80%)(39), cytomegalovirus (CMV) serostatus, glomerulonephritis, polycystic kidney disease, congenital/reflux renal disease, hypertensive renal disease, diabetes, and HCV seropositivity. Donor variables included age, sex, race (black or non-black), CMV, and donor type (live, deceased, expanded criteria deceased donor).(40) Allograft variables included human leukocyte antigen mismatch (zero or nonzero). We performed stratified analyses and tests for interaction between PF quartile and age.(39) We examined Schoenfeld residuals and log-log plots to confirm the proportional hazards assumption.
Outcome of three-year mortality and estimation of mortality reduction in a hypothetical scenario that transplant recipients experienced improvements in function
To express our results in a transparent way to clinicians and policymakers, we examined three-year survival. Pre-transplant characteristics would be expected to influence mortality within this time-frame, and three-year survival is a metric of quality for transplant institutions.(41) To have sufficient follow-up, this analysis was restricted to recipients transplanted before the year 2008 (n=9,307, 86% of total). We fit a multivariable logistic model for the outcome of three-year survival, using the same covariates used in the Cox model for mortality. The hypothesis of good fit using the Hosmer-Lemeshow test was not rejected (p=0.91).
To estimate the expected deaths if kidney allografts were distributed to a hypothetical population that had experienced improvements in function, we increased the PF quartile of recipients in the lower half of functional status by one quartile. Specifically, we raised the PF quartile for those in the third quartile to the second quartile, and we raised those in the fourth quartile to the third, leaving other recipient attributes unchanged. We applied our logistic regression model to this hypothetical population and summed the predicted probabilities of death by three years.
Secondary outcomes
We fit multivariable logistic regression models for the outcomes of delayed graft function (defined as dialysis in the first week after kidney transplantation), as well as six month and one year survival. We also fit a multivariable Cox regression model for all-cause allograft failure. These models included the same covariates used in the Cox model for mortality.
Missing Data
A minority of recipients had missing data on variables relevant to the primary analysis, including PRA (n=588, 5%). In primary analyses, for categorical values with >1% missing data, we created a separate category for missingness. In addition, we performed sensitivity analyses in which extreme values for missing data were assigned to individuals with missing data. The associations between PF quartile and the outcomes were unchanged and not shown.
Supplementary Material
Table 2.
Multivariable Cox regression analysis of patient survival after kidney transplantation*
| Recipient Functional Status alone |
Recipient Age alone |
Recipient Age and Functional Status |
Full Model: All Recipient and Donor Attributes |
|||||
|---|---|---|---|---|---|---|---|---|
| HR (CI) | p- value |
HR (CI) | p- value |
HR (CI) | p- value |
HR (CI) | p-value | |
| FUNCTIONAL STATUS | ||||||||
| Highest | Reference | Reference | Reference | |||||
| Near-highest | 1.28 (1.12, 1.47) | <0.001 | 1.15 (1.01, 1.33) | 0.04 | 1.09 (0.94, 1.77) | 0.25 | ||
| Near-lowest | 1.54 (1.36, 1.76) | <0.001 | 1.36 (1.20, 1.55) | <0.001 | 1.28 (1.12, 1.46) | <0.001 | ||
| Lowest | 2.30 (2.03, 2.61) | <0.001 | 1.88 (1.66, 2.14) | <0.001 | 1.66 (1.45, 1.89) | <0.001 | ||
| AGE | ||||||||
| <35 years | Reference | Reference | Reference | |||||
| ≥35 & <45 years | 1.54 (1.17, 2.04) | 0.002 | 1.49 (1.13, 1.96) | 0.005 | 1.25 (0.94, 1.67) | <0.001 | ||
| ≥45 & <55 years | 2.47 (1.89, 3.23) | <0.001 | 2.26 (1.73, 2.96) | <0.001 | 1.75 (1.32, 2.32) | <0.001 | ||
| ≥55 & <65 years | 3.84 (2.95, 5.00) | <0.001 | 3.40 (2.61, 4.43) | <0.001 | 2.55 (1.92, 3.38) | <0.001 | ||
| ≥65 years | 5.71 (4.37, 7.47) | <0.001 | 5.10 (3.90, 6.68) | <0.001 | 3.79 (2.84, 5.05) | <0.001 | ||
Full model also adjusted for: recipient attributes of race, sex, diabetes status, prior solid organ transplant, dialysis time, panel reactive antibody, hepatitis C serostatus, glomerulonephritis, polycystic kidney disease, congenital/reflex kidney disease, hypertensive kidney disease, and cytomegalovirus status; donor attributes of type (live, deceased versus extended criteria deceased), age, race (black versus other), and cytomegalovirus status; and allograft attributes of human leukocyte antigen mismatch.
Acknowledgements
Preliminary results were presented at the 2011 American Geriatrics Society Annual Meeting in Washington DC and the 2012 American Transplant Congress.
Abbreviations
- AUC
Area-Under-the-Curve
- CMV
Cytomegalovirus
- ESRD
End-Stage Renal Disease
- FMC
Fresenius Medical Care
- HCV
Hepatitis C Virus
- KTR
Kidney Transplant Recipient
- PF
Physical Function
- SSDMF
Social Security Death Master File
- UNOS
United Network for Organ Sharing
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Funding sources and participation:
Dr. Reese is supported by R01-DK090388-01A1 and by a grant from the Society of Specialty Professors, the American Society of Nephrology, the John A. Hartford Foundation and the Atlantic Philanthropies. He participated in research design, data analysis, and writing of the paper.
Dr. Bloom participated in research design and writing of the paper
Dr. Shults participated in research design, data analysis and writing of the paper
Dr. Thomasson participated in data analysis and writing of the paper
Mr. Mussell participated in writing of the paper
Dr. Rosas participated in research design and writing of the paper
Dr. Johansen participated in research design and writing of the paper
Dr. Abt participated in research design and writing of the paper
Dr. Levine participated in research design and writing of the paper
Dr. Caplan participated in writing of the paper
Dr. Feldman is supported by NIH grant K24-DK002651. He participated in research design and writing of the paper
Dr. Karlawish participated in research design and writing of the paper
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: UNOS Disclaimer: The data reported here have been supplied by the United Network for Organ Sharing (UNOS) as the contractor for the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN contractor. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the OPTN or the U.S. Government.
References
- 1.Ma X, Becker D, Arena VC, Vicini P, Greenbaum C. The effect of age on insulin sensitivity and insulin secretion in first-degree relatives of type 1 diabetic patients: a population analysis. J Clin Endocrinol Metab. 2009;94(7):2446. doi: 10.1210/jc.2008-2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Organ Procurement and Transplantation Network. [Accessed: 08/23/2012];Transplants in the U.S. by Recipient Age: Organ = Kidney. URL: http://optn.transplant.hrsa.gov/latestData/rptData.asp.
- 3.Machnicki G, Pinsky B, Takemoto S, et al. Predictive Ability of Pretransplant Comorbidities to Predict Long-Term Graft Loss and Death. Am J Transplant. 2008 doi: 10.1111/j.1600-6143.2008.02486.x. [DOI] [PubMed] [Google Scholar]
- 4.Painter P, Stewart AL, Carey S. Physical functioning: definitions, measurement, and expectations. Adv Ren Replace Ther. 1999;6(2):110. doi: 10.1016/s1073-4449(99)70028-2. [DOI] [PubMed] [Google Scholar]
- 5.Jylha M. What is self-rated health and why does it predict mortality? Towards a unified conceptual model. Soc Sci Med. 2009;69(3):307. doi: 10.1016/j.socscimed.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Johansen KL, Chertow GM, Jin C, Kutner NG. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18(11):2960. doi: 10.1681/ASN.2007020221. [DOI] [PubMed] [Google Scholar]
- 7.Hartmann EL, Kitzman D, Rocco M, et al. Physical Function in Older Candidates for Renal Transplantation: An Impaired Population. Clin J Am Soc Nephrol. 2009 doi: 10.2215/CJN.03860808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH. Association among SF36 quality of life measures and nutrition, hospitalization, and mortality in hemodialysis. J Am Soc Nephrol. 2001;12(12):2797. doi: 10.1681/ASN.V12122797. [DOI] [PubMed] [Google Scholar]
- 9.Kalantar-Zadeh K, Ikizler TA, Block G, Avram MM, Kopple JD. Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences. Am J Kidney Dis. 2003;42(5):864. doi: 10.1016/j.ajkd.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 10.Bologa RM, Levine DM, Parker TS, et al. Interleukin-6 predicts hypoalbuminemia, hypocholesterolemia, and mortality in hemodialysis patients. Am J Kidney Dis. 1998;32(1):107. doi: 10.1053/ajkd.1998.v32.pm9669431. [DOI] [PubMed] [Google Scholar]
- 11.Balakrishnan VS, Guo D, Rao M, et al. Cytokine gene polymorphisms in hemodialysis patients: association with comorbidity, functionality, and serum albumin. Kidney Int. 2004;65(4):1449. doi: 10.1111/j.1523-1755.2004.00531.x. [DOI] [PubMed] [Google Scholar]
- 12.Stenvinkel P, Ketteler M, Johnson RJ, et al. IL-10, IL-6, and TNF-alpha: central factors in the altered cytokine network of uremia--the good, the bad, the ugly. Kidney Int. 2005;67(4):1216. doi: 10.1111/j.1523-1755.2005.00200.x. [DOI] [PubMed] [Google Scholar]
- 13.Kutner NG, Zhang R, Bowles T, Painter P. Pretransplant physical functioning and kidney patients' risk for posttransplantation hospitalization/death: evidence from a national cohort. Clin J Am Soc Nephrol. 2006;1(4):837. doi: 10.2215/CJN.01341005. [DOI] [PubMed] [Google Scholar]
- 14.Hartmann EL, Kitzman D, Rocco M, et al. Physical function in older candidates for renal transplantation: an impaired population. Clin J Am Soc Nephrol. 2009;4(3):588. doi: 10.2215/CJN.03860808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zelle DM, Corpeleijn E, Stolk RP, et al. Low physical activity and risk of cardiovascular and all-cause mortality in renal transplant recipients. Clin J Am Soc Nephrol. 2011;6(4):898. doi: 10.2215/CJN.03340410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pham PT, Pham PA, Pham PC, Parikh S, Danovitch G. Evaluation of adult kidney transplant candidates. Semin Dial. 2010;23(6):595. doi: 10.1111/j.1525-139X.2010.00809.x. [DOI] [PubMed] [Google Scholar]
- 17.Kasiske BL, Ramos EL, Gaston RS, et al. The evaluation of renal transplant candidates: clinical practice guidelines. Patient Care and Education Committee of the American Society of Transplant Physicians. J Am Soc Nephrol. 1995;6(1):1. doi: 10.1681/ASN.V611. [DOI] [PubMed] [Google Scholar]
- 18.Ware JEKM, Keller SK. Physical and Mental Health Summary Scales: A User's Manual. Boston, MA: The Health Institute; 1994. [Google Scholar]
- 19.Jylha M, Guralnik JM, Balfour J, Fried LP. Walking difficulty, walking speed, and age as predictors of self-rated health: the women's health and aging study. J Gerontol A Biol Sci Med Sci. 2001;56(10):M609. doi: 10.1093/gerona/56.10.m609. [DOI] [PubMed] [Google Scholar]
- 20.Weng FL, Reese PP, Mulgaonkar S, Patel AM. Barriers to Living Donor Kidney Transplantation among Black or Older Transplant Candidates. Clin J Am Soc Nephrol. 2010 doi: 10.2215/CJN.03040410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grams ME, Kucirka LM, Hanrahan CF, Montgomery RA, Massie AB, Segev DL. Candidacy for kidney transplantation of older adults. J Am Geriatr Soc. 2012;60(1):1. doi: 10.1111/j.1532-5415.2011.03652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knoll GA. Is kidney transplantation for everyone? The example of the older dialysis patient. Clin J Am Soc Nephrol. 2009;4(12):2040. doi: 10.2215/CJN.04210609. [DOI] [PubMed] [Google Scholar]
- 23.Alexander GC, Sehgal AR. Barriers to cadaveric renal transplantation among blacks, women, and the poor. Jama. 1998;280(13):1148. doi: 10.1001/jama.280.13.1148. [DOI] [PubMed] [Google Scholar]
- 24.Kucirka LM, Grams ME, Balhara KS, Jaar BG, Segev DL. Disparities in provision of transplant information affect access to kidney transplantation. Am J Transplant. 2012;12(2):351. doi: 10.1111/j.1600-6143.2011.03865.x. [DOI] [PubMed] [Google Scholar]
- 25.Segev DL, Kucirka LM, Oberai PC, et al. Age and Comorbidities Are Effect Modifiers of Gender Disparities in Renal Transplantation. J Am Soc Nephrol. 2009 doi: 10.1681/ASN.2008060591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abecassis M, Bridges ND, Clancy CJ, et al. Solid-Organ Transplantation in Older Adults: Current Status and Future Research. Am J Transplant. 2012;12(10):2608. doi: 10.1111/j.1600-6143.2012.04245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garonzik-Wang JM, Govindan P, Grinnan JW, et al. Frailty and delayed graft function in kidney transplant recipients. Arch Surg. 2012;147(2):190. doi: 10.1001/archsurg.2011.1229. [DOI] [PubMed] [Google Scholar]
- 28.Knight EL, Ofsthun N, Teng M, Lazarus JM, Curhan GC. The association between mental health, physical function, and hemodialysis mortality. Kidney Int. 2003;63(5):1843. doi: 10.1046/j.1523-1755.2003.00931.x. [DOI] [PubMed] [Google Scholar]
- 29.Lowrie EG, Curtin RB, LePain N, Schatell D. Medical outcomes study short form-36: a consistent and powerful predictor of morbidity and mortality in dialysis patients. Am J Kidney Dis. 2003;41(6):1286. doi: 10.1016/s0272-6386(03)00361-5. [DOI] [PubMed] [Google Scholar]
- 30.Johansen KL, Painter P, Kent-Braun JA, et al. Validation of questionnaires to estimate physical activity and functioning in end-stage renal disease. Kidney Int. 2001;59(3):1121. doi: 10.1046/j.1523-1755.2001.0590031121.x. [DOI] [PubMed] [Google Scholar]
- 31.Centers for Medicare and Medicaid Services. Clinical Performance Measures (CPM) Project. URL: http://www.cms.gov/Medicare/End-Stage-Renal-Disease/CPMProject/index.html?redirect=/cpmproject.
- 32.Tonelli M, Sacks F, Pfeffer M, et al. Biomarkers of inflammation and progression of chronic kidney disease. Kidney Int. 2005;68(1):237. doi: 10.1111/j.1523-1755.2005.00398.x. [DOI] [PubMed] [Google Scholar]
- 33.Workeneh BT, Mitch WE. Review of muscle wasting associated with chronic kidney disease. Am J Clin Nutr. 2010;91(4):1128S. doi: 10.3945/ajcn.2010.28608B. [DOI] [PubMed] [Google Scholar]
- 34.McHorney CA, Haley SM, Ware JE., Jr Evaluation of the MOS SF-36 Physical Functioning Scale (PF-10): II. Comparison of relative precision using Likert and Rasch scoring methods. J Clin Epidemiol. 1997;50(4):451. doi: 10.1016/s0895-4356(96)00424-6. [DOI] [PubMed] [Google Scholar]
- 35.United Network for Organ Sharing. [Accessed: 06/15/2011];Data collection. URL: http://www.unos.org/donation/index.php?topic=data_collection. [Google Scholar]
- 36.Wolfe RA, McCullough KP, Schaubel DE, et al. Calculating life years from transplant (LYFT): methods for kidney and kidney-pancreas candidates. Am J Transplant. 2008;8(4 Pt 2):997. doi: 10.1111/j.1600-6143.2008.02177.x. [DOI] [PubMed] [Google Scholar]
- 37.Locke JE, Segev DL, Warren DS, Dominici F, Simpkins CE, Montgomery RA. Outcomes of kidneys from donors after cardiac death: implications for allocation and preservation. Am J Transplant. 2007;7(7):1797. doi: 10.1111/j.1600-6143.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- 38.Merion RM, Ashby VB, Wolfe RA, et al. Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA. 2005;294(21):2726. doi: 10.1001/jama.294.21.2726. [DOI] [PubMed] [Google Scholar]
- 39.Organ Procurement and Transplantation Network. [Accessed, 06/08/2011];Concepts for Kidney Allocation. Release Date: February 16, 2011. URL: http://optn.transplant.hrsa.gov/SharedContentDocuments/KidneyConceptDocument.PDF.
- 40.Metzger RA, Delmonico FL, Feng S, Port FK, Wynn JJ, Merion RM. Expanded criteria donors for kidney transplantation. Am J Transplant. 2003;3(Suppl 4):114. doi: 10.1034/j.1600-6143.3.s4.11.x. [DOI] [PubMed] [Google Scholar]
- 41.Scientific Registry of Transplant Recipients. Guide to the Program-Specific Reports v 13.5. 2011 Jun; URL: http://www.srtr.org/csr/current/all_csr_documentation.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.