Abstract
Objective
The effect of weight loss by diet or diet and exercise on salivary cortisol levels, a measure of hypothalamic pituitary adrenal activity, in overweight individuals is not known. To test the hypothesis that 24 weeks of moderate caloric restriction (CR) (25%) by diet or diet and aerobic exercise would alter morning and diurnal salivary cortisol levels.
Design and Setting
Randomized control trial in an institutional research center.
Participants
Thirty-five overweight (BMI:27.8±0.7kg/m2) but otherwise healthy participants (16M/19F).
Intervention
Participants were randomized to either calorie restriction (CR: 25% reduction in energy intake, n=12), calorie restriction+exercise (CR+EX: 12.5% reduction in energy intake+12.5% increase in exercise energy expenditure, n=12) or control (healthy weight-maintenance diet, n=11) for 6 months.
Main outcome measure
Salivary cortisol measured at 8:00, 8:30, 11:00, 11:30, 12:30, 1300, 16:00 and 16:30. Morning cortisol was defined as the mean cortisol concentration at 08:00 and 08:30. Diurnal cortisol was calculated as the mean of the 8 cortisol measures across the day.
Results
In the whole cohort, higher morning and diurnal cortisol levels were associated with impaired insulin sensitivity (morning: P=0.004, r2=0.24; diurnal: P=0.02, r2=0.15). Using mixed model analysis, there was no significant effect of group, time or sex on morning or diurnal cortisol levels.
Conclusion
A 10% weight loss with a 25% CR diet alone or with exercise did not impact morning or diurnal salivary cortisol levels.
Keywords: cortisol, obesity, calorie restriction, weight loss
Introduction
The hypothalamic pituitary adrenal (HPA) axis is an auto-regulating system with numerous modulatory functions including the secretion of cortisol from the adrenal glands. In healthy individuals, this central regulation results in a distinct diurnal rhythm, with high cortisol activity in the early morning and low activity in the afternoon/evening. Stress (physiological, social or physical) can cause disturbances in the HPA axis resulting in either elevated circulating cortisol levels or a blunted cortisol diurnal pattern. Such alterations in cortisol patterns have been observed with aging and obesity [1-3] as well as in athletes [4] and in patients with anorexia nervosa [5].
Caloric restriction (CR) increases health span and lifespan in most animal species and has been advocated as an anti-aging intervention in humans [6]. Given that excess weight and energy restricted conditions are separately associated with increased cortisol levels, the aims of this study were to test that 6 months of CR would a) decrease mean cortisol levels and diurnal cortisol variability and b) these decreases in cortisol levels would be associated with changes in body weight and composition and cardio-metabolic parameters. This was examined in frequently measured saliva samples obtained from young, overweight subjects that participated in the Pennington CALERIE study, the first randomized control trial of calorie restriction in humans, which was originally designed to examine whether CR improved biomarkers of longevity. The study population and metabolic outcomes and other results from the study have been extensively described [7;8].
Methods
The Comprehensive Assessment of Long-Term Effects of Reducing Intake of Energy (CALERIE) randomized control trial was approved by the Pennington Biomedical Research Center (PBRC) Institutional Review Board and subjects provided written, informed consent. Forty-six healthy, overweight (25≤BMI<30) men (n=20) and women (n=26) completed the study. Details of the screening process and the study population have been previously described [7].
Subjects were enrolled and randomized into one of four groups for 24 weeks: 1) Control= weight maintenance diet based on the American Heart Association Step 1 diet; 2) CR= 25% caloric restriction of energy expenditure (EE) requirements, 3) CR+ exercise (EX)= 12.5% caloric restriction and 12.5% increased EE by structured, supervised aerobic exercise sessions and 4) low calorie diet (LCD)= LCD until achievement of 15% weight loss followed by weight maintenance. Since the goal of the LCD treatment group was to achieve a specific weight loss followed by weight maintenance (and not to achieve a specific level of CR), this group was excluded from the current analysis. Details of the intervention have been extensively described [7].
All assessments were performed at baseline and at week 24 over 5-day inpatient stays in the Inpatient Unit of PBRC [7]. Body composition was measured using dual x-ray absorptiometry (Hologics QDR 4500A, Bedford, MA) and abdominal fat distribution by multi-slice computed tomography (GE Light Speed, General Electric, Milwaukee, WI). Insulin sensitivity (Si) and the acute insulin response to glucose (AIRg) were determined by the insulin-modified frequently sampled intravenous glucose tolerance test. Fasting blood samples were taken for the measurement of insulin, glucose, lipids, IGF1, T3 and T4 and leptin [7].
To assess diurnal cortisol dynamics at baseline and after 24 weeks of CR, frequent saliva samples were collected during the 4th inpatient day. Samples were collected at 8:00 and 8:30, 11:00, 11:30, 12:30, 1300, 16:00 and 16:30. Standard meals, calculated on the basis of calorie levels, were consumed at 09:00 (breakfast) and 11:30 (lunch) with water consumed ad libitum. Salivary cortisol samples were batched until the end of study and were measured by ELISA according to kit instructions (Salimetrics, State College, PA). Morning cortisol was defined as the mean cortisol concentration at 08:00 and 08:30. Diurnal cortisol was calculated as the mean of the 8 cortisol measures across the day [9]. Statistical analyses were performed using SAS 9.2 (SAS Institute Inc., Cary, NC) software and SPSS Version 20. Mixed model analysis and paired t-tests were used to examine group, sex and time-point differences in morning and diurnal cortisol levels. Associations between cortisol levels and body composition and metabolic parameters were examined using Pearsons Correlation Coefficient and Spearmans Rho for normal and not-normally distributed data, respectively. Data are presented as mean±SD and P values<0.05 considered statistically significant.
Results
Body composition, insulin sensitivity and biochemistry
As previously reported [10], body weight, fat mass and visceral adipose tissue were significantly reduced from baseline in CR and CR+EX groups (both P<0.05) compared to the control group, in which body weight and composition remained stable. Compared to controls, Si improved in the CR+EX groups (P=0.01) with a trend towards improved Si in the CR group (P=0.08) after 6 months [8] (Table 1). As previously reported [11], fasting leptin levels were significantly decreased in response to CR, independent of the type of CR; there were no significant changes in T3, T4 or IGF1 levels in any of the intervention groups.
Table 1.
Participant characteristics and morning and diurnal cortisol levels, thyroid hormone, IGF1 and leptin levels in control, CR and CR + EX groups
| Control (n=11) |
CR (n=11) |
CR+EX (n=12) |
CR & CR+EX groups combined (n=23) |
|||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Month 6 | Baseline | Month 6 | Baseline | Month 6 | Baseline | Month 6 | |
| Females/Males | 6/5 | 5/6 | 7/5 | 12/11 | ||||
| Age, y | 38.5 ± 7.4 | 39.7 ± 5.5 | 36.3 ± 5.7 | 38.1 ± 5.8 | ||||
| Weight, kg | 82.3 ± 9.5 | 81.5 ± 9.9 | 81.2 ±11.4 | 72.8 ± 10.8* | 82.1 ± 10.8 | 73.7 ± 9.6* |
80.6 ± 10.6 |
73.3 ± 10.6* |
| Fat mass, kg | 33.1 ± 1.8 | 33.2 ± 1.5 | 33.0 ± 1.5 | 31.3 ± 2.0* | 32.3 ± 3.0 | 31.2 ± 2.3* |
32.3 ± 2.0 | 31.9 ± 1.9* |
| VAT, kg | 2.9 ± 1.4 | 2.8 ± 1.4 | 3.2 ± 1.8 | 2.3 ± 1.4* | 2.8 ± 1.5 | 2.0 ± 1.1* | 2.8 ± 1.5 | 2.2 ± 1.2* |
| Insulin sensitivity, Si |
2.8 ± 1.2 | 2.5 ± 1.2 | 3.3 ± 1.6 | 4.2 ± 3.4 | 3.4 ± 1.2 | 5.3 ± 2.6* | 3.5 ± 1.7 | 4.6 ± 2.8* |
| AIRg | 750 ± 135 | 685 ± 98 | 815 ± 136 | 558 ± 71* | 729 ± 175 | 440 ± 115 * |
730.5 ± 486.6 |
526.6 ± 344.0* |
| Morning cortisol, nmol/la |
5.7 ± 2.6 | 9.0 ± 2.4* | 6.1 ± 3.3 | 7.7 ± 2.5 | 5.8 ± 2.5 | 7.6 ± 1.7 | 6.0 ± 2.9 | 7.6 ± 2.1* |
| Diurnal cortisol, nmol/lb |
4.9 ± 1.3 | 5.6 ± 1.1 | 4.5 ± 1.2 | 5.2 ± 1.3* | 4.9 ± 0.9 | 5.5 ± 1.0 | 4.7 ± 1.1 | 5.4 ± 1.1* |
| T3, ng/dl | 144 ± 26.8 | 149 ± 24.5 | 139 ± 23 | 130 ± 22 | 135 ± 21 | 120 ± 21 | 137 ± 22 | 125 ± 22 |
| T4, ng/dl | 7.2 ± 1.5 | 7.3 ± 1.5 | 7.4 ± 1.5 | 7.5 ± 1.6 | 7.4 ± 1.3 | 7.0 ± 1.2 | 7.4 ± 1.4 | 7.3 ± 1.4 |
| IGF1,μg/L | 424 ± 114 | 422 ± 110 | 420 ± 80 | 412 ± 64 | 380 ± 115 | 438 ± 124 | 399 ± 100 | 426 ± 98 |
| Fasting leptin, ng/ml |
20.8 ± 13.7 | 17.3 ± 10.9 | 15.6 ± 10.6 | 8.2 ± 6.2** | 18.8 ± 11.8 | 8.7 ± 7.3* | 17.3 ± 11.1 |
8.4 ± 6.6** |
Data are presented as mean ± SD
Morning cortisol was calculated as the mean of cortisol values at 08:00 and 08:30
Diurnal cortisol was calculated as the mean of cortisol values at 08:00, 08:30, 11:00, 11:30, 12:30, 1300, 16:00 and 16:30
Denotes the change is significantly different from baseline, P≤0.05
Abbreviations- VAT= visceral adipose tissue, AIRg= acute insulin response to glucose, CR= calorie restriction, CR+EX= calorie restriction and exercise, IGF=insulin growth factor
Note: Using mixed modelling analysis, there was no significant group (control, CR, CR+EX) , time (M0, M6) or sex effects on morning and diurnal cortisol levels.
Morning and diurnal cortisol levels
There was an equal distribution of males and females within the 3 groups and no sex differences in morning and diurnal cortisol levels at baseline. Using mixed modelling analysis, there was no significant group, time or sex effects on morning and diurnal cortisol levels.When the calorie restriction groups were combined (CR and CR+EX), morning (BL=6.0±2.9, W24=7.6±2.1nmol/l) and diurnal cortisol levels (BL=4.7±1.1, W24=5.4± 1.1nmol/l) were significant higher at week 24 compared to baseline (both P<0.01). Similar increases at week 24 were seen in the control group (BL=5.7 ± 2.6nmol/l, W24=9.0 ±2.4nmol/l; P=0.002) for morning cortisol levels.
Associations between cortisol levels and body composition and metabolic parameters
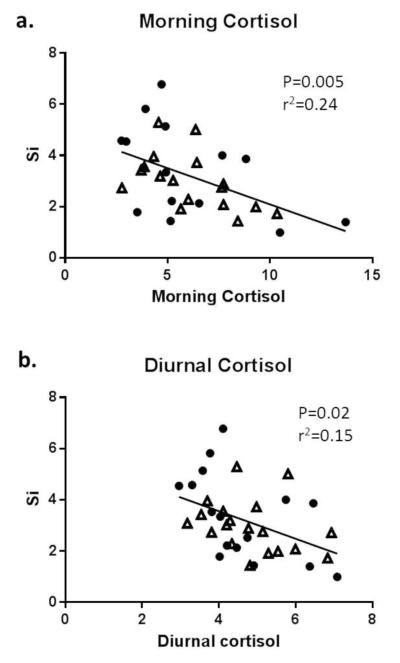
Similar to previous studies [9], morning and diurnal cortisol levels were highly correlated in the whole group (both P<0.001) before and after 24 weeks of CR. At baseline, higher morning and diurnal cortisol levels were associated with lower Si (both P<0.05) in the whole group (Figure 1). These associations were not apparent post CR. There were no significant associations between changes in Si and changes in morning and diurnal cortisol levels (24 weeks – baseline). At baseline and 24 weeks post CR, there were no associations between morning/diurnal cortisol levels and body composition (fat mass, visceral adipose tissue), or leptin levels in the whole group, or when groups were examined separately (data not shown). Next, we examined associations between morning and diurnal cortisol levels at baseline and change in body composition, insulin sensitivity, lipids and biochemistry levels in the CR and CR+EX groups combined. Interestingly, higher morning and diurnal cortisol levels at baseline were both negatively associated with change in the amount of visceral adipose tissue (both P<0.03); these correlations were not seen in the control group. No other significant associations between cortisol levels at baseline and change in body composition and metabolic parameters were observed.
Figure 1.
Associations between insulin sensitivity and a) morning and b) diurnal cortisol levels (nmol /l) in the whole cohort at baseline (circles= males, triangles= females)
Discussion
Our key findings were that higher salivary cortisol levels (morning and diurnal) were associated with impaired insulin sensitivity, and that 10% body weight loss induced with 25% sustained caloric deficit (by diet alone or diet and exercise) had little effect on salivary cortisol levels. Our finding that higher cortisol levels are associated with impaired insulin sensitivity is consistent with other studies linking higher cortisol levels with metabolic impairment independent of age and BMI [12].
Cortisol secretion is regulated by hypothalamic centers that receive stimulatory signals from the central nervous system and are modified by adrenergic, dopaminergic and serotoninergic systems in a complicated system that is only partly understood. There is also some evidence that leptin may act in a permissive manner in regulating the adrenal axis [13]. These regulatory events result in a characteristic diurnal pattern of cortisol secretion with high activity in the early morning hours and low activity in the afternoon, a pattern which is often blunted in obese individuals [2;9;14], although there is also evidence to suggest otherwise [15]. Several cross-sectional studies have demonstrated that lean individuals have higher basal cortisol and higher cortisol variability compared to obese individuals [1;9;16]. In our study, 10% weight loss (induced by CR or CR+EX) was insufficient to alter salivary cortisol levels (morning and diurnal) in overweight individuals. Interestingly, a study in obese men also found that 10% weight loss by VLCD resulted in no change in plasma cortisol concentrations but a substantial decline in cortisol production together with evidence of reduced cortisol metabolism of cortisol and cortisone, assessed by urinary cortisol metabolites [17]. Based on these findings, it may be that during calorie restriction, a decrease in peripheral cortisol clearance is associated with a compensatory fall in cortisol production in order to maintain systemic cortisol levels. Unfortunately, urinary cortisol metabolites and upstream regulators of cortisol secretion (corticotropin releasing hormone and adrenocorticotropic hormone) were not measured in the current study. The association between lower cortisol levels and visceral adiposity has similarly been demonstrated in several population-based studies [14] and may be explained by tissue-specific differences in cortisol metabolism. In obese Zucker rats, the reactivation of cortisone to cortisol by 11 -HSD1 is impaired in the liver but enhanced in adipose tissue, demonstrating tissue-specific cortisol metabolism [18]. Thus, local cortisol metabolism may potentially influence systemic cortisol concentrations. Future studies examining cortisol metabolism in visceral adipose tissue are required.
Alternatively, calorie restriction may be considered a physiological stressor resulting in increased HPA axis activity, supported by findings that energy restricted athletes have higher cortisol and ACTH secretion [4], and subjects with anorexia nervosa have higher cortisol levels [5]. Furthermore, in Biosphere 2, 8 participants underwent calorie restriction (1750-2100kcal/day) for 2 years in a closed ecological space and had significant increases in morning total cortisol levels and marginal increases in free cortisol [19].
Strengths of this study include the thorough clinical investigation of the participants and the use of frequent sampling to assess cortisol levels throughout the day. Limitations include that morning cortisol levels were not measured in ideal conditions, as subjects had already been awake for 1hour before the first blood sample was drawn. This variability in the method which cortisol is measured appears to be a recurring theme in the literature with variability in the way cortisol is measured (fasting or non-fasting conditions, within 1-4 hours of waking) and an issue to be mindful of when comparing studies [15]. Further, it should be noted that many factors can lead to acute changes in cortisol levels including physical and psychological stressors and even simple laboratory tasks (mental arithmetic, public speaking). While we made every possible attempt to create a relaxed environment subjects were being tested on a metabolic ward in a clinical research facility for consecutive days; these stressors may partially explain the increased cortisol levels at baseline in our control group. Our study also did not measure ACTH levels or 24hr urinary cortisol, which may shed further insight onto the diurnal regulation of the HPA axis.
In summary, our study found that 10% weight loss was insufficient to alter salivary morning and diurnal cortisol levels in overweight individuals. This does not exclude the possibility that peripheral cortisol clearance and/or cortisol production may be altered with weight loss. Our findings suggest that prolonged restriction of energy intake is not perceived by the body as a stressor, at least in our cohort of young, non-obese men and women, and thus CR may present a viable intervention for longevity studies in other populations.
Acknowledgments
Funding sources: This work was supported by the National Institutes of Health U01-AG20478, a NORC Center Grant 1P30 DK072476. CST is supported by NHMRC Early Career Fellowship (#1037275) and LMR is supported by R00HD060762.
Abbreviations
- ACTH
Adrenocorticotropic hormone
- AIRg
Acute insulin response to glucose
- BMI
Body Mass Index
- CALERIE
The Comprehensive Assessment of Long-Term Effects of Reducing Intake of Energy
- CR
Calorie restriction
- CR+EX
Calorie restriction + exercise
- EE
Energy expenditure
- ELISA
Enzyme-linked immunosorbant assay
- HPA
Hypothalamic pituitary adrenal
- IGF
Insulin-like growth factor
- LCD
Low calorie diet
- PBRC
Pennington Biomedical Research Center
- Si
Insulin sensitivity
- T3
Triiodothyronine
- T4
Thyroxine
- TSH
Thyroid stimulating hormone
- VLCD
Very low calorie diet
Footnotes
Author Contributions: CST, EAF, WX and LMR analysed the data, interpreted the results and wrote the paper. ER wrote the original grant for the study and contributed to interpretation of results. JR performed the cortisol analyzes in the laboratory. All authors reviewed the manuscript prior to publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Summary: The authors have nothing to disclose related to this study.
Clinical Trial Registration Number: CALERIE, NCT00099151 (www.clinicaltrials.gov)
Reference List
- 1.Travison TG, O’Donnell AB, Araujo AB, et al. Cortisol levels and measures of body composition in middle-aged and older men. Clin Endocrinol (Oxf) 2007;67:71–7. doi: 10.1111/j.1365-2265.2007.02837.x. [DOI] [PubMed] [Google Scholar]
- 2.Bjorntorp P, Rosmond R. Obesity and cortisol. Nutrition. 2000;16:924–36. doi: 10.1016/s0899-9007(00)00422-6. [DOI] [PubMed] [Google Scholar]
- 3.Bjorntorp P. Alterations in the ageing corticotropic stress-response axis. Novartis Found Symp. 2002;242:46–58. [PubMed] [Google Scholar]
- 4.Villanueva AL, Schlosser C, Hopper B, et al. Increased cortisol production in women runners. J Clin Endocrinol Metab. 1986;63:133–6. doi: 10.1210/jcem-63-1-133. [DOI] [PubMed] [Google Scholar]
- 5.Casper RC, Chatterton RT, Jr., Davis JM. Alterations in serum cortisol and its binding characteristics in anorexia nervosa. J Clin Endocrinol Metab. 1979;49:406–11. doi: 10.1210/jcem-49-3-406. [DOI] [PubMed] [Google Scholar]
- 6.Redman LM, Ravussin E. Caloric restriction in humans: impact on physiological, psychological, and behavioral outcomes. Antioxid Redox Signal. 2011;14:275–87. doi: 10.1089/ars.2010.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heilbronn LK, de Jonge L, Frisard MI, et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295:1539–48. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larson-Meyer DE, Heilbronn LK, Redman LM, et al. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006;29:1337–44. doi: 10.2337/dc05-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosmond R, Holm G, Bjorntorp P. Food-induced cortisol secretion in relation to anthropometric, metabolic and haemodynamic variables in men. Int J Obes Relat Metab Disord. 2000;24:416–22. doi: 10.1038/sj.ijo.0801173. [DOI] [PubMed] [Google Scholar]
- 10.Redman LM, Heilbronn LK, Martin CK, et al. Effect of calorie restriction with or without exercise on body composition and fat distribution. J Clin Endocrinol Metab. 2007;92:865–72. doi: 10.1210/jc.2006-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lecoultre V, Ravussin E, Redman LM. The fall in leptin concentration is a major determinant of the metabolic adaptation induced by caloric restriction independently of the changes in leptin circadian rhythms. Journal of Clinical Endocrinology & Metabolism. 2011 doi: 10.1210/jc.2011-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park SB, Blumenthal JA, Lee SY, et al. Association of cortisol and the metabolic syndrome in Korean men and women. J Korean Med Sci. 2011;26:914–8. doi: 10.3346/jkms.2011.26.7.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan SM, Hamnvik OP, Brinkoetter M, et al. Leptin as a modulator of neuroendocrine function in humans. Yonsei Med J. 2012;53:671–9. doi: 10.3349/ymj.2012.53.4.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Champaneri S, Xu X, Carnethon MR, et al. Diurnal salivary cortisol is associated with body mass index and waist circumference: The multiethnic study of atherosclerosis. Obesity (Silver Spring) 2013;21:E56–E63. doi: 10.1002/oby.20047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abraham SB, Rubino D, Sinaii N, et al. Cortisol, obesity, and the metabolic syndrome: A cross-sectional study of obese subjects and review of the literature. Obesity (Silver Spring) 2013;21:E105–E117. doi: 10.1002/oby.20083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Praveen EP, Sahoo JP, Kulshreshtha B, et al. Morning cortisol is lower in obese individuals with normal glucose tolerance. Diabetes Metab Syndr Obes. 2011;4:347–52. doi: 10.2147/DMSO.S23915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnstone AM, Faber P, Andrew R, et al. Influence of short-term dietary weight loss on cortisol secretion and metabolism in obese men. Eur J Endocrinol. 2004;150:185–94. doi: 10.1530/eje.0.1500185. [DOI] [PubMed] [Google Scholar]
- 18.Rask E, Olsson T, Soderberg S, et al. Tissue-specific dysregulation of cortisol metabolism in human obesity. J Clin Endocrinol Metab. 2001;86:1418–21. doi: 10.1210/jcem.86.3.7453. [DOI] [PubMed] [Google Scholar]
- 19.Walford RL, Mock D, Verdery R, et al. Calorie restriction in biosphere 2: alterations in physiologic, hematologic, hormonal, and biochemical parameters in humans restricted for a 2-year period. J Gerontol A Biol Sci Med Sci. 2002;57:B211–B224. doi: 10.1093/gerona/57.6.b211. [DOI] [PubMed] [Google Scholar]