Abstract
Purpose
To provide a detailed review of current clinical guidelines for the diagnosis, work-up and treatment of autoimmune retinopathy, and briefly preview possible future therapies.
Design
Perspective based on literature review and clinical expertise.
Methods
Interpretation of current literature, relying on the authors’ clinical experience.
Results
Autoimmune retinopathy is a rare immunologic disease characterized by the presence of circulating anti-retinal antibodies along with electroretinographic (ERG) and visual field abnormalities. Ophthalmic exam can be normal or show minimal findings. The diagnosis of autoimmune retinopathy is made difficult by diagnostic criteria which are both limited and non-standardized. Currently, the diagnosis is made based on the demonstration of serum antiretinal antibodies and the presence of clinical manifestations (including abnormal ERGs). The mere presence of these antibodies is not diagnostic. Lack of an accepted gold standard for antiretinal antibodies detection and poor inter-laboratory concordance makes the diagnosis challenging. There are anecdotal reports on immunosuppressive therapy in autoimmune retinopathy; however, the response to treatment is variable, with more favorable results achieved in paraneoplastic retinopathy, particularly cancer-associated retinopathy, with a combination of chemotherapy and immunosuppression. Whether an earlier attempt to treat non-paraneoplastic autoimmune retinopathy would be more beneficial is unknown. Early treatment attempts are limited by lack of sensitive and specific assays and definitive clinical criteria.
Conclusions
Little is known about the clinical course, prognosis and treatment of autoimmune retinopathy. Additional studies should examine the specificity and pathogenicity of antiretinal antibodies, screen for biomarkers, and should be conducted concurrently with studies seeking to identify appropriate treatment.
PERSPECTIVE
Introduction
Autoimmune retinopathy is an inflammatory mediated retinopathy characterized by vision loss, scotomas, visual field deficits, photoreceptor dysfunction, and the presence of circulating antiretinal antibodies. On clinical exam, the fundus usually appears unremarkable; however some patients may show retinal pigment epithelium abnormalities, vascular attenuation or optic disc pallor. There is minimal or no intraocular inflammation.1 The sine qua non of autoimmune retinopathy is the presence of circulating antiretinal antibodies which target retinal antigens and are believed to be responsible for the photoreceptor damage, though the precise mechanisms are not entirely understood.2–3 Autoimmune retinopathy can be divided into two groups: paraneoplastic and non-paraneoplastic, with paraneoplastic further subdivided into cancer-associated retinopathy (CAR) and melanoma-associated retinopathy (MAR).4 Nonparaneoplastic autoimmune retinopathy is probably more common than paraneoplastic retinopathies. CAR is more common than MAR, though the prevalence of MAR is increasing, while CAR prevalence is decreasing.5 Vision loss and photoreceptor dysfunction associated with cancer was first described by Sawyer et al. in 1976 and the term “paraneoplastic retinopathy” was coined by Klingele et al in 1984.6,7
Although it is believed to be rare, the prevalence of autoimmune retinopathy is currently unknown. It constitutes far less than 1% of all cases seen at our tertiary uveitis and ocular immunology clinic. The overlap of clinical features with other degenerative retinal disorders and lack of standardized clinical and laboratory diagnostic criteria may be contributing to an underestimation of its prevalence. In this article we will focus on pathophysiology, clinical manifestations and management of the nonparaneoplastic form of autoimmune retinopathy.
Pathophysiology
Multiple retinal proteins have been found to be antigenic, some of these are retina-specific (e.g. recoverin) and others can be found in nonretinal tissues as well (e.g. α-enolase). While recoverin, a 23kDa calcium binding protein found in photoreceptors, and α-enolase, a 48kDa ubiquitous glycolytic enzyme, are the most widely studied antigens in autoimmune retinopathy, associations with autoantibodies against carbonic anhydrase, arrestin, transducin-β, TULP1, neurofilament protein, heat shock protein-70, photoreceptor-cell-specific nuclear receptor (PNR), Müller-cell-specific antigen, transient receptor potential cation channel, subfamily M, member 1 (TRPM1) and a number of yet-unidentified putative antigen targets have been reported (Table 2).8–10 Evidence suggests that paraneoplastic autoimmune retinopathy may be triggered by molecular mimicry between tumor antigens and retinal proteins. Using immunohistochemical staining, serum from CAR patients labeled photoreceptors on human retinal sections and reacted with a 23 kDa protein on Western blot. The antigen was later identified as recoverin8,11–12, which is a calcium-binding protein found in photoreceptors and has been shown to be expressed in the tumor cells of patients with cancer associated retinopathy.8,12 A similar mechanism has been suggested in anti-alpha-enolase mediated CAR.8 It is possible that nonparaneoplastic forms may also be triggered by a cross-reaction between retinal proteins and presumed viral or bacterial proteins. Recoverin is most commonly associated with CAR but has also been found in nonparaneoplastic autoimmune retinopathy as well.13 Similarly, α-enolase has been associated with both paraneoplastic and nonparaneoplastic forms.3,8
Both In vitro and in vivo experiments have attempted to elucidate the pathogenic role of antiretinal antibodies. In vitro studies have shown that both recoverin and α-enolase induce apoptosis of retinal cells following cellular internalization via caspase pathways and intracellular calcium influx.2,14 An in vivo experiment in monkey eyes showed that intravitreal injection of human MAR IgG altered the b-wave in monkey ERGs mimicking the ON-bipolar cell dysfunction and negative ERG commonly seen in MAR patients. This experiment supports the hypothesis that circulating MAR IgG plays a role in MAR pathogenicity.15 In spite of this evidence supporting the pathogenic role of anti-retinal antibodies, it is still unclear why some patients with such antibodies develop retinopathy while others do not.
Antiretinal antibodies can target any retinal cell-type including photoreceptor cells, ganglion cells, or bipolar cells. However, the presence of these antibodies alone is not sufficient for the diagnosis of this ocular disorder, as they can also be found in a variety of retinal diseases, systemic autoimmune diseases as well as in the serum of healthy individuals.12,14,16
Clinical Features and Diagnosis
Patients with nonparaneoplastic autoimmune retinopathy typically present with subacute vision loss, scotomas, photopsias, nyctalopia or photoaversion and dyschromatopsia. Visual acuity can be deceivingly good in the early stages. On examination, the fundus may appear unremarkable or demonstrate retinal vascular attenuation, diffuse retinal atrophy, retinal pigment epithelial (RPE) changes and waxy disc pallor. The disease is usually bilateral but it can be asymmetric. Typically there are minimal or no intraocular inflammatory cells.3,17–18 Among nonparaneoplastic patients, there is a female predominance (63–66%), and a history of autoimmune disease is common.3,17 The typical autoimmune retinopathy patient would be an adult female in her fifth to sixth decade with no history of visual problems prior to the onset of photopsias, presence of scotomas, and no family history of retinitis pigmentosa (RP). If these features, and the circulating antiretinal antibodies are present, and if there is no malignancy at presentation or following a thorough investigation, a tentative diagnosis can be made.
In an effort to simplify and standardize the diagnostic criteria for nonparaneoplastic autoimmune retinopathy, the authors propose a set of four essential criteria along with five symptoms which serve as supportive criteria. Essential elements include: no evidence of malignancy after a thorough work-up, no evidence of degenerative eye disease, such as retinitis pigmentosa, a positive screen for serum anti-retinal antibodies, and an ERG abnormality with or without visual field abnormality Supportive criteria include the presence of symptoms such as photopsias, scotomas, nyctalopia or photoaversion, and dyschromatopsia.
Visual-field testing shows constriction, central or paracentral scotomas, and ERG can show abnormalities in dark adapted or light adapted responses, bipolar cell responses or a combination of these. Fluorescein angiography (FA) in autoimmune retinopathy rarely shows leakage in the macula and OCTs can show cystoid macular edema (CME), typically in the form of cystic spaces.17 Recent advances in imaging technology are promising. For instance, OCT and fundus autofluorescence (FAF) in patients with autoimmune retinopathy showed abnormal autofluorescence patterns, mainly in the form of hyperautofluorescent ring in the parafoveal region, that corresponds to loss of outer-retinal structures on spectral domain OCT. Loss or disruption of the photoreceptor layer with decreased central macular thickness have also been observed. Both FAF and OCT have the potential to aid in the diagnosis, to understand its pathogenesis, and to monitor disease progression.19,20 In our experience among 24 patients with nonparaneoplastic autoimmune retinopathy, the most common findings were loss of the inner/outer segment layer on SD-OCT and parafoveal hyperautofluorescent ring or at least mild speckling on FAF which were present in approximately half of our patients (unpublished data).
As might be expected for an entity with no consensus in diagnosis, retrospective studies in patients with nonparaneoplastic autoimmune retinopathy showed that clinical features vary considerably. In one study, diffuse retinal atrophy was seen in the majority of patients (83%) and pigment deposits in only a small proportion (13%), and macular edema was present in approximately half of these cases. In another study pigmentary changes were seen in approximately half of the patients and macular edema was present in only 24%.3,17 Given the nature of our referral center, the majority of our patients had more advanced disease and demonstrated clinical findings such as RPE mottling, pigment deposits, and attenuated vessels (unpublished data).
Demonstration of antiretinal antibodies is crucial for the diagnosis of autoimmune retinopathy. They can be detected using Western blot (WB), immunohistochemistry (IHC) or enzyme-linked immunosorbent assay (ELISA). Each approach has its corresponding advantages and disadvantages, and the most commonly performed techniques tend to be WB and IHC. WB identifies antibodies based on the size of the protein, and is both technically difficult and lacking in specificity. For example, the detection of a 23 kDa band on WB does not necessarily mean antibody against recoverin. Using IHC to detect antiretinal antibodies, on the other hand, involves fixing patient serum against frozen retina from a human donor, or monkey, or mouse. Sections are then analyzed using light microscopy to determine which layers of the retina the antibody binds to. The advantage with IHC is the ability to localize the specific site of binding within the retina. ELISA involves adding various dilutions of patient sera into wells coated with specific retinal antigens and binding is detected using secondary antibodies. The clear disadvantage of this approach is that one must know the antigen of interest for a specific antibody ahead of time, and thus it lacks sensitivity in identifying all potential antiretinal antibodies. Unfortunately, all of these techniques lack standardization. Furthermore, it must be emphasized that the mere detection of antiretinal antibodies is not sufficient for a diagnosis of autoimmune retinopathy, nor does it prove that the antibodies detected are pathogenic. Currently the only commercially available testing is through Oregon Health Sciences University (OHSU) (Accessed on http://www.ohsu.edu/xd/health/services/casey-eye/clinical-services/diagnostic-services/upload/Ocular-Immunology-Web-2.pdf).
A recent study examined the concordance rate of antiretinal antibody testing between 2 separate laboratories which commonly accept samples from outside their own institutions. They point out that though the sample shipment and handling were performed identically for both laboratories, the processing and detection methods employed by the laboratories are distinct (Laboratory A used human retinal extract with positive controls (serum samples of patients with a known antiretinal antibody) and negative controls (using only secondary antibody). Laboratory B used pig retinal extract with normal controls (serum samples of people with no antibody activity). Of note, they do not mention whether the dilutions used were the same at both laboratories – something that would undoubtedly affect the sensitivity of antibody detection. This study found that the overall concordance rate of any antiretinal antibody detection was 60% between the two laboratories, and that among these, just over half, or a mere 36% of the total cohort, showed antiretinal antibody-specific concordance (same band detected on Western Blot).21 The overall inter-observer agreement was very poor with a kappa value of −0.13. We agree with the authors’ conclusion that the lack of a gold standard has led to dramatic variability in laboratory detection of antiretinal antibodies, and standardized methods across laboratories are urgently needed in order to produce consistent results.
Differential Diagnosis
Due to the reasons discussed above, the diagnosis of autoimmune retinopathy is challenging. Currently, the diagnosis is made based on the presence of clinical manifestations (including abnormal ERGs) and the demonstration of serum ARAs. Differential diagnosis of autoimmune retinopathy includes white-dot syndrome spectrum disorders (particularly acute zonal occult outer retinopathy (AZOOR)), retinal degenerative disorders (such as retinitis pigmentosa (RP) and cone-rod dystrophy), and non-infectious and infectious uveitis syndromes. Because of significant implications, it is important to differentiate the paraneoplastic type from the nonparaneoplastic. An extensive investigation to rule out any malignancy should be undertaken in any patient that presents with signs and symptoms suggestive of autoimmune retinopathy. This investigation may be facilitated by an internist or primary care physician, who would take an inventory of the patients’ history, review of systems, physical exam findings and basic laboratory investigations in order to determine the patients’ individual risk factors and, as such, determine the need to image with brain MRI, chest, abdomen and pelvis CT, colonoscopy and other age and gender appropriate testing such as mammogram, etc. Paraneoplastic retinopathies, similar to nonparaneoplastic, are characterized by vision loss, photopsias, nyctalopia and scotomas with a more rapid decline. CAR is typically associated with anti-recoverin antibody, and most commonly associated with small-cell carcinoma of the lung but can occur with other cancers. ERG in CAR typically shows involvement of cone responses.11,13 MAR occurs most commonly in patients with cutaneous melanoma and is characterized by a negative waveform on standardized full-field ERG due to reduction in b-wave amplitudes. CAR can precede the diagnosis of cancer, whereas MAR typically presents after the diagnosis of melanoma, usually metastatic melanoma.18
The majority of autoimmune retinopathy patients may have more than one antibody.4 Diagnosis is made more difficult due to the fact that presence of antiretinal antibodies alone is not diagnostic, and that no standards for their detection exists.21 Moreover, antiretinal antibodies can be found in other systemic autoimmune diseases such as inflammatory bowel disease, Behcet’s disease, systemic lupus erythematosis, multiple sclerosis22–25 as well as retinal degenerations including age related macular degeneration (AMD)26, infectious and non-infectious uveitides18,27, and in up to 42% of normal controls.28
Retinitis pigmentosa patients can have similar clinical features to autoimmune retinopathy and approximately 10–37% of patients with RP may have circulating antiretinal antibodies, which makes differentiating these two entities with overlapping findings and symptoms difficult.4,29 Interestingly, RP patients with antiretinal antibodies have been found to be more likely to have macular edema, than those without antibodies.29 It is unclear if the antibodies in RP patients precede the onset of retinopathy or are simply a consequence of retinal damage.
As mentioned previously, antiretinal antibodies have been identified in AMD, along with other degenerative diseases.26 Though their role in the pathogenesis remains largely unknown, a number of studies have suggested a diagnostic and prognostic role for serum antiretinal antibodies in AMD.26,30
Some uveitis syndromes such as Vogt-Koyanagi Harada syndrome (VKH) and sympathetic ophthalmia (SO) can also demonstrate antiretinal antibodies.18,26 In patients with VKH, antibody reactivity to photoreceptors correlated with disease activity. Other rare cases of retinopathies associated with retinal antibodies include onchocerciasis and ocular toxoplasmosis. Antibodies to retinal pigment epithelium (RPE), neural retina or photoreceptor layer has been described in these infectious retinopathies.27–28 All these syndromes are characterized by significant intraocular inflammation in addition to their unique fundus findings, making the differentiation rather unproblematic.
AZOOR can present with similar symptoms, visual field and ERG findings as autoimmune retinopathy. It is typically bilateral but can be asymmetric. Most patients either stabilize or show partial recovery without treatment. Multiple evanescent white dot syndrome (MEWDS), despite having similar symptoms, is a unilateral retinopathy which is characterized by afferent pupillary defect, optic nerve swelling, and spontaneous recovery; and hence is more readily differentiated from autoimmune retinopathy. Both AZOOR and MEWDS may show enlarged blind spot on visual fields. In addition, the majority of eyes affected by AZOOR may show characteristic and striking fundus autofluorescence abnormalities with well-demarcated areas of hypoautofluorescence which have not been observed in autoimmune retinopathy.31–32
In summary, antiretinal antibodies can be found in a number of inflammatory or degenerative ocular diseases. In all of the aforementioned diseases, it is unclear if the antibodies precede the retinal disease or if the immune reactivity is simply a consequence of the retinal degenerative process regardless of the underlying etiology.
Treatment
Because of the presumed autoimmune nature of autoimmune retinopathy, various forms of immunomodulatory approaches have been tried. However, the ambiguity in diagnosis creates an enormous challenge in the management of this disease. For paraneoplastic retinopathies, decreasing tumor burden, using surgery, chemotherapy, or radiation, as applicable, is the best approach. Currently, most of the information on treatment of autoimmune retinopathy comes from reports on paraneoplastic retinopathy. Therefore, immunomodulatory therapy for nonparaneoplastic autoimmune retinopathy should be considered empiric at this time.
Common approaches to both para-neoplastic and non-paraneoplastic autoimmune retinopathy include systemic or local corticosteroids, intravenous immunoglobulin (IVIG), or plasmapheresis. Additionally, antimetabolites such as mycophenolate mofetil, azathioprine, and T-cell inhibitors such as cyclosporine have been used.17 Less frequently, targeted B-cell therapy, such as anti-CD20 monoclonal antibody (Rituximab), has also been used.33–35 Unfortunately, therapy is not helpful once widespread retinal degeneration occurs.4,17
In a cohort of 24 nonparaneoplastic autoimmune retinopathy patients that received therapy with various combinations of prednisone, cyclosporine, azathioprine, mycophenolate mofetil, periocular or intravitreal steroid injections, 15 of the 24 (62.5%) showed varying degrees of improvement in visual acuity or visual field, and CME improved in almost half of the patients.17 Decrease in antiretinal antibodies following treatment may be seen in some cases; however the clinical significance of this finding is unclear.17,27 Serial functional testing (e.g. with GVFs and ERGs) is commonly used as an indicator of treatment response. These can be repeated every 3–6 months to monitor the effect of treatment. In our experience with 24 autoimmune retinopathy patients, the clinical course of patients who received immunomodulatory therapy - compared with those who did not - was not significantly different. We observed that a proportion of patients remain stable even in the absence of treatment while some progress despite immunomodulatory therapy.
Conclusion
Given the current ambiguity in diagnosis, clinical and laboratory guidelines for the diagnosis of autoimmune retinopathy are needed. It is uncertain whether changes in autoantibody levels correlate with clinical improvement, and more importantly, if treatment significantly alters the natural course of the disease. The response to treatment is quite variable, with more favorable results achieved in paraneoplastic retinopathy, particularly CAR, with a combination of chemotherapy and immunosuppression. It is possible that an earlier attempt to treat could be more beneficial, but currently most patients go undiagnosed for a long time and have advanced disease at the time of treatment initiation. Early treatment attempts are limited by lack of sensitive and specific assays and more definitive clinical criteria. Additional investigations should examine the specificity and pathogenicity of antiretinal antibodies, screen for biomarkers, and should be conducted concurrently with studies seeking to identify appropriate treatment.
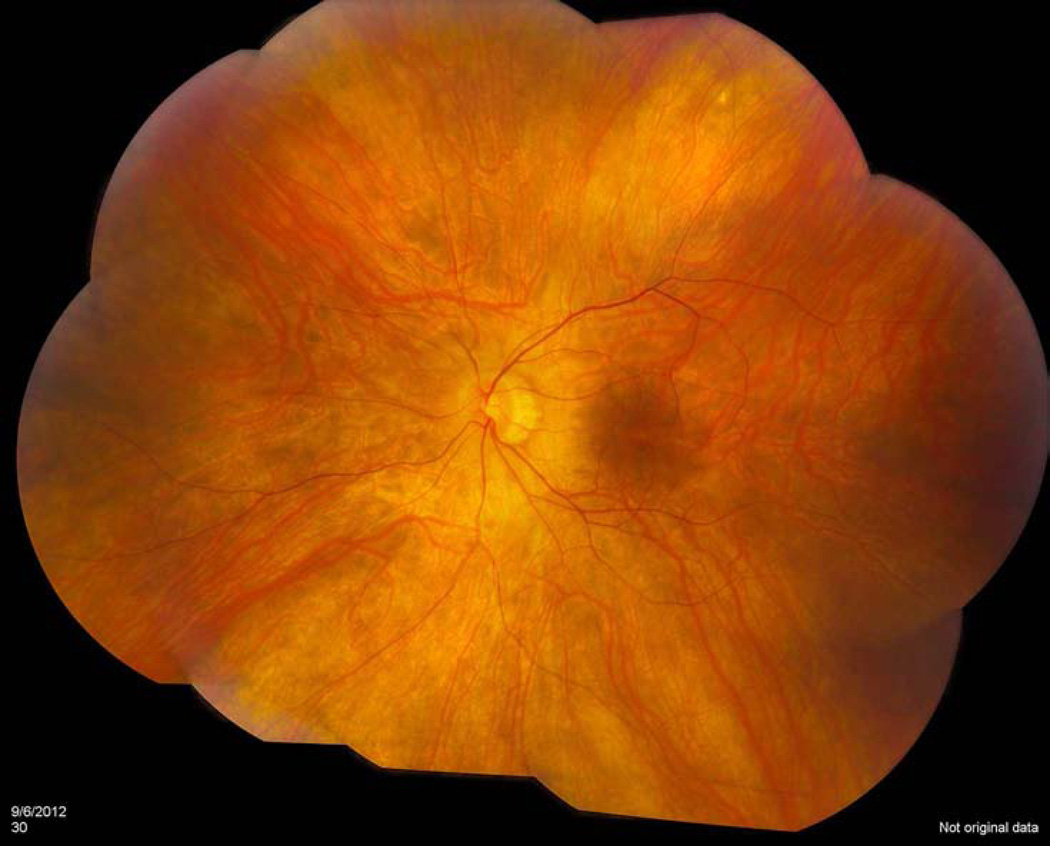
Figure 1.
Fundus photo of a nonparaneoplastic autoimmune retinopathy patient with 20/20 vision which demonstrates a poor foveal reflex with mild vascular attenuation, and an otherwise normal appearing fundus.
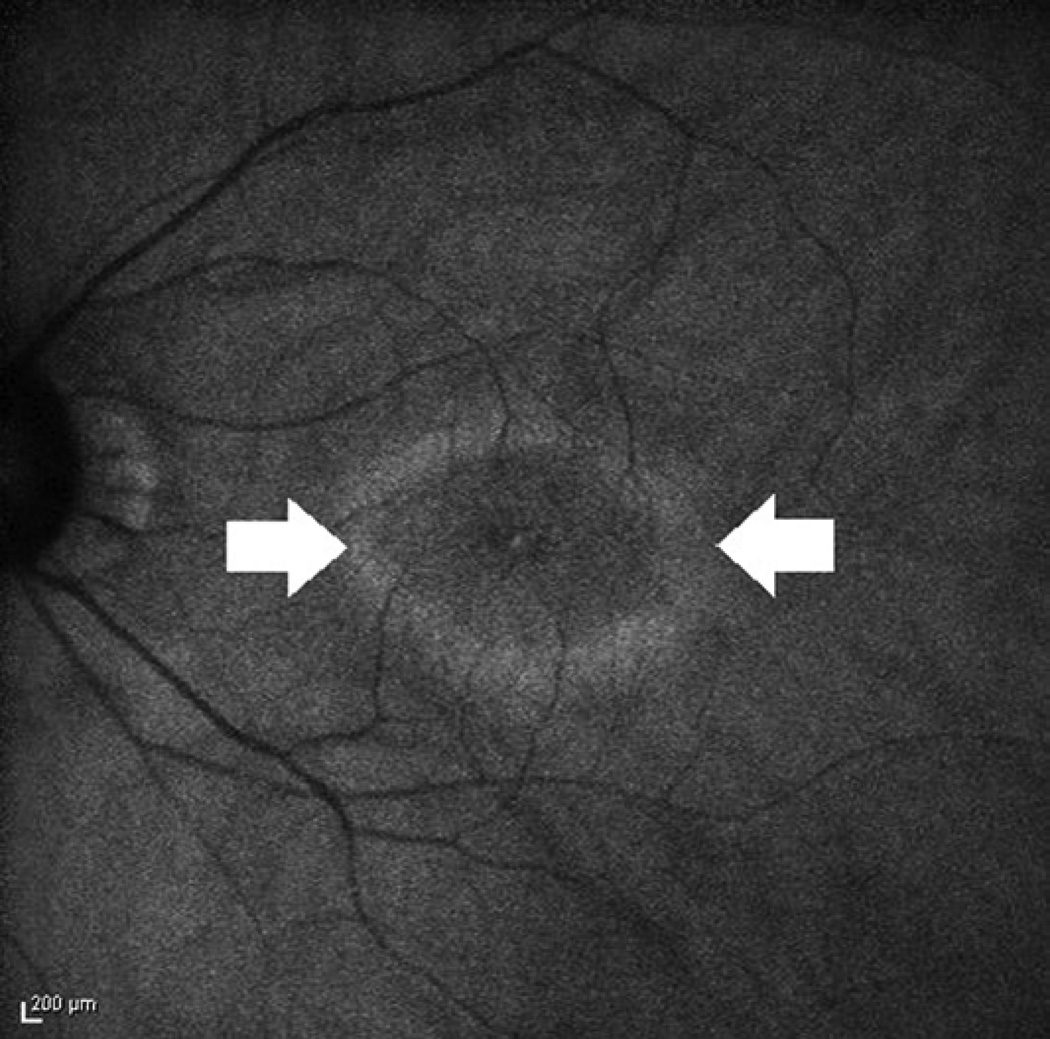
Figure 2.
Fundus autofluorescence of a patient with confirmed autoimmune retinopathy reveals a ring of outer hyperautofluorescence (arrows).
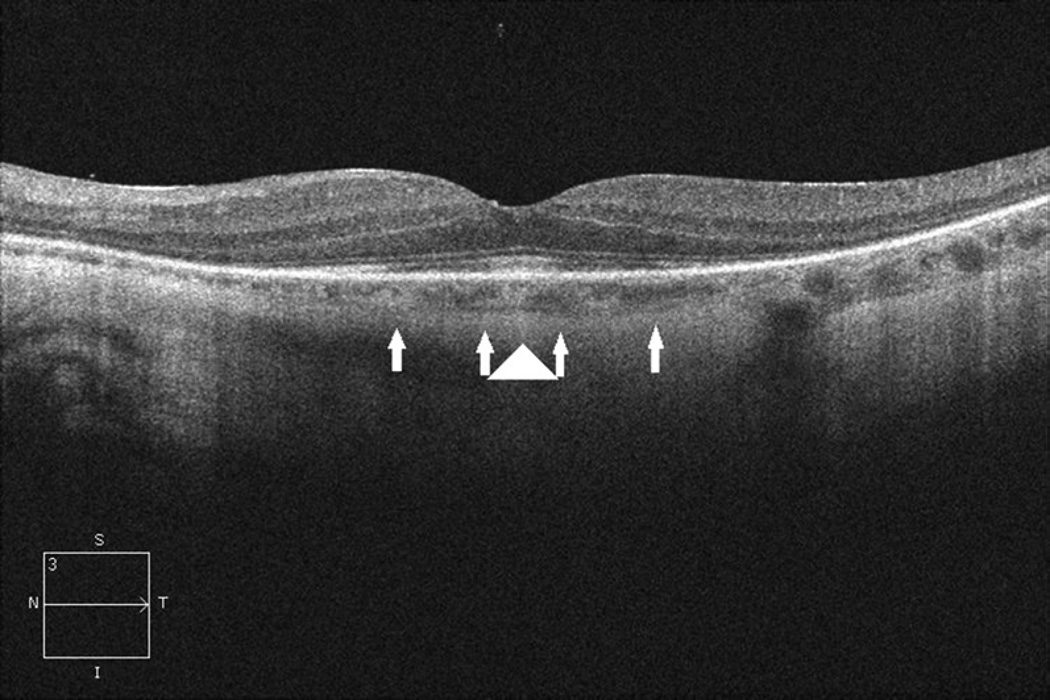
Figure 3.
Spectral Domain-OCT of the macula of a patient with confirmed autoimmune retinopathy reveals loss of IS-OS junction (between arrows) with preservation at the fovea (arrowhead).
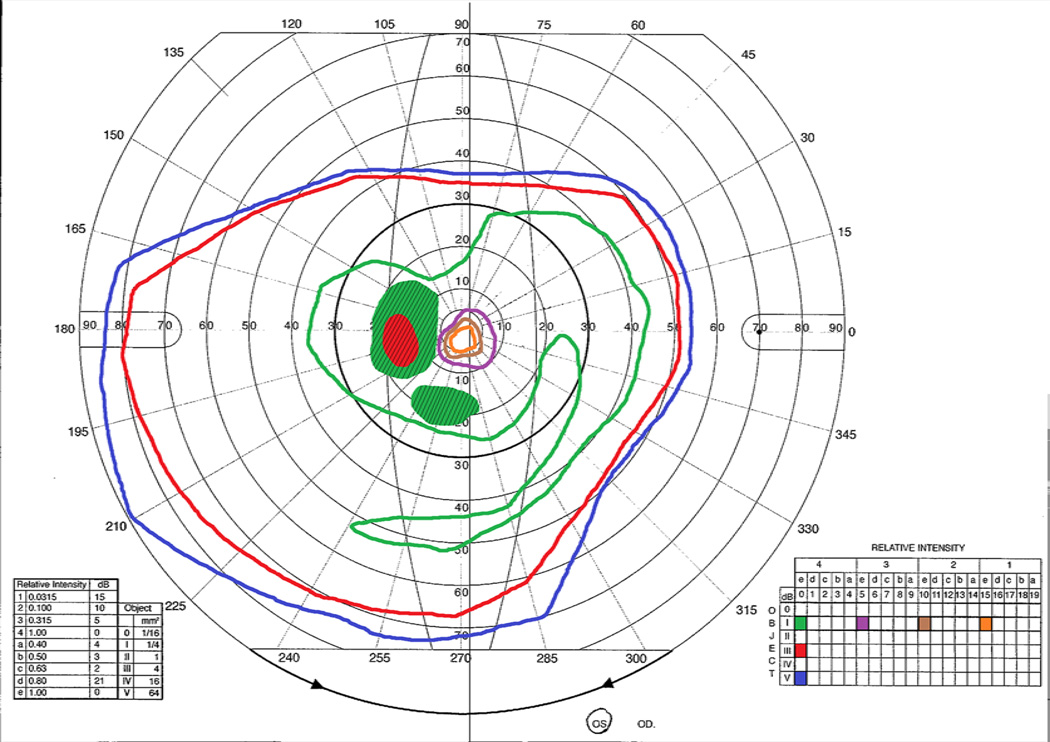
Figure 4.
Goldman visual field of the left eye for a patient with confirmed autoimmune retinopathy demonstrating marked constriction of the inner isopters and paracentral scotomas (shaded with diagonal lines).
ACKNOWLEDGEMENTS
Funding/Support: This work is supported by the NEI Intramural Research Program. This research was made possible through the National Institutes of Health (NIH) Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer Inc, The Leona M. and Harry B. Helmsley Charitable Trust, and the Howard Hughes Medical Institute, as well as other private donors. For a complete list, please visit the Foundation website at http://www.fnih.org/work/programs-development/medical-research-scholars-program.
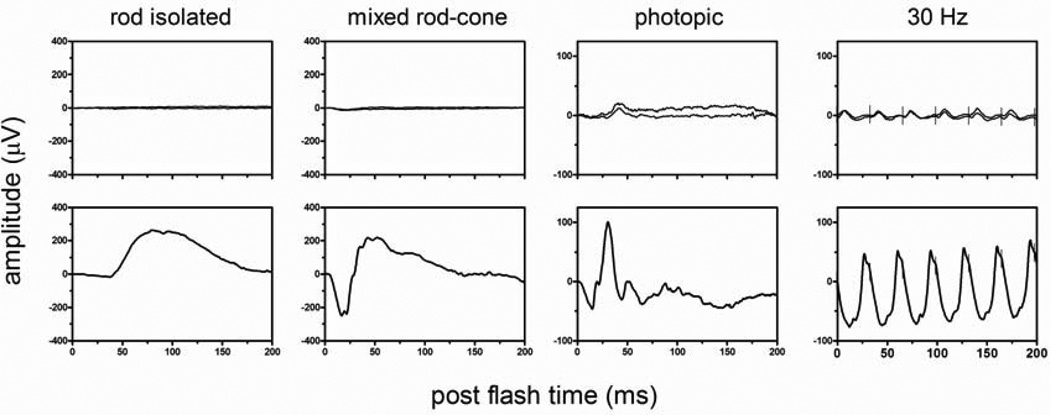
Other Acknowledgments: The authors wish to acknowledge the contribution of Brett Jeffrey, of the National Eye Institute in helping to prepare the ERG displayed as Figure 5.
Figure 5.
Full-field ERG responses – the top row is from a patient with confirmed autoimmune retinopathy and the bottom row represents a healthy control. Rod responses are absent from the patient. Photopic and 30 Hz tests reveal cone responses that are extremely reduced and delayed. (Courtesy of Brett Jeffrey, PhD)
Biography

Landon Kelly Grange is a medical student at the University of California, San Diego. He is currently a participant of the National Institutes of Health Medical Research Scholars Program (MRSP). He is conducting clinical research with the Uveitis section of the Ocular Immunology Laboratory of the National Eye Institute (NEI). He has published a case report of testicular lymphoma relapsing in the eye after 11 years of remission, and a review of Autoimmune Retinopathy in Review of Ophthalmology. His research interests include immune privilege and immunologic conditions affecting the eye.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE
Financial Disclosures: No financial disclosures to report.
Contributions of Authors in each of these areas: Preparation (LKG, MD, HNS), Review (LKG, MD, HNS, RBN), Approval of the Manuscript (LKG, MD, HNS, RBN).
REFERENCES
- 1.Jacobson DM, Thirkill CE, Tipping SJ. A clinical triad to diagnose paraneoplastic retinopathy. Ann Neurol. 1990;28(2):162–167. doi: 10.1002/ana.410280208. [DOI] [PubMed] [Google Scholar]
- 2.Adamus G. Autoantibody-induced apoptosis as a possible mechanism of autoimmune retinopathy. Autoimmun Rev. 2003;2(2):63–68. doi: 10.1016/s1568-9972(02)00127-1. [DOI] [PubMed] [Google Scholar]
- 3.Adamus G, Ren G, Weleber RG. Autoantibodies against retinal proteins in paraneoplastic and autoimmune retinopathy. BMC Ophthalmol. 2004;4:5. doi: 10.1186/1471-2415-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heckenlively JR, Ferreyra HA. Autoimmune retinopathy: a review and summary. Semin Immunopathol. 2008;30(2):127–134. doi: 10.1007/s00281-008-0114-7. [DOI] [PubMed] [Google Scholar]
- 5.Mizener JB, Kimura AE, Adamus G, Thirkill CE, Goeken JA, Kardon RH. Autoimmune retinopathy in the absence of cancer. Am J Ophthalmol. 1997;123(5):607–618. doi: 10.1016/s0002-9394(14)71073-6. [DOI] [PubMed] [Google Scholar]
- 6.Sawyer RA, Selhorst JB, Zimmerman LE, Hoyt WF. Blindness caused by photoreceptor degeneration as a remote effect of cancer. Am J Ophthalmol. 1976;81(5):606–613. doi: 10.1016/0002-9394(76)90125-2. [DOI] [PubMed] [Google Scholar]
- 7.Klingele TG, Burde RM, Rappazzo JA, Isserman MJ, Burgess D, Kantor O. Paraneoplastic retinopathy. J Clin Neuroophthalmol. 1984;4(4):239–245. [PubMed] [Google Scholar]
- 8.Forooghian F, Macdonald IM, Heckenlively JR, et al. The need for standardization of antiretinal antibody detection and measurement. Am J Ophthalmol. 2008;146(4):489–495. doi: 10.1016/j.ajo.2008.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kondo M, Sanuki R, Ueno S, et al. Identification of autoantibodies against TRPM1 in patients with paraneoplastic retinopathy associated with ON bipolar cell dysfunction. PLoS One. 2011;6(5):e19911. doi: 10.1371/journal.pone.0019911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sen HN, Nussenblatt RB. Chapter 77- Autoimmune Retinopathies. In: Ryan SJ, Schachat AP, Wilkinson CP, et al., editors. Retina. 5th ed. Elsevier, Inc; 2013. pp. 1381–1389. [Google Scholar]
- 11.Thirkill CE, Roth AM, Keltner JL. Cancer-associated retinopathy. Arch Ophthalmol. 1987;105(3):372–375. doi: 10.1001/archopht.1987.01060030092033. [DOI] [PubMed] [Google Scholar]
- 12.Polans AS, Witkowska D, Haley TL, Amundson D, Baizer L, Adamus G. Recoverin, a photoreceptor-specific calcium-binding protein, is expressed by the tumor of a patient with cancer-associated retinopathy. Proc Natl Acad Sci U S A. 1995;92(20):9176–9180. doi: 10.1073/pnas.92.20.9176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitcup SM, Vistica BP, Milam AH, Nussenblatt RB, Gery I. Recoverin-associated retinopathy: a clinically and immunologically distinctive disease. Am J Ophthalmol. 1998;126(2):230–237. doi: 10.1016/s0002-9394(98)00149-4. [DOI] [PubMed] [Google Scholar]
- 14.Ren G, Adamus G. Cellular targets of anti-alpha-enolase autoantibodies of patients with autoimmune retinopathy. J Autoimmun. 2004;23(2):161–167. doi: 10.1016/j.jaut.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Lei B, Bush RA, Milam AH, Sieving PA. Human melanoma-associated retinopathy (MAR) antibodies alter the retinal ON-response of the monkey ERG in vivo. Invest Ophthalmol Vis Sci. 2000;41(1):262–266. [PubMed] [Google Scholar]
- 16.Pratesi F, Moscato S, Sabbatini A, Chimenti D, Bombardieri S, Migliorini P. Autoantibodies specific for alpha-enolase in systemic autoimmune disorders. J Rheumatol. 2000;27(1):109–115. [PubMed] [Google Scholar]
- 17.Ferreyra HA, Jayasundera T, Khan NW, He S, Lu Y, Heckenlively JR. Management of autoimmune retinopathies with immunosuppression. Arch Ophthalmol. 2009;127(4):390–397. doi: 10.1001/archophthalmol.2009.24. [DOI] [PubMed] [Google Scholar]
- 18.Chan JW. Paraneoplastic retinopathies and optic neuropathies. Surv Ophthalmol. 2003;48(1):12–38. doi: 10.1016/s0039-6257(02)00416-2. [DOI] [PubMed] [Google Scholar]
- 19.Lima LH, Greenberg JP, Greenstein VC, et al. Hyperautofluorescent ring in autoimmune retinopathy. Retina. 2012;32(7):1385–1394. doi: 10.1097/IAE.0b013e3182398107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abazari A, Allam SS, Adamus G, Ghazi NG. Optical coherence tomography findings in autoimmune retinopathy. Am J Ophthalmol. 2012;153(4):750–756. doi: 10.1016/j.ajo.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faez S, Loewenstein J, Sobrin L. Concordance of antiretinal antibody testing results between laboratories in autoimmune retinopathy. JAMA Ophthalmol. 2013;131(1):113–115. doi: 10.1001/jamaophthalmol.2013.574. [DOI] [PubMed] [Google Scholar]
- 22.Rahi AH, Addison DJ. Autoimmunity and the outer retina. Trans Ophthalmol Soc U K. 1983;103(Pt 4):428–437. [PubMed] [Google Scholar]
- 23.Forooghian F, Adamus G, Sproule M, Westall C, O’Connor P. Enolase autoantibodies and retinal function in multiple sclerosis patients. Graefes Arch Clin Exp Ophthalmol. 2007;245(8):1077–1084. doi: 10.1007/s00417-006-0527-8. [DOI] [PubMed] [Google Scholar]
- 24.Lee JH, Cho SB, Bang D, et al. Human anti-alpha-enolase antibody in sera from patients with Behçet's disease and rheumatologic disorders. Clin Exp Rheumatol. 2009;27(2) Suppl 53:S63–S66. [PubMed] [Google Scholar]
- 25.Vermeulen N, Arijs I, Joossens S, et al. Anti-alpha-enolase antibodies in patients with inflammatory bowel disease. Clin Chem. 2008;54(3):534–541. doi: 10.1373/clinchem.2007.098368. [DOI] [PubMed] [Google Scholar]
- 26.Patel N, Ohbayashi M, Nugent AK, et al. Circulating anti-retinal antibodies as immune markers in age-related macular degeneration. Immunology. 2005;115(3):422–430. doi: 10.1111/j.1365-2567.2005.02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hooks JJ, Tso MO, Detrick B. Retinopathies associated with antiretinal antibodies. Clin Diagn Lab Immunol. 2001;8(5):853–858. doi: 10.1128/CDLI.8.5.853-858.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ko AC, Brinton JP, Mahajan VB, et al. Seroreactivity against aqueous-soluble and detergent-soluble retinal proteins in posterior uveitis. Arch Ophthalmol. 2011;129(4):415–420. doi: 10.1001/archophthalmol.2011.65. [DOI] [PubMed] [Google Scholar]
- 29.Heckenlively JR, Aptsiauri N, Nusinowitz S, Peng C, Hargrave PA. Investigations of antiretinal antibodies in pigmentary retinopathy and other retinal degenerations. Trans Am Ophthalmol Soc. 1996;94:179–200. [PMC free article] [PubMed] [Google Scholar]
- 30.Gu X, Meer SG, Miyagi M, et al. Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration. J Biol Chem. 2003;278(43):42027–42035. doi: 10.1074/jbc.M305460200. [DOI] [PubMed] [Google Scholar]
- 31.Fujiwara T, Imamura Y, Giovinazzo VJ, Spaide RF. Fundus autofluorescence and optical coherence tomographic findings in acute zonal occult outer retinopathy. Retina. 2010;30(8):1206–1216. doi: 10.1097/IAE.0b013e3181e097f0. [DOI] [PubMed] [Google Scholar]
- 32.Yeh S, Forooghian F, Wong WT, et al. Fundus autofluorescence imaging of the white dot syndromes. Arch Ophthalmol. 2010;128(1):46–56. doi: 10.1001/archophthalmol.2009.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sen HN, Chan CC, Caruso RC, Fariss RN, Nussenblatt RB, Buggage RR. Waldenström's macroglobulinemia-associated retinopathy. Ophthalmology. 2004;111(3):535–539. doi: 10.1016/j.ophtha.2003.05.036. [DOI] [PubMed] [Google Scholar]
- 34.Mahdi N, Faia LJ, Goodwin J, Nussenblatt RB, Sen HN. A case of autoimmune retinopathy associated with thyroid carcinoma. Ocul Imunol Inflamm. 2010;18(4):322–323. doi: 10.3109/09273941003802379. [DOI] [PubMed] [Google Scholar]
- 35.Or C, Collins DR, Merkur AB, Wang Y, Chan CC, Forooghian F. Intravenous rituximab for the treatment of cancer-associated retinopathy. Can J Ophthalmol. 2013;48(2):35–38. doi: 10.1016/j.jcjo.2012.11.010. [DOI] [PubMed] [Google Scholar]