Abstract
NY-ESO-1, a cancer testis antigen, is an ideal target for adoptive cell transfer immunotherapy. Evidence from several clinical trials in melanoma and other malignancies shows the potential value of targeting the NY-ESO-1 antigen in immune-based therapy of metastatic tumors. However, the incidence of NY-ESO-1 expression in metastatic melanoma is unknown, and thus it is unclear how many patients might benefit from this therapy. In this study, we analyzed NY-ESO expression in 222 melanoma specimens, including 16 primary and 206 metastatic tumors. Our results support previous findings showing higher expression of NY-ESO-1 in metastatic (58/206; 28.2%) versus primary (0/16) tumors. In addition, our results show that the epithelioid subtype of melanoma has the highest incidence of NY-ESO-1 expression. These findings provide evidence of the value of this specific adoptive cell transfer therapy for the treatment of metastatic melanoma.
Keywords: NY-ESO-1, Melanoma, Cancer testis antigen, Immunotherapy
1. Introduction
The diagnosis of melanocytic lesions is challenging because of their diverse morphology and similarity to many poorly differentiated metastatic malignancies. Diagnosis is primarily based on histopathologic analysis and ancillary studies such as immunohistochemistry of melanocytic markers [1]. New York esophageal squamous cell carcinoma-1 (NY-ESO-1), a member of the cancer testis antigen (CTA) family, has been suggested as a prognostic marker of advanced-stage disease because of its higher expression in metastatic than in primary melanomas [2].
Studies examining the diagnostic role of CTAs are ongoing, so far with discordant data. However, it has been suggested that expression of CTAs may be useful in differentiating between melanoma and benign melanocytic lesions with atypical features [3].
Immunotherapy is a promising therapeutic option for patients with metastatic melanoma. Cancer testis antigens have been studied as potential therapeutic targets and/or diagnostic/prognostic markers in melanoma and other malignant lesions [2–6]. Humoral and cellular responses to NY-ESO-1 have been analyzed extensively in patients with various cancers, such as gastrointestinal, hematogenous, and skin malignancies as well as ovarian and urothelial carcinomas [7–10].
The adoptive transfer of cultured melanoma-reactive T cells, isolated from autologous tumor-infiltrating lymphocytes (TILs) after lymphodepletion chemotherapy, mediates objective tumor regression in 49% to 72% of patients with metastatic melanoma [11,12]. By using genetically modified lymphocytes targeting a specific tumor marker, adoptive cell transfer has proved a highly effective treatment for patients with metastatic melanomas [13]. Rosenberg and coworkers from our institution [4,14] have also published findings that T cell receptor–based gene therapies targeting NY-ESO-1 represent a new and effective therapeutic approach for patients with metastatic melanoma. This study confirms NY-ESO-1 as an immunogenic tumor marker that could play an important role in the treatment of metastatic melanoma. The immunohistochemical staining profiling of these tumors is critical for patient selection and potential enrollment in targeted adoptive cell-transfer immunotherapy clinical trials.
In this study, we characterized the expression of NY-ESO-1 in primary and metastatic melanomas, including melanomas of various morphologic subtypes. To our knowledge, this study is one of the largest evaluating NY-ESO-1 expression in metastatic melanomas of different morphologies.
2. Materials and methods
2.1. Case selection
A series of 16 primary and 206 metastatic melanoma specimens from 186 patients were retrieved from the archives of the Laboratory of Pathology at the National Cancer Institute (NCI) between April 2008 and April 2009. All studies were carried out in accordance with the approved guidelines of the NCI Institutional Review Board. Cases with available paraffin blocks or unstained slides were selected, and all were reviewed histologically to confirm the diagnosis. The available clinicopathologic information of the primary melanomas is summarized in Table 1. For the 16 primary cancers, the median thickness was 3.12 mm (mean 4.93 mm; range 0.0–25.0 mm). Of these tumors, 2 (12.5%) were <1.0 mm in Breslow thickness (defined as thin), 8 (50.0%) were 1.01–4.0 mm thick (intermediate), and 6 (37.5%) were >4.0 mm thick. Ten tumors (62.5%) were axial, and 6 (37.5%) were on the extremities. The average patient age was 53.1 years with a male predominance (male-to-female ratio 2.2:1.0). Histologically, 5 (31.3%) of these primary melanomas were of the superficial spreading type, 7 (43.8%) were nodular, and 1 (6.3%) each were acral lentiginous, mucosal lentiginous, and lentigo maligna type. There was one case classified as T0 (MMIS, malignant melanoma in situ) and one case as T1b. Five cases were T3a, three cases T3b, and six T4b. The distribution of tumor stage among the cases was as follows: stage 0 (n = 1), stage II (n = 6), stage III (n = 3), and stage IV (n = 6).
Table 1.
Clinicopathologic features of primary melanoma
| Case | Age/Sex | Site | Thickness (mm) | Ulceration | Clark level | Subtype | Margin | Cytology | Growth | TIL | LVI | S | PNI | TNM | Stage | Metastasis
|
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | NY-ESO-1 | ||||||||||||||||
| 1 | 29/F | Back | 3.25 | − | IV | Nodular | + | Epith | Vert | − | − | − | − | T3aNxM1 | IV | Lungs | Pos |
| 2 | 77/M | L forearm | 0.5 | + | I | Super spread | + | Epith | Vert | − | − | − | − | T1bN2M0 | III | R neck LN | Neg |
| 3 | 58/M | Scalp | 3 | − | IV | Super spread | + | Epith | Vert | − | − | − | − | T3aN2M0 | III | Occipital LN | Neg |
| 4 | 32/M | L thigh | 4.6 | + | IV | Nodular | NA | Epith | Vert | − | + | − | − | T4bNxM1 | IV | Colon | Pos |
| 5 | 58/F | R thigh | 4.2 | + | IV | Nodular | + | Epith | Vert | − | − | − | − | T4bNxM1 | IV | R thigh | Neg |
| 6 | 77/F | Anus | 5 | + | IV | Mucosal lentiginous | + | Epith | Vert | + | NA | NA | NA | T4bN3M1 | IV | Rectum, LN | Neg |
| 7 | 29/F | Back | 3.25 | − | IV | Nodular | + | Epith | Vert | − | − | − | − | T3aNxM1 | IV | R chest wall | Pos |
| 8 | 52/M | Plantar foot | 4.5 | + | V | Acral lentiginous | + | Epith | Vert | − | − | − | + | T4bNxMx | II | -- | -- |
| 9 | 55/F | L leg | 3 | + | IV | Nodular | − | Epith | Vert | − | − | − | − | T3bNxMx | II | -- | -- |
| 10 | 61/M | L cheek | 13 | + | V | Lentigo maligna | + | Epith, spindle | Vert | + | NA | NA | NA | T4bNxMx | II | -- | -- |
| 11 | 51/M | L flank | 2.8 | + | IV | Nodular | + | Epith | Vert | + | − | − | − | T3bNxMx | II | -- | -- |
| 12 | 32/M | Back | 25 | + | IV | Super spread | − | Spindle | Vert | − | − | + | − | T4bNxMx | II | -- | -- |
| 13 | 63/M | L arm | 2.2 | + | IV | Nodular | NA | Epith | NA | NA | NA | NA | NA | T3bNxM1 | IV | Jejunum | Neg |
| 14 | 59/M | R upper back | 2.3 | − | IV | Super spread | − | Balloon | Vert | + | + | − | − | T3aNxMx | II | -- | -- |
| 15 | 51/M | R buttock | MMIS | − | NA | NA | NA | Epith | NA | NA | NA | NA | NA | T0 | 0 | -- | -- |
| 16 | 65/M | R upper back | 2.3 | − | IV | Super spread | − | Epith | Vert | + | + | − | − | T3aN2Mx | III | Supraclavicular LN | Neg |
NOTE. All cases showed >1 mitoses/mm2.
Abbreviations: TIL, tumor-infiltrating lymphocytes; LVI, lymphovascular invasion; S, satellitosis; PNI, perineural invasion; F, female; M, male; L, left; R, right; Epith, epithelioid; LN, lymph nodes; MMIS, malignant melanoma in situ; Spread, spreading; Super, superficial; Vert, vertical; NA, not available.
2.2. Classification of the lesions
All cases were classified into morphologic subtypes, namely, epithelioid, spindle cell, and mixed (epithelioid and spindle). Tumors with anaplastic, balloon cell, or desmoplastic features were specially annotated. Lesions had to have more than 50% of the tumor cells of either epithelioid or spindle cell morphology to be classified into either category. Lesions without predominant cell morphology were classified as mixed (epithelioid and spindle). The classification of cell morphology subtype was performed and agreed on by two pathologists (Y.C.L. and C.C.L.).
2.3. Immunohistochemical staining analysis
The primary antibodies, their sources, and the dilutions used were as follows:
NY-ESO-1 (Invitrogen, Carlsbad, CA, 1;100);
MART1 (Cell Marque, Rocklin, CA, #CMC756, 1:200);
Tyrosinase (Novocastra Division, Leica Microsystems, IL, #NCL-TYROS, 1:20); [AU: Where is this located in IL?]
HMB45 (Enzo Life Sciences, Farmingdale, NY, #30930, 1:4);
S100 (BioGenex, Fremont, CA, 1:8000).
Paraffin-embedded tissue sections of 5 mm were deparaffinized through xylene and graded alcohols. Immunohistochemical staining was performed following heat-induced epitope retrieval with target retrieval solution (low pH; DAKO). Slides were incubated in Tris with 3% goat serum for 15 min and then incubated at room temperature with primary antibody for 1–2 h. Detection was carried out using an automated slide stainer (Autostainer; DAKO) with a horseradish peroxidase/3,3′-diaminobenzidine polymer-based detection system (Envision +; DAKO). The immunohistochemical staining was classified as positive or negative by two pathologists (Y.C.L. and C.L.L.). Red chromogen was used in heavily pigmented melanomas. For cases with limited unstained material, the missing results were classified as unknown.
2.4. Statistics
The JMP 5.1 software (SAS, NC) was used for the statistical analyses. The χ2 test was used to characterize the immunohistochemical results. P < .05 was considered statistically significant.
3. Results
3.1. Melanoma-associated marker expression in primary and metastatic melanoma
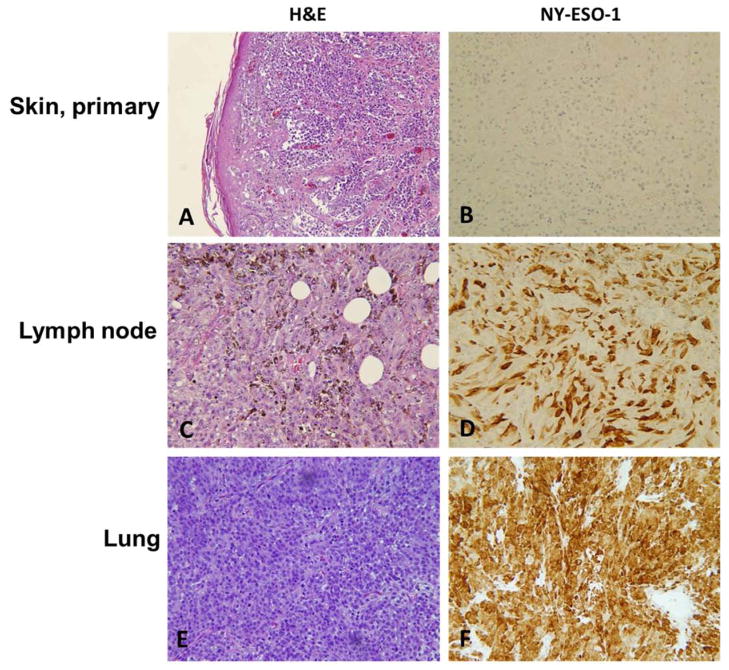
The NY-ESO-1 stain was negative in all the primary melanomas and positive in 58 (28.2%) of the metastatic melanomas (Table 2). In comparison, expression of S100 was detected in all primary and 201 (98.5%) of the metastatic melanoma specimens. Thus NY-ESO-1 is more likely to be expressed in metastatic than in primary lesions. The immunohistochemical staining patterns of NY-ESO-1 in primary and metastatic malignant melanomas are shown in Fig. 1. The representative metastatic malignant melanomas demonstrate strong cytoplasmic staining for NY-ESO-1 (Fig. 1D–F).
Table 2.
Expression of NY-ESO-1 and S100 in primary and metastatic melanoma
| Primary (n = 16) | Metastatic (n = 206) | Total (n = 222) | |
|---|---|---|---|
| S100 | |||
| Positive | 16 | 201 | 217 |
| Negative | 0 | 3 | 3 |
| Unknown | 0 | 2 | 2 |
| Sensitivity | 1.0 | 0.99 | 0.99 |
| NY-ESO-1 | |||
| Positive | 0 | 58 | 58 |
| Negative | 16 | 148 | 164 |
| Unknown | 0 | 0 | 0 |
| Sensitivity | 0 | 0.28 | 0.26 |
Fig. 1.
Representative examples of expression of NY-ESO-1 in primary and metastatic melanomas. A, Primary cutaneous melanoma (H&E). B, Negative expression in primary cutaneous melanoma. C, Metastatic melanoma in lymph node (H&E). D, NY-ESO-1 expression in metastasis in lymph node. E, Metastasis in lung (H&E). F, NY-ESO-1 expression in lung metastasis. A–C and E, ×10. D and F, ×20.
NY-ESO-1 positivity was found in metastases in almost all the organ sites evaluated (Table 3). However, metastases to the brain (n = 2), breast (n = 2), salivary gland (n = 1), and ureter (n = 1) were all negative for NY-ESO-1. The clinical significance of the finding is uncertain because of the small number of cases.
Table 3.
Expression of NY-ESO-1 in primary and metastatic melanoma lesions from various organ sites
| Positive | Negative | Total | |
|---|---|---|---|
| Primary | |||
| Skin | 0 | 15 | 15 |
| Rectum/anus | 0 | 1 | 1 |
| Metastasis | |||
| Lymph node | 19 | 49 | 68 |
| Skin | 5 | 35 | 40 |
| Dermis | 2 | 13 | 15 |
| Dermis/subcutis | 3 | 17 | 20 |
| Subcutis | 0 | 5 | 5 |
| Soft tissue | 10 | 26 | 36 |
| Lung | 7 | 12 | 19 |
| GI | 6 | 11 | 17 |
| Stomach | 0 | 1 | 1 |
| Small bowel | 5 | 10 | 15 |
| Colon | 1 | 0 | 1 |
| Gallbladder | 1 | 0 | 1 |
| Liver | 3 | 3 | 6 |
| Muscle | 1 | 2 | 3 |
| Brain | 0 | 2 | 2 |
| Spleen | 2 | 1 | 3 |
| Adrenal gland | 1 | 1 | 2 |
| Bone | 1 | 1 | 2 |
| Ovary | 1 | 1 | 2 |
| Breast | 0 | 2 | 2 |
| Omentum | 1 | 0 | 1 |
| Salivary gland | 0 | 1 | 1 |
| Ureter | 0 | 1 | 1 |
| Total | 58 | 164 | 222 |
3.2. NY-ESO-1 expression in melanoma of different morphologies
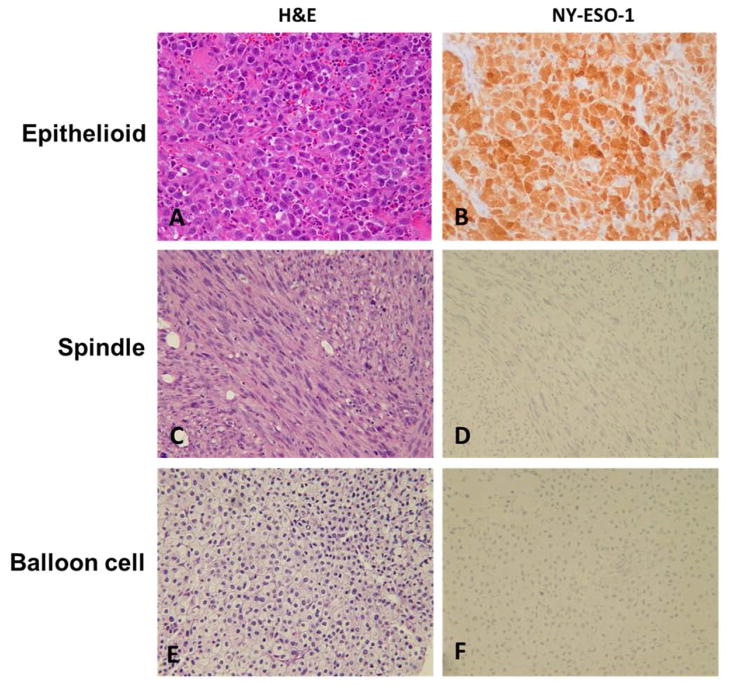
Most of the cases could be classified into one of the more common morphologic subtypes, epithelioid (n = 185; 83.3%) or spindle cell (n = 23; 10.4%). The remaining cases showed either a mixed or a balloon cell–type morphology (n = 14; 6.4%). Expression of NY-ESO-1 was seen in 32.6% of the metastatic melanomas of epithelioid morphology (n = 56) and 9.1% of the lesions of the spindle cell subtype (n = 2; Table 4). Although the sample size for tumors with spindle morphology is relatively low, the difference in the NY-ESO-1 expression between these two morphologic subtypes is statistically significant (P = .02). This result suggests that NY-ESO-1 expression is more likely to be associated with metastatic melanoma of epithelioid morphology. Fig. 2 shows positive staining for NY-ESO-1 in lesions of various morphologies.
Table 4.
NY-ESO-1 expression in malignant melanoma lesions according to morphology
| Morphology | NY-ESO-1 expression
|
|||
|---|---|---|---|---|
| Primary (%) | Metastatic (%) | Total (%) | ||
| Melanoma | ||||
| Epithelioid | 13 (65.0) | 172 (83.5) | 185 (83.3) | |
| With anaplastic features | 1 (50) | 5 (2.4) | ||
| Spindle | 1 (20.0) | 22 (10.7) | 23 (10.4) | |
| With desmoplastic features | 0 | 3 (1.5) | ||
| Mixed | 1 (6.25) | 8 (3.9) | 9 (4.1) | |
| Balloon | 1 (6.25) | 4 (1.9) | 5 (2.3) | |
| Total | 16 | 206 | 222 | |
| Metastatic melanoma | Positive (%) | Negative (%) | Total (%) | |
| Epithelioid | 56 (32.6) | 116 (67.4) | 172 | |
| With anaplastic features | 2 (1.1) | 3 | ||
| Spindle | 2 (9.1) | 20 (90.9) | 22 | |
| With desmoplastic features | 2 (9.1) | 1 | ||
| Mixed | 0 | 8 (100) | 8 | |
| Balloon | 0 | 4 (100) | 4 | |
| Total | 58 | 148 | 206 | |
| Metastatic melanoma excluding multiple specimens from same patient | ||||
| Epithelioid | 37 (30.8) | 83 (69.2) | 120 | |
| With anaplastic features | 2 (1.7) | 3 | ||
| Spindle | 2 (10.5) | 17 (89.5) | 19 | |
| With desmoplastic features | 2 (10.5) | 1 | ||
| Mixed | 0 | 7 (100) | 7 | |
| Balloon | 0 | 2 (100) | 2 | |
| Total | 39 | 109 | 148 | |
NOTE. Mixed is epithelioid and spindle cell subtypes.
Fig. 2.
Representative examples of NY-ESO-1 expression in metastatic melanoma of various morphologies (×20). A, Epithelioid subtype (H&E). B, Expression in epithelioid subtype. C, Spindle cell subtype (H&E). D, Negative expression in spindle cell subtype. E, Balloon cell subtype (H&E). F, Negative expression in balloon cell subtype.
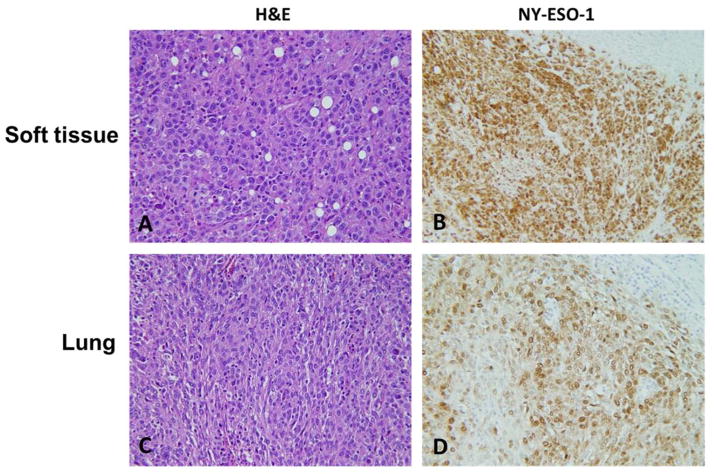
Samples used for the above analyses (n = 226) were collected from a total of 186 patients. Among them, 47 patients had a series of specimens included. Expression of NY-ESO-1 was consistent in 80.0% of the specimens from the same patient (Fig. 3A–D).
Fig. 3.
Representative examples of immunoexpression of NY-ESO-1 in paired specimens from different metastatic sites in same patient (×20). A, Metastasis in soft tissue (H&E). B, Metastasis in soft tissue positive for NY-ESO-1. C, Metastasis in lung (H&E). D, Metastasis in lung positive for NY-ESO-1.
In view of the potential overrepresentation bias introduced by incorporating multiple specimens from the same patient, we evaluated the NY-ESO-1 expression after excluding the additional specimens from the same patient. Positivity was still found to be associated with metastatic melanoma of epithelioid morphology. This finding is the same as the observation we made by analyzing all specimens (Table 4).
3.3. NY-ESO-1 Expression in paired primary and metastatic lesions from the same patient
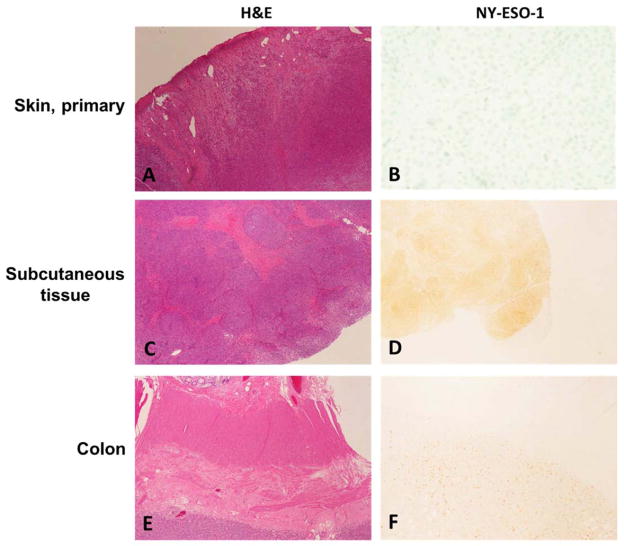
Nine of the cases in our series were paired primary and metastatic tumor samples from the individual patients. Whereas 6 of the cases showed negative NY-ESO-1 staining in both primary and metastatic lesions, the remaining 3 paired samples showed negative staining in the primary lesion (Fig. 4A and B) but positive staining in the metastatic lesions (Fig. 4C–F).
Fig. 4.
Representative examples of NY-ESO-1 expression in paired primary and metastatic lesions from the same patient. A, Primary cutaneous melanoma (H&E). B, Negative NY-ESO-1 expression in primary tumor (NY-ESO-1). C, Metastasis in subcutaneous tissue (H&E), D, Expression of NY-ESO-1 in metastasis in subcutaneous tissue. E, Metastasis in colon (H&E). F, Expression of NY-ESO-1 in metastasis in colon. A, C and E, ×4; B, D and F, ×10.
3.4. NY-ESO-1 expression in metastatic melanomas with rhabdoid and desmoplastic features
We analyzed the expression of NY-ESO-1 in 8 metastatic melanomas with rhabdoid features. Five primary lesions corresponding to the metastatic rhabdoid lesions were available for review. Intriguingly, none of the 5 primary lesions had recognizable rhabdoid morphology. Traditional markers, namely MART-1/Melan-A, and tyrosinase, were highly sensitive in detecting metastatic melanomas with rhabdoid features (S100 100%, MART-1/Melan-A 100%, tyrosinase 88.9%), but not HMB45 (45.8%). NY-ESO-1 was expressed in 37.5% of the metastatic lesions with rhabdoid features (n = 8). Among 5 metastases with desmoplastic features, NY-ESO-1 was positive in 50%. Further studies with a larger number of samples of the rare morphologic subtypes are required to confirm these findings.
4. Discussion
Cancer testis antigens (CTAs) have tissue-restricted expression to germ-line cells and frequent ectopic expression in a variety of tumors [15]. The nearly exclusive expression of CTAs in normal germ-line tissues makes them attractive therapeutic targets because germ cells do not express class I MHC molecules [15]. Moreover, CTAs are expressed in many cancers, especially in diseases of advanced stage, and immunotherapeutic vaccines targeting these antigens have shown variable effects in different malignancies [15,16]. To date, only a few CTAs (of which more than 40 have been identified) elicit both humoral and cell-mediated immune responses in humans, including SSX, melanoma-associated antigen (MAGE) A, and NY-ESO-1. These proteins are currently the most promising CTA targets for tumor immunotherapy and are expressed in a large variety of tumor types, including melanoma and carcinomas of the bladder, lung, liver, breast, ovary, and synovial cells with a frequency of 30%–80% [17]. Expression of certain CTAs is also a prognostic marker in specific types of cancers. The expression of MAGE-A adapter proteins involved in the p53 pathway has been associated with development of a malignant phenotype and worse clinical outcomes [18]. Among the synovial sarcoma X (SSX) chromosome breakpoint family of CTAs, SSX-2 mRNA expression has been associated with a worse prognosis in several types of cancer [19]. Clinical trials using MAGE-A3 and NY-ESO-1 as tumor targets are under way.
Lüftl and colleagues [3] reported high expression of CTAs in primary melanomas compared with benign melanocytic proliferations, suggesting their diagnostic utility in the differential diagnosis of benign and malignant melanocytic lesions. Those investigators performed immunohistochemistry studies using monoclonal antibodies against MAGE-A1, MAGE-A4, NY-ESO-1, MAGE-C1, MAGE-A3, and GAGE. All nevi (100%) were negative for all tested antibodies. Among 26 melanomas tested for the expression of 6 CTAs, 20 (77%) were positive for at least one. This confirms that CTAs may be useful in differentiating benign from malignant lesions. In this study, 24% (9/38) of primary melanoma cases were positive for NY-ESO-1.
T cell receptor–based gene therapies targeting NY-ESO-1 represent a novel and effective therapeutic approach for patients with metastatic melanoma [12,14]. Testing NY-ESO-1 expression is critical for patient selection, and it is also important for post-treatment monitoring. Although NY-ESO-1 expression in metastatic melanomas has been reported [4], the large sample size of our study provides a more reliable estimate of the prevalence of NY-ESO-1 expression in such tumors. Our study showed that NY-ESO-1 protein is more likely to be expressed in metastatic than in primary melanomas. This finding justifies the use of NY-ESO-1–targeted immune therapy for patients with metastatic disease but not patients with localized disease. In addition, in the paired primary and metastatic sample sets in our study, NY-ESO-1 positivity was seen in the metastatic lesions but not their primary counterparts in 33.3% of the paired samples, with the 3 cases being Stage IV (2 T3aNxM1 and 1 T4bNxM1). The finding of increased NY-ESO-1 expression in metastatic lesions of advanced stage compared with primary lesions, even in the limited number of paired samples, suggests that NY-ESO-1 expression is a marker for lesions with advanced stage/poor prognosis, as previously mentioned [2]. In the same previous study, NY-ESO-1 immunoexpression was seen in 18 of 56 metastatic (32%) and 8 of 61 primary (13%) melanoma cases. In our study, 77% of metastatic and none of the primary melanomas were positive for NY-ESO-1. Although the absolute numbers differ, perhaps because of differences in sample size, the finding that NY-ESO-1 expression is present in a higher percentage of metastatic versus primary lesions is in agreement in the two studies. We also identified that NY-ESO-1 expression is closely associated with the epithelioid subtype of metastatic melanomas but also is seen in a small subset of metastatic melanomas with spindle, rhabdoid, and desmoplastic features. This result suggests that patients with metastatic melanoma of epithelioid morphology are more likely to benefit from NY-ESO-1 adoptive cell-transfer therapy.
Footnotes
Disclosure: The authors declare that they have no conflicting financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ivan D, Prieto VG. Use of immunohistochemistry in the diagnosis of melanocytic lesions: applications and pitfalls. Future Oncol. 2010;6:1163–75. doi: 10.2217/fon.10.81. [DOI] [PubMed] [Google Scholar]
- 2.Velazquez EF, Jungbluth AA, Yancovitz M, et al. Expression of the cancer/testis antigen NY-ESO-1 in primary and metastatic malignant melanoma (MM): correlation with prognostic factors. Cancer Immun. 2007;7:11. [PMC free article] [PubMed] [Google Scholar]
- 3.Lüftl M, Schuler G, Jungbluth AA. Melanoma or not? Cancer testis antigens may help. Br J Dermatol. 2004;151:1213–8. doi: 10.1111/j.1365-2133.2004.06260.x. [DOI] [PubMed] [Google Scholar]
- 4.Robbins PF, Morgan RA, Feldman SA, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29:917–24. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai JP, Robbins PF, Raffeld M, et al. NY-ESO-1 expression in synovial sarcoma and other mesenchymal tumors: significance for NY-ESO-1-based targeted therapy and differential diagnosis. Mod Pathol. 2012;25:854–8. doi: 10.1038/modpathol.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawada J, Wada H, Isobe M, et al. Heteroclitic serological response in esophageal and prostate cancer patients after NY-ESO-1 protein vaccination. Int J Cancer. 2012;130:584–92. doi: 10.1002/ijc.26074. [DOI] [PubMed] [Google Scholar]
- 7.de Carvalho F, Vettore AL, Inaoka RJ, et al. Evaluation of LAGE-1 and NY-ESO-1 expression in multiple myeloma patients to explore possible benefits of their homology for immunotherapy. Cancer Immun. 2011;11:1. [PMC free article] [PubMed] [Google Scholar]
- 8.Walter A, Barysch MJ, Behnke S, et al. Cancer-testis antigens and immunosurveillance in human cutaneous squamous cell and basal cell carcinomas. Clin Cancer Res. 2010;16:3562–70. doi: 10.1158/1078-0432.CCR-09-3136. [DOI] [PubMed] [Google Scholar]
- 9.Gnjatic S, Cao Y, Reichelt U, et al. NY-CO-58/KIF2C is overexpressed in a variety of solid tumors and induces frequent T cell responses in patients with colorectal cancer. Int J Cancer. 2010;127:381–93. doi: 10.1002/ijc.25058. [DOI] [PubMed] [Google Scholar]
- 10.Chen YT, Chadburn A, Lee P, et al. Expression of cancer testis antigen CT45 in classical Hodgkin lymphoma and other B-cell lymphomas. Proc Natl Acad Sci USA. 2010;107:3093–8. doi: 10.1073/pnas.0915050107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–9. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21:233–40. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodi FS, Fisher DE. Adoptive transfer of antigen-specific CD4+ T cells in the treatment of metastatic melanoma. Nat Clin Pract Oncol. 2008;5:696–7. doi: 10.1038/ncponc1259. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–7. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scanlan MJ, Simpson AJ, Old LJ. The cancer/testis genes: review, standardization, and commentary. Cancer Immun. 2004;4:1. [PubMed] [Google Scholar]
- 16.Jäger D, Jäger E, Knuth A. Immune responses to tumour antigens: implications for antigen-specific immunotherapy of cancer. J Clin Pathol. 2001;54:669–74. doi: 10.1136/jcp.54.9.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scanlan MJ, Güre AO, Jungbluth AA, et al. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol Rev. 2002;188:22–32. doi: 10.1034/j.1600-065x.2002.18803.x. [DOI] [PubMed] [Google Scholar]
- 18.Feng Y, Gao J, Yang M. When MAGE meets RING: insights into biological functions of MAGE proteins. Protein Cell. 2011;2:7–12. doi: 10.1007/s13238-011-1002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith HA, McNeel DG. The SSX family of cancer-testis antigens as target proteins for tumor therapy. Clin Dev Immunol. 2010;2010:150591. doi: 10.1155/2010/150591. [DOI] [PMC free article] [PubMed] [Google Scholar]