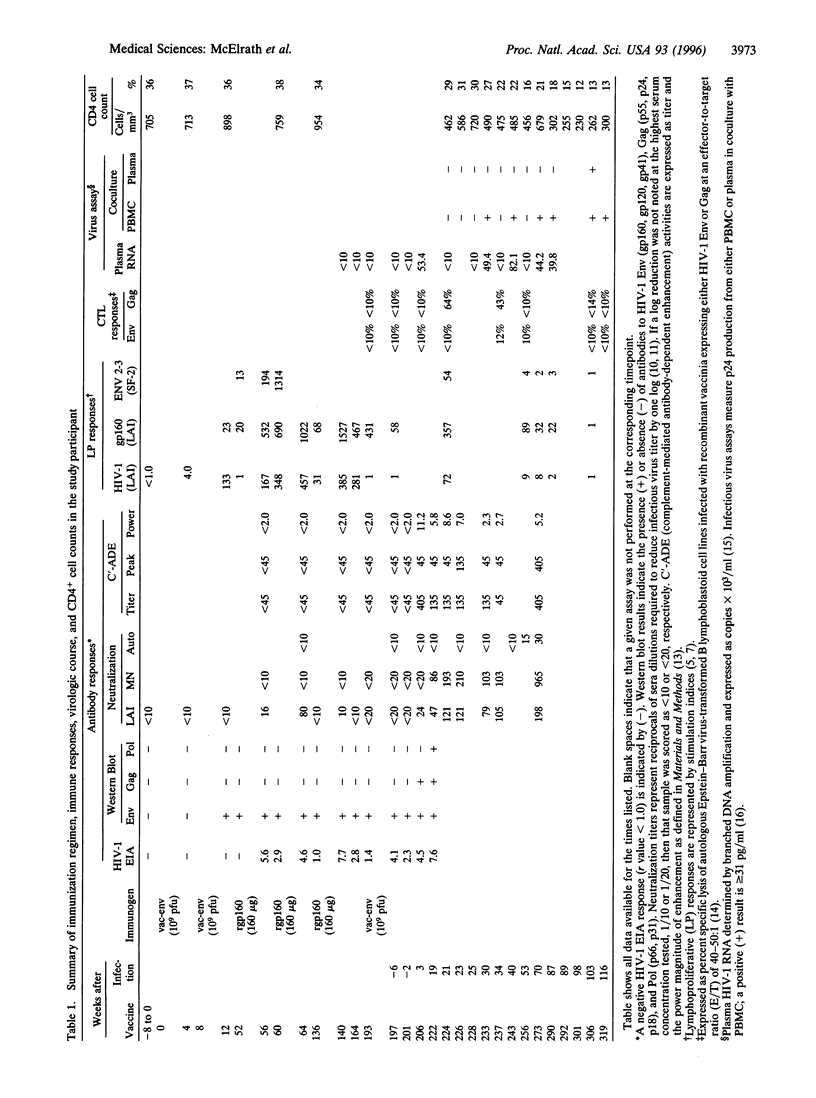
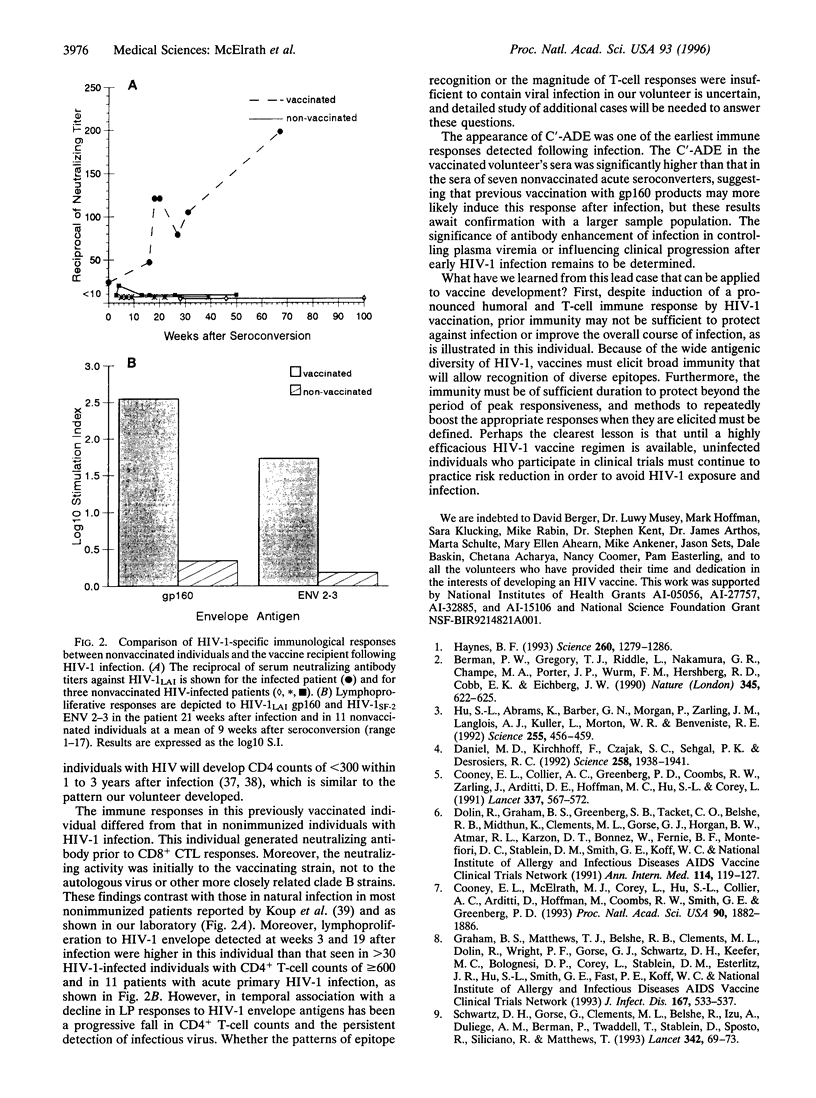
Abstract
With efforts underway to develop a preventive human immunodeficiency virus type 1 (HIV-1) vaccine, it remains unclear which immune responses are sufficient to protect against infection and whether prior HIV-1 immunity can alter the subsequent course of HIV-1 infection. We investigated these issues in the context of a volunteer who received six HIV-1LAI envelope immunizations and 10 weeks thereafter acquired HIV-1 infection through a high-risk sexual exposure. In contrast to nonvaccinated acutely infected individuals, anamnestic HIV-1-specific B- and T-cell responses appeared within 3 weeks in this individual, and neutralizing antibody preceded CD8+ cytotoxic responses. Despite an asymptomatic course and an initial low level of detectable infectious virus, a progressive CD4+ cell decline and dysfunction occurred within 2 years. Although vaccination elicited immunity to HIV-1 envelope, which was recalled upon HIV-1 exposure, it was insufficient to prevent infection and subsequent immunodeficiency.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad S., Lohman B., Marthas M., Giavedoni L., el-Amad Z., Haigwood N. L., Scandella C. J., Gardner M. B., Luciw P. A., Yilma T. Reduced virus load in rhesus macaques immunized with recombinant gp160 and challenged with simian immunodeficiency virus. AIDS Res Hum Retroviruses. 1994 Feb;10(2):195–204. doi: 10.1089/aid.1994.10.195. [DOI] [PubMed] [Google Scholar]
- Belshe R. B., Bolognesi D. P., Clements M. L., Corey L., Dolin R., Mestecky J., Mulligan M., Stablein D., Wright P. HIV infection in vaccinated volunteers. JAMA. 1994 Aug 10;272(6):431–431. doi: 10.1001/jama.272.6.431b. [DOI] [PubMed] [Google Scholar]
- Belshe R. B., Clements M. L., Dolin R., Graham B. S., McElrath J., Gorse G. J., Schwartz D., Keefer M. C., Wright P., Corey L. Safety and immunogenicity of a fully glycosylated recombinant gp160 human immunodeficiency virus type 1 vaccine in subjects at low risk of infection. National Institute of Allergy and Infectious Diseases AIDS Vaccine Evaluation Group Network. J Infect Dis. 1993 Dec;168(6):1387–1395. doi: 10.1093/infdis/168.6.1387. [DOI] [PubMed] [Google Scholar]
- Berman P. W., Gregory T. J., Riddle L., Nakamura G. R., Champe M. A., Porter J. P., Wurm F. M., Hershberg R. D., Cobb E. K., Eichberg J. W. Protection of chimpanzees from infection by HIV-1 after vaccination with recombinant glycoprotein gp120 but not gp160. Nature. 1990 Jun 14;345(6276):622–625. doi: 10.1038/345622a0. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Nishio J., Perryman S. Macrophage-tropic human immunodeficiency virus isolates from different patients exhibit unusual V3 envelope sequence homogeneity in comparison with T-cell-tropic isolates: definition of critical amino acids involved in cell tropism. J Virol. 1992 Nov;66(11):6547–6554. doi: 10.1128/jvi.66.11.6547-6554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs R. W., Collier A. C., Allain J. P., Nikora B., Leuther M., Gjerset G. F., Corey L. Plasma viremia in human immunodeficiency virus infection. N Engl J Med. 1989 Dec 14;321(24):1626–1631. doi: 10.1056/NEJM198912143212402. [DOI] [PubMed] [Google Scholar]
- Cooney E. L., Collier A. C., Greenberg P. D., Coombs R. W., Zarling J., Arditti D. E., Hoffman M. C., Hu S. L., Corey L. Safety of and immunological response to a recombinant vaccinia virus vaccine expressing HIV envelope glycoprotein. Lancet. 1991 Mar 9;337(8741):567–572. doi: 10.1016/0140-6736(91)91636-9. [DOI] [PubMed] [Google Scholar]
- Cooney E. L., McElrath M. J., Corey L., Hu S. L., Collier A. C., Arditti D., Hoffman M., Coombs R. W., Smith G. E., Greenberg P. D. Enhanced immunity to human immunodeficiency virus (HIV) envelope elicited by a combined vaccine regimen consisting of priming with a vaccinia recombinant expressing HIV envelope and boosting with gp160 protein. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1882–1886. doi: 10.1073/pnas.90.5.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel M. D., Kirchhoff F., Czajak S. C., Sehgal P. K., Desrosiers R. C. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992 Dec 18;258(5090):1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- Delwart E. L., Shpaer E. G., Louwagie J., McCutchan F. E., Grez M., Rübsamen-Waigmann H., Mullins J. I. Genetic relationships determined by a DNA heteroduplex mobility assay: analysis of HIV-1 env genes. Science. 1993 Nov 19;262(5137):1257–1261. doi: 10.1126/science.8235655. [DOI] [PubMed] [Google Scholar]
- Dolin R., Graham B. S., Greenberg S. B., Tacket C. O., Belshe R. B., Midthun K., Clements M. L., Gorse G. J., Horgan B. W., Atmar R. L. The safety and immunogenicity of a human immunodeficiency virus type 1 (HIV-1) recombinant gp160 candidate vaccine in humans. NIAID AIDS Vaccine Clinical Trials Network. Ann Intern Med. 1991 Jan 15;114(2):119–127. doi: 10.7326/0003-4819-114-2-119. [DOI] [PubMed] [Google Scholar]
- Gorham E. D., Garland F. C., Mayers D. L., Goforth R. R., Brodine S. K., Wiess P. J., McNally M. S. CD4 lymphocyte counts within 24 months of human immunodeficiency virus seroconversion. Findings in the US Navy and Marine Corps. The Navy Retroviral Working Group. Arch Intern Med. 1993 Apr 12;153(7):869–876. [PubMed] [Google Scholar]
- Graham B. S., Matthews T. J., Belshe R. B., Clements M. L., Dolin R., Wright P. F., Gorse G. J., Schwartz D. H., Keefer M. C., Bolognesi D. P. Augmentation of human immunodeficiency virus type 1 neutralizing antibody by priming with gp160 recombinant vaccinia and boosting with rgp160 in vaccinia-naive adults. The NIAID AIDS Vaccine Clinical Trials Network. J Infect Dis. 1993 Mar;167(3):533–537. doi: 10.1093/infdis/167.3.533. [DOI] [PubMed] [Google Scholar]
- Haynes B. F. Scientific and social issues of human immunodeficiency virus vaccine development. Science. 1993 May 28;260(5112):1279–1286. doi: 10.1126/science.8493572. [DOI] [PubMed] [Google Scholar]
- Hoth D. F., Bolognesi D. P., Corey L., Vermund S. H. NIH conference. HIV vaccine development: a progress report. Ann Intern Med. 1994 Oct 15;121(8):603–611. doi: 10.7326/0003-4819-121-8-199410150-00008. [DOI] [PubMed] [Google Scholar]
- Hu S. L., Abrams K., Barber G. N., Moran P., Zarling J. M., Langlois A. J., Kuller L., Morton W. R., Benveniste R. E. Protection of macaques against SIV infection by subunit vaccines of SIV envelope glycoprotein gp160. Science. 1992 Jan 24;255(5043):456–459. doi: 10.1126/science.1531159. [DOI] [PubMed] [Google Scholar]
- Israel Z. R., Edmonson P. F., Maul D. H., O'Neil S. P., Mossman S. P., Thiriart C., Fabry L., Van Opstal O., Bruck C., Bex F. Incomplete protection, but suppression of virus burden, elicited by subunit simian immunodeficiency virus vaccines. J Virol. 1994 Mar;68(3):1843–1853. doi: 10.1128/jvi.68.3.1843-1853.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurriaans S., Van Gemen B., Weverling G. J., Van Strijp D., Nara P., Coutinho R., Koot M., Schuitemaker H., Goudsmit J. The natural history of HIV-1 infection: virus load and virus phenotype independent determinants of clinical course? Virology. 1994 Oct;204(1):223–233. doi: 10.1006/viro.1994.1526. [DOI] [PubMed] [Google Scholar]
- Koot M., Vos A. H., Keet R. P., de Goede R. E., Dercksen M. W., Terpstra F. G., Coutinho R. A., Miedema F., Tersmette M. HIV-1 biological phenotype in long-term infected individuals evaluated with an MT-2 cocultivation assay. AIDS. 1992 Jan;6(1):49–54. doi: 10.1097/00002030-199201000-00006. [DOI] [PubMed] [Google Scholar]
- Koup R. A., Safrit J. T., Cao Y., Andrews C. A., McLeod G., Borkowsky W., Farthing C., Ho D. D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994 Jul;68(7):4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. H., Sheppard H. W., Reis M., Dondero D., Osmond D., Busch M. P. Circulating HIV-1-infected cell burden from seroconversion to AIDS: importance of postseroconversion viral load on disease course. J Acquir Immune Defic Syndr. 1994 Apr;7(4):381–388. [PubMed] [Google Scholar]
- Lin H. J., Myers L. E., Yen-Lieberman B., Hollinger F. B., Henrard D., Hooper C. J., Kokka R., Kwok S., Rasheed S., Vahey M. Multicenter evaluation of quantification methods for plasma human immunodeficiency virus type 1 RNA. J Infect Dis. 1994 Sep;170(3):553–562. doi: 10.1093/infdis/170.3.553. [DOI] [PubMed] [Google Scholar]
- Mann D. L., Carrington M., O'Donnell M., Miller T., Goedert J. HLA phenotype is a factor in determining rate of disease progression and outcome in HIV-1-infected individuals. AIDS Res Hum Retroviruses. 1992 Aug;8(8):1345–1346. doi: 10.1089/aid.1992.8.1345. [DOI] [PubMed] [Google Scholar]
- Marthas M. L., Sutjipto S., Higgins J., Lohman B., Torten J., Luciw P. A., Marx P. A., Pedersen N. C. Immunization with a live, attenuated simian immunodeficiency virus (SIV) prevents early disease but not infection in rhesus macaques challenged with pathogenic SIV. J Virol. 1990 Aug;64(8):3694–3700. doi: 10.1128/jvi.64.8.3694-3700.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascola J. R., Mathieson B. J., Zack P. M., Walker M. C., Halstead S. B., Burke D. S. Summary report: workshop on the potential risks of antibody-dependent enhancement in human HIV vaccine trials. AIDS Res Hum Retroviruses. 1993 Dec;9(12):1175–1184. doi: 10.1089/aid.1993.9.1175. [DOI] [PubMed] [Google Scholar]
- McElrath M. J., Corey L., Berger D., Hoffman M. C., Klucking S., Dragavon J., Peterson E., Greenberg P. D. Immune responses elicited by recombinant vaccinia-human immunodeficiency virus (HIV) envelope and HIV envelope protein: analysis of the durability of responses and effect of repeated boosting. J Infect Dis. 1994 Jan;169(1):41–47. doi: 10.1093/infdis/169.1.41. [DOI] [PubMed] [Google Scholar]
- McElrath M. J., Hoffman M., Kluckling S., Corey L., Greenberg P. D. HIV-infected macrophages as efficient stimulator cells for detection of cytotoxic T cell responses to HIV in seronegative and seropositive vaccine recipients. AIDS Res Hum Retroviruses. 1994 May;10(5):541–549. doi: 10.1089/aid.1994.10.541. [DOI] [PubMed] [Google Scholar]
- Mellors J. W., Kingsley L. A., Rinaldo C. R., Jr, Todd J. A., Hoo B. S., Kokka R. P., Gupta P. Quantitation of HIV-1 RNA in plasma predicts outcome after seroconversion. Ann Intern Med. 1995 Apr 15;122(8):573–579. doi: 10.7326/0003-4819-122-8-199504150-00003. [DOI] [PubMed] [Google Scholar]
- Montefiori D. C., Lefkowitz L. B., Jr, Keller R. E., Holmberg V., Sandstrom E., Phair J. P. Absence of a clinical correlation for complement-mediated, infection-enhancing antibodies in plasma or sera from HIV-1-infected individuals. Multicenter AIDS Cohort Study Group. AIDS. 1991 May;5(5):513–517. doi: 10.1097/00002030-199105000-00006. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Gulizia R. J., MacIsaac P. D., Corey L., Greenberg P. D. Resistance to human immunodeficiency virus 1 infection of SCID mice reconstituted with peripheral blood leukocytes from donors vaccinated with vaccinia gp160 and recombinant gp160. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2443–2447. doi: 10.1073/pnas.90.6.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen C., Pedersen C., Lundgren J. D., Gerstoft J. Biological properties of HIV isolates in primary HIV infection: consequences for the subsequent course of infection. AIDS. 1993 Aug;7(8):1035–1040. doi: 10.1097/00002030-199308000-00002. [DOI] [PubMed] [Google Scholar]
- Niu M. T., Jermano J. A., Reichelderfer P., Schnittman S. M. Summary of the National Institutes of Health workshop on primary human immunodeficiency virus type 1 infection. AIDS Res Hum Retroviruses. 1993 Sep;9(9):913–924. doi: 10.1089/aid.1993.9.913. [DOI] [PubMed] [Google Scholar]
- Schwartz D. H., Gorse G., Clements M. L., Belshe R., Izu A., Duliege A. M., Berman P., Twaddell T., Stablein D., Sposto R. Induction of HIV-1-neutralising and syncytium-inhibiting antibodies in uninfected recipients of HIV-1IIIB rgp120 subunit vaccine. Lancet. 1993 Jul 10;342(8863):69–73. doi: 10.1016/0140-6736(93)91283-r. [DOI] [PubMed] [Google Scholar]
- Sinicco A., Fora R., Sciandra M., Lucchini A., Caramello P., Gioannini P. Risk of developing AIDS after primary acute HIV-1 infection. J Acquir Immune Defic Syndr. 1993 Jun;6(6):575–581. [PubMed] [Google Scholar]
- Weiss P. J., Brodine S. K., Goforth R. R., Kennedy C. A., Wallace M. R., Olson P. E., Garland F. C., Hall F. W., Ito S. I., Oldfield E. C., 3rd Initial low CD4 lymphocyte counts in recent human immunodeficiency virus infection and lack of association with identified coinfections. J Infect Dis. 1992 Nov;166(5):1149–1153. doi: 10.1093/infdis/166.5.1149. [DOI] [PubMed] [Google Scholar]
- Zhang L. Q., MacKenzie P., Cleland A., Holmes E. C., Brown A. J., Simmonds P. Selection for specific sequences in the external envelope protein of human immunodeficiency virus type 1 upon primary infection. J Virol. 1993 Jun;67(6):3345–3356. doi: 10.1128/jvi.67.6.3345-3356.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T., Mo H., Wang N., Nam D. S., Cao Y., Koup R. A., Ho D. D. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science. 1993 Aug 27;261(5125):1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]




