Abstract
A better understanding of mucosal immunity is required to develop more protective vaccines against Mycobacterium tuberculosis. We developed a murine aerosol challenge model to investigate responses capable of protecting against mucosal infection. Mice received vaccinations intranasally with CpG-adjuvanted antigen 85B (Ag85B/CpG) and/or Bacillus Calmette-Guerin (BCG). Protection against aerosol challenge with a recombinant GFP- expressing BCG was assessed. Mucosal prime/boost vaccinations with Ag85B/CpG and BCG were protective, but did not prevent lung infection indicating more efficacious mucosal vaccines are needed. Our novel finding that protection correlated with increased airway dendritic cells early post-challenge could help guide the development of enhanced mucosal vaccines.
Keywords: Mycobacterium tuberculosis (Mtb), BCG, Vaccination, Mucosal Specific Immunity
1. Introduction
One third of the world’s population is infected with Mycobacterium tuberculosis (Mtb), the causative agent of tuberculosis (TB), leading to 1–2 million deaths annually [1]. Although the Bacille Calmette-Guérin (BCG) vaccine has been used for prevention of TB since 1921 efficacy rates of intradermal BCG vaccination are highly variable [2]. Meta-analyses suggest that BCG vaccination can reduce pulmonary TB by approximately 50% [3]. These partially protective effects appear to wane within 10–15 years of infant BCG vaccination, and there is little evidence to support prolonged benefits [4].
Heterologous prime/boost vaccination strategies induce robust T cell responses and may improve protection compared to BCG alone [5–7]. Therefore, many new TB vaccine approaches under development focus on “booster” vaccines to enhance and extend immunity acquired after primary BCG immunization [8]. Another potentially useful strategy is mucosal vaccination. BCG is usually delivered intradermally which is not expected to induce optimal mucosal TB immunity. Regional immunity in the lung may be important for enhanced protection at the site of initial infection, and intranasal (IN) or other mucosally delivered vaccines might induce Mtb specific mucosal immunity capable of preventing TB infection [9].
In the present work, we investigated whether prime/boosting intranasal vaccinations with BCG and Ag85B/CpG can induce mucosal protection against primary mycobacterial infection of the lung better than a single dose of BCG given intranasally. We use a recombinant BCG expressing green fluorescent protein (GFPrBCG) strain for aerosol challenge to allow sensitive detection of infection, and to maximize our ability to identify at least partially protective mucosal immune responses that can be iteratively improved.
2. Materials and methods
2.1 Animals and organisms
Studies using six to eight week old NCI/Charles River Laboratory C57BL/6 mice were carried out in AAALAC accredited facilities, and approved by the Institutional Animal Care and Use Committee of Saint Louis University (A3225-01). Mycobacterium bovis Bacillus Calmette- Guerin (BCG) and GFPrBCG were grown, frozen and titered as previously described [10].
2.2 Immunizations
Prior to IN immunization with BCG and/or CpG-adjuvanted protein (10μl volume split between naris), mice were anesthetized by intraperitoneal injection of ketamine (60 mg/kg) and xylazine (5mg/kg). Based on previously published data [11] and upon our preliminary dose-optimization studies, mice received 1×107 CFU of BCG Danish strain (Statens Serum Institut, Denmark) 8 twice on two consecutive days and/or 10μg Ag85B (provided by M.A. Horowitz [12]) mixed with 10μg CpG 1826 (Coley Pharmaceuticals).
2.3 Aerosol challenge and determination of BCG growth
Stock vials of GFPrBCG were thawed, sonicated (Digital Sonifier 450 sonicator) and diluted in 0.9% saline containing 0.04% Tween-80 to a final concentration of ~1×107 CFU/ml. Mice were challenged with aerosolized BCG using a nose-only inhalation exposure system (NOIES; CH Technologies) as described previously[13]. Animals were exposed to aerosolized BCG for 20 minutes with an air pressure of 20 psi, an air flow rate of 2.0 liters/min and a BCG suspension flow rate of 1.0 ml/min, followed by 5 min of air flow only. Growth of BCG in tissues was evaluated 24 hours to 6 months after aerosol challenge. Lungs and spleens were homogenized in albumin-dextrose-catalase (ADC) supplemented Middlebrook 7H9 media and plated on Middlebrook 7H10 agar containing oleic-acid-albumin-dextrose-catalase (OADC) enrichment ±Kanamycin (30μg/ml).
2.4 Bronchoalveolar lavage
Bronchoalveolar lavage (BAL) was performed to collect cells for use in IFN-γ ELISPOT and flow cytometric analyses. Briefly, mice were euthanized, and the trachea was exposed and cannulated. The lungs were lavaged three times with 1ml PBS. Red blood cells were lysed in NH4Cl lysis buffer, then cells washed with PBS, before being resuspended in complete media.
For flow cytometric analysis, cells were stained using the following antibodies: FITC-anti-CD40, PE-anti-MHCII, PerCP-anti-CD8, PE-Cy7-anti-CD11c, APC-anti-CD19 and Pacific Blue-anti-CD4 (BD) and analyzed using an LSR II Flow Cytometry unit (BD) and FlowJo7 software (TreeStar, Ashland, OR) [14].
2.5 Antigen-specific IFN-γ E LISPOT responses
IFN-γ production was measured by ELISPOT assay using splenocytes (5×105 cells/well) or cells obtained by BAL (5×104 cells/well). Cells were stimulated overnight with recombinant Ag85B protein (10μg/ml), Mtb culture filtrate proteins (MtbCF, 10μg/ml, Colorado State University), Mtb whole cell lysate (MtbWL, 10μg/ml) [15], live BCG (MOI of 2.0, 0.2 and 0.02), or medium alone. Results are reported as numbers of IFN-γ spot-forming cells (SFC) per million cells.
2.6 Statistical analysis
Mann–Whitney U tests and Spearman-Rank tests were performed using Statistica v6.0 software (Statsoft, Tulsa, OK). Probability values below 0.05 were considered significant.
3. Results
3.1 Intranasal BCG vaccination results in long-term infection and induces potent CD4+ and CD8+ T cell responses in the airways of mice
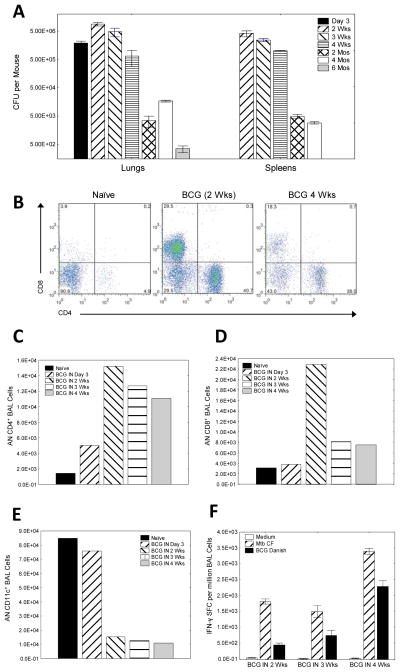
To investigate persistence of BCG after mucosal immunization, mice were vaccinated IN with 1×107 BCG on two consecutive days (optimal immunogenic dose determined in preliminary experiments; not shown) and kinetics of BCG persistence in lungs and spleens were examined at various time points post-vaccination (day 3, week 2, week 3, week 4, month 2, month 4 and month 6). We observed peak BCG CFU in the lungs and spleens 2 weeks following IN infection (>5×106 CFU/mouse), though high numbers of BCG persisted throughout the first month of infection (>5×105 CFU/mouse). Importantly, low levels of BCG persisted in the lungs (250–450 CFU/mouse), for at least 6 months post-vaccination (Fig. 1A).
Figure 1.
BCG persistence and recruitment of mycobacterial-specific T cells into the lungs of mice after intranasal BCG vaccination (A–F). C57BL6/J mice were sacrificed 3 days to 6 months after two administrations of 1×107 BCG on two consecutive days, and lung and spleen homogenates were plated on Middlebrook agar and incubated at 37°C for >3weeks to enumerate BCG (colony forming units, CFU) (A). C57BL6/J mice were immunized IN with 1×107 BCG two times on two consecutive days and bronchoalveolar lavage (BAL) cells harvested 3 days, 2 weeks, 3 weeks and 4 weeks later for analysis by flow cytometry (B–E) and IFN-γ ELISPOT (F). Shown in B are representative FACS plots of BAL cells from naïve and BCG vaccinated mice (2 and 4 weeks post immunization). Absolute numbers (AN) of BAL CD4+ T cells (C), BAL CD8+ T cells (D), and BAL CD11c+ cells (E), were determined 3 days to 4 weeks post-BCG vaccination. Additionally, BAL cells from naïve mice and IN BCG vaccinated mice were stimulated with Mtb culture filtrate (MtbCF) or live BCG in IFN-γ ELISPOT assays 2, 3, and 4 weeks post-vaccination (F). Bars represent mean values, and whiskers represent standard errors.
BAL cells are important for mucosal protection in the lungs, and responses by BAL cells serve as a potential surrogate marker for assessing new TB vaccines [16]. To examine the kinetics of BCG-specific T cell responses in the lungs, mice were vaccinated intranasally with BCG, and BAL cells were harvested at various time points for flow cytometry and IFN-γ ELISPOT assays. We observed an increase of both CD4+ and CD8+ T cells in the lungs of mice within the first four weeks following vaccination (Fig. 1B). The absolute numbers (AN) of BAL CD4+ and CD8+ T cells increased within 3 days post-immunization, peaked 2 weeks post-immunization, and remained increased through week 4 (Figs. 1C&D). It is noteworthy that during the time period of T cell influx into the lungs of BCG vaccinated mice (3 days to 4 weeks post-vaccination), a log decrease in absolute number of airway CD11c+ cells was observed (Fig. 1E). These CD11c+ cells expressed other markers characteristic of dendritic cells (DC) including high levels of MHC-II and CD40 (not shown).
IFN-γ responses produced by BAL cells after in vitro stimulation with MtbCF or BCG in ELISPOT assays confirmed that antigen-specific CD4+ and CD8+ T cells were recruited into the lungs (Fig. 1F), and also demonstrated that the frequencies of TB-specific T cells among BAL cells were 10 fold greater than the frequencies present among spleen cells (the numbers of Mtb culture filtrate- and live BCG-stimulated IFN-γ-producing SFC detectable in BAL were 3,386±93 and 1506±179/million cells, respectively; the matching results for splenocytes were more than 10 fold lower with 268±32 and 21±3/million cells, respectively).
3.2 Development of a sensitive challenge model system for detection of mucosal protection against initial lung infection
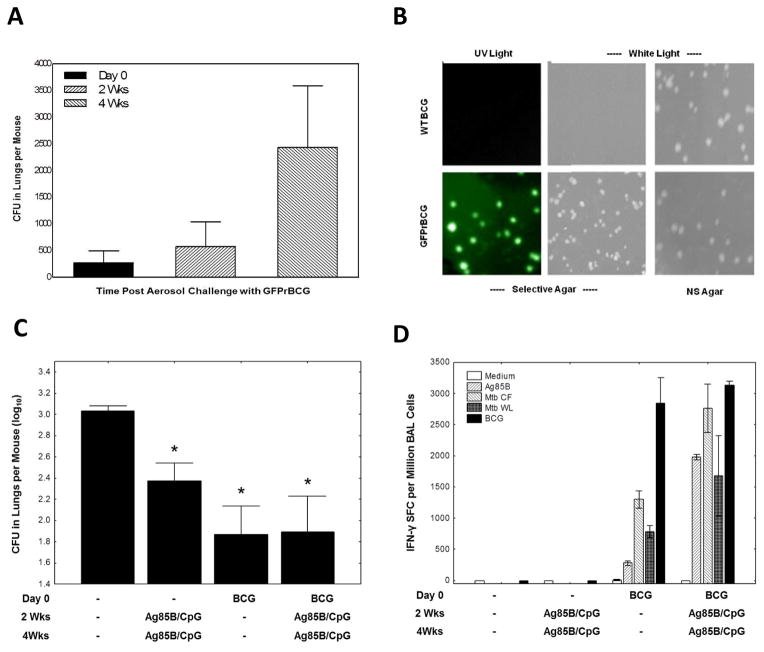
As an initial screen for mucosal immune responses relevant for protection against initial TB infection, we developed an aerosol BCG challenge model. We reasoned that if a mucosal vaccine does not protect against a BCG mucosal challenge it would not protect against an Mtb mucosal challenge. To distinguish BCG persisting in the lungs of mice after BCG vaccination from mycobacteria establishing a new infection, post-challenge naïve mice were challenged with aerosolized GFPrBCG. Numbers of CFUs in the lungs of GFPrBCG-infected mice increased 3 fold from day 0 to 2 weeks and were more than 10 fold higher 4 weeks post-infection (Fig. 2A). Homogenized lungs after wild type (WT) BCG vaccination and GFPrBCG challenge were plated on selective (kanamycin) and nonselective 7H10 Middlebrook agar. As shown (Fig. 2B), GFPrBCG grows on both types of media and is fluorescent under ultraviolet lighting, whereas WT BCG does not grow on selective agar and is not fluorescent under ultraviolet illumination.
Figure 2.
Mycobacterial growth and immune protection against aerosolized mycobacterial challenge after intranasal prime-boost vaccination (A–D). Naïve C57BL6/J mice were infected by aerosol with GFPrBCG with a challenge dose of ~250 GFPrBCG CFU/mouse, sacrificed at day 0, 2 weeks and 4 weeks post-infection and lung homogenates were plated on kanamycin selective 7H10 Middlebrook agar. BCG growth is reported as mycobacterial colony forming units (CFU) per animal. Each time point represents data from of 3–4 mice per group (A). To demonstrate our ability to properly discriminate between BCG used for vaccination and BCG used for challenge, wild type BCG (WT BCG) and GFP-expressing recombinant BCG (GFPrBCG) were plated on selective and nonselective (NS) 7H10 Middlebrook agar (with and without 30μg/ml kanamycin, respectively). After incubation at 37°C for ~3 weeks, colonies were visualized under white and ultraviolet (UV) light (B). The results of immunization and challenge experiments are shown in panel (C). C57BL6/J mice were vaccinated IN with 2 doses of a subunit vaccine containing Ag85B/CpG, with 2 consecutive days of BCG intranasal vaccination alone, or with heterologous prime/boost combinations of BCG and Ag85B/CpG (all mice received vaccinations or saline at time 0, week 2 and week 4) and subsequently challenged by aerosol with GFPrBCG two months after the initial vaccination. Mice were sacrificed 4 weeks post aerosol challenge and lung homogenates were plated on kanamycin selective 7H10 Middlebrook agar. Colonies of challenge organisms (GFPrBCG), growing on selective plates are reported as the average mycobacterial colony forming units (CFU) per animal. (Shown are means ± SE from 8 mice per group, *p < 0.05 compared with the control group by Mann-Whitney U test.) BAL cells from naïve mice and mice vaccinated IN with BCG and/or Ag85B/CpG (day 0, 2 weeks and 4 weeks) and subsequently challenged by aerosol with GFPrBCG two months after the initial vaccination were harvested 2 weeks after aerosol challenge and stimulated overnight with Mtb antigens or live BCG in IFN-γ ELISPOT assays (D). The results are presented as the numbers of IFN-γ spot forming cells per million BAL cells. Bars represent mean values, and whiskers represent standard errors.
3.3 Ag85B vaccinations with and without BCG priming partially protect against mucosal infection
The protective efficacy of IN immunization against aerosol infection was examined after immunizations with BCG alone, Ag85B/CpG alone, or various prime/boosting combinations of BCG and CpG ±Ag85B. Groups of 8 mice were vaccinated IN three times, two weeks apart with BCG and/or CpG±Ag85B. Two months after the first immunization, mice were challenged with aerosolized GFPrBCG. The numbers of GFPrBCG were assessed in the lungs one month post-challenge (Fig. 2C). All BCG and/or Ag85B/CpG vaccinated mice were significantly protected compared to controls (P<0.05). Although there was a trend for enhanced protection, BCG priming followed by Ag85B/CpG boosting did not significantly enhance protective mucosal immunity. Similar results were seen in additional experiments challenging mice 7.5 months after immunization (data not shown).
Figure 2D presents the results of IFN-γ ELISPOT assays conducted using BAL cells collected 2 weeks post-aerosol challenge. Immunization with BCG±Ag85B induced increased IFN-γproducing T cells specific for Mtb secreted proteins and live BCG when compared to naïve mice, while immunization with Ag85B protein alone failed to show differences after Mtb secreted protein and live BCG stimulation compared with the control group. The numbers of antigen-specific T cells recruited into the lungs and reactive with Ag85B, Mtb CF and Mtb WL were 2–8 fold higher when BCG priming was followed by Ag85B/CpG boosting (Fig. 2D). However, IFN-γ ELISPOT responses in BAL cells stimulated with live BCG were similar between BCG/Ag85B prime/boosted mice and mice vaccinated with BCG alone.
The failure to enhance overall responses to all antigens expressed by the challenge strain may explain why the prime/boost vaccination protocol was not significantly more protective than BCG vaccination alone. To assess potential bio-markers capable of guiding the development of more successful mucosal vaccine strategies, we studied correlations between immune subsets recruited to the lungs early (day 3) after aerosol challenge, and the levels of protection measured by CFUs of the challenge strain present 4 weeks post-aerosol challenge. The numbers of CD4+ and CD8+ airway T cells present on day 3 post-challenge did not correlate with protection, however, spearman rank tests confirmed significant (positive) correlations between the absolute numbers of CD11c+, CD11c+CD40+ and CD11c+MHC-II+ dendritic cells in BAL on day 3 post-challenge and the mean protective value in lungs (log10 GFPrBCG in lungs for naïve mice minus log10 GFPrBCG in lungs for immunized mice) 4 weeks post-aerosol challenge (Table 1).
Table 1.
Specific immune cell subsets detected in bronchoalveolar lavage cells 3 days after aerosol challenge were compared to the mean protective responses in lungs. This protection was determined based on number of GFPrBCG colony forming units (CFU) counted 4 weeks post-aerosol challenge (log10 CFU GFPrBCG for naïve mice minus log10 CFU GFPrBCG for immunized mice).
| Comparison* | Valid | Spearman-Rank Test | P-level |
|---|---|---|---|
| AN CD11c+ vs Protective Responses in Lungs | 8 | 0.7381 | 0.036 |
| AN CD11c+CD40+ vs Protective Responses in Lungs | 8 | 0.7381 | 0.036 |
| AN CD11c+ MHCII+ vs Protective Responses in Lungs | 8 | 0.7143 | 0.046 |
AN – Absolute number;
4. Discussion
BCG vaccination has not lowered the overall prevalence of TB infection and disease. In fact, it is thought that current BCG vaccines provide very limited to no protection against mucosal TB infection [17–19]. Thus, the development of a sensitive model to identify partially protective mucosal immune responses remains of great importance. We reasoned that an attenuated BCG strain would not overwhelm suboptimal mucosal immune responses allowing for their sensitive detection, and that mucosal immune responses unable to protect against BCG infection would not protect against more virulent Mtb challenges. Therefore, we have developed a sensitive model that can identify the mechanisms of immunity responsible for partial mucosal protection to allow for the iterative optimization of future mucosal vaccination strategies.
This study presents evidence that BCG administered intranasally to mice results in chronic infection and persists in host lungs for months. In addition, we developed a GFPrBCG aerosol challenge model, which can be used to distinguish between BCG persistence after vaccination and infection after aerosol challenge. Intranasal vaccinations with BCG and/or Ag85B/CpG were partially protective against mucosal infection. These IN vaccinations resulted in recruitment of both CD4+Th1 and CD8+ T cells into the airways of mice within two weeks of infection. Large granular CD11c+ DCs noticeably decreased in frequency in the lungs two weeks post-infection. However, the number of DCs present in the airways three days post-aerosol challenge significantly correlated with levels of protection measured 4 weeks post-challenge.
Mucosal vaccination with soluble antigens has been shown to enhance protection against mucosally invasive intracellular pathogens[20]. For example, IN vaccination of mice with dimethyl-dioctadecyl-ammonium-bromide-adjuvanted Ag85 induced protective immunity against virulent Mtb challenge [21]. Our results are consistent with these findings. Although Ag85B/CpG boosting of BCG immunity did not significantly enhance protective mucosal immunity, future subunit vaccines given IN may be useful in boosting immunity in BCG primed individuals. Our new model will be useful in assessing these possibilities. In addition, the protection provided by Ag85B/CpG IN vaccination alone in our model is important because subunit vaccines in general are safer for mucosal delivery than live vaccines.
Induction of potent immune responses at the initial site of mucosal TB infection in the lung is likely to be essential for inducing optimal protection. It has been demonstrated that mucosal immunization with BCG induces strong type-1 immune responses in mice, associated with protection against mycobacterial challenges [22]. Although it is known that BCG induces CD4+, CD8+, CD1-restricted αβ and γδ T cell responses, while soluble Ag85 protein likely induces only CD4+ and some CD8+ T cell responses, very little is known about the immune subsets important for vaccine-induced mucosal protection [5, 23]. It has been reported that mucosal vaccination with a recombinant adenovirus induces better protection against Mtb aerosolized challenges, and that the numbers of TB specific CD4+ Th1 cells within the airways correlate with optimal mucosal protection [24]. In our current study we did not find a correlation between airway T cells and protection. This may be related to the time (3d post-challenge) we performed BAL which may have been too early to see maximal T cell recruitment into the airways.
We observed a significant correlation between the number of airway DCs 3d post-challenge and protection. This early DC correlation with protection has not been reported previously. Infected DCs migrate from the lungs of Mtb-infected mice into the mediastinal lymph nodes, where they activate both memory and new antigen-specific T cells [25]. In addition, early post-challenge, infected DCs could rapidly activate resident airway antigen specific T cells already present in the airways. Both of these early effects mediated by DCs could partially explain the novel correlation we have observed. More DCs present early post-challenge could result in more relevant T cells being recruited earlier to the lungs. This hypothesis will need to be further studied. The relative loss of DCs in BAL samples later after mucosal challenge could be due to the development and recruitment of mycobacterial-specific T cells capable of eliminating BCG-infected DCs.
Our newly developed GFPrBCG aerosol challenge model has a sensitive capacity for identification of immune responses important for protective mucosal immunity against mycobacterial infection. The use of BCG should not overwhelm partial immunity, and can serve as an initial screen; providing the advantages of limiting biohazardous exposure to laboratory personnel and serving as an initial milestone for the evaluation of mucosal vaccines. This model will allow us to more carefully examine the efficacy of protection against infection in future prime-boost immunization experiments because immune responses unable to protect against BCG could not be expected to provide protection against virulent Mtb. Of course, mucosal vaccination strategies and immune subsets protective against aerosolized BCG will need to be further tested for the capacity to protect against aerosolized Mtb challenge in order to confirm mucosal protection against virulent Mycobacterium.
Acknowledgments
We are grateful to Drs. Liana Tsenova and Gilla Kaplan (Rockefeller University, New York, NY) for their advices with Mtb aerosol challenge. This work was supported by NIH R21 AI059362 (“Vaccine induced mucosal protection against TB infection”) to D.F.H. Mtb whole cell lysate was provided as part of NIH, NIAID Contract No. HSN266200400091C, entitled “Tuberculosis Vaccine Testing and Research Materials,” which was awarded to Colorado State University. Michael O’Donnell (University of Iowa) kindly provided the GFPrBCG strain.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Organization WH. Global Tuberculosis Control: A short update to the 2009 report. 2009. [Google Scholar]
- 2.Manissero D, Hollo V, Huitric E, Kodmon C, Amato-Gauci A. Analysis of tuberculosis treatment outcomes in the European Union and European Economic Area: efforts needed towards optimal case management and control. Euro Surveill. 2010;15 [PubMed] [Google Scholar]
- 3.Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, Mosteller F. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271:698–702. [PubMed] [Google Scholar]
- 4.Sterne JA, Rodrigues LC, Guedes IN. Does the efficacy of BCG decline with time since vaccination? Int J Tuberc Lung Dis. 1998;2:200–207. [PubMed] [Google Scholar]
- 5.Hoft DF. Tuberculosis vaccine development: goals, immunological design, and evaluation. Lancet. 2008;372:164–175. doi: 10.1016/S0140-6736(08)61036-3. [DOI] [PubMed] [Google Scholar]
- 6.McConkey SJ, Reece WH, Moorthy VS, Webster D, Dunachie S, Butcher G, Vuola JM, Blanchard TJ, Gothard P, Watkins K, Hannan CM, Everaere S, Brown K, Kester KE, Cummings J, Williams J, Heppner DG, Pathan A, Flanagan K, Arulanantham N, Roberts MT, Roy M, Smith GL, Schneider J, Peto T, Sinden RE, Gilbert SC, Hill AV. Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nature medicine. 2003;9:729–735. doi: 10.1038/nm881. [DOI] [PubMed] [Google Scholar]
- 7.Vuola JM, Keating S, Webster DP, Berthoud T, Dunachie S, Gilbert SC, Hill AV. Differential immunogenicity of various heterologous prime-boost vaccine regimens using DNA and viral vectors in healthy volunteers. J Immunol. 2005;174:449–455. doi: 10.4049/jimmunol.174.1.449. [DOI] [PubMed] [Google Scholar]
- 8.McShane H, Hill A. Prime-boost immunisation strategies for tuberculosis. Microbes Infect. 2005;7:962–967. doi: 10.1016/j.micinf.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Hoft DF, Brown RM, Belshe RB. Mucosal bacille calmette-Guerin vaccination of humans inhibits delayed-type hypersensitivity to purified protein derivative but induces mycobacteria-specific interferon-gamma responses. Clin Infect Dis. 2000;30(Suppl 3):S217–222. doi: 10.1086/313864. [DOI] [PubMed] [Google Scholar]
- 10.Luo Y, Szilvasi A, Chen X, DeWolf WC, O’Donnell MA. A novel method for monitoring Mycobacterium bovis BCG trafficking with recombinant BCG expressing green fluorescent protein. Clin Diagn Lab Immunol. 1996;3:761–768. doi: 10.1128/cdli.3.6.761-768.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langermann S, Palaszynski S, Sadziene A, Stover CK, Koenig S. Systemic and mucosal immunity induced by BCG vector expressing outer-surface protein A of Borrelia burgdorferi. Nature. 1994;372:552–555. doi: 10.1038/372552a0. [DOI] [PubMed] [Google Scholar]
- 12.Hoft DF, Blazevic A, Abate G, Hanekom WA, Kaplan G, Soler JH, Weichold F, Geiter L, Sadoff JC, Horwitz MA. A new recombinant bacille Calmette-Guerin vaccine safely induces significantly enhanced tuberculosis-specific immunity in human volunteers. J Infect Dis. 2008;198:1491–1501. doi: 10.1086/592450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schriewer J, Buller RM, Owens G. Mouse models for studying orthopoxvirus respiratory infections. Methods in molecular biology. 2004;269:289–308. doi: 10.1385/1-59259-789-0:289. [DOI] [PubMed] [Google Scholar]
- 14.Eickhoff CS, Vasconcelos JR, Sullivan NL, Blazevic A, Bruna-Romero O, Rodrigues MM, Hoft DF. Co-administration of a plasmid DNA encoding IL-15 improves long-term protection of a genetic vaccine against Trypanosoma cruzi. PLoS neglected tropical diseases. 2011;5:e983. doi: 10.1371/journal.pntd.0000983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoft DF, Brown RM, Roodman ST. Bacille Calmette-Guerin vaccination enhances human gamma delta T cell responsiveness to mycobacteria suggestive of a memory-like phenotype. J Immunol. 1998;161:1045–1054. [PubMed] [Google Scholar]
- 16.Santosuosso M, Zhang X, McCormick S, Wang J, Hitt M, Xing Z. Mechanisms of mucosal and parenteral tuberculosis vaccinations: adenoviral-based mucosal immunization preferentially elicits sustained accumulation of immune protective CD4 and CD8 T cells within the airway lumen. J Immunol. 2005;174:7986–7994. doi: 10.4049/jimmunol.174.12.7986. [DOI] [PubMed] [Google Scholar]
- 17.Xing Z, Santosuosso M, McCormick S, Yang TC, Millar J, Hitt M, Wan Y, Bramson J, Vordermeier HM. Recent advances in the development of adenovirus- and poxvirus-vectored tuberculosis vaccines. Curr Gene Ther. 2005;5:485–492. doi: 10.2174/156652305774329230. [DOI] [PubMed] [Google Scholar]
- 18.Brewer TF. Preventing tuberculosis with bacillus Calmette-Guerin vaccine: a meta-analysis of the literature. Clin Infect Dis. 2000;31(Suppl 3):S64–67. doi: 10.1086/314072. [DOI] [PubMed] [Google Scholar]
- 19.Glyn Hewinson R. TB vaccines for the World. Tuberculosis (Edinb) 2005;85:1–6. doi: 10.1016/j.tube.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Santosuosso M, McCormick S, Roediger E, Zhang X, Zganiacz A, Lichty BD, Xing Z. Mucosal luminal manipulation of T cell geography switches on protective efficacy by otherwise ineffective parenteral genetic immunization. J Immunol. 2007;178:2387–2395. doi: 10.4049/jimmunol.178.4.2387. [DOI] [PubMed] [Google Scholar]
- 21.Giri PK, Verma I, Khuller GK. Enhanced immunoprotective potential of Mycobacterium tuberculosis Ag85 complex protein based vaccine against airway Mycobacterium tuberculosis challenge following intranasal administration. FEMS Immunol Med Microbiol. 2006;47:233–241. doi: 10.1111/j.1574-695X.2006.00087.x. [DOI] [PubMed] [Google Scholar]
- 22.Lyadova IV, Vordermeier HM, Eruslanov EB, Khaidukov SV, Apt AS, Hewinson RG. Intranasal BCG vaccination protects BALB/c mice against virulent Mycobacterium bovis and accelerates production of IFN-gamma in their lungs. Clin Exp Immunol. 2001;126:274–279. doi: 10.1046/j.1365-2249.2001.01667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santosuosso M, Wang J, Xing Z. The Prospects Of Mucosal Vaccination Against Pulmonary Tuberculosis. In: Smithe LT, editor. Focus on tuberculosis research. Nova Science Publishers, Inc; Hauppauge, NY: 2005. pp. 141–164. [Google Scholar]
- 24.Xing Z, McFarland CT, Sallenave JM, Izzo A, Wang J, McMurray DN. Intranasal mucosal boosting with an adenovirus-vectored vaccine markedly enhances the protection of BCG-primed guinea pigs against pulmonary tuberculosis. PLoS One. 2009;4:e5856. doi: 10.1371/journal.pone.0005856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolf AJ, Linas B, Trevejo-Nunez GJ, Kincaid E, Tamura T, Takatsu K, Ernst JD. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J Immunol. 2007;179:2509–2519. doi: 10.4049/jimmunol.179.4.2509. [DOI] [PubMed] [Google Scholar]