Abstract
Aims
To assess the effect of brief motivational enhancement intervention postpartum alcohol use.
Design
Single-blinded, randomized controlled effectiveness trial in which pregnant women were assigned to receive usual care or up to 5 face-to-face brief motivational enhancement sessions lasting 10–30 minutes each and occurring at study enrollment, 4 and 8 weeks after enrollment, 32 weeks of gestation, and 6 weeks postpartum.
Setting
Large, urban, obstetrics clinic.
Participants
Women who were ≥18 years old, < 20 weeks of gestation, and consumed alcohol during pregnancy. Of 3438 women screened, 330 eligible women were assigned to usual care (n=165) or intervention (n=165). Due to missing data, we analyzed 125 in the intervention group and 126 in the usual care group.
Measurements
The proportion of women with any alcohol use and the number of drinks per day, reported via follow-up telephone interviews at 4 and 8 weeks after enrollment, 32 weeks of gestation, and 6 weeks, 6 months, and 12 months postpartum.
Findings
In random effects models adjusted for confounders, the intervention group was less likely to use any alcohol (odds ratio 0.50; 95% confidence interval [CI], 0.23 – 1.09; P=0.08) and consumed fewer drinks per day (coefficient −0.11; 95% CI −0.23 − 0.01; P=0.07) than the usual care group in the postpartum period but these differences were non-significant. Missing data during the prenatal period prevented us from modeling prenatal alcohol use.
Conclusions
Brief motivational enhancement intervention delivered in an obstetrical outpatient setting did not conclusively decrease alcohol use during the postpartum period.
INTRODUCTION
Alcohol use by women in the prenatal period is the most preventable cause of mental retardation in the United States [1]. Fetal alcohol spectrum disorder (FASD), a cluster of infant abnormalities including growth retardation, central nervous system impairment, and craniofacial anomalies, is the most severe manifestation of prenatal alcohol exposure. Alcohol-related birth defects and alcohol-related neurodevelopemental disorders represent effects that do not meet criteria for FASD but are associated with alcohol use during pregnancy [2, 3] Although the risk of adverse fetal effects rises with heavy and binge-drinking, there is no evidence to confidently support a safe, lower-limit of alcohol intake during pregnancy. As such, even light-to-moderate drinking during pregnancy is a recommended target for intervention [4].
Despite considerable attention from public health agencies over several decades, alcohol use during pregnancy continues to exceed Healthy People 2010 and 2020 targets. Although most women stop drinking during pregnancy, approximately 11–13% of pregnant women continue to drink and 2–5% binge drink [5–7]. More effective public health and clinical interventions, targeting both non-pregnant women of childbearing age and pregnant women, are needed to reduce the prevalence of alcohol use during pregnancy and improve fetal outcomes.
Brief motivational enhancement (ME) interventions to reduce unhealthy alcohol use are effective in some clinical settings [8–10]. Such interventions have been studied in non-pregnant women of childbearing age [11, 12], postpartum women [13], and pregnant women [14–18]. The five randomized trials that delivered brief interventions during pregnancy to decrease alcohol use provided some mixed evidence of effectiveness of brief interventions. Two of the five studies found that brief intervention with a self-help component decreased alcohol use [17, 18]. Interestingly, both of these studies were done in low income population. The other three clinical trials did not find any treatment effect when brief intervention was used [15, 16, 19]. The brief interventions varied across the studies and included approximately 1 hour motivational interviewing [15, 19], take home manuals [15, 17, 18], and brief intervention with the pregnant woman and her partner [16]. In a retrospective cohort study, Goler et al evaluated the “real-world” effectiveness of Early Start, a program of prenatal substance use screening and treatment linked to prenatal care visits in the Kaiser Permanente Northern California system. [20] Of the 49,985 participants in Early Start, women who screened positive and received substance use treatment had better neonatal and maternal outcomes than women who screened positive but did not receive treatment. However, the analysis did not evaluate alcohol separately and address alcohol consumption outcomes.
Here, we report the results of a randomized controlled effectiveness trial of brief ME to reduce alcohol use during pregnancy and for 12 months postpartum. We hypothesized that pregnant women who received the brief ME in an obstetrical clinical setting would be more likely to abstain or significantly reduce their alcohol use during and after pregnancy than would women who received usual care in the same setting.
METHODS
Study Design, Setting, and Participants
This study was a single-blinded, randomized controlled effectiveness trial of a brief ME to prevent or reduce prenatal and postpartum alcohol use. It was implemented in a large, urban, obstetrics clinic in Pittsburgh, Pennsylvania. Women were eligible to participate in the study if they met the following criteria: (1) 18 years or older; (2) pregnant, planned to continue their pregnancy, and were not over 20 weeks of gestation; (3) spoke English; and (4) had consumed at least 3 drinks a week between conception and recognition of pregnancy, consumed at least 1 drink a week after recognition of pregnancy, or had at least one episode of binge drinking, defined as drinking ≥4 drinks on one occasion, after conception. While it would be beneficial to include all women who consumed any alcohol during pregnancy, we selected this level of alcohol use because a lower threshold would dilute any observed effect and would be insufficient for detecting intervention effects during and after pregnancy
Study enrollment took place between April 2000 and October 2002, and study follow-up was completed on June 30, 2004. The institutional review boards of the University of Pittsburgh and the hospital that housed the clinic approved the project, and all participants provided written informed consent to be included in the study.
Study Procedures
We recruited pregnant women from a large urban prenatal clinic who were attending their first or second obstetric visit in two phases. During the first phase, we collaborated with another ongoing study on preeclampsia and combined efforts for screening. We had an abbreviated screening instrument to determine the participants’ initial eligibility for either study. The screening instrument was administered by clinic staff and consisted of two questions about pre-pregnancy frequency of alcohol intake and frequency of binge drinking. Women who were eligible for either study were approached by a research assistant for recruitment and informed consent. Patients who screened positive (initial screen was positive if patient used alcohol at least weekly before the pregnancy and/or reported any binge of 4 or more drinks on one occasion during the year before pregnancy) on the initial screen were given a brief informed consent to undergo a more complete assessment of eligibility. This eligibility assessment instrument was administered by the research assistant and took approximately 5–10 minutes to complete. Unlike the initial screen, this assessment focused on the inclusion and exclusion criteria listed above. Women who met complete eligibility criteria for the study were asked to complete informed consent for the clinical trial. The informed consent described the clinical trial as a study about whether advice and counseling about lifestyle changes, such as alcohol, drug, and tobacco use, during pregnancy can improve the health of pregnant women and their babies. Eligible women who gave their consent were randomized to receive usual care (usual care group) or to receive brief ME designed to decrease their alcohol use during and after pregnancy (intervention group). Women randomized to usual care received the standard warnings on alcohol use that are administered by the prenatal clinic staff but did not receive any other intervention.
Randomization was accomplished with the use of sealed envelopes that were prepared in 7 blocks of 64 by the study statistician according to standard randomization techniques and consecutively numbered in order to avoid temporal effects. Enrollment continued until the number of women assigned to groups reached 330. Our sample size calculations indicated that 150 subjects per group gave 80% power to detect a difference in abstinence of 14% (50% vs. 64%) at the 2nd trimester, 12% (15% vs. 27%) at 6 months postpartum, and 10% (9% vs. 19%) at 12 months postpartum (one-sided α = 0.05 and β =0.20).
Intervention
Participants in the intervention group were asked to attend 5 sessions that used motivational interviewing strategies [21]. We specifically modified the motivational enhancement therapy [22] of Project MATCH into a brief format suitable for an outpatient obstetrical setting and for a range of alcohol use. We used the FRAMES (feedback, responsibility, advice, menu, empathy, self-efficacy) structure for the brief intervention content [21, 23]. The content for the intervention was developed and approved by the investigative team which included expertise in motivational interviewing, psychology, internal medicine, addiction medicine, obstetrics-gynecology, and neonatology.
The 5 intervention sessions focused on alcohol use, provided specific feedback based on use and alcohol risks to the fetus, and included a plan for changes in behavior. The sessions took place at enrollment, 4 and 8 weeks later, at 32 weeks of gestation, and at 6 weeks postpartum during participants’ regular scheduled clinic visits with their obstetrical providers. For the 6-week postpartum visit only, the intervention was conducted by telephone if the participant missed the clinic visit. This intervention session focused on safe drinking behaviors. Otherwise, make-up intervention sessions were not scheduled if the participant missed the prenatal clinic visit or the intervention could not be done for another reason. The prenatal sessions lasted 10–15 minutes, and the postpartum session lasted 10–30 minutes. The main goals were to motivate the women to abstain from alcohol while pregnant, encourage alcohol-dependent women to accept referral to a specialized treatment program, reinforce safe prenatal alcohol use in women who had already eliminated alcohol, and encourage safe drinking behaviors after delivery to protect future pregnancies and to improve overall health.
The sessions were motivational, face-to-face, and led by a registered nurse or a lay counselor who had been trained by two study investigators. We selected these individuals for training because they were the types of providers who could be taught to deliver the same intervention in typical obstetric practices. One of the investigators trained the interventionists over two 8-hour training sessions by reviewing the treatment manual, reviewing concepts, and role playing. Interventionists were recorded during training and the audiotapes were reviewed in order to provide feedback.
Intervention fidelity during the trial was maintained by audiotaping all intervention sessions with subjects and regular weekly-to-biweekly meetings for the interventionists to review the audiotapes with two study investigators and receive feedback. Intervention fidelity was further maintained by having the interventionists complete a checklist of therapist actions (opening statement, providing feedback and information sheet, assessment of subject understanding and concerns, exploration of concerns, elicitation of self-motivational statements, assist with decision making, change plan, assessment of stage of change) after each intervention and by trying to have the same interventionist cover a specific subject throughout the study.
Study Outcomes and Instruments
A blinded member of the study team telephoned participants in the intervention group and the usual care group to assess their alcohol use at 4 and 8 weeks after enrollment, at 32 weeks of gestation, and at 6 weeks, 6 months, and 12 months postpartum. To determine alcohol use at baseline and each follow-up, we used a validated instrument developed by the Maternal Health Practices and Child Development Project [24, 25]. This instrument probes the usual, minimum, and maximum quantities and frequencies of drinking wine, beer, and liquor. It allows for calculation of quantity, frequency, minimum and maximum alcohol use on drinking days, average drinks per day, binge and frequent heavy drinking. At each assessment time, we characterized alcohol use behavior in terms of any alcohol use (proportion of women who consumed any alcohol) and number of drinks per day.
We used the alcohol module of the Composite International Diagnostic Interview (CIDI) to determine whether participants had an Alcohol Use Disorder at baseline [5]. This comprehensive, fully structured diagnostic interview uses algorithms to determine lifetime diagnoses of disorders according to the accepted definitions of the 10th revision of the International Classification of Diseases (ICD-10) and the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV).
We used the Edinburgh Postnatal Depression Scale, a validated, 10-item instrument developed specifically for use in pregnant and postnatal women to determine whether participants were depressed at baseline and at each follow-up [26–28]. This instrument is 86% sensitive and 78% specific for the diagnosis of depression and is sensitive to change over time [26].
Statistical Analyses
We used descriptive statistics to characterize the participants at baseline. To compare the usual care group with the intervention group in terms of sociodemographic characteristics and baseline alcohol use, we used analysis of variance (ANOVA) for continuous variables and chi-square tests for categorical variables.
Our primary analysis was intention-to-treat. Our two primary outcomes were any alcohol use (yes/no) and the number of drinks per day. To adjust for intra-patient correlation, we used random effects logistic regression to model any alcohol use. We modeled the log of drinks per day (+1) and used random effects tobit regression because drinks per day had a positive skewed distribution and the data were censored below 0. Given substantial missing data during the prenatal period, we only modeled alcohol use for the postpartum period. We modeled a quadratic trend across the three postpartum time periods. We only included observations that did not have any missing data on the covariates or if they had alcohol use data on any of the three postpartum time points, resulting in 125 participants in the intervention group and 126 in the usual care group.
In the regression analyses, we controlled for baseline alcohol use, age, race, education level, smoking, whether it was their first pregnancy, and depression. Because of the limited number of women who reported hazardous drinking (>1 drink per day on average) or binge drinking (≥4 drinks on one occasion) after baseline, we did not include these as separate outcomes in our regression analyses.
For all analyses, we used Stata V11 (StataCorp, College Station, Texas), and we considered a P value of <0.05 to be significant.
RESULTS
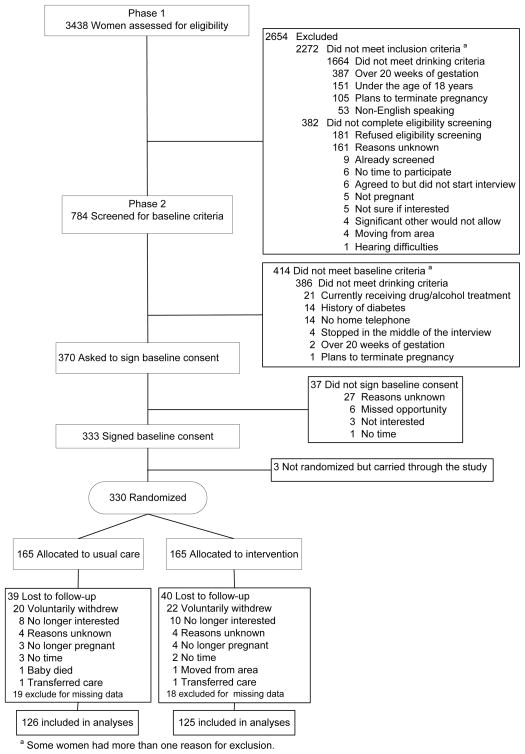
We initially screened 3438 women for the brief ME intervention study (Figure 1). Many of these women were not eligible for the study because they failed to meet the alcohol use criteria. The women who did not meet the alcohol use criteria were more likely to be younger, black, and have non-Medicaid insurance than were women who met the alcohol use criteria for our intervention study (P ≤ 0.001 for each).
Figure 1.
Flow Process for Screening, Enrollment, and Randomization
Randomization and Follow-Up of Participants
A total of 330 women who met the study criteria were randomized to receive usual care or a brief ME, with 165 in each group. Of the 330 participants, 38% (40% usual care vs. 37% intervention) completed the assessment at 4 weeks after enrollment, 52% (56% vs. 51%) at 8 weeks after enrollment, 56% (58% vs. 54%) at 32 weeks of gestation, 75% (73% vs. 76%) at 6 weeks postpartum, 71% (72% vs. 71%) at 6 months postpartum and 68% (70% vs. 66%) at 12 months postpartum. Twenty women from usual care and 22 women from the intervention group withdrew with the majority of the women no longer being interested (Figure 1). Seventy nine women (24%) were lost to follow-up.
We compared those who had some missing data (n=272) to those with no missing data (n=58) and found no differences on baseline drinking (any drinking P=0.95 or average daily drinking P=0.44). We also compared baseline characteristics of those that were included in the analyses by intervention group and usual care group and found no significant differences between those that were included in the analysis and those that had missing data.
In the intervention group, 25% of participants attended all 5 sessions of the intervention, 25% attended 4 sessions, 13% attended 3 sessions, 13% attended 2 sessions, 15% attended 1 session, and 9% attended no sessions.
Characteristics of Study Participants at Baseline
At baseline, the intervention and usual care groups were similar on sociodemographic and clinical characteristics (Table 1). The only significant difference was a higher average depression score in the usual care group than in the intervention group (8.69 versus 7.27; P= 0.01).
Table 1.
Sociodemographic Characteristics and Alcohol-Related Characteristics of the 330 Study Participants at Baselinea
| Characteristic | Usual Care Group (n = 165) | Intervention Group (n = 165) | P Value |
|---|---|---|---|
| Age, mean ± SD | 24.1 ± 5.40 | 23.5 ± 4.04 | 0.31 |
| Race (N = 330) | 0.66 | ||
| Black | 67 (40.6) | 75 (45.5) | |
| White | 92 (55.8) | 85 (51.5) | |
| Other | 6 (3.6) | 5 (3.0) | |
| Insurance (N = 310) | 0.31 | ||
| Non-Medicaid | 20 (12.7) | 14 (9.2) | |
| Medicaid | 137 (87.3) | 139 (90.8) | |
| Marital status (N = 296) | 0.29 | ||
| Never married | 97 (64.7) | 92 (63.0) | |
| Presently married | 17 (11.3) | 10 (6.8) | |
| Marriage-like relationship | 27 (18.0) | 29 (19.9) | |
| Divorced or separated | 6 (4.0) | 6 (4.1) | |
| Other | 3 (2.0) | 9 (6.2) | |
| Gestational age, mean ± SD | 9.7 ± 3.8 | 9.9 ± 4.3 | 0.68 |
| Education level (N = 293) | 0.054 | ||
| Less than a high school diploma | 38 (25.5) | 27 (18.8) | |
| High school diploma or GED | 51 (34.2) | 72 (50.0) | |
| Some post–high school education | 42 (28.2) | 33 (22.9) | |
| Degree past high school diploma | 18 (12.1) | 12 (8.3) | |
| Current employment status (N = 294) | 0.34 | ||
| Unemployed | 18 (12.2) | 12 (8.2) | |
| Full-time homemaker | 48 (32.4) | 45 (30.8) | |
| Part-time student | 3 (2.0) | 7 (4.8) | |
| Full-time student | 19 (12.8) | 18 (12.3) | |
| Part-time worker (not student) | 17 (11.5) | 27 (18.5) | |
| Full-time worker | 43 (29.1) | 37 (25.3) | |
| Smoker at baseline | 64 (42.7) | 63 (43.2) | 0.93 |
| Number of previous pregnancies (N = 295) | 0.63 | ||
| 0 | 37 (24.8) | 32 (21.9) | |
| 1 | 41 (27.5) | 33 (22.6) | |
| 2 | 29 (19.5) | 32 (21.9) | |
| ≥3 | 42 (28.2) | 49 (33.6) | |
| Planned pregnancy (N=294) | 16 (10.7) | 21 (14.5) | 0.33 |
| Depression score (EPDS), mean ± SD (N=296) | 8.7 ± 5.4 | 7.3 ± 4.4 | 0.01 |
| Number of drinks per day (N=330) | |||
| Before pregnancy, mean ± SD (median; range) | 3.4 ± 4.7 (2.0; 0–31.8) | 3.6 ± 5.4 (2.0; 0–48.7) | 0.65 |
| Before recognition of pregnancy, mean ± SD (median; range) | 2.1 ± 3.5 (1.2; 0–27.4) | 2.3 ± 4.2 (1.2; 0–43.2) | 0.89 |
| After recognition of pregnancy, mean ± SD (median; range) | 0.5 ± 3.4 (0; 0–31.8) | 0.2 ± 0.8 (0; 0–7) | 0.98 |
| Any alcohol use after recognition of Pregnancy (N=330) | 52 (31.5) | 55 (33.3) | 0.72 |
| Binge drinking (≥4 drinks on one occasion) (N=330) | |||
| Before pregnancy | 149 (90.3) | 153 (92.7) | 0.43 |
| Before recognition of pregnancy | 122 (73.9) | 129 (78.2) | 0.37 |
| After recognition of pregnancy | 17 (10.3) | 16 (9.7) | 0.85 |
| Alcohol disorder status, based on CIDI score (N = 250) | 0.73 | ||
| Alcohol dependence | 32 (25.0) | 27 (22.1) | |
| Alcohol abuse | 31 (24.2) | 27 (22.1) | |
| Neither dependence nor abuse | 65 (50.8) | 68 (55.7) |
Abbreviations: CIDI, alcohol module of the Composite International Diagnostic Interview based on the accepted definitions of the 10th revision of the International Classification of Diseases (ICD-10) and the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV); EPDS, Edinburgh Postnatal Depression Scale; GED, graduate equivalency degree; SD, standard deviation.
Data are presented as number (%) unless otherwise indicated.
There was no significant difference between the groups in terms of baseline alcohol use. In general, the study participants reported substantial alcohol use before pregnancy. The average prepregnancy rates were 3.4 drinks per day in the usual care group and 3.6 drinks per day in the intervention group (Table 1). In both groups, alcohol use dropped steadily after the pregnancy was recognized. Over 70% of participants reported binge behavior between conception and recognition of pregnancy. Fewer than 35% reported any alcohol use between the time they recognized they were pregnant and the time of study enrollment.
Any Alcohol Use in the Postpartum
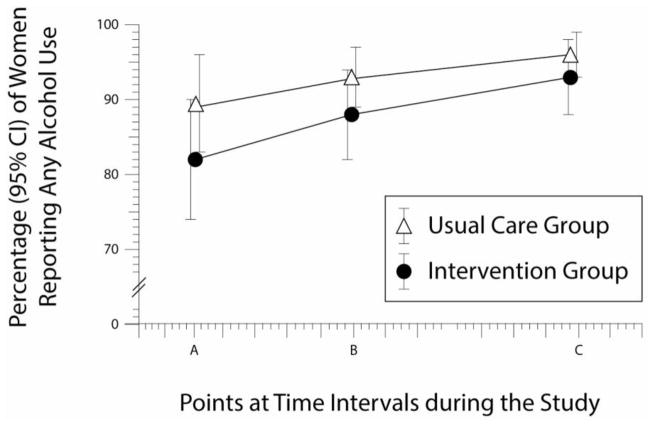
During the postpartum period, 89%–96% of the usual care group and 82%–93% of the intervention group drank at least some alcohol (Figure 2).
Figure 2. Any Drinking Between Brief Intervention and Usual Care.
Percentage of women reporting any alcohol use, with 95% confidence intervals (CIs), at the following points: (A) 6 weeks postpartum (P = 0.08); (B) 6 months postpartum (P = 0.09); and (B) 12 months postpartum (P = 0.11).
The random effects logistic regression model for any alcohol use (Table 2) showed a nonsignificant intervention effect during the postpartum period (OR 0.50; 95% CI 0.23–1.09; P=0.08). Younger age (P=0.02), higher education level (P≤0.001), and positive smoking status (P=0. 02) at baseline were associated with any alcohol use in the postpartum. No other variable was related to this outcome. First pregnancy approached (P=0.06) but did not meet the threshold for significance.
Table 2.
Random Effects Logistic Regression Model of Any Alcohol Use During Postpartum (n=251)
| Variable | Odds | Standard Error | P Value | 95% Confidence Interval |
|---|---|---|---|---|
| Intervention effect -prenatal | -- | -- | -- | -- |
| Intervention effect –postpartum | 0.502 | 0.395 | 0.081 | (0.231, 1.090) |
| Log drinking volume at baseline | 0.840 | 0.444 | 0.694 | (0.351, 2.007) |
| Age | 0.905 | 0.044 | 0.022 | (0.831, 0.986) |
| White | 1.112 | 0.412 | 0.797 | (0.495, 2.495) |
| Smoker | 2.867 | 0.431 | 0.015 | (1.232, 6.671) |
| Depression Scale | 1.008 | 0.041 | 0.836 | (0.931, 1.092) |
| First pregnancy | 0.400 | 0.486 | 0.059 | (0.154, 1.036) |
| Education | 1.426 | 0.101 | <0.001 | (1.169, 1.740) |
| # of intervention sessions | 1.020 | 0.181 | 0.911 | (0.715, 1.455) |
Drinks per Day after Baseline
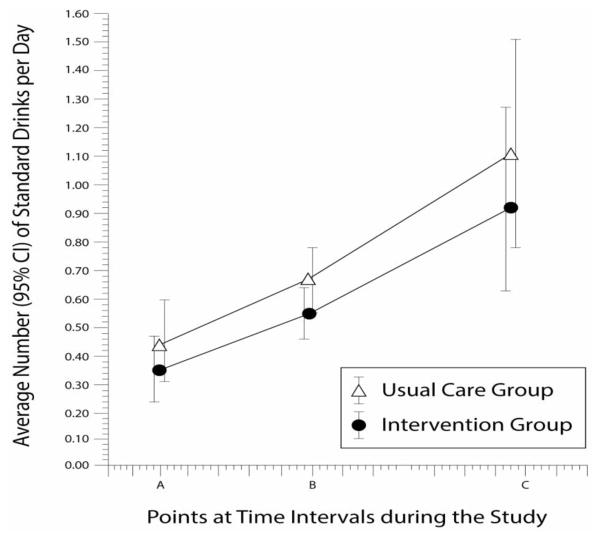
During the postpartum period, both the usual care and intervention groups showed an increase in drinks per day at each time point, with the usual care group consuming more alcohol than the intervention group (Figure 3). However, by the end of the study at 12 months post-partum, neither group had returned to their pre-pregnancy drinking levels.
Figure 3. Drinks Per Day Between Brief Intervention and Usual Care.
Average number of standard drinks per day, with 95% confidence intervals (CIs), at the following points: (A) 6 weeks postpartum (P = 0.07); (B) 6 months postpartum (P = 0.07); and (C) 12 months postpartum (P = 0.07).
The random effects tobit regression model (Table 3) showed differences between the groups with regard to drinks per day in the postpartum period (coefficient −0.11; 95% CI −0.23–0.01; P=0.07) with the intervention group reporting fewer drinks per day during the postpartum period than the usual care group but this fell just short of statistical significance. Smoking was associated with a higher number of drinks per day in this model (P=0.02), as it was in the previous model. This model found that African Americans reported a higher number of drinks per day (P<0.01) and unlike the previous model, age was not associated with drinks per day in the postpartum (P=0.61). We did not find any dose effects from number of intervention sessions completed (P=0.06).
Table 3.
Random Effects Tobit Regression Model of the Log of the Number of Drinks per Day During Postpartum (n=251)
| Variable | Coefficient | Standard Error | P Value | 95% Confidence Interval |
|---|---|---|---|---|
| Intervention effect - prenatal | -- | -- | -- | -- |
| Intervention effect – postpartum | −0.109 | 0.060 | 0.069 | (−0.227, 0.008) |
| Log drinking volume at baseline | −0.010 | 0.083 | 0.900 | (−0.174, 0.153) |
| Age | −0.004 | 0.007 | 0.611 | (−0.017, 0.010) |
| White | −0.237 | 0.065 | <0.001 | (−0.364, −0.109) |
| Smoker | 0.154 | 0.063 | 0.015 | (0.030, 0.277) |
| Depression | −0.001 | 0.006 | 0.934 | (−0.013, 0.012) |
| First pregnancy | −0.128 | 0.074 | 0.086 | (−0.273, 0.018) |
| Education | 0.024 | 0.014 | 0.087 | (−0.004, 0.052) |
| # of intervention sessions | −0.050 | 0.027 | 0.062 | (−0.103, 0.003) |
Table 4 shows the model estimates for both any drinking and average drinks per day for the 2 groups at each time point in the postpartum. As seen in the table, the probability of any drinking increases across the postpartum period for both groups, with the intervention group increasing at a slower rate. Similarly, the mean number of drinks per day increases across the postpartum period for both groups, with the intervention group consuming fewer drinks at each time point. While Table 4 shows a consistent intervention effect, that effect did not reach statistical significance in the multivariable models (Tables 2 & 3).
Table 4.
Model Estimates for Alcohol Use Outcomes at Each Time point
| Outcome | Time point | Brief Intervention | Usual Care | P value |
|---|---|---|---|---|
| Probability of any alcohol use (95% CI) | ||||
| Any Alcohol Use (Y/N) | 6 weeks postpartum | 0.82 (0.74 – 0.90) | 0.89 (0.83 – 0.96) | 0.084 |
| 6 months postpartum | 0.88 (0.82 – 0.94) | 0.93 (0.89 – 0.97) | 0.087 | |
| 12 months postpartum | 0.93 (0.88 – 0.98) | 0.96 (0.93 – 0.99) | 0.110 | |
| Mean drinks/day (95% CI)* | ||||
| Drinks Per Day | 6 weeks postpartum | 0.35 (0.24 – 0.47) | 0.44 (0.31 – 0.59) | 0.072 |
| 6 months postpartum | 0.55 (0.46 – 0.64) | 0.67 (0.57 – 0.78) | 0.069 | |
| 12 months postpartum | 0.92 (0.63 – 1.27) | 1.11 (0.78 – 1.51) | 0.069 | |
Antilog was used to calculate the means.
Newborn Infant Outcomes
At the time of delivery, we recorded the newborn infant’s head circumference, body weight, and body length. We controlled for gestational age and tested the difference between the intervention group and the usual group. Birthweight was slightly different between the two groups with the intervention group weighing less than the usual care group (3014 grams vs 3160 grams; P= 0.04). We found no differences between groups for head circumference or body length.
DISCUSSION
We found that brief ME did not reduce the percentage of pregnant women reporting any alcohol use and the number of drinks per day in the postpartum period when compared with usual care. While we found a trend toward an effect of the intervention on both outcomes in the postpartum period, neither reached statistical significance. Younger age, smoking, and higher education were associated with any alcohol use and smoking and being African American were associated with a greater number of drinks per day in the postpartum.
Although we observed a non-significant trend toward less postpartum alcohol use in the intervention group, both intervention and control groups returned to average levels of drinking postpartum that were substantially less than the heavy drinking they reported pre-pregnancy. Beyond potential effects of the intervention, this could have been a result of reactivity to our regular assessments of alcohol use [29, 30], underreporting of alcohol use in both groups due to social desirability bias, or actual decrease in alcohol use due to health, motivation, family duties, and other factors during the postpartum year. These factors may not have a differential effect between the intervention group and the usual care group and therefore contribute to the lack of a significant intervention effect.
Five prior clinical trials that focused on alcohol use in pregnant women had mixed results [12]. The earliest study showed that the intervention group had a higher quit rate, particularly among light drinkers [18]. Three subsequent studies found that a brief intervention of motivational interviewing did not significantly reduce alcohol use in pregnant women [14–16]. However, one study found that the women in the brief motivational intervention group were more likely than those in the assessment-only group to report abstinence from alcohol use in the third trimester of pregnancy when studying women in a nutrition program for women infants and children [14].
Our study and several prior studies did not exclude pregnant women who had already changed their alcohol use upon learning of their pregnancy. The rationale for including this group is that, although they might have stopped drinking at the time of enrollment, they are at risk for returning to drinking later in pregnancy and during postpartum. As such, the majority of our sample reported abstinence from alcohol at the time of enrollment. This may reflect the strong motivating effect that pregnancy can have on women to change their alcohol use but may have also resulted from underreporting due to social desirability bias. Regardless of the cause, abstinence at time of enrollment illustrates why it is difficult to demonstrate an intervention effect.
The impact of our findings for continuing brief ME through to the first post-partum visit is not clear. Our purpose in doing so was to continue engaging the new mother in discussion about safe alcohol use with the goal of providing a healthy family environment for the new child, optimizing the mother’s health, and protecting the next pregnancy from alcohol exposure. One other study that initiated brief intervention during pregnancy and also examined alcohol use in the postpartum period did not find a significant reduction in alcohol use when assessed at the first postpartum visit [12]. The Healthy Moms Study [13], did show a decrease in alcohol use after 4 brief intervention sessions, all delivered postpartum. Likewise, it is possible our study would have shown a stronger postpartum effect if additional postpartum sessions were added.
Our study has several strengths. First, we made this an “effectiveness” trial, meaning we took care to design the intervention as we thought it would occur in actual obstetrical practice. We used interventionists with no prior experience with brief ME. We delivered the interventions in clinic on the day of the women’s obstetrics visit, and did not require participants to have a minimum number of intervention sessions. In fact, only 50% of our intervention group had at least 4 of the 5 planned intervention sessions and 24% had 1 or fewer sessions. Second, participants in our study were rather heavy drinkers, averaging about 3.5 drinks per day pre-pregnancy which is well above the at-risk drinking cut-off for women, and many met criteria for alcohol dependence or abuse. That we observed a trend toward improvement in intervention subjects in the postpartum time period is encouraging but additional intervention sessions or a stronger intervention may be required for such a heavy drinking population. Lastly, our study population was racially diverse.
Our study had several limitations. First, we often had difficulty in reaching participants for follow-up assessments, particularly in the prenatal period. We made multiple attempts (up to 100 per participant per time point) to reach a participant by telephone for follow-up at a single time point. Despite this effort, our prenatal follow-up rates were much lower than expected, resulting in substantial missing data. The low follow-up rates affect the power of the study and also the validity of the study. With substantial missing data in the prenatal period, our estimates would likely be biased. Therefore, we only tested for differences between the two groups during the postpartum period when the follow-up rates consistently exceeded 70%. Second, because limited numbers of women reported hazardous drinking (>1 drink per day) and binge drinking (≥4 drinks on any one occasion) after baseline, we were unable to model changes in the study groups’ hazardous alcohol use and binge drinking across the perinatal period. Third, although we designed the study to be an effectiveness trial appropriate to “real-world” settings, we concede that delivering up to five 10–30 minute intervention sessions may not be feasible in many prenatal/postpartum care settings. Related to this, we did not formally assess the feasibility and acceptability of the intervention to the clinic staff and administrators nor assess the cost-effectiveness of the intervention. Finally, although the generalizability of the study is limited by its single site design, the results should be relevant to other prenatal clinics that care for a racially diverse, low-income pregnant population.
In summary, we found that brief ME may be effective for decreasing postpartum alcohol use among racially diverse, low-income pregnant women. The brief ME, initiated during pregnancy and carried on to the first postpartum visit, requires more research in order to determine the efficacy, effectiveness, and optimal implementation of the approach. Because implementation in real-life obstetrical practices is a major challenge, stepped care approaches that are flexible to severity of alcohol use and progress of the pregnant woman may be more efficient than the 5 planned sessions in our study. For example, in women who have risky prepregnancy or pre-recognition drinking but are abstinent after recognition, a potential approach is to have a single intervention during pregnancy and place more emphasis on the postpartum period with added booster sessions to prevent return to risky drinking and to protect the next pregnancy. Furthermore, computer-delivered and secure web-based approaches are worthy of study as they may facilitate accurate disclosure of alcohol use during and after pregnancy by blunting the social desirability bias and may improve efficiency of intervention delivery in busy clinical settings [31–33]. As a nonthreatening technology with proven efficacy in many clinical settings, brief ME has the potential to substantially reduce prenatal and postpartum drinking, improve the health of women and their infants, and reduce societal costs from fetal alcohol syndrome and other alcohol-related disorders.
Acknowledgments
This research was supported by grant AA012485 from the National Institute on Alcohol Abuse and Alcoholism, and UL1TR000005 from the National Center for Advancing Translational Sciences, National Institutes of Health.
Footnotes
Declarations of interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maier SE, West JR. Drinking patterns and alcohol-related birth defects. Alcohol Research & Health: the Journal of the National Institute on Alcohol Abuse & Alcoholism. 2001;25(3):168–74. [PMC free article] [PubMed] [Google Scholar]
- 2.Stratton KHC, Battaglia F, editors. Fetal Alcohol Syndrome: Diagnosis, Epidemiology, Prevention, and Treatment. National Academy Press; Washington DC: 1996. [Google Scholar]
- 3.Sokol RJ, Martier SS, Ager JW. The T-ACE questions: practical prenatal detection of risk-drinking. American Journal of Obstetrics & Gynecology. 1989;160(4):863–8. doi: 10.1016/0002-9378(89)90302-5. discussion 868–70. [DOI] [PubMed] [Google Scholar]
- 4.ACOG. At-Risk Drinking adn Alcohol Dependence: Obstetric and Gynecologic Implications. 2011 doi: 10.1097/AOG.0b013e31822c9906. ACOG Committee Opinion No. 496. [DOI] [PubMed] [Google Scholar]
- 5.Floyd RL, Sidhu JS. Monitoring prenatal alcohol exposure. Am J Med Genet C Semin Med Genet. 2004;127(1):3–9. doi: 10.1002/ajmg.c.30010. [DOI] [PubMed] [Google Scholar]
- 6.Morris DS, et al. Exploring pregnancy-related changes in alcohol consumption between black and white women. Alcoholism: Clinical & Experimental Research. 2008;32(3):505–12. doi: 10.1111/j.1530-0277.2007.00594.x. [DOI] [PubMed] [Google Scholar]
- 7.Control CfD. Alcohol use among pregnant and nonpregnant women of childbearing age --- United States, 1991—2005. MMWR. 2009:529–532. [PubMed] [Google Scholar]
- 8.Wilk AI, Jensen NM, Havighurst TC. Meta-analysis of randomized control trials addressing brief interventions in heavy alcohol drinkers. Journal of General Internal Medicine. 1997;12(5):274–83. doi: 10.1046/j.1525-1497.1997.012005274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitlock EP, et al. Behavioral counseling interventions in primary care to reduce risky/harmful alcohol use by adults: a summary of the evidence for the U.S. Preventive Services Task Force. Annals of Internal Medicine. 2004;140(7):557–68. doi: 10.7326/0003-4819-140-7-200404060-00017. [DOI] [PubMed] [Google Scholar]
- 10.Kaner EFS, et al. The effectiveness of brief alcohol interventions in primary care settings: a systematic review. Drug & Alcohol Review. 2009;28(3):301–23. doi: 10.1111/j.1465-3362.2009.00071.x. [DOI] [PubMed] [Google Scholar]
- 11.Floyd RL, et al. Preventing alcohol-exposed pregnancies: a randomized controlled trial.[Erratum appears in Am J Prev Med. 2007 Apr;32(4):360 Note: Johnson, Kenneth [added]] American Journal of Preventive Medicine. 2007;32(1):1–10. doi: 10.1016/j.amepre.2006.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manwell LB, et al. Treatment of problem alcohol use in women of childbearing age: results of a brief intervention trial. Alcoholism: Clinical & Experimental Research. 2000;24(10):1517–24. [PubMed] [Google Scholar]
- 13.Fleming MF, et al. The Healthy Moms Study: the efficacy of brief alcohol intervention in postpartum women. Alcoholism: Clinical & Experimental Research. 2008;32(9):1600–6. doi: 10.1111/j.1530-0277.2008.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Handmaker NS, Miller WR, Manicke M. Findings of a pilot study of motivational interviewing with pregnant drinkers. Journal of Studies on Alcohol. 1999;60(2):285–7. doi: 10.15288/jsa.1999.60.285. [DOI] [PubMed] [Google Scholar]
- 15.Chang G, et al. Brief intervention for alcohol use in pregnancy: a randomized trial. Addiction. 1999;94(10):1499–508. doi: 10.1046/j.1360-0443.1999.941014996.x. [DOI] [PubMed] [Google Scholar]
- 16.Chang G, et al. Brief intervention for prenatal alcohol use: a randomized trial. Obstetrics & Gynecology. 2005;105(5 Pt 1):991–8. doi: 10.1097/01.AOG.0000157109.05453.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Connor MJ, Whaley SE. Brief intervention for alcohol use by pregnant women. American Journal of Public Health. 2007;97(2):252–8. doi: 10.2105/AJPH.2005.077222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynolds KD, et al. Evaluation of a self-help program to reduce alcohol consumption among pregnant women. International Journal of the Addictions. 1995;30(4):427–43. doi: 10.3109/10826089509048735. [DOI] [PubMed] [Google Scholar]
- 19.Handmaker NS, Hester RK, Delaney HD. Videotaped training in alcohol counseling for obstetric care practitioners: a randomized controlled trial. Obstetrics & Gynecology. 1999;93(2):213–8. doi: 10.1016/s0029-7844(98)00377-9. [DOI] [PubMed] [Google Scholar]
- 20.Goler NC, et al. Substance abuse treatment linked with prenatal visits improves perinatal outcomes: a new standard.[Erratum appears in J Perinatol. 2009 Feb;29(2):181] Journal of Perinatology. 2008;28(9):597–603. doi: 10.1038/jp.2008.70. [DOI] [PubMed] [Google Scholar]
- 21.Miller W, Rollnick S. Motivational Interviewing: Preparing People to Change Addictive Behavior. New York: Guilford Press; 1991. [Google Scholar]
- 22.Miller W, Zweben A, DiClemente CC, Rychtarik RG. Project MATCH Monograph Series. Vol. 2. NIAAA; Rockville, MD: 1992. Motivational Enhancement Theory Manual. [Google Scholar]
- 23.Samet JH, Rollnick S, Barnes H. Beyond CAGE. A brief clinical approach after detection of substance abuse. Archives of Internal Medicine. 1996;156(20):2287–93. doi: 10.1001/archinte.156.20.2287. [DOI] [PubMed] [Google Scholar]
- 24.Day NL, et al. Prenatal exposure to alcohol: effect on infant growth and morphologic characteristics. Pediatrics. 1989;84(3):536–41. [PubMed] [Google Scholar]
- 25.Robles N, Day NL. Recall of alcohol consumption during pregnancy. J Stud Alcohol. 1990;51(5):403–7. doi: 10.15288/jsa.1990.51.403. [DOI] [PubMed] [Google Scholar]
- 26.Cox J, Holden J, Sagovsky R. Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression Scale. British Journal of Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 27.Schaper AM, et al. Use of the Edinburgh Postnatal Depression Scale to identify postpartum depression in a clinical setting. J Reprod Med. 1994;39(8):620–4. [PubMed] [Google Scholar]
- 28.Murray L, Carothers AD. The validation of the Edinburgh Post-natal Depression Scale on a community sample. British Journal of Psychiatry. 1990;157:288–290. doi: 10.1192/bjp.157.2.288. [DOI] [PubMed] [Google Scholar]
- 29.Bernstein JA, Bernstein E, Heeren TC. Mechanisms of change in control group drinking in clinical trials of brief alcohol intervention: implications for bias toward the null. Drug & Alcohol Review. 2010;29(5):498–507. doi: 10.1111/j.1465-3362.2010.00174.x. [DOI] [PubMed] [Google Scholar]
- 30.Kypri K. Methodological issues in alcohol screening and brief intervention research. Substance Abuse. 2007;28(3):31–42. doi: 10.1300/J465v28n03_04. [DOI] [PubMed] [Google Scholar]
- 31.Ondersma SJ, Svikis DS, Schuster CR. Computer-based brief intervention a randomized trial with postpartum women.[Erratum appears in Am J Prev Med. 2007 Jun;32(6):549] American Journal of Preventive Medicine. 2007;32(3):231–8. doi: 10.1016/j.amepre.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ondersma SJ, et al. Motivation Enhancement Therapy with pregnant substance-abusing women: does baseline motivation moderate efficacy? Drug & Alcohol Dependence. 2009;101(1–2):74–9. doi: 10.1016/j.drugalcdep.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tzilos GK, et al. Psychosocial factors associated with depression severity in pregnant adolescents. Archives of Women’s Mental Health. 2012;15(5):397–401. doi: 10.1007/s00737-012-0296-9. [DOI] [PMC free article] [PubMed] [Google Scholar]